Calcimycin restricts Wnt/β-catenin–regulated S100A4 expression, leading to reduced S100A4-mediated cell migration and invasion in colon cancer cells, as well as to inhibition of metastasis formation in xenografted mice.
Abstract
The calcium-binding protein S100A4 is a central mediator of metastasis formation in colon cancer. S100A4 is a target gene of the Wnt/β-catenin pathway, which is constitutively active in the majority of colon cancers. In this study a high-throughput screen was performed to identify small-molecule compounds targeting the S100A4-promoter activity. In this screen calcimycin was identified as a transcriptional inhibitor of S100A4. In colon cancer cells calcimycin treatment reduced S100A4 mRNA and protein expression in a dose- and time-dependent manner. S100A4-induced cellular processes associated with metastasis formation, such as cell migration and invasion, were inhibited by calcimycin in an S100A4-specific manner. Calcimycin reduced β-catenin mRNA and protein levels despite the expression of Δ45-mutated β-catenin. Consequently, calcimycin inhibited Wnt/β-catenin pathway activity and the expression of prominent β-catenin target genes such as S100A4, cyclin D1, c-myc, and dickkopf-1. Finally, calcimycin treatment of human colon cancer cells inhibited metastasis formation in xenografted immunodeficient mice. Our results demonstrate that targeting the expression of S100A4 with calcimycin provides a functional strategy to restrict cell motility in colon cancer cells. Therefore calcimycin may be useful for studying S100A4 biology, and these studies may serve as a lead for the development of treatments for colon cancer metastasis.
INTRODUCTION
S100A4 is a ubiquitous small, calcium-binding protein that enables cell migration and invasion to increase cell motility (Garrett et al., 2006). Consequently, S100A4 expression is up-regulated during wound healing, neurite outgrowth, fibrosis, or neovascularization—all physiological processes that rely on increased cell motility. However, overexpression of S100A4 is often correlated with pathological conditions such as epithelial–mesenchymal transition, tumor outgrowth, and metastasis formation (Schneider et al., 2008; Boye and Maelandsmo, 2010).
In colon cancer distant metastases are the major cause for cancer death, rendering this disease the second-most-frequent cause of cancer-related death in the Western world (Jemal et al., 2008). S100A4 expression is significantly associated with metastasis formation in colorectal cancer patients, and the expression of S100A4 represents a significant prognostic marker in colorectal carcinoma (Gongoll et al., 2002; Cho et al., 2005; Stein et al., 2006). Despite S100A4 increasing the metastatic potential of the cell, S100A4 transgenic mice are not phenotypically changed (Ambartsumian et al., 1996). However, when these are crossed with spontaneous tumor-forming mice, S100A4 overexpression leads to highly aggressive primary tumors and formation of metastasis (Davies et al., 1996). Highly metastatic mouse mammary carcinoma cells injected into S100A4 null mice are unable to form metastases (Grum-Schwensen et al., 2005). These observations suggest that S100A4 is not simply a marker for metastatic disease but rather has a causal role in mediating this process.
Tumor growth and metastasis depend on increased cell migration, invasion, and angiogenesis. S100A4 is involved in all of these processes by interaction with multiple proteins in the cytoplasm and extracellular space. For instance, intracellular S100A4 increases cell migration by rearranging proteins of the cytoskeleton. S100A4 binds tropomyosin and nonmuscle myosin II in a Ca2+-dependent manner and inhibits the actin-regulated ATPase activity of myosin II (Kriajevska et al., 1994; Ford et al., 1997). It thus promotes the disassembly of myosin filaments and inhibits their reassembly (Ford et al., 1997; Li et al., 2003). S100A4 is located at the leading edge of migrating cells (Kim and Helfman, 2003), where it induces the formation of flexible protrusions. Moreover, in the presence of a chemoattractant, S100A4 enhances directed migration (Li and Bresnick, 2006). Thus the S100A4–myosin II interaction not only increases cell motility, but it also enhances cell polarization and directed migration.
S100A4 expression is associated with reduced expression levels of E-cadherin, thus loosening epithelial cell adhesion (Keirsebilck et al., 1998). In addition, S100A4 reduces cell adhesion by interacting with liprin-β1 (Kriajevska et al., 2002). S100A4 can access the extracellular space by a yet-unknown mechanism, by which it interacts with endothelial-bound annexin II and thus enhances angiogenesis (Ambartsumian et al., 2001; Kriajevska et al., 2002). Extracellular S100A4 can also bind to the receptor for glycation end products and thereby activate matrix metalloproteinase-13 (MMP-13) expression (Yammani et al., 2006). Furthermore, up-regulation of MMP-2 and MMP-9 expression by extracellular S100A4 allows cell invasion and thus promotes metastasis formation (Mathisen et al., 2003; Saleem et al., 2006).
S100A4 gene transcription in colon cancer cells is regulated by the canonical Wnt/β-catenin pathway, one of the most frequently deregulated pathways in colon cancer (Stein et al., 2006). The pathway strictly controls the cytoplasmic level of β-catenin via a destruction complex. In this complex β-catenin is phosphorylated and thus marked for proteasomal degradation (Barker and Clevers, 2006). Mutations within this pathway lead to intracellular accumulation of β-catenin, which then translocates to the nucleus and activates β-catenin/T cell factor (TCF) target gene transcription (Giles et al., 2003; Stein et al., 2006).
Because S100A4 plays such a central role in the process of metastasis formation, the inhibition of S100A4 expression provides a promising strategy for novel antimetastastic treatments (Sack and Stein, 2009). To identify potential S100A4 transcription inhibitors, we performed a high-throughput screen for compounds that inhibited S100A4-promoter activity. Thus we identified calcimycin as a novel inhibitor of S100A4 transcription, which overcomes a constitutively active Wnt/β-catenin pathway and significantly restricts S100A4-induced cell motility in colon cancer cells.
RESULTS
Identification of S100A4 transcription inhibitor calcimycin
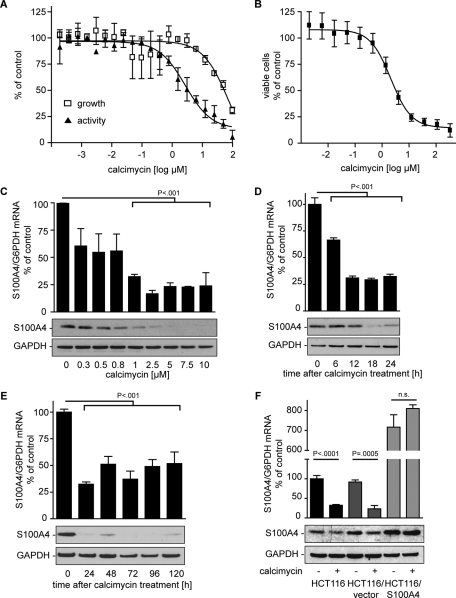
Calcimycin was identified as an inhibitor for S100A4 expression in a high-throughput screen of the Library of Pharmacologically Active Compounds (LOPAC) 1280, a collection of 1280 pharmacologically active compounds that have shown effects on major drug target classes such as kinases, G proteins, and ion channels. HCT116/S100A4pLUC cells expressing S100A4-promoter-driven firefly luciferase were exposed to 20 twofold dilutions of calcimycin. After 24 h, luciferase activity was determined as a read-out for S100A4-promoter activity (Figure 1A). From the high-throughput data it was found that calcimycin inhibited luciferase activity in a concentration-dependent manner, with an EC50 of 2.7 μM (95% confidence interval [CI], 1.0–4.0 μM). At the same time growth inhibition was assessed from the high-throughput data, yielding an IC50 of ∼80 μM (95% CI, 15.2–403.9 μM).
