Abstract
Semaphorin 3A (Sema3A) is a secreted factor known to guide axon/dendrite growth and neuronal migration. We found that it also acts as a polarizing factor for axon/dendrite development in cultured hippocampal neurons. Exposure of the undifferentiated neurite to localized Sema3A suppressed its differentiation into axon and promoted dendrite formation, resulting in axon formation away from the Sema3A source, and bath application of Sema3A to polarized neurons promoted dendrite growth but suppressed axon growth. Fluorescence resonance energy transfer (FRET) imaging showed that Sema3A elevated the cGMP but reduced cAMP and protein kinase A (PKA) activity, and its axon suppression is attributed to the down-regulation of PKA-dependent phosphorylation of axon determinants LKB1 and GSK-3β. Down-regulating Sema3A signaling in rat embryonic cortical progenitors via in utero electroporation of siRNAs against the Sema3A receptor neuropilin-1 also resulted in polarization defects in vivo. Thus, Sema3A regulates the earliest step of neuronal morphogenesis by polarizing axon/dendrite formation.
Introduction
The polarized architecture of axon and dendrites is critical for the neuron to function as a computational unit in the nervous system. During early development, the initial establishment of axon/dendrite polarity may depend on intrinsic determinants within the neuron and extrinsic factors from its environment (Arimura and Kaibuchi, 2007; Barnes et al., 2008). The intrinsic determinant may accumulate asymmetrically in the cytoplasm as a result of the last mitotic division (de Anda et al., 2005) or a self-amplification process (Arimura and Kaibuchi, 2007; Shelly et al., 2007) that acts upon local stochastic fluctuation of its distribution or activity (Jacobson et al., 2006), leading to axon/dendrite differentiation. Many extrinsic factors, including gradients of diffusible or bound chemical factors in the developing tissue, may polarize the neuron by setting the axis of the asymmetric division, the direction of axon/dendrite initiation from the soma, and the morphology and orientation of axonal and dendritic arbors (Polleux et al., 1998; Polleux et al., 2000; Noctor et al., 2004; Adler et al., 2006; Hilliard and Bargmann, 2006; Yi et al., 2010). In this study, we focused on the role of Sema3A, a secreted protein of the class III semaphorin superfamily, in axon/dendrite initiation during the early phase of neuronal polarization, prior to its effects as a chemotropic factor for axon/dendrite guidance (Polleux et al., 2000) and neuronal migration (Chen et al., 2008).
In the absence of asymmetric extrinsic cues, dissociated embryonic hippocampal neurons undergo spontaneous polarization in culture - from a cell exhibiting several morphologically similar neurites to a mature neuron exhibiting a single axon and multiple dendrites within few days and capable of forming functional synapses within 1-2 weeks (Dotti and Banker, 1987; Dotti et al., 1988). Using this culture system, previous studies have shown that local activation of either PI3-kinase (Shi et al., 2003; Yoshimura et al., 2005) or cAMP/PKA (Shelly et al., 2007; Shelly et al., 2010) signaling pathways can trigger axon differentiation, through recruitment or phosphorylation of proteins such as plasma membrane ganglioside sialidase (PMGS) (Da Silva et al., 2005), shootin1 (Toriyama et al., 2006), and LKB1 (Barnes et al., 2007; Shelly et al., 2007). These proteins, in turn regulate downstream effectors, such as the PAR3/PAR6/aPKC complex (Shi et al., 2003), GSK-3β (Yoshimura et al., 2005), Rho family of small GTPases (Schwamborn et al., 2004), CRMP2 (Inagaki et al., 2001), as well as PAR-1 related kinases, including SAD-A and SAD-B (Kishi et al., 2005), leading to local regulation of cytoskeletal dynamics that are required for axon growth. In these studies, axon differentiation of one neurite is accompanied by dendrite formation in other neurites, suggesting that dendrite formation may represent a default pathway in the absence of axon formation. However, the possibility remains for the existence of factors that specifically trigger dendrite formation either by actively suppressing axon differentiation or directly promoting dendrite formation and growth. In this study, we found that Sema3A is indeed a factor that promotes dendrite differentiation by suppressing axon development.
We have previously shown that local cAMP and cGMP activities exert antagonistic actions on axon/dendrite polarization (Shelly et al., 2010). These effects are attributed to reciprocal regulation between cAMP and cGMP, through the activation of specific phosphodiesterases (PDEs) and protein kinases, and differential regulation of PKA-dependent phosphorylation of LKB1 and GSK-3β, two proteins critical for axon formation (Shelly et al., 2010). The identity of cAMP/cGMP-modulating extracellular factors responsible for polarizing neurons in vivo remains unknown. In the developing cortex, Sema3A is expressed in a descending gradient across the cortical layers, with the highest expression at the pial surface, whereas its receptor neuropilin-1 (NP1) is expressed at a high level in developing cortical neurons (Polleux et al., 2000; Chen et al., 2008). The Sema3A gradient was shown to function as a chemoattractant for orienting apical dendrites of cortical pyramidal neurons towards the pial surface (Polleux et al., 2000), and for guiding radial migration of these neurons (Chen et al., 2008). These roles of Sema3A signaling in the developing cortex prompted us to examine whether Sema3A plays an early role in neuronal polarization by regulating axon/dendrite differentiation. Using cultured hippocampal neurons, we found that Sema3A regulates neuronal polarization by suppressing axon formation and promoting dendrite growth. These effects were mediated by reciprocal regulation between cAMP and cGMP signals - Sema3A elevated cytoplasmic cGMP, which in turn caused the reduction of cAMP/PKA activities via cGMP/PKG-dependent activation of cAMP-selective phosphodiesterase PDE4. The suppressive effect of Sema3A on axon formation was mediated by the antagonistic action of Sema3A-induced cGMP on PKA-dependent phosphorylation of LKB1 and GSK-3β. In polarized neurons, Sema3A also promoted the growth of dendrites and suppressed that of the axon. Furthermore, down-regulation of the NP1 in rat embryonic cortical progenitor cells via in utero electroporation of NP1-specific small interference RNA (siRNAs), resulted in polarization defects of postmitotic cortical neurons. Finally, we showed that Sema3A and BDNF exert antagonistic actions on axon/dendrite initiation, and these effects are mediated by reciprocal regulation of cAMP/cGMP signaling.
Results
Local Exposure to Sema3A Polarizes Axon/Dendrite Formation
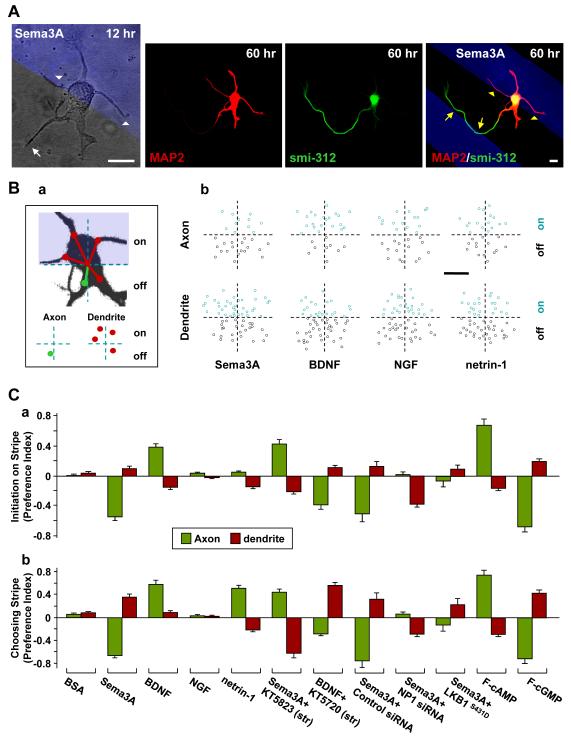
We first examined whether Sema3A serves as a polarizing factor for axon/dendrite differentiation in cultured hippocampal neurons (Dotti and Banker, 1987; Dotti et al., 1988). For comparison, we also tested the effect of netrin-1, BDNF, and NGF, secreted factors known to be involved in neuronal polarization in various systems. Dissociated hippocampal neurons from rat embryos were plated on substrates coated with stripes (50 μm wide with 50 μm gap) of the recombinant form of Sema3A, BDNF, NGF, or netrin-1 (see Experimental Procedures). To examine neuronal polarization, neurons were imaged at 12 and 60 hr after cell plating, before and after axon/dendrite differentiation, respectively. At 12 hr, the cells exhibited several short neurites of similar lengths without apparent polarity (Figure 1A), whereas most cells developed a single axon and multiple dendrites at 60 hr, as shown by immunostaining with axonal marker Smi-312 and somatodendritic marker MAP2 (Figure 1A). Strikingly, we found that axons were mostly formed off the Sema3A-coated stripe, whereas more dendrites were found to differentiate on than off the Sema3A stripe (Figure 1A). Furthermore, axonal growth cones often turned at the stripe boundary to stay away from the Sema3A stripe, whereas dendrites showed opposite tendency (Figure 1A), suggesting attractive and repulsive actions of Sema3A on dendritic and axonal growth cones, respectively.
Figure 1. Opposite Effects of Sema3A on Axon and Dendrite Differentiation.
(A) Images of an example hippocampal neuron cultured on substrates coated with stripes (blue) of Sema3A, taken at 12 and 60 hr after cell plating and immunostaining (at 60 hr) for axons and dendrites with smi-312 and MAP2 antibodies, respectively. White arrows and arrowheads mark neurites that later became the axon and dendrites, respectively. Yellow symbols mark axon/dendrite turning at the stripe boundary. Scale is 10 μm.
