Abstract
Pro-inflammatory cytokines released from activated microglia may be responsible for neuronal damage and resulting motor deficits associated with CNS disorders such as spinal cord injury, Parkinson’s disease, and multiple sclerosis. Estrogen (17β-estradiol) is capable of ameliorating motoneuron death following spinal cord injury, but has a number of deleterious side effects. Genistein (GEN), an estrogen receptor beta agonist and potent antioxidant, may represent an alternative to estrogen in treating neurodegenerative disorders. However, little is known about the neuroprotective effects of GEN. We therefore tested whether GEN would prevent apoptosis in cultured motoneurons following exposure to pro-inflammatory cytokines released from IFN-γ activated microglia. Exposure of ventral spinal cord 4.1 motoneurons to microglial cytokine supernatant in vitro caused significant apoptosis and reduced mitochondrial membrane potential. An increase in reactive oxygen species, intracellular Ca2+, calpain, caspases, cytochrome c, and the bax:bcl-2 ratio were also noted. GEN treatment reversed apoptotic death and cellular changes following cytokine exposure and was associated with increased expression of estrogen receptor β suggesting that GEN may promote neuroprotection via receptor-mediated pathways. The addition of ICI 182,780, an estrogen receptor antagonist following GEN treatment attenuated neuroprotection, suggesting that GEN may act mainly via estrogen receptor β to protect VSC4.1 motoneurons. We conclude that GEN protects cultured ventral spinal cord 4.1 cells from inflammatory insult and thus may represent a potential beneficial therapy in the treatment of neurodegenerative disorders.
Keywords: Genistein, Motoneurons, Microglia, Cytokines, Apoptosis, Neuroprotection
1. Introduction
Recent studies suggest that inflammation mediated by activated microglia may result in excitotoxic damage to motoneurons following spinal cord injury (SCI) (Gonzalez-Scarano and Baltuch, 1999). Microglia belong to the macrophage cell lineage and act as sensors during pathological events in the central nervous system (CNS) (Chan et al., 2007; Kreutzberg, 1996; Nakajima and Kohsaka, 2001). During injury and the subsequent inflammatory response, these cells are activated and proliferate (Kuno et al., 2005). We have found that activated microglia secrete a number of bioactive pro-inflammatory cytokines including TNF-α, IL-1α, IL-1β, IL-1ra, IL-2, IL-3, and IL-10. These proinflammatory cytokines may play an important role in mediating neuronal injury in many neurodegenerative diseases including SCI, Alzheimer’s disease (AD), Parkinson’s disease (PD), and multiple sclerosis (MS). In the particular case of SCI, pro-inflammatory cytokines may cause secondary damage to motoneurons, resulting in decreased function and recovery in SCI patients (Gonzalez-Scarano and Baltuch, 1999).
Previous studies have shown that estrogen (17β-estradiol) is capable of ameliorating neuronal death in SCI and other neurodegenerative diseases through activation of estrogen receptors (ERα and ERβ) (Brann et al., 2007; Sribnick et al., 2004a; Zhao et al., 2004). However, 17β-estradiol administration results in numerous side effects that prohibit its use in neurodegenative disorders including increased risk of thrombosis, stroke, heart disease, and feminizing effects in males (Nef and Parada, 2000; Paganini-Hill, 2001). Therefore, GEN, an ERβ agonist, has been proposed as an alternative to estrogen therapy with similar actions in neuronal protection following CNS insult (Liang et al., 2008; Reiter et al., 2009; Sawada and Shimohama, 2003).
GEN is a non-steroidal phyto-estrogen that influences cellular function by acting as an agonist at ERβ (Kuiper et al., 1998). It may be preferred over estrogen as a neuroprotective agent because it does not cause feminization in males (Messina, 2010; Nef and Parada, 2000). Furthermore, GEN is a potent cellular antioxidant, inhibits atherosclerosis, has anti-angiogenic effects, and is a tyrosine kinase inhibitor (Fotsis et al., 1998; Kousidou et al., 2006; Liang et al., 2008; Walker et al., 2001). It has also been shown to reduce reactive oxygen species (ROS) generation, decrease lipid peroxidation, and inhibit the apoptotic signaling cascade (Fuhrman and Aviram, 2001). These findings indicate that GEN may provide cellular protection to neurons following neurodegenerative insult. However, little is known about the mechanisms by which GEN may provide neuroprotection. Thus, we examined the effects of GEN on cell survival and the inflammatory response in ventral spinal cord 4.1 (VSC 4.1) motoneurons following microglial cytokine exposure.
2. Materials and Methods
2.1 Primary microglia culture and collection of microglial cytokine supernatant after activation with IFN-γ
All animal usage procedures were followed in strict accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals and approved by the Medical University of South Carolina. One day postnatal Sprague-Dawley rats were selected for primary cell culture. All efforts were made to minimize the number of experimental animals, and reduce suffering. Microglia were isolated from mixed glial cultures according to a modified procedure of McCarthy (McCarthy and de Vellis, 1980). Briefly, the cerebral cortices from one day postnatal Sprague-Dawley rats were dissected and digested with 0.25% trypsin and DNase for 5 min at 37°C (Sigma, St. Louis, MO, USA). The resulting cells were seeded in 75 cm2 uncoated culture flasks, and cultured with DMEM/F12 (1:1) (Sigma, St. Louis, MO, USA) supplemented with 100 U/ml penicillin, 100 ug/ml streptomycin (Gibco-Invitrogen, Grand Island, NY, USA), and 10% FBS (Hyclone, Logan, UT, USA) in a fully humidified incubator containing 5% CO2 at 37°C. Cells were allowed to grow for 2 weeks and obtain confluence. Once confluent, floating and weakly attached cells above the mixed glial cell layer were isolated by extracting the media. The resultant cell suspension was transferred to 75 cm2 poly-L-ornithine coated culture flasks (Sigma, St. Louis, MO, USA) and allowed to adhere at 37° Celsius for 24 hours. The unattached cells were then removed with the media, leaving behind strongly adherent microglia. To ensure that the microglia were purified, astrocytes were suppressed according to the procedure developed by Cole and de Vellis (Cole and de Vellis, 2001).
2.2 ELISpot Cytokine Array assay
Isolated microglia were incubated (activated) in buffer containing 500 units of IFN-γ (Sigma) in a time-dependent experiment (30 min, 2 hr, 6 hr, 24 hr) to determine at the time point at which pro-inflammatory cytokine release was greatest. After incubation in IFN-γ, the cells were centrifuged and the supernatant was collected. This separated supernatant was used in all subsequent experiments. Cytokines released in the supernatant were analyzed using an ELISpot Cytokine Array kit (R&D Biosystems,Minneapolis, MN, USA). The ELISpot Cytokine Array kit was used according to the manufacturer’s instructions. Each nitrocellulose membrane provided in the kit contained a marker for 29 different cytokines and chemokines. The membranes were first blocked for an hour at room temperature with 2 ml of blocking buffer. Then, the supernatant (1 mL) from each time point was mixed with 0.5 mL of the provided array buffer and 15μl of detection antibody cocktail and left to incubate at room temperature for an hour. The microglial cytokine supernatant/detection antibody cocktail was then poured over another nitrocellulose membrane and allowed to incubate on a rocking platform overnight at 4 ° C. Each membrane was then washed 3 times for 10 min with 20 mL of wash buffer. 1.5 ml of streptavidin-HRP was then added to each membrane and allowed to incubate for 30 min. at room temperature. The membranes were again washed 3 times for 10 min each with 20 mL of wash buffer. Membranes were then drained and exposed to 2 mL each of Chemiglow West Substrate (Alpha Innotech, San Leandro, CA, USA) for 3 min. The membranes were scanned using Photoshop software (Adobe Systems, Seattle, WA, USA).
