Abstract
BACKGROUND
Congenital cytomegalovirus (CMV) infection is an important cause of hearing loss, and most infants at risk for CMV-associated hearing loss are not identified early in life because of failure to test for the infection. The standard assay for newborn CMV screening is rapid culture performed on saliva specimens obtained at birth, but this assay cannot be automated. Two alternatives — real-time polymerase-chain-reaction (PCR)–based testing of a liquid-saliva or dried-saliva specimen obtained at birth — have been developed.
METHODS
In our prospective, multicenter screening study of newborns, we compared real-time PCR assays of liquid-saliva and dried-saliva specimens with rapid culture of saliva specimens obtained at birth.
RESULTS
A total of 177 of 34,989 infants (0.5%; 95% confidence interval [CI], 0.4 to 0.6) were positive for CMV, according to at least one of the three methods. Of 17,662 newborns screened with the use of the liquid-saliva PCR assay, 17,569 were negative for CMV, and the remaining 85 infants (0.5%; 95% CI, 0.4 to 0.6) had positive results on both culture and PCR assay. The sensitivity and specificity of the liquid-saliva PCR assay were 100% (95% CI, 95.8 to 100) and 99.9% (95% CI, 99.9 to 100), respectively, and the positive and negative predictive values were 91.4% (95% CI, 83.8 to 96.2) and 100% (95% CI, 99.9 to 100), respectively. Of 17,327 newborns screened by means of the dried-saliva PCR assay, 74 were positive for CMV, whereas 76 (0.4%; 95% CI, 0.3 to 0.5) were found to be CMV-positive on rapid culture. Sensitivity and specificity of the dried-saliva PCR assay were 97.4% (95% CI, 90.8 to 99.7) and 99.9% (95% CI, 99.9 to 100), respectively. The positive and negative predictive values were 90.2% (95% CI, 81.7 to 95.7) and 99.9% (95% CI, 99.9 to 100), respectively.
CONCLUSIONS
Real-time PCR assays of both liquid- and dried-saliva specimens showed high sensitivity and specificity for detecting CMV infection and should be considered potential screening tools for CMV in newborns. (Funded by the National Institute on Deafness and Other Communication Disorders.)
Cytomegalovirus (CMV) is a frequent cause of congenital infection and a leading nongenetic cause of sensorineural hearing loss.1–5 In most infants with congenital CMV infection, clinical abnormalities do not manifest at birth; rather, the infection is asymptomatic. However, sensorineural hearing loss eventually develops in approximately 10 to 15% of CMV-positive children,3,4,6–8 in a substantial proportion who are not diagnosed by means of newborn hearing screening.7–9 Screening of newborns for CMV infection will permit early identification of at-risk congenitally infected infants for purposes of targeted monitoring and intervention during critical stages of speech and language development.10,11
A variety of methods have been evaluated for use in the diagnosis of congenital CMV infection on the basis of saliva, urine, and dried-blood-spot specimens obtained from newborns.12–17 Culture-based testing of urine and saliva specimens has been the standard method to identify infants with congenital CMV infection.13,18,19 However, culture-based methods are not easily amenable to automation and, therefore, cannot be adapted for large-scale newborn screening.
Since dried-blood-spot specimens are obtained routinely in all infants, the usefulness of polymerase-chain-reaction (PCR) testing of dried-blood spots for the diagnosis of congenital CMV infection has been examined.15,16,20–23 In addition, our recent large-scale newborn-screening study of a dried-blood-spot PCR assay that was prospectively compared with the standard saliva rapid culture showed that real-time dried-blood-spot PCR assay fails to identify the majority of CMV-infected newborns.14 Therefore, challenges remain in achieving high sensitivity of dried-blood-spot testing to screen newborns for CMV infection.24 Urine specimens collected on filter disks have also been explored as samples for CMV screening in newborns, but urine samples are harder to collect than saliva samples; this approach has not been validated by direct comparison with culture.17,25
Because of their ease of collection and since high titers of CMV are shed in the saliva of infected newborns, saliva specimens appear to be a better and less invasive type of sample for newborn screening.24,26,27 The current study was designed to determine the usefulness of a real-time PCR assay of saliva specimens obtained from newborns for CMV screening. During phase 1 of the study, saliva specimens were placed in transport medium and stored at 4°C before testing. PCR testing of dried-saliva specimens (those that were not placed in transport medium and remained at ambient temperature during specimen storage and transport) was examined in phase 2 of the study, since dried specimens are easier to store and transport. Finally, all PCR assays were performed without a DNA-extraction step, to test an assay that would be more practical for screening all newborns.