(B) Preferential axon/dendrite differentiation was quantified retrospectively at 48-60 hr after plating, for polarized neurons with somata located at the stripe boundary. (a) Schematic diagram depicts the determination of the angular location of neurite initiation sites on the soma for all neurites that became the axon or dendrites at 48-60 hr. The initiation site (marked by the circle) was determined relative to the center of the stripe boundary intersecting the soma (axon, green; dendrites, red). (b) Angular locations of all initiation sites, either “on-stripe” (blue circles) or “off-stripe” (black circles), for axons (30 cells) and dendrites (10 cells) initiated on substrates striped with Sema3A, BDNF, NGF, or netrin1. Scale is 5 μm.
(C) Summary of preferential axon/dendrite differentiation (a) and pathfinding (b) for all neurons cultured on substrates striped with various factors indicated. Preference index (PI) was defined as [(% on stripe) − (% off stripe)] / 100%. Data shown are average PIs for axon/dendrite differentiation for neurons with somata located on the stripe boundary (a), and pathfinding for all neurons (b), determined at 48-60 hr after cell plating. Substrates were stripe-coated with BSA, Sema3A, BDNF, NGF, netrin1, F-cAMP, or F-cGMP, either alone or together with KT5720 or KT5823. Treatments included transfection with GFP-LKB1S431D, NP1-siRNA or control siRNA. Data represents average ± SD (n = 50 - 75 cells, 3 cultures each). Significant differences were found for all treatments from corresponding values found for BSA stripes (P < 0.001, two tailed t-test or Kolmogorov-Smirnov test).
The effect of Sema3A on axon/dendrite formation was quantified by determining the distribution of axon/dendrite initiation sites on the soma for all polarized cells with their somata located on the stripe boundary at 48-60 hr, when neurons had completed the polarization process (Figures 1Ba and 1Bb). Because the neurite initiation site on the soma does not move significantly during axon/dendrite differentiation (Figure 1A), this retrospective analysis allowed us to determine whether coated stripes influenced axon/dendrite differentiation after neurites had been initiated from the soma. We found that axon differentiation largely occurred for neurites initiated off the Sema3A stripe, whereas slightly more dendrites developed on the Sema3A stripe (Figure 1Bb). We also found that the preference of axon/dendrite formation on BDNF-coated stripes was opposite to that for Sema3A stripes (Figure 1Bb), consistent with a previous report (Shelly et al., 2007). In contrast, we found no preference of axon/dendrite differentiation for stripes coated with BSA or NGF, and a slight preference of dendrite differentiation away from the netrin-1 stripes (Figure 1Bb). In Figure 1Ca, these results on axon/dendrite formation are quantified by using the preference index (PI = [(% on stripe) − (% off stripe)] / 100%. Overall, the most striking effect of Sema3A on neuronal polarization is its suppression of axon differentiation, resulting in strong preference of axon formation away from Sema3A stripes (Figures 1Bb and 1Ca). The small but significant preference for dendrite formation on the Sema3A stripe (Figures 1Bb and 1Ca) may result indirectly from axon formation away from the stripe that reduced the overall frequency of dendrite formation off the stripe, or could be a direct result of Sema3A in promoting dendrite growth (see Figure 5 below). Retrospective analysis on the choice preference of axon/dendrite growth cones for the striped surface when they encountered the stripe boundary (Figures S1 and 1Cb) also showed strong preference for axon and dendrite to turn away or stay on the Sema3A stripe, respectively (Figure 1Cb), in agreement with the known function of Sema3A as a guidance factor for axon/dendrite pathfinding in the developing cortex (Polleux et al., 2000).
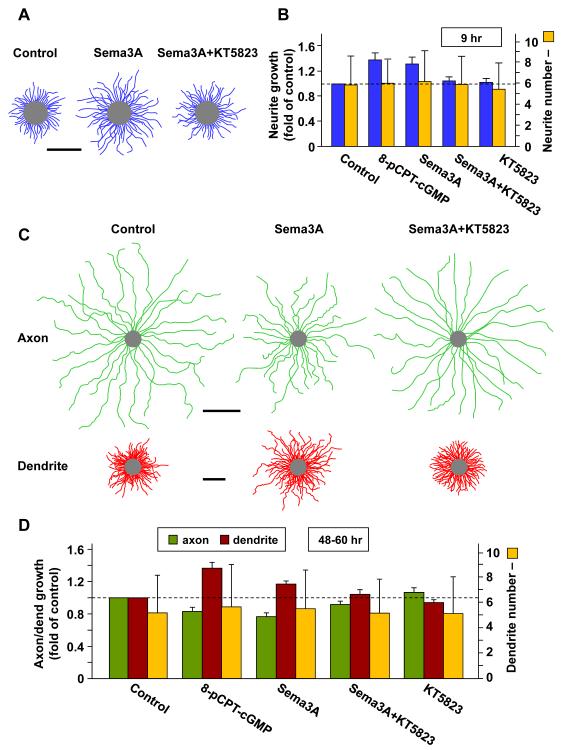
Figure 5. Sema3A Promotes Growth of Undifferentiated Neurites and Dendrites.
(A, B) Sema3A promotes uniform growth of undifferentiated neurites. Hippocampal neurons were incubated with Sema3A in the bath from 2 hr after plating, and neurite lengths were measured at 9 hr. (A) Composite tracings depict undifferentiated neurites of 10 randomly sampled neurons in control or Sema3A-treated cultures in the absence or presence of KT5823. Scale is 50 μm. (B) Average neurite length and average neurite number (± SD; n = 3 cultures, 50-75 cells each) in parallel control untreated cultures, and cultures treated with the conditions indicated, shown as fold of control.
(C, D) Sema3A promotes dendrite growth and suppresses axon growth in polarized neurons. Hippocampal neurons were incubated with Sema3A in the bath from 24 hr after plating. The average axon and dendrite lengths were measured at 48-60 hr following co-immunostaining with axon- and dendrite-specific marker smi-312 and MAP2, respectively. (C) Composite tracings depict randomly sampled cells (axons, 25 cells, green; dendrites, 15 cells, red) in control cultures or cultures treated with Sema3A, in the absence or presence of KT5823. Scale is 50 μm. (D) Histograms show the average axon and dendrite length and average dendrite number (± SD; n = 3 cultures, 50-75 cells each), in parallel control untreated cultures, and cultures treated with the conditions indicated, shown as fold of control.
The effects of Sema3A on both axon/dendrite initiation and pathfinding were mediated by its receptor NP1, because when NP1 was down-regulated in these neurons by transfection at 4 hr after plating with constructs expressing two specific siRNAs against NP1 (Chen et al., 2008), together with enhanced green fluorescent protein (EGFP) and examined at 60 hr, the polarized neurons showed a striking absence of preferential axon initiation at the stripe boundary and dendrite initiation preference away from the stripe (Figure 1Ca), whereas transfection with control siRNA had no effect. Down-regulation of NP1 also resulted in similar effects on the choice preference of axon/dendrite growth cones for the Sema3A striped surface (Figure 1Cb).
Sema3A/BDNF-Induced Neuronal Polarization is Mediated by cGMP/cAMP
Previous studies suggest that cAMP and cGMP signaling may transduce antagonistic actions of extracellular factors on axon/dendrite formation through their reciprocal regulation – elevating cAMP promotes axon differentiation and suppresses dendrite formation, whereas elevating cGMP has opposite effects (Shelly et al., 2010; Figure 1Ca). Notably, these cGMP and cAMP effects resemble those described above for stripes coated with Sema3A and BDNF, respectively (Figures 1Bb and 1Ca). We thus tested whether the axon/dendrite differentiation effects of Sema3A- and BDNF-striped substrates are indeed mediated by cGMP and cAMP elevation in the neurite, respectively. Using stripes coated with specific PKG inhibitor KT5823 and PKA inhibitor KT5720 together with Sema3A and BDNF, respectively, we found that axon differentiation and pathfinding showed preferences (Figure 1C) similar to those found for substrates coated with membrane-permeable fluoresecent analogue of cAMP (F-cAMP, see Experiemental Procedures) and F-cGMP alone, respectively (Figure 1C; Shelly et al., 2010). Local PKG and PKA inhibition thus had not only eliminated the effects of Sema3A and BDNF, respectively, but also produced axon/dendrite polarization effects similar to that resulted from elevating cAMP and cGMP. These results suggest that the Sema3A and BDNF effects on axon/dendrite differentiation and pathfinding are mediated by cGMP/PKG and cAMP/PKA activities, respectively.
