Abstract
One of the capabilities developed by bacteria is the ability to gain large fragments of DNA from other bacteria or to lose portions of their own genomes. Among these exchangeable fragments are the genomic islands (GIs). Nine GIs have been identified in Brucella, and genomic island 3 (GI-3) is shared by two pathogenic species, B. melitensis and B. abortus. GI-3 encodes mostly unknown proteins. One of the aims of this study was to perform pulsed-field gel electrophoresis (PFGE) on field isolates of B. abortus from Chile to determine whether these isolates are clonally related. Furthermore, we focused on the characterization of GI-3, studying its organization and the genetic conservation of the GI-3 sequence using techniques such as tiling-path PCR (TP-PCR) and restriction fragment length polymorphism-PCR (RFLP-PCR). Our results, after PFGE was performed on 69 field isolates of B. abortus from Chile, showed that the strains were genetically homogeneous. To increase the power of genetic discrimination among these strains, we used multiple locus variable-number tandem-repeat (VNTR) analysis with 16 loci (MLVA-16). The results obtained by MLVA-16 showed that the strains of B. abortus were genetically heterogeneous and that most of them clustered according to their geographic origin. Of the genetic loci studied, panel 2B was the one describing the highest diversity in the analysis, as well as locus Bruce19 in panel 2A. In relation to the study of GI-3, our experimental analysis by TP-PCR identified and confirmed that GI-3 is present in all wild strains of B. abortus, demonstrating the high stability of gene cluster GI-3 in Chilean field strains.
INTRODUCTION
Brucellosis is a zoonotic and endemic disease in many areas of the world (8). Similarly to other facultative intracellular parasites, Brucella strains survive outside cells, but they must infect and replicate intracellularly in animals to perpetuate themselves (11). Brucella strains are extremely well adapted to the intracellular niche, and therefore they should be described as facultative extracellular intracellular parasites (24). Brucella causes abortion in cattle, goats, and sheep and a febrile illness (undulant fever) in humans (28). The genus Brucella has six recognized species, all of which exhibit distinct host preferences. Common host-pathogen associations among the classical Brucella species are as follows: B. abortus, cattle; B. suis, swine; B. melitensis, goats; B. ovis, sheep; B. canis, dogs; and B. neotomae, desert wood rats (13). Recently, a new species, B. mari, isolated from marine mammals, was suggested (15). Since brucellosis threatens the food supply and causes a long, debilitating disease in humans, Brucella species are recognized as potential agricultural, civilian, and military bioterrorism agents (13).
Brucella infections are endemic in humans and livestock in Mediterranean, Asian, sub-Saharan African, and Latin American countries (32). In Chile, the disease is spread mainly in the southern area, which has the largest livestock herds (21, 31).
The genus Brucella shows a high degree of similarity among species (9, 25). Based on DNA-DNA hybridization, it was proposed that Brucella is a monospecific genus and that B. melitensis would be the only species in the genus. The remaining species should be considered B. melitensis biovars (37). The B. abortus genome consists of two circular chromosomes (18), with 2,124,242 bp in chromosome I (Chr I) and 1,162,780 bp in chromosome II (Chr II). Altogether, these chromosomes carry 3,296 open reading frames (ORFs) that are annotated as genes, 2,158 on Chr I and 1,138 on Chr II (13).
Bacteria, in the course of the evolution, have developed the ability to gain large fragments of exogenous DNA from other bacteria or to lose their own genome fragments, allowing their survival in new environments. Genomic islands (GIs) are found within these fragments (12). These islands can encode metabolic pathways and/or virulence factors, providing new features that can transform a nonpathogenic bacterium into a pathogenic bacterium (12). Nine GIs have been identified in B. melitensis, and two of them (GI-4 and GI-8) are missing in B. abortus (27). Subsequent studies reported that of these nine islands present in Brucella, GI-1, GI-5, and GI-6 do not contribute to Brucella virulence (26). On the other hand, GI-3, which is present in B. melitensis and B. abortus, both of which are pathogenic for humans, contains 29 genes, most of them with unknown function. GI-3 was characterized in two sequenced B. abortus strains, 2308 and 9-941.
Mancilla et al. (22), in a previous study in Chile, compared the genotypes of B. abortus by IS711 restriction fragment length polymorphism analysis (RFLP). Apart from their work, there is little information about the genetic characteristics of the B. abortus strains infecting fields in Chile. Hence, in this work we performed pulsed-field gel electrophoresis (PFGE) and multiple locus variable-number tandem-repeat (VNTR) analysis with 16 loci (MLVA-16) on field isolates of B. abortus from Chile in order to determine whether these isolates were genetically homogeneous. Furthermore, we focused our work on the characterization of GI-3 to study the organization and the genetic conservation degree of the GI-3 sequence, using techniques such as tiling-path PCR (TP-PCR) and RFLP-PCR.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
A total of 69 isolates, collected between 1997 and 2008 by the Servicio Agrícola Ganadero de Chile (SAG) were obtained. They were recovered from milk samples and fetal remains, mainly from regions in southern Chile. Details about the bacterial strains used for this study are provided in Table 1. B. abortus strains 2308, RB51, and 544 were included as references. All strains were characterized by classical microbiological methods as B. abortus biovar 1 and were cultured in brucella agar (Oxoid Limited, Cambridge, United Kingdom) at 37°C with 5% CO2.
Table 1.
Wild strains of Brucella abortus included in this study
| Place of isolation | Geographic location (latitude) | Isolation yr | No. of strains |
|---|---|---|---|
| Osorno | 40°34′00"S | 1997 | 3 |
| Purranque | 40°55′00"S | 1997 | 4 |
| Río Bueno | 40°19′00"S | 1997 | 2 |
| Río Negro | 40°47′00"S | 1997 | 1 |
| Puerto Montt | 41°28′18"S | 1997 | 2 |
| Valdivia | 39°48′30"S | 1997 | 1 |
| Paillaco | 40°04′06"S | 1997 | 1 |
| Osorno | 40°34′00"S | 1998 | 1 |
| Purranque | 40°55′00"S | 1998 | 1 |
| Río Bueno | 40°19′00"S | 1998 | 1 |
| Río Negro | 40°47′00"S | 1998 | 2 |
| Osorno | 40°34′00"S | 2007 | 2 |
| Puerto Octay | 40°58′00"S | 2007 | 1 |
| Paillaco | 40°04′06"S | 2007 | 1 |
| Máfil | 39°39′00"S | 2007 | 1 |
| Río Bueno | 40°19′00"S | 2007 | 4 |
| Santiago | 33°27′00"S | 2007 | 23 |
| Puerto Montt | 41°28′18"S | 2008 | 1 |
| Vilcún | 42°05′00"S | 2008 | 1 |
| Bulnes | 36°44′00"S | 2008 | 9 |
| San Carlos | 36°25′00"S | 2008 | 7 |
PFGE.