METHODS
STUDY DESIGN
Infants born at seven hospitals in the United States from June 2008 through November 2009 were enrolled prospectively in our National Institute on Deafness and Other Communication Disorders (NIDCD) CMV and Hearing Multicenter Screening (CHIMES) study. All live-born infants were eligible for participation. Infants with positive saliva-screening results (from rapid culture or PCR assay) were enrolled in the follow-up component of the study to monitor hearing outcome. Clinical decisions about evaluation and possible treatment of the CMV-infected infants were made by the physicians at each study site.
The NIDCD was the study sponsor and provided general oversight for the design and conduct of the study. However, the NIDCD had no role in the collection, management, analysis, and interpretation of the data or in the preparation, review, or approval of the manuscript. Institutional-review-board approval was obtained at each study site, and written informed consent was obtained from a parent or parents of all participating infants. The study was conducted according to the protocol (available with the full text of this article at NEJM.org). Race or ethnic group was reported by a parent. The study was designed by the CHIMES study investigators in consultation with NIDCD project officers. All authors vouch for the integrity of the data and data analyses and made the decision to submit the manuscript for publication. Members of the CHIMES study group are listed in the Supplementary Appendix, available at NEJM.org.
SPECIMEN COLLECTION
A real-time PCR protocol developed in our laboratory was adapted to test saliva specimens from newborns.14 Saliva specimens were collected by swabbing the inside of the baby’s mouth using a sterile polyester-fiber–tipped applicator (PurFybr) and transported to the central laboratory at the University of Alabama at Birmingham within 1 week after collection.14,19
Saliva swabs were placed in transport medium, transported to the central laboratory, and tested by means of rapid culture. During phase 1 of the study (beginning in June 2008), the specimens were also tested by means of liquid-saliva PCR assay. For phase 2 of the study (March through November 2009), an additional saliva swab collected at the same time was allowed to air-dry, placed in a sterile tube without transport medium, maintained and transported at ambient temperature to the central laboratory, and tested by means of dried-saliva PCR assay. Saliva specimens from some of the infants born between June 2008 and February 2009 were tested with the use of all three methods (rapid culture, liquid-saliva PCR assay, and dried-saliva PCR assay).
SPECIMEN PROCESSING AND TESTING
Liquid-saliva specimens were processed for rapid culture and PCR assay as described previously.14,19 Dried-saliva specimens were processed by adding 300 µl of PCR-grade water to the tubes containing the swabs, vortexing, and incubating for 20 minutes at room temperature. Then, 5 µl of the eluate containing saliva was used, without first undergoing DNA extraction, in the real-time PCR assay.
Rapid-Culture Assay
A rapid-culture assay for the detection of early-antigen fluorescent foci, involving a monoclonal antibody against the major immediate early antigen of CMV, was used to detect CMV in saliva specimens.14,18,19 Laboratory personnel performing the rapid culture were unaware of the results of PCR assay, and those performing the PCR assay were unaware of the results of the rapid culture.
Real-Time PCR Assay
A real-time PCR protocol described previously for dried-blood spots was performed to detect CMV DNA in saliva samples.14 A sample was considered positive if five or more copies per reaction were detected.
Follow-up Testing
Infants with positive rapid culture, PCR assay, or both were reevaluated to determine whether the PCR results were true or false positive results. This was done by testing saliva and urine18,19 specimens with the use of rapid culture and PCR assay (as described above).
STATISTICAL ANALYSIS
The results of the liquid- and dried-saliva real-time PCR assays were compared with those of saliva rapid culture (the standard method). Sensitivity, specificity, and predictive values for the PCR assays were calculated using standard methods for proportions and exact 95% confidence limits.
Likelihood ratios are based on the ratio of sensitivity and specificity and are independent of the prevalence of congenital CMV infection in the population; therefore, likelihood ratios can be used directly to estimate the probability of congenital CMV infection at the individual level.28 The positive likelihood ratio was calculated as the sensitivity divided by (1 − specificity), the negative likelihood ratio was calculated as (1 − sensitivity) divided by the specificity, and the 95% confidence intervals were calculated according to the method described by Simel and colleagues.24 All statistical analyses were performed using SAS software, version 9.2 (SAS Institute).
RESULTS
STUDY POPULATION AND SPECIMENS
During the study period, 34,989 infants were enrolled. The mean (±SD) age at the time of collection of saliva specimens was 1.0±1.2 days. Characteristics of the study population are shown in Table 1. Nearly all the infants (98.0%) were from well-baby nurseries. The median age at the time of collection of follow-up samples was 3.6 weeks (interquartile range, 2.6 to 6.6). Overall, 177 newborns (0.5%; 95% confidence interval [CI], 0.4 to 0.6) tested positive for CMV on screening by means of rapid culture, PCR assay, or both. No study-related adverse events were observed.
