Abstract
Dectin-1, the major β-glucan receptor in leukocytes, triggers an effective immune response upon fungal recognition. Here we use sortase-mediated transpeptidation, a technique that allows placement of a variety of probes on a polypeptide backbone, to monitor the behavior of labeled functional dectin-1 in live cells with and without fungal challenge. Installation of probes on dectin-1 by sortagging permitted highly specific visualization of functional protein on the cell surface and its subsequent internalization upon ligand presentation. Retrieval of sortagged dectin-1 expressed in macrophages uncovered a unique interaction between dectin-1 and galectin-3 that functions in the proinflammatory response of macrophages to pathogenic fungi. When macrophages expressing dectin-1 are exposed to Candida albicans mutants with increased exposure of β-glucan, the loss of galectin-3 dramatically accentuates the failure to trigger an appropriate TNF-α response.
Keywords: pattern-recognition receptors, sortase A, polysaccharide
Dectin-1, a C-type lectin, is the major receptor on macrophages for β-1,3-glucan, a polymer of glucose present in the fungal cell wall that stimulates phagocytosis and production of inflammatory cytokines (1, 2). Dectin-1 contains a single extracellular carbohydrate-recognition domain, an immunoreceptor tyrosine activation-like motif in its cytoplasmic tail (3), and is N-glycosylated, a modification that contributes to its surface expression and function (4). The cellular responses observed upon activation of dectin-1 include phagocytosis and an oxidative burst, as well as the production of eicosanoids, inflammatory cytokines, and chemokines (1, 5).
The specificity of dectin-1 for the fungal polysaccharide β-glucan is firmly established by in vitro experiments (1, 6–9), but most aspects of dectin-1 signaling in vivo remain obscure. Dectin-1–mediated responses may require collaboration with Toll-like receptors (TLR) (10–12), tetraspanins (13–15), and the DC-SIGN receptor (5). Establishing the association of dectin-1 with potential partners has been hindered by technical difficulties common to membrane proteins that must traffic to the surface of the cell to perform their function. Standard methods to label and visualize dectin-1 on the surface of live cells involve the use of fluorophore-conjugated antibodies or tags added to the protein (5, 14, 16, 17). Such fusions of membrane proteins with GFP or epitope tags can render the protein inactive, unable to transit through the secretory pathway to the surface, or affect cellular activation or antigen trafficking behavior upon binding to the molecule in question.
To avoid these obstacles we applied sortase-mediated transpeptidation (sortagging) (18), which selectively labels dectin-1 on living cells, a procedure that permitted the identification of a key partner in fungal recognition. Sortagging allows efficient and selective labeling of dectin-1 with affinity probes or fluorophores. This site-specific labeling allowed us to monitor the behavior of functional dectin-1 on the surface of living cells with and without a fungal challenge. Using sortagged dectin-1 we uncovered an association of dectin-1 with galectin-3, a pattern recognition receptor (PRR) that recognizes carbohydrates uniquely present in the cell wall of Candida albicans (19), triggering a pathogen-specific response (20). This dectin-1/galectin-3 association modulates the induction of TNF-α such that a reduction in galectin-3 levels lowers the induction of TNF-α. The dectin-1/galectin-3 interaction sheds light on fungal recognition and the mechanism by which the innate immune system discriminates nonpathogenic from pathogenic fungi.
Results
Dectin-1 Expressed on Mammalian Cells Can Be Labeled Through Sortagging Without Affecting Its Functionality.
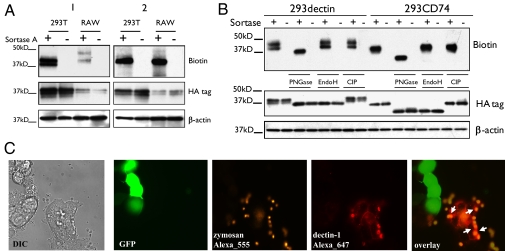
Murine dectin-1 equipped with an LPETG motif followed by an HA epitope tag at its C terminus is properly transported to the cell membrane and retains its function. Cells stably expressing tagged dectin-1 on the cell surface are capable of binding zymosan (SI Results and Fig. S1).To determine whether sortase A could act on tagged dectin-1 on intact cells, we incubated HEK 293T and RAW 264.7 cells that stably express dectin-1 with sortase A together with a biotinylated probe (18). The intact cells were then lysed, the lysates separated by SDS/PAGE, and biotinylated products revealed on blots with streptavidin-HRP. Dectin-1 was labeled selectively, because there were no other biotinylated polypeptides that originated either from cell surface or cytosolic proteins. When these lysates were subjected to immunoblotting to detect HA-tagged materials not cleaved by sortase, HA-tagged dectin-1 was present in all samples, both before and after incubation with sortase A and the biotinylated probe (Fig. 1A). Any cell-internal dectin-1 would be inaccessible to sortase and so retain its HA tag. Even if all dectin-1 were surface exposed, the sortase reaction may not proceed quantitatively, also accounting for the presence of HA-positive dectin-1. Cells that were first lysed, sortagged, and only then subjected to streptavidin-HRP immunoblotting also showed the presence of labeled dectin-1 (Fig. 1A).
