Abstract
Background
High levels of amyloid-β (Aβ) characterize Alzheimer’s disease.
Objective
To investigate whether longitudinal changes in Aβ deposition can be detected in vivo in older adults without dementia (hereafter referred to as nondemented).
Design
Prospective study.
Setting
Community-dwelling older adults.
Participants
Twenty-four nondemented participants (4 with a baseline Clinical Dementia Rating Scale score of 0.5; mean [SD] age 79.2 [8.1] years) in the Baltimore Longitudinal Study of Aging underwent serial carbon 11-labeled Pittsburgh Compound B- positron emission tomography ([11C]PiB-PET) (follow-up at a mean [SD] of 1.5 [0.5] years), with 5 participants undergoing a third [11C]PiB-PET examination.
Main Outcome Measures
Annual changes in distribution volume ratio (DVR) were evaluated using a global index of cortical DVR (cDVR) and region-of-interest analyses. Given the variability of cDVR at initial PiB-PET, annual changes in cDVR in those with minimal vs those with elevated initial cDVR were compared.
Results
In nondemented older adults, annual increase in [11C]PiB retention is 0.011 DVR per year (0.9%; P=0.01) which localizes to prefrontal, parietal, lateral temporal, and occipital cortices as well as anterior and posterior cingulate cortices. Annual change in cDVR is greater in older adults with elevated cDVR than in those with minimal initial cDVR (p=0.006).
Conclusions
Fibrillar Aβ detected by [11C]PiB-PET increases over time even in nondemented older adults. Individuals with higher initial [11C]PiB retention have greater rates of Aβ deposition, providing evidence for differential rates of Aβ deposition. Moreover, regional vulnerabilities to Aβ deposition allow for more targeted investigation of early Aβ changes.
INTRODUCTION
Positron emission tomography (PET) amyloid imaging radiotracers have enabled longitudinal investigation of changes in fibrillar amyloid (Aβ) in vivo 1. Although several studies2–4 have documented longitudinal changes in patients with Alzheimer’s disease (AD), information on serial changes in Aβ in demented older adults without dementia is limited4.
In vivo imaging and postmortem studies of nondemented adults older than 70 years show elevated Aβ levels in approximately one-third of individuals5–12. However, cross-sectional studies cannot determine whether trajectories of Aβ accumulation differ in individuals with elevated deposition compared with those with minimal initial Aβ deposition. Longitudinal investigations of individual differences in trajectories of Aβ accumulation in relation to cognitive outcomes are needed. Characterization of individuals with elevated Aβ but with normal cognition also provides an opportunity for investigation of factors that explain why some individuals with elevated Aβ deposition progress to AD and others remain cognitively normal13, 14. Furthermore, longitudinal studies in nondemented older adults will provide information about the spatial patterns of Aβ change, which may guide more focused neuropathological studies of the earliest regional changes.
To investigate longitudinal patterns of change in Aβ deposition, we evaluated 24 nondemented older participants in the Neuroimaging Substudy of the Baltimore Longitudinal Study of Aging (NI-BLSA) who underwent at least 2 carbon 11-labeled ([11C]PiB-PET) studies during intervals up to 2.6 years. We hypothesized that there is variation in the rates of Aβ deposition in cognitively normal individuals and that higher rates of Aβ deposition occur in those with higher Aβ levels at initial PiB-PET. In addition, we anticipated regional variation in rates of Aβ deposition, with regions showing early Aβ deposition, such as the precuneus or the prefrontal cotex8, 9, demonstrating the clearest evidence of longitudinal change. Understanding longitudinal Aβ changes will contribute to the understanding of the association between Aβ deposition and progression to cognitive decline and AD.
MATERIALS AND METHODS
Study Participants
Twenty-four nondemented NI-BLSA participants (4 with a Clinical Dementia Rating Scale [CDR] score=0.5 at baseline) who underwent both an initial [11C]PiB PET and at least 1 follow-up scan (a mean [SD] of 1.5(0.5) years after the initial scan) were included in the study. Five of the 24 participants also underwent a third [11C]PiB PET study a mean (SD) of 2.2 (SD 0.3) years after the initial scan. Exclusionary criteria at neuroimaging study entry included metastatic cancer, severe pulmonary disease or cardiovascular disease, and central nervous system disease (i.e. stroke). Sample characteristics are given in Table 1.
Table 1.