FIGURE 1:
S100A4-promoter activity is inhibited by calcimycin in a concentration- and time-dependent manner. Luciferase activity and cell viability of calcimycin-treated HCT116/S100A4pLUC cells were determined after 24 h. S100A4/G6PDH mRNA ratios were determined by qRT-PCR. S100A4 and GAPDH protein levels were measured by Western blot. (A) High-throughput screening data of calcimycin inhibiting S100A4 promoter–driven reporter activity and cell viability. (B) Cell viability of HCT116 cells treated with increasing concentrations of calcimycin for 24 h is reduced in s concentration-dependent manner (C) S100A4 mRNA and protein levels are reduced in HCT116 cells treated with increasing concentrations of calcimycin. (C, D) S100A4 expression is reduced in HCT116 cells treated with a single dose of 1 μM calcimycin for the time indicated. Data represent mean ± SE (n = 4). Statistical significance was analyzed by two-sided ANOVA and Bonferroni post hoc multiple comparison test. (E) CMV promoter–driven S100A4 expression is not affected by calcimycin treatment. Data represent mean ± SE (n = 3). Statistical significance was analyzed by Student's t test.
To define cytotoxic side effects potentially affecting further experiments, the effect of calcimycin on cell viability of HCT116 cells was analyzed more precisely by manual pipetting. Exposure of HCT116 cells to calcimycin concentrations ranging from 2 nM to 300 μM for 24 h resulted in a reduction of cell viability in a concentration-dependent manner with an IC50 of 2.1 μM (95% CI, 1.5–2.2 μM; Figure 1B).
We next analyzed the ability of calcimycin to reduce endogenous S100A4 expression in HCT116 cells. Exposure of HCT116 cells to increasing concentrations of calcimycin for 24 h resulted in a concentration-dependent reduction of S100A4 mRNA and protein. Treatment with 1 μM calcimycin reduced S100A4 expression to 30% of that for the solvent-treated control (Figure 1C). Cell viability was not affected by this concentration of calcimycin after 24 h. Therefore we chose the calcimycin concentration of 1 μM for use in the following studies.
S100A4 expression is inhibited in a time-dependent manner by calcimycin
To assess the kinetics of the calcimycin effect, HCT116 cells were exposed to 1 μM calcimycin, and S100A4 expression was analyzed every 6 h. S100A4 mRNA was reduced in a time-dependent manner. After 12 h of treatment a basal reduction level of ∼30% of the solvent-treated control was observed (Figure 1D). After 18 h of treatment the S100A4 protein levels were also reduced. S100A4 expression inhibition continued for 5 consecutive days after a single dose of 1 μM calcimycin (Figure 1E). Similar to HCT116 cells, HCT116/vector cells showed reduced S100A4 mRNA to ∼30% of solvent-treated control cells after 1 μM calcimycin treatment for 24 h (Figure 1F). In contrast, HCT116/S100A4 cells showed no significant reduction in S100A4 mRNA or protein upon calcimycin treatment. These cells overexpressed S100A4 cDNA under the control of a cytomegalovirus (CMV) promoter, which was not targeted by calcimycin treatment.
S100A4-induced cell migration and invasion is inhibited by calcimycin
We previously showed that S100A4 expression in colon tumors significantly correlates with metastasis formation (Stein et al., 2006). Moreover, S100A4 drives metastasis formation by increasing cell migration and invasion (Garrett et al., 2006). Therefore we next examined S100A4-induced cell motility in the presence of calcimycin. Exposure of HCT116 cells to calcimycin significantly reduced the number of migrated cells to 20% of that for solvent-treated HCT116 cells (Figure 2A). Similarly, calcimycin treatment inhibited cell migration of HCT116/vector cells to 16% of that for the control cells. In contrast, HCT116/S100A4 cells showed no significant reduction in cell migration upon calcimycin treatment. Cell invasion of calcimycin-treated HCT116 and HCT116/vector cells was inhibited to <30% of that for the solvent-treated control cells (Figure 2B). In contrast, cell invasion of HCT116/S100A4 cells was not significantly changed upon calcimycin treatment. Directed migration analyzed by wound-healing assay revealed that calcimycin clearly inhibited wound closure in HCT116 and HCT116/vector cells but not in HCT116/S100A4 cells (Figure 2C). In summary, calcimycin displayed an antimigratory and anti-invasive effect in HCT116 cells, and this effect was overcome by the exogenous overexpression of S100A4.
FIGURE 2:
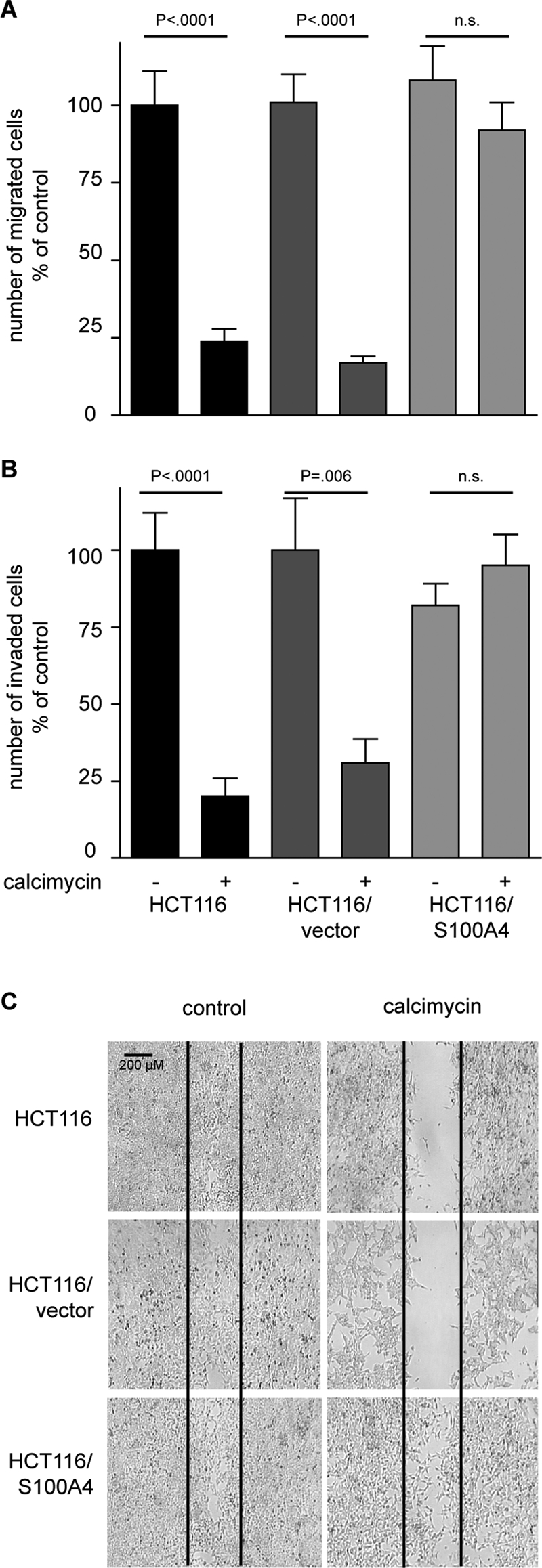
S100A4 induced cell motility is inhibited by calcimycin. Cell migration was determined with Boyden chamber and wound-healing assay. Cell invasion was measured with a Matrigel-covered Boyden chamber assay (A) Cell migration is inhibited in HCT116 and HCT116/vector cells but not in HCT116/S100A4 cells when treated with calcimycin. (B) Cell invasion is inhibited in calcimycin-treated HCT116 and HCT116/vector cells but not in HCT116/S100A4 cells. Data represent mean ± SE (n = 5). Statistical significance was analyzed with Student's t test. (C) Directed migration is inhibited in calcimycin-treated HCT116 and HCT116/vector cells but not in HCT116/S100A4 cells. Microphotographs were taken with 10× magnification 4 d after wound was entered.
