Abstract
Summary: Autosomal recessive IRAK-4 and MyD88 deficiencies predispose affected patients to recurrent invasive pyogenic bacterial infection. Both defects result in the selective impairment of cellular responses to Toll-like receptors (TLRs) other than TLR3 and of cellular responses to most interleukin-1 receptors (IL-1Rs), including IL-1R, IL-18R, and IL-33R. Hypomorphic mutations in the X-linked NEMO gene and hypermorphic mutations in the autosomal IKBA gene cause X-linked recessive and autosomal dominant anhidrotic ectodermal dysplasia with immunodeficiency (EDA-ID) syndromes. Both of these defects impair NF-κB-mediated cellular responses to multiple receptors, including TLRs, IL-1Rs, and tumor necrosis factor receptors (TNF-Rs). They therefore confer a much broader predisposition to infections than that for IRAK-4 and MyD88 deficiencies. These disorders were initially thought to be rare but have now been diagnosed in over 170 patients worldwide. We review here the infectious diseases affecting patients with inborn errors of NF-κB-dependent TLR and IL-1R immunity.
INTRODUCTION
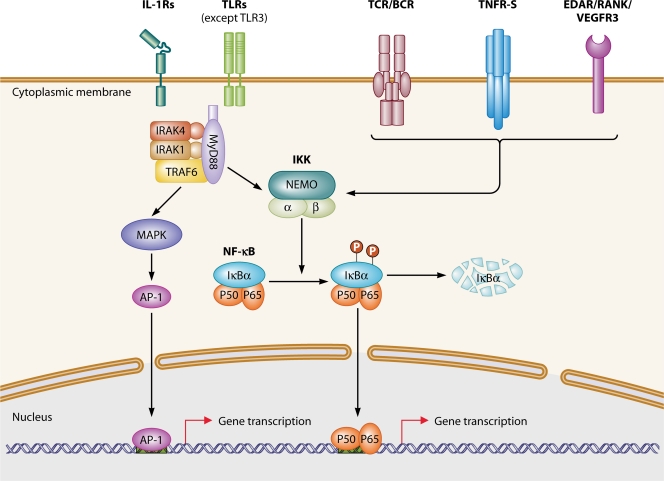
Toll-like receptors (TLRs) sense microbial products and play an important role in innate immunity. Ten TLR paralogs and up to 10 members of the interleukin-1 receptor (IL-1R) family have been identified in humans. IL-1Rs are also innate receptors important for the signaling of three cytokines, IL-1β, IL-18, and IL-33, which are thought to contribute to host defense in the early steps of the inflammatory response (17). TLRs and members of the IL-1R family contain an intracellular domain known as the Toll–IL-1R domain (TIR) (30). TIR-containing TLRs and IL-1Rs recruit the TIR-containing cytosolic adaptors MyD88, TRIF, TIRAP (also known as MAL), TRAM, and SARM (31, 47). The canonical TIR pathway depends on MyD88, which is used by all TLRs except for TLR3 and by at least three IL-1Rs: IL-1R, IL-18R, and IL-33R (Fig. 1). The alternative pathway is controlled by another key adaptor, TRIF, which is the only adaptor used by TLR3 and is also used by TLR4 (which can also use MyD88). The remaining three adaptors serve as coadaptors or negative regulators. The sorting adaptor TIRAP recruits MyD88 to TLR2 and TLR4, whereas TRAM recruits TRIF to TLR4. Finally, SARM appears to be a negative regulator of TRIF (5). The adaptors, in turn, recruit cytosolic kinases, including the IL-1R-associated kinase (IRAK) complex, which is recruited by MyD88 and seems to be the most TIR-specific kinase used in these pathways (38, 61).
Fig. 1.
TIR and NF-κB signaling pathways. Immune receptor signaling pathways leading to NF-κB activation can be grouped into four categories on the basis of the surface receptors involved: members of the TIR superfamily (IL-1Rs/TLRs), antigen receptors (TCR and BCR), members of the TNF-R superfamily (TNF-Rs), and RANK, VEGFR3, and EDAR. The two proteins of the TIR signaling pathway (MyD88 and IRAK-4) and the two proteins of the NF-κB signaling pathway (NEMO and IκBα) responsible for primary immunodeficiencies are shown.
The classical pathway results in the activation of both nuclear factor κB (NF-κB) and mitogen-activated protein kinases (MAPKs) via the IRAK complex (Fig. 1), which consists of two active kinases (IRAK-1 and IRAK-4) and two noncatalytic subunits (IRAK-2 and IRAK-3/M). NF-κB is a transcription factor sequestered in the cytoplasm of resting cells through association with the inhibitor of NF-κB (IκB) proteins. Upon cell stimulation, IκBs are phosphorylated at two conserved critical amino-terminal serine residues by the IκB kinase (IKK) complex, leading to their ubiquitination and subsequent degradation. The IKK complex is composed of at least two related catalytic subunits, IKKα and IKKβ, and IKKγ/NEMO (NF-κB essential modulator) (Fig. 1). The degradation of IκBs results in the translocation of NF-κB dimers to the nucleus, where they bind to DNA at cognate binding sites and regulate gene transcription (59). The classical proinflammatory TLR signaling pathway leads to the synthesis of inflammatory cytokines and chemokines, such as IL-1β, -6, -8, and -12 and tumor necrosis factor alpha (TNF-α). NF-κB dimers are also involved in various other immunological pathways (e.g., tumor necrosis factor receptor [TNF-R] superfamily member, T-cell receptor [TCR], and B-cell receptor [BCR] pathways) and developmental pathways (e.g., pathways with ectodysplasin [EDA], RANK, and VEGFR3, required for normal ectodermal, bone, and lymphatic development, respectively) (Fig. 1).