Demographic, Genetic, and Cognitive Data
| Whole Group | cDVR < 1.062 | cDVR ≥1.062 | P Value | |
|---|---|---|---|---|
| Number of Subjects | (N=24) | (n=14) | (n=10) | |
| Demographics | ||||
| Age at initial [11C]PiB PET, mean (SD), y | 79.2 (8.1) | 75.9 (9.7) | 83.8 (4.27) | .008a |
| Follow-up interval, subsequent [11C] PiB PET, mean (SD), y | 1.5 (0.5) | 1.7 (0.5) | 1.28 (0.5) | .08a |
| Sex (No.) | 10 | 6 | 4 | >.99b |
| Education (SD), y | 17.0 (2.6) | 16.5 (2.7) | 17.7 (2.5) | .28a |
| Race (No.) | 20 | 11 | 9 | .61b |
| Genetics | ||||
| Apo E (1+ allele)c | 5 | 1 | 4 | .13b |
| Cognitive status at baseline, No. | ||||
| CDR= 0.5, informant based | 4 | 0 | 4 | .02b |
| CDR-SB = 0 | 19 | 13 | 6 | .12b |
| Neuropsychological Testing | ||||
| At Initial [11C]PiB PET, mean (SD) | ||||
| MMSE | 29.0 (1.0) | 29.3 (0.7) | 28.7 (1.2) | .14a |
| CVLT Immediate Recall(total correct) score | 57.8 (13) | 60.8 (12.3) | 54.0 (13.5) | .22a |
| CVLT Long Delay Recall (total correct) score | 12.0 (3.0) | 12.6 (3.9) | 11.2 (2.7) | .28a |
| BVRT, No. of errors | 5.5 (3.4) | 5.2 (3.9) | 5.9 (2.9) | .62a |
| Preceding [11C]PiB PET | ||||
| Slope MMSE | −0.008 (0.02) | 0.02 (0.02) | −0.04 (0.03) | .14d |
| Slope CVLT immediate recall score | −0.12 (0.16) | 0.22 (0.22) | −0.55 (0.20) | .01d |
| Slope CVLT long delay recall score, no errors | −0.09 (0.04) | −0.02 (0.04) | −0.17 (0.05) | .04d |
| Slope BVRT | 0.17 (0.05) | 0.14 (0.06) | 0.20 (0.07) | .50d |
Abbreviations: BVRT – Benton Visual Retention Test (increased slopes reflect greater decline in performance); [11C]PiB-PET, carbon 11-labeled Pittsburgh Compound B-positron emission tomography; CDR, Clinical Dementia Rating Scale; CDR-SB, Clinical Dementia Rating Scale Sum of Boxes; cDVR, cortical distribution volume ratio; CVLT, California Verbal Learning Test, MMSE - Minimental Status Examination.
By t-test, 2-tailed.
By Fisher’s exact test.
One data point is missing.
From mixed models
Written informed consent was obtained from each participant at each imaging visit. This study was approved by the Institutional Review Boards of the National Institute on Aging Intramural Research Program and The Johns Hopkins Medical Institutions.
Cognitive Status and Neuropsychological Evaluation
Cognitive status was determined by consensus diagnosis according to established procedures11, 15. Consensus diagnosis was based on serial neuropsychological evaluations and the CDR16, which was typically informant based. The neuropsychological measures used for consensus diagnosis obtained between years 1986 and 2005 included tests of mental status, word knowledge and verbal ability, memory, language, verbal fluency, attention, executive function, and spatial ability. Individuals with CDR = 0.5 who do not meet criteria for mild cognitive impairment typically have only mild memory loss on CDR and do not show clear evidence of decline on objective testing or functional loss. In addition to the diagnostic test battery, we administered the California Verbal Learning Test and Benton Visual Retention Tests as outcome measures of verbal and visual episodic memory, respectively.
Dynamic [11C]PiB-PET studies were performed on a Advance scanner GE Advance; GE Healthcare, Waukesha, Wisconsin) in 3-dimensional mode, and 37 time frames (90-minute acquisition) were obtained during a resting state. Image acquisition started immediately after intravenous bolus injection of mean (SD) 14.5(0.7) mCi [11C]PiB with specific activity of 4.4(2.3) Ci/μmol (at initial PiB-PET); 14.8 (0.8) mCi [11C]PiB with specific activity of 8.2 (5.1) Ci/μmol (at second PiB-PET);14.9 (0.4) mCi with specific activity of 6.3 (1.6) Ci/μmol in five participants at third PiB-PET. Participants were fitted with a thermoplastic mask for PET imaging to minimize motion during scanning. Transmission scans in 2-dimensional mode using a Ge-68 source were used for attenuation correction. Dynamic images were reconstructed using filtered back projection with a ramp filter (image size=128×128, pixel size=2×2mm, slice thickness=4.25mm), yielding a spatial resolution of about 4.5mm full-width at half maximum at the center of field of view.
