Abstract
Emerging genetic and clinical evidence suggests a link between Gaucher disease and the synucleinopathies Parkinson disease and dementia with Lewy bodies. Here, we provide evidence that a mouse model of Gaucher disease (Gba1D409V/D409V) exhibits characteristics of synucleinopathies, including progressive accumulation of proteinase K-resistant α-synuclein/ubiquitin aggregates in hippocampal neurons and a coincident memory deficit. Analysis of homozygous (Gba1D409V/D409V) and heterozygous (Gba1D409V/+ and Gba1+/−) Gaucher mice indicated that these pathologies are a result of the combination of a loss of glucocerebrosidase activity and a toxic gain-of-function resulting from expression of the mutant enzyme. Importantly, adeno-associated virus-mediated expression of exogenous glucocerebrosidase injected into the hippocampus of Gba1D409V/D409V mice ameliorated both the histopathological and memory aberrations. The data support the contention that mutations in GBA1 can cause Parkinson disease-like α-synuclein pathology, and that rescuing brain glucocerebrosidase activity might represent a therapeutic strategy for GBA1-associated synucleinopathies.
Gaucher disease, the most prevalent lysosomal storage disease, is caused by mutations in the gene encoding glucocerebrosidase (GBA1) (1, 2). The disease is characterized by lysosomal accumulation of undegraded substrates, primarily glucosylceramide (GlcCer) and glucosylsphingosine (GlcSph). Routine administration of glycan-modified glucocerebrosidase (Cerezyme) has been shown to be effective in treating the hematological, skeletal, and visceral disease manifestations (3, 4). However, because the recombinant enzyme is unable to traverse the blood-brain barrier, this therapy does not address the increasingly recognized CNS manifestations (4, 5).
Emerging evidence suggests that mutations in GBA1 are the most common genetic risk factor for synucleinopathies, such as Parkinson disease (PD) and dementia with Lewy bodies (DLB), acting to modify the age of onset and disease progression (6–13). The nervous system of subjects with PD and DLB typically shows abnormal accumulation of α-synuclein (α-syn) in Lewy bodies (LB) and Lewy neurites (LN) (14). Aberrant processing of α-syn is thought to play a role in the development of these neurodegenerative diseases (15). Histopathological analyses of brains from Gaucher patients who developed Parkinsonism and from PD patients who harbored GBA1 mutations revealed the presence of α-syn–containing ubiquitinated LB and LN inclusions in the hippocampal region (11, 16–19). In addition, pathological accumulation of α-syn has recently been reported in double-mutant Gaucher mice carrying a hypomorphic prosaposin transgene to further decrease glucocerebrosidase activity (20, 21). Several hypotheses have been proposed to explain the relationship between GBA1 variants and the abnormal accumulation of α-syn in the CNS (13, 22). One intriguing possibility is that mutations in GBA1 can lead to an enzymatic loss-of-function and consequently decrease lysosomal activity and function, which might negatively affect α-syn processing and metabolism (23, 24). An alternative hypothesis posits that translation of a mutated GBA1 disrupts proteostasis processes involved in proper folding of proteins, leading to aggregation and accumulation of α-syn (25). These two hypotheses are not mutually exclusive and there is clinical and genetic evidence supporting each premise (2, 13).
The present study shows that a single point mutation in GBA1 can cause two characteristics of synucleinopathies (α-syn misprocessing and cognitive deficits). It also provides evidence that both enzymatic loss-of-function and toxic gain-of-function mechanisms contribute to development of Gaucher-related synucleinopathies. In addition, the data show that exogenous administration of glucocerebrosidase can overcome the pathological features in the Gaucher mice, suggesting CNS-delivered glucocerebrosidase replacement therapy as a putative therapeutic approach.
Results
Mouse Model of Gaucher Disease (Gba1D409V/D409V) Exhibits Hippocampal Accumulation of α-Syn/Ubiquitin Aggregates in Neurons.
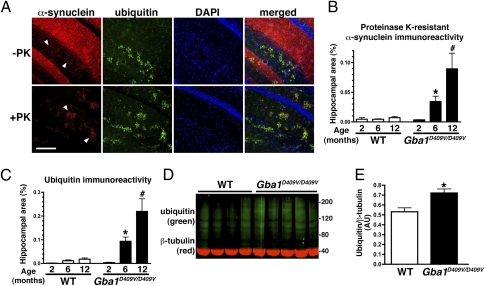
To determine if point mutations in Gba1 can cause α-syn and ubiquitin pathology similar to human patients (11, 16–18), brain sections of 12-mo-old Gba1D409V/D409V Gaucher mice and age-matched wild-type controls were examined. Although the high level of endogenous soluble α-syn precluded facile detection of aggregated α-syn, evidence of limited, punctate accumulation of α-syn was noted in the hippocampus of Gba1D409V/D409V mice but not control mice (Fig. 1A, arrowheads). Proteinase K treatment of tissue sections has been successfully used to unmask α-syn deposits in animal and human brains (26). In Gba1D409V/D409V mouse brain sections treated with proteinase K, the presence of punctate α-syn aggregates was readily revealed in the hippocampus (Fig. 1A), a region characteristically affected in humans with DLB or advanced PD and in Gaucher disease patients with Parkinsonism. Importantly, and also similar to LB and LN, the α-syn aggregates in the mice contained ubiquitin, as indicated by colocalization studies (Fig. 1A). Gba1D409V/D409V mice showed positive α-syn/ubiquitin immunoreactivity in the cerebral cortex and cerebellum as well, but to a lesser extent than in the hippocampus (Fig. S1).
Fig. 1.