Four Mendelian primary immunodeficiencies (PIDs) associated with impaired signaling of the TLR canonical pathway have been reported, with mutations in MyD88, IRAK4, NEMO, and IKBA (Fig. 1) (12, 18, 57, 70). Defects of NEMO and IKBA also impair the alternative, TRIF-dependent pathway. The dominant infectious phenotype of patients with any of these four defects is the occurrence of pyogenic bacterial infections. Alternatively, three other genetic defects, caused by mutations in TLR3, UNC93B, and TRAF3, principally affect the alternative pathway (8, 55, 73). In addition, mutations in UNC93B and TRAF3 also impair the TLR7-9 pathway without any overt clinical consequences. The dominant infectious phenotype of patients with TLR3, UNC93B, or TRAF3 deficiency is herpes simplex encephalitis. We summarize here the infectious diseases seen in patients with mutations predominantly impairing the canonical pathway (6). The infections striking patients with mutations in the alternative pathway have been reviewed elsewhere (55, 72). We also discuss the diagnostic and therapeutic options for such patients in an attempt to propose tentative guidelines for clinicians.
INBORN ERRORS OF THE TIR PATHWAY: IRAK-4 AND MyD88 DEFICIENCIES
Molecular Basis and Immunological Features
Autosomal recessive IRAK-4 deficiency was first discovered in 2003 (57). Up to 49 patients have since been identified, from 32 kindreds in 14 countries on 4 continents: the Americas (Canada, El Salvador, and the United States), Asia (Israel, Japan, Saudi Arabia, and Turkey), Australia, and Europe (France, Hungary, Portugal, Slovenia, Spain, and the United Kingdom) (2, 4, 10, 13, 15, 16, 21, 27, 32, 34–36, 41, 43, 58, 66, 68, 69, 71; our unpublished data). Autosomal recessive MyD88 deficiency was first discovered in 2008 (70). Up to 22 patients have since been identified, from seven kindreds in six countries in the Americas (United States), Asia (Turkey), and Europe (France, Portugal, Serbia, and Spain) (11, 58). MyD88- and IRAK4-deficient patients have homozygous or compound heterozygous mutations in the IRAK4 or MYD88 gene, while heterozygous carriers are asymptomatic. IRAK-4 is a serine-threonine kinase acting downstream from TLRs and IL-1Rs (TIRs) (Fig. 1). MyD88 is a cytosolic adapter molecule connecting TLRs and IL-1Rs to the IRAK complex (Fig. 1). The MyD88- and IRAK-4-dependent TIR pathway leads to the production of proinflammatory cytokines. All human TLRs other than TLR3 use both MyD88 and IRAK-4 (64, 65). Blood leukocytes derived from MyD88- and IRAK4-deficient patients display a lack of IL-6 production by whole blood or a lack of CD62 ligand (CD62L) shedding from granulocytes following activation with most of the TLR and IL-1R agonists tested, with the exception of agonists of TLR3, which uses a MyD88- and IRAK-4-independent pathway (69, 70, 73). MyD88 and IRAK-4 deficiencies are phenocopies in terms of their immunological phenotype (70). There seems to be no overt defect of leukocyte development in IRAK-4- and MyD88-deficient patients; antigen-specific T- and B-cell responses seem to be normal, as shown in routine immunological workups, with two notable exceptions (35, 70). First, the glycan-specific immunoglobulin G (IgG) and IgM antibody responses to pneumococcal and AB glycans (allohemagglutinins of the ABO system) are impaired in up to one-third of patients explored (58). Second, serum IgE and IgG4 concentrations are high in up to two-thirds and one-third, respectively, of patients tested (58). Nevertheless, none of the MyD88- and IRAK-4-deficient patients described thus far suffer from allergic asthma, and chronic eczematous skin disease has been reported for only one patient. Both IRAK-4 and MyD88 deficiencies confer a predisposition to severe bacterial infection, with impairment of the abilities to increase plasma C-reactive protein (CRP) concentrations and to mount fever at the beginning of infection; however, pus formation is observed at the various sites of infection (58). Only small amounts of IL-6 are produced by IRAK-4- and MyD88-deficient cells upon activation with IL-1 and TLR agonists, and CRP is an IL-6-inducible molecule. Likewise, small amounts of IL-8 are produced in response to the same agonists, yet pus is formed in the patients, although IL-8 is a major chemoattractant of granulocytes. This suggests that IL-8 is produced in response to other stimuli in vivo, that factors other than IL-8 recruit granulocytes locally, or both. Finally, delayed separation of the umbilical cord is observed in 20% of IRAK-4-deficient patients (58). The underlying mechanisms are unclear.