Table 1.
Baseline Characteristics of the 34,989 Study Newborns.*
| Characteristic | Value |
|---|---|
| Sex — no. (%) | |
| Female | 17,278 (49.4) |
| Male | 17,711 (50.6) |
| Race or ethnic group — no. (%)† | |
| Asian | 1,358 (3.9) |
| Black | 8,298 (23.7) |
| White, Hispanic | 11,356 (32.5) |
| White, non-Hispanic | 12,835 (36.7) |
| Other, including >1 category | 1,142 (3.3) |
| Insurance for hospital stay — no. (%) | |
| Private | 23,326 (66.7) |
| Public or no insurance | 11,663 (33.3) |
| Hospital nursery — no. (%) | |
| “Well-baby” nursery | 34,275 (98.0) |
| Neonatal intensive care | 714 (2.0) |
| Maternal age — yr | |
| Mean | 27.3±6.1 |
| Median (range) | 27 (12–52) |
Plus–minus values are means ±SD.
Race or ethnic group was reported by a parent.
NEWBORN CMV SCREENING WITH SALIVA RAPID CULTURE AND REAL-TIME PCR ASSAY
Rapid Culture and Liquid-Saliva PCR Assay
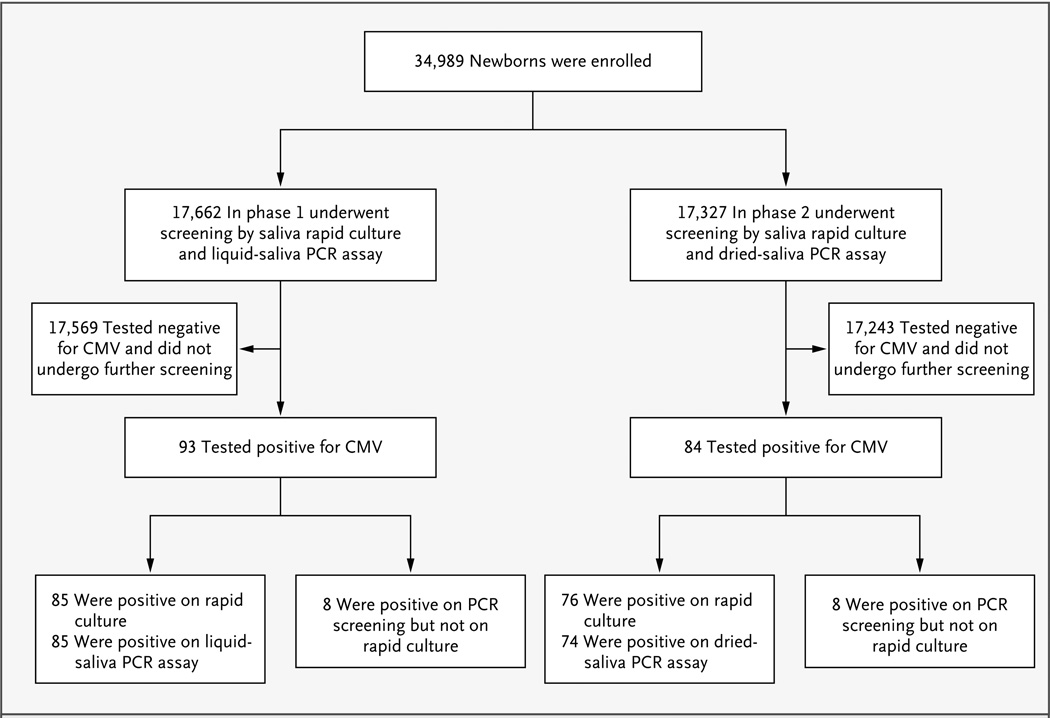
During phase 1, liquid-saliva specimens were collected from 17,662 newborns and tested for CMV with the use of rapid culture and liquid-saliva real-time PCR assay. A total of 93 infants (0.5%; 95% CI, 0.4 to 0.6) tested positive for CMV by any test (Fig. 1). All 85 infants with a positive rapid-culture result also had a positive liquid-saliva PCR assay, and the PCR assay also identified 8 additional infants as infected although their culture results were negative (Table 2). The sensitivity of liquid-saliva real-time PCR assay as compared with standard rapid culture was 100% (95% CI, 95.8 to 100) (based on 85 of 85 infants); the specificity was 99.9% (95% CI, 99.9 to 100) (based on 17,569 of 17,577 infants). The positive and negative predictive values for the saliva PCR assay were 91.4% (95% CI, 83.8 to 96.2) and 100% (95% CI, 99.9 to 100), respectively (based on 85 of 93 infants and 17,569 of 17,569 infants, respectively). The positive likelihood ratio for the liquid-saliva PCR assay was 2197 (95% CI, 1099 to 4393), and the negative likelihood ratio was 0 (95% CI, 0.0 to 0.1). Of the 93 newborns who were positive on screening, 79 (85%) were enrolled for follow-up, of whom 72 tested positive on both rapid culture and PCR assay, with 1 of the 72 found to be negative on retesting by means of rapid culture and PCR assay of both saliva and urine specimens. Of the 8 infants who tested positive on PCR assay only, 7 were enrolled in follow-up; of those, 6 were found to be negative for CMV on retesting by means of rapid culture and PCR assay of both saliva and urine specimens.