Progressive accumulation of α-syn/ubiquitin aggregates in Gba1D409V/D409V Gaucher mouse brains. (A) Frozen sections were pretreated without (Upper) or with (Lower) proteinase K (PK) to uncover endogenous α-syn aggregates (arrowheads). Images show α-syn (red) and ubiquitin (green) immunostaining and nuclear staining (blue) in the hippocampus of 12-mo-old Gba1D409V/D409V mice. (Scale bar, 200 μm.) (B) Quantification of α-syn immunoreactivity in wild-type (WT) and Gba1D409V/D409V hippocampi at 2, 6, and 12 mo following proteinase K treatment showed progressive accumulation of aggregates with age (n ≥ 5 per group). (C) Quantification of ubiquitin immunoreactivity in Gba1D409V/D409V hippocampus at 2, 6, and 12 mo (n ≥ 5 per group) showed progressive accumulation with age, similar to α-syn. (D) Increased ubiquitination of high molecular weight proteins was detected in the hippocampus of Gba1D409V/D409V mice. Shown is an immunoblot of hippocampal lysates from 12-mo-old Gba1D409V/D409V mice and age-matched controls: ubiquitin (green) and β-tubulin (red). Each lane represents an independent mouse brain. (E) Quantitative analysis of ubiquitin/β-tubulin immunoblots in 12-mo-old Gba1D409V/D409V mice and age-matched controls. All data represent mean ± SEM. Bars with * and # symbols are significantly different from each other (P < 0.05), as noted also in Table 1.
The onset and progression of the accumulation of proteinase K-resistant α-syn and ubiquitin aggregates in the hippocampus of Gba1D409V/D409V mice were studied by staining brains from 2-, 6-, and 12-mo-old mutant mice and age-matched wild-type controls. At 2 mo of age, immunoreactivity levels of α-syn and ubiquitin in wild-type and Gaucher mice were not significantly different (Fig. 1 B and C, and Fig. S2). However, the levels of both α-syn and ubiquitin were significantly higher in Gba1D409V/D409V mice than in age-matched controls at 6 mo. Accumulation was progressive, with 12-mo-old Gba1D409V/D409V mice showing even higher levels of α-syn/ubiquitin aggregates (Fig. 1 B and C). Hence, the CNS of Gba1D409V/D409V mice recapitulates the progressive accumulation of ubiquitinated aggregates containing proteinase K-resistant α-syn, a primary pathological feature noted in patients with PD and DLB. These results indicated that the degradation pathways might be disturbed in mutant mouse brains. Indeed, Western blot analysis of wild-type and Gba1D409V/D409V hippocampal lysates showed no differences in lysosomal and autophagy markers except for beclin (Fig. S3), suggesting that initiation of autophagosome formation might be disturbed in mutant Gba1 mice. In addition, the hippocampal lysates exhibited significantly increased amounts of ubiquitinated proteins compared with wild-type mice (Fig. 1 D and E). These results suggest that degradation of cellular proteins might be directly or indirectly hindered by the loss of glucocerebrosidase activity, the presence of the mutant glucocerebrosidase, or both.
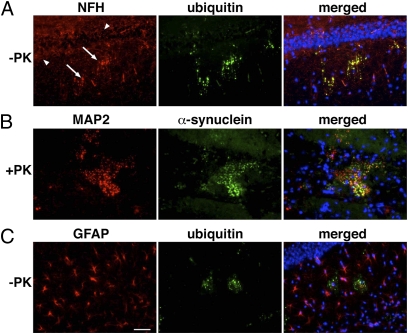
To establish the cellular localization of α-syn/ubiquitin aggregates in Gba1D409V/D409V mouse brains, sections were coimmunostained for neurofilament-H (NFH) and microtubule-associated protein (MAP2), two neuron-specific markers present in LB and LN (27), and for GFAP, an astrocyte marker. Staining for NFH in the Gba1D409V/D409V hippocampus showed a classic dendritic pattern (Fig. 2A, arrowheads) as well as some dendritic swellings, which colocalized with aggregated ubiquitin signal (Fig. 2A, arrows). In addition, the neuronal marker MAP2 colocalized with proteinase K-resistant α-syn aggregates in Gba1D409V/D409V hippocampus (Fig. 2B). Indeed, colocalization studies indicated that the majority of the ubiquitin and α-syn aggregates were present in NFH- and MAP2-positive cells (Fig. 2 A and B). None of the ubiquitinated aggregates in the Gba1D409V/D409V hippocampus were colocalized with GFAP (Fig. 2C). Thus, accumulation of α-syn/ubiquitin in Gba1D409V/D409V mice occurs primarily within neurites, like LN in human cases.
Fig. 2.
Ubiquitin and α-syn aggregates colocalize with neuronal markers. Sections of hippocampus in 12-mo-old Gba1D409V/D409V brains, either pretreated with proteinase K (PK) (B) or not (A and C) before immunostaining. Immunostaining for NFH (A) exposed the presence of normal (arrowheads) and dystrophic (arrows) hippocampal neurites. (A) Colabeling of NFH and ubiquitin showed that ubiquitin aggregates were located in neurites. (B) Pretreating sections with proteinase K showed MAP2 colocalized with α-syn in Gba1D409V/D409V hippocampus. (C) Immunostaining for GFAP and ubiquitin showed no colocalization of the astrocyte marker with the ubiquitin aggregates. DAPI nuclear staining is shown in blue. (Scale bar, 50 μm.)
Brains of Gba1D409V/D409V Mice Exhibit No Inflammation or Cell Death Despite Increased Levels of Glucosylsphingosine.