Magnetic Resonance Image–Based Region-of-Interest Definition
Spoiled gradient recalled (SPGR) MRI scans (124 slices, image matrix=256×256, pixel size=0.94×0.94mm, slice thickness=1.5 mm) were coregistered to the mean of the first 20 min dynamic PET images for each participant using the mutual information method in the Statistical Parametric Mapping software (SPM2; Wellcome Department of Cognitive Neurology, London, England). With the exception of one claustrophobic participant in whom structural MRI was obtained only 10 years prior to the initial [11C]PiB-PET study, participants had structural MRI scans in conjunction with each [11C]PiB-PET study. ROI definitions were based on the initial MRI, which was coregistered to the corresponding [11C]PiB PET. The cerebellar ROI, which was used as the reference region, and 15 additional ROIs were manually drawn on the initial MRI and then applied to the initial17, 18 and co-registered follow-up PET scans.
Quantification of [11C]PiB retention
Parametric DVR images were generated by simultaneous fitting of a reference tissue model and linear regression with spatial constraint to dynamic [11C]PiB-PET images17, 19. The DVR values for the 15 ROIs were then extracted from the parametric images. Mean cortical DVR (cDVR) was calculated by averaging DVR values from orbitofrontal, prefrontal (including middle and inferior frontal gyri), superior frontal, parietal, lateral temporal, occipital, and anterior and posterior cingulate regions. Parametric images were then spatially normalized using an R1 (=K1/K1(reference tissue), the target to reference tissue ratio of tracer transport rate constant from vascular space to tissue) template17 and smoothed with a gaussian filter of 8, 8, 8 mm in the x, y, and z planes, respectively.
Initial [11C]PiB assessment
The cDVR was used as an index of cortical [11C]PiB retention. In addition to evaluating the group of nondemented older adults as a whole, we also evaluated changes in [11C]PiB retention in individuals with minimal cDVR and elevated cDVR at initial PiB-PET. We defined minimal cDVR as values below DVR = 1.062, based on the test/retest variability for DVR using SRTM analysis of +/−6.2%20 and the fact that DVR = 1 denotes absence of specific binding.
Global and regional changes in [11C]PiB retention
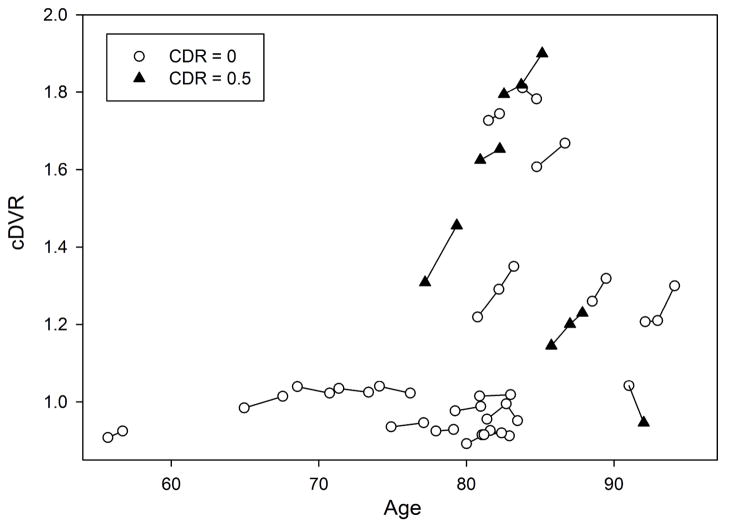
The cDVR at initial and follow-up PiB-PET were first examined in relation to age at initial PiB (Figure 1). Then, annual differences and annual percent differences were estimated as differences between cDVR at first follow-up and at initial PiB-PET, adjusted for interscan interval. Similarly, annual differences and percent differences were also estimated for the 15 ROI’s. The annual cDVR and regional changes in the whole group as well as in those with minimal and elevated initial cDVR were evaluated using Wilcoxon Signed-Rank Tests to test whether DVR values increased over time (one-sided tests). In addition, a regression model was used to assess whether age (continuous or dichotomized at age 80) was a predictor of longitudinal change in cDVR.
Figure 1.
Trajectories of longitudinal changes in carbon 11-labeled Pittsburgh Compound B retention in 24 nondemented older adults, including 5 individuals with a third follow-up scan. The Clinical Dementia Rating Scale (CDR) score at each time point is noted. cDVR indicates mean cortical distribution volume ratio.
Subsequently, we used the Wilcoxon Rank Sum tests to evaluate whether change in DVR differed between those with minimal and elevated cDVR at initial PiB-PET. We also repeated analyses examining whether baseline age (continuous or dichotomized at age 80) was an additional predictor of longitudinal change in cDVR, adding dichotomized baseline cDVR as an additional covariate.