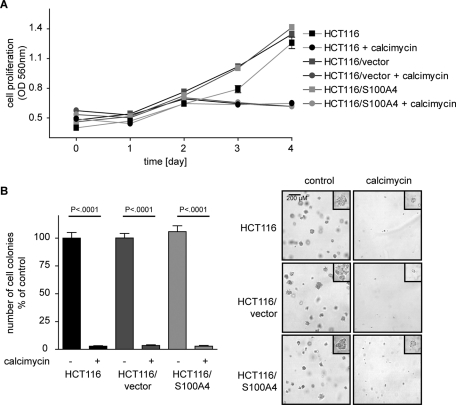
Calcimycin inhibits anchorage-dependent and anchorage-independent cell proliferation
We next analyzed cell proliferation under calcimycin treatment. Exposure of HCT116, HCT116/vector, and HCT116/S100A4 cells to a single dose of 1 μM calcimycin resulted in a clear inhibition of anchorage-dependent proliferation after day 3 (Figure 3A). Calcimycin further inhibited anchorage-independent growth as analyzed by colony formation. On exposure to calcimycin the number of colonies formed by HCT116, HCT116/vector, and HCT116/S100A4 cells was significantly reduced to <5% of that for solvent-treated control cells (Figure 3B). Furthermore, as shown in the insets of the microphotographs, the size of colonies was clearly reduced by calcimycin treatment. Proliferation of cells that exogenously overexpress S100A4 was also affected by calcimycin treatment. Thus the antiproliferative effect of calcimycin on anchorage-dependent and anchorage-independent growth was not overcome by an increased S100A4 expression level.
FIGURE 3:
Calcimycin inhibits anchorage-dependent and anchorage-independent cell growth. Anchorage-dependent growth was determined by MTT assay; anchorage-independent growth was measured in a soft-agar colony formation assay. (A) Anchorage-dependent cell proliferation of HCT116, HCT116/vector, and HCT116/S100A4 cells was reduced upon calcimycin treatment ( , solvent-treated HCT116;
, solvent-treated HCT116;  , calcimycin-treated HCT116;
, calcimycin-treated HCT116;  , solvent-treated HCT116/vector;
, solvent-treated HCT116/vector;  , calcimycin-treated HCT116/vector;
, calcimycin-treated HCT116/vector;  , solvent-treated HCT116/S100A4;
, solvent-treated HCT116/S100A4;  , calcimycin-treated HCT116/S100A4 cells). (B) Anchorage-independent cell proliferation of HCT116, HCT116/vector, and HCT116/S100A4 cells was inhibited by calcimycin. Number of colonies was counted on day 7, when microphotographs were taken with 10× and 40× (insets) magnification. Data represent mean ± SE (n = 3). Statistical significance was determined by Student's t test.
, calcimycin-treated HCT116/S100A4 cells). (B) Anchorage-independent cell proliferation of HCT116, HCT116/vector, and HCT116/S100A4 cells was inhibited by calcimycin. Number of colonies was counted on day 7, when microphotographs were taken with 10× and 40× (insets) magnification. Data represent mean ± SE (n = 3). Statistical significance was determined by Student's t test.
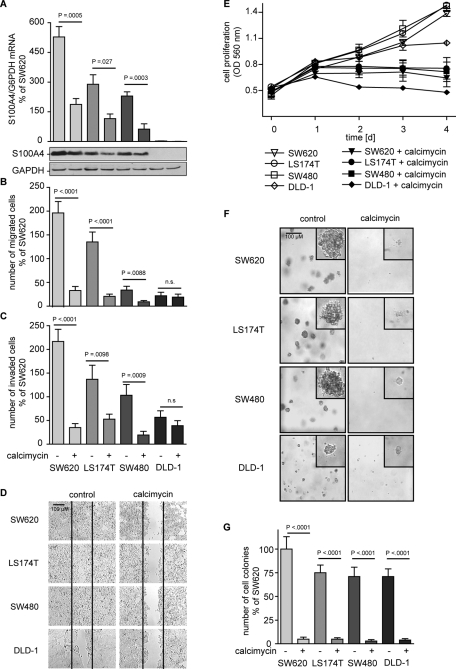
Calcimycin inhibits S100A4 expression, cell motility, and proliferation in other human colon cancer cell lines
We next analyzed calcimycin effects in other human colon cancer cell lines. Exposure of SW620, LS174T, and SW480 cells to 1 μM calcimycin for 24 h reduced the S100A4 mRNA level to <40% of that for the respective solvent-treated controls (Figure 4A). S100A4 protein in these cells was clearly reduced upon calcimycin treatment. In DLD-1 cells no S100A4 mRNA and protein expression was detected, and therefore no further effect by calcimycin was possible.
FIGURE 4:
Calcimycin inhibits S100A4 expression, cell motility, and proliferation in human colon cancer cell lines. S100A4/G6PDH mRNA ratios were determined by qRT-PCR. S100A4 and GAPDH protein levels were measured by Western blot. Cell migration was determined with Boyden chamber and wound-healing assay. Cell invasion was measured with a Matrigel-covered Boyden chamber assay. Anchorage-dependent growth was determined by MTT assay; anchorage-independent growth was measured in a soft-agar colony formation assay. (A) S100A4 expression was reduced upon calcimycin treatment. (B) Calcimycin treatment inhibited cell migration. (C) Cell invasion was inhibited upon calcimycin treatment. (D) Calcimycin inhibited directed migration. (E) Calcimycin inhibited cell proliferation. (F, G) Calcimycin reduced the size and number of colonies formed, respectively. Number of colonies was counted on day 7, when microphotographs were taken with 10× and 40× (insets) magnification. Data represent mean ± SE (n = 3). Statistical significance was determined by Student's t test.
Exposure of SW620, LS174T, and SW480 cells to 1 μM calcimycin for 24 h inhibited cell migration to <25% of that for the respective solvent-treated controls (Figure 4B). DLD-1 cells presented the lowest migration rate, which was not further affected by calcimycin treatment. Cell invasion of SW620, LS174T, and SW480 cells was reduced to <30% of that for the respective solvent-treated controls by calcimycin treatment (Figure 4C). The low cell invasion rate of DLD-1 cells was not significantly changed upon calcimycin treatment. In the wound-healing assay solvent-treated SW620, LS174T, and SW480 cells were able to close the wound by day 4 after wound insertion (Figure 4D). In contrast, in solvent-treated DLD-1 cells wound closure was not completed by day 4, which was consistent with their decreased migration and invasion rates. Following a single dose of 1 μM calcimycin, wound closure, as measurement for directed migration, was impaired in all four colon cancer cell lines for 4 d post–wound insertion.
Anchorage-dependent cell proliferation was inhibited upon calcimycin treatment in all four colon cancer cell lines as measured on day 4 (Figure 4E). In a colony formation assay solvent-treated SW620, LS174T, and SW480 cells formed large colonies by day 7 (Figure 4F). Colony formation was inhibited in all four colon cancer cell lines following calcimycin treatment. Colonies of solvent-treated DLD-1 cells were smaller than colonies of other solvent-treated colon cancer cells lines. However, the size of DLD-1 colonies was still reduced upon calcimycin treatment. Quantification of formed colonies revealed that calcimycin treatment significantly reduced the number of colonies in all four colon cancer cell lines to <10% of the respective-solvent treated controls (Figure 4G). In summary, calcimycin inhibited cell migration and invasion of various colon cancer cell lines in relation to their S100A4 expression level. Cell proliferation was inhibited independent of the S100A4 expression level of S100A4 in these cells.
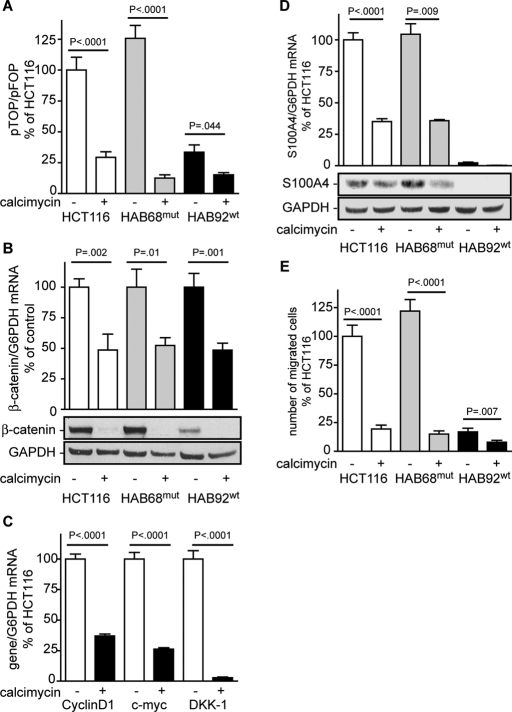
Calcimycin inhibits constitutively active Wnt/β-catenin pathway signaling
We previously reported that S100A4 is a β-catenin target gene (Stein et al., 2006). Therefore we investigated Wnt/β-catenin pathway activity using the TOP/FOPflash reporter assay to assess the mechanism underlying calcimycin inhibition of S100A4 expression. We used HCT116 cells that are heterozygous for mutated β-catenin. The mutated β-catenin lacks serine 45, the initial phosphorylation site needed for proteasomal degradation (Amit et al., 2002). Consistent with this mutation, in these cells expression of the TOPflash reporter is increased. Treatment of HCT116 cells with calcimycin reduced the TOPflash reporter activity to <30% upon calcimycin treatment (Figure 5A). In HAB68mut cells, which are homozygous for this β-catenin mutation, calcimycin treatment reduced TOPflash reporter activity to <10% of that for solvent-treated HCT116 cells. TOPflash reporter activity of solvent-treated HAB92wt cells, which are homozygous for wild-type β-catenin, was <30% of the activity found in cells that bear a constitutively active Wnt/β-catenin pathway due to mutated β-catenin. Moreover, calcimycin treatment of HAB92wt cells further reduced this TOPflash reporter activity to 12%.
