Abstract
Serum response factor (SRF) is a ubiquitously expressed transcription factor that regulates cell-specific functions such as muscle development and breast cancer metastasis. The myocardin-related transcription factors (MRTFs), which are transcriptional coactivators mediating cell-specific functions of SRF, are also ubiquitously expressed. How MRTFs and SRF drive cell-specific transcription is still not fully understood. Here we show that LIM domain only 7 (LMO7) is a cell-specific regulator of MRTFs and plays an important role in breast cancer cell migration. LMO7 activates MRTFs by relieving actin-mediated inhibition in a manner that requires, and is synergistic with, Rho GTPase. Whereas Rho is required for LMO7 to activate full-length MRTFs that have three RPEL actin-binding motifs, the disruption of individual actin-RPEL interactions is sufficient to eliminate the Rho dependency and to allow the strong Rho-independent function of LMO7. Mechanistically, we show that LMO7 colocalizes with F-actin and reduces the G-actin/F-actin ratio via a Rho-independent mechanism. The knockdown of LMO7 in HeLa and MDA-MB-231 cells compromises both basal and Rho-stimulated MRTF activities and impairs the migration of MDA-MB-231 breast cancer cells. We also show that LMO7 is upregulated in the stroma of invasive breast carcinoma in a manner that correlates with the increased expression of SRF target genes that regulate muscle and actin cytoskeleton functions. Together, this study reveals a novel cell-specific mechanism regulating Rho-MRTF-SRF signaling and breast cancer cell migration and identifies a role for actin-RPEL interactions in integrating Rho and cell-specific signals to achieve both the synergistic and Rho-dependent activation of MRTFs.
INTRODUCTION
Serum response factor (SRF) is a mammalian MADS-type transcription factor that recognizes the consensus sequence CC(A/T)2A(A/T)3GG known as the CArG element (8, 35, 42, 57, 58). SRF regulates the transcription of immediate-early genes (IEGs), such as c-fos, and muscle-specific genes as well as genes involved in the regulation of the cytoskeleton, motility, and adhesion. Depending on the promoter context, SRF utilizes different coactivators to regulate the transcription of target genes (see Fig. 1A). In the case of IEGs, such as c-fos, the promoter region often contains an additional binding site termed Ets. This facilitates the formation of a ternary complex containing CArG-bound SRF and Ets-bound ternary complex factor (TCF). TCF in this complex serves the coactivator function. The activity of TCF is regulated by mitogen-activated protein (MAP) kinase-dependent phosphorylation, which converts TCF from a repressor into a potent activator.
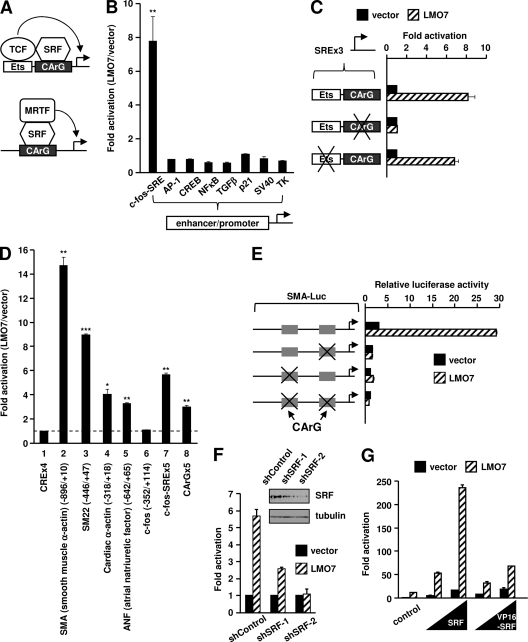
Fig. 1.
LMO7 specifically increases luciferase reporter transcription driven by the CArG-binding site of muscle-specific SRF target gene promoters. (A) Schematic models showing the two types of SRF coactivators involved in the regulation of IEGs and muscle-specific genes. (B) Luciferase assays of 293T cells showing the effect of ectopic LMO7 on the activities of various reporters. The fold activation by LMO7 was calculated as the ratio of LMO7 to empty vector for each reporter. (C) CArG, but not Ets, is important for LMO7-dependent activation. At the left is a schematic representation of wild-type and mutant SREx3-Luc reporters. “×” indicates mutated sites. The fold activation for each reporter was relative to the activation observed with the empty vector, which was set to 1. (D) Luciferase assays of 293T cells transfected with LMO7 or empty vector along with various reporters indicated at the bottom. For muscle gene and c-fos promoters, the numbers in the parentheses indicate the 5′ and 3′ boundaries of the promoter fragment relative to the transcription start site. The fold activation by LMO7 was normalized to the activation of CRE-Luc, which was set to 1. (E) Luciferase assays of 293T cells transfected with LMO7 or empty vector along with wild-type or CArG-deleted SMA reporters. (F) Luciferase assays of 293T cells transfected with the SMA-Luc reporter along with LMO7 or empty vector under conditions of cotransfection with either control shRNA (shControl) or SRF-specific shRNAs (shSRF-1 and shSRF-2). (G) Luciferase assays of 293T cells transfected with SMA-Luc, LMO7, or empty vector and increasing amounts of SRF or VP16-SRF.
A distinct type of SRF coactivators is involved in the regulation of muscle-specific genes. These include myocardin, a muscle-specific protein, and its related proteins, myocardin-related transcription factors (MRTFs), which are ubiquitously expressed proteins (29, 30, 42). MRTFs include two highly related members encoded by different genes, namely, MRTF-A (also called MAL, MKL1, or BSAC) and MRTF-B (also called MKL2). Like TCFs, MRTFs are also inactive in the basal state. The inactivation of MRTFs is achieved by their association with monomeric G-actin through the conserved actin-binding motifs (RPEL1 to RPEL3) located in the N-terminal region of MRTFs. The binding of MRTFs to G-actin promotes their nuclear export while inhibiting their nuclear import. Recent studies have also shown that in the nucleus, actin binding interferes directly with the ability of MRTFs to activate transcription (61). In addition to muscle-related genes, MRTFs have also been shown to regulate IEGs (7, 23, 50).
Rho signaling is responsible for the activation of MRTFs (29, 30, 42). Rho is activated in response to various extracellular stimuli such as growth factors. Activated Rho engages multiple downstream effectors to act collaboratively to stabilize actin filaments and to stimulate G-actin polymerization. These activities cause a depletion of G-actin, thereby allowing MRTF to activate SRF-dependent transcription. The deregulation of MRTF activity has been implicated in various diseases (48). For instance, it was proposed previously that aberrantly increased MRTF/SRF activity enhances the migration potential of breast cancer (BC) cells (5, 27).
LIM domain only 7 (LMO7) is a mammalian protein containing a LIM domain and other evolutionarily conserved domains, which suggests that LMO7 functions in the regulation of cell adhesion and signaling (36, 64). The expression of LMO7 is cell type specific (13, 19, 25, 44, 46, 47). Expressed early in muscle and heart, LMO7 is essential for the development of these tissues and has been implicated in Emery-Dreifuss muscular dystrophy (17, 37, 44). Another important aspect of the biological function of LMO7 is its potential involvement in cancer metastasis. LMO7 is upregulated in multiple cancers, especially at the metastatic stage, whereas its normal expression is low and limited to very few tissues (13, 19, 47). In cultured rat ascites hepatoma cells, the upregulation of LMO7 correlates with the ability of transforming growth factor β (TGFβ) to enhance the invasiveness of these cells (33). In addition, LMO7 has been identified as a breast cancer signature gene specifically expressed in metastatic breast cancer cells (38).
Here, using a candidate-based approach, we tested the hypothesis that LMO7 exerts its function by regulating signal-dependent transcription in a cell-specific manner. Our study unexpectedly reveals a role for LMO7 in the regulation of actin dynamics and links LMO7 to the Rho-dependent MRTF-SRF signaling pathway. The LMO7 expression level is elevated in MDA-MB-231 breast cancer cells, where it plays an important role in the migration of these cells. LMO7 is also upregulated in the stroma of invasive breast tumors, and this upregulation correlates with increased expression levels of a large network of SRF target genes involved in the regulation of the actin cytoskeleton, motility, migration, and adhesion. In addition, our study reveals a novel function of the actin-binding RPEL regions in integrating distinct upstream signals to allow the synergistic activation of MRTFs without a loss of the Rho-dependent control of MRTF activity.
MATERIALS AND METHODS
Cloning of human full-length LMO7.
By aligning LMO7 sequences available in the GenBank database, we found that the putative human LMO7 (Swiss-Prot database accession number Q8WWI1) was not a full-length protein. It lacks an evolutionarily conserved N-terminal region that exists in LMO7 proteins from other species (see Fig. S1 at http://cellbiology.uc.edu/files/jinsongzhanglab/Hu_MCB_supplemental_figures_tables.pdf). Human full-length LMO7 cDNA was then constructed from three partial cDNA clones (GenBank accession numbers BE270452, N31885, and AB020665) by using overlapping PCR.
Plasmids.
LMO7 was fused to a hemagglutinin (HA) tag at the N terminus and was expressed from the cytomegalovirus (CMV) promoter. Expression vectors encoding MAL(met) and C630 were gifts of R. Prywes (Columbia University) and were previously described (7). Full-length Elk-1 and its Ets domain-deleted derivative (Elk-1ΔEts) were gifts of A. Sharrocks (University of Manchester) and were previously described (65). The expression vector encoding ΔN100 was derived from Addgene plasmid 19848 and was previously described (7). The expression vector encoding full-length MAL [MAL(fl)] was constructed by overlapping PCR using the following two templates: (i) the MAL(met) plasmid described above and (ii) a MAL expressed sequence tag (EST) clone containing an intact N terminus. RPEL domain mutants were generated by PCR. The expression vectors for MRTF-B and myocardin were derived from full-length EST cDNA clones. Expression vectors for RhoA-L63 and the C3 exoenzyme were gifts of Deniz Toksoz (Tufts Medical Center) (28). VP16-SRF and VP16-LMO7 have the VP16 activation domain fused to the N terminus of full-length SRF and full-length LMO7, respectively. Luciferase reporters for smooth muscle (SM) α-actin (SMA), SM22, cardiac α-actin, atrial natriuretic factor, and c-fos promoters were gifts of R. Prywes and were previously described (7). SREx5-Luc, AP1-Luc, NFκB-Luc, cyclic AMP response element (CRE)-Luc, and TGFβ-Luc (also called TARE-Luc) reporters were obtained from Stratagene. p21-Luc contains the 2.1-kb promoter of the p21WAF1 gene (43). SV40-Luc and TK-Luc are driven by the simian virus 40 (SV40) early promoter and the herpes simplex virus (HSV) TK promoter, respectively (15, 66). For the disruption of Ets- and CArG-binding sites in the c-fos serum response element (SRE) (5′-AGGATGTCCATATTAGGACATCT-3′), the wild-type Ets sequence (AGGA) was mutated to AAAA, and the wild-type CArG sequence (CCATATTAGG) was mutated to AAATATTAAA. Three consecutive copies of the wild-type or mutant c-fos SRE sequences were inserted into the multiple-cloning site of the pLuc-MCS empty vector (Stratagene). The SM α-actin promoters with deletions in the two CArG boxes were constructed by overlapping PCR. All constructs and mutations were confirmed by direct DNA sequencing.
Antibodies and chemicals.