Figure 1. Enrollment and Prospective Screening of 34,989 Newborns.
CMV denotes cytomegalovirus, and PCR polymerase chain reaction.
Table 2.
Real-Time Polymerase-Chain-Reaction (PCR) Assays of Liquid- and Dried-Saliva Specimens, vs. Rapid Culture, Used to Screen for Congenital Cytomegalovirus Infection.
| Rapid Culture | Liquid-Saliva PCR Assay | Dried-Saliva PCR Assay | ||||
|---|---|---|---|---|---|---|
| Positive | Negative | Total | Positive | Negative | Total | |
| Positive | 85 | 0 | 85 | 74 | 2 | 76 |
| Negative | 8 | 17,569 | 17,577 | 8 | 17,243 | 17,251 |
| Total | 93 | 17,569 | 17,662 | 82 | 17,245 | 17,327 |
| Sensitivity (95% CI) — % | 100 (95.8–100) | 97.4 (90.8–99.7) | ||||
| Specificity (95% CI) — % | 99.9 (99.9–100) | 99.9 (99.9–100) | ||||
| Positive likelihood ratio (95% CI) | 2197 (1099–4393) | 2100 (1049–4202) | ||||
| Negative likelihood ratio (95% CI) | 0 (0.0–0.1) | 0.03 (0.0–0.1) | ||||
| Positive predictive value (95% CI) — % | 91.4 (83.8–96.2) | 90.2 (81.7–95.7) | ||||
| Negative predictive value (95% CI) — % | 100 (99.9–100) | 99.9 (99.9–100) | ||||
Rapid Culture and Dried-Saliva PCR Assay
During phase 2, a dried-saliva specimen was also collected from 17,327 newborns. Of the 84 (0.5%; 95% CI, 0.3 to 0.5) newborns who were positive for CMV on either type of screening assay, 76 (90%) were positive on rapid culture (Fig. 1). The dried-saliva real-time PCR assay yielded positive results for 74 of the 76 samples that were positive on rapid culture and an additional 8 samples that were negative on rapid culture (Table 2). As compared with rapid culture, the sensitivity of the dried-saliva PCR assay was 97.4% (95% CI, 90.8 to 99.7) (based on 74 of 76 infants) and the specificity was 99.9% (95% CI, 99.9 to 100) (based on 17,245 of 17,253 infants), respectively. The positive and negative predictive values for the dried-saliva PCR assay were 90.2% (95% CI, 81.7 to 95.7) and 99.9% (95% CI, 99.9 to 100), respectively (based on 74 of 82 infants and 17,243 of 17,245 infants, respectively). The positive likelihood ratio for the dried-saliva PCR assay was 2100 (95% CI, 1049 to 4202), and the negative likelihood ratio was 0.03 (95% CI, 0.0 to 0.1) (Table 2). Of the 84 infants who were positive for CMV on either test, 74 (88%) were enrolled in follow-up. All 66 infants whose specimens were positive by means of both rapid culture and PCR assay and were enrolled in follow-up were positive for CMV on retesting. The 2 infants who were positive on rapid culture but negative on PCR assay were found to still be positive for CMV on retesting with the use of rapid culture and PCR assay. Of the 8 infants who were found to be CMV-positive on PCR assay but not rapid culture, 2 were lost to follow-up and 6 underwent retesting with the use of rapid culture: 4 were found to be CMV-negative and 2 were found to still be CMV-positive.
Liquid-Saliva vs. Dried-Saliva PCR Assay
Between June 2008 and February 2009, all three screening methods (saliva rapid culture, liquid-saliva PCR assay, and dried-saliva PCR assay) were carried out on saliva specimens obtained from 5276 newborns. There was 100% agreement between the results of the liquid-saliva and the dried-saliva PCR assays (Table 3). Both types of PCR assay confirmed the CMV-positive status of all 42 infants with positive rapid-culture results and identified 1 additional infant as being CMV-positive after receiving negative results on rapid culture and positive results on retesting.
