Abstract
Purpose
Adenosine kinase (ADK) represents the key metabolic enzyme for the regulation of extracellular adenosine levels in the brain. In adult brain, ADK is primarily present in astrocytes. Several lines of experimental evidence support a critical role of ADK in different types of brain injury associated with astrogliosis, which is also a prominent morphological feature of temporal lobe epilepsy (TLE). We hypothesized that dysregulation of ADK is an ubiquitous pathological hallmark of TLE.
Methods
Using immunocytochemistry and western blot analysis, we investigated ADK protein expression in a rat model of TLE during epileptogenesis and the chronic epileptic phase and compared those findings with tissue resected from TLE patients with mesial temporal sclerosis (MTS).
Key findings
In rat control hippocampus and cortex, a low baseline expression of ADK was found with mainly nuclear localization. One week after the electrical induction of status epilepticus (SE), prominent up-regulation of ADK became evident in astrocytes with a characteristic cytoplasmic localization. This increase in ADK persisted at least for 3-4 months after SE in rats developing a progressive form of epilepsy. In line with the findings from the rat model, expression of astrocytic ADK was also found to be increased in the hippocampus and temporal cortex of TLE patients. In addition, in vitro experiments in human astrocyte cultures showed that ADK expression was increased by several pro-inflammatory molecules (interleukin-1β and LPS).
Significance
These results suggest that dysregulation of ADK in astrocytes is a common pathological hallmark of TLE. Moreover, in vitro data suggest the existence of an additional layer of modulatory crosstalk between the astrocyte-based adenosine cycle and inflammation. Whether this interaction also can play role in vivo needs to be further investigated.
Keywords: rat, human, hippocampus, ADK, epilepsy, astrocytes
Introduction
Mesial temporal sclerosis (MTS) represents a frequent pathophysiological substrate of temporal lobe epilepsy (TLE), a common neurological disorder (Engel 2001, Wieser 2004). MTS represents the pathological substrate of a process that occurs over a long period of time following an initial injury (i.e. febrile seizures, status epilepticus (SE) of several causes, cerebral trauma) (Engel, et al. 2003, Pitkanen 2010).
Large-scale analysis of gene expression studies performed in different experimental models of TLE revealed that the biological process that emerges as the most prominent during epileptogenesis is related to glial activation (Lukasiuk, et al. 2006, Aronica & Gorter 2007). Astrogliosis is indeed a major pathological feature of MTS (Wieser 2004, Blümcke, et al. 2009) and recent data suggest a role for astroglial cells in epilepsy, indicating that astroglia can display different functional phenotypes within the epileptic focus, contributing to seizure development (Binder & Steinhauser 2006, Wetherington, et al. 2008, Seifert, et al. 2010).
Recently attention has focused on the role of astrocytic adenosine kinase (ADK). This enzyme is responsible for the phosphorylation of adenosine into AMP and has been shown to critically regulate the extracellular adenosine levels in brain (Boison 2006, Etherington, et al. 2009). In particular, increased levels of astrocytic ADK have been reported in the kainic acid-induced mouse model of TLE and inhibition of ADK has been shown to be an effective anticonvulsant strategy in this model (Gouder, et al. 2004, Fedele, et al. 2005a; reviewed in Boison 2008, Boison 2010). A key challenge in translating the link between ADK expression and epilepsy into the clinic is represented by the generalization of these findings to different animal models and validation of those findings in appropriate patient populations.
In order to understand the dynamics of ADK expression during development and progression of epilepsy we evaluated the expression and cellular distribution of ADK in a rat model of TLE (post-SE model induced by electrical stimulation), as well as in hippocampal and cortical specimens of MTS patients. Moreover, we investigated whether ADK could be regulated by IL-1β, a pro-inflammatory cytokine that has been shown to be activated in epileptic tissue (Vezzani & Baram 2007).
Material and methods
Experimental animals
Adult male Sprague Dawley rats (Harlan CPB laboratories, Zeist, The Netherlands) weighing 300-500 grams were used in this study which was approved by the Animal Welfare committee of the University of Amsterdam and which is in accordance with the NIH guidelines for the care and use of experimental animals. All experimental protocols followed the European Communities Council directive 86/609/EEC and the Dutch Experiments on Animal Act (1997), and were approved by the Dutch animal welfare committee (DEC).
The rats were housed individually in a controlled environment (21±1°C; humidity 50-60%; lights on 08:00 AM - 8:00 PM; food and water available ad libitum).
Electrode implantation and seizure induction
Adequate measures were taken to minimize pain or discomfort. Rats were anaesthetized with an intramuscular injection of ketamine (57 mg/kg; Alfasan, Cuyk, The Netherlands) and xylazine (9 mg/kg; Bayer AG, Germany) and placed in a stereotactic apparatus. In order to record hippocampal EEG, a pair of insulated stainless steel electrodes (70 μm wire diameter, tips were 80 μm apart) were implanted into the left dentate gyrus (DG) under electrophysiological control, using the following co-ordinates: 3.9 mm anterior-posterior (AP), 1.7 mm mediolateral, nosebar −3.9 mm, as previously described (Gorter, et al. 2001). A pair of stimulation electrodes was implanted in the angular bundle (7.2 mm anterior-posterior, 4.5 mm mediolateral). Two weeks after implantation rats underwent tetanic stimulations (50 Hz) of the hippocampus in the form of a succession of trains of pulses every 13 seconds. Each train had a duration of 10 seconds and consisted of biphasic pulses (pulse duration 0.5 ms, maximal intensity 500 μA). Stimulation was stopped when the rats displayed sustained forelimb clonus and salivation for minutes, which usually occurred within 1 hour. However, stimulation never lasted longer than 90 minutes. Differential EEG signals were amplified (10x) via a FET transistor that connected the headset to a differential amplifier (20x; CyberAmp, Axon Instruments, Burlingame, CA, USA), filtered (1-60 Hz), and digitized by a computer. A seizure detection program (Harmonie, Stellate Systems, Montreal, Canada) sampled the incoming signal at a frequency of 200 Hz per channel. EEG recordings were monitored also visually and screened for seizure activity. Behavior was observed during electrical stimulation and several hours thereafter. Immediately after termination of the stimulation, periodic epileptiform discharges (PEDs) occurred at a frequency of 1-2 Hz and they were accompanied by behavioral and EEG seizures (status epilepticus; SE). Rats were monitored continuously from the induction of SE to the time of sacrifice (24 h to 1 week). The chronic epileptic group (3-4 months after SE) was monitored during and shortly after SE and during 3-5 days before sacrifice in order to determine the frequency of spontaneous seizures. Sham-operated control rats were handled and recorded identically, but did not receive electrical stimulation. None of these rats needed to be reimplanted.