Genomic DNA (gDNA) suitable for pulsed-field gel electrophoresis (PFGE) was prepared according to the method described by Ridler et al. (30), with some modifications. Briefly, B. abortus isolates were grown on brucella agar (Oxoid Limited, Cambridge, United Kingdom) for 48 h at 37°C with 5% CO2. The colonies were harvested into 3 ml of brain heart infusion broth (Difco Laboratories, Detroit, MI), and the optical density was measured and adjusted to 1.4 at 610 nm. A 100-μl aliquot of cells was pelleted by centrifugation at 13,000 rpm for 5 min. Cells were washed once with 150 ml of Pett IV buffer (1 M NaCl, 10 mM Tris-HCl [pH 8.0], 10 mM EDTA [pH 8.0]) and then centrifuged and resuspended in 50 ml of Pett IV buffer. The bacterial suspension was mixed with 100 ml of 1% low-melting-point preparative-grade agarose (Bio-Rad Laboratories, Richmond, CA) and dispensed into plug molds. The agarose plugs were placed on ice for 1 h to solidify. The plugs were lysed overnight at 56°C in a buffer solution (50 mM EDTA [pH 8.0], 50 mM Tris-HCl [pH 8.0], 1% sodium lauroyl sarcosine) containing 1 mg of proteinase K (Sigma Chemical Company, St. Louis, MO) per ml. Following lysis, the plugs were washed five times for 1 h each time with 10 ml of TE buffer (10 mM Tris-HCl, 1 mM EDTA) on ice. A 3-mm slice of each plug was equilibrated in 100 μl of XbaI restriction buffer (New England BioLabs, Beverly, MA) for 45 min on ice. The restriction buffer was removed and replaced with 100 μl of fresh restriction buffer containing 30 units of XbaI (New England BioLabs, Beverly, MA). The plug slices were held on ice for an additional 45 min before an overnight incubation at 37°C. The macrorestriction fragments were separated by pulsed-field gel electrophoresis on a CHEF-DRIII system (Bio-Rad Laboratories, Richmond, CA) in a 1% agarose gel (pulsed-field certified agarose; Bio-Rad) in 0.5× TBE buffer (45 mM Tris, 45 mM boric acid, 1 mM EDTA [pH 8.0]) at 6 V/cm and 14°C. Macrorestriction fragments produced with XbaI were separated according the method described by Ridler (30), with ramped pulse times of 0.5 to 10 s for 24 h. The lambda ladder PFG marker and low-range PFG marker (New England BioLabs, Beverly, MA) were included as molecular size standards. Gels were stained with ethidium bromide, and images were captured under UV illumination, The macrorestriction patterns were analyzed visually using the criteria of Tenover et al. (35).
MLVA-16 analysis.
In addition to the B. abortus 2308, RB51, and 544 reference strains, MLVA-16 analysis also included the S19 vaccine strain and 67 field strains studied by PFGE (two strains could not be recovered). The genomic DNA was extracted from one loopful of bacterial cells grown for 48 h at 37°C with 5% CO2 on brucella agar medium using the UltraClean microbial DNA isolation kit (Mo Bio Laboratories, Carlsbad, CA). We studied 16 locus grouped into three panels (panel 1, Bruce06, Bruce08, Bruce11, Bruce12, Bruce42, Bruce43, Bruce45, and Bruce55; panel 2A, Bruce18, Bruce19, and Bruce21; and panel 2B, Bruce04, Bruce07, Bruce09, Bruce16, and Bruce30). The selected primers and PCR amplification protocol for each of the loci comprising panels 1, 2A, and 2B were as described by Le Flèche et al. (20) and Al Dahouk et al. (1), with the addition of Bruce19 in panel 2A. Briefly, the amplification was performed in a MyCycler thermal cycler (Bio-Rad) with an initial step of denaturation at 96°C for 5 min followed by 30 cycles of denaturation at 96°C for 30 s, annealing at 60°C for 30 s, and elongation at 70°C for 1 min. The final extension was performed at 72°C for 5 min. Products were analyzed by gel electrophoresis on a 3% standard agarose gel using 100-bp and 20-bp ladders (Fermentas, Lithuania) as molecular size markers depending on the tandem repeat unit length. Gel images and PCR product sizes were determined using Gel Compar II software (Applied Maths). Allele numbers were established according to product size ranges (see Table 3). Different alleles for each strain were represented as a numerical matrix, and a dendrogram was constructed using the unweighted-pair group method using average linkages (UPGMA) method and Treecon software version 1.3b (36).
Table 3.
Summary of MLVA-16 results for 71 animal isolates of Brucella abortus
| Panel and VNTR locus | Size (bp) of allelea: |
HGDIb |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | Mean | 95% CI | |
| 1 | |||||||||
| Bruce06 | 542 | 0 | |||||||
| Bruce08 | 348 | 366 | 0.155 | 0.049–0.261 | |||||
| Bruce11 | 383 | 0 | |||||||
| Bruce12 | 375 | 390 | 405 | 0.227 | 0.106–0.349 | ||||
| Bruce42 | 289 | 0 | |||||||
| Bruce43 | 170 | 182 | 194 | 206 | 0.5 | 0.383–0.618 | |||
| Bruce45 | 151 | 169 | 0.407 | 0.315–0.499 | |||||
| Bruce55 | 273 | 0 | |||||||
| 2A | |||||||||
| Bruce18 | 146 | 154 | 162 | 0.393 | 0.282–0.504 | ||||
| Bruce19 | 178 | 184 | 190 | 196 | 0.710 | 0.671–0.748 | |||
| Bruce21 | 148 | 156 | 164 | 0.453 | 0.353–0.553 | ||||
| 2B | |||||||||
| Bruce04 | 160 | 168 | 176 | 184 | 192 | 0.689 | 0.612–0.766 | ||
| Bruce07 | 134 | 142 | 150 | 158 | 166 | 174 | 182 | 0.743 | 0.697–0.790 |
| Bruce09 | 116 | 124 | 132 | 140 | 148 | 0.736 | 0.689–0.783 | ||
| Bruce16 | 152 | 160 | 168 | 176 | 192 | 0.620 | 0.531–0.709 | ||
| Bruce30 | 127 | 135 | 143 | 151 | 159 | 0.765 | 0.724–0.806 | ||
Allele size determined in B. abortus isolates from animals by using MLVA-16. The expected size in the B. abortus 9-941 reference strain is in bold.