Table 3.
Real-Time Polymerase-Chain-Reaction (PCR) Assays of Liquid- and Dried-Saliva Specimens, vs. Rapid Culture, in 5276 Newborns Who Underwent All Three Assays Used to Screen for Congenital Cytomegalovirus Infection.
| Rapid Culture | Liquid-Saliva PCR Assay | Dried-Saliva PCR Assay | Total | ||
|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | ||
| number of newborns | |||||
| Positive | 42 | 0 | 42 | 0 | 42 |
| Negative | 1 | 5233 | 1 | 5233 | 5234 |
| Total | 43 | 5233 | 43 | 5233 | 5276 |
DISCUSSION
Our large, prospective study of CMV screening in newborns shows that the real-time PCR assay of both liquid-saliva and dried-saliva samples has excellent sensitivity (>97%) and specificity (99.9%) as compared with the standard saliva rapid culture. This indicates that the saliva PCR assays, which can easily be adapted for large-scale screening of newborns, will identify most infants who have congenital CMV infection.
The majority of infants with congenital CMV infection will not be identified by means of clinical examination during the newborn period. In addition, sensorineural hearing loss can develop after birth and continue to progress during early childhood in a significant proportion of children with CMV-associated sensorineural hearing loss.1,6–8,29 Thus, the availability of rapid and reliable diagnostic methods that can be adapted for high-throughput screening is essential for early identification of children at risk for CMV-associated sensorineural hearing loss. Testing dried-blood-spot specimens with the use of PCR-based methods appeared to be a promising strategy for CMV screening in newborns, because several previous studies reported that dried-blood-spot PCR assay is highly sensitive in identifying infants with congenital CMV infection.15,20,21,30
However, the results of our recent multicenter study comparing dried-blood-spot real-time PCR assays with saliva rapid culture in more than 20,000 infants revealed that dried-blood-spot PCR assays identified fewer than 40% of CMV-infected newborns.14 In addition, the performance of the dried-blood-spot PCR assay has been shown to vary according to the size of the filter-paper punch, the DNA-extraction methods, and the PCR-assay protocols used.16,22,23,31 These findings, in addition to demonstrating the challenges in developing sensitive high-throughput assays for testing dried-blood spots, suggest that many newborns with congenital CMV infection may not have detectable CMV DNA in peripheral blood. Further advances in PCR methods might improve the sensitivity of the dried-blood-spot PCR assay, however, allowing for acceptable levels of detection of infants with congenital CMV infection in the future.
The data reported here show that the same dried-blood-spot PCR protocol applied to saliva14 identified more than 97% of CMV-infected newborns. In addition, these findings show that saliva is a more reliable type of specimen than dried-blood spots for identifying congenital CMV infection by means of PCR assay and can be an effective tool for mass screening of newborns for CMV. Although testing of urine specimens collected on filter disks inserted into diapers of newborns was recently shown to be a promising approach for newborn CMV screening, urine specimen collection is not without challenges.17,32 Obtaining urine specimens from infants requires additional steps and time that are not needed for collecting saliva, and validation of methods of urine collection and urine PCR assay are needed before the practicality of urine-sample screening can be evaluated for large-scale CMV screening in newborns.
In 16 infants, saliva specimens were positive on screening by means of real-time PCR assay but not rapid culture. To determine whether these PCR results were false positives, retesting was performed with the use of PCR assay of saliva and rapid culture of saliva and urine specimens obtained at the time of enrollment into the follow-up study. If these tests were negative, we considered the screening results to be false positives. Three infants who were found to be CMV-positive only at birth, one by means of liquid-saliva PCR assay and two by means of dried-saliva PCR assay, had positive results on rapid culture and PCR assay during follow-up. These findings indicate that PCR assays identified additional CMV-infected newborns missed when tested with the use of rapid culture.
In 10 infants who had negative rapid culture results but positive PCR results (6 on liquid-saliva PCR assay and 4 on dried-saliva PCR assay), retesting yielded false positive PCR results: the follow-up saliva and urine specimens were negative for CMV. As CMV is occasionally shed in the genital tract secretions of seropositive women at delivery and in the breast milk of most seropositive mothers, these false positive results could be due to CMV-containing maternal secretions present in the infants’ saliva samples.33–38 Although false positive saliva PCR results could lead to unwarranted parental anxiety and additional testing in infants to confirm or rule out congenital CMV infection, the overall frequency of false positive results for both liquid-saliva and dried-saliva PCR assays was less than 0.03%. In addition, the small negative likelihood ratios for both saliva PCR assays indicate that a negative result on these assays does rule out congenital CMV infection (Table 2).28 Nevertheless, when saliva PCR assay is used to screen newborns, a positive screening result should be confirmed within the first 3 weeks of age to avoid false positive screening results.