Anti-HA and anti-Myc antibodies were obtained from Covance. Anti-SRF and anti-MKL2 antibodies for Western blotting were obtained from Santa Cruz. Anti-MAL antibody for Western blotting was obtained from Bethyl Laboratories. Mouse monoclonal antitubulin antibody was obtained from Active Motif. Mouse polyclonal anti-LMO7 antibody was obtained from Abnova. Anti-MAL, anti-MRTF-B, and anti-LMO7 antibodies for immunofluorescence staining were prestige antibodies from Sigma. Antiactin antibodies were obtained from Millipore and Sigma. Anti-Rho antibody was obtained from Cell Signaling Technology. Anti-polymerase II (anti-Pol II) (catalog number 05-623B) and anti-trimethyl-histone H3K4 (catalog number CS200580) antibodies were obtained from Millipore. Alexa 488-DNase I, Alexa 488-phalloidin and Texas Red-X–phalloidin were obtained from Invitrogen. l-α-Lysophosphatidic acid (LPA) was obtained from Sigma. Latrunculin B (LatB) was obtained from Enzo Life Sciences.
Cell culture, transfection, and luciferase activity assay.
293T, MCF7, HeLa, and MDA-MB-231 cells were maintained in Dulbecco's modified Eagle's medium (DMEM) with 10% fetal bovine serum (FBS). For luciferase assays of 293T cells grown in 24-well plates, FuGENE 6 transfection reagent (Roche) was used. 293T cells were transfected with 15 ng of reporter, 10 ng of β-galactosidase expression vector, and 340 ng of LMO7, unless otherwise noted. For luciferase assays with MCF7, HeLa, and MDA-MB-231 cells, TurboFect transfection reagent (Fermentas) was used. Empty vectors were used to achieve equal amounts of total plasmid DNA. The luciferase activity was measured at 24 h posttransfection and normalized to β-galactosidase activity, which served as the internal control for transfection efficiency. Fold activation was determined relative to the activity of empty vector controls unless otherwise indicated. For serum stimulation, at 16 h posttransfection, the cells were serum starved for 24 h in DMEM containing 0.2% FBS. This was followed by stimulation with various concentrations of fetal bovine serum for 5 h. All assays were performed in duplicate and were repeated at least three times. The averages and standard errors of data from duplicate samples from representative assays are shown.
Immunofluorescence.
293T cells grown on 18-mm glass coverslips were transiently transfected with 680 ng of the LMO7 construct or the empty vector, along with 6.5 ng FLAG-tagged MAL(fl), MAL(met), or MALΔN100, using TurboFect transfection reagent (Fermentas). For serum starvation, cells were switched to 0.2% FBS at 16 h posttransfection and starved for at least 24 h before the assay. For LatB treatment, 1.5 μM LatB was added to the medium 12 h before the assay. For the C3 effect, 7.5 ng C3 was cotransfected unless otherwise noted. Immunofluorescence staining was performed by using anti-FLAG antibody on cells fixed with 4% formaldehyde. DAPI (4′,6′-diamino-2-phenylindole) was used to stain nuclear DNA. Cells were examined and imaged by using a Zeiss Axioplan 2 imaging fluorescence microscope equipped with AxioVision software. Immunofluorescence staining of HeLa and MDA-MB-231 cells was performed similarly, except that MDA-MB-231 cells were fixed with methanol-acetone (1:3) for staining with LMO7 antibody. Confocal microscopy was performed with a Carl Zeiss LSM 700 laser scanning microscope.
ChIP.
Chromatin immunoprecipitation (ChIP) experiments were performed by using the EZ-ChIP kit (Millipore), with minor modifications. In brief, transfected 293T cells grown in 15-cm tissue dishes were cross-linked with 1% formaldehyde for 10 min at room temperature, and the reaction was then stopped by the addition of glycine to a final concentration of 0.125 M and the incubation of the mixture for 15 min at room temperature. Fixed cells were rinsed twice with phosphate-buffered saline (PBS) and scraped into 2 ml cell lysis buffer [5 mM piperazine-N,N′-bis(2-ethanesulfonic acid) (PIPES) (pH 8), 85 mM KCl, 0.5% NP-40]. Following centrifugation, the pellets were resuspended in 1 ml SDS lysis buffer. About 1.5 × 106 cell equivalents of chromatin were used for each immunoprecipitation. Quantitative PCR (qPCR) was performed by using Power SYBR green PCR master mix (Applied Biosystems). Chromatin binding was calculated as the percentage of immunoprecipitated DNA relative to the amount of input. The following primers were used for qPCR: 5′-AGCAGAACAGAGGAATGCAGTGGAAGAGAC-3′ and 5′-CCTCCCACTCGCCTCCCAAACAAGGAGC-3′ for SMA and 5′-TGCTCACGAGATTAGGACACGCGCCAAG-3′ and 5′-CCAACCGCATCTGCAGCGAGCATCT-3′ for c-fos.
Quantitative reverse transcription-PCR (RT-qPCR).
RNA was extracted by using Trizol reagent (Invitrogen). One microgram of total RNA was reverse transcribed into cDNA by using the High Capacity cDNA reverse transcription kit (Applied Biosystems). qPCR was performed by using Power SYBR green PCR master mix (Applied Biosystems). Primer sequences are listed in Table S1 at http://cellbiology.uc.edu/files/jinsongzhanglab/Hu_MCB_supplemental_figures_tables.pdf.
shRNA-mediated knockdown.
pLKO.1 short hairpin RNA (shRNA) plasmids were obtained from Sigma. For the infection of HeLa or MDA-MB-231 cells, lentiviruses were produced in 293T cells by calcium phosphate transfection. Forty-eight hours later, lentiviruses were collected to infect HeLa or MDA-MB-231 cells. The infected cells were split after 24 h and selected in 3 μg/ml puromycin for 1 week. For the knockdown of SRF in 293T cells, cells grown in 6-well dishes were transfected with SRF shRNAs and selected in 3 μg/ml puromycin for 1 day. The cells were then split into a 24-well plate and used for luciferase assays. shRNA sequences are listed in Table S2 at http://cellbiology.uc.edu/files/jinsongzhanglab/Hu_MCB_supplemental_figures_tables.pdf.
Cell migration assay.
The cell migration assay was performed by using the BD Falcon HTS multiwell insert system (pore size, 8 μm). The assay was initiated by the addition of 1 × 105 MDA-MB-231 cells in serum-free DMEM to the insert, and DMEM containing 10% FBS was added to the bottom wells. After 6 h of incubation, the cells remaining in the insert were scraped off by using cotton swabs. Migrated cells at the bottom of the insert were fixed with 4% formaldehyde for 20 min, stained with 0.1% crystal violet for 30 min, and counted by microscopic examination using a 20× objective.
FACS quantitation of F-actin and G-actin contents.
The protocol for fluorescence-activated cell sorter (FACS) quantitation of F-actin and G-actin contents was previously described (41). Briefly, 293T cells grown in 60-mm plates were transfected with 6 μg of LMO7 or the control vector alone, or cotransfected with 100 ng of C3, by using TurboFect transfection reagent (Fermentas). At 20 h posttransfection, cells were trypsinized, fixed with 4% paraformaldehyde, permeabilized with 0.2% Triton X-100, and stained with Alexa 488-phalloidin or Alexa 488-DNase I. FACS analysis was performed by using a FACSCalibur instrument (BD Biosciences) with CellQuest Pro software. The mean Alexa 488 fluorescence was calculated by using FlowJo.
Actin fractionation.
The experiments for actin fractionation were performed according to a previously described protocol (41). The transfection of 293T cells was performed as described above for the FACS quantitation of F-actin and G-actin contents. F-actin-enhancing solution, which contains phalloidin, and actin antibody were obtained from Cytoskeleton. The levels of G-actin and F-actin were quantified by using NIH ImageJ software (http://rsb.info.nih.gov/ij/) (1).
Immunoprecipitation.
The immunoprecipitation procedure was previously described (15). TurboFect transfection reagent (Fermentas) was used to transfect MAL(Fl) (180 ng), LMO7 (6 μg), and RhoA-L63 (900 ng) into 293T cells grown in 60-mm plates.
Statistical analysis.
A Student t test was used to test for the statistical significance of the differences between the different group parameters.
Identification and analysis of genes that exhibit expression patterning that is highly similar to that of LMO7 in invasive BC stroma.
Raw data from NCBI GEO data set GSE9014 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE9014) (10) were used to carry out detailed microarray expression analyses. This data set consists of 123 microarrays derived from laser capture-isolated tumor and normal stroma samples from 53 invasive breast cancer (BC) samples and 6 controls. Probes were identified which exhibited an Agilent Feature Extraction raw signal intensity of 500 or greater with at least 5 samples (33,545 probes), a tumor-versus-normal P value false discovery rate (FDR) of <5% (27,691 probes) (Benjamini-Hochberg FDR), and a Pearson correlation to LMO7 probe A_24_P301146 of greater than 0.717 (376 probes), to TAGLN probe A_23_P87011 of greater than 0.734 (376 probes), or to CNN1 probe A_23_P125233 of greater than 0.821 (354 probes). A total of 1,152 probes, 60 of which were shared by two of the three inputs, corresponding to 846 unique Entrez genes whose expression correlations were then calculated relative to that of LMO7, were identified (http://cellbiology.uc.edu/files/jinsongzhanglab/Hu_MCB_supplemental_excel_file.xls). Enrichments of gene- and protein-associated features of the LMO7-correlated genes were performed with the Toppgene server (http://Toppgene.cchmc.org/). A multidimensional network view of LMO7-correlated genes associated with actin cytoskeleton function was generated by using enrichment analysis through the Toppcluster server (http://Toppcluster.cchmc.org/) (18) and rendered in Cytoscape.
Nucleotide sequence accession number.
The derived full-length cDNA sequence, which encodes 1,631 amino acids, has been deposited in the GenBank database under accession number FJ711162.
RESULTS
LMO7 specifically enhances reporter gene transcription driven by the CArG-binding site.
A full-length cDNA encoding human LMO7 was constructed to facilitate the study of LMO7 function in human cells (for details, see Materials and Methods and see Fig. S1 at http://cellbiology.uc.edu/files/jinsongzhanglab/Hu_MCB_supplemental_figures_tables.pdf). The ectopic expression of LMO7 in 293T cells, which do not express endogenous LMO7 (see Fig. 6A), specifically increased reporter activity controlled by tandem c-fos serum response elements (SREs) but showed no effect on other randomly selected reporters (Fig. 1B). The specific activation of c-fos SRE was further confirmed by a dose-titration experiment (see Fig. S2 at the URL mentioned above).
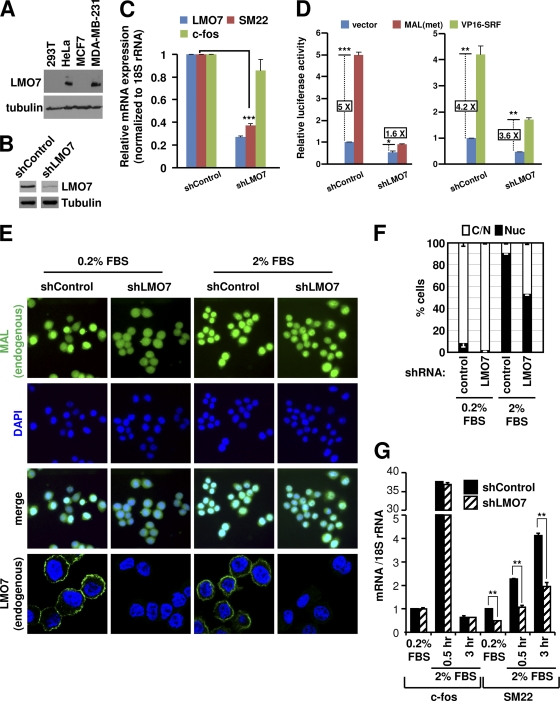
Fig. 6.