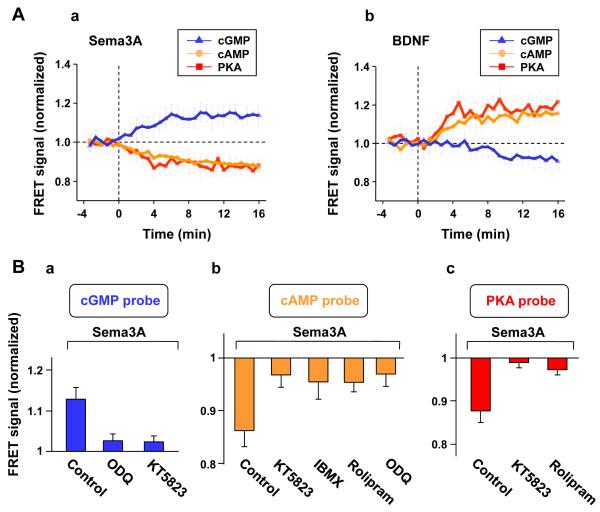
Using FRET sensors expressed individually in these cultured hippocampal neurons, we have directly measured changes in the level of cGMP, cAMP, or PKA activity induced by bath-applied Sema3A and BDNF. The cells were transfected at 2 hr after cell plating with one of the constructs encoding the FRET reporter for cGMP (cGES-DE5) (Nikolaev et al., 2006), cAMP (ICUE) (DiPilato et al., 2004), or PKA activity (AKAR) (Zhang et al., 2001). The FRET measurements were performed at 10-16 hr after cell plating, when most neurons had extended multiple neurites of similar morphology without apparent axon/dendrite differentiation. The FRET signals at the neurite, as indicated by the ratio of YPF to CFP fluorescence for AKAR and cGES-DE5, and the ratio of CPF to YFP fluorescence for ICUE (see Experimental Procedures), showed that bath application of Sema3A (1 μg/ml) induced a gradual elevation of the cGMP level, as well as a gradual reduction of the cAMP level and PKA activity (Figure 2Aa). Interestingly, bath application of BDNF (50 ng/ml) resulted in effects opposite to that induced by Sema3A – increasing cAMP/PKA activity while decreasing cGMP (Figure 2Ab), whereas similar treatment with NGF (50 ng/ml) did not cause any change in FRET signals (data not shown), consistent with the lack of effect of NGF on axon/dendrite polarization (Figures 1Bb and 1Ca). The opposite actions of Sema3A and BDNF on the cAMP/cGMP level support the notion that their opposite axon/dendrite polarization effects are mediated directly by these cyclic nucleotides, which exhibit reciprocal down-regulation in these neurons (Shelly et al., 2010).
Figure 2. Sema3A Induces Elevation of cGMP and Reduction of cAMP/PKA in a PKG- and PDE-Dependent Manner.
(A) Summary of the average FRET signal for all 16-hr hippocampal neurons expressing the FRET sensors for cGMP (cGES-DE5), cAMP (ICUE), or PKA activity (AKAR), before and after bath application of Sema3A (a) or BDNF (b) observed at the neurite. The YFP/CFP fluorescence intensity ratio for the PKA-activity and the cGMP probes and the CFP/YFP fluorescence intensity ratio for the cAMP sensor representing the FRET signal were averaged over 40s for each cell and normalized by the mean control value before Sema3A or BDNF application. Data points are average ± SEM (n = 5 cells each, up to two neurites per cell).
(B) The role of PDEs and PKG in Sema3A-induced regulation of cGMP and cAMP/PKA. The data represent average Sema3A-induced (± SEM, n = 5 cells each, up to two neurites per cell) FRET signals for cGMP (a), cAMP (b), or PKA activity (c), measured at the neurite, at 10-20 min after bath application of Sema3A following pre-incubation with various drugs, as compared to the FRET signal prior to Sema3A application. Drug treatments included: sGC inhibitor ODQ, PKG inhibitor KT5823, general PDE inhibitor IBMX, or cAMP-specific PDE4 inhibitor rolipram. Data include those shown in A.
Mechanisms Underlying Sema3A-Induced Changes in cGMP and cAMP
The reciprocal regulation between cAMP and cGMP levels in these cultured neurons is mediated by activation of PKA/PKG and cyclic nucleotide-specific PDEs (Shelly et al., 2010). Given the finding that Sema3A/BDNF effects on dendrite/axon formation depend on PKG/PKA activities (Figure 1Ca), we further inquired whether Sema3A-induced reduction of cAMP depends on the activation of PKG and cAMP-specific PDE. Further measurements using FRET sensors yielded the following two findings. First, pre-incubation of these cultured neurons with PKG inhibitor KT5823 (200 nM) prevented the effect of Sema3A on cGMP elevation (Figure 2Ba) as well as the reciprocal down-regulation of cAMP and PKA activity (Figures 2Bb and 2Bc). Second, this reduction of cAMP/PKA activity was also prevented by pre-incubation of the cells with either the non-specific PDE inhibitor 3-Isobutyl-1-methylxanthine (IBMX, 50 μM) or cAMP-selective PDE4 inhibitor rolipram (1 μM) (Figures 2Bb and 2Bc). Finally, FRET measurements also showed that pre-incubation with the soluble guanylate cyclase (sGC) inhibitor ODQ (1 μM) abolished the Sema3A-induced elevation and reduction of cGMP and cAMP levels, respectively (Figures 2Ba and 2Bb). Together, these results are consistent with the notion that Sema3A-induced changes in cGMP/cAMP are due to a PKG-dependent regulation of sGC (Figure 2B; Polleux et al., 2000; Togashi et al., 2008) and cAMP-selective PDE activities, in a manner consistent with the reciprocal regulation between cAMP and cGMP reported previously (Shelly, et al. 2010).
Sema3A Antagonizes PKA-Dependent Phosphorylation of LKB1 and GSK-3β
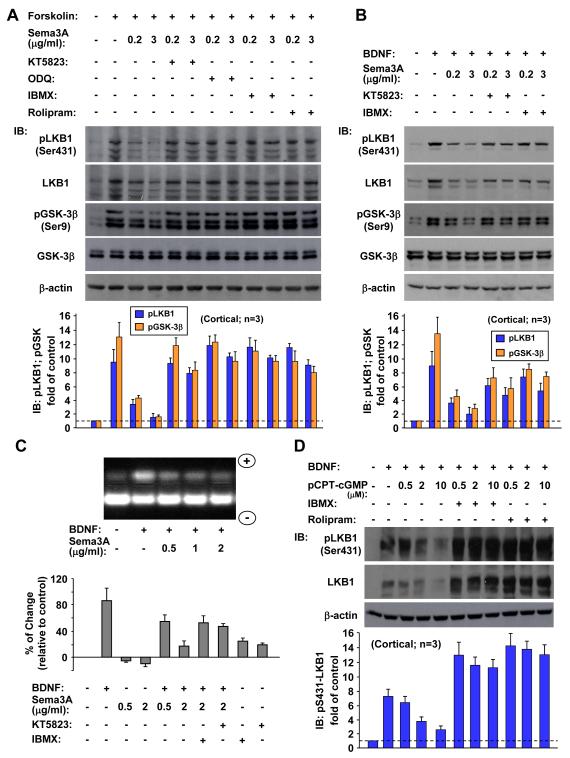
Previous studies have shown that axon initiation triggered by localized exposure to cAMP analogue or BDNF requires PKA-dependent phosphorylation of LKB1, a serine/threonine kinase that is essential for axon formation (Shelly et al., 2007). We have also shown that GSK-3β, a crucial axon determinant downstream of BDNF/PI3-kinase signaling, is also phosphorylated upon elevation of cAMP/PKA activity and that BDNF-induced GSK-3β phosphorylation may depend on both PI3K and PKA signaling pathways (Shelly et al., 2010). Furthermore, cGMP elevation antagonizes the PKA-mediated LKB1 and GSK-3β phosphorylation by down-regulation of cAMP, through the activation of PDE4 (Shelly et al., 2010). Because Sema3A increased the cGMP level (Figure 2), the polarizing effect of Sema3A on axon/dendrite differentiation may be attributed directly to the suppressive action of the Sema3A-induced cGMP on cAMP-dependent LKB1 and GSK-3β phosphorylation. This idea was tested by the following experiments using immunoblotting of lysates of cultured cortical neurons with phosphorylation site-specific antibodies. The results showed that elevating cAMP synthesis in these neurons with forskolin induced LKB1 phosphorylation at serine 431 (S431) and GSK-3β phosphorylation at serine 9 (S9) (Figure 3A; Shelly et al., 2010), and such phosphorylation was prevented in a dose-dependent manner by co-application of Sema3A (Figure 3A). The time course of the Sema3A-dependent reduction of forskolin-induced LKB1 and GSK-3β phosphorylation (Figure S2) correlated well with the Sema3A-induced elevation of cGMP activity (Figure 2). The Sema3A treatment also diminished dose-dependently the BDNF-induced phosphorylation of these proteins (Figure 3B) in a similar manner to the antagonistic effect of 8-pCPT-cGMP on the BDNF action (Figure 3D). Of note, the elevation of pLKB1-S431 correlated with that of the total level of LKB1, consistent with previous report (Shelly et al., 2007). The increased accumulation of LKB1 caused by forskolin- or BDNF-induced PKA-dependent phosphorylation of LKB1 (Figure 3) or by LKB1-STRAD interaction (Shelly et al., 2007) could be attributed to the reduction in LKB1 ubiquitination (Figure S3; Cheng et al., 2011) and the consequent reduced degradation. Peptide-based PKA activity assay in cultured hippocampal neurons also showed that Sema3A dose-dependently reduced the basal as well as BDNF-induced PKA activity (Figure 3C).
Figure 3. Sema3A Antagonizes cAMP- and BDNF-Induced Phosphorylation of LKB1 and GSK-3β in a PKG- and PDE-Dependent Manner.
(A, B) Immunoblots of total cell lysates of 5-d cultures of cortical neurons, with phosphorylation site-specific antibodies targeted at the PKA site-S431 of LKB1, S9 of GSK-3β, and with protein-specific antibodies. Cells were treated with forskolin (A) or BDNF (B) either alone or together with increasing concentrations of Sema3A, in the absence or presence of KT5823, ODQ, IBMX, or rolipram. Note that the phosphorylation of LKB1 at S431 correlated with an elevated LKB1 level. Histograms below show the average phosphorylation level of pLKB1-S431 and pGSK-3β, normalized to β-actin, shown as fold of control (± SD, n = 3). All values are significantly higher than the control (p < 0.01, t-test).