HGDI, Hunter-Gaston diversity index; CI, confidence interval.
Data analysis.
Polymorphism was quantified by the Hunter-Gaston diversity index (HGDI) (14).
Genetic characterization of GI-3 by TP-PCR.
A technique called tiling-path PCR scanning (TP-PCR), previously described by Ren et al. (29), was used with some modifications. Briefly, the method consists of several interlocking PCR-based approaches able to characterize large genomic regions. A series of primers was designed to amplify fragments of ∼5 kb spanning the region of interest (Fig. 1) but also having short overlaps of a few hundred base pairs. The DNA sequences of the TP-PCR primers used to amplify regions of the GI-3 were designed using Primer 3 software on the basis of the sequence of chromosome I (GenBank accession no. NC_006932, B. abortus biovar 1 strain 9-941). The primer sequences are listed in Table 2. Long PCRs were performed to amplify the region in question. Any negative results by long PCR for a given primer pair were followed up by amplification of the relevant short overlaps with the same primers and/or a deletion-scanning long PCR that employed primers flanking the lost segments. Long PCRs were performed in 50 μl of reaction mixture containing 1 mM deoxynucleoside triphosphate (dNTP) mix, 10 pmol of each primer, 1.5 mM MgCl2, 1× reaction buffer supplied by the manufacturer, 0.5 U of High Fidelity DNA polymerase (Fermentas Life Sciences, CA), and 5 μl of DNA as the template. The genomic DNA was obtained with the UltraClean microbial DNA isolation kit (Mo Bio Laboratories, Carlsbad, CA). Long-PCR conditions were 30 cycles of 10 s at 94°C, 30 s at 62°C, and 10 min at 68°C, followed by a 10-min extension at 68°C. For short PCRs, each multiplex PCR assay was performed in 25 μl of reaction mixture containing 1 mM deoxynucleoside triphosphate mix, 10 pmol of each primer, 2.0 mM MgCl2, 1× reaction buffer (10 mM Tris-HCl, 50 mM KCl), 0.25 U of Taq DNA polymerase (Fermentas Life Sciences, CA), and 2 μl of DNA as the template. The samples were amplified for 35 cycles, and each cycle consisted of 1.5 min at 94°C, 1.5 min at 64°C, and 1.5 min at 72°C. The PCR products were separated by electrophoresis in 1.5% agarose gels in a 0.5× TBE buffer system for 2 h. Following electrophoresis, the gel was stained with ethidium bromide (1 μg/ml) and photographed under UV light.
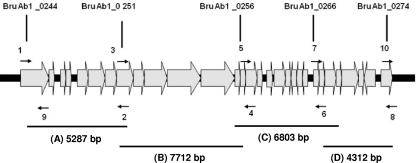
Fig. 1.
Organization of open reading frames in GI-3 in B. abortus. 1, 2, 3, 4, 5, 6, 7, 8, and 9 are primers (listed in Table 2) used to amplify regions A, B, C, and D. Only relevant features are shown; the figure is not drawn to scale.
Table 2.
Primers used for genetic characterization of GI-3 by tiling-path PCR
| Primer(s) | Annealing site | Sequence, 5′ → 3′ | GI-3 fragment amplified (bp) |
|---|---|---|---|
| 1 | 260022–260043 | CGACCAAAATCTGAATTGACCT | A (5,287) |
| 2 | 265308–265288 | TTATTTCGCCTCCCTGATTTT | |
| 3 | 263807–263829 | ATCTTCGAGAACCTGGCTAAAGA | B (7,712) |
| 4 | 271518–271498 | AGCGCGTAAGTAAGCCTTGAG | |
| 5 | 269673–269694 | ATGTGATGCGAACGTTCATTAC | C (6,804) |
| 6 | 276476–276454 | TATTGTTGGAAACGGCTTTGATA | |
| 7 | 276029–276052 | CTCTAATGTTTCGGTGTAATTGAA | D (4,312) |
| 8 | 280340–280318 | TAAAGAGAAACAAGCCCAATCAA | |
| 9 + 1 | 260274–260252 | GCACCATTATTTTACCAAGCAAT | BruAb_0244 (252) |
| 10 + 8 | 279596–279618 | GATAAGGGAATGCAGTTCTTTCG | BruAb_0274 (744) |
Restriction analysis of fragments generated by tiling-path PCR.
DNA generated by tiling-path PCR of each fragment was digested with 10 U of restriction enzymes (Promega, Madison, WI) (AvaII to digest fragment A, EaeI to digest fragment B, BanI to digest fragment C, and BspHI to digest fragment D) and incubated at 37°C for 2 h according to the manufacturer's instructions. Restriction DNA fragments were separated by electrophoresis at 80 V in a horizontal gel containing 1.5% agarose in a 0.5× TBE buffer system for 2 h. Following electrophoresis, the gel was stained in ethidium bromide (1 μg/ml) and photographed under UV light.
RESULTS
PFGE analyses shows a low variability in the Chilean B. abortus strains.