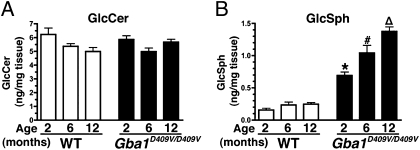
The residual glucocerebrosidase activity in the hippocampus of Gba1D409V/D409V mice was determined to be 25 ± 4% of the wild-type controls (n = 5, P < 0.05), similar to previous findings on whole-brain lysates (28). A deficiency of glucocerebrosidase activity led to increased lysosomal storage of GlcCer and GlcSph in brains of patients with neuronopathic Gaucher disease (29, 30). Mass spectrometry analysis of glycosphingolipids in the Gba1D409V/D409V hippocampus showed normal GlcCer levels (Fig. 3A) but marked and progressive accumulation of GlcSph starting at 2 mo of age (Fig. 3B). Similar results were obtained in the cerebral cortex and cerebellum of the Gba1D409V/D409V mice (Fig. S4). Hence, the residual enzymatic activity in Gba1D409V/D409V mice was sufficient to prevent the accumulation of GlcCer but not of the neurotoxic lipid, GlcSph.
Fig. 3.
Analysis of glucocerebrosidase substrates in Gba1D409V/D409V mouse hippocampus shows progressive accumulation of glucosylsphingosine. The levels of GlcCer and GlcSph substrates in hippocampal lysates of 2-, 6-, and 12-mo-old wild-type and Gba1D409V/D409V mice were quantified by mass spectrometry (n ≥ 6 per group). (A) No differences in the levels of GlcCer were noted between normal and Gaucher mice. (B) However, Gba1D409V/D409V mice exhibited a marked and progressive accumulation of GlcSph. Data shown represent mean ± SEM. Bars with different symbols are significantly different from each other and from wild-type controls (P < 0.05).
In a different set of experiments, hippocampal sections of 12-mo-old Gba1D409V/D409V mice showed no evidence of inflammation or neuronal death by staining for GFAP (astroglial marker), IbaI (microglial marker), CD68 (macrophage and microglial marker), or Fluoro-Jade C (neuronal degeneration marker) (Fig. S5). Furthermore, staining for tyrosine hydroxylase showed no overt nigrostriatal cell loss in Gba1D409V/D409V mice up to one year of age (Fig. S6). Thus, despite the presence of marked α-syn/ubiquitin pathology and increased GlcSph levels, no neuroinflammatory reaction or neuronal cell death was found in the brains of the Gba1D409V/D409V Gaucher mice.
Gba1D409V/D409V Gaucher Mice Exhibit Impaired Memory.
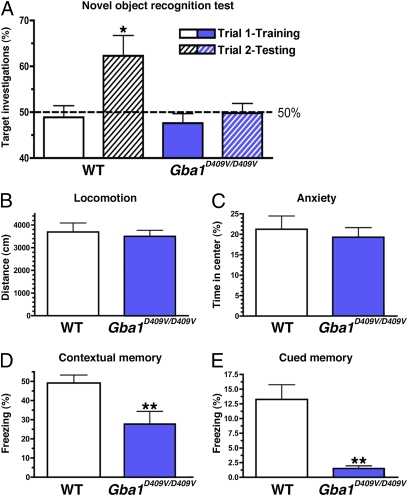
As the brains of Gba1D409V/D409V mice exhibited progressive α-syn/ubiquitin pathology and elevated GlcSph levels in the hippocampus, it was pertinent to assess memory function. Six-month-old Gba1D409V/D409V mice and wild-type littermates were subjected to novel object recognition and contextual fear-conditioning behavioral tests. For the novel object-recognition test, after a habituation period of 3 d, mice were presented with two identical objects (trial 1, training). As expected, both wild-type and Gba1D409V/D409V Gaucher mice displayed no preference for either object during this first exposure (Fig. 4A). Twenty-four hours later, the same mice were presented with a familiar object and a novel object (trial 2, testing). Although wild-type mice showed a higher preference for investigating the novel object (P = 0.02), Gba1D409V/D409V mice showed no significant novelty discrimination (Fig. 4A) (P = 0.92), suggestive of memory impairment. This deficit in the Gba1D409V/D409V mice was observed also in a 12-mo-old independent cohort and was not related to a loss of ambulatory activity or an increase in anxiety, a judgment based on similar performance to control mice in an open-field test (Fig. 4 B and C; P = 0.69 and P = 0.62, respectively).
Fig. 4.
Hippocampal memory deficits in Gba1D409V/D409V mice. (A) Six-month-old wild-type and Gba1D409V/D409V mice showed no object preference when exposed to two identical objects. Results from Trial 1 (training) are shown as white (wild-type mice) and blue (Gba1D409V/D409V mice) solid bars. After a 24-h retention period, mice were presented with a novel object. In trial 2 (testing), wild-type mice (white hatched bar) investigated the novel object significantly more times (P < 0.05). In contrast, Gba1D409V/D409V mice (blue hatched bar) showed no preference for the novel object, indicating a cognitive impairment (P = 0.92). The horizontal line demarcates 50% target investigations, which represents no preference for either object. (B and C) Open-field analysis showed no differences in ambulation or anxiety between Gba1D409V/D409V and age-matched wild-type controls. (D and E) The memory impairment of Gba1D409V/D409V mice was confirmed by decreased freezing responses in contextual and cued fear conditioning testing. All data represent mean ± SEM (*P < 0.05; **P < 0.01).
This observed memory impairment was confirmed with another behavioral paradigm, fear conditioning. Another cohort of 6-mo-old Gba1D409V/D409V mice and wild-type littermates was first exposed to a conditioned (tone) stimulus followed by an unconditioned (shock) stimulus. After 24 h, context-specific and tone-related freezing behaviors were measured consecutively (see Materials and Methods). Gba1D409V/D409V Gaucher mice exhibited significant deficits in both contextual and cued fear tasks (P < 0.01 and P < 0.001, respectively), confirming the memory impairment in these animals (Fig. 4 D and E).