Clinical Manifestations
Despite having a broad and profound immunological phenotype, patients with IRAK-4 and MyD88 deficiencies present narrow susceptibility ranges for invasive (meningitis, sepsis, arthritis, osteomyelitis, and abscesses) pyogenic bacterial infections and have normal resistance to common fungi, parasites, viruses, and many bacteria. In one study, meningitis occurred in 63% of IRAK-4-deficient patients, sepsis in 37%, arthritis in 29%, osteomyelitis in 14%, and deep inner organ/tissue abscesses in 29% (Table 1) (58; unpublished data). Meningitis occurred in 45% of MyD88-deficient patients, sepsis in 50%, arthritis in 14%, osteomyelitis in 9%, and deep inner organ/tissue abscesses in 14% (Table 1). Only five IRAK-4-deficient patients have never developed invasive bacterial infection, including four patients diagnosed at birth (siblings of previously diagnosed patients with IRAK-4 deficiency) who have remained asymptomatic on prophylactic treatment (58). Only two MyD88-deficient patients have never developed invasive bacterial infection (11). For both IRAK-4 and MyD88 deficiencies, most of the invasive bacterial infections observed are caused by Streptococcus pneumoniae, Staphylococcus aureus, and Pseudomonas aeruginosa. In IRAK-4-deficient patients, S. pneumoniae was involved in 54% of documented invasive episodes, whereas S. aureus and P. aeruginosa were found in 14% and 19% of such episodes, respectively (Table 2). Other Gram-positive and Gram-negative bacteria also cause invasive disease in IRAK-4-deficient patients (Table 2). In MyD88-deficient patients, S. pneumoniae was involved in 41% of documented invasive episodes, whereas S. aureus and P. aeruginosa were found in 20% and 16% of such episodes, respectively (Table 2). Other Gram-positive and Gram-negative bacteria also cause invasive disease in MyD88-deficient patients (Table 2). The first bacterial infection occurred before the age of 2 years in 90% of IRAK-4- and MyD88-deficient patients. Twenty-seven patients (38%) died of invasive bacterial infections (37% of IRAK-4- and 41% of MyD88-deficient patients), all before the age of 8 years and most before the age of 2 years (11, 58). Eighteen of these patients died of invasive pneumococcal disease. However, both PIDs improved with age, and patients with IRAK-4 and MyD88 deficiencies presented no further invasive bacterial infections after their teens (58).
Table 1.
Percentages of IRAK-4- and MyD88-deficient patients with bacterial infections at various sitesa
| Infection | % of patients with infection |
||
|---|---|---|---|
| All patients (n = 71) | IRAK-4-deficient patients (n = 49) | MyD88-deficient patients (n = 22) | |
| Meningitis | 58 | 63 | 45 |
| Sepsis | 41 | 37 | 50 |
| Arthritis | 24 | 29 | 14 |
| Osteomyelitis | 13 | 14 | 9 |
| Abscess | 24 | 29 | 14 |
| Lymphadenitis | 27 | 29 | 23 |
| Skin infection | 35 | 45 | 14 |
| Pneumonia | 17 | 18 | 14 |
| ENT infection | 28 | 35 | 14 |
Table 2.
Documented bacterial infections in IRAK-4- and MyD88-deficient patientsa
| Infection organism | % of patients with invasive infection (n = 71 patients) |
% of patients with noninvasive infection (n = 71 patients) |
||
|---|---|---|---|---|
| IRAK-4-deficient patients (105 infections) | MyD88-deficient patients (44 infections) | IRAK-4-deficient patients (63 infections) | MyD88-deficient patients (15 infections) | |
| S. aureus | 14 | 20 | 43 | 53 |
| S. pneumoniae | 54 | 41 | 16 | 20 |
| Other Streptococcus spp. (A and B groups) | 6 | 11 | 8 | |
| P. aeruginosa | 19 | 16 | 22 | 13 |
| Other Gram-negative bacteria | 7 | 11 | 10 | 13 |
| Shigella sonnei | 2 | |||
| Neisseria meningitidis | 2 | |||
| Haemophilus influenzae | 2 | 2 | ||
| Salmonella enterica serovar Enteritidis | 7 | |||
| Klebsiella pneumoniae | 7 | |||
| Escherichia coli | 5 | 7 | ||
| Serratia marcescens | 2 | |||
| Moraxella catarrhalis | 2 | 2 | ||
| Clostridium septicum | 1 | |||
| Citrobacter freundii | 2 | |||
| Mycobacterium avium | 1 | |||
Patients with IRAK-4 and MyD88 deficiencies also present noninvasive pyogenic bacterial infections, mostly affecting the skin and upper respiratory tract sites, where necrotizing infections are particularly common. Recurrent, localized skin infections (furunculosis, folliculitis, cellulitis, omphalitis, and orbital cellulitis or endophthalmitis) have been found in 35% of patients, lymphadenitis in 27% of patients, and ear, nose, and throat (ENT) infections (otitis, sinusitis, tonsillar abscesses, necrotizing epiglotitis, pharyngitis, and palate infection) in 28% of patients (Table 1) (58; unpublished data). Intriguingly, only 17% of patients have had pneumonia, and none have developed chronic bronchopulmonary disease. The principal bacterial species isolated during noninvasive infections were S. aureus, in 43% of episodes in IRAK-4-deficient patients and 53% of episodes in MyD88-deficient patients; P. aeruginosa, in 22% of episodes in IRAK-4-deficient patients and 13% of episodes in MyD88-deficient patients; and S. pneumoniae, in 16% of episodes in IRAK-4-deficient patients and 20% of episodes in MyD88-deficient patients (Table 2). Other Gram-positive and Gram-negative bacteria have also caused noninvasive disease in IRAK-4-deficient patients, whereas only a few other Gram-negative bacteria have been shown to cause noninvasive disease in MyD88-deficient patients (Table 2). Infections caused by agents other than pyogenic bacteria did not include severe mycobacterial, viral, parasitic, and fungal diseases. Only one IRAK-4-deficient patient developed otitis and pneumonia caused by Mycobacterium avium. All IRAK-4-deficient and MyD88-deficient patients have presented noninvasive bacterial infections, with more than half of these patients suffering from their first noninvasive bacterial infection before the age of 2 years and with all patients continuing to suffer from skin infections, sinusitis, or pneumonia, including those who have reached adulthood (58; unpublished data).