FIGURE 5:
Calcimycin inhibits constitutively active Wnt/β-catenin pathway. Activity of the Wnt/β-catenin pathway was analyzed by TOP/FOPflash reporter assay. Quantification of mRNA was performed with qRT-PCR; protein expression was determined by Western blot. (A) Wnt/β-catenin pathway was reduced in calcimycin-treated HCT116 cells and in derivative cell lines HAB68mut and HAB92wt. (B) The expression of β-catenin mRNA and protein was reduced in calcimycin-treated in HCT116, HAB68mut, and HAB92wt cells. (C) mRNA levels of β-catenin/TCF transcription target genes cyclin D1, c-myc, and DKK-1 were reduced upon calcimycin treatment. (D) S100A4 expression was inhibited in HCT116, HAB68mut, and HAB92wt cells treated with calcimycin. (E) Cell migration of HCT116, HAB68mut, and HAB92wt cells was decreased upon calcimycin treatment. Data represent mean ± SE (n = 3). Statistical significance was analyzed by Student's t test.
We next analyzed the expression level of β-catenin, which represents the central player of this pathway. In calcimycin-treated HCT116, HAB68mut, and HAB92wt cells the β-catenin mRNA levels were reduced to <50% of that for solvent-treated HCT116 cells (Figure 5B). Consistent with this result, the β-catenin protein level was diminished upon calcimycin treatment of these cells.
Reduced β-catenin levels should result in reduced target gene transcription. Therefore we analyzed the expression levels of prominent β-catenin/TCF target genes such as cyclin D1 (Shtutman et al., 1999), c-myc (He et al., 1998), and dickkopf-1 (DKK-1; Gonzalez-Sancho et al., 2005). In calcimycin-treated HCT116 cells the expression of cyclin D1, c-myc, and DKK-1 was inhibited to 35, 25, and 2% of the solvent-treated controls, respectively (Figure 5C).
We analyzed the effect of calcimycin on S100A4 expression in the presence of mutated or wild-type β-catenin and found that S100A4 mRNA was reduced to 35% in HAB68mut and HCT116 cells and was hardly detectable in HAB92wt cells (Figure 5D). In parallel, calcimycin treatment reduced S100A4 protein levels in HCT116 and HAB68mut cells, whereas no S100A4 protein was detectable in HAB92wt cells. Cell migration in HCT116 and HAB68mut cells was inhibited to the extent of HAB92wt cells, which was <25% of that for solvent-treated HCT116 cells (Figure 5E). These results provide evidence that calcimycin restricts the Wnt/β-catenin pathway–driven S100A4 expression and thus S100A4-induced cell motility.
Calcimycin inhibits the metastatic potential of human colon cancer cells in xenografted mice
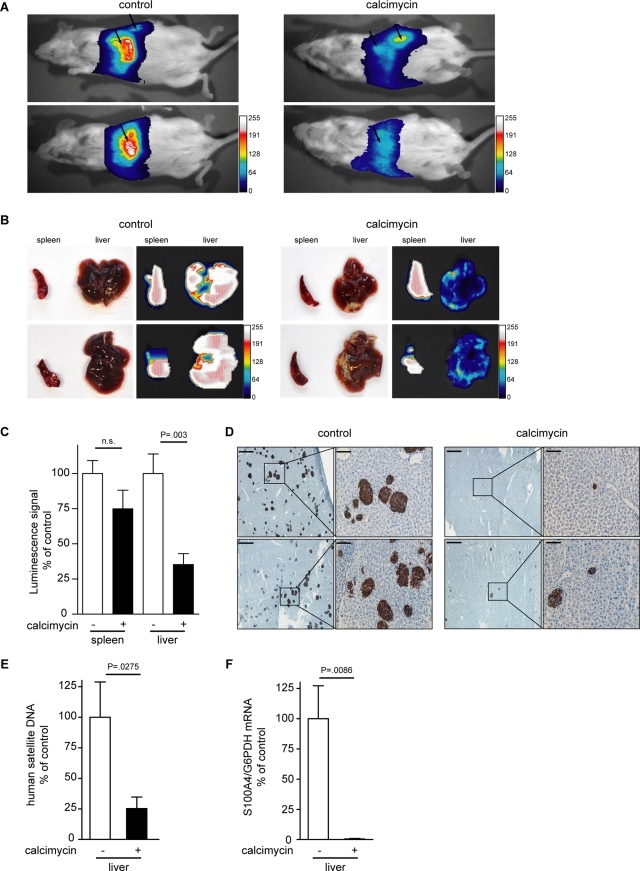
To test the effect of calcimycin on the ability of cells to form metastasis in vivo, we used HCT116/LUC cells, which stably expressed the firefly luciferase protein and therefore allowed the detection of metastases via noninvasive in vivo luminescence imaging. Cells were treated with 1 μM calcimycin or solvent for 24 h and subsequently inoculated intrasplenically into nonobese diabetic/severe immune-deficient (NOD/SCID)–IL2R− mice. Six days posttransplantation, luminescence signals were detected in the lateral and ventral abdominal cavity, where the spleen and liver are situated, respectively (Figure 6A). In control mice, the ventral luminescence signal was stronger than in mice from the calcimycin group.
FIGURE 6:
Calcimycin reduces the metastatic potential of human colon cancer cells in xenografted mice. Mice were intrasplenically injected with calcimycin- or solvent-treated HCT116/LUC cells. Two representative mice per group are shown. (A) Bioluminescence was measured 6 d posttransplantation in the region of spleen and liver (metastases target organ). (B) Spleen tumor and liver metastases are visualized by bioluminescence imaging. (C) No significant difference in the luminescence signal of spleen tumors was found between mice injected with control or calcimycin-treated cells. The liver luminescence signal was significantly reduced in the calcimycin group. Data represent mean ± SE (n = 6). Statistical significance was analyzed by Student's t test. (D) Immunohistochemistry for human cytokeratin-19 identified smaller and fewer micrometastases in the calcimycin group. (E) The amount of human satellite DNA was reduced in livers from the calcimycin group. (F) The S100A4 mRNA expression normalized to hG6PDH was absent in livers of mice of the calcimycin group. Data represent mean ± SE (n > 3). Statistical significance was analyzed by Student's t test.
To investigate the origin of luminescence, the spleen and the liver were dissected. All animals developed tumors in the spleen, which were clearly visible in the bright-field analysis (Figure 6B). A strong luminescence signal originated from the spleen tumor, indicating that the tumor was formed by HCT116/LUC cells. Bright-field analysis of the resected livers revealed no clear differences between mice of the control and calcimycin groups. However, the luminescence signal from livers of control mice was clearly stronger than that from livers of calcimycin mice.
Quantification of the luminescence signal revealed no significant differences in the signal intensity of spleen tumors from mice of the control and calcimycin groups (Figure 6C). In contrast, the luminescence signal of livers was reduced to 30% in mice of the calcimycin group compared with control mice.