2.3 VSC4.1 motoneuron cell culture
Ventral spinal cord 4.1 (VSC 4.1) motor neurons were formed by fusion of dissociated embryonic rat ventral spinal cord neuron with mouse N18TG2 neuroblastoma cell (Smith et al., 1994). VSC4.1 cells were grown in a monolayer to sub-confluence in 75-cm2 flasks containing 10 mL of Dulbecco’s modified Eagle’s media (DMEM)/F12 medium with 15 mM HEPES, pyridoxine, and NaHC03 (Sigma, St. Louis, MO, USA), supplemented with 2% Sato’s components, 1% penicillin/streptomycin (Gibco-Invitrogen, Grand Island, NY, USA), and 2% heat-inactivated fetal bovine serum (Hyclone, Logan, UT, USA). Eight treatment groups were used: control (CTL); 50 nM GEN (24 hr); 10μM ICI 182,780 (24 hr); 50 nM GEN + 10μM ICI 182,780 (24 hr); 12.5 μl/ml microglia cytokine supernatant (24 hr); 12.5 μl/ml microglia cytokine supernatant + 50nM GEN (24 hr); 12.5 μl/ml microglia cytokine supernatant + 10μM ICI 182,780 (24 hr); 12.5 μl/ml microglia cytokine supernatant + 50 nM GEN + 10μM ICI 182,780 (24 hr). All treatments were given at 15 min post microglial supernatant exposure.
2.4 Trypan blue dye exclusion test for residual cell viability after treatment
The viability of VSC4.1 motoneurons in each treatment group was estimated by trypan blue dye exclusion test as previously described (Das et al., 2010). Viable cells did not take up trypan blue (Sigma) and maintained membrane integrity. Dead cells with compromised cell membranes took up trypan blue. At least 500 cells were counted in four different fields. The percentage of residual cell viability was calculated using the following formula: percentage of residual cell viability = [number of trypan blue negative cells/ (number of trypan blue positive cells + number of trypan blue negative cells )] × 100.
2.5 Wright staining and ApopTag assay
VSC4.1 cells from each of the eight previously described treatment groups were collected, washed with 1x PBS (pH 7.4; Sigma), sedimented onto microscope slides, and fixed as previously described (Das et al., 2007a, b; Das et al., 2005). Wright staining and ApopTag assay were performed as previously described to examine the morphological and biochemical features of apoptosis, respectively (Das et al., 2005). After Wright staining and ApopTag assay, cells were counted under the light microscope to determine percentage of apoptosis.
2.6 Fura-2 Assay for determination of intracellular free [Ca2+]
The level of intracellular free [Ca2+] was measured using the fluorescence Ca2+ indicator fura-2/AM, as described previously (Das et al., 2007a, b; Das et al., 2005). The value of Kd, a cell-specific constant, was determined experimentally to be 0.164 μM for the VSC 4.1 motor neurons, using standards of the Calcium Calibration Buffer Kit with Magnesium (Molecular Probes, Eugene, OR, USA).
2.7 Colorimetric assays for the measurement of caspase-8, caspse-9, and caspase-3
Caspase activities in cells from each of the eight treatment groups were measured with commercially available caspase-8 (Abcam, Cambridge, MA, USA), caspase-9 (Abcam), and caspase-3 assay kits according to the manufactuer’s instructions (Sigma) and as previously described (Das et al., 2010). The colorimetric assays were based on the hydrolysis of the amino acid chains acetyl-Ile-Glu-Thr-Asp p-nitroanilide (Ac-IETD-pNA) by caspase-8, acetyl-Leu-Glu-His-Asp p-nitroanilide (Ac-LEHD-pNA) by caspase-9, and acetyl-Asp-Glu-Val-Asp p-nitroanilide (Ac-DEVD-pNA) by caspase-3, resulting in the release of the p-nitroaniline (pNA). Proteolytic reactions were carried out in extraction buffer containing 200 μg of cytosolic protein extract and 40 μM Ac-IETD-pNA, 40 μM Ac-LEHD-pNA, or 40 μM Ac-DEVD-pNA. The reaction mixtures were incubated at room temperature for 2 hours and the formation of pNA was measured at 405 nm in a colorimeter. The concentration of the pNA released from the substrate was calculated from the absorbance values. Experiments were performed in triplicate.
2.8 Antibodies
Monoclonal antibody against β-actin (Sigma) was used to standardize cytosolic protein loading on SDS-PAGE gels. Anti-cytochrome c oxidase subunit IV (COX4) antibody (Molecular Probes) was used to standardize mitochondrial protein levels. COX 4 is a membrane protein in the inner mitochondrial membrane and it remains in the mitochondria regardless of activation of apoptosis. Antibodies against ERα and ERβ were purchased from Santa Cruz Biotech (Santa Cruz, CA, USA). All other primary IgG antibodies were purchased from Santa Cruz Biotech or Calbiochem (Gibbstown, NG, USA). Secondary antibodies were horseradish peroxidase-conjugated goat anti-mouse IgG (ICN Biomedicals, Aurora, OH, USA) and horseradish peroxidase-conjugated goat anti-rabbit IgG (ICN Biomedicals, Solon, OH, USA).
2.9 Western Blotting
The isolation of cytosolic, mitochondrial, and nuclear fractions was performed by standard procedures (Das et al., 2007a, b; Das et al., 2005). Western blotting was used to analyze cytochrome c, 21 kD bax, 24 kD bax, bcl-2, calpain, calpastatin, ERs (α and β), and caspase-3, -8, and -9 expression in VSC4.1 cells from each treatment group. The films were scanned using Photoshop software (Adobe Systems, Seattle, WA, USA) and the optical density (OD) of each band was determined using Quantity One software (Bio-Rad, Hercules, CA, USA).
2.10 Detection of reactive oxygen species and mitochondrial membrane potential
The fluorescence probe 2′,7′- dichlorofluorescin diacetate (DCF-DA) was used for the assessment of intracellular reactive oxygen species (ROS) production in VSC 4.1 motoneurons as described previously (Das et al., 2007a, b; Das et al., 2005). Briefly, cells were seeded (2 × 105 cells/well) in 6-well culture plates. Forty-eight hours after seeding, cells were treated, incubated at 37 □C, and collected at multiple time points from 30 to 1440 min following treatment. At each measured time point, plates were washed twice with Hank’s balanced salt solution (GIBCO-BRL, Grand Island, NY, USA) and loaded with 1000 μl DMEM/F12 containing 5μM of DCF-DA. The fluorescence intensity was measured at 530 nm after excitation at 480 nm in a Spectramax Gemini XPS fluorescence microplate reader (Molecular Devices). The increase in fluorescence intensity was used to determine intracellular ROS production.
Mitochondrial potential loss was measured by using the fluorescent probe JC-1. Cells from each of the eight treatment groups were incubated in DMEM/F12 medium containing 5μg/ml JC-1 and collected at multiple time points (0 to 1440 min following treatment). After staining, cells were washed twice with PBS (pH 7.4). When excited at 488 nm, the fluorescence emission of JC-1 was measured at wavelengths corresponding to its monomer (530 ± 15 nm) and J aggregate (> 590 nm) forms. A decrease in the ratio of 590 nm: 530 nm forms indicated loss of mitochondrial membrane potential. Fluorescence was measured in a fluorescent plate reader (Molecular Devices).
2.11 Statistical Analysis
Results obtained from different experiments were analyzed using StatView software (Abacus Concepts). Data were expressed as mean ± standard error of mean (S.E.M.) of separate experiments (n >3) and compared by one-way analysis of variance (ANOVA) followed by Fisher’s post hoc test. Significant differences between the control group and other treatment groups were indicated by *P < 0.05 or **P < 0.01. Significant differences between cytokine exposure and all subsequent treatment groups was indicated by #P < 0.05 or ##P < 0.01. *P ≤ 0.05 in Figure 3 is used to show significant increase in ER expression when cells are exposed to GEN.