Treatment of IRAK-4 and MyD88 Deficiencies
Patients with IRAK-4 and MyD88 deficiencies should be immunized with S. pneumoniae conjugated and nonconjugated vaccines, Haemophilus influenzae conjugated vaccine, and Neisseria meningitidis conjugated and nonconjugated vaccines. A preventive treatment including antibiotic prophylaxis with cotrimoxazole plus penicillin V (in the absence of allergy to one of these antibiotics) should be administered throughout the life of the patient. Regarding the severity of bacterial infection during childhood and the defect of antibody production found in some IRAK-4-deficient patients, we also recommend empirical intravenous or subcutaneous IgG injections until the patient is at least 10 years old. This prophylaxis seems to decrease the incidence of invasive bacterial infections (58). The most important advice for the families and physicians of IRAK-4-deficient and MyD88-deficient patients is to initiate empirical parenteral antibiotic treatment against S. pneumoniae, S. aureus, and P. aeruginosa as soon as an infection is suspected or if the patient develops a moderate fever, without taking inflammatory parameters into account, because patients may die from rapid invasive bacterial infection despite appropriate prophylaxis. Secondary adaptation of antibiotic treatment should be done once the causal bacterium has been documented.
Outcomes of IRAK-4 and MyD88 Deficiencies
Both IRAK-4 and MyD88 deficiencies confer a predisposition to invasive bacterial infections, mostly caused by S. pneumoniae, S. aureus, and P. aeruginosa. These two deficiencies also confer a predisposition to noninvasive bacterial infection, with severe skin infections, mostly caused by S. aureus, and severe forms of ENT infections caused by P. aeruginosa frequently observed. Clinical status and outcome improve with age, and prophylactic treatment seems to be beneficial in these patients. The dramatic improvement with age may be accounted for by the development of adaptive antigen-specific T- and B-lymphocyte responses. Thus, in both IRAK-4 and MyD88 deficiencies, S. pneumoniae, S. aureus, and P. aeruginosa are by far the most commonly isolated pathogens causing invasive infection, and S. aureus is by far the most commonly isolated pathogen causing noninvasive infection. Of course, with only 71 patients from 15 countries, we cannot draw firm and definitive conclusions regarding the range and severity of infectious diseases in such patients. Indeed, similar patients exposed to other microorganisms may develop an as yet unknown infectious phenotype. For example, two patients with shigellosis and two others with late-onset group B streptococcal disease have been identified. Nevertheless, we think that the phenotype described is sufficiently robust that the discovery of new infections would have no major effect on the phenotypic description of these disorders.
INBORN ERRORS OF NF-κB-MEDIATED IMMUNITY: NEMO AND IκBα DEFICIENCIES
Molecular Basis and Immunological Features
X-linked recessive anhidrotic ectodermal dysplasia with immunodeficiency (XR-EDA-ID) caused by hypomorphic IKBKG/NEMO mutations impairing NF-κB activation was first described in 2000 (74) and 2001 (18). NEMO is a regulatory subunit of the IKK complex (59). Up to 100 male patients with hypomorphic mutations of NEMO have been reported, and about 43 different mutations leading to impaired NF-κB activation have been identified (1, 9, 14, 18, 20, 22, 24–26, 28, 33, 34, 37, 40, 45, 46, 48–53, 60, 62, 63, 74; unpublished data). Patients with this deficiency have been identified in 14 countries on 4 continents: Africa (South Africa), North America (Canada and the United States), Asia (Japan and Turkey), and Europe (Belgium, France, Germany, Italy, Poland, Netherlands, Norway, Switzerland, and the United Kingdom). In 2003, an autosomal dominant form of EDA-ID (AD-EDA-ID) was identified, caused by a hypermorphic heterozygous mutation of NFKBIA/IKBA, impairing the phosphorylation and degradation of IκBα and resulting in the partial retention of NF-κB dimers in the cytoplasm (Fig. 1) (12). Five patients with three different hypermorphic mutations of IKBA were subsequently identified in 2003 (12, 19, 29, 39, 42). The patients originated from three countries on two continents: North America (United States) and Europe (Italy and the Netherlands). NF-κB dimers are involved in several pathways, including those triggered by the many members of the TNF-R, IL-1R, TCR, BCR, and TLR families. IκBα deficiency involves a severe impairment of TCR signaling (12). For NEMO deficiency, the degree of impairment of the various pathways depends on the mutation, with anything from one to all of these pathways being affected (59). NEMO-deficient patients generally display a lack of IL-10 production in response to activation with TNF-α in whole-blood assays (25, 59). Most patients bearing NEMO mutations have an impaired antibody response, in particular that to glycans, including pneumococcal capsules (59). IκBα-deficient patients have hypogammaglobulinemia with no production of specific antibodies; some of them also have low proportions of memory CD4 and CD8 T cells and no TCRγ/δ T cells and display severe impairment of T-cell proliferation in response to anti-CD3. All IκBα-deficient patients without mosaicism and about 90% of the NEMO-deficient patients described to date have EDA, with sparse hair, abnormal teeth (conical teeth, tooth agenesis), and hypohidrosis (a lack of sweating) (25, 59). These features result from defective signaling via the ectodysplasin receptor (EDA-R) signaling pathway. One IκBα-deficient patient with complex mosaicism does not display features of EDA (29). In some NEMO-deficient patients, associated osteopetrosis and/or lymphedema has been described in addition to EDA (18, 25). About 10% of NEMO-deficient patients have no developmental phenotype (25, 45, 60).