Rat tissue preparation
Rats were disconnected from the EEG recording set-up and deeply anesthetized with pentobarbital (Nembutal, intraperitoneally, 60 mg/kg). For immunocytochemistry, the animals were perfused through the ascending aorta with 300 ml of 0.37 % Na2S solution, followed by 300 ml 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4. Thereafter, the brains were removed, incubated for 72 hours in 0.3 M EDTA, pH 6.7 (Merck, Amsterdam, The Netherlands) and paraffin embedded. Paraffin-embedded tissue was sectioned at 6 μm, mounted on pre-coated glass slides (Star Frost, Waldemar Knittel GmbH, Brunschweig, Germany) and were used for immunocytochemistry. Horizontal sections were analyzed at a midlevel of the brain (5300–6100 μm below cortex surface). Immunocytochemistry was performed on two adjacent serial sections from each group [control, n=6; 24 h, n= 5; 1 week, n=6; 3-4 months, long term (LT) epilepsy, progressive pLT, n= 5 and non-progressive, npLT, n=5 and non-SE rats, n=3). Progressive epileptic rats (pLT) were characterized by frequent daily seizures that had progressed over time until epilepsy had developed with a rather stable frequency (~10 seizures/day). Non-progressive epileptic rats (npLT) were characterized by exhibiting only a few occasional seizures per week, the frequency of which did not increase over time. In these rats the last seizure had been detected >1 day before sacrifice. Non-SE rats did not develop a SE during stimulation, had no chronic seizures detected and were sacrificed 3-4 months after stimulation. Two additional serial slices were used for the double staining, as described below.
For Western blot analysis, animals were decapitated in the acute phase (one day after SE, n=4), in the latent period (1 week after SE, n=6; tissue was selected from rats that did not yet exhibit spontaneous seizures as verified by EEG recordings) and in the chronic epileptic phase, LT (progressive pLT n=5 and non-progressive, npLT n=4). Control rats (n=6) were implanted but not stimulated. The temporal cortex was dissected and immediately frozen on dry ice and stored at −80 °C until use (Western blot analysis).
Human material
The human cases included in this study were obtained from the files of the Departments of Neuropathology of the Academic Medical Center (AMC, University of Amsterdam) and the VU University Medical Center (VUMC). Eleven patients underwent resection of the hippocampus for medically intractable TLE. Informed consent was obtained for the use of brain tissue and for access to medical records for research purposes. All samples were obtained and used in a manner compliant with the Declaration of Helsinki. Two neuropathologists reviewed all cases independently. In 6 cases a pathological diagnosis of HS (without extra-hippocampal pathology) was made. The HS specimens included 4 cases of classical HS (grade 3, mesial temporal sclerosis type 1a) and 2 cases of severe HS (grade IV; mesial temporal sclerosis type 1b; (Wyler, et al. 1992, Blumcke, et al. 2007). Five non-HS cases, in which a focal lesion [ganglioglioma not involving the hippocampus proper] was identified, were also included to provide a comparison group to HS cases. Control hippocampal tissue was obtained at autopsy from 6 patients without history of seizures or other neurological diseases. All autopsies were performed within 12 hours after death. Table 1 (supplementary) summarizes the clinical features of TLE and control cases.
Tissue was fixed in 10% buffered formalin and embedded in paraffin. Paraffin-embedded tissue was sectioned at 6 μm, mounted on organosilane-coated slides (Star Frost, Waldemar Knittel GmbH, Brunschweig, Germany) and used for in situ hybridization and immunocytochemistry as described below.
Autopsy temporal cortex from control patients (n=5) and surgical cortex from MTS (n=5) and non-MTS (n=4) patients was snap frozen in liquid nitrogen and stored at −80°C until further use (western blot analysis).
Immunocytochemistry
Glial fibrillary acidic protein (GFAP; polyclonal rabbit, DAKO, Glostrup, Denmark; 1:4000), vimentin (mouse clone V9, DAKO; 1:1000), neuronal nuclear protein (NeuN; mouse clone MAB377, IgG1; Chemicon, Temecula, CA, USA; 1:2000) and (HLA)-DP, DQ, DR (mouse clone CR3/43; DAKO, Glostrup, Denmark, 1:400) were used in the routine immunocytochemical analysis of TLE human specimens.
For the detection of ADK, we used a polyclonal rabbit antibody (1:500;Studer, et al. 2006; Ren, et al. 2007). Single-label immunocytochemistry was performed with Powervision (Immunologic, Duiven, The Netherlands). 3,3-Diaminobenzidine was used as chromogen. Sections were counterstained with Haematoxylin. For double-labeling studies, sections, after incubation with primary antibodies, were incubated for 2 h at RT with Alexa Fluor® 568 and Alexa Fluor® 488 (anti-rabbit IgG or anti-mouse IgG; 1:200; Molecular probes, Eugene, USA).
For double labeling we also used the anti-doublecortin (DCX; Abcam 1:4000; Cambridge, UK). Sections were analyzed by means of a laser scanning confocal microscope (Bio-Rad, Hercules, CA, USA; MRC1024) equipped with an argon-ion laser.
Quantitative analysis was also performed in rat hippocampus and the number of positive cells was quantified as previously described (Maroso, et al. 2010). Briefly, two representative adjacent non-overlapping fields of the hippocampus (CA1, CA3 and hilar region of the dentate gyrus, DG) were captured (magnification 40x; total area of each field: 171,600 μm2) and digitized using a laser scanning confocal microscope (Bio-Rad, Hercules, CA, USA; MRC1024). We counted the total number of GFAP-positive cells, and those showing nuclear or extra-nuclear ADK staining.