Studies in Gaucher Heterozygous Mice Suggest That a Loss of Glucocerebrosidase Activity and Expression of Mutant Glucocerebrosidase Conspire to Generate the α-Syn/Ubiquitin Pathology and Memory Deficit.
In the search for a relationship between glucocerebrosidase activity and Parkinsonism, different knock-in or knock-out Gaucher mice were examined for hippocampal accumulation of α-syn/ubiquitin and memory function. In addition to the Gba1D409V/D409V mice, GBA1 knockout mice (Gba1−/−) and heterozygous mice (Gba1D409V/+, Gba1+/−) were studied (Table 1; see also Materials and Methods). The brains of young Gba1−/− mice contained less than 10% residual glucocerebrosidase activity and accumulate both GlcCer and GlcSph. Concomitant with these changes was the development of neurodegenerative disease leading to death by postnatal day 14 (31, 32). Consequently, further studies were restricted to heterozygous Gba1+/− mice that retain ∼54% of wild-type GBA1 activity (Table 1).
Table 1.
Characteristics of different mouse models of Gaucher disease at 6 mo of age
| Gba+/+ | Gba1D409V/+ | Gba1D409V/D409V | Gba1+/− | |
| Glucocerebrosidase activity (ng/h/mg of protein) | 19.2 ± 1.3 (n = 8) | 11.3 ± 1.2 (n = 7)* | 3.6 ± 0.4 (n = 7)# | 10.4 ± 0.8 (n = 7)* |
| GlcCer (ng/mg wet tissue) | 5.9 ± 0.5 (n = 7) | 6.2 ± 0.8 (n = 7) | 6.3 ± 0.8 (n = 7) | 6.1 ± 0.6 (n = 7) |
| GlcSph (ng/mg wet tissue) | 0.32 ± 0.06 (n = 7) | 0.29 ± 0.08 (n = 7) | 0.73 ± 0.21 (n = 7)* | 0.29 ± 0.09 (n = 7) |
| α-Synuclein aggregates† | 0.04 ± 0.01% (n = 8) | 0.15 ± 0.03% (n = 5)* | 0.23 ± 0.04% (n = 7) # | 0.05 ± 0.02% (n = 5) |
| Hippocampal memory‡ | 71 ± 2% (n = 7)§ | 72 ± 4% (n = 6)§ | 47 ± 5% (n = 7) | 71 ± 3% (n = 7)§ |
*,#Groups with different symbols are significantly different from each other using one-way ANOVA followed by Newman-Keuls (P < 0.05). All data are expressed as mean ± SEM.
†Proteinase K-resistant α-syn aggregates are represented as percent-threshold area in the hippocampus.
‡Data for memory function represents the percentage of target investigations on the testing day (T2).
§Wild-type (Gba1+/+), Gba1D409V/+, and Gba1+/− mice showed a significant preference for the novel object (target investigations > 50%) on the testing day using one-sample t test (P < 0.01).
In contrast to Gba1D409V/D409V mice, neither the heterozygous Gba1+/− nor the Gba1D409V/+ mice accumulated GlcSph, indicating that carrying one copy of the wild-type Gba1 allele is sufficient to prevent buildup of this toxic substrate (Table 1). Surprisingly, despite retaining similar levels of glucocerebrosidase activity, Gba1D409V/+ but not Gba1+/− mice exhibited α-syn/ubiquitin aggregates at 6 mo of age. However, the amount of aggregates in heterozygous Gba1D409V/+ mice was ∼50% of the level detected in Gba1D409V/D409V mice at the same age (Table 1), suggesting that in addition to the loss of enzymatic activity, a single dose of a mutant glucocerebrosidase (D409V) might also contribute to the generation of misprocessed α-syn.
To determine if the heterozygous mice exhibited hippocampal memory deficits, 6-mo-old wild-type, Gba1+/−, Gba1D409V/+, and Gba1D409V/D409V mice were subjected to the novel object-recognition test. In contrast to Gba1D409V/D409V mice, animals carrying at least one copy of the wild-type Gba1 allele showed intact memory function (Table 1). Hence, hippocampal accumulation of α-syn/ubiquitin aggregates per se is not sufficient to cause a loss in memory recognizable with the tests used; loss of more than 75% glucocerebrosidase activity appears to be an additional requirement.
Hippocampal Administration of Recombinant scAAV1 Vector Expressing Glucocerebrosidase Corrects Biochemical and Memory Deficits in Gba1D409V/D409V Mice.
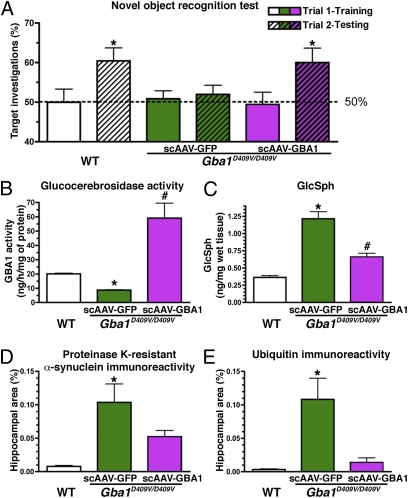
To determine if expression of exogenous glucocerebrosidase could lower the amount of α-syn/ubiquitin aggregates and correct the memory deficits of Gba1D409V/D409V mice, recombinant self-complementary adeno-associated viral (scAAV1) vectors expressing human glucocerebrosidase (scAAV1-GBA1) or green fluorescent protein (scAAV1-GFP) were administered bilaterally into the hippocampus of 2-mo-old mice. Memory function was evaluated 2 mo later with the novel object-recognition test. The administration of scAAV1-GBA1 but not scAAV1-GFP into the hippocampus corrected the memory deficit in Gba1D409V/D409V mice (Fig. 5A) (P < 0.05).