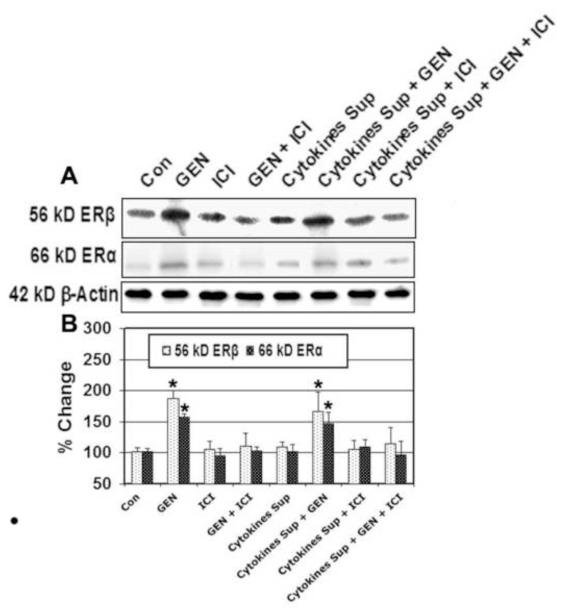
Figure 3. Western Blotting for Determining Expression of Estrogen Receptors in VSC4.1 Cells.
VSC4.1 cells were subjected to activated microglial cytokines for 15 min. and treated with GEN and/or ICI 182,780 for 24 hr. ER expression was then determined by Western blot analysis. (A) Representative pictures to show levels of ER-β, ER-α, and β-actin protein levels. (B) Bar graph to indicate percentage of change in ER-β and ER-α protein expression relative to control (CTL). Eight treatment groups: control (CTL); 50 nM GEN; 10μM ICI; 50 nM GEN + 10μM ICI; 12.5 μl/ml microglia cytokine supernatant; 12.5 μl/ml microglia cytokine supernatant + 50nM GEN; 12.5 μl/ml microglia cytokine supernatant + 10μM ICI; 12.5 μl/ml microglia cytokine supernatant + 50 nM GEN + 10μM ICI. *P < 0.05 compared to control.
3. Results
3.1 Microglial Activation with IFN-γ Results in Release of Pro-inflammatory Cytokines
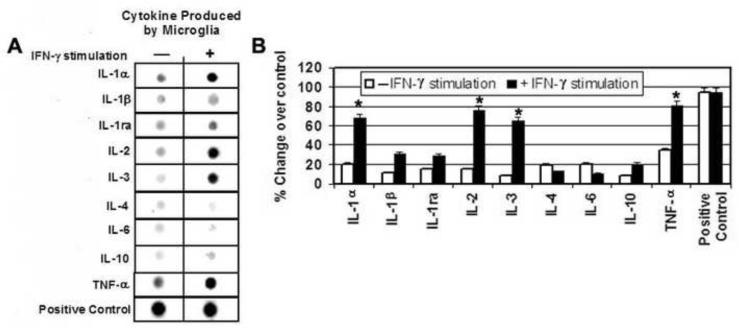
An ELISpot Cytokine Array was used to determine the time point (30min, 2 hr, 6 hr, 24 hr) of maximal cytokine release from microglia activated with IFN-γ. The maximum amount of cytokine release was noted at 30 min following activation. At this time point, increased release of pro-inflammatory cytokines including TNF-α, IL-1α, IL-1β, IL-1ra, IL-2, and IL-3 was noted (Figure 1). IL-1α, IL-2, IL-3, and TNF-α levels were seen to increase by ~50%, while IL-1ra levels increased by ~ 20%, and IL-1β levels increased by ~ 20% Furthermore, release of the anti-inflammatory cytokines IL-4 and IL-6 decreased by ~ 10% following IFN-γ activation. Interestingly, release of IL-10, another anti-inflammatory cytokine appeared to increase by ~10%.
Figure 1. ELISpot Cytokine Array Analysis of Cytokine Supernatant Released from IFN-γActivated Microglia.
Primary microglia were exposed to IFN-γ (500 units) for 30 min. and cytokine production was measured by ELISpot analysis. (A) Representative images to show increased levels of the proinflammatory cytokines TNF-α, IL-1α, IL-1β, IL-1ra, IL-2, IL-3, IL-10 following IFN-γ exposure indicated by a darker spot in the right column compared to inactivated microglia with a lighter spot in the left column. (B) Bar graph quantifying the percent change in proinflammatory cytokine expression compared to inactivated microglia. *P < 0.05 compared to control; **P < 0.01 compared to control.
3.2 GEN Inhibits Apoptotic Death of VSC4.1 Cells Following Cytokine Insult
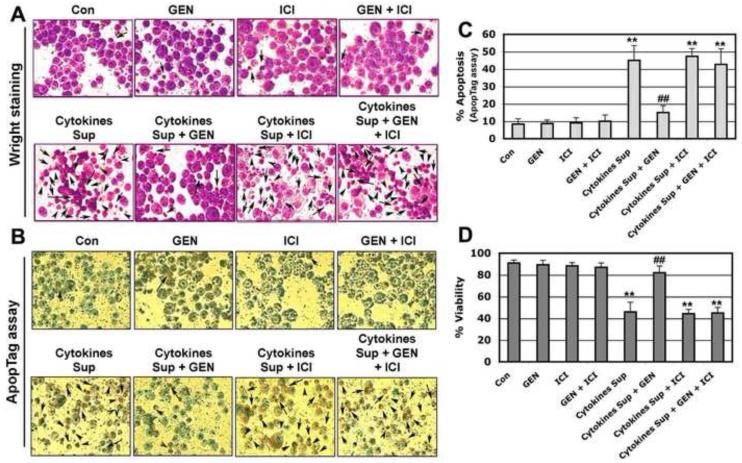
To determine the extent of apoptotic cell death following microglial cytokine exposure, Wright staining and the ApopTag assay were used. Morphological features of apoptosis were detected using the Wright staining procedure (Figure 2A). Apoptotic death was confirmed based on characteristic morphological features such as nuclear condensation, cytoplasmic blebbing, and apoptotic body formation. Results obtained from Wright staining were further supported by the ApopTag assay (Figure 2B). Apoptotic death was confirmed by the presence of ApopTag-positive cells which stained brown. All treatment groups were examined under light microscopy and cells were counted to determine the percentage of apoptotic cells (Figure 2C). Compared with control, cells exposed to microglial cytokines showed a significant increase (greater than 50%) in apoptotic cell death (P ≤ 0.01). GEN treatment of VSC4.1 motoneurons 15 min. post-exposure to microglial cytokine supernatant attenuated apoptosis associated with cytokine insult (P ≤ 0.01). The ER antagonist ICI 182,780 inhibited GEN-mediated neuronal survival, indicating a role for ERβ-mediated pathways in neuroprotection.
Figure 2. Post-treatment with GEN Prevented Apoptotic Death of VSC4.1 Cells Damaged by Microglia Cytokine Supernatant.
VSC4.1 motoneurons were exposed to microglial supernatant for 15 min. and treated with GEN and/or ICI 182,780 for 24 hr. Cell viability and apoptotic death was measured by Wright staining, ApopTag assay, and typan blue dye exclusion test. (A) Photomicrographs showing representative cells from each treatment groups following Wright staining. The arrows indicate apoptotic cells. (B) Photomicrographs showing representative cells from each treatment group following ApopTag assay. The arrows indicate apoptotic cells. (C) Bar graph indicating the percentage of apoptotic cells in each treatment group (based on Wright staining). (D) GEN prevented microglia cytokine supernatant mediated decrease in VSC4.1 cell viability. The trypan blue dye exclusion test was used to assess cell viability in VSC4.1 cells. Eight treatment groups: control (CTL); 50 nM GEN; 10μM ICI; 50 nM GEN + 10μM ICI; 12.5 μl/ml microglia cytokine supernatant; 12.5 μl/ml microglia cytokine supernatant + 50nM GEN; 12.5 μl/ml microglia cytokine supernatant + 10μM ICI; 12.5 μl/ml microglia cytokine supernatant + 50 nM GEN + 10μM ICI. **P < 0.01 compared to control; ##P < 0.05 compared to cytokine exposure. (Reproduce in color on the Web, black and white in print).