Fig. 1.
Sortagged dectin-1 with a biotinylated probe is functional. (A) Stably transduced HEK 293T and RAW 264.7 cells were incubated with sortase A together with a biotinylated probe. Cell lysates from the sortagged cells were analyzed by immunoblotting. The only detectable biotinylated species has the molecular characteristics of dectin-1 (Biotin). HA immunoblotting showed persistence of HA-tagged dectin-1 (HA tag) in all samples. 1: Intact cells were lysed after sortagging. 2: Cells were first lysed, and lysates were subsequently sortagged. (B) Stably transduced HEK 293T cells expressing tagged dectin (293dectin) were sortagged with a biotinylated probe, lysed, and treated with PNGase F, EndoH, or CIP. Streptavidin-HRP immunobloting reveals deglycosylated dectin-1 exclusively when treated with PNGase F (Biotin). Anti-HA blotting shows deglycosylation of dectin-1 when treated with either PNGase F or EndoH (HA tag). HEK 293T cells transfected with similarly tagged CD74 was the positive control (293CD74). (C) A mixed population of stably transduced HEK 293T cells expressing tagged dectin-1 and control HEK 293T cells transfected with GFP were incubated with sortase A and the Alexa Fluor 647 (red) probe, then incubated with Alexa Fluor 555 (orange)-labeled particles of zymosan (10 particles per cell, 30 min). Red-labeled sortagged dectin-1 recognized β-glucan and bound Alexa Fluor 555-labeled zymosan particles (arrowheads). Control GFP+ cells without sortase A treatment and incubated with the fluorophore showed no red fluorescence.
Dectin-1 analyzed by immunoblotting presents itself as a doublet. Murine dectin-1 is glycosylated (4) and phosphorylated, modifications that could yield multiple electrophoretically distinct species. We sortagged dectin-1 in stably transduced HEK 293T cells with a biotinylated probe and subjected the cell lysates to digestion with the glycosidases N-Glycosidase F (PNGase F) or endoglycosidase H (EndoH), as well as with calf intestinal alkaline phosphatase (CIP). PNGase F hydrolyzes all types of N-glycans, whereas EndoH is specific for high mannose-type N-linked glycans. The presence of high mannose-type N-linked glycans is usually diagnostic of localization to the endoplasmic reticulum (ER) and/or cis Golgi. After PNGase F treatment, a single biotinylated polypeptide remained; samples treated with either EndoH or CIP still presented as two distinct polypeptides (Fig. 1B), which must therefore be the result of differential carbohydrate modifications. The exclusive presence of EndoH-resistant, biotinylated dectin-1 when intact cells were labeled indicates that sortase A installed the biotinylated probe only on dectin-1 that had undergone complex glycan modification before transit to the cell surface.
To investigate whether sortagged dectin-1 is functional on live cells, we installed an Alexa Fluor 647 probe on dectin-1 and determined whether the tagged protein retained its ability to bind zymosan. The specificity of sortagging was evaluated by labeling a mixture of dectin-1–expressing HEK 293T cells and control HEK 293T cells transfected with a plasmid encoding GFP. Treatment of the cells with sortase A installed the fluorophore only on the surface of cells that did not express GFP; none of the GFP+ cells was labeled. Furthermore, only those cells that displayed the Alexa 647 fluorophore bound zymosan (Fig. 1C).
We conclude that a fraction of dectin-1 is accessible at the cell surface and amenable to sortagging. This labeling is highly selective: no biotinylated materials are detected on empty vector controls, and the only biotinylated species detectable in cells expressing dectin-1 has the molecular characteristics of dectin-1. Moreover, surface-disposed sortagged dectin-1 retains its function.
Internalization of Dectin-1 upon Zymosan Presentation.
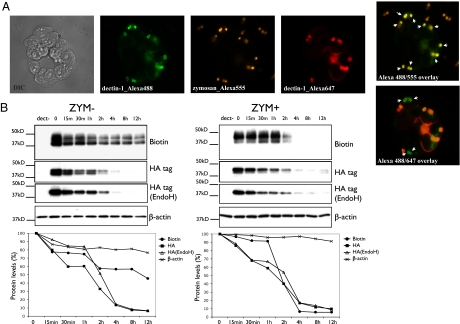
Because sortase A installs a label specifically on surface-exposed dectin-1, we could follow the location of sortagged dectin-1 after exposure of cells to zymosan. We first installed an Alexa Fluor 488 probe on surface-disposed dectin-1 in HEK 293T cells and then exposed the cells to zymosan for 30 min. Then an Alexa Fluor 647 probe was installed on the surface to mark any newly surface exposed dectin-1 that appeared after the first round of sortagging. Cells were then fixed and examined by fluorescence microscopy. The fluorophore installed first showed the presence of dectin-1, either bound to zymosan particles on the surface or internalized. However, dectin-1 sortagged in a second round of labeling with the Alexa Fluor 647 was found only on the cell surface (Fig. 2A).
Fig. 2.