From the reduced liver luminescence signal in mice of the calcimycin group, we hypothesized that the calcimycin treatment hampered HCT116/LUC cells from forming metastases in the liver tissue. We therefore performed immunohistological staining for human cytokeratin-19 in liver cryosections of mice injected with control and calcimycin-treated cells. In the livers of control mice the incidence of micrometastases was clearly higher than in those of mice of the calcimycin group (Figure 6D). Moreover, the size of the micrometastases was reduced in livers of mice injected with calcimycin-treated cells.
We next quantified the amount of human DNA in the liver sections of control and calcimycin mice by quantitative PCR amplification of human satellite DNA as previously described (Becker et al., 2002). In livers from mice of the calcimycin group the amount of human DNA was reduced to ∼25% of that of control mice, indicating that the calcimycin treatment indeed inhibited human colon cancer cells from forming liver metastases (Figure 6E). Analysis of the human-specific S100A4 expression in liver metastases of the xenografted mice revealed that in livers from mice of the calcimycin group the S100A4 mRNA was hardly detectable, in contrast to the control mice (Figure 6F). From these data we conclude that the treatment of colon cancer cells with calcimycin restricted their potential to form liver metastases in vivo.
DISCUSSION
Intensive research has demonstrated the central role of S100A4 in the process of cancer metastasis, which qualifies S100A4 as a potentially promising target for therapeutic intervention against metastasis (Sherbet, 2009; Boye and Maelandsmo, 2010). Most of the work concerning S100A4 has concentrated on the elucidation of the mechanism by which this molecule drives metastasis. Less work has focused on the inhibition of S100A4 to reduce S100A4-induced cell motility. Here we report the calcium ionophore calcimycin as an inhibitor of S100A4 transcription in colon cancer cells. We show that calcimycin treatment inhibits a constitutively active Wnt/β-catenin pathway, thereby inhibiting S100A4 expression and leading to restricted S100A4-induced cell migration and invasion in vitro and in vivo.
In a high-throughput screen we identified calcimycin as one of the most effective inhibitors of S100A4-promoter activity. In this screen we used HCT116 cells in which the S100A4-promoter was highly active. Inhibition of S100A4-promoter activity by calcimycin indicated that calcimycin targets S100A4 at the transcription level. Calcimycin reduced S100A4 mRNA levels in a concentration- and time-dependent manner in colon cancer cell lines with increased basal S100A4 expression levels. In line with our observation in human colon cancer cells, calcimycin has been reported to reduce the S100A4 mRNA level in mouse mammary adenocarcinoma cells, as well as in human monocytes and lymphocytes (Grigorian et al., 1994). We further showed that S100A4 protein levels were also suppressed in a concentration- and time-dependent manner following calcimycin treatment in several colon cancer cell lines.
Calcimycin is an ionophorous, polyether antibiotic isolated from Streptomyces chartreusensis. Calcimycin facilitates the transport of divalent cations across the membrane, which makes it a useful tool to study calcium signaling (Pressman, 1976). Because calcimycin elevates intracellular calcium levels (Gwak et al., 2006), one would expect that calcimycin treatment would increase S100A4 protein activity, which depends on calcium ions (Santamaria-Kisiel et al., 2006). However, in our study, we did not see an increased migratory or invasive phenotype in exogenously S100A4-overexpressing cells that were treated with calcimycin. Although we cannot completely exclude that calcimycin caused increased S100A4 protein activity, we definitely show here that reducing the overall expression level of S100A4 significantly inhibits S100A4-induced cell motility.
S100A4 protein drives metastasis by interaction with a multitude of partner proteins, leading to increased cell migration and invasion (Belot et al., 2002; Stein et al., 2006). Consequently, down-regulation of S100A4 expression by calcimycin restricted cell migration in colon cancer cells. This is in line with observations from RNA interference experiments in which knockdown of S100A4 mRNA was shown to reduce cell migration (Gao et al., 2005; Tabata et al., 2009). Of interest, calcimycin was not able to suppress cell migration or invasion in cells that exogenously overexpressed S100A4. From these observations we conclude that the antimigratory and anti-invasive effect of calcimycin was caused by the down-regulation of S100A4 expression from its native promoter.
Knockdown of S100A4 mRNA levels with short hairpin RNA (shRNA) was shown to inhibit cell proliferation in vitro and to reduce tumor growth and metastasis in vivo (Shi et al., 2006). Moreover, in gastric cancer cells, shRNA knockdown of S100A4 increased the occurrence of apoptosis (Hua et al., 2009). In our study, knockdown of S100A4 expression by treatment with calcimycin was accompanied by reduced cell proliferation. Exogenous overexpression of S100A4 was ineffective in overcoming the antiproliferative effect of calcimycin, which suggests that this effect is independent of the S100A4 expression level.
S100A4 is a target gene of the Wnt/β-catenin pathway (Stein et al., 2006). We found that calcimycin treatment inhibits β-catenin transcription, which leads to the inhibition of the Wnt/β-catenin pathway activity in colon cancer cells and explains its inhibitory effect on S100A4 expression. Of interest, calcimycin was able to overcome a constitutively active Wnt/β-catenin pathway, since it reduced β-catenin also in cells that are heterozygous or even homozygous for Δ45-mutated β-catenin. In line with our findings, calcimycin treatment also abolished Wnt/β-catenin pathway activity in HEK293 cells that stably expressed the TOPflash reporter plasmid (Gwak et al., 2006).
In line with reduced Wnt/β-catenin pathway activity, we found that S100A4 and other β-catenin/TCF target genes such as cyclin D1, c-myc, and DKK-1 were down-regulated following calcimycin treatment. Cyclin D1 and c-myc are known oncogenes, and their overexpression causes increased cell proliferation (He et al., 1998; Shtutman et al., 1999). Knockdown of β-catenin expression levels by shRNA was shown to result in reduced levels of cyclin D1 and c-myc, and this reduction was associated with reduced cell proliferation (Huang et al., 2007). Consistent with this observation, in our study calcimycin reduced the cyclin D1 and c-myc mRNA levels, which might explain the observed inhibition of anchorage-dependent and anchorage-independent cell proliferation following calcimycin treatment.
Intrasplenic inoculation of S100A4 overexpressing cells was shown to induce liver metastases in xenografted mice (Stein et al., 2006). Because S100A4 is a major regulator of colon cancer metastasis, its inhibition should result in restricted metastasis formation. Indeed, we found in this study that calcimycin treatment of human colon cancer cells restricted their potential to form liver metastases in vivo. The number and size of liver metastases formed by calcimycin treated cells were significantly reduced. Bioluminescence imaging, as well as immunohistochemistry, visualized larger and more frequent micrometastases in livers of control animals than in livers of mice from the calcimycin group. The amount of human satellite DNA was reduced in livers of calcimycin mice, supporting the observation that fewer human colon cancer cells invaded the liver tissue. Thus calcimycin treatment restricted the metastatic potential of colon cancer cells in vivo.
We are aware that by interfering with the Wnt/β-catenin pathway, calcimycin will have several different functions and actions within the cell. Among these functions we show that calcimycin inhibits S100A4 expression and that this inhibition leads to reduced cell motility. We showed that HCT116/S100A4 cells migrated and invaded despite the calcimycin treatment. The inhibitory effect of calcimycin on cell migration and invasion was overcome by exogenous overexpression of S100A4. Thus we provide evidence that the antimigratory and anti-invasive effect of calcimycin was specific to the calcimycin-mediated S100A4 expression inhibition.
In conclusion, our study reports calcimycin as a novel inhibitor of S100A4-promoter activity, which leads to reduced S100A4 expression and thus impairs S100A4-induced cell motility and metastasis. Because metastasis is the major cause of colon cancer death, there is an urgent need for antimetastatic treatment. S100A4, as a mediator of this disease progression, provides a promising therapeutic target. We provide evidence that targeting S100A4 expression by application of calcimycin restricts S100A4-induced cell migration and invasion in vitro and in vivo. Therefore we show that calcimycin is a useful compound not only to study S100A4 biology, but also to form the basis for the development of treatments against colon cancer metastasis.