Endogenous LMO7 is important for MRTF function in HeLa cells. (A) Western blot analysis of LMO7 expression levels in different cells using anti-LMO7 antibody. (B) Western blot analysis of LMO7 levels in knockdown and control HeLa cells using anti-LMO7 antibody. (C) RT-qPCR analysis of expressions of LMO7, SM22, and c-fos in LMO7 knockdown or control HeLa cells. (D) Luciferase assays of LMO7 knockdown and control HeLa cells transfected with SM22-Luc and the indicated plasmids. (E) Subcellular localization of endogenous MAL and LMO7 in control or LMO7 knockdown HeLa cells under serum-starved and serum-stimulated conditions. (F) Percentage of cells with whole-cell (C/N) or nuclear (Nuc) localization of endogenous MAL in control or LMO7 knockdown HeLa cells under serum-starved or serum-stimulated conditions. Averages and standard errors were derived from two independent experiments. For each experiment, a total of 200 cells were examined. (G) RT-qPCR analysis of SM22 expression in LMO7 knockdown and control HeLa cells under serum-starved or serum-stimulated conditions. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
We next determined that the CArG element of the c-fos SRE was responsible for LMO7-mediated activation. CArG and Ets binding sites were mutated to disrupt their respective bindings to SRF and TCF (12, 24). Mutations of CArG, but not of Ets, completely abolished LMO7-dependent activation (Fig. 1C). These results indicate that the binding of SRF to CArG, but not the binding of TCF to Ets, was required for LMO7-dependent activation. In another experiment, we found that the overexpression of a TCF protein (Elk-1) abolished LMO7-mediated activation (see Fig. S3 at http://cellbiology.uc.edu/files/jinsongzhanglab/Hu_MCB_supplemental_figures_tables.pdf). A similar inhibition was observed with an Elk-1 derivative (Elk-1ΔEts) that cannot bind DNA but retains the ability to bind SRF (see the URL mentioned above). We conclude that the binding of TCF to SRF disables the ability of SRF to respond to LMO7.
LMO7 activates muscle-specific gene promoters.
In view of the known involvement of LMO7 in the regulation of muscle and heart, we reasoned that LMO7 should similarly activate muscle- and cardiac-specific SRF target promoters. This was tested by using luciferase reporter assays (Fig. 1D). LMO7 increased the activities of muscle and heart gene promoters known to be regulated by SRF (50, 62), with the smooth muscle α-actin (SMA) promoter showing the greatest effect (Fig. 1D, lane 2). We also tested a natural c-fos promoter that contains only one SRE. Interestingly, unlike SREx5-Luc containing multiple c-fos SRE sites, the natural c-fos promoter was not activated by LMO7 (Fig. 1D, lane 6). This result suggests that LMO7 preferentially targets cell-specific, rather than ubiquitous, SRF target promoters. The lack of an LMO7-mediated activation of the c-fos promoter could be due to two reasons. First, the single SRE in the c-fos promoter may favor the recruitment of a TCF-SRF complex, which cannot be activated by LMO7, as shown above. Second, LMO7 may preferentially activate promoters containing multiple CArGs. Indeed, we confirmed that LMO7 can activate a reporter driven by five CArG elements (Fig. 1D, lane 8) but was unable to activate a reporter driven by a single c-fos SRE (data not shown).
A multiplicity of CArG sites is a common feature of muscle-related SRF target genes (30, 39). In the case of the SMA promoter, we confirmed that both of its CArG elements were important for the promoter's responsiveness to LMO7 (Fig. 1E). Interestingly, deletions of CArG sites in the SMA promoter not only abolished its responsiveness to LMO7 but also reduced its basal level of transcription (Fig. 1E, black bars). This indicates that SRF, possibly by responding to basal cellular signaling, also plays a role in maintaining basal promoter activity. The critical role for SRF in mediating LMO7-dependent promoter activation was also confirmed by knockdown experiments (Fig. 1F).
LMO7 activates MRTFs by targeting the actin-binding RPEL motifs.
Western blot analysis showed that LMO7 did not change the expression level of endogenous SRF (see Fig. S4 at http://cellbiology.uc.edu/files/jinsongzhanglab/Hu_MCB_supplemental_figures_tables.pdf). We therefore tested whether LMO7 increases SRF's transcriptional activity or potentiates its intrinsic ability to bind to DNA. The data supported the former but not the latter possibility. When SRF was fused to the potent VP16 activation domain, the resulting fusion protein was much less sensitive to LMO7-mediated activation (Fig. 1G), although LMO7 was still able to weakly stimulate VP16-SRF, which could reflect a contribution of the increased SRF activity induced by LMO7 to the overall activity of the fusion protein.
We also ruled out the possibility that LMO7 regulates SRF-dependent transcription by directly binding to the promoter region. Thus, the fusion of VP16 to LMO7 did not increase LMO7's ability to stimulate transcription (see Fig. S5 at http://cellbiology.uc.edu/files/jinsongzhanglab/Hu_MCB_supplemental_figures_tables.pdf). The absence of promoter binding by LMO7 was also confirmed by ChIP (see Fig. 5C and see Fig. S11 at the URL mentioned above). These results, along with the lack of TCF involvement, led us to explore the role of MRTFs in LMO7-mediated activation. Two MAL derivatives were used for this purpose (Fig. 2A, right). The first was C630, a potent dominant negative mutant of MAL created by the deletion of the C-terminal activation domain (7). The second derivative, designated MAL(met), lacks the actin-binding RPEL1 motif and is partially active in transcription (7, 31). Confirming our hypothesis, C630 abolished the ability of LMO7 to activate reporter transcription similarly to the inhibition observed with MAL(met) (Fig. 2A). Using a previously validated shRNA that targets both forms of MRTFs (27), we further showed that the knockdown of endogenous MRTFs strongly reduced LMO7-mediated activation (Fig. 2B). Together, these results confirmed the critical importance of MRTFs in the LMO7-mediated activation of SRF-dependent transcription.
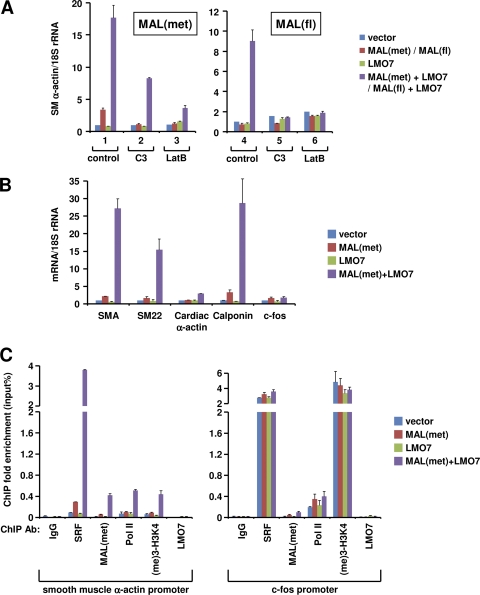
Fig. 5.
LMO7 enhances the ability of MAL to activate endogenous SRF target genes. (A) RT-qPCR analysis of endogenous SMA expression in 293T cells transfected with the indicated plasmids in the absence or presence of C3 or LatB. SMA expression was normalized to 18S rRNA levels. (B) RT-qPCR analysis of SRF target genes in 293T cells transfected with the same plasmids as those used for panel A. (C) ChIP analysis of SMA and c-fos promoters to determine the binding of SRF, MAL(met), Pol II, and LMO7 and the level of histone H3K4 trimethylation in transfected 293T cells.
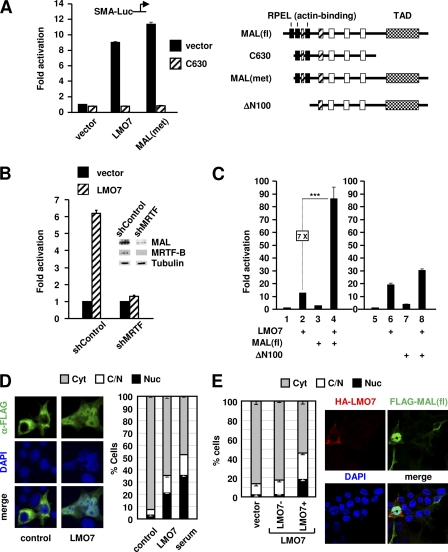
Fig. 2.
LMO7 activates MAL by targeting the RPEL motifs. (A) Luciferase assays of 293T cells transfected with SMA-Luc along with the plasmids indicated at the bottom as well as C630 or vector, as indicated. At the right is a schematic representation of full-length MAL [MAL(fl)] and derivatives used in panels A and C. Black rectangles represent RPEL motifs. TAD, C-terminal transcriptional activation domain. Also shown are other conserved domains of MRTFs. (B) Knockdown of MRTFs abolished LMO7-mediated activation of SMA-Luc. (C) Luciferase assays of 293T cells transfected with SMA-Luc along with expression vectors indicated at the bottom. Lanes 1 to 4 and 5 to 8 were from two different experiments. ***, P < 0.001. (D, left) Representative immunofluorescence images using anti-FLAG antibody showing subcellular localization of FLAG-tagged MAL(fl) in 293T cells cotransfected with LMO7 or empty vector (control). DAPI was used to stain the nucleus. (Right) Percentage of cells with predominant cytoplasmic (Cyt), whole-cell (C/N), or nuclear (Nuc) localization of MAL(fl) under various conditions, as indicated. Averages and standard errors were derived from two independent experiments. Upon transfection, the cells were serum starved for 24 h, followed by staining. For the effect of serum stimulation, cells were treated with 10% serum for 1 h before staining. For each experiment, a total of 150 transfected cells were examined. (E) HA-LMO7- and FLAG-MAL(fl)-cotransfected cells were stained with both anti-HA and anti-FLAG antibodies. The subcellular localization of MAL(fl) in the presence or absence of LMO7 expression was determined as described above (D). Vector-transfected cells were similarly assayed.
To determine whether LMO7 induces MRTF activity directly, we transfected 293T cells with MAL(fl) (Fig. 2A, right) alone or together with LMO7. Despite the weak activity of MAL(fl) (Fig. 2C, lane 3), which is consistent with the presence of three RPELs, the coexpression of MAL(fl) and LMO7 resulted in a robust and synergistic activation of reporter transcription (lane 4). A similar result was obtained when MRTF-B and LMO7 were coexpressed (see Fig. S6 at http://cellbiology.uc.edu/files/jinsongzhanglab/Hu_MCB_supplemental_figures_tables.pdf). Together with the C630 and the MRTF knockdown data (Fig. 2A and B), our results show that LMO7 can activate both MTRF proteins and that the activity observed with LMO7 alone reflects its activation of endogenous MRTFs.
We next determined that the ability of MAL to respond to LMO7 was dependent on its actin-binding domains, as shown by the lack of a synergistic effect between LMO7 and ΔN100, a MAL derivative lacking all actin-binding RPEL domains (Fig. 2A, right, and C, lanes 5 to 8). These results led to the hypothesis that LMO7 activates MAL by relieving its binding by actin. To further test this hypothesis, we asked whether LMO7 can facilitate the nuclear translocation of MAL. In serum-starved cells, MAL(fl) was localized predominantly in the cytoplasm (Fig. 2D), consistent with its high level of actin binding. The expression of LMO7 dramatically enhanced MAL(fl) nuclear localization, similar to the effect induced by serum stimulation (Fig. 2D). Further confirming that the increased nuclear localization of MAL(fl) was due to LMO7 expression, we found that increased MAL(fl) nuclear localization was restricted to the LMO7-expressing cell population (“LMO7+”) (Fig. 2E) in LMO7-transfecetd cells.
LMO7 and Rho cooperatively activate MAL.