RESULTS
Cortical [11C]PiB retention at initial evaluation
The mean (SD) cDVR at initial PiB evaluation was 1.179 (SD 0.305) for the entire sample, 0.97 (SD 0.046) for the group with cDVR<1.062, and 1.514 (SD 0.246) in the group with cDVR of 1.062 or greater.
Changes in global [11C]PiB retention
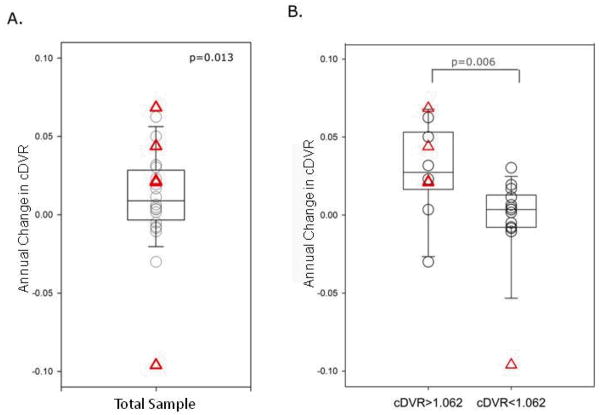
The mean (SD) annualized change in cDVR was 0.011(SD 0.033), with a median 0.009 DVR per year (P=0.01) (Figure 2). This represents a mean 0.9% annual increase in cDVR from baseline. Four older adults with CDR=0.5 and five of 19 older adults with CDR=0 had annualized change in cDVR greater than 0.02 DVR per year. The greatest increase in cDVR was observed in an 84 year-old man with 1 ApoE e4 allele who did not meet clinical consensus criteria for mild cognitive impairment 21 but had a CDR score of 0.5 (CDR Sum of Boxes (SOB) = 1.0). This participant’s cDVR increased from 1.309 to 1.456 (11.2%) over 2.1 years. In 6 participants, cDVR was lower at follow-up than at initial scan (mean (SD) annual change in cDVR of −0.026 (SD 0.035)). In five of the 6 participants, cDVR decreased by less than 0.062 at follow-up PiB, with trends in this low DVR range likely reflecting random variation.
Figure 2.
Annual changes in mean cortical carbon 11-labeled Pittsburgh Compound B ([11C]PiB) retention. A, Nondemented older adults as a group. B, Older adults with minimal vs elevated initial [11C]PiB retention. Triangles represent individuals with a Clinical Dementia Rating (CDR) Scale total score of 0.5. Two individuals with CDR=0.5 have an annual change in mean cortical distribution volume ratio (cDVR) of 0.02. The horizontal line in the middle of each box indicates the median, and the top and bottom borders of the box mark the 90th and 10th percentiles, respectively. The points beyond the whiskers are outliers beyond the 90th or 10th percentiles.
Annualized change in cDVR was significantly higher in those with elevated compared with those with a minimal cDVR at the initial evaluation (P =0.006) (Figure 2). In participants with minimal cDVR at initial PiB-PET, cDVR at follow-up did not significantly differ from the initial cDVR (P>0.05). In contrast, the group with elevated cDVR at initial PiB-PET showed significant increases in cDVR( p=0.02), representing a 2.3% increase in cDVR from baseline.
Baseline age was not a significant predictor of annual change in cDVR, with or without baseline cDVR in the model. Also, change in cDVR was not significantly associated with change in specific activity.
Changes in [11C]PiB retention in individuals with 3 [11C]PiB PET Studies
Of the 5 participants with 3 [11C]PiB studies each, the largest increase of 0.13 DVR (11.1 %) was observed over 2.45 years of follow-up in a participant with an initial cDVR of 1.22 (Figure 1). Overall, the participants with an elevated initial cDVR showed mean (SD) increases of 0.045 (SD 0.005) cDVR per year. Except for 1 individual who showed a nonlinear increase in cDVR, cDVR increases were linear over the three [11C]PiB-PET assessments (Figure 1). The cDVR of the 1 individual with minimal cDVR at initial evaluation decreased slightly during 2 year follow-up.
Regional Changes in [11C]PiB retention
ROI analysis revealed increases in DVR in the prefrontal, superior frontal, parietal, lateral temporal, occipital, and anterior and posterior cingulate corteces (P<0.05) (Table 2). Overall, significant regional differences in annual change in DVR between those with minimal vs those with an elevated cDVR at the initial PiB-PET were observed in the frontal, parietal, lateral temporal, occipital, anterior cingulated as well as in the caudate, and thalamus (P<0.05, Table 2). Except for the thalamus and midbrain, no significant changes in regional DVR were observed in those with a minimal initial cDVR. In contrast, in participants with an elevated cDVR at initial PiB-PET, increases in Aβ deposition were observed in the prefrontal cortex, superior frontal cortex, parietal, lateral temporal, occipital, and anterior cingulate cortices (P<0.05, Table 2).