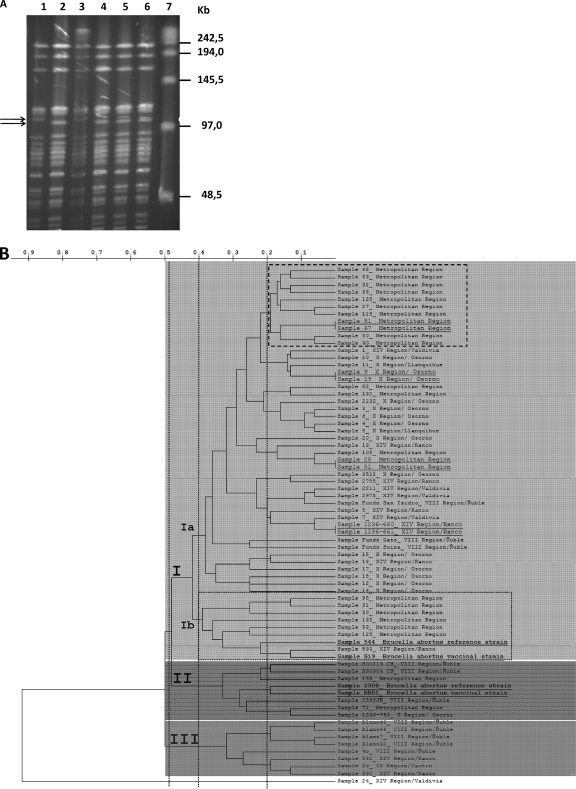
We analyzed a total of 69 field strains of B. abortus isolated from milk and fetuses by pulsed-field gel electrophoresis (PFGE) in order to elucidate the genetic diversity of the Chilean strains. We were able to see two patterns, consisting of about 20 or 21 bands with a size ranging from less than 48.5 to 242.5 kb. In this report, a gel with 6 strains (3 reference strains and 3 field strains) is shown (Fig. 2 A). The most prevalent pattern was designated PFGE type A1 (Fig. 2A, lane 3) and was the same as that of B. abortus biovar 1 strain 544 (Fig. 2A, lane 1), being recognized in 67 of 69 isolates. Therefore, all of these 67 strains exhibited a genetic homogeneity that was independent of the city and period of isolation, suggesting that they were of a unique clonal origin. The second pattern was designated PFGE type A2 (Fig. 2A, lane 5 and 6), and it was found in just 2 of the 69 isolates from southern Chile. PFGE types A1 and A2 could be differentiated by the absence of a 109-kb band and the presence of another 104-kb band in type A2, with this pattern being the same for strains RB51 and 2308 (Fig. 2A, lanes 2 and 4). The pulse times used were 0.5 to 10 s for 24 h at 6 V/cm, and these pulses resolved the larger restriction fragments, but RB51 and 2308 strain patterns were indistinguishable. Since RB51 and 2308 showed different rifampin susceptibilities, this feature allowed us to recognize both wild strains (rifampin resistant) as RB51 strains. Restriction analysis with XbaI showed a low variability among strains. These results are consistent with previous findings of other authors (13, 22), However, the genetic homogeneity observed in most B. abortus isolates showed the need to complement this study with a methodology able to provide a higher resolution power, incorporating MLVA analysis and specifically MLVA-16 (comprising 16 loci). MLVA-16 has been shown to have good discriminatory power to determine genetic relationships among strains of the same species of Brucella (19).
Fig. 2.
(A) PFGE (pulse time, 0.5 to 10 s) of XbaI digests of DNAs from B. abortus strains. Lane 1, strain 544; lane 2, strain RB51; lanes 3, 5, and 6, Chilean field strains; lane 4, strain 2308; lane 7, ladder. The positions of size markers are indicated on the right. Arrows indicate differentiating bands. (B) Dendrogram based on MLVA-16 genotyping analysis, showing the relationships of the 67 field strains of B. abortus from animal reservoirs and reference strains 2308, 544, S19, and RB51. The last two strains are vaccine strains. All strains analyzed using panels 1, 2A, and 2B allowed us to distinguish three groups of genetically related strains in clusters I, II, and III (featured with different gray background intensities). The group that harbored the greatest number of field strains was B. abortus group I, which in turn is divided into Ia and Ib. Similarity cutoff values of 51%, 60%, and 80% are shown with vertical dotted lines. It is also possible to distinguish clonal strains (underlined) and reference strains (highlighted in bold). Each individual strain was coded as follows: Chilean geographic region/province.
When the cutoff value was 51% similarity, cluster analysis of the VNTR data recognized one major group and two minor groups (clusters, I, II and III) (Fig. 2B). When the 60% cutoff value was used, cluster I was divided into two groups, a larger one (named Ia) containing 45 out of 52 B. abortus strains and a minor group (named Ib) including 9 out of 52 strains, with 2 of them being reference strains (B. abortus 544 and S19). When the 80% cutoff value of similarity was applied, the dendrogram showed that subgroup Ia was subdivided into several other small clusters, with variable numbers of members. However, this cutoff highlighted the presence of one group of 11/52 strains of B. abortus, all of them isolated in the metropolitan region, which also contained two clonal strains (Fig. 2B, upper dashed box).
The MLVA-16 analysis allowed us to recognize a genetically heterogeneous population of B. abortus, which could be grouped into three main clusters named I, II, and III. Group I contained the largest number of strains tested (54/71), with individuals from different regions of southern Chile, such as region XIV (11/54), region X (16/54), and region VIII (3/54). It is also possible to observe that a large group of strains in this group originated from the central part of Chile (metropolitan region, 22/54). These 54 field strains belonging to group I in turn could be subdivided into subgroups Ia and Ib. Subgroup Ia included representatives of all the above-mentioned regions, with four pairs of clonally related strains and each of them with a common geographic origin. We also observed that a large group of strains isolated in the central area of Chile (11/22) (Fig. 2B, upper dashed box) were closely related strains (>80% similarity), and the rest of them were related to different groups of strains isolated from southern Chile. In contrast, subgroup Ib (>60% similarity) included mainly individuals from the central area (6/22) (Fig. 2B, lower dashed box) and were genetically less related than those strains belonging to the metropolitan area of group Ia. Interestingly, in this group, there was one isolate from the south of Chile (region XIV) which was genetically more closely related to B. abortus reference strains 544 and S19 and in particular to S19, with more than 80% similarity. In summary, group I included most of the strains studied and could be subdivided into two genetically heterogeneous subgroups (Ia and Ib), with similarity higher than 60%. Only in subgroup Ia were there pairs of clonally related strains, with those bacterial isolates forming clusters mainly corresponding to the same geographic origin. Finally, two reference strains used in this study (544 and S19) were associated with group I (mainly Ib).