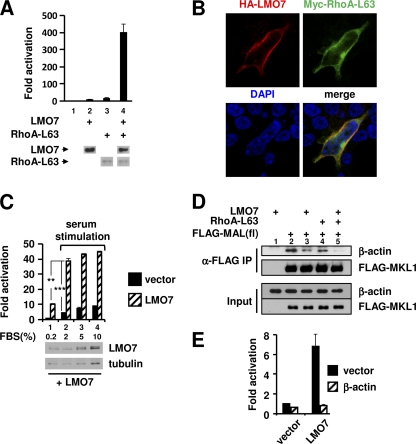
Our results thus far indicated that, like Rho, LMO7 also targeted the actin-binding domains of MRTFs to regulate their activity. It was thus important to explore whether there is functional cross talk between LMO7 and Rho in the regulation of MRTF/SRF-dependent transcription. RhoA-L63 is a constitutively active form of Rho. When expressed at suboptimal amounts, RhoA-L63 and LMO7 independently activated the transcriptional activity of SMA-Luc by 17.7-fold and 9.8-fold, respectively (Fig. 3A, lanes 3 and 2, respectively). Remarkably, the coexpression of RhoA-L63 and LMO7 led to a 402-fold activation without significantly changing the expression level of either RhoA-L63 or LMO7 (Fig. 3A, lane 4). These results demonstrated strong functional cross talk between LMO7 and RhoA-L63, which was also supported by their colocalization at the cell surface, as shown by confocal microscopic images (Fig. 3B).
Fig. 3.
LMO7 and Rho synergistically activate SRF-dependent transcription and reduce actin binding to MAL. (A) Luciferase assays performed on lysates of 293T cells transfected with SMA-Luc along with empty vector, LMO7, and/or RhoA-L63. (Bottom) Western blot showing expression levels of LMO7 and RhoA-L63 in the same lysates used in luciferase assays. Loading amounts were normalized to the β-galactosidase transfection control. LMO7 and RhoA-L63 were tagged with HA and Myc, respectively, for Western blot detection. (B) Confocal microscopic images of 293T cells cotransfected with HA-LMO7 and Myc-RhoA-L63 and stained with anti-HA and anti-Myc antibodies. (C) Luciferase assays of 293T cells transfected with SMA-Luc along with the empty vector or LMO7 under different serum conditions. At the bottom LMO7 expression (detected by an anti-HA antibody) in serum-starved cells (0.2% FBS) and in cells stimulated with 2%, 5%, or 10% serum is shown. **, P < 0.01; ***, P < 0.001. (D) Coimmunoprecipitation (IP) analysis of β-actin–MAL(fl) interactions in 293T cells transfected with the plasmids indicated at the top. (E) Luciferase assays of 293T cells transfected with SMA-Luc and the expression vectors indicated at the bottom along with β-actin or an empty vector.
The observed synergy between LMO7 and RhoA-L63 suggested that a similar synergy might occur between LMO7 and serum stimulation. In cells that had been serum starved for 24 h, LMO7 was still able to activate transcription (Fig. 3C, lane 1), consistent with its ability to promote MAL nuclear localization in serum-starved cells (Fig. 2D). As expected, serum treatment also increased the reporter activity (Fig. 3C, black bars). When LMO7 expression was combined with serum stimulation, a strong synergistic effect was indeed observed (Fig. 3C, lanes 2 to 4). The effect was most evident with 2% serum stimulation, under which condition the level of LMO7 expression was not increased.
To explain the synergy between LMO7 and Rho, we explored their effect on the binding of actin to MAL(fl) in 293T cells. Endogenous actin coimmunoprecipitated with FLAG-MAL(fl) in transfected cells (Fig. 3D, lane 2). The binding of actin was significantly reduced in cells expressing either LMO7 or RhoA-L63 (Fig. 3D, lanes 3 and 4 versus lane 2), and a synergistic reduction was observed for cells expressing both proteins (lane 5). These results indicate that LMO7 and RhoA-L63 function cooperatively to reduce actin binding to MAL. Further supporting the idea that LMO7 targets actin binding, the overexpression of actin completely blocked the ability of LMO7 to activate SRF-dependent transcription (Fig. 3E).
Actin-RPEL interactions regulate the functional cross talk between LMO7 and Rho.
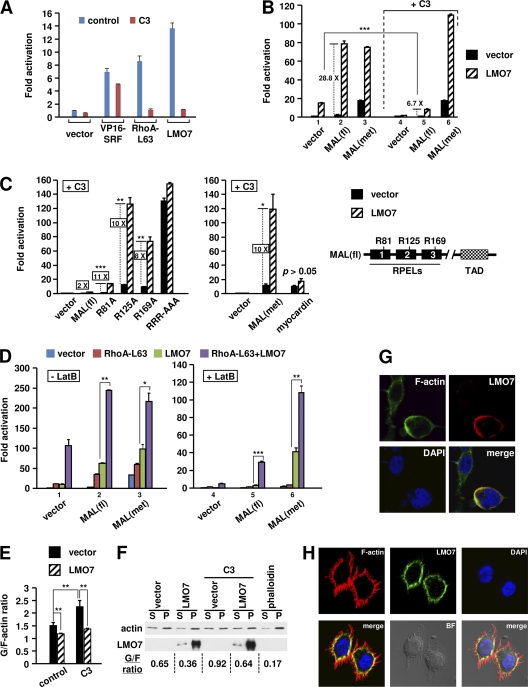
We next sought to understand the mechanism that underlies the functional cooperation between LMO7 and Rho. An initial study showed that LMO7 lost the ability to stimulate SRF-dependent transcription when endogenous Rho was inactivated by its specific inhibitor C3 (Fig. 4A and B, lane 1 versus lane 4, hatched bars), indicating that Rho activity was required for LMO7 to regulate endogenous MRTFs. Rho activity was also required for LMO7 to activate ectopically expressed MAL(fl) (Fig. 4B), as the strong activation of MAL(fl) in the absence of C3 (28.8-fold) (Fig. 4B, lane 2) was reduced to only 6.7-fold in the presence of C3 (lane 5). An even greater inhibition was achieved by increasing the concentration of C3 (Fig. 4C), indicating that the weak activation observed here might result from an incomplete inhibition of Rho. LMO7, however, did not increase cellular Rho activity, as shown by glutathione S-transferase (GST)–rhotekin–Rho binding domain (RBD) pulldown assays (see Fig. S7 at http://cellbiology.uc.edu/files/jinsongzhanglab/Hu_MCB_supplemental_figures_tables.pdf). Together, these results indicated that LMO7 requires basal Rho activity to overcome a limiting step toward the activation of the full-length MRTF proteins.
Fig. 4.
LMO7 and Rho cooperatively relieve actin inhibition of MRTFs. (A to D) Luciferase assays performed on lysates of 293T cells transfected with SMA-Luc and the indicated plasmids. (A and B) Two nanograms of C3 expression vector or empty vector was used. (C) A total of 7.5 ng of C3 expression vector or empty vector was used. (D) Cells were treated with LatB (1.5 μM) or vehicle. In panels B and D, C3 or LatB treatment reduced the basal activity of the reporter. The fold activation under each treatment condition (i.e., control, C3, or LatB) was normalized to the respective basal activity observed with the empty vector under the same treatment conditions. In panels B to D, 0.4 ng of MAL(fl) or its Arg mutants, 0.1 ng of MAL(met), and 1 ng of myocardin were used. (E) FACS analysis of the G-actin/F-actin ratio in 293T cells transfected with the indicated plasmids in the absence or presence of C3. *, P < 0.05; **, P < 0.01; ***, P < 0.001. (F) Western blot analysis of G-actin (S, supernatant) and F-actin (P, pellet) levels in 293T cells transfected with the indicated plasmids in the absence or presence of C3. (G) Confocal microscopic images of 293T cells transfected with HA-LMO7 and stained with both anti-HA antibody and Alexa 488-phalloidin. (H) Confocal microscopic images of HeLa cells stained with both anti-LMO7 antibody and Texas Red-X–phalloidin. BF, bright-field cell image.
LMO7 stimulated the partially active MAL(met) to a level similar to that observed with MAL(fl) (Fig. 4B, lane 3 versus lane 2). However, unlike MAL(fl), the activation of MAL(met) by LMO7 was not affected by C3 (Fig. 4B, lane 3 versus lane 6). These results indicate that LMO7 has an intrinsic Rho-independent function that is sufficient to strongly activate MAL(met). In the context of full-length MAL, however, a Rho-dependent step is required. The critical importance of Rho in the activation of full-length MAL is also underscored by the ability of C3 to abolish the weak basal activity of MAL(fl) (Fig. 4B, lane 5 versus lane 2, black bars).
To determine whether the ability of MAL(met) to undergo Rho-independent activation by LMO7 is due to its reduced actin binding or a specific involvement of RPEL1, we generated three MAL(fl) mutants (R81A, R125A, and R169A) (Fig. 4C, right). In these mutants, the Arg residues known to be important for actin binding were changed to Ala in each respective RPEL domain (14). A larger amount of C3 (7.5 ng) was used to minimize the LMO7-mediated activation of wild-type MAL(fl) (∼2-fold) (Fig. 4C). Under this condition, LMO7 strongly activated the R81A, R125A, and R169A mutants by 11-, 10-, and 8-fold, respectively (Fig. 4C). Notably, the maximal activity of the R81A mutant in the presence of LMO7 was much lower than those of the other mutants, which is consistent with an incomplete inhibition of actin binding to the RPEL1 domain by the R81A mutation (14). The mutation of all Arg residues (RRR-AAA) resulted in high levels of Rho-independent activity that could no longer be significantly increased by LMO7 (Fig. 4C). We also tested MAL(met) and myocardin under the same conditions (Fig. 4C). When transfected at a suboptimal amount, myocardin showed a Rho-independent activity similar to that of MAL(met). However, only MAL(met) was strongly activated by LMO7, consistent with absence of significant actin-myocardin interactions (14). Taken together, our results here indicate that whereas actin binding is a prerequisite for the responsiveness of MRTFs to LMO7, the strength of such binding dictates the Rho dependency. Under conditions of high binding affinity [e.g., in MAL(fl)], Rho acts as a rate-limiting factor to control the activation. In the situation of reduced binding affinity [e.g., in MAL(met) and MAL Arg mutants], Rho is dispensable, and the activation can be driven by other proteins, such as LMO7.
We next further explored the idea that actin-MRTF binding can influence the functional cooperation between Rho and LMO7. Latrunculin B (LatB), a reversible actin-depolymerizing compound (32, 52–54), was used to increase the cellular G-actin level, thereby enhancing actin binding to MAL. Since LatB reduced the basal reporter activity, to compare the abilities of ectopically expressed proteins to activate reporter transcription under different treatment conditions (i.e., with or without LatB), luciferase activities were normalized to the respective basal activities observed under the same treatment conditions (Fig. 4D). Compared to untreated cells, the synergistic effect between LMO7 and RhoA-L63 was generally greater in LatB-treated cells. The most dramatic effect was observed with MAL(fl), in that the activation of MAL(fl) in LatB-treated cells required the coexpression of both LMO7 and RhoA-L63 (Fig. 4D, lane 5). In addition, a strong synergy was also observed for the activation of MAL(met) in LatB-treated cells (Fig. 4D, lane 6). These results underscore the mutual dependence of LMO7 and RhoA-L63 for overcoming the increased actin inhibition of MAL(fl) in these cells. It was also evident from these experiments that LMO7 was more capable than RhoA-L63 of activating MAL(met) (Fig. 4D, lanes 3 and 6). On the other hand, whereas the activation of MAL(fl) by LMO7 requires a Rho-dependent step (see above), RhoA-L63 by itself is sufficient to activate MAL(fl) although to a lesser extent than the induction observed with the expression of both RhoA-L63 and LMO7 (Fig. 4D, lane 2). Thus, Rho and LMO7 may differentially target actin-RPEL interactions, which may underlie their functional cooperation.