Table 2.
Mean Cortical and Regional DVR at Initial PiB Study and Annual Change in DVR
| Baseline DVR Mean (SD) N=24 | Annual Change in DVR | Annual Change in DVR by Baseline cDVR | ||||
|---|---|---|---|---|---|---|
| All Mean(SD)[%] | Statistic al TestaPValue | Baseline DVR <1.062 Mean (SD) [%] | Baseline DVR≥ 1.062 Mean (SD) [%] | Group difference statistical testa P Value | ||
| Overall cortical DVR | ||||||
| cDVR | 1.179 (0.305) | 0.011 (0.9%) (0.033) | 0.013b | −0.002 (−0.1%) (0.029) | 0.03b (2.3%) (0.029) | 0.006b |
| Regional DVR | ||||||
| Orbitofrontal Cortex | 1.104 (0.292) | 0.008 (0.6%) (0.032) | 0.125 | −0.002 (−0.2%) (0.022) | 0.023 (1.7%) (0.040) | 0.04b |
| Prefrontal Cortexc | 1.109 (0.329) | 0.014 (1.1%) (0.035) | 0.041b | −0.002 (−0.1%) (0.03) | 0.036b (2.7%) (0.029) | 0.009b |
| Superior Frontal Cortex | 1.2 (0.38) | 0.010 (0.7%) (0.033) | 0.049b | −0.004 (−0.4%) (0.02) | 0.03b (2.3%) (0.038) | 0.005b |
| Anterior Cingulate Cortex | 1.29 (0.39) | 0.012 (0.8%) (0.039) | 0.014b | −0.002 (−0.2%) (0.037) | 0.032b (2.2%) (0.034) | 0.03b |
| Posterior Cingulate Cortex | 1.329 (0.399) | 0.017 (1.3%) (0.047) | 0.018b | 0.004 (0.4%) (0.033) | 0.034d (2.6%) (0.058) | 0.05 |
| Parietal Cortex | 1.145 (0.285) | 0.009 (0.8%) (0.036) | 0.025b | −0.003 (−0.2%) (0.03) | 0.026b (2.2%) (0.037) | 0.007b |
| Lateral Temporal Cortex | 1.133 (0.279) | 0.010 (0.8%) (0.035) | 0.003b | −0.002 (−0.2%) (0.032) | 0.026b (2.2%) (0.033) | 0.003b |
| Medial Temporal Cortex | 1.021 (0.109) | −0.003 (−0.4%) (0.022) | 0.268 | −0.005 (−0.5%) (0.022) | −0.001 (−0.2%) (0.022) | 0.33 |
| Occipital Cortex | 1.123 (0.194) | 0.009 (0.9%) (0.045) | 0.010b | −0.005 (−0.1%) (0.051) | 0.028b (2.3%) (0.026) | 0.03b |
| Caudate | 1.244 (0.302) | 0.008 (0.4%) (0.062) | 0.402 | −0.015 (−1.3%) (0.05) | 0.041 (2.7%) (0.065) | 0.03b |
| Putamen | 1.342 (0.239) | 0.010 (0.8%) (0.055) | 0.066 | −0.0004 (0.1%) (0.044) | 0.024 (1.8%) (0.067) | 0.13 |
| Thalamus | 1.398 (0.122) | −0.010 (−0.8%) (0.033) | 0.056 | −0.021b,e (−1.5%) (0.03) | 0.004 (0.4%) (0.032) | 0.049b |
| Pons | 1.571 (0.086) | 0.001 (0.1 %) (0.047) | 0.024b,e | 0.005 (0.3%) (0.056) | −0.004 (−0.3%) (0.033) | 0.13 |
| Midbrain | 1.503 (0.11) | 0.007 (0.6%) (0.068) | 0.088 | 0.015b,e (1.2%) (0.079) | −0.003 (−0.2%) (0.053) | 0.09 |
| White Matter | 1.384 (0.132) | −0.017 (−1.1%) (0.070) | 0.252 | −0.007 (−0.2%) (0.068) | −0.030 (−2.3%) (0.074) | 0.35 |
Abbreviations: DVR; distribution volume ratio; PiB, Pittsburgh Compound B.