Cell cultures
For cell culture experiments (astrocyte-enriched human cultures), fetal brain tissue (22–23 weeks of gestation) was obtained from spontaneous or medically induced abortions with appropriate maternal written consent for brain autopsy. Resected tissue samples were collected in Dulbecco's modified Eagle's medium, DMEM/HAM F10 (1:1) medium (Gibco, Grand Island, NJ). Cell isolation was performed as previously described (Aronica et al., 2003; Aronica et al., 2005). Briefly, after removal of meninges and blood vessels, tissue was dissociated by incubation at 37°C for 20 min in a Hank's balanced salt solution containing 2.5 mg/ml trypsin (Sigma, St. Louis, MO, USA) and 0.1 mg/ml bovine pancreatic Dnase I (Boehringer Mannheim, Germany). Tissue was triturated and washed with DMEM/HAM F10 medium, supplemented with 50 units/ml penicillin and 50 μg/ml streptomycin and 10% fetal calf serum (FCS). Cell suspensions (containing ~0.5 g wet weight tissue/10 ml culture medium) were passed through a 70 μm cell sieve (Becton Dickinson, USA) and plated into poly-L-lysine (PLL; 15 μg/ml, Sigma), pre-coated 25 cm2 flasks (Falcon, Lincoln Park, NJ) and maintained in a 5% CO2 incubator at 37°C. After 48 h the culture medium was replaced by fresh medium and cultures were subsequently fed twice a week. Cultures reached confluence after 2-3 weeks. Secondary astrocyte cultures were established by trypsinizing confluent cultures and sub-plating onto PLL-precoated 6 wellplates or 75 cm2 flasks (0.5-1 × 106 cell/ml; for Western blot analysis or for the generation of serial passages respectively). More than 98% of the cells in primary culture, as well as in the successive 12 passages were strongly immunoreactive for the astrocytic marker GFAP. In the present study astrocytes were used for Western blot analysis at passage 3-4. IL-1 ß (10 ng/ml; IL-1b, Peprotech, NJ, USA) or LPS (lipo-polysaccharide; 100 ng/ml; Sigma, St. Louis, MO, USA) were applied and maintained in serum free medium for 24 h before harvesting them for Western blot analysis. As previously shown (Aronica et al., 2005), the viability of human astrocytes in culture was not influenced by the treatments.
Western blot analysis
For immunoblot analysis, samples were homogenized in lysis buffer containing 10 mM Tris (pH 8.0), 150 mM NaCl, 10% glycerol, 1% NP-40, Na-orthevanadate (10.4 mg/ml), 5 mM EDTA (pH 8.0), 5 mM NaF and protease inhibitor cocktail (Boehringer Mannheim, Germany). Protein content was determined using the bicinchoninic acid method. For electrophoresis, equal amounts of proteins (30 μg/lane) were separated by sodium dodecylsulfate-polyacrylamide gel electrophoretic (SDS-PAGE) analysis. Separated proteins were transferred to nitrocellulose paper for 1 h and 30 min, using a semi-dry electroblotting system (BioRad, Transblot SD, Hercules, CA, USA). Blots were incubated overnight in TTBS (20 mM Tris, 150 mM NaCl, 0.1% Tween, pH 7.5)/5% non fat dry milk, containing the primary antibody (1:5000). After several washes in TTBS, the membranes were incubated in TTBS/5% non fat dry milk/1% BSA, containing the goat anti-rabbit or goat anti-mouse antibodies coupled to horse radish peroxidase (1:2500; Dako, Denmark) for 1 h. After washes in TTBS, immunoreactivity was visualized using Lumi–light PLUS western blotting substrate (Roche Diagnostics, Mannheim, Germany) and digitized using a Luminescent Image Analyzer (LAS-3000, Fuji Film, Japan). Expression of β-actin (monoclonal mouse, Sigma, St. Louis, MO, USA 1:50.000) was used as reference. Statistical analyses were performed with SPSS for Windows (SPSS 11.5, SPSS Inc., Chicago, IL, USA) using two-tailed Student's t-test; to assess differences between more than two groups, ANOVA and a non-parametric Kruskal–Wallis test followed by Mann–Whitney U-test were performed. P < 0.05 was considered significant. Correlations between Western blot data (optical density values) and different clinical variables (supplemental Table 1: duration of epilepsy, seizure frequency, seizure type, age at surgery, age at seizure onset) were assessed using the Spearman's rank correlation test.
Results
Overexpression of ADK in hippocampus of rats with progressive course of TLE
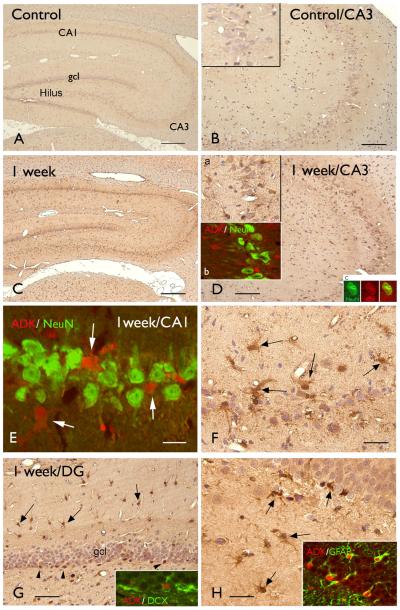
To determine the temporal-spatial expression and cellular distribution of ADK we performed immunocytochemistry in tissue samples of control rats and rats that were sacrificed at different time points after SE (1 day, 1 week and 3-4 months post-SE). Control hippocampus displayed weak ADK immunoreactivity (IR) in the different hippocampal subfields (Fig. 1 A-B). No ADK IR was detected in neurons, however nuclear ADK IR was observed in resting glial cells. Only sparse nuclear ADK IR was observed at one day post-SE (not shown). At 1 week post-SE (Fig. 1 C-H), ADK IR (with cytoplasmic staining) was detected within the different hippocampal regions in glial cells with the morphology of reactive astrocytes (Fig. 2 D-H). Double labelling confirmed ADK expression in GFAP-positive reactive astrocytes (Figure 1 H). The large majority of pyramidal neurons in CA1 and CA3 regions did not display ADK expression (Fig. 1 D-E, insets), however, occasionally neuronal IR was observed (Fig. 1 D, inset). Strongly stained ADK positive cells in the subgranular zone of the DG did not appear to co-localize with DCX (doublecortin; Fig. 1 G, inset).
Figure 1. Immunostaining of ADK in hippocampal tissue of control rat and after induction of status epilepticus (SE).
Panels A-B: Control hippocampus showing low ADK immunoreactivity (IR) in the different hippocampal subfields; B: CA3 region showing no detectable IR in neurons and sparse nuclear IR in glial cells (inset).
Panels C-H: hippocampus 1 week post-SE showing increased ADK expression in glial cells within the different hippocampal subfields, including CA3 (D), CA1 (E-F; arrows), DG (G-H, arrows; arrowheads in G indicate ADK positive cells in the subgranular zone) regions; inset a in D shows ADK positive astrocytes in CA3; inset b in D: merged confocal image, showing ADK positive glial cells (red) surrounding the NeuN (green) positive neuronal cells in CA3. Inset C in D: ADK (red) NeuN (green) co-localization. Panel E: merged confocal image, showing ADK positive glial cells (red) surrounding the NeuN (green) in CA1; inset in G: absence of co-localization of ADK (red) and DCX (green) in the subgranular zone; inset in H shows expression of ADK (red) in astrocytes (GFAP positive, green). Scale bars: A, C: 560 μm; B, D: 140 μm. E: 20 μm; G: 70 μm; F, H: 40 μm.