Fig. 5.
CNS administration of scAAV1-GBA1 ameliorates accumulation of GlcSph and ubiquitin/α-syn aggregates and corrects memory deficits in Gba1D409V/D409V mice. Two-month-old Gba1D409V/D409V mice were injected with either scAAV1-GFP (n = 8) or scAAV1-GBA1 (n = 12) bilaterally into the hippocampus. Age-matched wild-type (WT) uninjected mice were used as a positive control (n = 10). (A) Animals were subjected to the novel object-recognition test at 2 mo postinjection, as outlined in the legend to Fig. 4. scAAV1-GBA1–treated Gba1D409V/D409V mice (purple hatched bar) but not scAAV1-GFP–treated animals (green hatched bar) showed improvement in the memory test. Four months postinjection, tissues were collected for biochemical and pathological analysis. Hippocampal administration of scAAV1-GBA1 to Gba1D409V/D409V mice increased glucocerebrosidase activity (B), promoted clearance of GlcSph (C), and reduced the accumulation of hippocampal α-syn (D) and ubiquitin (E) aggregates. Results represent mean ± SEM. (A) Horizontal line demarcates 50% target investigations, which represents no preference for either object (*, significantly different from 50%, P < 0.05); (B–E) Bars with different symbols are significantly different from each other (P < 0.05).
Immunohistochemical examination of brains of scAAV1-GBA1–treated Gba1D409V/D409V mice at 4 mo posttreatment showed abundant hippocampal expression of glucocerebrosidase (Fig. S7) that was ∼10-fold higher than in scAAV1-GFP–injected littermates (Fig. 5B). Provision of exogenous glucocerebrosidase significantly reduced the amount of GlcSph in the hippocampus of Gba1D409V/D409V but not in the scAAV1-GFP–injected controls (Fig. 5C). Notably, the hippocampal levels of α-syn and ubiquitin aggregates were also significantly reduced by scAAV1-GBA1 treatment compared with scAAV1-GFP–treated littermates (Fig. 5 D and E). These data established that augmenting glucocerebrosidase activity in the CNS can abate the levels of GlcSph and α-syn/ubiquitin aggregates and, importantly, correct the memory impairment in Gba1D409V/D409V mice.
Discussion
Genetic, pathological, and clinical studies have implicated variants in GBA1 as a risk factor for development of the synucleinopathies PD and DLB (13). Our studies in mice clearly support the contention that there is a link between mutations in GBA1 and the development of synucleinopathies. The primary pathologic feature of the neuronal synucleinopathies is the deposition of ubiquitinated LB and LN rich in α-syn in neurons (27, 33, 34). Both murine models of Gaucher disease (Gba1D409V/D409V and Gba1D409V/+) exhibited a similar CNS phenotype. The increase in α-syn aggregates associated with ubiquitin immunoreactivity was progressive, particularly in the hippocampus of the Gba1D409V/D409V mice. Hippocampal localization of LB and LN has also been noted in Gaucher carriers and in Gaucher patients with symptoms of Parkinsonism (16, 17), as well as in the unexpectedly high percentage of PD patients carrying GBA1 mutations (11, 18). Similar α-syn aggregates have been recently described in GbaV394L/V394L and GbaD409H/D409H Gaucher mice carrying a prosaposin hypomorphic transgene to reduce glucocerebrosidase activity (20, 21). Importantly, the α-syn aggregates in Gba1D409V/D409V mice were proteinase K-resistant, like those reported for misfolded α-syn inclusions in transgenic mice overexpressing α-syn and in human subjects with synucleinopathies (26). Coincidentally, Gba1D409V/D409V hippocampi show increased α-syn concentration in membrane-associated fractions (21). Moreover, as with the α-syn inclusions in PD and DLB patients, and in Gaucher patients who developed Parkinsonism (13), the inclusions in the Gaucher mice were located primarily in neurons, as indicated by their colocalization with the neuronal markers MAP2 and NFH.
Another feature shared by human synucleinopathies and neuropathic Gaucher disease is inflammation in the brain (3, 4, 17, 35), but despite the presence of overt neuropathology and accumulation of GlcSph, no indication of astrogliosis, microgliosis, or neuronal death was observed in Gba1D409V/D409V mice. This difference suggests that an alternative or more complex pathogenic mechanism underlies the neuroinflammatory process in synucleinopathies and neuropathic Gaucher disease. Another explanation might be that the pathological insult in Gba1D409V/D409V mice is relatively mild, as more severely affected Gaucher mice and transgenic mice overexpressing α-syn do display glial activation, particularly at late stages of disease after neuronal death has occurred (20, 26, 31, 32).
PD patients with GBA1 mutations, in addition to exhibiting α-syn–positive hippocampal LB-like inclusions, also have pronounced cognitive impairments (11, 16, 17, 36). Our finding of similar neuronal aggregates of α-syn/ubiquitin in the hippocampus of Gba1D409V/D409V mice prompted an evaluation of memory function. Using two different behavioral paradigms, novel object recognition and fear conditioning, memory impairment was demonstrated in this mouse model.