(C) Peptide-based assays of PKA activity show that the antagonistic effect of Sema3A on BDNF-induced PKA activation depended on PDE and PKG activation. Total cell lysates of 16-hr cultures of hippocampal neurons were assayed for PKA activation using a fluorescent, PKA-specific peptide substrate (see Experimental Procedures). Cultures were treated with BDNF together with different concentrations of Sema3A, either alone or in combination, or left untreated. Above: peptide migration assay, with PKA activity indicated by the intensity of phosphorylated peptide migrating towards the cathode (“+”). Histograms show the average phosphorylated fluorescent peptide in cultures treated with BDNF either alone or together with increasing concentrations of Sema3A, in the absence or presence of KT5823 or IBMX, shown as the average percentage (± SD, n = 3) of change relative to control untreated cultures.
(D) Antagonistic effect of cGMP activity on BDNF-induced LKB1 phosphorylation is mediated by cAMP-specific PDEs. Immunoblotting as in (A, B), with antibodies targeted at the phospho-S431 of LKB1 or the LKB1 protein. Cells were treated with BDNF, either alone or in combination with increasing concentrations of 8-pCPT-cGMP, and in the absence or presence of IBMX or rolipram. LKB1 phosphorylation at S431 (normalized to β-actin) is shown as fold of control (± SD, n = 3).
The reciprocal regulation between cAMP and cGMP and Sema3A/BDNF-induced reciprocal regulation of these cyclic nucleotides are both modulated by specific PDEs and PKA/PKG activities (Figure 2B; Shelly et al., 2010). Consistently, we found that the suppressive effect of Sema3A or cGMP on forskolin or BDNF-induced LKB1/GSK-3β phosphorylation and PKA activity was greatly reduced by inhibiting PDEs with IBMX (Figures 3A, 3B, 3C and 3D) and abolished by cAMP-specific PDE4 inhibitor rolipram (Figures 3A and 3D), indicating that Sema3A induced-cGMP activity reduced cAMP-mediated signaling primarily via activation of cAMP-specific PDE4. Inhibition of PKG with KT5823 had similar effects as PDE inhibitors (Figures 3A, 3B and 3C). Finally, inhibiting cGMP synthesis with ODQ prevented the antagonistic effect of Sema3A on forskolin-induced LKB1 and GSK-3β phosphorylation (Figure 3A). Thus, axon suppression and neuron polarizing effects of Sema3A could be accounted for by its elevation of cGMP, which reduced cAMP/PKA activity by activating PKG and cAMP-selective PDEs, leading to the suppression of PKA-dependent LKB1/GSK-3β phosphorylation that is critical for axon formation.
To test further whether the suppression of LKB1-S431 phosphorylation is critical for Sema3A effect on axon initiation, we performed Sema3A stripe assay for neurons transfected with a construct expressing LKB1 with S431 site mutated to aspartic acid (LKB1S431D), mimicking the phosphorylated LKB1 (at S431). As shown in Figure 1Ca, preferential axon initiation was indeed abolished for neurons over-expressing LKB1S431D, consistent with the notion that LKB1S431D is no longer subjected to suppression by Sema3A.
Sema3A Prevents Preferential pLKB1-S431 Accumulation
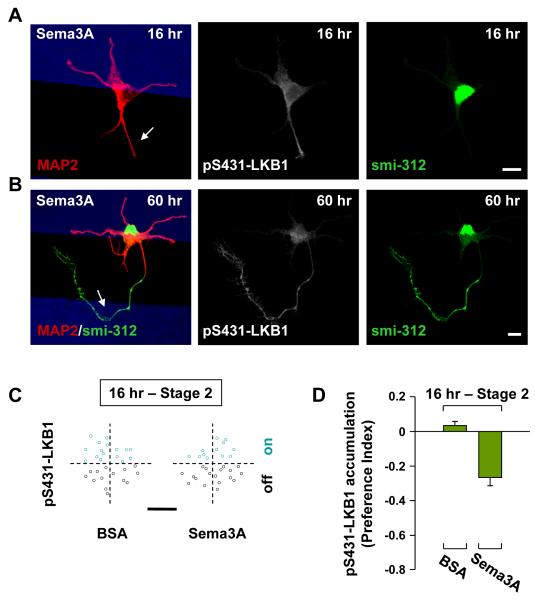
Localized elevation of cAMP activity is sufficient to initiate axon differentiation through PKA-dependent phosphorylation and accumulation of LKB1 (Shelly et al., 2007), an essential protein for axon formation in vivo (Barnes et al., 2007; Shelly et al., 2007). Consistent with this critical function of PKA-dependent LKB1 phosphorylation and the antagonistic effect of Sema3A on cAMP activity (Figure 3), we found that phosphorylated LKB1 (pLKB1-S431) showed early accumulation (at 10-16 hr after cell plating) in undifferentiated neurites off the Sema3A stripe (Figure 4A) and the accumulation persisted in axons after neuronal polarization (Figure 4B). The effect of Sema3A on LKB1 phosphorylation and on early pLKB1-S431 accumulation was quantified for all cells with their somata located on the stripe boundary, by determining the distribution of initiation sites on the soma of the most prominent pLKB1-S431-enriched neurite in all un-polarized cells at 16 hr. We found that pLKB1-S431 expression was largely associated with undifferentiated neurites initiated off the Sema3A stripe (Figure 4C). Preferential pLKB1-S431 accumulation were quantified by using the preference index (PI = [(% on stripe) − (% off stripe)] / 100% and the result further supports the notion that the polarizing action of Sema3A depends on local prevention of PKA-dependent phosphorylation and accumulation of LKB1 (Figure 4D). Finally, we note that at 60 hr when neurons became polarized, most axons showed highest accumulation of pLKB1-S431 regardless of the location of axon on or off the Sema3A stripe, whereas dendrites mostly showed low pLKB1-S431 expression.
Figure 4. Polarized Distribution of pLKB1-S431 Induced by Local Sema3A Exposure.
(A, B) Localized Sema3A inhibited LKB1 phosphorylation in undifferentiated neurites and axons. Example images of hippocampal neurons at 16 (A) and 60 (B) hr after cell plating, on substrates striped (blue) with Sema3A, co-immunostained with axon- and dendrite-specific marker smi-312 and MAP2, respectively, together with pLKB1-S431. Arrows: neurites or axons showing accumulation of pLKB1-S431 off the Sema3A stripe. Scale is 10 μm.
(C) Preferential expression of pLKB1-S431 was quantified by the distribution of the initiation site for the neurite with highest accumulation of pLKB1-S431 among all neurites of the same neuron at 16 hr, for all neurons with somata located at the BSA or the Sema3A stripe boundary. Scale is 5 μm.
(D) Summary of preferential pLKB1-S431 accumulation in neurites at 16 hr, using Preference Index (PI), as described in Figure 1.
Regulation of Neurite and Axon/Dendrite Growth by Sema3A
Our results described above (Figures 1-3) showed that the opposing effects of Sema3A and BDNF on axon/dendrite initiation could be attributed directly to the antagonism between the cGMP and cAMP pathways at the level of cyclic nucleotides and their effector enzymes. Axon formation in cultured hippocampal neurons is also tightly linked to the preferential growth acceleration of a single undifferentiated neurite (Dotti and Banker, 1987; Dotti et al., 1988). We have previously shown that cAMP and cGMP may regulate the growth of neurites and axon/dendrite in distinct manners (Shelly et al., 2010). We thus examined the effect of Sema3A on the growth of both undifferentiated neurites and axon/dendrite of polarized neurons. For effects on neurite growth, we bath-applied Sema3A at 2 hr after cell plating in the culture medium and measured the average neurite length at 9 hr. We found that the Sema3A treatment resulted in a uniform increase in the growth of all neurites (Figures 5A and 5B), similar to that found by bath-applied 8-pCPT-cGMP (Figure 5B; Shelly et al., 2010). The Sema3A effect was PKG-dependent because it was prevented by the presence of KT5823 in the bath (Figures 5A and 5B), whereas application of KT5823 alone had a minor effect on neurite growth (Figure 5B).
Next, we examined the effect of Sema3A on axon/dendrite growth after neuronal polarity is established. We added Sema3A to the culture 1 d after cell plating and measured the length of axon and dendrite at 48-60 hr. We found that Sema3A treatment resulted in increased dendrite length but reduced axon length, as compared to that found in parallel control cultures not treated with Sema3A (Figures 5C and 5D). The Sema3A effect was similar to that found for 8-pCPT-cGMP treatment (Figure 5D) and was largely prevented by the presence of the PKG inhibitor KT5873 (Figures 5C and 5D), whereas KT5823 alone had no significant effect on axon/dendrite growth (Figure 5D). We note that various treatments had no effect on the number of neurites at 9 hr or of axon/dendrites at 48-60 hr (Figures 5B and 5D). Taken together, these results indicate that Sema3A-induced PKG activity promotes neurite growth and differentially activates effectors of cytoskeletal dynamics in dendrites that are distinct from those in the axon, leading to promotion of dendrite growth and suppression of axon growth.
Polarization Defects Induced by Down-Regulating Sema3A Signaling In Vivo
The mechanisms that determine neuronal polarization in vivo remain largely unknown. Given the polarizing effects of Sema3A on cultured hippocampal neurons (Figure 1), the existence of Sema3A gradient in the developing cortex (Polleux et al., 2000), and the expression of NP1 in cortical pyramidal neurons (Chen et al., 2008), we tested whether Sema3A also serves as a neuron polarizing factor in vivo by perturbing Sema3A signaling in newly generated cortical neurons in rat embryos. The expression of NP1 in a subpopulation of neural progenitor cells was down-regulated by in utero electroporation at embryonic day 18 (E18) with two constructs expressing specific siRNAs against NP1 (Chen et al., 2008) together with EGFP. When developing cortical neurons derived from siRNA-expressing progenitor cells were examined in E21 embryos, we found that neurons expressing the NP1-siRNA exhibited impaired radial migration to the cortical plate (CP) (Figures 6A and 6B), and displayed miss-orientation with respect to the CP (Figure 6B, arrows), as compared to neurons expressing control siRNA, as previously demonstrated (Chen et al., 2008). This was quantified by the percentage of transfected cells located in the ventricular zone (VZ) / subventricular zone (SVZ), in the intermediate zone (IZ), and the CP (Figure 6C), with much higher and lower fraction of NP1-siRNA transfected neurons located at the VZ/SVZ and CP, respectively, as compared to control cells.