Sortagged dectin-1 visualized on the cell surface or internalized upon zymosan binding. (A) Stably transduced HEK 293T cells were incubated with sortase A and the Alexa Fluor 488 (green) probe, incubated with Alexa Fluor 555-labeled (orange) zymosan (10 particles per cell, 30 min), and then incubated with sortase A and the Alexa Fluor 647 (red) probe. Fluorophore-labeled dectin-1 appears bound to Alexa Fluor 555-labeled (orange) zymosan particles (arrowheads, Alexa 488/555 overlay). Alexa Fluor 647-labeled (red) dectin-1 appears only on the cell surface. Alexa Fluor 488-labeled (green) dectin-1 appears on the cell surface or internalized (arrowheads) (Alexa 488/647 overlay). (B) A biotinylated probe was installed on dectin-1 in stably transduced HEK 293T cells. Cells inhibited with cycloheximide (50 μg/mL) were untreated or presented with zymosan. The levels of biotinylated dectin-1 on the cell surface and intracellular HA-tagged dectin-1 were analyzed by immunoblotting at different times. Without zymosan, biotinylated dectin-1 levels on the cell surface decrease gradually to near 50% of the initial levels (ZYM-). With zymosan, the levels of biotinylated dectin-1 decrease rapidly after 1 h (ZYM+). The degradation rate of the intracellular HA-tagged dectin-1 in cytoplasm, cis Golgi, or ER (HA tag, EndoH) was constant (with or without zymosan). Biotin, biotinylated dectin-1; HA, HA-tagged dectin-1; EndoH, EndoH-treated HA-tagged dectin-1; dect-, control cells with no tagged-dectin-1 expression.
These data establish that, upon contact with zymosan, dectin-1 is internalized. As expected, regardless of zymosan-induced internalization, dectin-1 continues to emerge at the cell surface via the biosynthetic pathway. To follow dectin-1 internalization in time, a biotinylated probe was installed on dectin-1 in intact HEK 293T cells. Cells were then incubated with cycloheximide to arrest protein synthesis, and the culture was divided in two. One sample received zymosan, the other served as a control, and both were then sampled at different time points. Two different fractions of dectin-1 were assayed: dectin-1 located on the cell surface as inferred from the presence of the biotin label, and intracellular dectin-1 visualized by means of the HA tag. In the presence of zymosan, the rate of internalization and degradation of biotinylated dectin-1 on the cell surface increases dramatically after 1 h of incubation (Fig. 2B). The half-life of surface-located dectin-1, estimated to be between 8 h and 12 h in the absence of zymosan, decreases to <2 h in the presence of zymosan. The rate of degradation of intracellular HA-tagged dectin-1 was higher than that of biotinylated dectin-1, always showing a rapid decrease after 1 h of incubation, regardless of the presence of zymosan (Fig. 2B). This observation suggests that a significant fraction of newly synthesized dectin-1 fails to mature and is degraded instead.
Dectin-1 Associates with Galectin-3 in Macrophages.
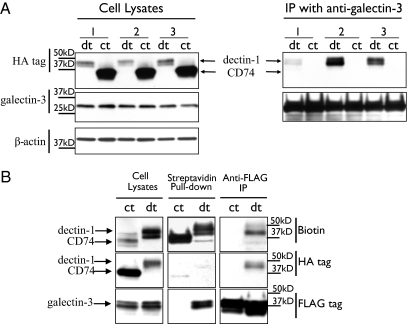
The installation of a biotinylated probe on surface-exposed dectin-1 and its subsequent retrieval with streptavidin beads uncovered an association between dectin-1 and galectin-3 in RAW 264.7 macrophages (SI Results and Fig. S2). We further analyzed the interaction between dectin-1 and galectin-3 in RAW 264.7 macrophages by coimmunoprecipitation. Cell lysates from RAW 264.7 cells stably expressing HA-tagged dectin-1 (and not exposed to sortase, in this case) were immunoprecipitated with an anti–galectin-3 antibody. Western blot analysis using anti-HA antibodies revealed HA-tagged dectin-1, confirming the interaction between galectin-3 and dectin-1 (Fig. 3A). We also assayed the effect of zymosan presentation on this association. Intact RAW 264.7 cells with stable HA-tagged dectin-1 expression were incubated with zymosan particles for 30 min, lysed, and then treated with the anti–galectin-3 antibody. As expected, the HA-immunoblotting analysis of the immunoprecipitates showed higher amounts of dectin-1 compared with samples without zymosan presentation. We obtained similar results when we lysed RAW 264.7 cells stably expressing dectin-1 and subsequently incubated the lysates with zymosan particles for 30 min. The anti–galectin-3 immunoprecipitates from control cells expressing HA-tagged CD74 did not show any HA-tagged protein when analyzed by immunoblotting with anti-HA antibodies (Fig. 3A), confirming the specificity of the dectin-1/galectin-3 association.
Fig. 3.