Brains of patients with neuronopathic Gaucher disease contain increased GlcCer and GlcSph (29, 30), and mice with severe Gaucher disease (Gba1−/−), which have a median lifespan of only 14 d, present a similar increase in these glycolipids (31, 32). The less severe Gba1D409V/D409V Gaucher mice do not accumulate GlcCer, even at 12 mo of age, and have a normal lifespan, presumably because these mice retain higher glucocerebrosidase activity (28). However, this article is unique in reporting that brains of Gba1D409V/D409V mice progressively accumulate GlcSph, and have impairment in cognitive memory. The divergent genotype-phenotype correlation between human Gaucher and mouse models has been previously documented (28). GlcSph is a putative neurotoxin that can affect neuronal calcium mobilization (2, 37) and might contribute to the development of cognitive deficits in Gba1D409V/D409V mice.
The Gaucher Gba1D409V/D409V mouse model, recapitulating some of the cardinal features of synucleinopathies, is a valuable tool to address questions of disease mechanism and potential therapy. Two fundamental hypotheses have evolved to explain the molecular link between Gaucher disease and synucleinopathies: a toxic gain-of-function and an enzymatic deficit (13, 22). In support of the former, it was noted that α-syn/ubiquitin inclusions were apparent in the brains of Gba1D409V/D409V and Gba1D409V/+ mice, but not in age-matched Gba1+/− mice. Because Gba1D409V/+ and Gba1+/− mice exhibited similar levels of residual glucocerebrosidase activity and no glycolipid accumulation, the mutant (D409V) enzyme might have contributed to the formation of the α-syn/ubiquitin aggregates. This finding would argue that the Parkinsonism noted in Gaucher patients might have been caused, at least in part, by a toxic gain-of-function. However, Gba1D409V/D409V mice exhibited lower levels of glucocerebrosidase activity and higher amounts of α-syn/ubiquitin aggregates than Gba1D409V/+ littermates, which suggests that loss-of-function (glucocerebrosidase activity) also might accelerate the accumulation of α-syn. Consistent with this notion, pharmacological inhibition of glucocerebrosidase activity negatively affects α-syn metabolism (24), and glucocerebrosidase augmentation reduces α-syn misprocessing in Gba1D409V/D409V mice (Fig. 5D). In addition, GBA1-null mutations (84GG or IVS2+1) are associated with the highest risk for synucleinopathies, whereas a putative folding mutation N370S having the highest residual activity presents the lowest risk (38). Hence, the data suggest that mutations in GBA1 are sufficient to initiate aberrant α-syn folding but decrease in glucocerebrosidase activity (regardless of mutations) seems to accelerate misprocessing, and thereby increase the susceptibility and cause earlier onset of PD-like changes (8, 39).
Interestingly, the memory defect was observed only in Gba1D409V/D409V mice, not in heterozygous Gba1D409V/+ or Gba1+/− Gaucher mice, suggesting that accumulation of α-syn aggregates (detected in Gba1D409V/+ mice) is not sufficient per se to produce a memory deficit. Rather, it appears that a mutation-associated reduction of glucocerebrosidase activity to below 25% of normal is the more critical determinant of memory function.
As a reduction in glucocerebrosidase activity correlated with both the accumulation of α-syn/ubiquitin aggregates and memory impairment in the Gba1D409V/D409V mice, one might predict that rescuing this activity would correct these aberrations. Indeed, scAAV1-mediated expression of glucocerebrosidase in the CNS of 2-mo-old Gba1D409V/D409V mice promoted clearance of GlcSph, reduced α-syn/ubiquitin aggregates, and improved mouse performance in the novel object-recognition test. Hence, increasing glucocerebrosidase activity in the CNS by gene therapy or direct infusion of recombinant proteins or small molecule modulators of enzyme activity might result in slowing α-syn misprocessing and may result in slower disease progression in Gaucher-related synucleinopathies.
In summary, the present study demonstrates that mutations in GBA1 can promote α-syn misprocessing and memory deficits, two characteristics of PD and DLB. An extant mouse model of Gaucher, Gba1D409V/D409V, exhibited progressive histopathology with accumulation of proteinase K-resistant α-syn/ubiquitin aggregates reminiscent of LN, increased brain concentration of the neurotoxin GlcSph, and associated impairment of memory. These aberrations appeared to be partly because of a loss of lysosomal glucocerebrosidase activity and to a gain-of-function associated with production of mutant glucocerebrosidase. Importantly, reconstituting the CNS of Gba1D409V/D409V mice with wild-type glucocerebrosidase ameliorated the histopathological, biochemical, and behavioral alterations. Hence, augmenting glucocerebrosidase activity in the CNS of patients with Gaucher-related synucleinopathies might represent a viable therapeutic strategy.
Materials and Methods
Animals.
All procedures were approved by the Institutional Animal Care and Use Committee at Genzyme Corporation. The Gba1D409V/D409V mouse model of Gaucher disease harbors a D409V point mutation in the murine glucocerebrosidase (Gba1) gene (28). Gba1D409V/D409V mice were crossed with C57BL/6 mice to generate Gba1D409V/+ animals. Gba1−/− and Gba1+/− mice used in this study express a truncated and unstable glucocerebrosidase in all tissues except in the skin and have been described in detail (31).
Western Blotting.
These procedures are described in SI Materials and Methods.
Measurements of Glucocerebrosidase Activity and Glycosphingolipid Levels.
Brain and hippocampal glucocerebrosidase activities were determined as previously described, with 4-methylumbelliferyl-β-d-glucoside as the artificial substrate (32). Tissue GlcCer and GlcSph levels were measured by mass spectrometry, as previously described (32).
Immunohistochemistry and Morphometric Analysis.
These procedures are described in SI Materials and Methods. Some tissue sections were pretreated with proteinase K (1:4 dilution; DAKO) for 7 min at room temperature to expose α-syn and other aggregated proteins (26).
Mice Behavioral Tests.
A detailed description of the behavioral tests is available in SI Materials and Methods.
Self-Complementary AAV Vectors.
A detailed description of the AAV vectors used in these studies is available in SI Materials and Methods.