LMO7 has a Rho-independent function to regulate actin dynamics by reducing the G-actin/F-actin ratio.
We next pursued mechanistic insight into the Rho-independent function of LMO7 in the activation of MAL. In LatB-treated cells, the ability of LMO7 to stimulate MAL(met) also relied on RPEL domains (see Fig. S8 at http://cellbiology.uc.edu/files/jinsongzhanglab/Hu_MCB_supplemental_figures_tables.pdf) and correlated with the ability of LMO7 to restore MAL(met) nuclear translocation (see the URL mentioned above). These results support the hypothesis that the Rho-independent function of LMO7, which may act cooperatively with Rho, reflects its ability to regulate actin dynamics. To further test this hypothesis, we asked whether the G-actin-to-F-actin ratio can be affected by the expression of LMO7 in 293T cells. FACS analysis was used to quantitate DNase I- and phalloidin-stainable actin (Fig. 4E). LMO7 expression reduced the G-actin/F-actin ratio. C3 increased the G-actin/F-actin ratio, expectedly. Remarkably, in C3-expressing cells, LMO7 was still able to reduce the G-actin/F-actin ratio, reaching a level comparable to the reduced level seen in the absence of C3. These results demonstrate the ability of LMO7 to regulate the G-actin/F-actin ratio independent of Rho, which is consistent with the ability of LMO7 to mediate the Rho-independent activation of MAL mutants in the presence of C3 (Fig. 4C). Western blot analysis of fractionated G-actin and F-actin (Fig. 4F) confirmed the FACS results. Further supporting the ability of LMO7 to regulate actin dynamics, LMO7 cofractionated with F-actin in transfected cells (Fig. 4F), and both ectopic and endogenous LMO7 colocalized with F-actin specifically at the cell membrane (Fig. 4G and H). No direct interaction, however, was observed between ectopically expressed LMO7 and β-actin (data not shown). Together, these results demonstrated that LMO7 has an intrinsic ability to regulate actin dynamics, providing further mechanistic insight into how LMO7 regulates MRTF/SRF-dependent transcription.
LMO7 enhances the ability of MAL to activate a subset of endogenous SRF target genes.
We next extended our studies to the role of LMO7 in regulating the MAL-dependent activation of endogenous SRF target genes. An initial study showed that in 293T cells, neither LMO7 nor RhoA-L63 could induce endogenous SMA expression (see Fig. S9 at http://cellbiology.uc.edu/files/jinsongzhanglab/Hu_MCB_supplemental_figures_tables.pdf), underscoring the stringent control of the activation of endogenous cell-specific promoters. This was reminiscent of a previous report showing that Rho by itself was insufficient to activate integrated chromosomal SRF reporters (2). Interestingly, the overexpression of MAL(met) produced a modest enhancement of SMA expression, and this activation was strongly enhanced by the coexpression of LMO7 (Fig. 5A and see Fig. S9 at the URL mentioned above). Compared to LMO7, RhoA-L63 produced a weaker effect in stimulating MAL(met)-dependent activation (see the URL mentioned above), consistent with their differential abilities in regulating MAL(met), as described above for reporter assays (Fig. 4D).
The stimulatory effect of LMO7 was even more dramatic with respect to MAL(fl). In this case, neither MAL(fl) nor LMO7 was able to induce SMA expression, but their coexpression led to a strong activation of SMA expression (Fig. 5A, lane 4). C3 or LatB abolished this activation (Fig. 5A, lanes 5 and 6), confirming our above-described results showing that LMO7 required Rho activity to relieve the actin inhibition of MAL(fl). This finding contrasts with the results for MAL(met), which was activated by LMO7 even in the presence of C3 or LatB (Fig. 5A, lanes 2 and 3), consistent with the reporter assay results described above (Fig. 4B to D).
As expected from previous studies, MAL(met) activated both muscle-specific genes and IEGs (Fig. 5B and see Fig. S10 at http://cellbiology.uc.edu/files/jinsongzhanglab/Hu_MCB_supplemental_figures_tables.pdf). Interestingly, LMO7 was found to enhance the MAL(met)-dependent activation of only some SRF target genes, including all muscle-specific genes tested here, and a few IEGs, such as TPM1 (49, 63) (Fig. 5B and see the URL mentioned above). Most IEGs, such as c-fos, were not affected by LMO7. To provide mechanistic insight into this differential regulation by LMO7, we measured SRF and Pol II occupancies and histone modifications on SMA and c-fos promoters in 293T cells transfected with the empty vector, MAL(met), LMO7, or MAL(met) plus LMO7. We found that in empty vector-transfected cells, the c-fos promoter, but not the SMA promoter, was associated with high levels of SRF, Pol II, and active histone modifications (Fig. 5C). LMO7 expression markedly increased MAL(met) binding to the SMA promoter, accompanied by a concurrent increase in SRF binding, Pol II recruitment, and histone modifications (Fig. 5C, left). In contrast, at the c-fos promoter, LMO7 only modestly increased MAL(met) binding and did not affect SRF binding, Pol II binding, or histone modifications (Fig. 5C, right), consistent with the lack of an effect of LMO7 on c-fos transcription. Of note, the observed increased binding of SRF to the endogenous SMA promoter in the presence of LMO7 is not in conflict with our above-described data showing that LMO7 did not enhance the binding of VP16-SRF to the transfected SMA-Luc template (Fig. 1G). In the latter case, the binding of VP16-SRF to transfected SMA-Luc was independent of MRTFs, as evidenced by its constitutive activity in the absence or presence of C3 (Fig. 4A). These results underscore the difference in the chromatin structures between transiently transfected reporters and endogenous promoters as well as the stringency of their transcriptional regulation, as noted above.
LMO7 is important for MRTF function in cells expressing endogenous LMO7.
To address the in vivo function of LMO7, we focused our initial studies on HeLa cells, which, unlike 293T cells, express a high level of endogenous LMO7 (Fig. 6A). An initial RT-qPCR analysis showed that SMA was not expressed in HeLa cells (see Fig. S12 at http://cellbiology.uc.edu/files/jinsongzhanglab/Hu_MCB_supplemental_figures_tables.pdf), precluding its use in assessments of the function of LMO7. SM22 and c-fos, however, were expressed in HeLa cells at levels even higher than those in 293T cells (see the URL mentioned above). The knockdown of LMO7 in HeLa cells (Fig. 6B) significantly reduced the level of expression of SM22 but not that of c-fos (Fig. 6C). The reduced SM22 expression level correlated with reduced MAL(met) activity in knockdown cells, as shown by the SM22-Luc reporter assays (Fig. 6D). As a control, the ability of VP16-SRF to stimulate the SM22 promoter was only modestly affected by the LMO7 knockdown (3.6-fold versus 4.2-fold) (Fig. 6D), consistent with the weak stimulation of VP16-SRF-dependent transcription by LMO7, as shown above (Fig. 1G).
In HeLa cells, MAL was either enriched in the nucleus or evenly distributed between the cytoplasm and the nucleus. Serum stimulation shifted MAL to the nucleus but did not change the cell membrane localization of LMO7 (Fig. 6E and F). The knockdown of LMO7 essentially blocked MAL nuclear accumulation in serum-starved cells (7.73% to 1.59%) and significantly reduced MAL nuclear accumulation under serum-stimulated conditions. These effects correlated with the reduced SM22 expression levels under both conditions (Fig. 6G). Further confirmation of a gene-specific role of LMO7 was provided by the finding that the expression of c-fos and its induction by serum were not affected by the LMO7 knockdown in HeLa cells (Fig. 6G). Together, these results indicate that LMO7 is important for the optimal levels of both basal and signal-dependent activities of MAL in HeLa cells.
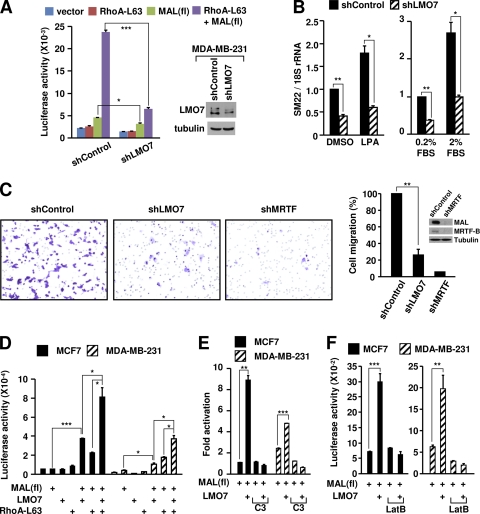
Consistent with a potential involvement of LMO7 in metastasis, LMO7 was expressed in the metastatic breast cancer cell line MDA-MB-231 but was absent in nonmetastatic MCF7 cells (Fig. 6A). In MDA-MB-231 cells, the high level of MAL activity remains Rho dependent, and the inhibition of either Rho or MRTF abrogates cell migration (5, 27). We therefore asked whether LMO7 expression is also important for the Rho-dependent activity of MRTF in MDA-MB-231 cells. We first confirmed that the knockdown of LMO7 reduced the steady-state SM22 expression level without affecting the levels of MRTFs (see Fig. S13 at http://cellbiology.uc.edu/files/jinsongzhanglab/Hu_MCB_supplemental_figures_tables.pdf). In SM22-Luc reporter assays, the reduction of LMO7 strongly reduced both basal and Rho-dependent MAL activities (Fig. 7A). Consistent with these results, RT-qPCR assays showed that the reduction of LMO7 similarly reduced the basal expression level of SM22 and its Rho-dependent activation induced by either lysophosphatidic acid (LPA), a specific Rho inducer, or serum stimulation (Fig. 7B). Finally, since the Rho-MRTF-SRF pathway is critically involved in the migration of MDA-MB-231 cells (5, 27), we asked whether LMO7 is important for the high motility of these cells. Using transwell migration assays, we showed that the knockdown of LMO7 markedly reduced the migration of MDA-MB-231 cells (Fig. 7C), which is consistent with the reduced MRTF activity in LMO7 knockdown cells described above. We also confirmed that MRTF activity is critical for the migration of these cells (Fig. 7C).
Fig. 7.
Endogenous LMO7 regulates Rho-dependent MRTF/SRF activity in MDA-MB-231 and MCF7 cells and is required for the migration of MDA-MB-231 cells. (A) Luciferase assays using the SM22-Luc reporter in control and LMO7 knockdown MDA-MB-231 cells. (Right) Western blot analysis of LMO7 expression in LMO7 knockdown and control MDA-MB-231 cells using anti-LMO7 antibody. (B) RT-qPCR analysis of SM22 expression in LMO7 knockdown and control MDA-MB-231 cells under basal and LPA- or serum-stimulated conditions. (C) Transwell migration assays of control, LMO7 knockdown, or MRTF knockdown MDA-MB-231 cells. At the right is a quantitation of migrated cells in transwell migration assays shown on the left and Western blot analysis of MAL and MRTF-B levels in MRTF knockdown and control MDA-MB-231 cells using anti-MAL and anti-MRTF-B antibodies. (D to F) Luciferase assays using the SM22-Luc reporter. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
To further substantiate the ability of LMO7 to regulate the Rho-MRTF-SRF pathway in MDA-MB-231 cells, and also to test whether this pathway remains sensitive to LMO7 in MCF7 cells that do not express endogenous LMO7, we performed SM22-Luc reporter assays with both MCF7 and MDA-MB-231 cells. Interestingly, in these cells, the basal reporter activity was not significantly increased by either LMO7 or RhoA-L63 (Fig. 7D). This is reminiscent of the lack of an effect of LMO7 or RhoA-L63 on endogenous muscle gene promoters in 293T cells described above (see Fig. S9 at http://cellbiology.uc.edu/files/jinsongzhanglab/Hu_MCB_supplemental_figures_tables.pdf), suggesting that the transfected reporter may be more stringently controlled in these cells. MAL(fl) showed a weak activity in MDA-MB-231 cells but not in MCF7 cells, consistent with a higher signaling activity in MDA-MB-231 cells. Nevertheless, in both types of cells, the activity of MAL(fl) was markedly increased by the expression of LMO7 or RhoA-L63, and a cooperative effect of LMO7 and RhoA-L63 in the activation of MAL(fl) was also evident (Fig. 7D). Interestingly, MCF7 cells appeared to be more sensitive to ectopic LMO7 than MDA-MB-231 cells, which may reflect the presence of endogenous LMO7 in MDA-MB-231 cells. In both types of cells, the ability of LMO7 to activate MAL(fl) was Rho dependent (Fig. 7E) and was sensitive to LatB treatment (Fig. 7F). Together, these results showed that LMO7 expression in breast cancer cells can generally increase Rho-dependent MRTF activity. Given that MDA-MB-231 is a mesenchymal cell-like cell line, the acquired expression of LMO7 in nonmetastatic breast cancer cells such as MCF7 cells may therefore promote the epithelial-to-mesenchymal cell transition, an important step toward metastasis.