3.3 GEN Restores Cell Viability in VSC4.1 Motoneurons Exposed to Microglial Cytokines
To further confirm the neuroprotective effects of GEN, the viability of VSC4.1 motoneurons was evaluated under a light microscope using the trypan blue dye exclusion test. Cells incubated with microglial cytokines demonstrated a significant reduction in cell viability compared to control (Figure 2D). GEN treatment partially reversed changes in cell viability following exposure to microglial cytokines. ICI 182,780, an estrogen receptor antagonist, attenuated protection of VSC4.1 neurons by GEN, indicating the involvement of ERβ in this process.
3.4 GEN Treatment Up-regulates Estrogen Receptor Expression in VSC4.1 Motoneurons
To determine the effects of GEN on estrogen receptor (ERα and ERβ) expression, we performed Western blotting using specific antibodies for ERα and ERβ (Figure 3). Our results indicated that both ERα and ERβ were expressed at significantly higher levels (P≤ 0.05) in motoneurons treated with GEN when compared to control. Additionally, protein expression of ERβ following GEN treatment was greater than ERα expression. The expression of estrogen receptors was not significantly altered by exposure to microglial cytokines or treatment with ICI 182,780 when compared to control.
3.5 GEN Inhibits ROS production in VSC4.1 Motoneurons Exposed to Pro-inflammatory Cytokines
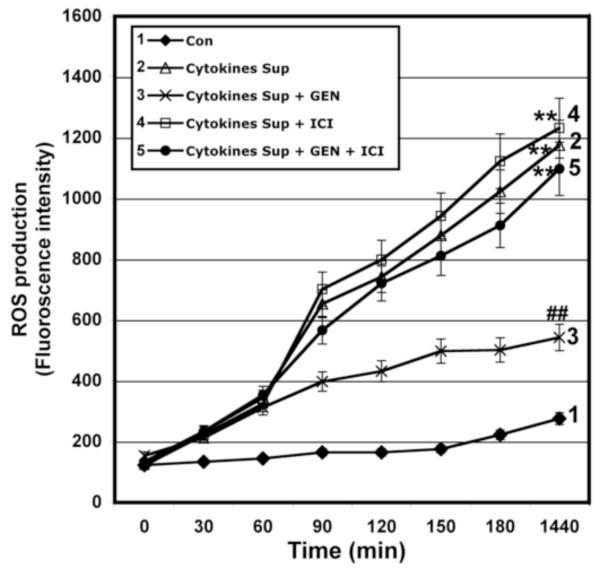
To examine the effects of GEN on intracellular ROS production, we measured oxidation of dichlorofluorescin (DCF) in VSC4.1 cells in a time-dependent manner. Cells exposed to microglial cytokine supernatant demonstrated a significant increase in ROS production at 24h post-exposure (P ≤ 0.01; Figure 4). GEN treatment attenuated increased production of ROS associated with exposure to pro-inflammatory cytokines (P ≤ 0.01). However, the ER antagonist ICI 182,780 reversed the effects of GEN on ROS generation following cytokine insult. These results indicate that GEN may act as a potent antioxidant by inhibiting ROS production via receptor-mediated pathways.
Figure 4. Determination of ROS Production in VSC4.1 Cells.
ROS production was measured in VSC4.1 cells exposed to microglial supernatant (15 min.) and treated with GEN and/or ICI 182,780 (24 hr) demonstrating inhibition of ROS production following treatment with GEN. ROS production was determined at various time points post-treatment (0, 30, 60, 90, 120, 150, 180, and 1440 min) in the presence of 5 μM 2′, 7′- dichlorofluorescin diacetate (DCF-DA). Eight treatment groups: control (CTL); 50 nM GEN; 10μM ICI; 50 nM GEN + 10μM ICI; 12.5 μl/ml microglia cytokine supernatant; 12.5 μl/ml microglia cytokine supernatant + 50nM GEN; 12.5 μl/ml microglia cytokine supernatant + 10μM ICI; 12.5 μl/ml microglia cytokine supernatant + 50 nM GEN + 10μM ICI. **P < 0.01 compared to control; ##P < 0.01 compared to cytokine exposure.
3.6 GEN Prevents Activation of the Extrinsic Apoptotic Pathway in Motoneurons Exposed to Activated Microglial Cytokines
In order to investigate the effects of GEN on the extrinsic pathway of apoptosis, caspase-8 activation and expression was examined in VSC4.1 cells. Motoneurons exposed to activated microglial supernatant demonstrated an increase in caspase-8 activity and expression (P ≤ 0.01). However, post-exposure treatment of these cells with GEN blocked both caspase-8 activity and expression (P ≤ 0.01) (Figures 5A & B). The inhibitory effects of GEN on caspase-8 activation and expression were reversed by ICI 182,780 (ER antagonist) treatment, indicating the involvement of receptor-mediated pathways. Caspase-8 activation may induce the proteolytic cleavage of Bid to tBid, resulting in tBid translocation from the cytosol to the mitochondrial membrane to stimulate more efficient oligomerization of Bax and thereby activation of the intrinsic apoptotic pathway (Das et al., 2010). Therefore, these findings suggest GEN may protect VSC4.1 motoneurons from cytokine-induced damage by inhibiting activation of the extrinsic apoptotic pathway and its effects on the extrinsic pathway.
Figure 5. Examination of Components Involved in Mitochondrial Pathways of Apoptosis in VSC4.1 Cells.
VSC4.1 cells were subjected to activated microglial cytokines (15 min.), treated with GEN and/or ICI 182,780 (24 hr.), and assayed for various components involved mitochondria-mediated apoptosis. (A) Western blotting for active caspase-8 protein compared to β-actin as a positive control. (B) Bar graph to show percent change in caspase-8 activity as determined by Western blotting and colorimetric assay. (C) Western blotting for 24 kD Bax, 21 kD Bax, and Bcl-2 compared to β-actin as a positive control. (D) Bar graph demonstrating the Bax: Bcl-2 ratio in each of the eight treatment groups. (E) JC-1 ratio (590 nm/530 nm) in cells after the treatments for different times (30, 60, 90, 120, 240, 540, 600, 1080, and 1440 min). (F) Western blotting for cytochrome c active caspase-9 compared to β-actin as a positive control. (G) Bar graph showing the percent changes in cytochrome c and caspase-9 activity. Eight treatment groups: control (CTL); 50 nM GEN; 10μM ICI; 50 nM GEN + 10μM ICI; 12.5 μl/ml microglia cytokine supernatant; 12.5 μl/ml microglia cytokine supernatant + 50nM GEN; 12.5 μl/ml microglia cytokine supernatant + 10μM ICI; 12.5 μl/ml microglia cytokine supernatant + 50 nM GEN + 10μM ICI. **P < 0.01 compared to control; #P < 0.05 compared to cytokine insult; ##P < 0.01 compared to cytokine exposure.
3.7 GEN Reverses Changes in the Bax:Bcl-2 Ratio Following Exposure of VSC4.1 Motoneurons to Activated Microglial Supernatant
The effects of GEN on the intrinsic apoptotic pathway at the mitochondrial level were investigated by determination of the Bax:Bcl-2 ratio. Exposure of motoneurons to activated microglial supernatant increased Bax and decreased Bcl-2 protein expression, resulting in an overall increase in the Bax:Bcl-2 ratio (P ≤ 0.01; Figure 5C & D). Addition of GEN following cytokine insult significantly attenuated increases in the Bax:Bcl-2 ratio (P ≤ 0.01). The actions of GEN in restoring Bax:Bcl-2 were inhibited using the ER antagonist ICI 182,780.
3.8 GEN Restores Mitochondrial Membrane Potential Following VSC4.1 Motoneuron Exposure to Activated Microglial Cytokine Supernatant
Mitochondrial swelling is often associated with the loss of mitochondrial membrane potential, a phenomenon readily measured after staining with the mitochondrial dye JC-1. As expected, control cells showed a high JC-1 ratio indicative of intact mitochondrial membrane potential. After exposure to activated microglial cytokine supernatant, the mean red and green fluorescence ratios of the mitochondria slowly decreased in a biphasic matter, indicating the loss of mitochondrial membrane potential (Figure 5E). GEN treatment significantly attenuated loss of mitochondrial membrane potential following exposure of VSC4.1 cells to pro-inflammatory cytokines (P ≤ 0.01). Furthermore, ICI 182,780 significantly inhibited the protective actions of GEN on mitochondrial membrane potential (P ≤ 0.01).