Stereotaxic Injections.
Two-month-old Gba1D409V/D409V mice anesthetized with isoflurane received bilateral injections into the hippocampus (A–P: −2.00; M–L: ±1.5; D–V: −1.5 from bregma and dura; incisor bar: 0.0) with 2 μL/site of AAV1-GFP (n = 8) or AAV1-GBA1 (n = 12) with a 10-μL Hamilton syringe (rate of 0.5 μL/min for a total of 4 × 1010 DRP per injection site). One hour before surgery and 24 h after surgery, mice were given ketoprofen (5 mg/kg s.c.) for analgesia.
Statistical Analysis.
Statistical analyses were performed by Student's t-test or analysis of variance (ANOVA) followed by Newman-Keuls’ post hoc test. Preference for novelty was defined as investigating the novel object more than 50% of the times using a one-sample t test. All statistical analyses were performed with GraphPad Prism v4.0 (GraphPad Software). Values with P < 0.05 were considered significant.
Supplementary Material
Acknowledgments
The authors thank Monyrath Chan, Eric Roskelley, Tatyana Taksir, Kuma Misra, Denise Woodcock, Shelley Nass, Brenda Burnham, Maryellen O'Neill, Mario Cabrera, John Marshall, Ron Scheule, Matthew DeRiso, Michael Phipps, Leah Curtin, JoAnne Fagan, David Lee-Parritz, and Douglas Matthews; and Dr. Naomi Kleitman for critical reading of the manuscript.
Footnotes
Conflict of interest statement: S.P.S., J.C., C.K., T.J.T., L.L., L.M.S., M.A.P., S.H.C. and L.S.S. are employees of Genzyme Corporation. M.G.S., S.H.C and L.S.S. have been listed as co-inventors of an application to the US Patent Office on the “Treatment of synucleinopathies.”
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1108197108/-/DCSupplemental.
References
- 1.Brady RO, Kanfer JN, Bradley RM, Shapiro D. Demonstration of a deficiency of glucocerebroside-cleaving enzyme in Gaucher's disease. J Clin Invest. 1966;45:1112–1115. doi: 10.1172/JCI105417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sidransky E. Gaucher disease: Complexity in a “simple” disorder. Mol Genet Metab. 2004;83:6–15. doi: 10.1016/j.ymgme.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 3.Cox TM. Gaucher's disease—An exemplary monogenic disorder. QJM. 2001;94:399–402. doi: 10.1093/qjmed/94.8.399. [DOI] [PubMed] [Google Scholar]
- 4.Grabowski GA. Phenotype, diagnosis, and treatment of Gaucher's disease. Lancet. 2008;372:1263–1271. doi: 10.1016/S0140-6736(08)61522-6. [DOI] [PubMed] [Google Scholar]
- 5.Grabowski GA, Leslie N, Wenstrup R. Enzyme therapy for Gaucher disease: The first 5 years. Blood Rev. 1998;12:115–133. doi: 10.1016/s0268-960x(98)90023-6. [DOI] [PubMed] [Google Scholar]
- 6.Aharon-Peretz J, Rosenbaum H, Gershoni-Baruch R. Mutations in the glucocerebrosidase gene and Parkinson's disease in Ashkenazi Jews. N Engl J Med. 2004;351:1972–1977. doi: 10.1056/NEJMoa033277. [DOI] [PubMed] [Google Scholar]
- 7.Goker-Alpan O, et al. The spectrum of parkinsonian manifestations associated with glucocerebrosidase mutations. Arch Neurol. 2008;65:1353–1357. doi: 10.1001/archneur.65.10.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clark LN, et al. Mutations in the glucocerebrosidase gene are associated with early-onset Parkinson disease. Neurology. 2007;69:1270–1277. doi: 10.1212/01.wnl.0000276989.17578.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mata IF, et al. Glucocerebrosidase gene mutations: A risk factor for Lewy body disorders. Arch Neurol. 2008;65:379–382. doi: 10.1001/archneurol.2007.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clark LN, et al. Association of glucocerebrosidase mutations with dementia with lewy bodies. Arch Neurol. 2009;66:578–583. doi: 10.1001/archneurol.2009.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neumann J, et al. Glucocerebrosidase mutations in clinical and pathologically proven Parkinson's disease. Brain. 2009;132:1783–1794. doi: 10.1093/brain/awp044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sidransky E, et al. Multicenter analysis of glucocerebrosidase mutations in Parkinson's disease. N Engl J Med. 2009;361:1651–1661. doi: 10.1056/NEJMoa0901281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Velayati A, Yu WH, Sidransky E. The role of glucocerebrosidase mutations in Parkinson disease and Lewy body disorders. Curr Neurol Neurosci Rep. 2010;10:190–198. doi: 10.1007/s11910-010-0102-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spillantini MG, et al. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 15.Vila M, Przedborski S. Genetic clues to the pathogenesis of Parkinson's disease. Nat Med. 2004;10(7) Suppl:S58–S62. doi: 10.1038/nm1068. [DOI] [PubMed] [Google Scholar]
- 16.Tayebi N, et al. Gaucher disease with parkinsonian manifestations: Does glucocerebrosidase deficiency contribute to a vulnerability to parkinsonism? Mol Genet Metab. 2003;79:104–109. doi: 10.1016/s1096-7192(03)00071-4. [DOI] [PubMed] [Google Scholar]