MATERIALS AND METHODS
Cell lines and treatments
The human colon cancer cell line HCT116 and its derivatives HAB-68mut and HAB-92wt were kindly provided by Todd Waldman (Georgetown University, Washington, DC). HCT116 cells are heterozygous for gain-of-function mutated Δ45-β-catenin. In HAB-68mut and HAB-92wt the wild-type or mutated allele of β-catenin was deleted, respectively, by homologous recombination (Kim et al., 2002). These cell lines were expanded briefly in culture and cryopreserved in multiple replicate vials. The cell banks were tested by PCR and culture methods and found to be free of mycoplasma. To authenticate the HAB-68mut and HAB-92wt cell lines as HCT116 derivatives, short tandem repeat (STR) genotyping was performed in August 2010 using the ABI Identifier Kit (Life Technologies, Darmstadt, Germany). The STR genotypes were consistent with published genotypes for HCT116. All cells were tested for the correct β-catenin genotype by restriction fragment length polymorphism as previously described (Stein et al., 2006). Cells were cultured in RPMI-1640 medium supplemented with 10% fetal calf serum (PAA Laboratories, Pasching, Austria) in a humidified incubator at 37°C and 5% CO2. HCT116 cells were transfected to express S100A4-promoter controlled firefly luciferase (HCT116/S100A4pLUC cells), CMV promoter–controlled firefly luciferase (HCT116/LUC), CMV promoter–controlled S100A4 cDNA (HCT116/S100A4 cells), or the empty vector as control (HCT116/vector cells). The S100A4 promoter was a kind gift from David Allard (University of Exeter and Plymouth, Exeter, United Kingdom) and comprised the sequence from −1487 to +33 base pairs around the S100A4 transcription start site (Hernan et al., 2003). The S100A4 cDNA was kindly provided by Claus Heizmann (University of Zurich, Zurich, Switzerland; Engelkamp et al., 1992).
Substances from the LOPAC 1280 and additional samples of calcimycin (both Sigma-Aldrich, St. Louis, MO) were dissolved in dimethylsulfoxide. Dilutions were performed with RPMI-1640 medium.
High-throughput screening and cell cytotoxicity assay
A BIOMEK2000 automatic pipetting system (Beckman Coulter, Brea, CA) was used to seed 2.5 × 103 HCT116/S100A4pLUC cells/well into 384-well plates. Cells were exposed to dilutions of each compound of the LOPAC 1280 for 24 h. Luciferase expression was determined using Britelite reagent in a Wallac Victor reader (both PerkinElmer, Waltham, MA). In parallel, cell cytotoxicity of the compounds was measured by Alamar blue cytotoxicity assay. Briefly, after 24 h of incubation with cells, plates were treated with Alamar blue (Sigma-Aldrich) solution for 4 h and then read on a fluorescence plate reader at excitation and emission wavelengths of 530 and 590 nm, respectively. Compounds showing inhibition in the S100A4 promoter screen were counterscreened using an HIF-1 promoter screen to establish selective activity (Rapisarda et al., 2002).
Quantitative real-time PCR, Western blot analysis, and immunohistochemistry
Quantitative real-time (qRT) PCR was carried out using the LightCycler480 (Roche, Mannheim, Germany) as described previously (Stein et al., 2006) with the primers and probes summarized in Table 1. For β-catenin, S100A4, and DKK-1 cDNA quantification HybProbe Faststart master mix (Roche) was used. Cyclin D1 and c-myc cDNAs were quantified with SYBR green (Roche). For quantification of the housekeeping gene glucose-6-phosphate dehydrogenase (G6PDH) cDNA the hG6PDH Roche Kit (Roche) was used.
TABLE 1:
Primers and probes used for qRT-PCR.
| Gene | Primer/probe | Sequence 5′–3′ |
|---|---|---|
| β-Catenin | Forward primer | gtg cta tct gtc tgc tct agt a |
| Reverse primer | ctt cct gtt tag ttg cag cat c | |
| FITC probe | agg act tca cct gac aga tcc aag tca-FITC | |
| Lcred640-probe | LCRed640-cgt ctt gtt cag aac tgt ctt tgg act ctc-phosphate | |
| Cyclin D1 | Forward primer | ctg ttt ggc gtt tcc cag agt cat c |
| Reverse primer | agc ctc ctc ctc aca cct cct c | |
| c-myc | Forward primer | acc ctt gcc gca tcc acg aaa c |
| Reverse primer | cgt agt cga ggt cat agt tcc tgt tgg | |
| DKK-1 | Forward primer | tag cac ctt gga tgg gta ttc |
| Reverse primer | ata ttt cta gtc cat gag agc c | |
| FITC probe | gtc tcc ggt cat cag act gtg cc-FITC | |
| Lcred640 probe | LCRed640-agg att gtg ttg tgc tag aca ctt ctg g-phosphate | |
| S100A4 | Forward primer | ctc agc gct tct tct ttc |
| Reverse primer | ggg tca gca gct cct tta | |
| FITC probe | tgt gat ggt gtc cac ctt cca caa gt-FITC | |
| Lcred640 probe | LCred640-tcg ggc aaa gag ggt gac aag t-phosphate | |
| Human satellite DNA | Forward primer | ggg ata att tca gct gac taa aca g |
| Reverse primer | aaa cgt cca ctt gca gat tct ag | |
| FITC probe | ctt cac ata aaa act aca cag atg cat tct cag g-FITC | |
| LCRed640 probe | LCred640-ctt ttt ggt gat gtt tgt att caa ctc cca g-phosphate |
FITC, fluorescein isothiocyanate.
Western blot was performed with the following antibodies: a polyclonal rabbit anti–human S100A4 antibody (Dako, Glostrup, Denmark), a polyclonal goat anti–human glycerin-aldehyde-3-phosphate dehydrogenase (GAPDH) antibody (Santa Cruz Biotechnology, Santa Cruz, CA), and a monoclonal mouse anti–human β-catenin antibody. Immunoblotting for GAPDH served as loading control.
Immunohistochemistry was performed as previously described (Stein et al., 2011). Briefly, cryosections were stained with rabbit anti–human-specific cytokeratin-19 antibody (dilution 1:50; Acris Antibodies, Herford, Germany) and horseradish peroxidase–coupled anti-rabbit antibody (dilution 1:1000; Promega, Madison, WI).
Cell migration, cell invasion, and wound-healing assay
For the Boyden chamber assay (Boyden, 1962), 2.5 × 105 cells were seeded into transwell filter membrane chambers (pore size, 12.0 μm; Millipore, Billerica, MA) and allowed to accommodate for 15 h. Cells were treated with 1 μM calcimycin for 18 h. The number of cells that migrated to the lower chamber was counted in a Neubauer chamber. For the invasion assays, transwell membranes were coated with 1:3 diluted Matrigel (BD Biosciences, Heidelberg, Germany). For the wound-healing assay, 2.5 × 105 cells were seeded at 60% confluence 24 h before a wound of ∼300 μM width was made with a pipette tip. Medium was exchanged daily, and microphotographs were taken on day 4.
Cell proliferation and colony formation assay
For determination of cell proliferation, 8 × 103 cells were treated daily for 4 d with 1 μM calcimycin. For determination of viable cells 3-(4,5-dimethyl-2-thiazol)-2,5-diphenyl-2H-tetrazolium bromide (MTT; Sigma-Aldrich) was added to a final concentration of 0.5 mg/ml, incubated for 3 h, and dissolved by 10% SDS in 10 mM HCl. The optical density was measured at 560 nm. For colony formation assays 1 × 103 cells were resuspended in 1 μM calcimycin- or solvent-containing medium supplemented with 0.33% (wt/vol) agarose and seeded as single cells. After incubation in a humidified incubator at 37°C and 5% CO2 for 7 d, colonies were analyzed by light microscopy. Colonies were counted if they consisted of more than three cells.
TOP/FOPflash reporter assay
Transfection of 8 × 105 cells with TOPflash or FOPflash plasmids (Promega) occurred 24 h before the cells were treated with 1 μM calcimycin. After 18 h of treatment luciferase activity was measured by the Steady Glow Luciferase Assay System (Promega). TOPflash reporter gene expression (representing the Wnt/β-catenin pathway activity) was normalized to FOPflash reporter gene expression (representing basal reporter gene expression).