Cluster II includes strains from southern (regions VIII and X) and central Chile. It must be noted that this particular group was related to two other reference strains of B. abortus used in this study, strains 2308 and RB51. The number of strains grouped in cluster II was 8/71. The genetic similarities among cluster II strains were also higher than 60%.
Cluster III consisted of 8/71 B. abortus field strains isolates, all of them obtained only from the south of Chile, with similarities higher than 60%. Region VIII has a significant number of representatives (5/8). However, there were no clonally related strains. Finally, this study describes one isolate from the region XIV with a VNTR amplification profile completely different from those of the rest of the strains (1/71). Using MLVA-16, this strain showed a similarity of below 10% to the rest of the strains studied. This strain corresponded to the Sample 24_XIVRegion/Valdivia, and only the Bruce04 and Bruce19 loci were positive for PCR amplification. It was impossible to obtain amplification products for the rest of the loci analyzed.
The different allele sizes found at each locus are shown in Table 3; allele size is given in base pairs for the PCR product. There was little diversity in the loci studied in panel 1, with Hunter-Gaston diversity index (HGDI) values of less than 0.50 and loci such as Bruce06, Bruce11, Bruce42, and Bruce55 having values equal to 0.0. A similar result of little diversity was observed for panel 2A, in which only the locus Bruce19 was highlighted with an HGDI value equal to 0.71. However, the diversity results observed for panel 2B were promising for the analysis of B. abortus strains, where PFGE provided little resolution in establishing genetic relationships. In our study, the average HGDI value obtained was higher than 0.70, highlighting the diversity observed in the loci Bruce07, Bruce09, and Bruce30, with an average value close to 0.75. At the same time, we could see that in addition to the expected sizes of B. abortus amplification products used to characterize panel 2B, an average of four or five different alleles in the reference strain B. abortus 9-941 could be included (Table 3) (20).
GI-3 is present in all isolated Brucella abortus strains.
In order to determine the conservation status of GI-3, we first performed in silico analyses of the region comprising GI-3 gene clusters and the neighboring region, available in the GenBank database, from genome sequences of chromosome I (accession no. NC_006932 for of B. abortus biovar 1 strain 9-941). Hence, 29 ORFs (BruAb1_0245 to BruAb1_0274) were included in GI-3. Based on this genomic sequence, we designed 5 pairs of primers for PCR amplification of 4 fragments (A, B, C, and D) of 4.3 to 7.7 kb (Table 2). Using the external primers neighboring GI-3 BruAb1_0244 (forward) and BruAb1_0274 (reverse), the presence of the island in the genome of the reference strain 544 and all field strains was verified. These results confirmed that all strains possessed the GI-3 gene cluster (data not shown).
To construct a complete tiling path through GI-3 and to determine the genetic organization within the island for the 69 strains, we used the TP-PCR method (29), which exploits short- and long-PCR protocols; this method allowed us to construct a complete tiling path through the GI-3 gene cluster for 69 strains and for type strain 544. Initially, we confirmed that reference strain 544 possessed GI-3 gene clusters by short and long PCR, first amplifying ORFs BruAb1_0251, BruAb1_0256, BruAb1_0266, and BruAb1_0274 by short PCR (data not shown) and then amplifying fragments A, B, C, and D by long PCR. It was also detected by short PCR of gene BruAb1_0244, a neighbor of GI-3.
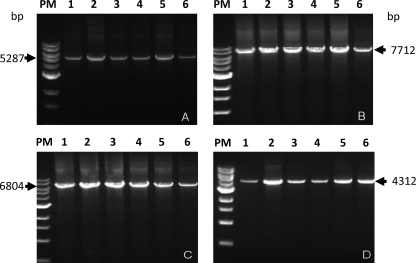
The next step was to amplify each of the 4 fragments (A, B, C, and D) in the 69 field strains. The result was that 100% of these B. abortus field strains studied (n = 69) showed all genes within the island. In all B. abortus strains (69/69), fragments A, B, C, and D of the expected sizes (5,287 bp, 7,712 bp, 6,804 bp, and 4,312 bp, respectively) were obtained. These results confirmed that the complete GI-3 gene cluster was present in all strains sampled (69 of 69), independently of the city or their period of isolation. Figure 3 shows the products obtained for 5 strains chosen at random and the reference strain 544. This indicates that GI-3 is stable in the Chilean field isolates.
Fig. 3.
GI-3 gene cluster fragments amplified by long PCR. Fragment A, 5,287 bp; fragment B, 7,712 bp; fragment C, 6,804 bp; fragment D, 4,312 bp. Lanes 1, amplification product of B. abortus 544; lanes PM, molecular size markers (range, 0.5 to 10 kb); lanes 2 to 6, amplicons obtained from Chilean field strains (see text for details).
RFLP analysis of fragments generated by TP-PCR of GI-3 from all isolated Brucella abortus strains shows results similar to the B. abortus 544 banding profile.
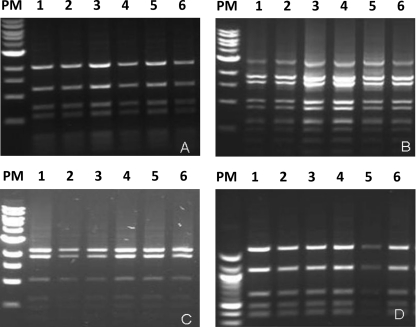
The next step was to characterize the degree of conservation of the structure of each constituent fragment of GI-3. For this, DNA generated by long PCR of each fragment (A, B, C, and D; 5,287, 7,712, 6,804 and 4,312 bp, respectively) from all 69 samples was digested using restriction enzymes AvaII for fragment A, EaeI for fragment B, BanI for fragment C, and BspHI for fragment D. The results of digestion were analyzed using gel electrophoresis (Fig. 4). The banding patterns of restriction fragment length polymorphism (RFLP)-PCR for all fragments of the isolates were similar to each banding pattern of the B. abortus biotype 1. These results indicate once again that GI-3 is conserved in Chilean field strains.
Fig. 4.