Coimmunoprecipitation of dectin-1 with galectin-3. (A) RAW 264.7 cells stably expressing tagged dectin-1 were split in three groups: one lysed with 0.5% Nonidet P-40 (1), a second incubated with zymosan (10 particles per cell, 30 min), then lysed (2), and a third group lysed and then incubated with zymosan (3). Cell extracts were immunoprecipitated with anti–galectin-3 antibody (5 μg) and analyzed by immunoblotting. Left: Immunoblots of lysate detecting dectin-1 and control CD74 (HA tag). Right: Immunoblot of precipitates from lysates with anti–galectin-3 antibody. (B) HEK 293T cells expressing tagged dectin-1 were transfected with a plasmid encoding 3xFLAG-tagged galectin-3. One fraction was sortagged, labeling exposed dectin-1 with a biotinylated probe, lysed with 0.5% Nonidet P-40, and lysates immunoblotted (Cell Lysates). A second fraction was immunoprecipitated with magnetic streptavidin Dynabeads and immunoblotted (Streptavidin Pull-down). A third fraction incubated with anti-FLAG–coated magnetic beads was immunoblotted to detect biotinylated and HA-tagged dectin-1 (anti-FLAG IP). HEK 293T cells cotransfected with tagged CD74 and tagged galectin-3 were used as a control. dt, cells expressing tagged dectin-1; ct, control cells expressing tagged CD74.
This specificity of the interaction between dectin-1 and galectin-3 was also confirmed in dectin-1–expressing HEK 293T cells transfected with a plasmid encoding 3xFLAG-tagged galectin-3. Using sortase, we labeled surface-exposed dectin-1 in intact cells with a biotinylated probe to differentiate surface disposed dectin-1 from the intracellular HA-tagged fraction. Cells were lysed, and a fraction of the lysate was then incubated with streptavidin beads to retrieve biotinylated dectin-1. Bound proteins were removed from beads, separated by SDS gel electrophoresis, and analyzed by immunoblotting. When we incubated the blot with an anti-FLAG antibody, we observed a polypeptide of the expected size for FLAG-tagged galectin-3. We also incubated a part of the cell lysates with an anti-FLAG antibody and analyzed the immunoprecipitates by streptavidin or HA-immunoblotting. Both biotin-labeled and HA-tagged dectin-1 were detected in all cases, confirming the interaction of galectin-3 with the fraction of dectin-1 located on the surface as well as the fraction inside the cell. Neither biotinylated nor HA-tagged CD74, the type II membrane protein used for comparison, from control cells interacted with galectin-3 (Fig. 3B).
Galectin-3 in Association with Dectin-1 Modulates the Induction of TNF-α Expression.
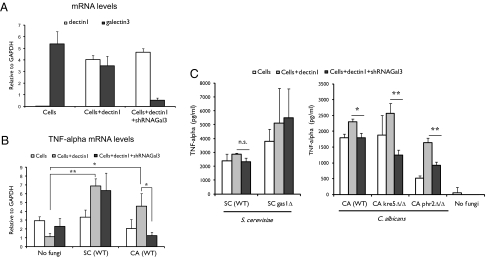
To study the biological consequences of the dectin-1/galectin-3 interaction, we performed siRNA-based knockdowns of galectin-3 in RAW 264.7 macrophages and determined the effect of its reduced expression on the proinflammatory response after fungal presentation. A comparison of the response to Saccharomyces cerevisiae and Candida albicans required that both be in the yeast form. However, C. albicans produces filaments under the conditions of this assay. For this reason we inactivated fungi with UV (both C. albicans and S. cerevisiae) because UV-irradiated Candida cells do not present as filaments and remain in the yeast form. We first incubated galectin-3–competent RAW 264.7 cells with UV-treated S. cerevisiae or C. albicans at a ratio of 1:5 and measured TNF-α mRNA expression after 4 h. There was no significant increase in the level of induction of TNF-α expression, compared with cells without fungal presentation. These cells have very low expression levels of dectin-1 (Fig. 4A), so we next studied the response in cells that stably express dectin-1. In these cells we observed a significant increase in TNF-α mRNA levels after fungal presentation (P < 0.05). This observation confirms the key role of dectin-1 in the induction of TNF-α when confronted with either S. cerevisiae or C. albicans. We then studied the response in RAW 264.7 cells in which the level of galectin-3 was reduced by an RNA hairpin directed against galectin-3 mRNA. This hairpin successfully reduced the level of galectin-3 mRNA (Fig. 4A). Cells with and without the hairpin had similar mRNA levels of TNF-α when they were incubated with S. cerevisiae. However, when cells were incubated with C. albicans, there is a nearly fourfold decrease (P < 0.05) compared with galectin-3–competent cells (Fig. 4B). These data show that galectin-3 functions in concert with dectin-1 to modulate differentially the response to these two fungi. Significantly, the intensity of the response is much greater for the pathogen C. albicans than the nonpathogen S. cerevisiae.
Fig. 4.