Clinical Manifestations
The broad and profound immunological phenotypes of patients with IκBα and NEMO deficiencies are responsible for their broad susceptibility to infections with invasive pyogenic bacteria (meningitis, sepsis, arthritis, osteomyelitis, and abscesses), environmental mycobacteria, and, to a lesser extent, parasites, viruses, and fungi. All five IκBα-deficient patients have developed recurrent bacterial infections, with pneumonia in five cases, sepsis or meningitis in three cases, and arthritis in one case (Table 3) (19, 29, 39, 42). They are also prone to opportunistic infections, with three of them having had pulmonary pneumocystosis and chronic mucocutaneous candidiasis (Table 4). Finally, four of these patients have presented recurrent diarrhea and/or colitis. One-third of NEMO-deficient patients have had sepsis, one-third have had deep tissue abscesses, one-third have had recurrent pneumonia with bronchiectasis, 18% have had meningitis or encephalitis, 24% have had gut infection, 16% have had osteomyelitis, and 22% have had ENT infections (Table 3) (18, 25, 59; unpublished data). Pyogenic bacterial infection was identified in about 90% of NEMO-deficient patients, and the bacteria involved included S. pneumoniae, H. influenzae, and S. aureus. Mycobacterial infection was found in about 40% of NEMO-deficient patients (cellulitis, osteomyelitis, lymphadenitis, pneumonia, and disseminated infections) and was caused by M. avium or Mycobacterium kansasii (18, 25; unpublished data). Serious viral infection occurred in 19% of NEMO-deficient patients (herpes simplex virus encephalitis, severe adenoviral gastroenteritis, or severe cytomegalovirus infection) (Table 4). Finally, the opportunistic infections pneumocystosis and chronic candidiasis occurred in fewer than 10% of patients (25; unpublished data). In summary, the spectrum of infectious diseases is broad in NEMO-deficient and IκBα-deficient patients, as most patients present multiple infections (3). Almost all patients have presented infections caused by pyogenic bacteria, with only some patients suffering from mycobacterial, fungal, and/or viral diseases.
Table 3.
Percentages of IκBα- and NEMO-deficient patients with infections at various sitesa
| Infection | % of patients with infection |
|
|---|---|---|
| IκBα-deficient patients (n = 5) | NEMO-deficient patients (n = 67) | |
| Meningitis/encephalitis | 20 | 18 |
| Sepsis | 40 | 31 |
| Arthritis/osteomyelitis | 20 | 16 |
| Abscess | 20 | 28 |
| Gut infection/diarrhea | 80 | 24 |
| Pneumonia | 80 | 34 |
| ENT infection | 20 | 22 |
Table 4.
Documented infections in IκBα- and NEMO-deficient patients
| Infection organism | % of patients with infection (no. of infected patients/total no. of patients)a |
|
|---|---|---|
| IκBα-deficient patients (n = 5) | NEMO-deficient patients (n = 67) | |
| Bacteria | 100 | 88 |
| S. aureus | 20 | >10 |
| S. pneumoniae | >10 | |
| Streptococcus (A group) | 20 | |
| P. aeruginosa | 20 | >10 |
| Haemophilus influenzae | >10 | |
| Salmonella enterica serovar Typhimurium | 20 | |
| Klebsiella pneumoniae | 20 | |
| Serratia marcescens | 20 | |
| Environmental mycobacteria | 39 | |
| Fungi | 80 | |
| Candida albicans | 100 | 10 |
| Pneumocystis jirovecii | 60 | 7 |
| Severe viral infection (herpes simplex virus, cytomegalovirus, or adenovirus) | 19 | |
Treatment and Outcomes of IκBα and NEMO Deficiencies
A preventive treatment including antibiotic prophylaxis with cotrimoxazole and/or penicillin V should be proposed (in the absence of allergy) and intravenous or subcutaneous IgG substitution should be carried out for patients with IκBα and NEMO deficiencies presenting an impairment of B-cell immunity. Patients with IκBα and NEMO deficiencies with functional B-cell immunity should be immunized with S. pneumoniae conjugated and nonconjugated vaccines, H. influenzae conjugated vaccine, and N. meningitidis conjugated and nonconjugated vaccines. Important advice for the families and physicians of IκBα- and NEMO-deficient patients is to initiate empirical parenteral antibiotic treatment against S. pneumoniae, S. aureus, P. aeruginosa, and H. influenzae as soon as infection is suspected or the patient develops a moderate fever, without taking inflammatory parameters into account, because patients may die from rapid invasive bacterial infection despite appropriate prophylaxis. Secondary adaptation of antibiotic treatment should be done once the causal bacterium has been documented. Hematopoietic stem cell transplantation (HSCT) has been reported for two patients with severe IκBα deficiency causing combined immunodeficiency (19, 23). One of these patients is alive and well, with no treatment, 8 years after haploidentical HSCT, whereas the other patient died of bacterial sepsis during the period of aplasia (19, 23). Seven NEMO-deficient patients with severe clinical and immunological phenotypes have undergone transplantation, with various conditioning regimens (ranging from myeloablative to reduced-intensity conditioning) and with a related matched donor or an unrelated partially matched donor (20, 23, 44, 54, 56, 67; unpublished data). Two patients died after HSCT, one from veno-occlusive disease and the other from parainfluenzavirus type III infection (20, 23). Five NEMO-deficient patients presented engraftment and correction of their immunodeficiency, but the preexisting colitis was not cured in two of these patients (23, 54, 56, 67; unpublished data). HSCT can correct these PIDs, but some inflammatory signs may persist and the EDA phenotype remains unmodified. This difficult procedure should be proposed only for selected patients who have severe immunodeficiency and for whom a donor of the best possible match is available. A large international clinical survey of NEMO-deficient patients is under way and should increase our understanding of the clinical and immunological outcomes for these patients. This study may facilitate the development of treatment guidelines for this heterogenous genetic disorder (C. Picard and J. S. Orange, unpublished data).