RFLP-PCR results for the GI-3 gene cluster. (A) Fragment of 5,287 bp digested with AvaII. (B) Fragment of 7,712 bp digested with EaeI. (C) Fragment of 6,804 bp digested with BanI. (D) Fragment of 4,312 bp digested with BspHI. Lanes 1, restriction fragment of B. abortus 544; lanes PM, molecular size markers (range in panels A, B, and C, 0.5 to 10 kb; range in panel D, 100 to 1,517 bp); lanes 2 to 6, amplicons obtained from Chilean field strains (see text for details).
DISCUSSION
The three analyses performed in this study revealed a very limited GI-3 diversity in the wild Chilean strains of B. abortus. Therefore, they allowed us to confirm the well-known lack of genetic heterogeneity of Brucella spp. and high degree of DNA similarity in Brucella, as previously reported (37). Despite this, the addition of a fourth genetic methodology, the multilocus VNTR analysis with 16 loci (MLVA-16), to study the genetic relationships between B. abortus strains provided an adequate variability to improve the discrimination power and allowed the characterization of field strains. This allowed us to establish the genetic relationships associated with a particular geographic distribution. This finding becomes of great importance when the researcher wants to know the origin of the spread of a pathogen and also to determine whether a bacterium is imported from other geographic locations where it is endemic (19, 20, 22).
The first analysis in this study was to perform pulsed-field gel electrophoresis (PFGE) on 69 Chilean field strains of B. abortus to determine possible genomic variability among the isolates. It should be noted that this study is the first report of PFGE analysis of Chilean isolates of B. abortus. Genomic macrorestriction fingerprinting using PFGE considers the overall organization of the bacterial genome and may be an indicator of clonal origin and genetic relatedness by comparing DNA restriction patterns of the isolates among strains (35). In this study, in order to determine possible genetic variability among the 69 analyzed strains from various regions of Chile, the endonuclease XbaI was selected because PFGE of genomic DNA digested with this restriction enzyme, which has infrequent cutting, has been used to discriminate among Brucella species (17). Also, it supported the designation of additional genomic groups of Brucella (16) and allowed differentiation of the currently licensed B. abortus vaccine strain RB51 from other brucellae, providing easily distinguishable results (17).
The PFGE patterns produced in the present study for the 69 strains digested with XbaI contained approximately 20 or 21 visible bands, with two distinct PFGE patterns. PFGE type A1 was the most prevalent pattern and was the same as that of B. abortus strain 544 biovar 1, being recognized in 67 of 69 isolates. This finding is not surprising, since in Chile and in countries such as the United States (2), Argentina (33), and countries in Central America (23), B. abortus biovar 1 was responsible for the greatest percentages of cases of bovine brucellosis. Our results also confirmed the report of Mancilla et al. on a previous study with Chilean isolates (22). Pattern A1 showed no appreciable banding differences among the 67 isolates, indicating, according to the interpretive criteria of PFGE patterns published by Tenover et al. (35), that these 67 isolates were genetically indistinguishable and therefore were clonally related.
The PFGE type A2 pattern was found just in 2 of the 69 isolates from southern Chile, and it was the same as the one described in previous studies corresponding to strain RB51 (17). In this study, PFGE type A1 was different from PFGE type A2 by the absence of one band of 109 kb and the presence of one band of 104 kb in type A2. Therefore, according to the criteria of Tenover et al. (35), the two groups would be closely related.
The differences in the patterns presented by these two strains correlated with their rifampin resistance, characteristic of strain RB51, confirming that they were closely related to RB51. RB51 is presently used in the national program in Chile to eradicate brucellosis, replacing strain S19, which was used from 1975 to 1995. The use of strain S19 decreased the disease prevalence in livestock from 7.5% to 2.5%. The use of vaccination with RB51, which started in 1996, together with appropriate management plans decreased the prevalence even further in Chilean herds, allowing some areas in southern Chile areas to be declared brucellosis-free zones (31).
Summarizing the results obtained using the PFGE technique, the low genetic variability of Chilean B. abortus strains is confirmed. Although most genomes of the Brucella strains we studied were identified as members of the same clone and are identical or nearly so, several of them might have, based on the proposition by Jensen et al. (16), different ancestries and be derived by horizontal (transmissible) transfer from other clones of the same or different species. This finding for Chilean strains confirmed the report by Mancilla et al., who in a previous study compared genotypes of B. abortus by IS711-RFLP analysis (22). Besides this, our goal of incorporating greater variability and discriminatory power was met by introducing MLVA-16 analysis (20) into the study.
We were able to recover 67 of the 69 field strains studied by PFGE, and using MLVA-16 analysis we succeeded in resolving 52 genotypes, which were grouped into three genetically related clusters. At the same time, a greater diversity was observed by incorporating this methodology through the different variants of VNTRs associated with each locus. MLVA-16 allowed grouping of most B. abortus strains according to their geographic origin. These results allowed us to conclude that there is a large group of B. abortus strains (clusters I and II) that brings together strains from southern and central Chile, which can be differentiated by their geographic origin. Distinct is cluster III, in which all strains have their origin in the south of Chile and are less related to the reference strains 544 and S19 (cluster I) and 2308 and RB51 (cluster II). This very close genetic association between the reference strains used in this study was described by Mancilla et al. (22) using IS711-RFLP. In summary, our results on the diversity (HGDI) for each of those loci studied by MLVA-16 (panel 1, panel 2, and panel 2B) (Table 3) were similar to those previously described by other authors (10, 19). Panel 2B contained those markers that displayed the greatest diversity and therefore would be those that also had a higher mutation rate (along with locus Bruce19 in panel 2A), all of them with HGDIs near or greater than 0.70. This observation would allow us to hypothesize that in the study of the genetic relationships among B. abortus field strains the power of discrimination of panel 2B would be enough to ascertain differences in bacterial strains defined as closely related or clonal by PFGE. However, we believe that a highly variable locus group, we might have the risk of erroneously defining all our strains as different or genetically unrelated, making us unable to establish relationships between them. From this point of view, we believe that the greater degree of conservation in the rest of those loci, with HGDI values of <0.50, allows us to provide the balance needed for this methodology to be a successful molecular tool with B. abortus field strains.
Finally we note that one of the strains studied (Sample 24_XIVRegion/Valdivia) amplified only 2 of the 16 loci studied (Bruce04 and Bruce19), showing only 10% similarity with the other strains, a situation that deserves further investigation.