Association between dectin-1 and galectin-3 modulates the TNF-α response in RAW 264.7 macrophages. (A) Galectin-3 siRNA knockdown in RAW 264.7 cells. mRNA levels in RAW 264.7 cells (Cells), stably dectin-1–expressing RAW 264.7 cells (Cells+dectin1), and stably dectin-1–expressing cells transduced with galectin-3 shRNA (Cells+dectin1+shRNAGal3) were determined by quantitative RT-PCR, normalized to GAPDH, and expressed as fold induction. (B) Macrophages were incubated for 4 h at 37 °C with wild-type S. cerevisiae or C. albicans at a 1:5 (macrophage:yeast) ratio. TNF-α mRNA levels were determined by quantitative RT-PCR. (C) Cells were incubated for 12 h with S. cerevisiae (wild-type) or gas1Δ mutant and C. albicans (wild-type) or phr2Δ/Δ (= C. albicans gas1) or kre5Δ/Δ mutants at a 1:5 (macrophage:yeast) ratio and TNF-α (supernatants) was measured. Mean values (± SEM) from three independent experiments are shown. **P < 0.01; *P < 0.05. n.s., not significant; SC, S. cerevisiae; CA, C. albicans.
Dectin-1/Galectin-3 Interaction Contributes to TNF-α Induction upon Recognition of β-glucan.
The functional consequences of the dectin-1/galectin-3 interaction could be muted by masking of the β-glucan by the outer coat of mannoproteins on yeast cell walls (21). Therefore, we analyzed the proinflammatory response using mutant yeast strains that unmask the underlying cell wall β-glucan. The S. cerevisiae gas1Δ and C. albicans phr2Δ/Δ and kre5Δ/Δstrains have increased β-glucan exposure on their surface compared with wild-type cells. The GAS1 gene encodes a β-glucan transglycosylase required for β-glucan branching and cell wall integrity. The PHR2 gene is the C. albicans homolog of the S. cerevisiae GAS1 gene. KRE5, essential for S. cerevisiae viability, is involved in the initiation of β-1,6-glucan synthesis. RAW 264.7 cells were incubated with wild-type and mutant yeast at a ratio of 1:5, and, after 12 h incubation, levels of TNF-α were quantified. When challenged with either wild-type or the mutants, macrophages that express higher levels of dectin-1 produce more TNF-α. When challenged with wild-type C. albicans, cells that have reduced galectin-3 expression have a significant decrease (P < 0.05) in TNF-α levels. In contrast, there was no significant decrease in the TNF-α levels when cells were exposed to S. cerevisiae cells (Fig. 4C).
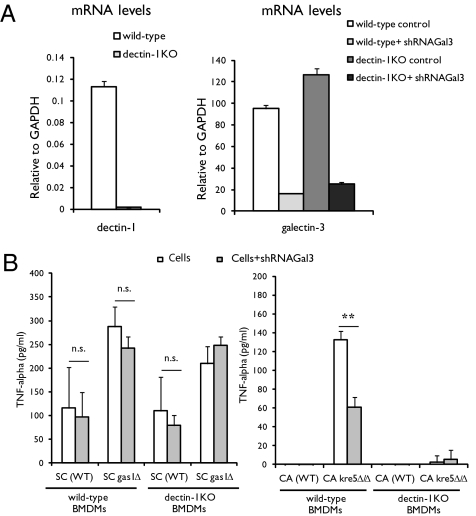
Yeast mutants with more exposed β-glucan showed a much greater reduction in TNF-α secretion (P < 0.01) by macrophages with reduced galectin-3 expression. We observed similar results when we exposed bone marrow-derived macrophages (BMDMs) from wild-type mice and dectin-1 knockout (dectin-1KO) mice that do not express dectin-1 (Fig. 5A) to yeast. When challenged with C. albicans, wild-type BMDMs elicited TNF-α only when exposed to C. albicans kre5Δ/Δ, whereas dectin-1KO BMDMs failed to trigger a TNF-α response with either wild-type or the mutant. Wild-type BMDMs with reduced galectin-3 expression showed a significant reduction in TNF-α secretion (P < 0.01) when exposed to the C. albicans mutant. We did not detect significant changes in TNF-α secretion between BMDMs with different levels of expression of either dectin-1 or galectin-3 when challenged with S. cerevisiae (Fig. 5B). These data show (i) that the observed proinflammatory response elicited by C. albicans is dectin-1 dependent and (ii) that a decrease in galectin-3 expression drastically affects the proinflammatory response to C. albicans but not that to S. cerevisiae.
Fig. 5.
Association between dectin-1 and galectin-3 is required for fungal recognition and TNF-α response in BMDMs. (A) Galectin-3 knockdown in BMDMs. mRNA levels from BMDMs from wild-type and dectin-1KO mice were determined by quantitative RT-PCR (normalized to GAPDH, fold induction). (B) BMDMs from wild-type and dectin-1KO mice (Cells) as well as cells transduced with galectin-3 shRNA (Cells+shRNAGal3) were incubated with wild-type yeast or with S. cerevisiae gas1Δ or C. albicans kre5Δ/Δ mutants at a 1:5 (macrophage:yeast) ratio. TNF-α (supernatants) was measured after 12 h. Data are the mean values (± SEM) from three independent experiments. **P < 0.01. n.s., not significant; SC, S. cerevisiae; CA, C. albicans.