CONCLUSIONS
The clinical and biological phenotypes of IRAK-4, MyD88, NEMO, and IκBα deficiencies are listed in Table 5. IRAK-4 and MyD88 deficiencies define a novel group of PIDs characterized by a selective and profound defect of the TIR canonical signaling pathway (3). Patients with these two deficiencies are highly susceptible to invasive bacterial infections caused by S. pneumoniae and, to a lesser extent, S. aureus and to noninvasive bacterial infections largely restricted to the skin (S. aureus) and the upper respiratory tract (P. aeruginosa). Infections typically run an acute course and may be difficult to diagnose due to the inflammatory signs being weak or occurring late. The sites of infection also provide us with unique information about the anatomic role of the TIR pathway in host defense (6, 7).
Table 5.
Clinical and biological phenotypes of IRAK-4, MyD88, NEMO, and IκBα deficiencies
| Phenotype | Presence of phenotype for deficiencyb |
|||
|---|---|---|---|---|
| IRAK-4 | MyD88 | NEMO | IκBα | |
| Pyogenic bacterial infection | + | + | + | + |
| Severe viral infection | − | + | ||
| Environmental mycobacterial infection | +/− | − | + | − |
| Opportunistic infections | − | − | + | + |
| EDA | − | − | + or −a | + |
| Colitis | − | − | + | + |
| Hypogammaglobulinemia | − | − | + | + |
| Specific protidic antibody defect | − | − | + | + |
| Specific polysaccharide antibody defect | +/− | ND | + | + |
| Low T-cell proliferation in response to anti-CD3 | − | +/− | + | |
| No IL-6 production by whole blood after activation with IL-1 or TLR agonists (except TLR3) | + | + | +/− | + |
| No IL-10 production by whole blood after activation with TNF-α | − | − | + | + |
Ten percent of NEMO-deficient patient have no EDA phenotype.
−, absent; +, present, +/−, present in some patients.
Hypomorphic NEMO deficiency is associated with susceptibility to various bacteria, including mycobacteria, and occasionally to other microbes, such as fungi and viruses. A wide range of infectious phenotypes is observed for patients with NEMO deficiency, reflecting the diversity of NEMO genotypes. IκBα deficiency has been identified in only five patients and has been associated with multiple bacterial and fungal infections. Delays in the development of inflammatory signs are also observed in patients with NEMO and IκBα deficiencies, who have a broader susceptibility to infections, including those caused by pyogenic bacteria (3). Thus, the bacterial diseases seen in NEMO-deficient patients are probably due in part to the impact of NEMO mutations on the TIR signaling pathway. Conversely, the infections seen in NEMO- and IκBα-deficient patients but not in IRAK-4-deficient and MyD88-deficient patients probably reflect the impairment of other signaling pathways.
ACKNOWLEDGMENTS
We thank all of the members of the Laboratory of Human Genetics of Infectious Diseases for helpful discussions, especially Jacinta Bustamante, Stéphanie Boisson-Dupuis, Pegah Ghandil, and Maya Chrahieb.
J.-L.C. was an International Scholar of the Howard Hughes Medical Institute. The Laboratory of Human Genetics of Infectious Diseases is supported by grants from The Rockefeller University Center for Clinical and Translational Science (grant 5UL1RR024143-03) and The Rockefeller University. The Laboratory of Human Genetics of Infectious Diseases is also supported by the March of Dimes, the Dana Foundation, the ANR, the INSERM, and the PHRC.
Biographies
 Capucine Picard is co-group leader of the bacterial team of the INSERM U980 Laboratory of Human Genetics of Infectious Diseases and leader of the Hospital Laboratory Study Center of Primary Immunodeficiency, Necker Hospital, University Paris Descartes, Paris, France. She is an expert in primary immunodeficiencies, molecular immunology, and human genetics of infectious diseases. She has made outstanding contributions to the field of human genetic predispositions to infectious diseases, including bacterial diseases, with the discoveries of IRAK-4 and MyD88 deficiencies. She has published more than 120 papers in peer-reviewed scientific journals and books.