Regarding the study of the genomic island GI-3, our experimental analysis by TP-PCR identified and confirmed that this island is present in all wild B. abortus strains, which demonstrated the high stability of gene cluster GI-3 in Chilean field strains. This might suggest that the island cannot be spontaneously separated from the genome. GI-3 is flanked by direct repeats of 18 bp (27), and the low number of base pairs might explain the low mobility of this genetic element in wild strains, despite the fact that this island displayed characteristics typical of GIs (12). The stability of GI-3 confirms, as reported by other authors, that the genomes of Brucella species are very stable and similar (37).
We were also able to verify that GI-3 maintained its structure. When each of the 4 fragments constituting GI-3 was amplified by PCR from samples of genomic DNA from each strain of the collection, it was found that all strains under study generated an amplification product of the expected size for each of the fragments. These results indicated that the structure in the island was maintained. There was no evidence of large insertions or rearrangements within this cluster, allowing us to conclude that the island was preserved in the Chilean strains, independently of their sources and period of isolation.
Regarding techniques that allow us to discriminate different strains within a species, molecular techniques that have been applied to many organisms on the basis of their discriminatory capacity to fully differentiate species and biovars have not been able to be used for Brucella strains because of their high DNA similarity (37). However, recently several molecular strategies have been described and employed in an attempt to find DNA markers for molecular identification and differentiation between Brucella species (39) and biovars (4, 7, 40, 41). Among these techniques are the use of DNA markers detected by PCR on restriction endonuclease polymorphism of genes encoding the major outer membrane proteins of brucellae (7, 34, 38). Other authors have analyzed insertion sequences, including IS711 elements, which had been described as a useful target for molecular characterization of Brucella species and biovars based on the number and distribution of copies within the bacterial genomes (3, 5, 6). Recently Zygmunt et al. (41) examined the polymorphism of O-polysaccharide genes wbkE, manAO-Ag, manBO-Ag, manCO-Ag, wbkF, wbkD, and wbo (wboA and wboB) and core genes manBcore. They reported that although most genes were highly conserved, species- and biovar-specific restriction patterns were found. In that study, after checking by TP-PCR that GI-3 was preserved and maintained its structure, the RFLP technique was applied to each of the 69 isolates of B. abortus. This technique was applied to characterize the degree of conservation of each of the fragments (A, B, C, and D) generated by TP-PCR, since there may be events such as simple mutations, deletions, or insertions which may not be noticeable by the prior technique. For this, the products A, B, C, and D were restricted with the AvaII (A), EaeI (B), BanI (C), and BspHI (D) endonucleases. It was observed that each fragment has the same cutting pattern presented by type strain 544, which would indicate to us again that GI-3 molecular structure would be preserved in the Chilean field strains.
ACKNOWLEDGMENTS
This work was supported by grant ADI 08/2006 from Programma Bicentenario de Ciencia y Technología, CONICYT, Chile.
We thank Minie Villarroel and Patricia Lopetegui from Servicio Agricola y Ganadero (SAG) for providing the B. abortus field strains.
Footnotes
Published ahead of print on 4 May 2011.
REFERENCES
- 1. Al Dahouk S., et al. 2007. Evaluation of Brucella MLVA typing for human brucellosis. J. Microbiol. Methods 69:137–145 [DOI] [PubMed] [Google Scholar]
- 2. Bricker B. 2004. Molecular diagnostics of animal brucellosis: a review of PCR-based assays and approaches, p. 25–51 In López-Goñi I., Moriyón I. (ed.), Brucella: molecular and cellular biology. Horizon Bioscience, Wymondham, United Kingdom [Google Scholar]
- 3. Bricker B. J., Ewalt D. R., MacMillan A. P., Foster G., Brew S. 2000. Molecular characterization of Brucella strains isolated from marine mammals. J. Clin. Microbiol. 38:1258–1262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bricker B. J., Halling S. M. 1994. Differentiation of Brucella abortus bv. 1, 2, and 4, Brucella melitensis, Brucella ovis, and Brucella suis bv. 1 by PCR. J. Clin. Microbiol. 32:2660–2666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bricker B. J., Halling S. M. 1995. Enhancement of the Brucella AMOS PCR assay for differentiation of Brucella abortus vaccine strains S19 and RB51. J. Clin. Microbiol. 33:1640–1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cloeckaert A., Grayon M., Grepinet O. 2000. An IS711 element downstream of the bp26 gene is a specific marker of Brucella spp. isolated from marine mammals. Clin. Diagn. Lab. Immunol. 7:835–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cloeckaert A., Verger J. M., Grayon M., Grepinet O. 1995. Restriction site polymorphism of the genes encoding the major 25 kDa and 36 kDa outer-membrane proteins of Brucella. Microbiology 141:2111–2121 [DOI] [PubMed] [Google Scholar]
- 8. Corbel M. J. 1997. Brucellosis: an overview. Emerg. Infect. Dis. 3:213–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gandara B., Merino A. L., Rogel M. A., Martinez-Romero E. 2001. Limited genetic diversity of Brucella spp. J. Clin. Microbiol. 39:235–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Garcia-Yoldi D., et al. 2007. Comparison of MLVA with other PCR-based methods for typing Brucella suis isolates. J. Clin. Microbiol. 45:4070–4072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Guzman-Verri C., et al. 2002. The two-component system BvrR/BvrS essential for Brucella abortus virulence regulates the expression of outer membrane proteins with counterparts in members of the Rhizobiaceae. Proc. Natl. Acad. Sci. U. S. A. 99:12375–12380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hacker J., Kaper J. B. 2000. Pathogenicity islands and the evolution of microbes. Annu. Rev. Microbiol. 54:641–679 [DOI] [PubMed] [Google Scholar]