The dried-saliva PCR assay failed to detect CMV infection in two newborns, leading to slightly lower sensitivity (97.4%; 95% CI, 90.8 to 99.7) than for the liquid-saliva PCR assay. Nevertheless, the simplified procedures for specimen collection, storage, and transport, combined with the high sensitivity, support dried-saliva PCR assay as a reasonable approach to CMV screening in newborns. Although the need for collection of an additional specimen adds to the complexity of the existing newborn-screening programs, the saliva PCR assays described in this study have four main advantages for CMV screening in newborns. These are reasonable sensitivity and specificity, noninvasive specimen collection, elimination of the DNA-extraction step (which simplifies the laboratory procedures, thus providing considerable cost savings), and the fact that dried-saliva specimens can be stored and transported at room temperature, further simplifying specimen handling and transport.
A limitation of this study is that the 34,812 infants found to be CMV-negative on both rapid culture and PCR assay of saliva samples obtained at the screening visit were not enrolled in follow-up to definitively exclude congenital CMV infection (by retesting with the use of rapid culture of saliva or urine). Therefore, it is possible that CMV-infected newborns may have been missed by the rapid culture, affecting our determination of the sensitivity and specificity of saliva PCR assay. However, we believe this possibility is quite low, since the saliva rapid culture has been shown to have a sensitivity of at least 98%.14,19 At present, although imperfect, rapid culture of saliva or urine specimens remains the most widely accepted standard method for identification of infants with congenital CMV infection.14,19,27
In summary, the usefulness of saliva specimens for identification of CMV by means of PCR assay was shown. The screening methods have been further simplified, with the use of dried specimens and processing that does not require a DNA-extraction step, without significant loss of sensitivity or specificity. This strategy appears to be suitable for a high-throughput assay for large-scale screening to identify newborns with congenital CMV infection.
Supplementary Material
Acknowledgments
Supported by a grant (N01 DC50008) from the National Institute on Deafness and Other Communication Disorders.
Dr. Boppana reports receiving consulting fees from GlaxoSmithKline; Dr. Palmer, grant support from Roche; Dr. Ahmed, manuscript preparation fees from Mead Johnson; Dr. Berstein, consulting fees from Vical and Novartis; and Dr. Fowler, consulting fees from GlaxoSmithKline and Merck.
We thank our medical and nursing colleagues and the infants and their parents who agreed to take part in this study.
Footnotes
Members of the CMV and Hearing Multicenter Screening (CHIMES) study group are listed in the Supplementary Appendix, available at NEJM.org.
Presented in part at the 47th Annual Meeting of the Infectious Diseases Society of America, Philadelphia, October 29–November 1, 2009, and at the 21st Annual Meeting of the Pediatric Academic Societies, Vancouver, BC, Canada, May 1–4, 2010.
No other potential conflict of interest relevant to this article was reported.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
REFERENCES
- 1.Dahle AJ, Fowler KB, Wright JD, Boppana SB, Britt WJ, Pass RF. Longitudinal investigation of hearing disorders in children with congenital cytomegalovirus. J Am Acad Audiol. 2000;11:283–290. [PubMed] [Google Scholar]
- 2.Morton CC, Nance WE. Newborn hearing screening — a silent revolution. N Engl J Med. 2006;354:2151–2164. doi: 10.1056/NEJMra050700. [DOI] [PubMed] [Google Scholar]
- 3.Demmler GJ. Infectious Diseases Society of America and Centers for Disease Control: summary of a workshop on surveillance for congenital cytomegalovirus disease. Rev Infect Dis. 1991;13:315–329. doi: 10.1093/clinids/13.2.315. [DOI] [PubMed] [Google Scholar]
- 4.Stagno S. Cytomegalovirus. In: Remington JS, Klein JO, Wilson CB, Baker CJ, editors. Infectious diseases of the fetus and newborn infant. Philadelphia: W.B. Saunders; 2006. pp. 389–424. [Google Scholar]