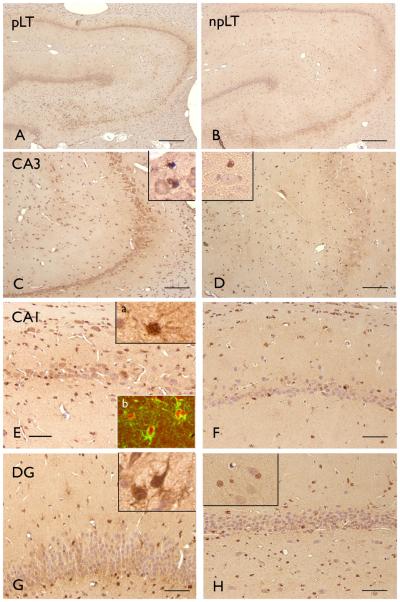
Figure 2. ADK immunoreactivity (IR) in rat hippocampus 3-4 month after SE.
Panels A, C, E, G: hippocampus of rats with long term epilepsy, progressive (pLT; ~10 seizures/day; last seizure less than 2 hours before sacrifice) showing both nuclear and cytoplasmic ADK IR in different hippocampal subfields, including CA3 (C; inset), CA1 (E; inset a) and dentate gyrus (DG, G; inset); Inset in C shows ADK positive glial cells surrounding a neuron with weak ADK. inset b in E shows expression of ADK (red) in astrocytes (GFAP positive, green). Panels B, D, F, H: hippocampus of rats with long term epilepsy, non-progressive (npLT; 1 seizure every other day; last seizure at least 1 day before sacrifice), showing low ADK IR in different hippocampal subfields; mainly nuclear IR is observed in sparse glial cells in CA3 (D, inset), CA1 (F) and DG (H; inset). Scale bars: A, C: 280 μm; C, D: 140 μm. E-H: 70 μm.
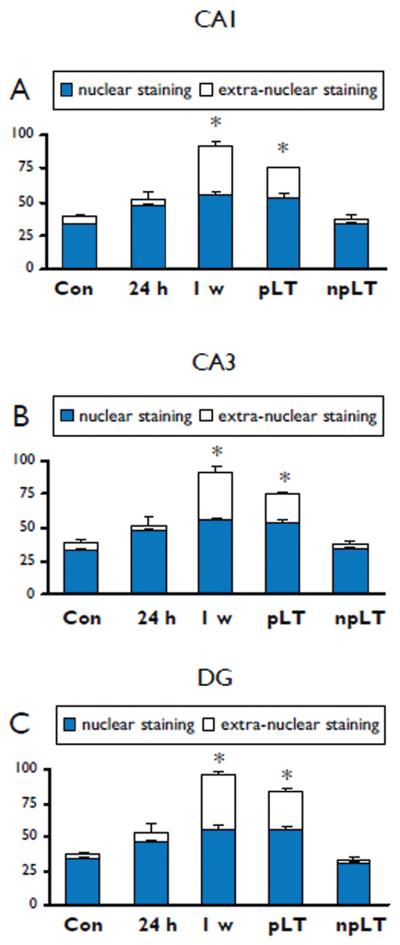
Chronic epileptic rats can be divided in rats with frequent daily seizures and a progressive form of epilepsy (pLT; range 4-16 seizures per day) and rats that had rare seizures that did not progress over time (npLT; ~ 1 seizure every other day; last seizure before sacrifice > 1 day). In chronic epileptic rats that had a progressive form of epilepsy (3-4 months post-SE; pLT), ADK was still increased in astrocytes and a weak ADK IR was detected in some neurons (Fig. 2 A, C, E, G). Co-localization studies indicated that ADK was expressed in GFAP positive cells (astrocytes; Fig. 2 E, inset b). In contrast, in chronic epileptic rats that had a non-progressive form of epilepsy (3-4 months post-SE; npLT) the expression pattern was similar to control hippocampus, with no neuronal expression and a few positive glial cells with nuclear IR (Fig. 2 B, D, F, H). Similar IR pattern, without neuronal expression, was observed in non-SE rat hippocampus (not shown). Quantitative analysis confirmed the increased expression of ADK (nuclear and extra-nuclear staining) at both 1 week and 3-4 months (chronic epileptic rats with progressive form of epilepsy) post SE in astrocytes within different hippocampal regions (Fig. 3 A-C).
Figure 3. Evaluation of ADK astroglial immunoreactivity in hippocampal regions of control rat and after induction of status epilepticus (SE).
Bar diagrams of ADK-positive cells in CA1 (A), CA3 (B) and dentate gyrus (hilar region; C) of control hippocampus and hippocampus at 24 h, 1 week and 3-4 months post-SE (rats with long term epilepsy, progressive, pLT (~10 seizures/day; last seizure less than 2 hours before sacrifice) and rats with long term epilepsy, non-progressive, npLT (1 seizure every other day; last seizure at least 1 day before sacrifice). Nuclear and extranuclear staining: *p< 0.05 vs control, one-way ANOVA followed by Tukey's test.
ADK expression in rat temporal cortex
To determine the temporal-spatial expression and cellular distribution of ADK in temporal cortex, we performed immunocytochemistry in tissue samples of control rats and rats that were sacrificed at different time points after SE (1 day, 1 week and 3-4 months post-SE). Control cortex displayed weak ADK IR (Supplemental Fig. 1 A). No ADK IR was detected in neurons, however sparse, mainly nuclear ADK IR was observed in resting glial cells (inset in A). Only sparse nuclear ADK IR was observed at one day post-SE (not shown). At 1 week post-SE (Supplemental Fig. 1 B-C), increased ADK IR was observed (with cytoplasmic staining) in glial cells. ADK IR was also observed in a few neuronal cells (Supplemental Fig. 1 C, insets a,c). In chronic epileptic rats that had a progressive form of epilepsy (3-4 months post-SE; pLT; Supplemental Fig. 1 D-E) increased ADK IR, with cytoplasmic localization, was still detected in glial cells. Co-localization studies confirmed ADK-expression in GFAP positive cells (astrocytes; Supplemental Fig. 1 E, inset). In contrast, in chronic epileptic rats that had a non-progressive form of epilepsy (3-4 months post-SE; npLT) only sparse nuclear IR was detected in glial cells (Supplemental Fig. 1 F).