3.9 GEN Reverses Changes in Mitochondrial Cytochrome C and Activation of Caspase-9 Associated with Loss of Mitochondrial Membrane Potential in VSC4.1
The loss of mitochondrial membrane potential following cytokine insult in VSC4.1 motoneurons was associated with the disappearance of 15kD cytochrome c from the mitochondrial fraction and its subsequent appearance in the cytosolic fraction (Figure 5F & G). The release of mitochondrial cytochrome c has been associated with the activation of caspase-9. Likewise, we detected a significant increase in caspase-9 activity and expression via colorimetric assay and Western blotting in motoneurons exposed to activated microglial supernatant (P ≤ 0.01; Figure 5F & 5G). GEN treatment prevented release of cytochrome c for these cells. In addition, GEN inhibited caspase-9 activity and expression following exposure to microglial cytokines. However, the restoration of mitochondrial integrity by GEN was reversed by the ER antagonist ICI 182,780. Together, these results suggest that GEN prevents cytokine-induced mitochondrial damage and activation of apoptosis via receptor-mediated pathways.
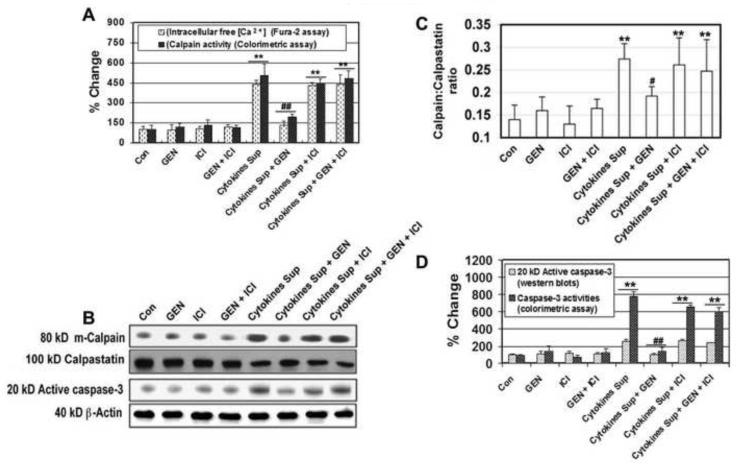
3.10 GEN Mediates Changes in Intracellular Calcium, Calpain, Calpastatin, and Caspase-3 Following Pro-inflammatory Cytokine Exposure in VSC4.1 Motoneurons
Changes in intracellular Ca2+ (ic[Ca2+]) in all treatment groups were measured using the Fura-2 assay (Figure 6A). Twenty-four hour exposure of VSC4.1 motoneurons to activated microglial supernatant resulted in a significant increase in ic[Ca2+] (P ≤ 0.01). Increases in ic[Ca2+] were attenuated following treatment with GEN. Additionally, the ER antagonist ICI 182,780 reversed GEN-mediated reduction of ic[Ca2+] influx, suggesting receptor-mediated pathways may be responsible for the protective actions of GEN.
Figure 6. Examination of the Increase in Intracellular Free [Ca+2] and Determination of Calpain, Calpastatin, and Caspase-3 Activities.
VSC4.1 cells were subjected to activated microglial cytokines for 15 min. and treated with GEN and/or ICI 182,780 for 24 hr to determine changes in intracellular Ca2+ and activities of calpain, calpastatin, and caspase-3. (A) Bar graph showing percentage change in intracellular free [Ca+2] (Fura-2 assay) and calpain activity (colorimetric assay) in each treatment group. (B) Western blotting for calpain, calpastatin, and caspase-3 protein levels compared to β-actin as a positive control. (C) Bar graph showing calpain: calpastatin ratio in each of the eight treatment groups. (D) Bar graph showing percent change in caspase-3 activity via Western blotting and colorimetric assay in each of the eight treatment groups. Eight treatment groups: control (CTL); 50 nM GEN; 10μM ICI; 50 nM GEN + 10μM ICI; 12.5 μl/ml microglia cytokine supernatant; 12.5 μl/ml microglia cytokine supernatant + 50nM GEN; 12.5 μl/ml microglia cytokine supernatant + 10μM ICI; 12.5 μl/ml microglia cytokine supernatant + 50 nM GEN + 10μM ICI. **P < 0.01 compared to control; #P < 0.05 compared to cytokine insult; ##P < 0.01 compared to cytokine exposure.
Increased ic[Ca2+] may result in the activation of a number cell death proteases including the Ca2+ dependent protease calpain and caspase-3. Therefore, we measured activity and expression of calpain via colorimetric assay and Western blotting respectively (Figure 6 A & B). Furthermore, expression of calpastatin, the endogenous inhibitor of calpain, was also determined. The calpain:calpastatin ratio was significantly increased in cells exposed to activated microglial supernatant, indicating a shift towards death in the cells (P ≤ 0.01; Figure 6C). GEN treatment partially reversed increases in the calpain:calpastatin ratio associated with cytokine insult.
The effects of GEN on caspase-3 expression and activity were also examined (Figure 6 A &B). VSC4.1 motoneurons exposed to activated microglial cytokines demonstrated up-regulated caspase-3 expression and activity (P ≤ 0.01). However, increased expression and activity of caspase-3 in these cells was attenuated following addition of GEN. Overall, GEN-mediated reduction of proteolytic enzyme (calpain, caspase-3) expression and activity was reversed by the ER antagonist ICI 182,780 (Figure 6D). Taken together, these findings indicate that GEN may act through ERβ to alter changes in Ca2+ influx, calpain activity, and caspase-3 activity following exposure of cells to pro-inflammatory cytokines.
3.11 GEN Attenuates Expression of Pro-inflammatory Factors COX-2 and NFκB in VSC4.1 Motoneurons Exposed to Activated Microglial Cytokines
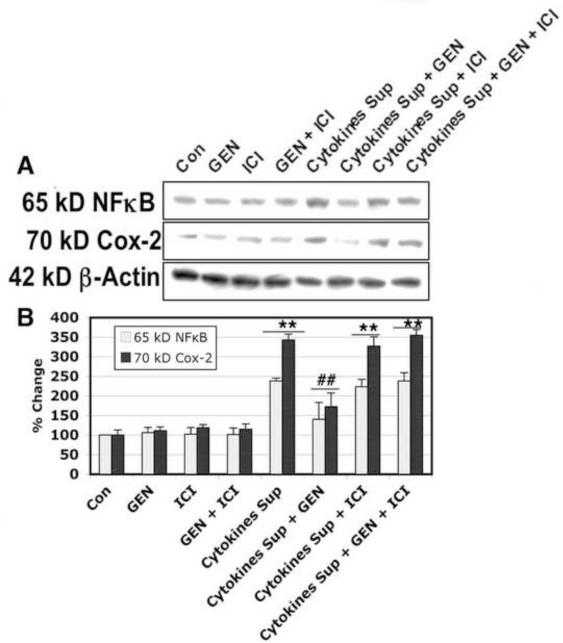
To determine whether GEN may reverse pro-inflammatory changes in neurons following IFN-γ-activated supernatant insult, we measured expression of cyclooxygenase-2 (COX-2) and nuclear factor κB (NFκB). Exposure of VSC4.1 motoneurons to activated microglial supernatant resulted in increased expression of COX-2 and NFκB. Treatment of these cells with GEN reversed changes in COX-2 and NFκB protein levels following cytokine insult (Figure 7). In addition, the estrogen receptor agonist ICI 182,780 appeared to inhibit the anti-inflammatory effects of GEN on COX-2 and NFκB expression. These results suggest GEN may act through receptor-mediated pathways to control the inflammatory response in motoneurons subjected to microglial factors released in the supernatant.