- 5.Stehel EK, Shoup AG, Owen KE, et al. Newborn hearing screening and detection of congenital cytomegalovirus infection. Pediatrics. 2008;121:970–975. doi: 10.1542/peds.2006-3441. [DOI] [PubMed] [Google Scholar]
- 6.Williamson WD, Demmler GJ, Percy AK, Catlin FI. Progressive hearing loss in infants with asymptomatic congenital cytomegalovirus infection. Pediatrics. 1992;90:862–866. [PubMed] [Google Scholar]
- 7.Fowler KB, McCollister FP, Dahle AJ, Boppana S, Britt WJ, Pass RF. Progressive and fluctuating sensorineural hearing loss in children with asymptomatic congenital cytomegalovirus infection. J Pediatr. 1997;130:624–630. doi: 10.1016/s0022-3476(97)70248-8. [DOI] [PubMed] [Google Scholar]
- 8.Foulon I, Naessens A, Foulon W, Casteels A, Gordts F. A 10-year prospective study of sensorineural hearing loss in children with congenital cytomegalovirus infection. J Pediatr. 2008;153:84–88. doi: 10.1016/j.jpeds.2007.12.049. [DOI] [PubMed] [Google Scholar]
- 9.Hicks T, Fowler K, Richardson M, Dahle A, Adams L, Pass R. Congenital cytomegalovirus infection and neonatal auditory screening. J Pediatr. 1993;123:779–782. doi: 10.1016/s0022-3476(05)80859-5. [DOI] [PubMed] [Google Scholar]
- 10.American Academy of Pediatrics, Joint Committee on Infant Hearing. Year 2007 position statement: principles and guidelines for early hearing detection and intervention programs. Pediatrics. 2007;120:898–921. doi: 10.1542/peds.2007-2333. [DOI] [PubMed] [Google Scholar]
- 11.Korver AMH, Konings S, Dekker FW, et al. Newborn hearing screening vs later hearing screening and developmental outcomes in children with permanent childhood hearing impairment. JAMA. 2010;304:1701–1708. doi: 10.1001/jama.2010.1501. [DOI] [PubMed] [Google Scholar]
- 12.Yamamoto AY, Mussi-Pinhata MM, Marin LJ, Brito RM, Oliveira PF, Coelho TB. Is saliva as reliable as urine for detection of cytomegalovirus DNA for neonatal screening of congenital CMV infection? J Clin Virol. 2006;36:228–230. doi: 10.1016/j.jcv.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 13.Yamamoto AY, Mussi-Pinhata MM, Pinto PCG, Figueiredo LTM, Jorge SM. Usefulness of blood and urine samples collected on filter paper in detecting cytomegalovirus by the polymerase chain reaction technique. J Virol Methods. 2001;97:159–164. doi: 10.1016/s0166-0934(01)00347-0. [DOI] [PubMed] [Google Scholar]
- 14.Boppana SB, Ross SA, Novak Z, et al. Dried blood spot real-time polymerase chain reaction assays to screen newborns for congenital cytomegalovirus infection. JAMA. 2010;303:1375–1382. doi: 10.1001/jama.2010.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barbi M, Binda S, Primache V, et al. Cytomegalovirus DNA detection in Guthrie cards: a powerful tool for diagnosing congenital infection. J Clin Virol. 2000;17:159–165. doi: 10.1016/s1386-6532(00)00089-5. [DOI] [PubMed] [Google Scholar]
- 16.Soetens O, Vauloup-Fellous C, Foulon I, et al. Evaluation of different cytomegalovirus (CMV) DNA PCR protocols for analysis of dried blood spots from consecutive cases of neonates with congenital CMV infections. J Clin Microbiol. 2008;46:943–946. doi: 10.1128/JCM.01391-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nozawa N, Koyano S, Yamamoto Y, Inami Y, Kurane I, Inoue N. Real-time PCR assay using specimens on filter disks as a template for detection of cytomegalovirus in urine. J Clin Microbiol. 2007;45:1305–1307. doi: 10.1128/JCM.02502-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boppana SB, Smith RJ, Stagno S, Britt WJ. Evaluation of a microtiter plate fluorescent antibody assay for rapid detection of human cytomegalovirus infections. J Clin Microbiol. 1992;30:721–723. doi: 10.1128/jcm.30.3.721-723.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Balcarek KB, Warren W, Smith RJ, Lyon MD, Pass RF. Neonatal screening for congenital cytomegalovirus infection by detection of virus in saliva. J Infect Dis. 1993;167:1433–1436. doi: 10.1093/infdis/167.6.1433. [DOI] [PubMed] [Google Scholar]
- 20.Halwachs-Baumann G, Genser B, Pailer S, et al. Human cytomegalovirus load in various body fluids of congenitally infected newborns. J Clin Virol. 2002;25 Suppl:S81–S87. doi: 10.1016/s1386-6532(02)00188-9. [DOI] [PubMed] [Google Scholar]