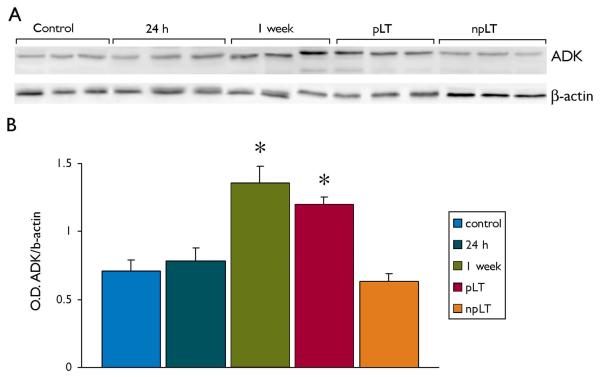
Western blot analysis of total homogenates of rat temporal cortex revealed a band at molecular weight of approximately 40 kDa (Fig. 4 A). Densitometric analysis (Fig. 4 B) of control cortex and cortex from rats at different time points after SE (1 day, 1 week and 3-4 months post-SE) revealed a significant increase in ADK expression at 1 week and in chronic epileptic rats that had a progressive form of epilepsy (pLT).
Figure 4. Western blot analysis of ADK in temporal cortex of control rat and after induction of status epilepticus (SE).
Panel A: representative immunoblots of total homogenates from control rat cortex and after induction of SE (24 h, 1 week, 3-4 months long term progressive epilepsy, pLT, and non-progressive, n-pLT).
Panel B: densitometric analysis: values (optical density units, O.D.) are mean ± SEM , (control, n=6; 24 h, n=4; 1 week, n=6; pLT, n=5; npLT, n=4) , relative to the optical density of β-actin; *, p < 0.05 compared to controls.
ADK expression in hippocampus and cortex of TLE patients with mesial temporal sclerosis (MTS)
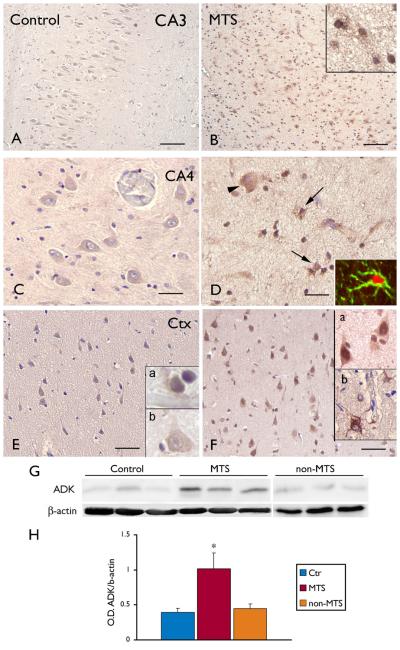
The expression and cellular distribution of ADK was studied by immunocytochemistry in hippocampal specimens of control and TLE patients with MTS. In control (autopsy) hippocampus, ADK displayed weak staining in the different hippocampal subfields, including CA3-CA4 regions (Fig. 5 A, C) and cortex, (Fig. 5 E). A similar immunoreactivity pattern was observed in non-MTS specimens (not shown). In HS specimens, ADK IR was observed in cells with typical astroglial morphology (Fig. 5 B, F). Double labeling confirmed ADK expression in GFAP-positive reactive astrocytes (Figure D, inset). Weak to moderate ADK IR was also observed in a few neuronal cells (Fig. 5 F). No detectable ADK IR was observed in cells of the microglial/ macrophage lineage. Western Blot analysis was also performed to quantify the total amount of ADK in total homogenates of control autopsy cortex and surgical cortex from MTS and non-MTS patients (Fig. 5 G). Densitometric analysis revealed a significant increase of ADK expression in MTS compared to control and non-MTS specimens (Fig. 5 H). No significant correlation was observed between Western blot data (optical density values) and different clinical variables.
Figure 5. Distribution of ADK immunoreactivity in the hippocampus and temporal cortex (Ctx) of control and TLE patients with mesial temporal sclerosis (MTS).
Panels A, C, E: control hippocampus (A, CA3; C, dentate gyrus, hilar region) and Ctx (E) showing weak ADK immunoreactivity (IR) in both glial and neuronal cells (insets a, b in E). Sections are counterstained with hematoxylin. Histologically normal surgical hippocampus and cortex displayed a pattern of IR similar to that observed in control autopsy hippocampus (not shown). Panels B and F: MTS, hippocampal sclerosis (B, CA3; F, dentate gyrus, hilar region), showing increased ADK expression. Expression was observed in residual neurons (arrowhead in D) and in reactive astrocytes (arrows in D).
Panel F: MTS, temporal cortex showing increased ADK expression with both neuronal and glial IR (insets). Scale bars: A, B: 160 μm; C, D 40 μm; E, F 40 μm. Western blot analysis of ADK in temporal cortex: representative immunoblots (G) and densitometric analysis (H) of total homogenates from autopsy control temporal cortex and surgical cortex from MTS and non-MTS specimens. H: densitometric analysis: values (optical density units, O.D.) are mean ± SEM, 5 controls, 5 MTS and 4 non-MTS, relative to the optical density of β-actin; * P< 0.05.
ADK in cultured astrocytes
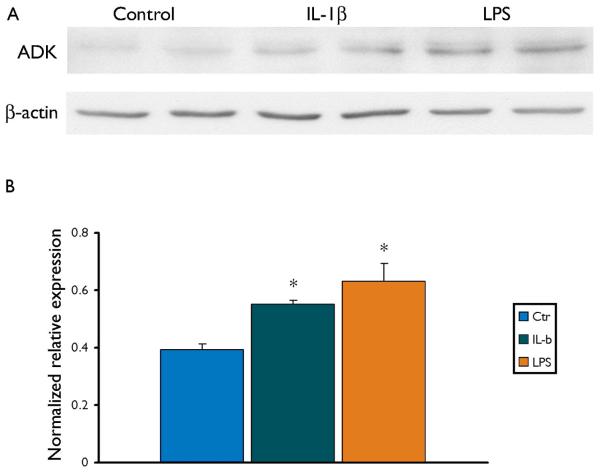
Since IL-1β is known to be activated in both experimental and human TLE (Ravizza, et al. 2008b, Vezzani, et al. 2008), we also investigated whether this inflammatory cytokine could play a role in the regulation of the expression of ADK. Astrocyte-enriched human cell cultures were exposed to LPS and to IL-1β. Western Blot analysis performed in total homogenates demonstrated that both IL-1β and LPS increased the expression of ADK in cultured human astrocytes (Fig. 6 A, B).