Capucine Picard is co-group leader of the bacterial team of the INSERM U980 Laboratory of Human Genetics of Infectious Diseases and leader of the Hospital Laboratory Study Center of Primary Immunodeficiency, Necker Hospital, University Paris Descartes, Paris, France. She is an expert in primary immunodeficiencies, molecular immunology, and human genetics of infectious diseases. She has made outstanding contributions to the field of human genetic predispositions to infectious diseases, including bacterial diseases, with the discoveries of IRAK-4 and MyD88 deficiencies. She has published more than 120 papers in peer-reviewed scientific journals and books.
 Jean-Laurent Casanova was appointed in 1999 as Professor of Pediatrics at Necker Hospital, where he cofounded and codirected, with Laurent Abel, the Laboratory of Human Genetics of Infectious Diseases. He was an international research scholar with the Howard Hughes Medical Institute from 2005 to 2008. He joined the faculty at The Rockefeller University as Professor of Pediatrics and Head of the St. Giles Laboratory of Human Genetics of Infectious Diseases in 2009. He is a leading international expert in the field of human genetics of infectious diseases and primary immunodeficiencies, as attested by his discoveries of the molecular genetic basis of various pediatric infectious diseases, including mycobacterial diseases (mutations in IFNGR1, IFNGR2, STAT1, IL12B, IL12RB1, and NEMO), invasive pneumococcal disease (mutations in NEMO, IKBA, IRAK4, and MYD88), and herpes simplex encephalitis (mutations in UNC93B1, TLR3, and TRAF3). He has published more than 300 papers in peer-reviewed scientific journals and books.
Jean-Laurent Casanova was appointed in 1999 as Professor of Pediatrics at Necker Hospital, where he cofounded and codirected, with Laurent Abel, the Laboratory of Human Genetics of Infectious Diseases. He was an international research scholar with the Howard Hughes Medical Institute from 2005 to 2008. He joined the faculty at The Rockefeller University as Professor of Pediatrics and Head of the St. Giles Laboratory of Human Genetics of Infectious Diseases in 2009. He is a leading international expert in the field of human genetics of infectious diseases and primary immunodeficiencies, as attested by his discoveries of the molecular genetic basis of various pediatric infectious diseases, including mycobacterial diseases (mutations in IFNGR1, IFNGR2, STAT1, IL12B, IL12RB1, and NEMO), invasive pneumococcal disease (mutations in NEMO, IKBA, IRAK4, and MYD88), and herpes simplex encephalitis (mutations in UNC93B1, TLR3, and TRAF3). He has published more than 300 papers in peer-reviewed scientific journals and books.
 Anne Puel is co-group leader, with Capucine Picard, of the bacterial team of the INSERM U980 Laboratory of Human Genetics of Infectious Diseases, University Paris Descartes, Paris, France. She is an expert in human genetics of infectious diseases. She has made substantial contributions to the field of PID (discovery of IL7RA deficiency) and human genetic predispositions to infectious diseases, including bacterial and fungal diseases (discovery of IL17RA and IL-17F deficiencies). She has published more than 80 papers in peer-reviewed scientific journals and books.
Anne Puel is co-group leader, with Capucine Picard, of the bacterial team of the INSERM U980 Laboratory of Human Genetics of Infectious Diseases, University Paris Descartes, Paris, France. She is an expert in human genetics of infectious diseases. She has made substantial contributions to the field of PID (discovery of IL7RA deficiency) and human genetic predispositions to infectious diseases, including bacterial and fungal diseases (discovery of IL17RA and IL-17F deficiencies). She has published more than 80 papers in peer-reviewed scientific journals and books.