It should be mentioned that in MDA-MB-231 cells, whereas LMO7 remained cell membrane localized (see Fig. S14 at http://cellbiology.uc.edu/files/jinsongzhanglab/Hu_MCB_supplemental _figures_tables.pdf), the knockdown of LMO7 did not significantly affect the subcellular localization of endogenous MAL (data not shown). In these cells, as previously reported (27), MAL was constitutively localized in the nucleus, even under serum starvation conditions (data not shown), indicating that other mechanisms may dominantly regulate the nuclear import or export of MAL in MDA-MB-231 cells. Nevertheless, our finding that the knockdown of LMO7 reduced the transcriptional activity of MAL is consistent with the notion that actin binding, which is the target of LMO7, rather than subcellular localization, is the ultimate determinant for the activity of MRTFs (61).
The LMO7 expression level is strongly increased in invasive breast cancer stroma samples and correlates with increased expression levels of SRF target genes.
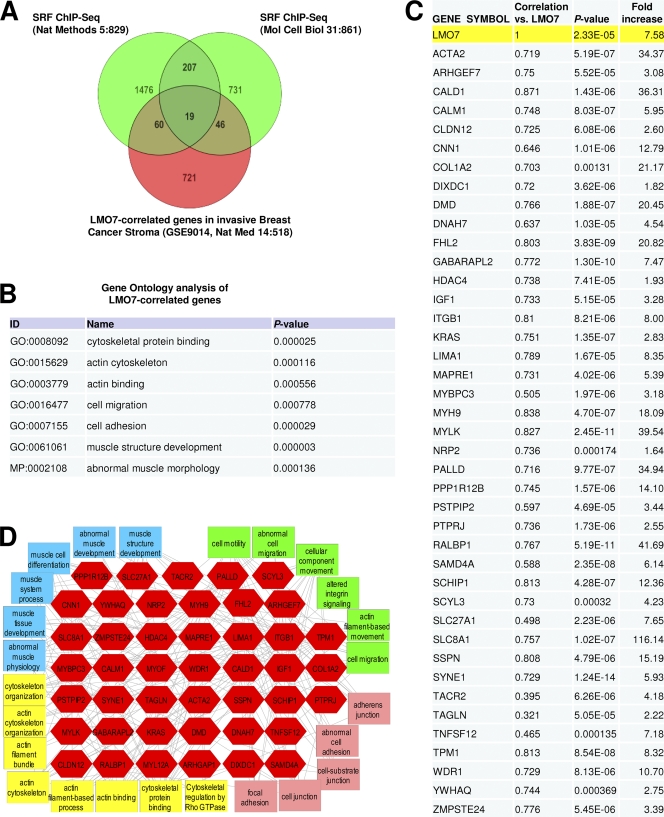
Given the highly invasive and stroma-like phenotype of MDA-MB-231 cells (22) and the importance of stromal cells in the development of breast cancer metastasis (10, 20), we asked whether the level of expression of LMO7 can also be increased in the stroma of invasive breast carcinomas. To do this, we analyzed the microarray data set GSE9014 (10), comprising a series of 123 microarrays from 53 invasive BC samples and 6 normal stroma controls, and found that LMO7 was strongly upregulated in nearly all BC stroma samples compared to normal control samples (mean increase, 7.58-fold; P < 0.0000233) (Fig. 8C and see http://cellbiology.uc.edu/files/jinsongzhanglab/Hu_MCB_supplemental_excel_file.xls). Subsequent correlation and clustering analyses identified 846 genes whose expression levels were also increased and correlated with that of LMO7 (Fig. 8A and see the URL mentioned above). Toppgene-based feature enrichment (Fig. 8B) and multidimensional network analyses (see Fig. S15 at http://cellbiology.uc.edu/files/jinsongzhanglab/Hu_MCB_supplemental_figures_tables.pdf) showed that a large number of LMO7-correlated genes were involved in the regulation of actin cytoskeleton-, cell migration-, and muscle-related processes, which are characteristic functions of SRF-controlled genes. We further compared LMO7-correlated genes and SRF target genes identified by ChIP plus sequencing (ChIP-seq) studies from two different cell lines (55, 59). The results confirmed an extensive overlap between these genes (Fig. 8A) and also suggested that LMO7 may preferentially regulate cell-specific SRF target genes (60 and 46 versus 19 genes) (Fig. 8A). Based on the ChIP-seq results and previously characterized SRF target genes, a total of 130 LMO7-corerelated genes fell into the group of direct SRF target genes (see http://cellbiology.uc.edu/files/jinsongzhanglab/Hu_MCB_supplemental_excel_file.xls). Many of these SRF target genes are involved directly in the regulation of actin cytoskeleton, muscle biology, migration, and cell adhesion processes (Fig. 8C and D). These genes also include the SMA/ACTA2, SM22/TAGLN, CNN1, and TPM1 genes, which we have shown to be induced by LMO7. Together, these studies provide further support for the ability of LMO7 to regulate SRF-dependent transcription and an important role for LMO7 in breast cancer metastasis through the regulation of SRF target genes. Our results also imply that the upregulation of smooth muscle genes, a previously identified signature of cancer metastasis (45), could be a result of an increased expression level of LMO7.
Fig. 8.
LMO7 is expressed at high levels in invasive breast cancer stroma samples, and other genes that exhibit correlated expression patterning across those samples map to an extensive network of SRF target genes. (A) Venn diagram showing overlaps among LMO7-correlated genes in stroma samples from invasive BC and SRF target genes identified from studies with macrophages and Jurkat T cells. ChIP-seq studies of SRF target genes in macrophage and Jurkat cells were previously described (55, 59). The list of genes bound by SRF in macrophages was reported previously (55). To obtain the list of SRF target genes in Jurkat cells, the genomic location of SRF binding peaks (available from http://mendel.stanford.edu/sidowlab/downloads/quest/) (59) was used to identify the closest genes located within 2,000 bp of the SRF-binding peak using Avadis NGS software. (B) Gene Ontology (GO) analysis of LMO7-correlated genes showing highly enriched biological functions that include cytoskeleton-, muscle-, motility-, and cell migration-associated gene groups. (C) List of LMO7-correlated genes that overlap with either ChIP-seq-identified SRF target genes or previously characterized SRF target genes and that regulate the various biological processes shown in D. Pearson's correlation coefficients, P values, and fold increases were obtained as described in Materials and Methods. (D) Network view of the genes shown in C. The associated features and functions of these genes were identified by using enrichment analysis through the Toppcluster server (http://Toppcluster.cchmc.org/) (18) and rendered in Cytoscape, which demonstrates the extensive interconnectivity of LMO7-correlated genes that are associated with SRF regulation and biological functions involving the actin cytoskeleton, muscle regulation, and the regulation of cell migration and adhesion.
DISCUSSION
In this study, we have shown that LMO7 is a novel cell-specific and gene-specific activator of the Rho-MRTF-SRF pathway. The overexpression of LMO7 enhances the Rho-dependent activation of MRTF by relieving actin-mediated inhibition. Consistently, the depletion of LMO7 impairs MRTF function in cells that express LMO7. Mechanistically, LMO7 does not increase Rho activity. Rather, it has an intrinsic Rho-independent ability to reduce the G-actin/F-actin ratio. This reduction, however, is insufficient to activate MRTF without a Rho-dependent step. Whereas actin binding is the target of LMO7, the reduction of the affinity of this binding eliminates the Rho dependency and allows strong Rho-independent activation by LMO7. Physiologically, the increased expression of LMO7 is important for the migration of metastatic breast cancer cells, and the upregulation of LMO7 correlates with the activation of SRF target genes in invasive breast tumors. Our study thus elucidates a novel cell-specific mechanism for the regulation of the Rho-MRTF-SRF pathway, which is important for breast cancer cell migration, and a previously unrecognized role for actin-binding motifs in the integration of Rho-dependent and Rho-independent signals to synergistically activate MRTFs while maintaining a robust control of gene activation.
The functional cooperation between LMO7 and Rho occurs at both basal and activated Rho levels. Whereas basal Rho activity allowed LMO7 to moderately activate SRF-dependent transcription, a synergistic effect was evident when the function of LMO7 was joined with that of RhoA-L63 or with serum stimulation. Our results indicate that the cooperative function of LMO7 and Rho can be attributed to their joint action in relieving the actin-mediated inhibition of MRTFs, as evidenced by (i) the requirement for Rho and RPEL regions in LMO7-mediated activation (Fig. 2C and 4A to D), (ii) the synergistic effect of LMO7 and RhoA-L63 in activating transcription and reducing actin binding to MAL (Fig. 3), (iii) the joint requirement for LMO7 and RhoA-L63 to overcome the LatB-imposed inhibition of MAL(fl) (Fig. 4D), (iv) the ability of LMO7 to reduce the G-actin/F-actin ratio both in the absence and in the presence of C3 (Fig. 4E and F), and (v) the ability of LMO7 to restore MAL(met) nuclear translocation in LatB-treated cells (see Fig. S8 at http://cellbiology.uc.edu/files/jinsongzhanglab/Hu_MCB_supplemental_figures_tables.pdf).
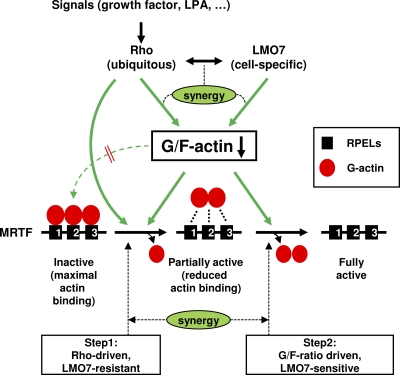
We propose a mechanistic model to explain the cooperative function between LMO7 and Rho (Fig. 9). First, given that both LMO7 and Rho can reduce the G-actin/F-actin ratio, they could elicit a synergistic effect in the regulation of the G-actin level. The observed colocalization of LMO7 and Rho at the cell membrane may further facilitate this cooperation. Such synergy may be particularly important for LMO7 and Rho to reduce the G-action/F-actin ratio under conditions of high levels of G-actin, such as those imposed by LatB (Fig. 4D). Under such conditions, the basal Rho activity, although dispensable for LMO7 to activate MAL(met) in the absence of LatB, may be important for LMO7 to activate MAL(met) when LatB is present. However, given the differential abilities of LMO7 and RhoA-L63 to regulate MAL(fl) versus MAL(met), the regulation of actin dynamics may not be the only mechanism underlying the cooperative function of LMO7 and Rho. Thus, whereas Rho can activate MAL(fl), it is less effective in stimulating MAL(met). In contrast, LMO7 has a strong effect on partially activated MAL mutants but is insufficient to activate MAL(fl) without Rho. These results are consistent with a fundamental difference between LMO7 and Rho in their regulations of MRTF. We propose that the initial release of actin from one of the RPELs is Rho dependent (Fig. 9), possibly through a function of Rho independent of regulating the G-actin/F-actin ratio. This explains why, in the absence of Rho activity, MAL(fl) is resistant to the reduced G-actin/F-actin ratio triggered by LMO7 expression. It is possible that the complex formed between actin and the three RPELs is sufficiently stable such that it is insensitive to a change in the G-actin level. A reduction of the G-actin level, however, may act in conjunction with Rho to promote the initial release of actin (Fig. 9). Upon the release of actin from one of the RPELs, further actin dissociation can be driven by the reduced G-actin/F-actin ratio, thus explaining the sensitivity of partially activated MAL derivatives to LMO7 (Fig. 4B and C). Thus, by targeting a step downstream of the initial Rho-dependent step, LMO7 is able to increase the magnitude of activation without compromising Rho-dependent control. Physiologically, the requirement for Rho can be an important checkpoint in the regulation of MRTF, which could ensure that MRTF and SRF target genes would not be inappropriately activated by Rho-independent signals, such as that mediated by LMO7. The disruption of this control can lead to diseases, as exemplified by leukemias caused by constitutively active MAL fusion proteins.