Discussion
Using sortagging we identify an association between dectin-1 and galectin-3 that is required for the proinflammatory response in macrophages exposed to fungi. Dectin-1 suitably modified for sortagging is active in live macrophages; it is inserted into the plasma membrane, where it recognizes its ligand, β-glucan, and initiates the signal for an inflammatory response when challenged with fungi. Cells expressing tagged dectin-1 recognized and bound zymosan, a particulate preparation of S. cerevisiae cell walls that contains large amounts of β-glucan. Sortagging showed that a fraction of dectin-1 exposed at the cell surface is accessible to sortase-mediated labeling in a specific manner. This finding also extends previous work in which the efficiency and specificity of the method were tested in complex protein mixtures, including mixtures of soluble proteins, and on live cells (18). We observed these results in transfected HEK 293T cells, a cell line that does not show phagocytic activity unless dectin-1 is expressed (22). The ability of tagged dectin-1 to enable binding and internalization of zymosan in otherwise nonphagocytic cells is similar to that reported for NIH 3T3 fibroblasts transfected with dectin-1 (7, 16, 23) and suggests that the tagged protein retains this function as well.
Visualization of dectin-1 on live cells without compromising its ability to recognize β-glucan—and avoiding the use of antibodies—was possible with site-specific installation of fluorophores on the protein. This fluorophore-labeled dectin-1 binds zymosan on the cell surface and is then internalized. Upon recognition of β-glucan, levels of surface-derived, biotinylated dectin-1 dropped precipitously after 1 h of incubation. In the absence of zymosan exposure, dectin-1 was more stable on the cell surface. The fraction of preexisting cell-internal dectin-1 was not affected by zymosan exposure. The degradation rate of intracellular dectin-1 was faster than that of the surface-disposed fraction, suggesting that a fraction of newly synthesized, cell-internal dectin-1 fails to mature and is degraded instead. Such retention may be similar to that reported for other glycoproteins such as the KIR3DL1 natural killer cell receptor, in which only a minor fraction escapes the ER, whereas the surface-exposed fraction is fully functional (24). The internalization of dectin-1 upon its interaction with ligand may attenuate pathways involved in induction of the innate immune response (25). Once dectin-1 is internalized, the route of intracellular processing may well depend on the nature of the ligand. For example, dectin-1 is directed to lysosomes after uptake of zymosan but recycled to the membrane when confronted with the soluble ligand laminarin (16).
We uncovered a previously undiscovered association between dectin-1 and galectin-3 in macrophages, which was confirmed by coimmunoprecipitation in RAW 264.7 cells and also in HEK 293T transfectants expressing both proteins. This association connects two signature molecules on the surface of fungi, β-glucan and oligomannans. Dectin-1 recognizes β-glucan, a carbohydrate that accounts for a major fraction of the cell wall of most fungi, and it is covalently linked to an outer layer of mannoproteins (1, 8, 9). Galectin-3 has been described as a PRR that recognizes specific β-1,2 oligomannans from the cell wall of certain fungi (20, 26). Galectin-3 binds to four Candida species expressing different combinations of β-1,2 oligomannans but does not bind S. cerevisiae, which lacks this specific oligosaccharide in its cell wall. Moreover, galectin-3 binding to C. albicans is fungicidal in a complement-independent fashion (20). There are specialized PRRs, such as TLRs, DC-SIGN, or the mannose receptor, that also recognize exposed cell wall mannose (19, 27–29), but only galectin-3 discriminates between pathogenic and nonpathogenic fungi that lack specific β-linked mannosides (20, 30).
Consistent with a role for the association between these molecules in fungal recognition, we found that galectin-3 modulates the inflammatory response of macrophages in cooperation with dectin-1. RAW 264.7 macrophages express galectin-3 but express little dectin-1 and do not show induction of TNF-α regardless of fungal presentation. This result shows that the presence of galectin-3 alone is insufficient to elicit TNF-α production. When dectin-1 expression is increased, RAW 264.7 macrophages show a significant TNF-α response upon exposure to either S. cerevisiae or the pathogen C. albicans, as previously reported (10). Therefore, dectin-1 must be present for TNF-α induction. If the levels of galectin-3 are reduced using RNAi, RAW 264.7 macrophages fail to generate this dectin-1–driven TNF-α response when confronted with the pathogen C. albicans. Thus, both dectin-1 and galectin-3 must be present for the proinflammatory response. Remarkably, the loss of galectin-3 does not affect the TNF-α response to the nonpathogen S. cerevisiae.
The functional significance of the dectin-1/galectin3 association was further supported using fungal mutants with increased β-glucan exposure. When RAW 264.7 macrophages or BMDMs expressing dectin-1 are exposed to C. albicans mutants with increased exposure of β-glucan, the loss of galectin-3 dramatically accentuates the failure to trigger an appropriate TNF-α response. Dectin-1 requires the association with galectin-3 to discriminate the nonpathogen S. cerevisiae from the pathogen C. albicans. The dectin-1/galectin3 association thus plays an important role in the recognition of C. albicans, distinct from the mode of recognition of S. cerevisiae. Our functional data provide a clue as to how cells differentiate between fungi that are pathogens and those that are not.