Statistical test is performed on the difference values, P value is one-sided
P<0.05
Middle and inferior frontal gyri
Change in PiB retention in areas of nonspecific binding that is within test-retest variability
Cognitive status, Cognitive Performance, and Changes in Aβ Deposition
None of the participants met the diagnostic criteria for mild cognitive impairment at the time of imaging or at followup. At the initial PiB study, 4 of the 24 participants had CDR=0.5 with 1 additional participant having CDR=0.5 only at follow-up (Figure 1). The cognitive status of this latter participant fluctuated over time, with CDR reaching 0.5 at only 3 of 6 annual visits preceding the initial [11C]PiB study. Although this participant’s test scores were below the sample mean, declines in performance were inconsistent across memory outcomes. Except for this individual, participants in the sample with CDR=0.5 showed increases over time in global cortical and regional in [11C]PiB retention (Figure 1 and 2). Furthermore, individuals with an elevated PiB retention at the initial PiB-PET had worse longitudinal episodic memory performance in the years preceding PiB-PET (Table 1).
DISCUSSION
In this prospectively observed cohort of nondemented older adults, we found longitudinal increases in fibrillar Aβ deposition as detected by [11C]PiB-PET. Change in Aβ varied across individuals, with some showing no change and others showing annual increase as high as 11.2 % over 2.1 year follow-up. Variability in the annual rate of change was affected by global cDVR at initial PiB-PET, and increases were greater in nondemented older adults with elevated Aβ level compared with minimal Aβ level at the initial evaluation. The ROI analysis showed that longitudinal increases in [11C]PiB retention were observed in the prefrontal, parietal, lateral temporal, occipital and anterior and posterior cingulate regions.
Using cDVR as a global index of [11C]PiB retention, we found increases in fibrillar Aβ deposition over time. This finding, together with a prior report of serial changes in [11C]PiB retention4 provides evidence of longitudinal increases in Aβ deposition in nondemented older adults. The mean overall rate of increase in cortical [11C]PiB retention was only 0.011 DVR per year, a 0.9% increase from baseline DVR. Combined with findings by Jack et. al.4, this suggests that the overall magnitude of change in [11C]PiB retention in older adults, at least over short follow-up, is small.
However, we observed variability in rates of change in [11C]PiB retention. On an individual level, we observed increases up to 11.2% DVR over 2.1 years, exceeding the +/− 6.2 % test-retest variability reported for the simplified reference tissue model in [11C]PiB-PET studies20, 22. On the other hand, some nondemented older adults show no increases in [11C]PiB retention. To further investigate this variability, we evaluated whether increases in [11C]PiB retention over time differ from the initial [11C]PiB retention. Annual change in cDVR was significantly greater in older adults with an elevated [11C]PiB retention compared to minimal [11C]PiB retention at the initial PET scan. Cortical distribution volume ratio increased by a mean of 0.03 per year in older adults with elevated cDVR at initial evaluation, whereas those with minimal initial [11C]PiB retention showed no significant increase over time. These differential rates of [11C]PiB retention are consistent with models of longitudinal change proposing variable rates of Aβ deposition in nondemented older adults13, 23.
Understanding factors that explain the variability in level and change over time in [11C]PiB retention may help differentiate between normal aging and cognitive impairment. Several models propose that accelerated Aβ deposition predicts which individuals will convert to AD13, 14, 23, 24. However, in the present study, 5 of 19 individuals who remain cognitively healthy (e.g. CDR = 0) show longitudinal increases greater than 0.02 DVR per year, values comparable to increases in [11C]PiB retention in the 4 older adults with CDR=0.5. Continued prospective follow-up of this cohort will determine whether individuals with greater change in [11C]PiB retention will ultimately show accelerated cognitive decline and will clarify the relationships between trajectories of Aβ deposition, age, and cognitive status.
Investigation of the regional patterns of longitudinal increases in [11C]PiB retention is especially important in the group of nondemented older adults with lower and more localized regions of [11C]PiB retention. Except for the medial temporal gyrus, annual increases in [11C]PiB retention were observed in most cortical regions. These increases were detected not only in those with elevated baseline cDVR but also in the whole group of nondemented older adults. Of the cortical regions, the posterior cingulate gyrus had the highest annual increase in [11C]PiB retention of 1.3% DVR. Increases in [11C]PiB retention in the orbitofrontal gyrus were significant only when older adults with elevated vs minimal baseline cDVR were compared, suggesting that at least in this sample, the magnitude of increase in [11C]PiB retention in the orbitofrontal gyrus may be relatively low compared with that in other regions. These findings extend those of previous cross-sectional studies8, 9 of early Aβ deposition and may provide insights into the relationships of global and regional Aβ deposition with cognitive decline25 and changes in brain networks10, 26.