- 17.Wong K, et al. Neuropathology provides clues to the pathophysiology of Gaucher disease. Mol Genet Metab. 2004;82:192–207. doi: 10.1016/j.ymgme.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 18.Nishioka K, et al. Glucocerebrosidase mutations in diffuse Lewy body disease. Parkinsonism Relat Disord. 2011;17:55–57. doi: 10.1016/j.parkreldis.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goker-Alpan O, Stubblefield BK, Giasson BI, Sidransky E. Glucocerebrosidase is present in α-synuclein inclusions in Lewy body disorders. Acta Neuropathol. 2010;120:641–649. doi: 10.1007/s00401-010-0741-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu YH, et al. Accumulation and distribution of alpha-synuclein and ubiquitin in the CNS of Gaucher disease mouse models. Mol Genet Metab. 2011;102:436–447. doi: 10.1016/j.ymgme.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cullen V, et al. Acid beta-glucosidase mutants linked to Gaucher disease, Parkinson disease, and Lewy body dementia alter alpha-synuclein processing. Ann Neurol. 2011;69:940–953. doi: 10.1002/ana.22400. [DOI] [PubMed] [Google Scholar]
- 22.Goldin E. Gaucher disease and Parkinsonism, a molecular link theory. Mol Genet Metab. 2010;101:307–310. doi: 10.1016/j.ymgme.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schlossmacher MG, Cullen V, Müthing J. The glucocerebrosidase gene and Parkinson's disease in Ashkenazi Jews. N Engl J Med. 2005;352:728–731. author reply 728–731. [PubMed] [Google Scholar]
- 24.Manning-Boğ AB, Schüle B, Langston JW. Alpha-synuclein-glucocerebrosidase interactions in pharmacological Gaucher models: A biological link between Gaucher disease and Parkinsonism. Neurotoxicology. 2009;30:1127–1132. doi: 10.1016/j.neuro.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 25.Ron I, Rapaport D, Horowitz M. Interaction between parkin and mutant glucocerebrosidase variants: A possible link between Parkinson disease and Gaucher disease. Hum Mol Genet. 2010;19:3771–3781. doi: 10.1093/hmg/ddq292. [DOI] [PubMed] [Google Scholar]
- 26.Neumann M, et al. Misfolded proteinase K-resistant hyperphosphorylated alpha-synuclein in aged transgenic mice with locomotor deterioration and in human alpha-synucleinopathies. J Clin Invest. 2002;110:1429–1439. doi: 10.1172/JCI15777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shults CW. Lewy bodies. Proc Natl Acad Sci USA. 2006;103:1661–1668. doi: 10.1073/pnas.0509567103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu YH, Quinn B, Witte D, Grabowski GA. Viable mouse models of acid beta-glucosidase deficiency: The defect in Gaucher disease. Am J Pathol. 2003;163:2093–2101. doi: 10.1016/s0002-9440(10)63566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nilsson O, Svennerholm L. Accumulation of glucosylceramide and glucosylsphingosine (psychosine) in cerebrum and cerebellum in infantile and juvenile Gaucher disease. J Neurochem. 1982;39:709–718. doi: 10.1111/j.1471-4159.1982.tb07950.x. [DOI] [PubMed] [Google Scholar]
- 30.Nilsson O, Grabowski GA, Ludman MD, Desnick RJ, Svennerholm L. Glycosphingolipid studies of visceral tissues and brain from type 1 Gaucher disease variants. Clin Genet. 1985;27:443–450. doi: 10.1111/j.1399-0004.1985.tb00229.x. [DOI] [PubMed] [Google Scholar]
- 31.Enquist IB, et al. Murine models of acute neuronopathic Gaucher disease. Proc Natl Acad Sci USA. 2007;104:17483–17488. doi: 10.1073/pnas.0708086104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cabrera-Salazar MA, et al. Intracerebroventricular delivery of glucocerebrosidase reduces substrates and increases lifespan in a mouse model of neuronopathic Gaucher disease. Exp Neurol. 2010;225:436–444. doi: 10.1016/j.expneurol.2010.07.023. [DOI] [PubMed] [Google Scholar]
- 33.Galvin JE, Lee VM, Trojanowski JQ. Synucleinopathies: Clinical and pathological implications. Arch Neurol. 2001;58:186–190. doi: 10.1001/archneur.58.2.186. [DOI] [PubMed] [Google Scholar]
- 34.Schlossmacher M. α–Synuclein and synucleinopathies. In: Rossor MN, Growdon JH, editors. The Dementias 2. Vol 30. Oxford: Butterworth Heinemann, Inc.; 2007. pp. 186–215. [Google Scholar]
- 35.Hirsch EC, Hunot S. Neuroinflammation in Parkinson's disease: A target for neuroprotection? Lancet Neurol. 2009;8:382–397. doi: 10.1016/S1474-4422(09)70062-6. [DOI] [PubMed] [Google Scholar]
- 36.Goker-Alpan O, et al. Glucocerebrosidase mutations are an important risk factor for Lewy body disorders. Neurology. 2006;67:908–910. doi: 10.1212/01.wnl.0000230215.41296.18. [DOI] [PubMed] [Google Scholar]
- 37.Lloyd-Evans E, et al. Glucosylceramide and glucosylsphingosine modulate calcium mobilization from brain microsomes via different mechanisms. J Biol Chem. 2003;278:23594–23599. doi: 10.1074/jbc.M300212200. [DOI] [PubMed] [Google Scholar]
- 38.Gan-Or Z, et al. Genotype-phenotype correlations between GBA mutations and Parkinson disease risk and onset. Neurology. 2008;70:2277–2283. doi: 10.1212/01.wnl.0000304039.11891.29. [DOI] [PubMed] [Google Scholar]
- 39.Nichols WC, et al. Parkinson Study Group-PROGENI Investigators Mutations in GBA are associated with familial Parkinson disease susceptibility and age at onset. Neurology. 2009;72:310–316. doi: 10.1212/01.wnl.0000327823.81237.d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.