Figure 7. Determination of COX-2 and NFκB Expression in VSC4.1 Motoneurons Exposed to Microglial Cytokines.
VSC4.1 cells were exposed to microglial supernatant for 15 min. and treated with GEN and/or ICI 182,780 for 24 hr. COX-2 and NFκB expression was determined by Western blot analysis. (A) Representative Western blots showing changes in COX-2 and NFκB expression following cytokine exposure and GEN treatment. β-actin was used as a positive control for even loading. (B) Bar graph quantifying changes in COX-2 and NFκB protein levels in each treatment group. Eight treatment groups: control (CTL); 50 nM GEN; 10μM ICI; 50 nM GEN + 10μM ICI; 12.5 μl/ml microglia cytokine supernatant; 12.5 μl/ml microglia cytokine supernatant + 50nM GEN; 12.5 μl/ml microglia cytokine supernatant + 10μM ICI; 12.5 μl/ml microglia cytokine supernatant + 50 nM GEN + 10μM ICI. **P < 0.01 compared to control; ##P < 0.01 compared to cytokine exposure.
4. Discussion
Activated microglia are generally recognized as cytotoxic effector cells and are thought to play a major role in the inflammatory mechanisms underlying neurodegenerative disorders (Kreutzberg, 1996; Kuno et al., 2005; Nakajima and Kohsaka, 2001). Although the mechanisms by which cells are damaged in CNS disorders remains unclear, it is suggested that activation of microglia may result in release of factors that are detrimental to neuronal/glial survival. We examined one of the factors, cytokines, that may play a critical role in cell death mechanisms. Our results suggest that numerous pro-inflammatory cytokines are released following activation of microglia with IFN-γ, with maximum cytokine release occurring 30 min post-activation. The pro-inflammatory cytokines that were released from the IFN-γ activated microglia included TNF-α, IL-1α, IL-1β, IL-1ra, IL-2, and IL-3. IL-1α, IL-2, IL-3, and TNF-α were seen to increase by ~50%. IL-1ra increased by ~ 20%, IL-1β increased by ~ 20%, and IL-10 increased by ~ 10%. Furthermore, the anti-inflammatory cytokines IL-4 and IL-6 decreased by ~ 10% following IFN-γ activation. While pro-inflammatory cytokines mediate the cytotoxic effects of microglia within the CNS and act as the essential mediators in the progression of neurodegenerative diseases, the anti-inflammatory cytokines may be useful in combating disease (Gonzalez-Scarano and Baltuch, 1999; Kreutzberg, 1996; Kuno et al., 2005; Nakajima and Kohsaka, 2001). In view of this, a pleotrophic action of microglia has recently been suggested (Sawada et al., 2010). Thus, supernatant from activated microglia containing released cytokines was found to damage VSC4.1 motoneurons, suggesting that cytokines are among the detrimental factors released following activation of microglia in CNS diseases and may be partly responsible for neuronal degeneration. It is important to note, however, that residual IFN-γ exogenously added in order to activate microglia may have also been present in the supernatant. We have previously demonstrated that IFN-γ exposure alone causes apoptotic death in VSC4.1 cells (Smith et al., 2009). Additionally, TNF-α and IFN-γ released from activated microglia may cooperatively act to induce further microglial activation and motoneuron death (Mir et al., 2009; Mir et al., 2008).
Understanding neurodegenerative mechanisms associated with microglial activation may be beneficial in the development of strategies or agents for neuroprotection in various CNS diseases and injuries. One of the agents found to be neuroprotective following CNS insult was estrogen (Brann et al., 2007; Sribnick et al., 2004a; Sribnick et al., 2010; Zhao et al., 2004). However, estrogen has serious side effects which may limit its use in humans. Since the beneficial effects of estrogen are mediated by its receptors, we have examined the effects of GEN, a multi-active ERβ agonist, as a neuroprotectant for motoneurons in this study. GEN is a powerful antioxidant, which scavenges highly toxic hydroxyl radicals and other ROS that initiate lipid peroxidation, DNA damage, and protein oxidation via receptor- and non-receptor mediated pathways (Fotsis et al., 1998; Kousidou et al., 2006; Kuiper et al., 1998; Walker et al., 2001). In addition, GEN may represent a promising agent in the treatment of neurodegenerative disorder due to its ability to cross the blood-brain barrier, relatively long half-life (15 to 22 hr.), and low oral in vivo toxicity (Liu et al., 2008; McClain et al., 2005; Takimoto et al., 2003; Tsai, 2005). Our results demonstrated that activated microglial supernatant caused significant loss of cell viability due to apoptotic cell death in VSC4.1 motoneurons. Treatment of cytokine-insulted motoneurons with GEN demonstrated marked neuroprotection. The protection of cells seen with GEN is likely by prevention of ROS production, Ca+2 influx, and reduced activation of pro-apoptoic factors. Similar protection of primary cortical neurons has been reported with estrogen (Sribnick et al., 2004b). In this light, GEN demonstrated significant attenuation of oxidative stress similar in extent to melatonin, a well-known anti-oxidant previously studied in the VSC cell line (Das et al., 2010). Our studies indicated that expression of both estrogen receptors (ERα and ERβ) in VSC4.1 motoneurons was up-regulated following GEN treatment. Furthermore, the ERβ:ERα ratio was also increased. ERβ is a nuclear receptor that is found in mammals including humans and has varying concentrations throughout the body including the brain (Kuiper and Gustafsson, 1997; Kuiper et al., 1998; Mitra et al., 2003). The ER antagonist ICI 182,780 significantly reduced the beneficial effects of GEN, indicating primary involvement of ERβ in GEN-mediated neuroprotection. Thus, these results suggest that GEN worked via ERβ to prevent apoptosis in VSC4.1 motoneurons, though not undermining the anti-inflammatory effect mediated by ERα. The neuroprotective effect of GEN through ERβ was further confirmed by determining the integrity of mitochondrial membrane potential in VSC4.1 cells following cytokine exposure (Figure 5E). In line with this, estrogen receptor-mediated neuroprotection has recently been shown in experimental autoimmune encephalomyelitis (EAE) (Tiwari-Woodruff et al., 2007).
Abnormal Ca+2 influx following neuronal injury may cause the activation of caspase-3 and the Ca+2-dependent protease calpain, both of which are detrimental to cell survival (Das et al., 2010; Das et al., 2005; Kass and Orrenius, 1999; McConkey and Orrenius, 1996; Simon et al., 2000). We found that microglia supernatant induced increases in intracellular free Ca+2, expression of calpain, and activation of caspase-3 in VSC4.1 cells. Our data suggests that GEN treatment suppresses increases in intracellular Ca2+ in VSC4.1 motoneurons following exposure to activated microglial, which consequently may prevent the activation of Ca+2-mediated cell death pathways. This is not surprising since estrogen has been found to modulate L-type Ca+2 channels which will help to reduce elevated calpain activity following neurodegenerative insult (Sribnick et al., 2009). To this effect, we noted that GEN caused a decrease in calpain and caspase-3 expression and activity. GEN also appeared to prevent activation of upstream mediators of the intrinsic apoptotic pathway. In particular, we have demonstrated that GEN reversed increases in the Bax:Bcl-2 ratio following cytokine insult. An increase in the Bax:Bcl-2 ratio has been suggested as an early indicator of apoptotic cell death via the intrinsic apoptotic pathway (Arends and Wyllie, 1991; Fesus et al., 1991). These findings support that GEN-mediated neuroprotection is due, in part, to inhibition of the mitochondrial apoptotic pathway.