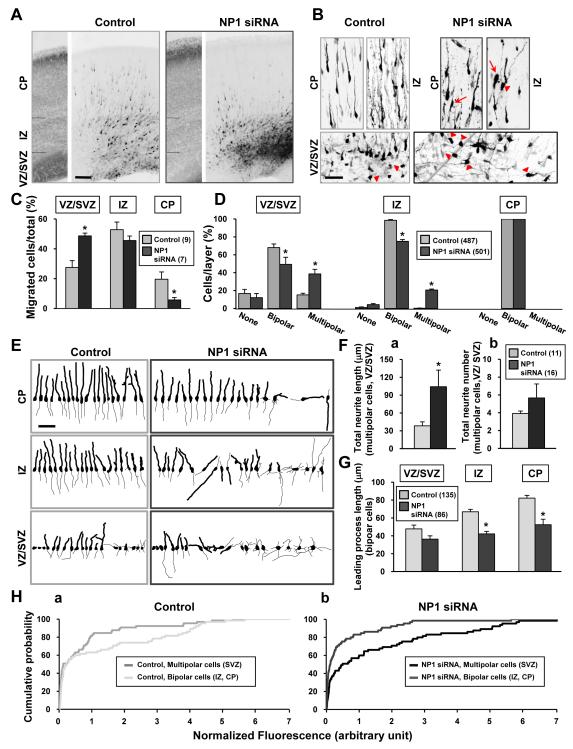
Figure 6. Down-Regulation of neuropilin-1 Disrupted Polarity Formation of Cortical Neurons In Vivo.
(A) Confocal images of GFP fluorescence of E21 rat cortices transfected via in utero electroporation at E18 (see Experimental Procedures) with the pRNAT-U6.3 vector expressing EGFP, together with either control siRNA or siRNAs directed against NP1. The panel on the left shows Hoechst staining for layer determination. Scale, 100 μm.
(B) Higher magnification images of transfected cortical neurons, taken at the level of the ventricular zone (VZ) / subvemtricular zone (SVZ), the intermediate zone (IZ), and the cortical plate (CP), from similar slices as in (A). Arrows mark cells with wrong orientation with respect to the CP. Arrow heads mark cells with multipolar morphology. Scale is 50 μm.
(C) Neuronal migration from the VZ/SVZ to the CP was quantified by determining the percentage of transfected cells located in the different layers for each treatment, from similar sections as in (A, B). Impeded radial migration was found for neurons expressing NP1 siRNAs at VZ/SVZ and CP levels (One Way Analysis of Variance, P < 0.001; post-hoc Student-Newman-Keuls Method P < 0.05). Histograms show the average percentage (± SEM). Numbers of animals analyzed are shown in parenthesis.
(D) Quantitative measurements of the polarity of transfected cortical neurons, at the level of the VZ/SVZ, the IZ, and the CP, from similar sections as in (A, B). Histograms show the average percentage (± SEM, n = 8 animals) of neurons exhibiting unipolar/bipolar, multipolar or no process morphology in each layer. Numbers of cells analyzed are shown in the parenthesis. Neurons expressing NP1 siRNAs showed higher frequency of cells with multipolar morphology than control neurons at VZ/SVZ and IZ (One Way Analysis of Variance, P < 0.001; post-hoc Student-Newman-Keuls Method P < 0.05).
(E) Tracings of representative neurons from similar sections as in (A, B), showing 2-D projection of the neuritic arbor of neurons transfected with different constructs, with neuronal orientation relative to cortical layers preserved. Scale is 50 μm.
(F) Quantitation of the total neuritic length (a) and neuritic branch number (b) of multipolar neurons residing at the VZ/SVZ transfected with control or NP1 siRNAs. Data represent mean ± SEM. Only the total length of NP1 siRNA transfected cells (a) differed from control values (Mann-Whitney test, p = 0.002). Numbers in parentheses refer to the total number of cells analyzed.
(G) Quantitation of the length of the leading process in bipolar neurons transfected with control or NP1 siRNAs at different cortical layers. Data represent mean ± SEM. Data for NP1 siRNA were significantly different from those for controls at IZ and CP (One Way Analysis of Variance on ranks, p < 0.001; post-hoc Dunn’s method, p < 0.05). Numbers in the parenthesis refer to the total number of cells analyzed.
(H) The average total somatic EGFP-fluorescence in transfected multipolar or bipolar neurons at VZ/SVZ or IZ/CP respectively, was quantified as a measure of the extent of expression of control (a) or NP1 (b) siRNAs, normalized to the total fluorescence of all cells in the slice/experimental case, and plotted as a cumulative probability distribution curve. Data for NP1 siRNA transfected multipolar neurons at VZ/SVZ significantly differed from that of NP1 siRNA transfected bipolar neurons at IZ/CP (Kolmogorov-Smirnov test).
Further examination of cortical neurons expressing NP1-siRNA showed that a large fraction of them exhibited multiple neurites (multipolar) in the VZ/SVZ and IZ, whereas most neurons in these regions of control embryos had a single neurite (unipolar) or two neurites (bipolar), with only a small fraction of control cells in the VZ/SVZ exhibiting multipolar morphology (Figures 6A, 6B, arrowheads, and 6D****). Furthermore, NP1-siRNA-expressing multipolar neurons located in the VZ/SVZ displayed a higher total neuritic length (Figure 6Fa) than control multipolar neurons in these regions, although the average neurite number per cell was not significantly different (Figure 6Fb). These polarization defects were illustrated by microscopic tracings of 20 randomly sampled control-siRNA and NP1-siRNA transfected neurons in various layers (Figure 6E).
Our studies in cultured hippocampal neurons showed that Sema3A might regulate neuronal polarization by selectively promoting dendrite growth and suppressing axon growth (Figure 5). We thus have also examined the length of the leading process that becomes the apical dendrite in control or NP1-siRNA transfected bipolar neurons. As shown in Figure 6G, NP1 down-regulation resulted in significant reduction of the growth of the leading process in cells located at the IZ and CP, consistent with the promotion of dendrite growth by Sema3A signaling. In addition, the increased total neurite length of multipolar NP1-siRNA-expressing cells in the VZ/SVZ (Figure 6Fa) is also consistent with the Sema-3A-suppression on axon growth, although immunostaining of the abnormal processes in multipolar neurons for axon-specific markers was not successful due to intense axon staining from non-transfected cells in these regions. We have also performed in utero electroporation using either one of the two NP1-siRNAs alone, and found similar neuronal polarization results as to that described above for electroporation with both NP1-siRNAs together.
Since neuronal polarization occurs in VZ/SVZ prior to radial migration, where down-regulation of NP1 resulted in pronounced polarity defect, the failure of radial migration may be attributed in part to the polarization defect (see Discussion). In support of this idea, we note that only cells displaying bipolar morphology were located at the CP for both control and NP1-siRNA transfected neurons (Figure 6D). This suggests that polarization defects had impeded the radial migration of these neurons. The polarization defects resulting from down-regulation of NP1 may depend on the level of NP1-siRNA expression in various progenitor cells. Assuming that the level of EGFP expression correlated with that of NP1-siRNA, we measured the EGFP fluorescence intensity of individual neuronal somata of various morphologies at different cortical layers in E21 rat embryos. The results (Figure 6H) suggest that the level of NP1-siRNA expression correlated well with the severity of the polarization defects, with neurons that exhibited multipolar morphology at the SVZ showing higher levels of GFP expression, in comparison to those exhibiting bipolar morphology at the IZ and CP (Figure 6Hb). Interestingly, in cells expressing control siRNA, the opposite was found for GFP expression - higher in bipolar cells in the IZ/CP than multipolar cells in the SVZ (Figure 6Ha). The latter finding suggests that for NP1-siRNA expressing neurons, the difference in the level of NP1-siRNA expression between normally migrating bipolar cells and polarization-defective multipolar cells could be even higher than that indicated by the EGFP expression.
Discussion
In this study, we examined the role of Sema3A in polarizing axon/dendrite differentiation in cultured hippocampal neurons and showed that localized exposure of an undifferentiated neurite to Sema3A induces its differentiation into the dendrite, via local suppression of axon development. This suppression is mediated by Sema3A-induced elevation of cGMP/PKG signaling that down-regulates cAMP/PKA-dependent LKB1 and GSK-3β phosphorylation, which is essential for axon formation. In addition to this local axon suppression effect, Sema3A also promotes dendrite growth. Furthermore, down-regulation of Sema3A signaling in developing cortical neurons in vivo resulted in severe polarization defects and reduced length of the leading process, the apical dendrite, in support of the notion that Sema3A may regulate axon/dendrite polarity during the early phase of neuronal development by both suppressing axon-specific cAMP/PKA-dependent processes as well as promoting dendrite-specific cGMP/PKG-dependent functions.