Figure 6. ADK in human astrocytes induced by IL-1β and LPS.
Panel A: Representative immunoblot of total homogenates from human fetal astrocytes untreated and treated for 24 h with 10 ng/ml IL-1β or 100 ng/ml LPS.
Panel B: Densitometric analysis: values (optical density units, O.D.) are mean ± SEM, (control, n=3; IL-1β, n=4 and LPS, n=4 treated astrocytes), relative to the optical density of β-actin; *, p < 0.05, compared to controls.
Discussion
Astrocytes play a major role in the regulation of brain levels of adenosine, a nucleoside and inhibitory neuromodulator that can act as an endogenous neuroprotectant and anticonvulsant (Boison 2006, Etherington, et al. 2009, Boison, et al. 2010). Mouse models of epilepsy support a role for astrocytic ADK regulation in epileptogenesis (Boison 2008, Boison 2010). Until now however it has not yet been established whether dysregulation of ADK is a common mechanism being operative in several forms of epilepsy. We therefore assessed the cellular distribution and expression of ADK in both epileptic tissue of TLE patients and in the TLE rat model.
ADK is known to be rapidly downregulated under different conditions of acute brain injury (Pignataro, et al. 2008). Accordingly, in a previous micro-array study, which was performed in the electrical post-SE rat model, we observed that the ADK gene is down-regulated 24 hrs after induction of SE in the CA3 region of the hippocampus (Gorter, et al. 2006). This regulation likely represents an attempt to increase the protective levels of adenosine (Pignataro, et al. 2008), but may also contribute, in concert with the regulation of glial adenosine receptor (AR) expression, to the development of astrogliosis (Boison 2010, Hask, et al. 2005).
In the post-SE rat model (which resembles the mouse models of epileptogenesis (Boison 2010), we detected an upregulation of ADK protein, both in hippocampus and temporal cortex, during the latent phase (1 week post SE), which precedes the development of spontaneous electrographic seizures and is characterized by prominent astrogliosis. Immunocytochemical analysis showed ADK expression in reactive astrocytes with both nuclear and cytoplasmic labeling. Two isoforms of ADK have been identified in mammalian organisms and they have a different subcellular localization (ADK-long and –short isoforms with respectively nuclear and cytoplasmic localization) and different functions (Cui, et al. 2009). Nuclear ADK is likely involved in epigenetic mechanisms, such as methylation reactions, whereas the cytoplasmic isoform is thought to regulate the extracellular levels of adenosine. Accordingly, mice constitutively overexpressing a transgene for the cytoplasmic isoform of ADK are characterized by a reduced adenosine tone, and display spontaneous seizures as well as increased susceptibility to seizure-induced neuronal cell death (Fedele, et al. 2005b, Pignataro, et al. 2007, Li, et al. 2008a, Li, et al. 2008b). This dual functionality is also suggested by previous developmental studies (Studer, et al. 2006). In addition, a more recent study confirms the differential role of ADK isoforms, showing that the cytoplasmic, but not the nuclear isoform of ADK is implicated in sleep regulation (Palchykova, et al. 2010).
The upregulation of ADK in activated astrocytes was also observed in the temporal cortex and persisted in both hippocampus and cortex into the chronic epileptic phase in rats with a progressive form of epilepsy. ADK expression in rats with a non-progressive form of epilepsy was very similar to control expression. These observations support the implication of glial ADK expression in the progression of epilepsy, increasing seizure severity.
In addition, ADK expression in reactive astrocytes has been confirmed in human MTS specimens of patients undergoing surgery for pharmacologically refractory TLE. Increased ADK in human astrocytes may explain the relatively low adenosine baseline levels detected in microdialysis samples of epileptic patients compared to control human hippocampus (During & Spencer 1992).
ADK was predominantly expressed in astrocytes, although neuronal expression was occasionally observed in both rat and human epileptic tissue. Interestingly, ADK is known to be developmentally regulated and ADK expression in neurons has been observed at early postnatal stages in both cerebral cortex and hippocampus (Studer, et al. 2006). Whether this expression may reflect a return to an earlier developmental state, as part of the process of epileptogenesis, and may contribute to a reduction of neuronal adenosine release, supporting a prolonged excitatory activity, requires further investigation. However, DCX positive neuronal progenitor cells, within the dentate gyrus of adult epileptic hippocampus did not express ADK. This observation is in agreement with the previously reported absence of ADK in neuronal progenitor cells in adult mouse hippocampus (Studer, et al. 2006).
We acknowledge limitations to the interpretation of these results. Since we analyzed a relatively small cohort of patients and experimental data were obtained from male rats, we cannot exclude the influence of clinical variables, including gender. Moreover, the expression patterns and regulation of adenosine receptors (A1, A2A, A2B, and A3) deserves further investigation.
We also show that inflammatory molecules, such as LPS and IL-1β may induce increased expression of ADK in human cultured astroglial cells. Inflammatory responses are known to be activated during epileptogenesis and IL-1β is up-regulated in epileptogenic tissue from TLE patients (Ravizza, et al. 2008b, Vezzani, et al. 2008). Thus, this cytokine could play a role in the regulation of ADK levels in astrocytes, providing a potential additional layer of modulatory crosstalk between the astrocyte-based adenosine cycle and inflammation. Further more detailed studies are needed to explore the relevance of this interaction.
In conclusion, the critical role of astroglial ADK in epileptic tissue is supported by these findings, providing additional evidence of ADK regulation in astrocytes during epileptogenesis, as well as during chronic epilepsy in both rat and human TLE. Our findings suggest that overexpression of ADK is a common pathological hallmark of medically intractable chronic epilepsy
Supplementary Material
Panels A: control cortex showing low ADK immunoreactivity (IR); inset in A shows nuclear ADK IR in glial cells.
Panels B-F: ADK IR in cortex after SE. B-C: 1 week post-SE showing increased ADK expression in glial cells (arrows in C) and neurons (arrowheads in C); inset a in C shows ADK positive neurons; inset b in C shows positive glial cells; inset c in C: merged confocal image (ADK, red; NeuN, green); arrow indicates ADK expression in a neuron; arrowheads indicate ADK positive glial cells surrounding negative neurons.