Our finding of differential recognition of S. cerevisiae and C. albicans is supported by previous work on galectin-3 knockout mice. Galectin-3 associates with TLR2 at the cell membrane. Jouault et al. (30) reported coprecipitation of galectin-3 and TLR2 in THP-1 cells. TLR2 recognizes phospholipomannan and activates inflammatory genes that trigger cytokine production (29, 31). The association with galectin-3 seems to modulate this TLR2-driven inflammatory response. Macrophages from galectin-3 knockout mice resulted in lower levels of TNF-α in response to C. albicans compared with cells from wild-type mice, whereas there was no significant difference in TNF-α induction between wild-type and mutant when cells were incubated with S. cerevisiae (30). Galectin-3 seems to display a similar role in association with TLR2, so it is likely that the association of galectin-3 with different cell surface receptors may well be a mechanism that would allow the cell to adjust the appropriate inflammatory response toward a specific fungal challenge, primarily after sensing C. albicans. The association between galectin-3 and dectin-1 can thus modulate the proinflammatory response to help distinguish between pathogenic and nonpathogenic fungi.
Materials and Methods
RAW 264.7 (ATCC TIB-71) and HEK 293T (ATCC CRL-1573) cells were obtained from the American Type Culture Collection. C57BL/6 mice were obtained from Charles River. Dectin-1−/− mice were a gift from Stuart M. Levitz (University of Massachusetts Medical School, Worcester, MA) and Gordon D. Brown (University of Cape Town, South Africa) (32). BMDMs were differentiated from murine monocyte progenitor cells and treated as described in SI Materials and Methods. Information on all experimental procedures is provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. Constance L. Cepko for the gift of the pCAG-GFP plasmid, Stuart M. Levitz and Gordon D. Brown for providing knockout mice, and Eric Spooner for his technical assistance with mass spectrometry. This work was funded by National Institutes of Health Grant GM040266 (to G.R.F.) and by National Institute of Allergy and Infectious Diseases Grant 5 R01 AI087879 (to H.L.P.). A.E. received funding from the Fulbright/Spanish Ministry of Education and Science Visiting Scholar Program (FU2006-0983). V.K.V. received a National Institutes of Health National Research Service Award (F32 AI729353) and the Margaret and Herman Sokol Fellowship in Biomedical Research.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1111415108/-/DCSupplemental.
References
- 1.Brown GD. Dectin-1: A signalling non-TLR pattern-recognition receptor. Nat Rev Immunol. 2006;6:33–43. doi: 10.1038/nri1745. [DOI] [PubMed] [Google Scholar]
- 2.Brown GD, Gordon S. Immune recognition. A new receptor for beta-glucans. Nature. 2001;413:36–37. doi: 10.1038/35092620. [DOI] [PubMed] [Google Scholar]
- 3.Fuller GL, et al. The C-type lectin receptors CLEC-2 and Dectin-1, but not DC-SIGN, signal via a novel YXXL-dependent signaling cascade. J Biol Chem. 2007;282:12397–12409. doi: 10.1074/jbc.M609558200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kato Y, Adachi Y, Ohno N. Contribution of N-linked oligosaccharides to the expression and functions of beta-glucan receptor, Dectin-1. Biol Pharm Bull. 2006;29:1580–1586. doi: 10.1248/bpb.29.1580. [DOI] [PubMed] [Google Scholar]
- 5.Valera I, et al. Costimulation of dectin-1 and DC-SIGN triggers the arachidonic acid cascade in human monocyte-derived dendritic cells. J Immunol. 2008;180:5727–5736. doi: 10.4049/jimmunol.180.8.5727. [DOI] [PubMed] [Google Scholar]
- 6.Brown GD, et al. Dectin-1 is a major beta-glucan receptor on macrophages. J Exp Med. 2002;196:407–412. doi: 10.1084/jem.20020470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herre J, Gordon S, Brown GD. Dectin-1 and its role in the recognition of beta-glucans by macrophages. Mol Immunol. 2004;40:869–876. doi: 10.1016/j.molimm.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 8.Palma AS, et al. Ligands for the beta-glucan receptor, Dectin-1, assigned using “designer” microarrays of oligosaccharide probes (neoglycolipids) generated from glucan polysaccharides. J Biol Chem. 2006;281:5771–5779. doi: 10.1074/jbc.M511461200. [DOI] [PubMed] [Google Scholar]
- 9.Adams EL, et al. Differential high-affinity interaction of dectin-1 with natural or synthetic glucans is dependent upon primary structure and is influenced by polymer chain length and side-chain branching. J Pharmacol Exp Ther. 2008;325:115–123. doi: 10.1124/jpet.107.133124. [DOI] [PubMed] [Google Scholar]
- 10.Brown GD, et al. Dectin-1 mediates the biological effects of beta-glucans. J Exp Med. 2003;197:1119–1124. doi: 10.1084/jem.20021890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Underhill DM. Collaboration between the innate immune receptors dectin-1, TLRs, and Nods. Immunol Rev. 2007;219:75–87. doi: 10.1111/j.1600-065X.2007.00548.x. [DOI] [PubMed] [Google Scholar]