Sema3A-Induced Neuronal Polarization and Dendrite Growth In Vitro
Axon/dendrite differentiation during neuronal polarization is a coordinated process, as exemplified by the formation of a single axon and multiple dendrites in cultured hippocampal neurons (Dotti et al., 1988). In most studies using these cells, the focus has been on the process of axon differentiation, with the implicit assumption that specification of the axon of one neurite determines the fate of all other neurites as dendrites. In this “axon dominance” view, the first event of neuronal polarization is the emergence of a localized signal for axon specification in one neurite. This axon-specifying signal could be pre-existing in the cytoplasm (de Anda et al., 2005) or become stably accumulated or activated locally via a local autocatalytic process. Axon specification is likely to be accompanied by a global long-range signal in the neuron to inhibit axon formation or to promote dendrite formation in all other neurites. Our results are consistent with the axon dominance view of polarization - the polarizing effect of Sema3A is to direct axon formation away from the localized Sema3A action in the neuron (Figure 1), and the higher frequency of dendrite formation on the Sema3A stripe might be a secondary consequence of axon specification. Our biochemical results support this notion by showing the effect of Sema3A in suppressing cAMP/PKA-dependent phosphorylation of axon-determinants LKB1 and GSK-3β via elevation of cGMP/PKG activity that activates cAMP-selective PDEs (Figures 2 and 3). Furthermore, we showed that prior to axon formation, neurite growing away from the Sema3A-stripes exhibits accumulation of pLKB1-S431 (Figure 4), the activated form of LKB1 known to trigger downstream effectors for axon formation (Barnes et al., 2007).
Axon determination is tightly linked to the selective growth acceleration of an undifferentiated neurite. An extracellular factor that promotes the growth of undifferentiated neurites could polarize the neuron simply by promoting growth of one neurite. Thus it is difficult to distinguish the polarity effect from the growth effect of a putative “axon determinant”. However, in the case of Sema3A, it uniformly promoted the growth of undifferentiated neurites (Figures 5A and 5B), yet axon differentiation was suppressed for those neurites in contact with the Sema3A stripe. Thus, Sema3A exerts the polarity effect besides its effect on neurite growth - it must act on the undifferentiated neurite in a manner that suppresses axon formation (e.g., by suppressing LKB1/GSK3β phosphorylation) and permits dendrite formation. As LKB1 and GSK3β play a key role in axon determination, the inhibitory effect of Sema3A on the PKA-dependent phosphorylation of these proteins shown here (Figure 3) further confirm that it acts as a polarity determinant in the early stage of neuronal polarization, in addition to its action at a later stage in promoting and suppressing dendrite and axon growth, respectively (Figure 5). Of note, it is the fact that neurite initiation sites do not move during axon/dendrite differentiation that allowed us to use the retrospective assay of polarity determination on the striped substrates to separate the early polarity effect from the later growth effect. Finally, cytoskeletal organizations are different between the axon and dendrites, including differences in the microtubule orientation and its associated proteins (Baas et al., 1988; Hirokawa and Takemura, 2005). Selective action of cGMP/PKG effectors in trafficking and modification of cytoskeletal components associated with axon vs. dendrite may account for the selective Sema3A actions on axon vs. dendrite growth after the polarity is established. Of note, down-regulation of the Sema3A receptor NP1 in these cultured neurons resulted in an increased axon formation, as shown by the increased percentage of multiple axon (MA) and reduced percentage of single axon (SA) population in 48-60 hr cultures (Supplementary Figure 4). These findings suggest that basal NP1 signaling may operate constitutively in these cultured neurons to facilitate neuron polarization and that secreted Sema3A may be present in these cultures. Furthermore, autocrine action of endogenous BDNF plays a significant role in axon differentiation in these cultured neurons (data not shown).
Down-Regulation of cAMP Signaling Underlies Axon Suppression Effect of Sema3A
Many extracellular factors known to regulate neuron polarization, including BDNF (Yoshimura et al., 2005; Shelly et al., 2007), NGF (Da Silva et al., 2005), Sema3A (Polleux et al., 2000), netrin-1 (Adler et al., 2006; Mai et al., 2009), and Wnt (Hilliard and Bargmann, 2006), could modulate cAMP or cGMP level in neurons (Polleux et al., 2000; Gao et al., 2003; Shelly et al., 2007; Togashi et al., 2008). We have previously shown that cAMP/cGMP activities exert antagonistic actions on axon/dendrite polarization (Shelly et al., 2010) through reciprocal down-regulation. In the present study, we showed that Sema3A causes cGMP elevation in the undifferentiated neurite, with accompanying reduction of cAMP/PKA activity (Figure 2). Thus, localized exposure with Sema3A could reduce cAMP level and suppress axon formation of Sema3A-exposed neurite, whereas a spontaneous elevation of cAMP activity could lead to axon formation in other neurites. The rule of one axon and multiple dendrites still operates under the restriction that the Sema3A-exposed neurite could not become the axon. Spontaneous axon formation away from the Sema3A may depend on stochastic local elevation of cAMP/PKA (Shelly et al., 2007) and local activation and accumulation of putative axon determinants (Shi et al., 2003; Yoshimura et al., 2005; Jacobson et al., 2006; Toriyama et al., 2006; Shelly et al., 2007), amplified by local autocatalytic process. The accompanying long-range suppression of cAMP (Shelly et al., 2010) would further ensure the low cAMP level (and the reciprocal high cGMP level) and the dendrite differentiation of the Sema3A-exposed neurite. It is also possible that cGMP elevation locally in the neurite is by itself sufficient for the dendrite development. This was supported by the finding that in developing cortical neurons with down-regulation of LKB1 expression or LKB1S431A overexpression, the defective axon formation did not affect the formation of relatively normal dendritic arbors (Barnes et al., 2007; Shelly et al. 2007). Nevertheless, our findings indicate that the main action of Sema3A-induced cGMP elevation is to suppress axon formation, although it could also promote selective dendrite growth after the dendrite is formed. The latter direct effect on dendrite growth is consistent with the finding in vivo that down-regulation of Sema3A signaling in newly-generated cortical neurons resulted in growth inhibition of the leading process (Figure 6).
Sema3A as a Neuronal Polarizing Factor in Developing Cortex In Vivo
For the development of ordered neural network in vivo, the polarity of cortical neurons must be established with respect to the coordinates of the surrounding tissue. Following mitosis, the newborn neuron acquires a bipolar morphology in the VZ with the long axis perpendicular to the cortical layers. With a brief transition to multipolar morphology in the SVZ, the neuron resumes its bipolar morphology prior to the onset of radial migration (Noctor et al., 2004). The leading process of the migrating cell becomes the apical dendrite whereas the trailing process becomes the axon and grows rapidly towards the target. The exact time of axon/dendrite specification, whether it begins during the pre-migratory or migratory phase, remains unclear.
The Sema3A is present in a descending gradient across the developing cortical layers, with highest expression at the pial surface (Polleux et al., 2000; Chen et al., 2008), whereas its receptor neuropilin-1 (NP1) is expressed in migrating cortical neurons (Chen et al., 2008). The Sema3A is responsible for orienting apical dendrites of developing cortical neurons towards the pial surface and guiding axon formation in the opposite direction (Polleux et al., 2000). In mice with Sema3 gene deletion, axon/dendrite formation in cortical pyramidal neurons appeared to be unaffected (Behar et al., 1996; Polleux et al., 1998), arguing against the idea that Sema3A plays a major role in neuronal polarization in vivo, although the possibility of compensatory effects in the Sema3 gene knockout mice cannot be excluded. Of note, a recent study demonstrated that in cultured Xenopus spinal commissural interneurons, Sema3A converted axons to dendrites by activating the CaV2:3 channels in a cGMP/PKG dependent manner (Nishiyama et al., 2011). The findings that Sema3A acts as a chemoattractant for directing radial migration of cortical neurons along the radial glia (Chen et al. 2008), together with the findings that Sema3A exerts polarizing action on cultured hippocampal neurons (Figure 1) and cortical neurons (Polleux, et al., 2000), support the idea that the cortical Sema3A gradient acts simultaneously as an axon/dendrite polarizing factor as well as a chemoattractant for radial migration.
By down-regulating the Sema3A signaling in newly-generated cortical neurons in vivo with NP1 siRNA, we showed a loss of the stereotypical bipolar morphology of these neurons in most cortical layers, with the most predominant effect in the neuronal populations in the VZ/SVZ (Figure 6). This early polarity defect found in the VZ/SVZ suggests that the Sema3A effects on neuronal polarization may occur prior to the onset of neuronal migration. However, our result cannot exclude the possibility that the failure in radial migration of these neurons to the CP due to the reduced chemoattractive action of Sema3A signaling (Figure 6; Chen et al., 2008) also contributes to the impaired polarization phenotype, because defective migration may prevent proper reception of other polarizing factors along their migratory route. Furthermore, down-regulation of Sema3A signaling in these cortical progenitors resulted in significant reduction of the growth of the leading process in cells located at the IZ and CP (Figure 6). Together with our findings on the effect of Sema3A on the selective promotion of dendrite growth and suppression of axon growth in cultured hippocampal neurons (Figure 5), these results would support the notion that Sema3A might regulate neuronal polarization and dendrite development by acting directly to promote dendrite growth. We note that the identity of the specific Plexin co-receptors that mediate the Sema3A effects on neuronal polarization together with NP1 remain to be determined.