In vivo bioluminescence imaging of xenograft mice
All experiments were performed in accordance with the United Kingdom Coordinating Committee for Cancer Research guidelines and approved by the State Office of Health and Social Affairs, Berlin, Germany. HCT116/LUC cells were treated with 1 μM calcimycin or solvent 24 h before 2 × 106 cells were intrasplenically injected into eight NOD/SCID-IL2R− mice per group. Mice were anesthetized by intraperitoneal injection of 35 mg/kg Hypnomidate (Jassen-Cilag, Neuss, Germany) and received 150 mg/kg d-luciferin (Biosynth, Staad, Switzerland) intraperitoneally for bioluminescence imaging. Imaging was performed with the NightOWL LB 981 system (Berthold Technologies, Bad Wildbad, Germany). ImageJ, version 2.3, was used for color coding of signal intensity (presenting a 256 grayscale) and for quantification of the luminescence signal. Mice were killed 6 d posttransplantation, when a clear liver signal was detected. The spleen (the tumor implantation site) and the liver (the metastasis target organ) were shock frozen in liquid nitrogen, and cryosections were performed for isolation of genomic DNA (Qiagen, Hilden, Germany) and mRNA (Roboklon, Berlin, Germany) and for immunohistochemistry.
Statistical analysis
Statistical analyses were performed with GraphPad Prism, version 4.01. Comparison of two groups was done by Student's t test. Comparison of a control versus several treated groups was performed by one-way analysis of variance (ANOVA) and Bonferroni post hoc multiple comparison. The inhibiting concentration 50 (IC50) was defined as the concentration that reduced cell viability to 50% of solvent-treated control cells. The effective concentration 50 (EC50) was defined to be the concentration at which reporter activity was reduced to 50% of solvent-treated control cells. The IC50 and EC50 were calculated by sigmoidal dose–response curve fit of × = log(x) transformed data. IC50 and EC50 values were given as geometric means with 95% confidence interval. All significance tests were two-sided. p < 0.05 was defined as statistically significant.
Acknowledgments
We are very grateful to Pia Hermann and Margit Lemm for technical assistance and to Franziska Siegel and Dennis Kobelt for methodological and scientific advice. This work was supported by the German Research Association (STE 671/8-1, to U.S. and P.M.S.), the Alexander von Humboldt Foundation (to U.S. and W.W.), and a Max-Delbrück-Center for Molecular Medicine Helmholtz Association Fellowship (to U.S.).
Abbreivations used:
- ANOVA
one-way analysis of variance
- DKK-1
dickkopf-1
- GAPDH
glycerin-aldehyde-3-phosphate dehydrogenase
- G6PDH
glucose-6-phosphate dehydrogenase
- LOPAC
library of pharmacologically active compounds
- MMP
matrix metalloproteinase
- NOD/SCID
nonobese diabetic/severe immune deficient
- TCF
T cell factor
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E10-09-0739) on July 27, 2011.
REFERENCES
- Ambartsumian N, et al. The metastasis-associated Mts1(S100A4) protein could act as an angiogenic factor. Oncogene. 2001;20:4685–4695. doi: 10.1038/sj.onc.1204636. [DOI] [PubMed] [Google Scholar]
- Ambartsumian NS, Grigorian MS, Larsen IF, Karlstrom O, Sidenius N, Rygaard J, Georgiev G, Lukanidin E. Metastasis of mammary carcinomas in GRS/A hybrid mice transgenic for the mts1 gene. Oncogene. 1996;13:1621–1630. [PubMed] [Google Scholar]
- Amit S, Hatzubai A, Birman Y, Andersen JS, Ben-Shushan E, Mann M, Ben-Neriah Y, Alkalay I. Axin-mediated CKI phosphorylation of beta-catenin at Ser 45: a molecular switch for the Wnt pathway. Genes Dev. 2002;16:1066–1076. doi: 10.1101/gad.230302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker N, Clevers H. Mining the Wnt pathway for cancer therapeutics. Nat Rev Drug Discov. 2006;5:997–1014. doi: 10.1038/nrd2154. [DOI] [PubMed] [Google Scholar]
- Becker M, Nitsche A, Neumann C, Aumann J, Junghahn I, Fichtner I. Sensitive PCR method for the detection and real-time quantification of human cells in xenotransplantation systems. Br J Cancer. 2002;87:1328–1335. doi: 10.1038/sj.bjc.6600573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belot N, Pochet R, Heizmann CW, Kiss R, Decaestecker C. Extracellular S100A4 stimulates the migration rate of astrocytic tumor cells by modifying the organization of their actin cytoskeleton. Biochim Biophys Acta. 2002;1600:74–83. doi: 10.1016/s1570-9639(02)00447-8. [DOI] [PubMed] [Google Scholar]
- Boyden S. The chemotactic effect of mixtures of antibody and antigen on polymorphonuclear leucocytes. J Exp Med. 1962;115:453–466. doi: 10.1084/jem.115.3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boye K, Maelandsmo GM. S100A4 and metastasis: a small actor playing many roles. Am J Pathol. 2010;176:528–535. doi: 10.2353/ajpath.2010.090526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho YG, Kim CJ, Nam SW, Yoon SH, Lee SH, Yoo NJ, Lee JY, Park WS. Overexpression of S100A4 is closely associated with progression of colorectal cancer. World J Gastroenterol. 2005;11:4852–4856. doi: 10.3748/wjg.v11.i31.4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies MP, Rudland PS, Robertson L, Parry EW, Jolicoeur P, Barraclough R. Expression of the calcium-binding protein S100A4 (p9Ka) in MMTV-neu transgenic mice induces metastasis of mammary tumours. Oncogene. 1996;13:1631–1637. [PubMed] [Google Scholar]
- Engelkamp D, Schafer BW, Erne P, Heizmann CW. S100 alpha, CAPL, and CACY: molecular cloning and expression analysis of three calcium-binding proteins from human heart. Biochemistry. 1992;31:10258–10264. doi: 10.1021/bi00157a012. [DOI] [PubMed] [Google Scholar]
- Ford HL, Silver DL, Kachar B, Sellers JR, Zain SB. Effect of Mts1 on the structure and activity of nonmuscle myosin II. Biochemistry. 1997;36:16321–16327. doi: 10.1021/bi971182l. [DOI] [PubMed] [Google Scholar]
- Gao XN, Tang SQ, Zhang XF. S100A4 antisense oligodeoxynucleotide suppresses invasive potential of neuroblastoma cells. J Pediatr Surg. 2005;40:648–652. doi: 10.1016/j.jpedsurg.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Garrett SC, Varney KM, Weber DJ, Bresnick AR. S100A4, a mediator of metastasis. J Biol Chem. 2006;281:677–680. doi: 10.1074/jbc.R500017200. [DOI] [PubMed] [Google Scholar]
- Giles RH, van Es JH, Clevers H. Caught up in a Wnt storm: Wnt signaling in cancer. Biochim Biophys Acta. 2003;1653:1–24. doi: 10.1016/s0304-419x(03)00005-2. [DOI] [PubMed] [Google Scholar]
- Gongoll S, Peters G, Mengel M, Piso P, Klempnauer J, Kreipe H, von Wasielewski R. Prognostic significance of calcium-binding protein S100A4 in colorectal cancer. Gastroenterology. 2002;123:1478–1484. doi: 10.1053/gast.2002.36606. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Sancho JM, Aguilera O, Garcia JM, Pendas-Franco N, Pena C, Cal S, Garcia de Herreros A, Bonilla F, Munoz A. The Wnt antagonist DICKKOPF-1 gene is a downstream target of beta-catenin/TCF and is downregulated in human colon cancer. Oncogene. 2005;24:1098–1103. doi: 10.1038/sj.onc.1208303. [DOI] [PubMed] [Google Scholar]