D: 3 months post SE (long term epilepsy, progressive, pLT) showing both nuclear and cytoplasmic ADK IR in glial cells; inset in E: merged confocal image showing ADK (red) expression in astrocytes (GFAP positive, green).
F: 3 months post SE (long term epilepsy, non-progressive, npLT) showing low ADK IR with mainly nuclear IR is observed in sparse glial cells (arrows in inset). Scale bars: A, B, F 140 μm;C -D: 70 μm; E: 40 μm.
Acknowledgements
We are grateful to J.T. van Heteren for her technical help. This work has been supported by National Epilepsy Funds, NEF 09-05 (EA), NEF07-19 (JAG); EU FP7 project NeuroGlia, Grant Agreement N° 202167, and the National Institutes of Health (DB, NIH, USA) through grant R01-NS061844.
We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Footnotes
Disclosure
None of the authors has any conflict of interest to disclose.
References
- Aronica E, Gorter JA, Ijlst-Keizers H, Rozemuller AJ, Yankaya B, Leenstra S, Troost D. Expression and functional role of mGluR3 and mGluR5 in human astrocytes and glioma cells: opposite regulation of glutamate transporter proteins. Eur J Neurosci. 2003;17:2106–2118. doi: 10.1046/j.1460-9568.2003.02657.x. [DOI] [PubMed] [Google Scholar]
- Aronica E, Gorter JA, Rozemuller AJ, Yankaya B, Troost D. Interleukin-1 beta down-regulates the expression of metabotropic glutamate receptor 5 in cultured human astrocytes. J Neuroimmunol. 2005;160:188–194. doi: 10.1016/j.jneuroim.2004.11.015. [DOI] [PubMed] [Google Scholar]
- Aronica E, Gorter J. Gene Expression Profile in Temporal Lobe Epilepsy. Neuroscientist. 2007;13:1–9. doi: 10.1177/1073858406295832. [DOI] [PubMed] [Google Scholar]
- Binder DK, Steinhauser C. Functional changes in astroglial cells in epilepsy. GLIA. 2006;54:358–368. doi: 10.1002/glia.20394. [DOI] [PubMed] [Google Scholar]
- Blumcke I, Pauli E, Clusmann H, Schramm J, Becker A, Elger C, Merschhemke M, Meencke HJ, Lehmann T, von Deimling A, Scheiwe C, Zentner J, Volk B, Romstock J, Stefan H, Hildebrandt M. A new clinico-pathological classification system for mesial temporal sclerosis. Acta Neuropathol. 2007;113:235–244. doi: 10.1007/s00401-006-0187-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blümcke I, Kistner I, Clusmann H, Schramm J, Becker AJ, Elger CE, Bien CG, Merschhemke M, Meencke HJ, Lehman T, Buchfelder M, Weigel, Buslei R, Stefan H, Pauli E, Hildebrandt M. Towards a clinico-pathological classification of granule cell dispersion in human mesial temporal lobe epilepsies. Acta Neuropathol. 2009;117:535–544. doi: 10.1007/s00401-009-0512-5. [DOI] [PubMed] [Google Scholar]
- Boison D. Adenosine kinase, epilepsy and stroke: mechanisms and therapies. Trends Pharmacol Sci. 2006;27:652–658. doi: 10.1016/j.tips.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Boison D. The adenosine kinase hypothesis of epileptogenesis. Prog Neurobiol. 2008;84:249–262. doi: 10.1016/j.pneurobio.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boison D. Adenosine Dysfunction and Adenosine Kinase in Epileptogenesis. Open Neuroscience Journal. 2010;4:93–101. doi: 10.2174/1874082001004020093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boison D, Chen JF, Fredholm BB. Adenosine signaling and function in glial cells. Cell Death Differ. 2010;17:1071–1082. doi: 10.1038/cdd.2009.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui XA, Singh B, Park J, Gupta RS. Subcellular localization of adenosine kinase in mammalian cells: The long isoform of AdK is localized in the nucleus. Biochem Biophys Res Commun. 2009;388:46–50. doi: 10.1016/j.bbrc.2009.07.106. [DOI] [PubMed] [Google Scholar]
- During MJ, Spencer DD. Adenosine: a potential mediator of seizure arrest and postictal refractoriness. Ann Neurol. 1992;32:618–624. doi: 10.1002/ana.410320504. [DOI] [PubMed] [Google Scholar]
- Engel J., Jr. Mesial temporal lobe epilepsy: what have we learned? Neuroscientist. 2001;7:340–352. doi: 10.1177/107385840100700410. [DOI] [PubMed] [Google Scholar]
- Engel J, Jr., Wilson C, Bragin A. Advances in understanding the process of epileptogenesis based on patient material: what can the patient tell us? Epilepsia. 2003;44(Suppl 12):60–71. doi: 10.1111/j.0013-9580.2003.12002.x. [DOI] [PubMed] [Google Scholar]
- Etherington LA, Patterson GE, Meechan L, Boison D, Irving AJ, Dale N, Frenguelli BG. Astrocytic adenosine kinase regulates basal synaptic adenosine levels and seizure activity but not activity-dependent adenosine release in the hippocampus. Neuropharmacol. 2009;56:429–437. doi: 10.1016/j.neuropharm.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedele DE, Gouder N, Gabernet L, Scheurer L, Crestani F, Boison D. Astrogliosis in epilepsy leads to overexpression of adenosine kinase, resulting in seizure aggravation. Brain. 2005a;128:2383–2395. doi: 10.1093/brain/awh555. [DOI] [PubMed] [Google Scholar]
- Fedele DE, Gouder N, Guttinger M, Gabernet L, Scheurer L, Rulicke T, Crestani F, Boison D. Astrogliosis in epilepsy leads to overexpression of adenosine kinase, resulting in seizure aggravation. Brain. 2005b;128:2383–2395. doi: 10.1093/brain/awh555. [DOI] [PubMed] [Google Scholar]
- Gorter JA, Vliet van EA, Aronica E, Lopes da Silva FH. Progression of spontaneous seizures after status epilepticus is related with extensive bilateral loss of hilar parvalbumin and somatostatin immunoreactive neurons. E. J. Neurosci. 2001;13:657–669. doi: 10.1046/j.1460-9568.2001.01428.x. [DOI] [PubMed] [Google Scholar]