- 12.Ferwerda G, Meyer-Wentrup F, Kullberg BJ, Netea MG, Adema GJ. Dectin-1 synergizes with TLR2 and TLR4 for cytokine production in human primary monocytes and macrophages. Cell Microbiol. 2008;10:2058–2066. doi: 10.1111/j.1462-5822.2008.01188.x. [DOI] [PubMed] [Google Scholar]
- 13.Mantegazza AR, et al. CD63 tetraspanin slows down cell migration and translocates to the endosomal-lysosomal-MIICs route after extracellular stimuli in human immature dendritic cells. Blood. 2004;104:1183–1190. doi: 10.1182/blood-2004-01-0104. [DOI] [PubMed] [Google Scholar]
- 14.Meyer-Wentrup F, et al. Dectin-1 interaction with tetraspanin CD37 inhibits IL-6 production. J Immunol. 2007;178:154–162. doi: 10.4049/jimmunol.178.1.154. [DOI] [PubMed] [Google Scholar]
- 15.van Spriel AB, et al. The tetraspanin protein CD37 regulates IgA responses and anti-fungal immunity. PLoS Pathog. 2009;5:e1000338. doi: 10.1371/journal.ppat.1000338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herre J, et al. Dectin-1 uses novel mechanisms for yeast phagocytosis in macrophages. Blood. 2004;104:4038–4045. doi: 10.1182/blood-2004-03-1140. [DOI] [PubMed] [Google Scholar]
- 17.Kennedy AD, et al. Dectin-1 promotes fungicidal activity of human neutrophils. Eur J Immunol. 2007;37:467–478. doi: 10.1002/eji.200636653. [DOI] [PubMed] [Google Scholar]
- 18.Popp MW, Antos JM, Grotenbreg GM, Spooner E, Ploegh HL. Sortagging: A versatile method for protein labeling. Nat Chem Biol. 2007;3:707–708. doi: 10.1038/nchembio.2007.31. [DOI] [PubMed] [Google Scholar]
- 19.Jouault T, et al. Candida albicans phospholipomannan is sensed through toll-like receptors. J Infect Dis. 2003;188:165–172. doi: 10.1086/375784. [DOI] [PubMed] [Google Scholar]
- 20.Kohatsu L, Hsu DK, Jegalian AG, Liu FT, Baum LG. Galectin-3 induces death of Candida species expressing specific beta-1,2-linked mannans. J Immunol. 2006;177:4718–4726. doi: 10.4049/jimmunol.177.7.4718. [DOI] [PubMed] [Google Scholar]
- 21.Wheeler RT, Fink GR. A drug-sensitive genetic network masks fungi from the immune system. PLoS Pathog. 2006;2:e35. doi: 10.1371/journal.ppat.0020035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gantner BN, Simmons RM, Underhill DM. Dectin-1 mediates macrophage recognition of Candida albicans yeast but not filaments. EMBO J. 2005;24:1277–1286. doi: 10.1038/sj.emboj.7600594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heinsbroek SE, et al. Expression of functionally different dectin-1 isoforms by murine macrophages. J Immunol. 2006;176:5513–5518. doi: 10.4049/jimmunol.176.9.5513. [DOI] [PubMed] [Google Scholar]
- 24.Taner SB, et al. Interactions of NK cell receptor KIR3DL1*004 with chaperones and conformation-specific antibody reveal a functional folded state as well as predominant intracellular retention. J Immunol. 2011;186:62–72. doi: 10.4049/jimmunol.0903657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hernanz-Falcón P, Joffre O, Williams DL, Reis e Sousa C. Internalization of Dectin-1 terminates induction of inflammatory responses. Eur J Immunol. 2009;39:507–513. doi: 10.1002/eji.200838687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fradin C, Poulain D, Jouault T. beta-1,2-linked oligomannosides from Candida albicans bind to a 32-kilodalton macrophage membrane protein homologous to the mammalian lectin galectin-3. Infect Immun. 2000;68:4391–4398. doi: 10.1128/iai.68.8.4391-4398.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koppel EA, van Gisbergen KP, Geijtenbeek TB, van Kooyk Y. Distinct functions of DC-SIGN and its homologues L-SIGN (DC-SIGNR) and mSIGNR1 in pathogen recognition and immune regulation. Cell Microbiol. 2005;7:157–165. doi: 10.1111/j.1462-5822.2004.00480.x. [DOI] [PubMed] [Google Scholar]
- 28.Taylor PR, Gordon S, Martinez-Pomares L. The mannose receptor: Linking homeostasis and immunity through sugar recognition. Trends Immunol. 2005;26:104–110. doi: 10.1016/j.it.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 29.Willment JA, Brown GD. C-type lectin receptors in antifungal immunity. Trends Microbiol. 2008;16:27–32. doi: 10.1016/j.tim.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 30.Jouault T, et al. Specific recognition of Candida albicans by macrophages requires galectin-3 to discriminate Saccharomyces cerevisiae and needs association with TLR2 for signaling. J Immunol. 2006;177:4679–4687. doi: 10.4049/jimmunol.177.7.4679. [DOI] [PubMed] [Google Scholar]
- 31.Underhill DM, et al. The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature. 1999;401:811–815. doi: 10.1038/44605. [DOI] [PubMed] [Google Scholar]
- 32.Taylor PR, et al. Dectin-1 is required for beta-glucan recognition and control of fungal infection. Nat Immunol. 2007;8:31–38. doi: 10.1038/ni1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.