Other Extracellular Signals for Neuronal Polarization
Intracellular signaling pathways involved in neuronal polarization have been extensively examined in cultured neurons, but the extracellular polarizing factors that activate these signaling pathways in vivo remain largely unknown. Secreted molecules such as BDNF (Yoshimura et al., 2005; Shelly et al., 2007), NGF (Da Silva et al., 2005), Insulin-like growth factor-1 (IGF-1) (Sosa et al., 2006), netrin-1 (Mai et al., 2009), and transforming growth factor beta (TGF-β) (Yi et al., 2010), were shown to promote axon initiation and growth in cultured hippocampal neurons, although our stripe assay failed to show the polarizing effect of NGF and netrin-1 (Figure 1). The latter discrepancy may be caused by the differences in the culture conditions or the sensitivity of the methods for assaying polarization. However, no axon formation defect was detected in mice with targeted deletion of genes for NGF (Crowley et al., 1994) and BDNF (Jones et al., 1994), or for their respective receptors TrkA (Smeyne et al., 1994) and TrkB (Klein et al., 1993). A notable exception is TGF-β, whose receptors are essential for axon formation in embryonic cortical neurons in vivo (Yi et al., 2010). We note that in utero electroporation was used in the latter study to perturb the signaling of TGF-β receptors in a subpopulation of cortical neurons, unlike the earlier studies with genetic deletion over entire population of neurons throughout prolonged developmental period. Differences between the methods used to assay effects of gene down-regulation may account for the lack of apparent effects in some of these studies.
In this study, we provided evidence that Sema3A may also regulate neuronal polarization in vivo. However, neuronal polarization in the developing brain is likely to depend on the coordinated actions of many extracellular factors. In addition to Sema3A (Polleux et al., 2000; Chen et al. 2008), the spatially regulated expression of other secreted molecules in the developing cortex has been reported. The TGF-β2 and TGF-β3 ligands are highly expressed in the VZ and SVZ of the embryonic neocortex (Yi et al., 2010), whereas Slits (Whitford et al., 2002) and ephrins (Liebl et al., 2003) are highly enriched at the CP, similar to the expression of Sema3A. Furthermore, extracellular factors may also influence neuronal polarization by modulating the expression and action of other polarizing factors. For examples, Wnt4 and TGF-β1 may regulate Sema3A expression (Kettunen et al., 2005), and Semaphorins may control TGF-β and ephrin signaling (Ikegami et al., 2004). The antagonistic effect of Sema3A and BDNF in polarizing axon/dendrite differentiation shown here (Figure 1), mediated by reciprocal cGMP/cAMP signaling in the neuron (Figure 2; Shelly, et al., 2010), further underscores the possibility that synergistic and antagonistic actions of extracellular factors may work in concert to polarize neurons in vivo. The involvement of multiple factors in vivo may account for the observations that disruption of the signaling of a single factor results in only subtle polarity defects.
Experimental Procedures
Cell cultures, transfection, immunostaining, immunoblotting, and PKA-activity detection
Cultures of dissociated hippocampal and cortical neurons were prepared as previously described (Shelly et al., 2007) and as presented in Supplementary Experimental Procedures. Live images for stripe assays were acquired 12 hr following plating, and immunostaining was performed as described in Supplementary Experimental Procedures. For FRET assays, transfections were carried out 2 hr after plating. For analysis of LKB1, GSK-3β and Akt phosphorylation by immunoblotting, cells were treated with forskolin (20 μM; 20 min) or BDNF (50 ng/ml, 15 min), either alone or together with the PKG inhibitor KT5823 (200 nM), the PDE inhibitor IBMX (50 μM), the PDE4 inhibitor rolipram (1 μM), or the sGC inhibitor ODQ (1 μM). To test for the antagonistic effects of Sema3A or 8-pCPT-cGMP, increasing concentrations of these factors were incubated together with forskolin or BDNF for 20 min. Whole-cell extracts were prepared at 5 DIV for cortical neurons, before subjected to immunoblotting. HEK-293T cells were grown in DMEM medium supplemented with 10% FBS, and transiently transfected using calcium-phosphate method. The ubiquitination assay and detection of PKA activity using a fluorescent peptide based “PepTag” assay is described in Supplementary Experimental Procedures.
Micro-fabrication and substrate patterning
Substrates were patterned as previously described (Shelly et al., 2007) and as presented in Supplementary Experimental Procedures. Microfluidic patterning of the following substrates alone or together with fluorescently conjugated BSA (5 μg/ml) as a marker was performed as follows: F-cAMP, F-cGMP, KT5720 or KT5823 (2 nM); NGF or BDNF (0.5 ng/ml), netrin-1 (0.5 and 0.05 ng/ml); Sema3A (0.5 and 0.05 μg/ml).
In Utero electroporation and quantitation of neuronal polarization, migration, and fluorescence
The method of in utero electroporation was performed as previously described (Shelly et al., 2007) and as presented in detail in Supplementary Experimental Procedures. Control embryos were electroporated with control siRNA and experimental embryos were electroporated with NP1, PlexinA2 or PlexinA4 siRNAs. Cortical neuronal development was analyzed at E21, as follows. For analysis of neuronal polarization, cells in different cortical layers were categorized according to the number of neuritic processes, exhibiting single (unipolar), double (bipolar), or multiple (multipolar) morphology, or exhibiting no (none) processes. To compare between control and NP1 siRNA transfected multipolar neuron population residing at the VZ/SVZ, the total neuritic branch number and neuritic length was quantified. To examine the effect of Sema3A signaling on dendrite growth in vivo, the length of the leading process was quanitifed in bipolar neurons at different cortical layers. For analysis of neuronal migration, cells were quantified according to their location in the different cortical layers based on Nissl staining of the slice. The total somatic EGFP-fluorescence in transfected multipolar or bipolar neurons at VZ/SVZ or IZ/CP respectively, was quantified as a measure of the extent of expression of control or NP1 siRNAs, normalized to the total fluorescence of all cells in the slice /experimental case, and plotted as a cumulative probability distribution curve. All parameters for image acquisition were kept constant while keeping emission levels below saturation.
FRET imaging and analysis
The FRET imaging and analysis was performed as previously described (Shelly et al., 2010) and as presented in Supplementary Experimental Procedures. Briefly, live images were acquired for 140-170 ms at 10-s intervals. For global manipulation of cAMP/cGMP signaling, Sema3A (1 μg/ml), BDNF (50 ng/ml), or NGF (50 ng/ml) were applied to the bath after 5 min of baseline recording. For the ratiometric FRET analysis, the CFP and YFP signal from the neurite was background-subtracted (with background intensity taken from a cell-free region), normalized by the control value (averaged over 5 min of baseline recording), and FRET value was calculated as a ratio of YFP signal to that of CFP signal for the PKA-activity and cGMP sensors, and as a ratio of CFP signal to that of YFP signal for the cAMP sensor. Concentrations of bath-applied pharmacological agents are described in Supplementary Experimental Procedures.
Supplementary Material
Acknowledgements
We thank R. Thakar, S. Li, M. Nasir, and D. Liepmann (University of California, Berkeley, CA) for help with PDMS microfluidic molds, J. Zhang (Johns Hopkins University, Baltimore, MD) for the ICUE and AKAR FRET probes, M. J. Lohse (University of Wurzburg, Germany) for the cGES-DE5 FRET probe, and M. Feller (University of California, Berkeley, CA) for advice on FRET imaging. This work was supported by a grant from USNIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adler CE, Fetter RD, Bargmann CI. UNC-6/Netrin induces neuronal asymmetry and defines the site of axon formation. Nat Neurosci. 2006;9:511–518. doi: 10.1038/nn1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arimura N, Kaibuchi K. Neuronal polarity: from extracellular signals to intracellular mechanisms. Nat. Rev. Neurosci. 2007;8:194–205. doi: 10.1038/nrn2056. [DOI] [PubMed] [Google Scholar]
- Baas PW, Deitch JS, Black MM, Banker GA. Polarity orientation of microtubules in hippocampal neurons: uniformity in the axon and nonuniformity in the dendrite. Proc. Natl Acad. Sci. USA. 1988;85:8335–8339. doi: 10.1073/pnas.85.21.8335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes AP, Lilley BN, Pan YA, Plummer LJ, Powell AW, Raines AN, Sanes JR, Polleux F. LKB1 and SAD kinases define a pathway required for the polarization of cortical neurons. Cell. 2007;129:549–563. doi: 10.1016/j.cell.2007.03.025. [DOI] [PubMed] [Google Scholar]