- 13. Halling S. M., et al. 2005. Completion of the genome sequence of Brucella abortus and comparison to the highly similar genomes of Brucella melitensis and Brucella suis. J. Bacteriol. 187:2715–2726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hunter P., Gaston M. 1988. Numerical index of the discriminatory ability of typing systems: an application of Simpson's index of diversity. J. Clin. Microbiol. 26:2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jahans K., Foster G., Broughton E. 1997. The characterisation of Brucella strains isolated from marine mammals. Vet. Microbiol. 57:373–382 [DOI] [PubMed] [Google Scholar]
- 16. Jensen A. E., Cheville N. F., Thoen C. O., MacMillan A. P., Miller W. G. 1999. Genomic fingerprinting and development of a dendrogram for Brucella spp. isolated from seals, porpoises, and dolphins. J. Vet. Diagn. Invest. 11:152–157 [DOI] [PubMed] [Google Scholar]
- 17. Jensen A. E., Ewalt D. R., Cheville N. F., Thoen C. O., Payeur J. B. 1996. Determination of stability of Brucella abortus RB51 by use of genomic fingerprint, oxidative metabolism, and colonial morphology and differentiation of strain RB51 from B. abortus isolates from bison and elk. J. Clin. Microbiol. 34:628–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jumas-Bilak E., Michaux-Charachon S., Bourg G., O'Callaghan D., Ramuz M. 1998. Differences in chromosome number and genome rearrangements in the genus Brucella. Mol. Microbiol. 27:99–106 [DOI] [PubMed] [Google Scholar]
- 19. Kattar M., et al. 2008. Evaluation of a multilocus variable-number tandem-repeat analysis scheme for typing human Brucella isolates in a region of brucellosis endemicity. J. Clin. Microbiol. 46:3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Le Flèche P., et al. 2006. Evaluation and selection of tandem repeat loci for a Brucella MLVA typing assay. BMC Microbiol. 6:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lopetegui P. 2005. Avances de la erradicación de la brucelosis bovina en Chile, vol. 3, p. 1–14 Boletín Veterinario Oficial, Servicio Agrícola y Ganadero, Santiago, Chile [Google Scholar]
- 22. Mancilla M., Villarroel M., Saldías M., Soto J., Zárraga A. 2008. Genotipos de aislados de campo de Brucella abortus de distintas regiones geográficas de Chile. Arch. Med. Vet. 40:187–192 [Google Scholar]
- 23. Moreno E. 2002. Brucellosis in Central America. Vet. Microbiol. 90:31–38 [DOI] [PubMed] [Google Scholar]
- 24. Moreno E., Moriyon I. 2002. Brucella melitensis: a nasty bug with hidden credentials for virulence. Proc. Natl. Acad. Sci. U. S. A. 99:1–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Paulsen I., et al. 2002. The Brucella suis genome reveals fundamental similarities between animal and plant pathogens and symbionts Proc. Natl. Acad. Sci. U. S. A. 99:13148–13153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rajashekara G., Covert J., Petersen E., Eskra L., Splitter G. 2008. Genomic island 2 of Brucella melitensis is a major virulence determinant: functional analyses of genomic islands. J. Bacteriol. 190:6243–6252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rajashekara G., Glasner J. D., Glover D. A., Splitter G. A. 2004. Comparative whole-genome hybridization reveals genomic islands in Brucella species. J. Bacteriol. 186:5040–5051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ratushna V. G., et al. 2006. Molecular targets for rapid identification of Brucella spp. BMC Microbiol. 6:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ren C. P., et al. 2004. The ETT2 gene cluster, encoding a second type III secretion system from Escherichia coli, is present in the majority of strains but has undergone widespread mutational attrition. J. Bacteriol. 186:3547–3560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ridler A. L., Leyland M. J., Fenwick S. G., West D. M. 2005. Demonstration of polymorphism among Brucella ovis field isolates by pulsed-field gel electrophoresis. Vet. Microbiol. 108:69–74 [DOI] [PubMed] [Google Scholar]
- 31. Rivera S. A., Ramirez M. C., Lopetegui I. P. 2002. Eradication of bovine brucellosis in the 10th region of Los Lagos, Chile. Vet. Microbiol. 90:45–53 [DOI] [PubMed] [Google Scholar]
- 32. Roth F., et al. 2003. Human health benefits from livestock vaccination for brucellosis: case study. Bull. World Health Organ. 81:867–876 [PMC free article] [PubMed] [Google Scholar]
- 33. Samartino L. E. 2002. Brucellosis in Argentina. Vet. Microbiol. 90:71–80 [DOI] [PubMed] [Google Scholar]
- 34. Sangari F. J., Aguero J. 1994. Identification of Brucella abortus B19 vaccine strain by the detection of DNA polymorphism at the ery locus. Vaccine 12:435–438 [DOI] [PubMed] [Google Scholar]
- 35. Tenover F. C., et al. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233–2239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Van de Peer Y., De Wachter R. 1994. TREECON for windows: a software package for the construction and drawing of evolutionary trees for Microsoft Windows environment Comput. Appl. Biosci. 10:569–570 [DOI] [PubMed] [Google Scholar]
- 37. Verger J., Grimont F., Grimont P. A. D., Grayon M. 1985. Brucella a monospecific genus as shown by deoxyribonucleic acid hybridization. Int. J. Syst. Bacteriol. 35:292–295 [Google Scholar]
- 38. Vizcaino N., Caro-Hernandez P., Cloeckaert A., Fernandez-Lago L. 2004. DNA polymorphism in the omp25/omp31 family of Brucella spp.: identification of a 1.7-kb inversion in Brucella cetaceae and of a 15.1-kb genomic island, absent from Brucella ovis, related to the synthesis of smooth lipopolysaccharide. Microbes Infect. 6:821–834 [DOI] [PubMed] [Google Scholar]
- 39. Vizcaino N., Verger J. M., Grayon M., Zygmunt M. S., Cloeckaert A. 1997. DNA polymorphism at the omp-31 locus of Brucella spp.: evidence for a large deletion in Brucella abortus, and other species-specific markers. Microbiology 143:2913–2921 [DOI] [PubMed] [Google Scholar]
- 40. Whatmore A. M., et al. 2005. Use of amplified fragment length polymorphism to identify and type Brucella isolates of medical and veterinary interest. J. Clin. Microbiol. 43:761–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zygmunt M. S., Blasco J. M., Letesson J. J., Cloeckaert A., Moriyon I. 2009. DNA polymorphism analysis of Brucella lipopolysaccharide genes reveals marked differences in O-polysaccharide biosynthetic genes between smooth and rough Brucella species and novel species-specific markers. BMC Microbiol. 9:92. [DOI] [PMC free article] [PubMed] [Google Scholar]