GEN may also have a similar effect on caspase-8 and the extrinsic apopotic pathway. Following activation of the extrinsic pathway, caspase-8 may affect mitochondrial damage resulting in the release of cytochrome c (Arends and Wyllie, 1991; Fesus et al., 1991; Kass and Orrenius, 1999; Simon et al., 2000). We have shown herein that exposure of VSC4.1 motoneurons to activated microglial supernatant resulted in significantly increased expression and activity of caspase-8. GEN treatment of activated supernatant-insulted neurons restored caspase-8 to levels below those of control cells. Changes in caspase-8 activity following GEN treatment were also associated with a reduction in cytochrome c release in injured neurons. The protective effects of GEN related to inhibition of the extrinsic apoptotic pathway were reversed using the ER antagonist ICI 182,780. Taken together, these findings indicate that GEN may act through receptor-mediated pathways to prevent activation of caspase-8 and release of cytochrome c following cytokine insult. Although both ERα and ERβ receptors may be involved in this mechanism, ERβ may be more preferentially protective as also suggested by previous findings (Tiwari-Woodruff et al., 2007; Tiwari-Woodruff and Voskuhl, 2009).
Our studies also suggest that GEN may attenuate the inflammatory response in motoneurons exposed to microglial cytokines. In particular, we examined COX-2 and NFκB as indicators of a pro-inflammatory response in VSC4.1 cells. Exposure of neurons to microglial cytokines resulted in increased expression of COX-2 and NFκB which was ameliorated by GEN treatment. The anti-inflammatory effects of GEN were reversed by the ER antagonist ICI 182,780, indicating that ERβ activation may play a role in reducing the inflammatory response in VSC4.1 motoneurons.
6. Conclusions
In conclusion, we have presented substantial evidence that GEN is neuroprotective in motoneurons exposed to activated microglial supernatant. Our study indicates that the cytoprotective effects of GEN may be due in part to the reduction of Ca+2 influx, inhibition of the activation of pro-apoptotic factors (such as calpain, caspase-3, caspase-8, and caspase-9), reduction of the inflammatory response, and maintenance of mitochondrial integrity following neuronal insult. Furthermore, the beneficial effects of GEN were inhibited by the ER antagonist ICI 182,780, suggesting a role for ERβ in GEN-mediated neuronal protection. Overall, these results suggest that GEN may have potential as a therapeutic agent for the treatment of CNS injuries and neurodegenerative diseases.
Research Highlights.
Studies have shown that estrogen is protective against neurodegenerative processes.
Genistein, an ERβ agonist, may be a safer clinical alternative to estrogen.
IFN-γ-activated microglia release a number of pro-inflammatory cytokines.
Activated microglial cytokines caused apoptotic death in VSC4.1 motoneurons.
Genistein reversed motoneuron apoptosis following microglial cytokine insult.
Acknowledgments
Acknowledgements and Funding Completion of this project was made possible by funding from the National Institutes of Health (NIH) and National Institute of Neurological Disorders and Stroke (NINDS): (NS-31622, NS-38146, and NS-41088) and the State of South Carolina Spinal Cord Injury Research Fund (SCSCIRF)
Abbreviations
- GEN
Genistein
- ERα
estrogen receptor alpha
- ERβ
estrogen receptor beta
- SCI
spinal cord injury
- PD
Parkinson’s Disease
- MS
multiple sclerosis
- AD
Alzheimer’s disease
- ROS
reactive oxygen species
- IFN-γ
interferon-γ
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arends MJ, Wyllie AH. Apoptosis: mechanisms and roles in pathology. Int Rev Exp Pathol. 1991;32:223–254. doi: 10.1016/b978-0-12-364932-4.50010-1. [DOI] [PubMed] [Google Scholar]
- Brann DW, Dhandapani K, Wakade C, Mahesh VB, Khan MM. Neurotrophic and neuroprotective actions of estrogen: basic mechanisms and clinical implications. Steroids. 2007;72:381–405. doi: 10.1016/j.steroids.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan WY, Kohsaka S, Rezaie P. The origin and cell lineage of microglia: new concepts. Brain Res Rev. 2007;53:344–354. doi: 10.1016/j.brainresrev.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Cole R, de Vellis J. Preparation of Astrocyte, Oligodendrocyte, and Microglial Cultures from Primary Rat Cerebral Cultures. In: Fedoroff S, Richardson A, editors. Protocols for Neural Cell Culture. Humana Press Inc.; Totowa, New Jersey: 2001. pp. 117–127. [Google Scholar]
- Das A, Banik NL, Ray SK. Differentiation decreased telomerase activity in rat glioblastoma C6 cells and increased sensitivity to IFN-gamma and taxol for apoptosis. Neurochem Res. 2007a;32:2167–2183. doi: 10.1007/s11064-007-9413-y. [DOI] [PubMed] [Google Scholar]
- Das A, Banik NL, Ray SK. Garlic compounds generate reactive oxygen species leading to activation of stress kinases and cysteine proteases for apoptosis in human glioblastoma T98G and U87MG cells. Cancer. 2007b;110:1083–1095. doi: 10.1002/cncr.22888. [DOI] [PubMed] [Google Scholar]
- Das A, McDowell M, Pava MJ, Smith JA, Reiter RJ, Woodward JJ, Varma AK, Ray SK, Banik NL. The inhibition of apoptosis by melatonin in VSC4.1 motoneurons exposed to oxidative stress, glutamate excitotoxicity, or TNF-alpha toxicity involves membrane melatonin receptors. J Pineal Res. 2010;48:157–169. doi: 10.1111/j.1600-079X.2009.00739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A, Sribnick EA, Wingrave JM, Del Re AM, Woodward JJ, Appel SH, Banik NL, Ray SK. Calpain activation in apoptosis of ventral spinal cord 4.1 (VSC4.1) motoneurons exposed to glutamate: calpain inhibition provides functional neuroprotection. J Neurosci Res. 2005;81:551–562. doi: 10.1002/jnr.20581. [DOI] [PubMed] [Google Scholar]
- Fesus L, Davies PJ, Piacentini M. Apoptosis: molecular mechanisms in programmed cell death. Eur J Cell Biol. 1991;56:170–177. [PubMed] [Google Scholar]
- Fotsis T, Pepper MS, Montesano R, Aktas E, Breit S, Schweigerer L, Rasku S, Wahala K, Adlercreutz H. Phytoestrogens and inhibition of angiogenesis. Baillieres Clin Endocrinol Metab. 1998;12:649–666. doi: 10.1016/s0950-351x(98)80009-8. [DOI] [PubMed] [Google Scholar]
- Fuhrman B, Aviram M. Flavonoids protect LDL from oxidation and attenuate atherosclerosis. Curr Opin Lipidol. 2001;12:41–48. doi: 10.1097/00041433-200102000-00008. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Scarano F, Baltuch G. Microglia as mediators of inflammatory and degenerative diseases. Annu Rev Neurosci. 1999;22:219–240. doi: 10.1146/annurev.neuro.22.1.219. [DOI] [PubMed] [Google Scholar]
- Kass GE, Orrenius S. Calcium signaling and cytotoxicity. Environ Health Perspect. 1999;107(Suppl 1):25–35. doi: 10.1289/ehp.99107s125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kousidou O, Tzanakakis GN, Karamanos NK. Effects of the natural isoflavonoid genistein on growth, signaling pathways and gene expression of matrix macromolecules by breast cancer cells. Mini Rev Med Chem. 2006;6:331–337. doi: 10.2174/138955706776073420. [DOI] [PubMed] [Google Scholar]
- Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Gustafsson JA. The novel estrogen receptor-beta subtype: potential role in the cell- and promoter-specific actions of estrogens and anti-estrogens. FEBS Lett. 1997;410:87–90. doi: 10.1016/s0014-5793(97)00413-4. [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der Burg B, Gustafsson JA. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139:4252–4263. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- Kuno R, Wang J, Kawanokuchi J, Takeuchi H, Mizuno T, Suzumura A. Autocrine activation of microglia by tumor necrosis factor-alpha. J Neuroimmunol. 2005;162:89–96. doi: 10.1016/j.jneuroim.2005.01.015. [DOI] [PubMed] [Google Scholar]
- Liang HW, Qiu SF, Shen J, Sun LN, Wang JY, Bruce IC, Xia Q. Genistein attenuates oxidative stress and neuronal damage following transient global cerebral ischemia in rat hippocampus. Neurosci Lett. 2008;438:116–120. doi: 10.1016/j.neulet.2008.04.058. [DOI] [PubMed] [Google Scholar]
- Liu LX, Chen WF, Xie JX, Wong MS. Neuroprotective effects of genistein on dopaminergic neurons in the mice model of Parkinson’s disease. Neurosci Res. 2008;60:156–161. doi: 10.1016/j.neures.2007.10.005. [DOI] [PubMed] [Google Scholar]
- McCarthy KD, de Vellis J. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J Cell Biol. 1980;85:890–902. doi: 10.1083/jcb.85.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClain RM, Wolz E, Davidovich A, Pfannkuch F, Bausch J. Subchronic and chronic safety studies with genistein in dogs. Food Chem Toxicol. 2005;43:1461–1482. doi: 10.1016/j.fct.2005.02.017. [DOI] [PubMed] [Google Scholar]
- McConkey DJ, Orrenius S. The role of calcium in the regulation of apoptosis. J Leukoc Biol. 1996;59:775–783. [PubMed] [Google Scholar]
- Messina M. Soybean isoflavone exposure does not have feminizing effects on men: a critical examination of the clinical evidence. Fertil Steril. 2010;93:2095–2104. doi: 10.1016/j.fertnstert.2010.03.002. [DOI] [PubMed] [Google Scholar]
- Mir M, Asensio VJ, Tolosa L, Gou-Fabregas M, Soler RM, Llado J, Olmos G. Tumor necrosis factor alpha and interferon gamma cooperatively induce oxidative stress and motoneuron death in rat spinal cord embryonic explants. Neuroscience. 2009;162:959–971. doi: 10.1016/j.neuroscience.2009.05.049. [DOI] [PubMed] [Google Scholar]
- Mir M, Tolosa L, Asensio VJ, Llado J, Olmos G. Complementary roles of tumor necrosis factor alpha and interferon gamma in inducible microglial nitric oxide generation. J Neuroimmunol. 2008;204:101–109. doi: 10.1016/j.jneuroim.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Mitra SW, Hoskin E, Yudkovitz J, Pear L, Wilkinson HA, Hayashi S, Pfaff DW, Ogawa S, Rohrer SP, Schaeffer JM, McEwen BS, Alves SE. Immunolocalization of estrogen receptor beta in the mouse brain: comparison with estrogen receptor alpha. Endocrinology. 2003;144:2055–2067. doi: 10.1210/en.2002-221069. [DOI] [PubMed] [Google Scholar]
- Nakajima K, Kohsaka S. Microglia: activation and their significance in the central nervous system. J Biochem. 2001;130:169–175. doi: 10.1093/oxfordjournals.jbchem.a002969. [DOI] [PubMed] [Google Scholar]
- Nef S, Parada LF. Hormones in male sexual development. Genes Dev. 2000;14:3075–3086. doi: 10.1101/gad.843800. [DOI] [PubMed] [Google Scholar]
- Paganini-Hill A. Hormone replacement therapy and stroke: risk, protection or no effect? Maturitas. 2001;38:243–261. doi: 10.1016/s0378-5122(01)00167-0. [DOI] [PubMed] [Google Scholar]
- Reiter E, Beck V, Medjakovic S, Jungbauer A. Isoflavones are safe compounds for therapeutical applications - evaluation of in vitro data. Gynecol Endocrinol. 2009;25:554–580. doi: 10.1080/09513590802596461. [DOI] [PubMed] [Google Scholar]
- Sawada H, Shimohama S. Estrogens and Parkinson disease: novel approach for neuroprotection. Endocrine. 2003;21:77–79. doi: 10.1385/ENDO:21:1:77. [DOI] [PubMed] [Google Scholar]
- Sawada H, Suzuki H, Nagatsu T, Sawada M. Neuroprotective and neurotoxic phenotypes of activated microglia in neonatal mice with respective MPTP- and ethanol-induced brain injury. Neurodegener Dis. 2010;7:64–67. doi: 10.1159/000285508. [DOI] [PubMed] [Google Scholar]
- Simon HU, Haj-Yehia A, Levi-Schaffer F. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis. 2000;5:415–418. doi: 10.1023/a:1009616228304. [DOI] [PubMed] [Google Scholar]
- Smith JA, Zhang R, Varma AK, Das A, Ray SK, Banik NL. Estrogen partially down-regulates PTEN to prevent apoptosis in VSC4.1 motoneurons following exposure to IFN-gamma. Brain Res. 2009;1301:163–170. doi: 10.1016/j.brainres.2009.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RG, Alexianu ME, Crawford G, Nyormoi O, Stefani E, Appel SH. Cytotoxicity of immunoglobulins from amyotrophic lateral sclerosis patients on a hybrid motoneuron cell line. Proc Natl Acad Sci U S A. 1994;91:3393–3397. doi: 10.1073/pnas.91.8.3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sribnick EA, Del Re AM, Ray SK, Woodward JJ, Banik NL. Estrogen attenuates glutamate-induced cell death by inhibiting Ca2+ influx through L-type voltage-gated Ca2+ channels. Brain Res. 2009;1276:159–170. doi: 10.1016/j.brainres.2009.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sribnick EA, Ray SK, Banik NL. Estrogen as a multi-active neuroprotective agent in traumatic injuries. Neurochem Res. 2004a;29:2007–2014. doi: 10.1007/s11064-004-6874-0. [DOI] [PubMed] [Google Scholar]
- Sribnick EA, Ray SK, Nowak MW, Li L, Banik NL. 17beta-estradiol attenuates glutamate-induced apoptosis and preserves electrophysiologic function in primary cortical neurons. J Neurosci Res. 2004b;76:688–696. doi: 10.1002/jnr.20124. [DOI] [PubMed] [Google Scholar]
- Sribnick EA, Samantaray S, Das A, Smith J, Matzelle DD, Ray SK, Banik NL. Postinjury estrogen treatment of chronic spinal cord injury improves locomotor function in rats. J Neurosci Res. 2010;88:1738–1750. doi: 10.1002/jnr.22337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takimoto CH, Glover K, Huang X, Hayes SA, Gallot L, Quinn M, Jovanovic BD, Shapiro A, Hernandez L, Goetz A, Llorens V, Lieberman R, Crowell JA, Poisson BA, Bergan RC. Phase I pharmacokinetic and pharmacodynamic analysis of unconjugated soy isoflavones administered to individuals with cancer. Cancer Epidemiol Biomarkers Prev. 2003;12:1213–1221. [PubMed] [Google Scholar]
- Tiwari-Woodruff S, Morales LB, Lee R, Voskuhl RR. Differential neuroprotective and antiinflammatory effects of estrogen receptor (ER)alpha and ERbeta ligand treatment. Proc Natl Acad Sci U S A. 2007;104:14813–14818. doi: 10.1073/pnas.0703783104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari-Woodruff S, Voskuhl RR. Neuroprotective and anti-inflammatory effects of estrogen receptor ligand treatment in mice. J Neurol Sci. 2009;286:81–85. doi: 10.1016/j.jns.2009.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai TH. Concurrent measurement of unbound genistein in the blood, brain and bile of anesthetized rats using microdialysis and its pharmacokinetic application. J Chromatogr A. 2005;1073:317–322. doi: 10.1016/j.chroma.2004.10.048. [DOI] [PubMed] [Google Scholar]
- Walker HA, Dean TS, Sanders TA, Jackson G, Ritter JM, Chowienczyk PJ. The phytoestrogen genistein produces acute nitric oxide-dependent dilation of human forearm vasculature with similar potency to 17beta-estradiol. Circulation. 2001;103:258–262. doi: 10.1161/01.cir.103.2.258. [DOI] [PubMed] [Google Scholar]
- Zhao L, Wu TW, Brinton RD. Estrogen receptor subtypes alpha and beta contribute to neuroprotection and increased Bcl-2 expression in primary hippocampal neurons. Brain Res. 2004;1010:22–34. doi: 10.1016/j.brainres.2004.02.066. [DOI] [PubMed] [Google Scholar]