Fig. 9.
Model showing the cooperative function of LMO7 and Rho in the activation of MRTFs. The cell-specific expression of LMO7 potentiates the Rho-dependent activation of MRTFs by relieving actin-mediated inhibition. We propose a stepwise model for the activation of MRTFs. Rho is required at an initial rate-limiting step to convert inactive MRTF into a partially activated form, represented by the loss of a G-actin molecule from the RPEL domains. This step is insensitive to a reduced G-actin/F-actin ratio. After this initial step, Rho and LMO7 can synergistically reduce the G-actin/F-actin ratio to facilitate the complete activation of the partially activated MRTF. Whereas a reduced G-actin/F-actin ratio alone is insufficient to drive the initial activation step, it may play a role in conjunction with a Rho-dependent activity in promoting the initial step. See the text for details.
The finding that LMO7 induced similar levels of Rho-independent activation of different RPEL-mutated MAL derivatives (Fig. 4C) suggests that the overall actin-binding affinity, rather than a specific RPEL-actin interaction, confers Rho dependency at the initial step. This is also consistent with the importance of all three RPELs for the proper Rho-dependent regulation of MRTFs (14). The MAL-R81A mutant is particularly interesting. Its activation by LMO7 is intermediate between those of MAL(fl) and MAL(met). This may reflect the fact that this mutation only partially disrupts actin binding to RPEL1, and therefore, only a fraction of the MAL-R81A population has actin dissociated from RPEL1 and could respond to LMO7.
How might LMO7 reduce the G-actin/F-actin ratio? LMO7 contains CH (calponin homology), PDZ, and LIM domains. In addition, we found that LMO7 colocalizes with F-actin at the cell surface, which is consistent with its previously reported associations with F-actin-binding proteins such as α-actinin at cell adhesion sites (36). Proteins that share similar domains and features have been reported to affect the G-actin/F-actin ratio by various mechanisms. LMO7 may act similarly. For instance, it may act like the PDZ/LIM domain protein ALP (actinin-associated LIM protein) to stabilize F-actin by increasing F-actin cross-linking and bundling (40). Alternatively, given the presence of multiple protein-protein interaction domains in LMO7, we favor the idea that LMO7 may act as a scaffold protein at the cell membrane or adhesion sites to coordinate the function of Rho and Rho-related cdc42 and Rac1 as well as their downstream effectors in the regulation of actin dynamics. This would resemble the function of zyxin and IQGAP (6, 11, 34), both of which also contain the LIM or CH domain and colocalize with F-actin at the cell surface or adhesion sites. It should be noted that these possibilities are not necessarily mutually exclusive. Future structure-function analyses of LMO7 and characterization of its interacting proteins should help fully elucidate the precise mechanism(s) by which LMO7 regulates actin dynamics.
Another interesting finding of this study is the gene-specific function of LMO7. Although MRTFs can stimulate both IEGs and muscle-specific genes, LMO7 preferentially enhanced the function of MRTFs on muscle-specific genes (Fig. 1D and 5B and see http://cellbiology.uc.edu/files/jinsongzhanglab/Hu_MCB_supplemental_figures_tables.pdf). This finding is in agreement with the reported role for LMO7 in regulating muscle and heart functions. Since LMO7 does not bind to gene promoters, the different responsivenesses of MRTF/SRF target genes to LMO7 should be explained by their different responsivenesses to MRTF/SRF. LMO7-sensitive genes are repressed at a step prior to MRTF/SRF recruitment (Fig. 5C). Such a recruitment is likely the predominant (if not only) limiting step in the activation of these genes (26, 67). LMO7 may likely induce other SRF target genes whose activation is dependent on the recruitment of SRF and MRTFs. The ability of LMO7, in conjunction with MRTFs, to regulate these genes is consistent with data from previous studies showing that MRTFs can remodel the chromatin of muscle-specific gene promoters and facilitate SRF binding to the promoter region (26, 67). The binding of MRTFs to these promoters may compete directly with the binding of repressors. In contrast, the binding of MRTFs to IEG promoters does not necessarily cause the release of TCF repressors (23), thus explaining the lack of sensitivity of these promoters to LMO7.
The ability of LMO7 to activate muscle-specific gene expression is reminiscent of that of STARS, a cardiac and skeletal muscle-specific activator of MRTFs (3, 21). Similar to LMO7, STARS activates MRTFs by relieving actin-mediated inhibition. Whereas STARS binds directly to F-actin, LMO7 may associate with F-actin via adaptor proteins, such as afadin and α-actinin, that are known to bind to both F-actin and LMO7 (36). It will be interesting to determine whether LMO7 and STARS have overlapping expression patterns in muscle and heart and, if so, whether they function cooperatively in the regulation of MRTF and SRF target genes.
Our results indicate that endogenous LMO7 contributes to the Rho-MRTF-SRF activity in LMO7-expressing cells. These results are consistent with previous observations that the depletion of LMO7 affects muscle gene expression (17) and compromises muscle and cardiac development (37, 51). In breast cancer cells, evidence for the physiological importance of LMO7 in regulating MRTF function is also provided by our observation that the knockdown of LMO7 impaired the migration of MDA-MB-231 cells. Although MCF7 cells do not express LMO7, they nevertheless showed a robust response to LMO7 expression. Thus, the acquired expression of LMO7 in breast cancer cells may generally enhance MRTF/SRF activity, which could promote the epithelial-to-mesenchymal cell transition of these cells. The increased expression levels of LMO7 may not only affect breast cancer cells but also regulate stromal cells, as supported by our finding that LMO7 is upregulated in the stroma of invasive breast tumors, and this upregulation correlates with the increased expressions of SRF target genes. Together, our study further substantiates an important role for LMO7 in breast cancer metastasis. Therapeutically, although an increased expression of Rho was also demonstrated in metastatic breast cancer (4, 5, 9, 16, 27, 56, 60), Rho may not be a specific target, given its expression in normal cells and its involvement in regulating other important cell functions. LMO7, however, is a cell-specific protein and is not expressed in normal breast tissues (19, 47). Thus, LMO7 can be a promising target for the treatment of metastatic breast cancers.
ACKNOWLEDGMENTS
This work was supported by funding from the University of Cincinnati College of Medicine and the Department of Cancer and Cell Biology (to J.Z.) and National Institutes of Health grants R01HL093195-01A1 (to J.Z.) and U54RR025216 (to B.J.A.). FACS data were acquired using equipment maintained by the Research Flow Cytometry Core in the Division of Rheumatology at Cincinnati Children's Hospital Medical Center, supported in part by NIH grant AR-47363.
We thank Ron Prywes, Deniz Toksoz, and Andrew Sharrocks for plasmids; Sohaib Khan, Jerry Lingrel, and Peter Stambrook for critical reading and helpful comments; and Birgit Ehmer for technical help.
Footnotes
Published ahead of print on 13 June 2011.
REFERENCES
- 1. Abramoff M. D., Magelhaes P. J., Ram S. J. 2004. Image processing with Image. J. Biophoton. Int. 11:36–42 [Google Scholar]
- 2. Alberts A. S., Geneste O., Treisman R. 1998. Activation of SRF-regulated chromosomal templates by Rho-family GTPases requires a signal that also induces H4 hyperacetylation. Cell 92:475–487 [DOI] [PubMed] [Google Scholar]
- 3. Arai A., Spencer J. A., Olson E. N. 2002. STARS, a striated muscle activator of Rho signaling and serum response factor-dependent transcription. J. Biol. Chem. 277:24453–24459 [DOI] [PubMed] [Google Scholar]
- 4. Bellizzi A., et al. 2008. RhoA protein expression in primary breast cancers and matched lymphocytes is associated with progression of the disease. Int. J. Mol. Med. 22:25–31 [PubMed] [Google Scholar]
- 5. Brandt D. T., et al. 2009. SCAI acts as a suppressor of cancer cell invasion through the transcriptional control of beta1-integrin. Nat. Cell Biol. 11:557–568 [DOI] [PubMed] [Google Scholar]
- 6. Brandt D. T., Grosse R. 2007. Get to grips: steering local actin dynamics with IQGAPs. EMBO Rep. 8:1019–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cen B., et al. 2003. Megakaryoblastic leukemia 1, a potent transcriptional coactivator for serum response factor (SRF), is required for serum induction of SRF target genes. Mol. Cell. Biol. 23:6597–6608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cen B., Selvaraj A., Prywes R. 2004. Myocardin/MKL family of SRF coactivators: key regulators of immediate early and muscle specific gene expression. J. Cell. Biochem. 93:74–82 [DOI] [PubMed] [Google Scholar]
- 9. Clark E. A., Golub T. R., Lander E. S., Hynes R. O. 2000. Genomic analysis of metastasis reveals an essential role for RhoC. Nature 406:532–535 [DOI] [PubMed] [Google Scholar]
- 10. Finak G., et al. 2008. Stromal gene expression predicts clinical outcome in breast cancer. Nat. Med. 14:518–527 [DOI] [PubMed] [Google Scholar]
- 11. Fradelizi J., et al. 2001. ActA and human zyxin harbour Arp2/3-independent actin-polymerization activity. Nat. Cell Biol. 3:699–707 [DOI] [PubMed] [Google Scholar]
- 12. Fujii M., et al. 1994. Serum response factor has functional roles both in indirect binding to the CArG box and in the transcriptional activation function of human T-cell leukemia virus type I Tax. J. Virol. 68:7275–7283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Furuya M., et al. 2002. A novel gene containing PDZ and LIM domains, PCD1, is overexpressed in human colorectal cancer. Anticancer Res. 22:4183–4186 [PubMed] [Google Scholar]
- 14. Guettler S., Vartiainen M. K., Miralles F., Larijani B., Treisman R. 2008. RPEL motifs link the serum response factor cofactor MAL but not myocardin to Rho signaling via actin binding. Mol. Cell. Biol. 28:732–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Guo C., Hu Q., Yan C., Zhang J. 2009. Multivalent binding of the ETO corepressor to E proteins facilitates dual repression controls targeting chromatin and the basal transcription machinery. Mol. Cell. Biol. 29:2644–2657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hakem A., et al. 2005. RhoC is dispensable for embryogenesis and tumor initiation but essential for metastasis. Genes Dev. 19:1974–1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Holaska J. M., Rais-Bahrami S., Wilson K. L. 2006. Lmo7 is an emerin-binding protein that regulates the transcription of emerin and many other muscle-relevant genes. Hum. Mol. Genet. 15:3459–3472 [DOI] [PubMed] [Google Scholar]