This study has several limitations. Given the small magnitude of annual change in [11C]PiB retention and its variability, investigation of large numbers of nondemented older adults is needed to understand the role of Aβ deposition in the context of neuropsychological, genetic, and biomarker data. Longer term follow-up is needed to investigate the trajectories of [11C]PiB retention and provide data about progression of disease. Nevertheless, this study of a well-characterized, prospectively observed community based sample provides detailed evaluation of [11C]PiB retention changes in nondemented older adults, including the regional patterns of changes in [11C]PiB retention.
The findings of increased [11C]PiB retention over time have several implications. First, the study suggests that over short-term follow-up, Aβ deposition may be a gradual process, at least in nondemented adults. Second, there is substantial variability in the rates of [11C]PiB retention among nondemented older adults, which underscores the potential utility of the measure. Older adults with minimal baseline [11C]PiB deposition have little increase in [11C]PiB retention over time and, as such, may represent the 20% to 56% of nondemented individuals with no or minimal amounts of Aβ on postmortem evaluation11, 27. Third, given the small magnitude of overall change over time, regionally directed investigations may provide a better understanding of the interrelationship of AD biomarkers, cognition, and ultimately the molecular and cellular mechanism underlying the earliest stage of Aβ deposition. Larger samples with longer follow-up will be neeeded to better characterize the trajectories of fibrillar Aβ deposition in vivo and to define factors that render some individuals vulnerable and others resilient to Aβ deposition.
Acknowledgments
Support: This research was supported by the Intramural Research Program of the NIH, National Institute on Aging, N01-AG-3-2124, and K24 DA000412.
We are grateful to the BLSA participants for their dedication to these studies. We also thank Andrew Crabb, MS for data management, Beth Nardi, MA and Wendy Elkins, MS for study management, as well as the staff of the PET facility at Johns Hopkins University, and the neuroimaging staff of the NIA for their assistance.
References
- 1.Klunk WE, Engler H, Nordberg A, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol. 2004 Mar;55(3):306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 2.Scheinin NM, Aalto S, Koikkalainen J, et al. Follow-up of [11C]PIB uptake and brain volume in patients with Alzheimer disease and controls. Neurology. 2009 Oct 13;73(15):1186–1192. doi: 10.1212/WNL.0b013e3181bacf1b. [DOI] [PubMed] [Google Scholar]
- 3.Engler H, Forsberg A, Almkvist O, et al. Two-year follow-up of amyloid deposition in patients with Alzheimer’s disease. Brain. 2006 Nov;129(Pt 11):2856–2866. doi: 10.1093/brain/awl178. [DOI] [PubMed] [Google Scholar]
- 4.Jack CR, Jr, Lowe VJ, Weigand SD, et al. Serial PIB and MRI in normal, mild cognitive impairment and Alzheimer’s disease: implications for sequence of pathological events in Alzheimer’s disease. Brain. 2009 May;132(Pt 5):1355–1365. doi: 10.1093/brain/awp062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jack CR, Jr, Lowe VJ, Senjem ML, et al. 11C PiB and structural MRI provide complementary information in imaging of Alzheimer’s disease and amnestic mild cognitive impairment. Brain. 2008 Mar;131(Pt 3):665–680. doi: 10.1093/brain/awm336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pike KE, Savage G, Villemagne VL, et al. Beta-amyloid imaging and memory in non-demented individuals: evidence for preclinical Alzheimer’s disease. Brain. 2007 Nov;130(Pt 11):2837–2844. doi: 10.1093/brain/awm238. [DOI] [PubMed] [Google Scholar]
- 7.Rowe CC, Ng S, Ackermann U, et al. Imaging beta-amyloid burden in aging and dementia. Neurology. 2007 May 15;68(20):1718–1725. doi: 10.1212/01.wnl.0000261919.22630.ea. [DOI] [PubMed] [Google Scholar]
- 8.Aizenstein HJ, Nebes RD, Saxton JA, et al. Frequent amyloid deposition without significant cognitive impairment among the elderly. Arch Neurol. 2008 Nov;65(11):1509–1517. doi: 10.1001/archneur.65.11.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mintun MA, Larossa GN, Sheline YI, et al. [11C]PIB in a nondemented population: potential antecedent marker of Alzheimer disease. Neurology. 2006 Aug 8;67(3):446–452. doi: 10.1212/01.wnl.0000228230.26044.a4. [DOI] [PubMed] [Google Scholar]