- Gorter JA, Van Vliet E, Aronica E, Rauwerda H, Breit T, Lopes da Silva FH, Wadman WJ. Potential New Antiepileptogenic Targets Indicated by Microarray Analysis in a Rat Model for Temporal Lobe Epilepsy. J Neurosci. 2006;26:11083–11110. doi: 10.1523/JNEUROSCI.2766-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouder N, Scheurer L, Fritschy JM, Boison D. Overexpression of adenosine kinase in epileptic hippocampus contributes to epileptogenesis. J Neurosci. 2004;24:692–701. doi: 10.1523/JNEUROSCI.4781-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hask F, Pacher P, Vizi ES, Illes P. Adenosine receptor signaling in the brain immune system. Trends Pharmacol Sci. 2005;26:511–516. doi: 10.1016/j.tips.2005.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Lan JQ, Boison D. Uncoupling of astrogliosis from epileptogenesis in adenosine kinase (ADK) transgenic mice. Neuron GLIA Biology. 2008a;4:91–99. doi: 10.1017/S1740925X09990135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Ren G, Lusardi T, Wilz A, Lan JQ, Iwasato T, Itohara S, Simon RP, Boison D. Adenosine kinase is a target for the prediction and prevention of epileptogenesis in mice. J Clinical Inv. 2008b;118:571–582. doi: 10.1172/JCI33737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukasiuk K, Dabrowski M, Adach A, Pitkanen A. Epileptogenesis-related genes revisited. Prog Brain Res. 2006;158:223–241. doi: 10.1016/S0079-6123(06)58011-2. [DOI] [PubMed] [Google Scholar]
- Maroso M, Balosso S, Ravizza T, Liu J, Aronica E, Iyer AM, Rossetti C, Molteni M, Manfredi AA, BM E, Vezzani A. Toll-like receptor 4 and high-mobility group box-1 are involved in ictogenesis and can be targeted to reduce seizures. Nature Med 1. 2010;16:413–419. doi: 10.1038/nm.2127. [DOI] [PubMed] [Google Scholar]
- Palchykova S, Winsky-Sommerer R, Shen HY, Boison D, Gerling A, Tobler I. Manipulation of adenosine kinase affects sleep regulation in mice. Jf Neurosci. 2010;30:13157–13165. doi: 10.1523/JNEUROSCI.1359-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignataro G, Maysami S, Studer FE, Wilz A, Simon RP, Boison D. Downregulation of hippocampal adenosine kinase after focal ischemia as potential endogenous neuroprotective mechanism. J. Cereb Blood Flow Metab. 2008;28:17–23. doi: 10.1038/sj.jcbfm.9600499. [DOI] [PubMed] [Google Scholar]
- Pignataro G, Simon RP, Boison D. Transgenic overexpression of adenosine kinase aggravates cell death in ischemia. J J. Cereb Blood Flow Metab. 2007;27:1–5. doi: 10.1038/sj.jcbfm.9600334. [DOI] [PubMed] [Google Scholar]
- Pitkanen A. Therapeutic approaches to epileptogenesis—Hope on the horizon. Epilepsia. 2010;52:2–17. doi: 10.1111/j.1528-1167.2010.02602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravizza T, Gagliardi B, Noe F, Boer K, Aronica E, Vezzani A. Innate and adaptive immune mechanisms during epileptogenesis and spontaneous seizures: evidence from experimental models and human temporal lobe epilepsy. Neurobiol Dis. 2008a;29:142–160. doi: 10.1016/j.nbd.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Ravizza T, Gagliardi B, Noe F, Boer K, Aronica E, Vezzani A. Innate and adaptive immunity during epileptogenesis and spontaneous seizures: evidence from experimental models and human temporal lobe epilepsy. Neurobiol Dis. 2008b;29:142–160. doi: 10.1016/j.nbd.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Ren G, Li T, Lan JQ, Wilz A, Simon RP, Boison D. Lentiviral RNAi-induced downregulation of adenosine kinase in human mesenchymal stem cell grafts: a novel perspective for seizure control. Exp Neurol. 2007;208:26–37. doi: 10.1016/j.expneurol.2007.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert G, Carmignoto G, Steinhäuser C. Astrocyte dysfunction in epilepsy. Brain Res Rev. 2010;63:212–221. doi: 10.1016/j.brainresrev.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Studer FE, Fedele DE, Marowsky A, Schwerdel C, Wernli K, Vogt K, Fritschy JM, Boison D. Shift of adenosine kinase expression from neurons to astrocytes during postnatal development suggests dual functionality of the enzyme. Neuroscience. 2006;142:125–137. doi: 10.1016/j.neuroscience.2006.06.016. [DOI] [PubMed] [Google Scholar]
- Vezzani A, Baram TZ. New roles for interleukin-1 Beta in the mechanisms of epilepsy. Epilepsy Curr. 2007;7:45–50. doi: 10.1111/j.1535-7511.2007.00165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezzani A, Ravizza T, Balosso S, Aronica E. Glia as a source of cytokines: implications for neuronal excitability and survival. Epilepsia. 2008;49(Suppl 2):24–32. doi: 10.1111/j.1528-1167.2008.01490.x. [DOI] [PubMed] [Google Scholar]
- Wetherington J, Serrano G, Dingledine R. Astrocytes in the epileptic brain. Neuron. 2008;58:168–178. doi: 10.1016/j.neuron.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieser HG. ILAE Commission Report. Mesial temporal lobe epilepsy with hippocampal sclerosis. Epilepsia. 2004;45:695–714. doi: 10.1111/j.0013-9580.2004.09004.x. [DOI] [PubMed] [Google Scholar]
- Wyler AR, Dohan C, Schweitzer JB, Berry AD., III A grading system for mesial temporal pathology (hippocampal sclerosis) from anterior temporal lobectomy. J Epilepsy. 1992;5:220–225. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Panels A: control cortex showing low ADK immunoreactivity (IR); inset in A shows nuclear ADK IR in glial cells.
Panels B-F: ADK IR in cortex after SE. B-C: 1 week post-SE showing increased ADK expression in glial cells (arrows in C) and neurons (arrowheads in C); inset a in C shows ADK positive neurons; inset b in C shows positive glial cells; inset c in C: merged confocal image (ADK, red; NeuN, green); arrow indicates ADK expression in a neuron; arrowheads indicate ADK positive glial cells surrounding negative neurons.
D: 3 months post SE (long term epilepsy, progressive, pLT) showing both nuclear and cytoplasmic ADK IR in glial cells; inset in E: merged confocal image showing ADK (red) expression in astrocytes (GFAP positive, green).
F: 3 months post SE (long term epilepsy, non-progressive, npLT) showing low ADK IR with mainly nuclear IR is observed in sparse glial cells (arrows in inset). Scale bars: A, B, F 140 μm;C -D: 70 μm; E: 40 μm.