- 18. Kaimal V., Bardes E. E., Tabar S. C., Jegga A. G., Aronow B. J. 2010. ToppCluster: a multiple gene list feature analyzer for comparative enrichment clustering and network-based dissection of biological systems. Nucleic Acids Res. 38:W96–W102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kang S., et al. 2000. PCD1, a novel gene containing PDZ and LIM domains, is overexpressed in several human cancers. Cancer Res. 60:5296–5302 [PubMed] [Google Scholar]
- 20. Karnoub A. E., et al. 2007. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature 449:557–563 [DOI] [PubMed] [Google Scholar]
- 21. Kuwahara K., Barrientos T., Pipes G. C., Li S., Olson E. N. 2005. Muscle-specific signaling mechanism that links actin dynamics to serum response factor. Mol. Cell. Biol. 25:3173–3181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lacroix M., Guy L. 2004. An updated view on cell lines as in vitro models for breast tumors, p. 131–182In Yao A. P. (ed.), Focus on breast cancer research. Nova Science Publishers, Commack, NY [Google Scholar]
- 23. Lee S. M., Vasishtha M., Prywes R. 2010. Activation and repression of cellular immediate early genes by serum response factor cofactors. J. Biol. Chem. 285:22036–22049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liao J., et al. 1997. Growth hormone regulates ternary complex factors and serum response factor associated with the c-fos serum response element. J. Biol. Chem. 272:25951–25958 [DOI] [PubMed] [Google Scholar]
- 25. Lindvall J. M., Blomberg K. E., Wennborg A., Smith C. I. 2005. Differential expression and molecular characterisation of Lmo7, Myo1e, Sash1, and Mcoln2 genes in Btk-defective B-cells. Cell. Immunol. 235:46–55 [DOI] [PubMed] [Google Scholar]
- 26. McDonald O. G., Wamhoff B. R., Hoofnagle M. H., Owens G. K. 2006. Control of SRF binding to CArG box chromatin regulates smooth muscle gene expression in vivo. J. Clin. Invest. 116:36–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Medjkane S., Perez-Sanchez C., Gaggioli C., Sahai E., Treisman R. 2009. Myocardin-related transcription factors and SRF are required for cytoskeletal dynamics and experimental metastasis. Nat. Cell Biol. 11:257–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Merdek K. D., et al. 2008. Alpha(E)-catenin induces SRF-dependent transcriptional activity through its C-terminal region and is partly RhoA/ROCK-dependent. Biochem. Biophys. Res. Commun. 366:717–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Miano J. M. 2003. Serum response factor: toggling between disparate programs of gene expression. J. Mol. Cell. Cardiol. 35:577–593 [DOI] [PubMed] [Google Scholar]
- 30. Miano J. M., Long X., Fujiwara K. 2007. Serum response factor: master regulator of the actin cytoskeleton and contractile apparatus. Am. J. Physiol. Cell Physiol. 292:C70–C81 [DOI] [PubMed] [Google Scholar]
- 31. Miralles F., Posern G., Zaromytidou A. I., Treisman R. 2003. Actin dynamics control SRF activity by regulation of its coactivator MAL. Cell 113:329–342 [DOI] [PubMed] [Google Scholar]
- 32. Morton W. M., Ayscough K. R., McLaughlin P. J. 2000. Latrunculin alters the actin-monomer subunit interface to prevent polymerization. Nat. Cell Biol. 2:376–378 [DOI] [PubMed] [Google Scholar]
- 33. Nakamura H., et al. 2005. Transforming growth factor-beta1 induces LMO7 while enhancing the invasiveness of rat ascites hepatoma cells. Cancer Lett. 220:95–99 [DOI] [PubMed] [Google Scholar]
- 34. Nguyen T. N., Uemura A., Shih W., Yamada S. 2010. Zyxin-mediated actin assembly is required for efficient wound closure. J. Biol. Chem. 285:35439–35445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Olson E. N., Nordheim A. 2010. Linking actin dynamics and gene transcription to drive cellular motile functions. Nat. Rev. Mol. Cell Biol. 11:353–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ooshio T., et al. 2004. Involvement of LMO7 in the association of two cell-cell adhesion molecules, nectin and E-cadherin, through afadin and alpha-actinin in epithelial cells. J. Biol. Chem. 279:31365–31373 [DOI] [PubMed] [Google Scholar]
- 37. Ott E. B., et al. 2008. The lim domain only protein 7 is important in zebrafish heart development. Dev. Dyn. 237:3940–3952 [DOI] [PubMed] [Google Scholar]
- 38. Perou C. M., et al. 2000. Molecular portraits of human breast tumours. Nature 406:747–752 [DOI] [PubMed] [Google Scholar]
- 39. Pipes G. C., Creemers E. E., Olson E. N. 2006. The myocardin family of transcriptional coactivators: versatile regulators of cell growth, migration, and myogenesis. Genes Dev. 20:1545–1556 [DOI] [PubMed] [Google Scholar]
- 40. Pomies P., et al. 2007. The cytoskeleton-associated PDZ-LIM protein, ALP, acts on serum response factor activity to regulate muscle differentiation. Mol. Biol. Cell 18:1723–1733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Posern G., Miralles F., Guettler S., Treisman R. 2004. Mutant actins that stabilise F-actin use distinct mechanisms to activate the SRF coactivator MAL. EMBO J. 23:3973–3983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Posern G., Treisman R. 2006. Actin' together: serum response factor, its cofactors and the link to signal transduction. Trends Cell Biol. 16:588–596 [DOI] [PubMed] [Google Scholar]
- 43. Prabhu S., Ignatova A., Park S. T., Sun X. H. 1997. Regulation of the expression of cyclin-dependent kinase inhibitor p21 by E2A and Id proteins. Mol. Cell. Biol. 17:5888–5896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Putilina T., et al. 1998. Analysis of a human cDNA containing a tissue-specific alternatively spliced LIM domain. Biochem. Biophys. Res. Commun. 252:433–439 [DOI] [PubMed] [Google Scholar]
- 45. Ramaswamy S., Ross K. N., Lander E. S., Golub T. R. 2003. A molecular signature of metastasis in primary solid tumors. Nat. Genet. 33:49–54 [DOI] [PubMed] [Google Scholar]
- 46. Rozenblum E., et al. 2002. A genomic map of a 6-Mb region at 13q21-q22 implicated in cancer development: identification and characterization of candidate genes. Hum. Genet. 110:111–121 [DOI] [PubMed] [Google Scholar]
- 47. Sasaki M., et al. 2003. PCD1, a novel gene containing PDZ and LIM domains, is overexpressed in human breast cancer and linked to lymph node metastasis. Anticancer Res. 23:2717–2721 [PubMed] [Google Scholar]
- 48. Scharenberg M. A., Chiquet-Ehrismann R., Asparuhova M. B. 2010. Megakaryoblastic leukemia protein-1 (MKL1): increasing evidence for an involvement in cancer progression and metastasis. Int. J. Biochem. Cell Biol. 42:1911–1914 [DOI] [PubMed] [Google Scholar]
- 49. Selvaraj A., Prywes R. 2004. Expression profiling of serum inducible genes identifies a subset of SRF target genes that are MKL dependent. BMC Mol. Biol. 5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Selvaraj A., Prywes R. 2003. Megakaryoblastic leukemia-1/2, a transcriptional co-activator of serum response factor, is required for skeletal myogenic differentiation. J. Biol. Chem. 278:41977–41987 [DOI] [PubMed] [Google Scholar]
- 51. Semenova E., Wang X., Jablonski M. M., Levorse J., Tilghman S. M. 2003. An engineered 800 kilobase deletion of Uchl3 and Lmo7 on mouse chromosome 14 causes defects in viability, postnatal growth and degeneration of muscle and retina. Hum. Mol. Genet. 12:1301–1312 [DOI] [PubMed] [Google Scholar]
- 52. Sotiropoulos A., Gineitis D., Copeland J., Treisman R. 1999. Signal-regulated activation of serum response factor is mediated by changes in actin dynamics. Cell 98:159–169 [DOI] [PubMed] [Google Scholar]
- 53. Spector I., Shochet N. R., Blasberger D., Kashman Y. 1989. Latrunculins—novel marine macrolides that disrupt microfilament organization and affect cell growth. I. Comparison with cytochalasin D. Cell Motil. Cytoskeleton 13:127–144 [DOI] [PubMed] [Google Scholar]
- 54. Spector I., Shochet N. R., Kashman Y., Groweiss A. 1983. Latrunculins: novel marine toxins that disrupt microfilament organization in cultured cells. Science 219:493–495 [DOI] [PubMed] [Google Scholar]
- 55. Sullivan A. L., et al. 2011. Serum response factor utilizes distinct promoter- and enhancer-based mechanisms to regulate cytoskeletal gene expression in macrophages. Mol. Cell. Biol. 31:861–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Suwa H., et al. 1998. Overexpression of the rhoC gene correlates with progression of ductal adenocarcinoma of the pancreas. Br. J. Cancer 77:147–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Treisman R., Alberts A. S., Sahai E. 1998. Regulation of SRF activity by Rho family GTPases. Cold Spring Harb. Symp. Quant. Biol. 63:643–651 [DOI] [PubMed] [Google Scholar]
- 58. Treisman R., Ammerer G. 1992. The SRF and MCM1 transcription factors. Curr. Opin. Genet. Dev. 2:221–226 [DOI] [PubMed] [Google Scholar]
- 59. Valouev A., et al. 2008. Genome-wide analysis of transcription factor binding sites based on ChIP-Seq data. Nat. Methods 5:829–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. van Golen K. L., et al. 1999. A novel putative low-affinity insulin-like growth factor-binding protein, LIBC (lost in inflammatory breast cancer), and RhoC GTPase correlate with the inflammatory breast cancer phenotype. Clin. Cancer Res. 5:2511–2519 [PubMed] [Google Scholar]
- 61. Vartiainen M. K., Guettler S., Larijani B., Treisman R. 2007. Nuclear actin regulates dynamic subcellular localization and activity of the SRF cofactor MAL. Science 316:1749–1752 [DOI] [PubMed] [Google Scholar]
- 62. Wang Y., et al. 1998. Activation of the c-fos serum response element by phosphatidyl inositol 3-kinase and rho pathways in HeLa cells. Cell Growth Differ. 9:513–522 [PubMed] [Google Scholar]
- 63. Wick M., Burger C., Brusselbach S., Lucibello F. C., Muller R. 1994. Identification of serum-inducible genes: different patterns of gene regulation during G0→S and G1→S progression. J. Cell Sci. 107(Pt. 1):227–239 [DOI] [PubMed] [Google Scholar]
- 64. Yamada A., Irie K., Fukuhara A., Ooshio T., Takai Y. 2004. Requirement of the actin cytoskeleton for the association of nectins with other cell adhesion molecules at adherens and tight junctions in MDCK cells. Genes Cells 9:843–855 [DOI] [PubMed] [Google Scholar]
- 65. Yang S. H., Bumpass D. C., Perkins N. D., Sharrocks A. D. 2002. The ETS domain transcription factor Elk-1 contains a novel class of repression domain. Mol. Cell. Biol. 22:5036–5046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zhang J., Zamir I., Lazar M. A. 1997. Differential recognition of liganded and unliganded thyroid hormone receptor by retinoid X receptor regulates transcriptional repression. Mol. Cell. Biol. 17:6887–6897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zhang M., Fang H., Zhou J., Herring B. P. 2007. A novel role of Brg1 in the regulation of SRF/MRTFA-dependent smooth muscle-specific gene expression. J. Biol. Chem. 282:25708–25716 [DOI] [PubMed] [Google Scholar]