- 10.Sperling RA, Laviolette PS, O’Keefe K, et al. Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron. 2009 Jul 30;63(2):178–188. doi: 10.1016/j.neuron.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Driscoll I, Resnick SM, Troncoso JC, An Y, O’Brien R, Zonderman AB. Impact of Alzheimer’s pathology on cognitive trajectories in nondemented elderly. Ann Neurol. 2006 Dec;60(6):688–695. doi: 10.1002/ana.21031. [DOI] [PubMed] [Google Scholar]
- 12.Galvin JE, Powlishta KK, Wilkins K, et al. Predictors of preclinical Alzheimer disease and dementia: a clinicopathologic study. Arch Neurol. 2005 May;62(5):758–765. doi: 10.1001/archneur.62.5.758. [DOI] [PubMed] [Google Scholar]
- 13.Klunk WE, Mathis CA, Price JC, Lopresti BJ, DeKosky ST. Two-year follow-up of amyloid deposition in patients with Alzheimer’s disease. Brain. 2006 Nov;129(Pt 11):2805–2807. doi: 10.1093/brain/awl281. [DOI] [PubMed] [Google Scholar]
- 14.Jack CR, Jr, Knopman DS, Jagust WJ, et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010 Jan;9(1):119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawas C, Gray S, Brookmeyer R, Fozard J, Zonderman A. Age-specific incidence rates of Alzheimer’s disease: the Baltimore Longitudinal Study of Aging. Neurology. 2000 Jun 13;54(11):2072–2077. doi: 10.1212/wnl.54.11.2072. [DOI] [PubMed] [Google Scholar]
- 16.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993 Nov;43(11):2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 17.Zhou Y, Resnick SM, Ye W, et al. Using a reference tissue model with spatial constraint to quantify [11C]Pittsburgh compound B PET for early diagnosis of Alzheimer’s disease. Neuroimage. 2007 Jun;36(2):298–312. doi: 10.1016/j.neuroimage.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Price JC, Klunk WE, Lopresti BJ, et al. Kinetic modeling of amyloid binding in humans using PET imaging and Pittsburgh Compound-B. J Cereb Blood Flow Metab. 2005 Nov;25(11):1528–1547. doi: 10.1038/sj.jcbfm.9600146. [DOI] [PubMed] [Google Scholar]
- 19.Zhou Y, Endres CJ, Brasic JR, Huang SC, Wong DF. Linear regression with spatial constraint to generate parametric images of ligand-receptor dynamic PET studies with a simplified reference tissue model. Neuroimage. 2003 Apr;18(4):975–989. doi: 10.1016/s1053-8119(03)00017-x. [DOI] [PubMed] [Google Scholar]
- 20.Lopresti BJ, Klunk WE, Mathis CA, et al. Simplified quantification of Pittsburgh Compound B amyloid imaging PET studies: a comparative analysis. J Nucl Med. 2005 Dec;46(12):1959–1972. [PubMed] [Google Scholar]
- 21.Petersen RC. Mild cognitive impairment: current research and clinical implications. Semin Neurol. 2007 Feb;27(1):22–31. doi: 10.1055/s-2006-956752. [DOI] [PubMed] [Google Scholar]
- 22.Yaqub M, Tolboom N, Boellaard R, et al. Simplified parametric methods for [11C]PIB studies. Neuroimage. 2008 Aug 1;42(1):76–86. doi: 10.1016/j.neuroimage.2008.04.251. [DOI] [PubMed] [Google Scholar]
- 23.Ingelsson M, Fukumoto H, Newell KL, et al. Early Abeta accumulation and progressive synaptic loss, gliosis, and tangle formation in AD brain. Neurology. 2004 Mar 23;62(6):925–931. doi: 10.1212/01.wnl.0000115115.98960.37. [DOI] [PubMed] [Google Scholar]
- 24.Jagust WJ, Landau SM, Shaw LM, et al. Relationships between biomarkers in aging and dementia. Neurology. 2009 Oct 13;73(15):1193–1199. doi: 10.1212/WNL.0b013e3181bc010c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Resnick SM, Sojkova J, Zhou Y, et al. Longitudinal cognitive decline is associated with fibrillar amyloid-beta measured by [11C]PiB. Neurology. 2010 Mar 9;74(10):807–815. doi: 10.1212/WNL.0b013e3181d3e3e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sheline YI, Raichle ME, Snyder AZ, et al. Amyloid Plaques Disrupt Resting State Default Mode Network Connectivity in Cognitively Normal Elderly. Biol Psychiatry. 2009 Oct 13; doi: 10.1016/j.biopsych.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bennett DA, Schneider JA, Arvanitakis Z, et al. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology. 2006 Jun 27;66(12):1837–1844. doi: 10.1212/01.wnl.0000219668.47116.e6. [DOI] [PubMed] [Google Scholar]