- Barnes AP, Solecki D, Polleux F. New insights into the molecular mechanisms specifying neuronal polarity in vivo. Curr. Opin. Neurobiol. 2008;18:44–52. doi: 10.1016/j.conb.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behar O, Golden JA, Mashimo H, Schoen FJ, Fishman MC. Semaphorin III is needed for normal patterning and growth of nerves, bones and heart. Nature. 1996;383:525–528. doi: 10.1038/383525a0. [DOI] [PubMed] [Google Scholar]
- Chen G, Sima J, Jin M, Wang KY, Xue XJ, Zheng W, Ding YQ, Yuan XB. Semaphorin-3A guides radial migration of cortical neurons during development. Nat. Neurosci. 2008;11:36–44. doi: 10.1038/nn2018. [DOI] [PubMed] [Google Scholar]
- Cheng PL, Lu H, Shelly M, Gao HF, Poo MM. Phosphorylation of E3 ligase Smurf1 switches its substrate preference in support of axon development. Neuron. 2011;69:231–243. doi: 10.1016/j.neuron.2010.12.021. [DOI] [PubMed] [Google Scholar]
- Crowley C, Spencer SD, Nishimura MC, Chen KS, Pitts-Meek S, Armanini MP, Ling LH, McMahon SB, Shelton DL, Levinson AD, Phillips HS. Mice lacking nerve growth factor display perinatal loss of sensory and sympathetic neurons yet develop basal forebrain cholinergic neurons. Cell. 1994;76:1001–1011. doi: 10.1016/0092-8674(94)90378-6. [DOI] [PubMed] [Google Scholar]
- Da Silva JS, Hasegawa T, Miyagi T, Dotti CG, Abad-Rodriguez J. Asymmetric membrane ganglioside sialidase activity specifies axonal fate. Nat. Neurosci. 2005;8:606–615. doi: 10.1038/nn1442. [DOI] [PubMed] [Google Scholar]
- de Anda FC, Pollarolo G, Da Silva JS, camoletto PG, feiguin F, Dotti CG. Centrosome localization determines neuronal polarity. Nature. 2005;436:704–708. doi: 10.1038/nature03811. [DOI] [PubMed] [Google Scholar]
- DiPilato LM, Cheng X, Zhang J. Fluorescent indicators of cAMP and Epac activation reveal differential dynamics of cAMP signaling within discrete subcellular compartments. Proc. Natl. Acad. Sci. USA. 2004;101:16513–16518. doi: 10.1073/pnas.0405973101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotti CG, Banker GA. Experimentally induced alteration in the polarity of developing neurons. Nature. 1987;330:254–256. doi: 10.1038/330254a0. [DOI] [PubMed] [Google Scholar]
- Dotti CG, Sullivan CA, Banker GA. The establishment of polarity by hippocampal neurons in culture. J Neurosci. 1988;8:1454–1468. doi: 10.1523/JNEUROSCI.08-04-01454.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Nikulina E, Mellado W, Filbin MT. Neurotrophins elevate cAMP to reach a threshold required to overcome inhibition by MAG through extracellular signal-regulated kinase-dependent inhibition of phosphodiesterase. J. Neurosci. 2003;23:11770–11777. doi: 10.1523/JNEUROSCI.23-37-11770.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilliard MA, Bargmann CI. Wnt signals and frizzled activity orient anterior-posterior axon outgrowth in C. elegans. Dev. Cell. 2006;10:379–390. doi: 10.1016/j.devcel.2006.01.013. [DOI] [PubMed] [Google Scholar]
- Hirokawa N, Takemura R. Molecular motors and mechanisms of directional transport in neurons. Nat. Rev. Neurosci. 2005;6:201–214. doi: 10.1038/nrn1624. [DOI] [PubMed] [Google Scholar]
- Ikegami R, Zheng H, Ong SH, Culotti J. Integration of semaphorin-2A/MAB-20, ephrin-4, and UNC-129 TGF-beta signaling pathways regulates sorting of distinct sensory rays in C. elegans. Dev. Cell. 2004;6:383–395. doi: 10.1016/s1534-5807(04)00057-7. [DOI] [PubMed] [Google Scholar]
- Inagaki N, Chihara K, Arimura N, Menager C, Kawano Y, Matsuo N, Nishimura T, Amano M, Kaibuchi K. CRMP-2 induces axons in cultured hippocampal neurons. Nat. Neurosci. 2001;4:781–782. doi: 10.1038/90476. [DOI] [PubMed] [Google Scholar]
- Jacobson C, Schnapp B, Banker GA. A change in the selective translocation of the Kinesin-1 motor domain marks the initial specification of the axon. Neuron. 2006;49:797–804. doi: 10.1016/j.neuron.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Jones KR, Farinas I, Backus C, Reichardt LF. Targeted disruption of the BDNF gene perturbs brain and sensory neuron development but not motor neuron development. Cell. 1994;76:989–999. doi: 10.1016/0092-8674(94)90377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettunen P, Løes S, Furmanek T, Fjeld K, Kvinnsland IH, Behar O, Yagi T, Fujisawa H, Vainio S, Taniguchi M, Luukko K. Coordination of trigeminal axon navigation and patterning with tooth organ formation: epithelial-mesenchymal interactions, and epithelial Wnt4 and Tgfbeta1 regulate semaphorin 3a expression in the dental mesenchyme. Development. 2005;132:323–334. doi: 10.1242/dev.01541. [DOI] [PubMed] [Google Scholar]
- Kishi M, Pan YA, Crump JG, Sanes JR. Mammalian SAD kinases are required for neuronal polarization. Science. 2005;307:929–932. doi: 10.1126/science.1107403. [DOI] [PubMed] [Google Scholar]
- Klein R, Smeyne RJ, Wurst W, Long LK, Auerbach BA, Joyner AL, Barbacid M. Targeted disruption of the trkB neurotrophin receptor gene results in nervous system lesions and neonatal death. Cell. 1993;75:113–122. [PubMed] [Google Scholar]
- Liebl DJ, Morris CJ, Henkemeyer M, Parada LF. mRNA expression of ephrins and Eph receptor tyrosine kinases in the neonatal and adult mouse central nervous system. J. Neurosci. Res. 2003;71:7–22. doi: 10.1002/jnr.10457. [DOI] [PubMed] [Google Scholar]
- Mai J, Fok L, Gao H, Zhang X, Poo MM. Axon initiation and growth cone turning on bound protein gradients. J. neurosci. 2009;29:7450–7458. doi: 10.1523/JNEUROSCI.1121-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolaev VO, Gambaryan S, Lohse MJ. Fluorescent sensors for rapid monitoring of intracellular cGMP. Nat. Methods. 2006;3:23–25. doi: 10.1038/nmeth816. [DOI] [PubMed] [Google Scholar]
- Nishiyama M, Kazunobu T, von Schimmelmann MJ, Lim CS, Maeda SI, Yamashita N, Goshima Y, Ishii S, Hong K. Semaphorin 3A induces CaV2:3 channel-dependent conversion of axons to dendrites. Nat. Cell Biol. 2011;13:676–686. doi: 10.1038/ncb2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noctor SC, Martinez-Cerdeno V, Ivic L, Kriegstein AR. Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat. Neurosci. 2004;7:136–144. doi: 10.1038/nn1172. [DOI] [PubMed] [Google Scholar]
- Polleux F, Giger RJ, Ginty DD, Kolodkin AL, Ghosh A. Patterning of cortical efferent projections by semaphorin-neuropilin interactions. Science. 1998;282:1904–1906. doi: 10.1126/science.282.5395.1904. [DOI] [PubMed] [Google Scholar]
- Polleux F, Morrow T, Ghosh A. Semaphorin 3A is a chemoattractant for cortical apical dendrites. Nature. 2000;404:567–573. doi: 10.1038/35007001. [DOI] [PubMed] [Google Scholar]
- Schwamborn JC, Puschel AW. The sequential activity of the GTPases Rap1B and Cdc42 determines neuronal polarity. Nat. Neurosci. 2004;7:923–929. doi: 10.1038/nn1295. [DOI] [PubMed] [Google Scholar]
- Shelly M, Cancedda L, Heilshorn S, Sumbre G, Poo MM. LKB1/STRAD promotes axon initiation during neuronal polarization. Cell. 2007;129:565–577. doi: 10.1016/j.cell.2007.04.012. [DOI] [PubMed] [Google Scholar]
- Shelly M, Lim BK, Cancedda L, Heilshorn SC, Gao H, Poo MM. Local and long-range reciprocal regulation of cAMP and cGMP in axon/dendrite formation. Science. 2010;327:547–552. doi: 10.1126/science.1179735. [DOI] [PubMed] [Google Scholar]
- Shi SH, Jan LY, Jan YN. Hippocampal neuronal polarity specified by spatially localized mPar3/mPar6 and PI 3-kinase activity. Cell. 2003;112:63–75. doi: 10.1016/s0092-8674(02)01249-7. [DOI] [PubMed] [Google Scholar]
- Smeyne RJ, Klein R, Schnapp A, Long LK, Bryant S, Lewin A, Lira SA, Barbacid M. Severe sensory and sympathetic neuropathies in mice carrying a disrupted Trk/NGF receptor gene. Nature. 1994;368:246–249. doi: 10.1038/368246a0. [DOI] [PubMed] [Google Scholar]
- Sosa L, Dupraz S, Laurino L, Bollati F, Bisbal M, Ca’ceres A, Pfenninger KH, Quiroga S. IGF-1 receptor is essential for the establishment of hippocampal neuronal polarity. Nat. Neurosci. 2006;9:993–995. doi: 10.1038/nn1742. [DOI] [PubMed] [Google Scholar]
- Togashi K, von Schimmelmann MJ, Nishiyama M, Lim CS, Yoshida N, Yun B, Molday RS, Goshima Y, Hong K. Cyclic GMP-gated CNG channels function in Sema3A-induced growth cone repulsion. Neuron. 2008;58:694–707. doi: 10.1016/j.neuron.2008.03.017. [DOI] [PubMed] [Google Scholar]
- Toriyama M, Shimada T, Kim KB, Mitsuba M, Nomura E, Katsuta K, Sakumura Y, Roepstorff P, Inagaki N. Shootin1: a protein involved in the organization of an asymmetric signal for neuronal polarization. J. Cell Biol. 2006;175:147–157. doi: 10.1083/jcb.200604160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitford KL, Marillat V, Stein E, Goodman CS, Tessier-Lavigne M, Chédotal A, Ghosh A. Regulation of cortical dendrite development by Slit-Robo interactions. Neuron. 2002;33:47–61. doi: 10.1016/s0896-6273(01)00566-9. [DOI] [PubMed] [Google Scholar]
- Yi JJ, Barnes AP, Hand R, Polleux F, Ehlers MD. TGF-beta signaling specifies axons during brain development. Cell. 2010;142:144–157. doi: 10.1016/j.cell.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura T, Kawano Y, Arimura N, Kawabata S, Kikuchi A, Kaibuchi K. GSK-3 regulates phosphorylation of CRMP-2 and neuronal polarity. Cell. 2005;120:137–149. doi: 10.1016/j.cell.2004.11.012. [DOI] [PubMed] [Google Scholar]
- Zhang J, Ma Y, Taylor SS, Tsien RY. Genetically encoded reporters of protein kinase A activity reveal impact of substrate tethering. Proc. Natl. Acad. Sci. USA. 2001;98:14997–15002. doi: 10.1073/pnas.211566798. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.