- 21.Revello MG, Gerna G. Diagnosis and management of human cytomegalovirus infection in the mother, fetus, and newborn infant. Clin Microbiol Rev. 2002;15:680–715. doi: 10.1128/CMR.15.4.680-715.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bale JF., Jr Screening newborns for congenital cytomegalovirus infection. JAMA. 2010;303:1425–1426. doi: 10.1001/jama.2010.424. [DOI] [PubMed] [Google Scholar]
- 23.Deeks JJ, Altman DG. Diagnostic tests 4: likelihood ratios. BMJ. 2004;329:168–169. doi: 10.1136/bmj.329.7458.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simel DL, Samsa GP, Matchar DB. Likelihood ratios with confidence: sample size estimation for diagnostic studies. J Clin Epidemiol. 1991;44:763–770. doi: 10.1016/0895-4356(91)90128-v. [DOI] [PubMed] [Google Scholar]
- 25.Dahle AJ, McCollister FP, Stagno S, Reynolds DW, Hoffman HE. Progressive hearing impairment in children with congenital cytomegalovirus infection. J Speech Hear Disord. 1979;44:220–229. doi: 10.1044/jshd.4402.220. [DOI] [PubMed] [Google Scholar]
- 26.Johansson PJH, Jönsson M, Ahlfors K, Ivarsson SA, Svanberg L, Guthenberg C. Retrospective diagnosis of congenital cytomegalovirus infection performed by polymerase chain reaction in blood stored on filter paper. Scand J Infect Dis. 1997;29:465–468. doi: 10.3109/00365549709011855. [DOI] [PubMed] [Google Scholar]
- 27.Scanga L, Chiang S, Powell C, et al. Diagnosis of human congenital cytomegalovirus infection by amplification of viral DNA from dried blood spots on perinatal cards. J Mol Diagn. 2006;8:240–245. doi: 10.2353/jmoldx.2006.050075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamagishi Y, Miyagawa H, Wada K, et al. CMV DNA detection in dried blood spots for diagnosing congenital CMV infection in Japan. J Med Virol. 2006;78:923–925. doi: 10.1002/jmv.20642. [DOI] [PubMed] [Google Scholar]
- 29.de Vries JJC, Claas ECJ, Kroes ACM, Vossen ACTM. Evaluation of DNA extraction methods for dried blood spots in the diagnosis of congenital cytomegalovirus infection. J Clin Virol. 2009;46 Suppl:S37–S42. doi: 10.1016/j.jcv.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 30.Göhring K, Dietz K, Hartleif S, Jahn G, Hamprecht K. Influence of different extraction methods and PCR techniques on the sensitivity of HCMV-DNA detection in dried blood spot (DBS) filter cards. J Clin Virol. 2010;48:278–281. doi: 10.1016/j.jcv.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 31.Barbi M, MacKay WG, Binda S, van Loon AM. External quality assessment of cytomegalovirus DNA detection in dried blood spots. BMC Microbiol. 2008;8:2. doi: 10.1186/1471-2180-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Inoue N, Koyano S. Evaluation of screening tests for congenital cytomegalovirus infection. Pediatr Infect Dis J. 2008;27:182–184. doi: 10.1097/INF.0b013e318161a2d5. [DOI] [PubMed] [Google Scholar]
- 33.Dworsky M, Yow M, Stagno S, Pass RF, Alford CA. Cytomegalovirus infection of breast milk and transmission in infancy. Pediatrics. 1983;72:295–299. [PubMed] [Google Scholar]
- 34.Hamprecht K, Maschmann J, Vochem M, Dietz K, Speer CP, Jahn G. Epidemiology of transmission of cytomegalovirus from mother to preterm infants by breastfeeding. Lancet. 2001;357:513–518. doi: 10.1016/S0140-6736(00)04043-5. [DOI] [PubMed] [Google Scholar]
- 35.Pass RF, Stagno S, Dworsky ME, Smith RJ, Alford CA. Excretion of cytomegalovirus in mothers: observation after delivery of congenitally infected and normal infants. J Infect Dis. 1982;146:1–6. doi: 10.1093/infdis/146.1.1. [DOI] [PubMed] [Google Scholar]
- 36.Reynolds DW, Stagno S, Hosty TS, Tiller M, Alford CA., Jr Maternal cytomegalovirus excretion and perinatal infection. N Engl J Med. 1973;289:1–5. doi: 10.1056/NEJM197307052890101. [DOI] [PubMed] [Google Scholar]
- 37.Knox GE, Pass RF, Reynolds DW, Stagno S, Alford CA. Comparative prevalence of subclinical cytomegalovirus and herpes simplex virus infections in the genital and urinary tracts of low income, urban females. J Infect Dis. 1979;140:419–422. doi: 10.1093/infdis/140.3.419. [DOI] [PubMed] [Google Scholar]
- 38.Mosca F, Pugni L, Barbi M, Binda S. Transmission of cytomegalovirus. Lancet. 2001;357:1800. doi: 10.1016/S0140-6736(00)04914-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.