- Grigorian M, Tulchinsky E, Burrone O, Tarabykina S, Georgiev G, Lukanidin E. Modulation of mts1 expression in mouse and human normal and tumor cells. Electrophoresis. 1994;15:463–468. doi: 10.1002/elps.1150150163. [DOI] [PubMed] [Google Scholar]
- Grum-Schwensen B, Klingelhofer J, Berg CH, El-Naaman C, Grigorian M, Lukanidin E, Ambartsumian N. Suppression of tumor development and metastasis formation in mice lacking the S100A4(mts1) gene. Cancer Res. 2005;65:3772–3780. doi: 10.1158/0008-5472.CAN-04-4510. [DOI] [PubMed] [Google Scholar]
- Gwak J, et al. Protein-kinase-C-mediated beta-catenin phosphorylation negatively regulates the Wnt/beta-catenin pathway. J Cell Sci. 2006;119:4702–4709. doi: 10.1242/jcs.03256. [DOI] [PubMed] [Google Scholar]
- He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B, Kinzler KW. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- Hernan R, Fasheh R, Calabrese C, Frank AJ, Maclean KH, Allard D, Barraclough R, Gilbertson RJ. ERBB2 up-regulates S100A4 and several other prometastatic genes in medulloblastoma. Cancer Res. 2003;63:140–148. [PubMed] [Google Scholar]
- Hua J, Chen D, Fu H, Zhang R, Shen W, Liu S, Sun K, Sun X. Short hairpin RNA-mediated inhibition of S100A4 promotes apoptosis and suppresses proliferation of BGC823 gastric cancer cells in vitro and in vivo. Cancer Lett. 2009;292:41–47. doi: 10.1016/j.canlet.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Huang WS, Wang JP, Wang T, Fang JY, Lan P, Ma JP. ShRNA-mediated gene silencing of beta-catenin inhibits growth of human colon cancer cells. World J Gastroenterol. 2007;13:6581–6587. doi: 10.3748/wjg.v13.i48.6581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- Keirsebilck A, Bonne S, Bruyneel E, Vermassen P, Lukanidin E, Mareel M, van Roy F. E-cadherin and metastasin (mts-1/S100A4) expression levels are inversely regulated in two tumor cell families. Cancer Res. 1998;58:4587–4591. [PubMed] [Google Scholar]
- Kim EJ, Helfman DM. Characterization of the metastasis-associated protein, S100A4Roles of calcium binding and dimerization in cellular localization and interaction with myosin. J Biol Chem. 2003;278:30063–30073. doi: 10.1074/jbc.M304909200. [DOI] [PubMed] [Google Scholar]
- Kim JS, Crooks H, Dracheva T, Nishanian TG, Singh B, Jen J, Waldman T. Oncogenic beta-catenin is required for bone morphogenetic protein 4 expression in human cancer cells. Cancer Res. 2002;62:2744–2748. [PubMed] [Google Scholar]
- Kriajevska M, Fischer-Larsen M, Moertz E, Vorm O, Tulchinsky E, Grigorian M, Ambartsumian N, Lukanidin E. Liprin beta 1, a member of the family of LAR transmembrane tyrosine phosphatase-interacting proteins, is a new target for the metastasis-associated protein S100A4 (Mts1) J Biol Chem. 2002;277:5229–5235. doi: 10.1074/jbc.M110976200. [DOI] [PubMed] [Google Scholar]
- Kriajevska MV, Cardenas MN, Grigorian MS, Ambartsumian NS, Georgiev GP, Lukanidin EM. Non-muscle myosin heavy chain as a possible target for protein encoded by metastasis-related mts-1 gene. J Biol Chem. 1994;269:19679–19682. [PubMed] [Google Scholar]
- Li ZH, Bresnick AR. The S100A4 metastasis factor regulates cellular motility via a direct interaction with myosin-IIA. Cancer Res. 2006;66:5173–5180. doi: 10.1158/0008-5472.CAN-05-3087. [DOI] [PubMed] [Google Scholar]
- Li ZH, Spektor A, Varlamova O, Bresnick AR. Mts1 regulates the assembly of nonmuscle myosin-IIA. Biochemistry. 2003;42:14258–14266. doi: 10.1021/bi0354379. [DOI] [PubMed] [Google Scholar]
- Mathisen B, Lindstad RI, Hansen J, El-Gewely SA, Maelandsmo GM, Hovig E, Fodstad O, Loennechen T, Winberg JO. S100A4 regulates membrane induced activation of matrix metalloproteinase-2 in osteosarcoma cells. Clin Exp Metastasis. 2003;20:701–711. doi: 10.1023/b:clin.0000006819.21361.03. [DOI] [PubMed] [Google Scholar]
- Pressman BC. Biological applications of ionophores. Annu Rev Biochem. 1976;45:501–530. doi: 10.1146/annurev.bi.45.070176.002441. [DOI] [PubMed] [Google Scholar]
- Rapisarda A, Uranchimeg B, Scudiero DA, Selby M, Sausville EA, Shoemaker RH, Melillo G. Identification of small molecule inhibitors of hypoxia-inducible factor 1 transcriptional activation pathway. Cancer Res. 2002;62:4316–4324. [PubMed] [Google Scholar]
- Sack U, Stein U. Wnt up your mind—intervention strategies for S100A4-induced metastasis in colon cancer. Gen Physiol Biophys. 2009;28:F55-F64. [PubMed] [Google Scholar]
- Saleem M, et al. S100A4 accelerates tumorigenesis and invasion of human prostate cancer through the transcriptional regulation of matrix metalloproteinase 9. Proc Natl Acad Sci USA. 2006;103:14825–14830. doi: 10.1073/pnas.0606747103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santamaria-Kisiel L, Rintala-Dempsey AC, Shaw GS. Calcium-dependent and -independent interactions of the S100 protein family. Biochem J. 2006;396:201–214. doi: 10.1042/BJ20060195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M, Hansen JL, Sheikh SP. S100A4: a common mediator of epithelial-mesenchymal transition, fibrosis and regeneration in diseases? J Mol Med. 2008;86:507–522. doi: 10.1007/s00109-007-0301-3. [DOI] [PubMed] [Google Scholar]
- Sherbet GV. Metastasis promoter S100A4 is a potentially valuable molecular target for cancer therapy. Cancer Lett. 2009;280:15–30. doi: 10.1016/j.canlet.2008.10.037. [DOI] [PubMed] [Google Scholar]
- Shi Y, Zou M, Collison K, Baitei EY, Al-Makhalafi Z, Farid NR, Al-Mohanna FA. Ribonucleic acid interference targeting S100A4 (Mts1) suppresses tumor growth and metastasis of anaplastic thyroid carcinoma in a mouse model. J Clin Endocrinol Metab. 2006;91:2373–2379. doi: 10.1210/jc.2006-0155. [DOI] [PubMed] [Google Scholar]
- Shtutman M, Zhurinsky J, Simcha I, Albanese C, D'Amico M, Pestell R, Ben-Ze'ev A. The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proc Natl Acad Sci USA. 1999;96:5522–5527. doi: 10.1073/pnas.96.10.5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein U, Arlt F, Smith J, Sack U, Herrmann P, Walther W, Lemm M, Fichtner I, Shoemaker RH, Schlag PM. Intervening in β-catenin signaling by sulindac inhibits S100A4-dependent colon cancer metastasis. Neoplasia. 2011;13:131–144. doi: 10.1593/neo.101172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein U, et al. The metastasis-associated gene S100A4 is a novel target of beta-catenin/T-cell factor signaling in colon cancer. Gastroenterology. 2006;131:1486–1500. doi: 10.1053/j.gastro.2006.08.041. [DOI] [PubMed] [Google Scholar]
- Tabata T, et al. RNA interference targeting against S100A4 suppresses cell growth and motility and induces apoptosis in human pancreatic cancer cells. Biochem Biophys Res Commun. 2009;390:475–480. doi: 10.1016/j.bbrc.2009.09.096. [DOI] [PubMed] [Google Scholar]
- Yammani RR, Carlson CS, Bresnick AR, Loeser RF. Increase in production of matrix metalloproteinase 13 by human articular chondrocytes due to stimulation with S100A4: Role of the receptor for advanced glycation end products. Arthritis Rheum. 2006;54:2901–2911. doi: 10.1002/art.22042. [DOI] [PubMed] [Google Scholar]