REFERENCES
- 1. Aradhya S., et al. 2001. Atypical forms of incontinentia pigmenti in male individuals result from mutations of a cytosine tract in exon 10 of NEMO (IKK-gamma). Am. J. Hum. Genet. 68:765–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bouma G., et al. 2009. Impaired neutrophil migration and phagocytosis in IRAK-4 deficiency. Br. J. Haematol. 147:153–156 [DOI] [PubMed] [Google Scholar]
- 3. Bustamante J., et al. 2008. Novel primary immunodeficiencies revealed by the investigation of paediatric infectious diseases. Curr. Opin. Immunol. 20:39–48 [DOI] [PubMed] [Google Scholar]
- 4. Cardenes M., et al. 2006. Autosomal recessive interleukin-1 receptor-associated kinase 4 deficiency in fourth-degree relatives. J. Pediatr. 148:549–551 [DOI] [PubMed] [Google Scholar]
- 5. Carty M., et al. 2006. The human adaptor SARM negatively regulates adaptor protein TRIF-dependent Toll-like receptor signaling. Nat. Immunol. 7:1074–1081 [DOI] [PubMed] [Google Scholar]
- 6. Casanova J. L., Abel L. 2005. Inborn errors of immunity to infection: the rule rather than the exception. J. Exp. Med. 202:197–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Casanova J. L., Abel L. 2007. Primary immunodeficiencies: a field in its infancy. Science 317:617–619 [DOI] [PubMed] [Google Scholar]
- 8. Casrouge A., et al. 2006. Herpes simplex virus encephalitis in human UNC-93B deficiency. Science 314:308–312 [DOI] [PubMed] [Google Scholar]
- 9. Chang T. T., Behshad R., Brodell R. T., Gilliam A. C. 2008. A male infant with anhidrotic ectodermal dysplasia/immunodeficiency accompanied by incontinentia pigmenti and a mutation in the NEMO pathway. J. Am. Acad. Dermatol. 58:316–320 [DOI] [PubMed] [Google Scholar]
- 10. Chapel H., Puel A., von Bernuth H., Picard C., Casanova J. L. 2005. Shigella sonnei meningitis due to interleukin-1 receptor-associated kinase-4 deficiency: first association with a primary immune deficiency. Clin. Infect. Dis. 40:1227–1231 [DOI] [PubMed] [Google Scholar]
- 11. Conway D. H., Dara J., Bagashev A., Sullivan K. E. 2010. Myeloid differentiation primary response gene 88 (MyD88) deficiency in a large kindred. J. Allergy Clin. Immunol. 126:172–175 [DOI] [PubMed] [Google Scholar]
- 12. Courtois G., et al. 2003. A hypermorphic IkappaBalpha mutation is associated with autosomal dominant anhidrotic ectodermal dysplasia and T cell immunodeficiency. J. Clin. Invest. 112:1108–1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Currie A. J., et al. 2004. Primary immunodeficency to pneumococcal infection due to a defect in Toll-like receptor signaling. J. Pediatr. 144:512–518 [DOI] [PubMed] [Google Scholar]
- 14. Dai Y. S., et al. 2004. Characteristics of mycobacterial infection in patients with immunodeficiency and nuclear factor-kappaB essential modulator mutation, with or without ectodermal dysplasia. J. Am. Acad. Dermatol. 51:718–722 [DOI] [PubMed] [Google Scholar]
- 15. Davidson D. J., et al. 2006. IRAK-4 mutation (Q293X): rapid detection and characterization of defective post-transcriptional TLR/IL-1R responses in human myeloid and non-myeloid cells. J. Immunol. 177:8202–8211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Day N., et al. 2004. Interleukin receptor-associated kinase (IRAK-4) deficiency associated with bacterial infections and failure to sustain antibody responses. J. Pediatr. 144:524–526 [DOI] [PubMed] [Google Scholar]
- 17. Dinarello C. A. 2009. Immunological and inflammatory functions of the interleukin-1 family. Annu. Rev. Immunol. 27:519–550 [DOI] [PubMed] [Google Scholar]
- 18. Doffinger R., et al. 2001. X-linked anhidrotic ectodermal dysplasia with immunodeficiency is caused by impaired NF-kappaB signaling. Nat. Genet. 27:277–285 [DOI] [PubMed] [Google Scholar]
- 19. Dupuis-Girod S., et al. 2006. Successful allogeneic hemopoietic stem cell transplantation in a child who had anhidrotic ectodermal dysplasia with immunodeficiency. Pediatrics 118:e205–e211 [DOI] [PubMed] [Google Scholar]
- 20. Dupuis-Girod S., et al. 2002. Osteopetrosis, lymphedema, anhidrotic ectodermal dysplasia, and immunodeficiency in a boy and incontinentia pigmenti in his mother. Pediatrics 109:e97. [DOI] [PubMed] [Google Scholar]
- 21. Enders A., et al. 2004. Two siblings with lethal pneumococcal meningitis in a family with a mutation in interleukin-1 receptor-associated kinase 4. J. Pediatr. 145:698–700 [DOI] [PubMed] [Google Scholar]
- 22. Filipe-Santos O., et al. 2006. X-linked susceptibility to mycobacteria is caused by mutations in NEMO impairing CD40-dependent IL-12 production. J. Exp. Med. 203:1745–1759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fish J. D., Duerst R. E., Gelfand E. W., Orange J. S., Bunin N. 2009. Challenges in the use of allogeneic hematopoietic SCT for ectodermal dysplasia with immune deficiency. Bone Marrow Transplant. 43:217–221 [DOI] [PubMed] [Google Scholar]
- 24. Fusco F., et al. 2008. Alterations of the IKBKG locus and diseases: an update and a report of 13 novel mutations. Hum. Mutat. 29:595–604 [DOI] [PubMed] [Google Scholar]
- 25. Hanson E. P., et al. 2008. Hypomorphic nuclear factor-kappaB essential modulator mutation database and reconstitution system identifies phenotypic and immunologic diversity. J. Allergy Clin. Immunol. 122:1169–1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Haverkamp M. H., Arend S. M., Lindeboom J. A., Hartwig N. G., van Dissel J. T. 2004. Nontuberculous mycobacterial infection in children: a 2-year prospective surveillance study in the Netherlands. Clin. Infect. Dis. 39:450–456 [DOI] [PubMed] [Google Scholar]
- 27. Hoarau C., et al. 2007. TLR9 activation induces normal neutrophil responses in a child with IRAK-4 deficiency: involvement of the direct PI3K pathway. J. Immuno1. 179:4754–4765 [DOI] [PubMed] [Google Scholar]
- 28. Jain A., et al. 2001. Specific missense mutations in NEMO result in hyper-IgM syndrome with hypohydrotic ectodermal dysplasia. Nat. Immunol. 2:223–228 [DOI] [PubMed] [Google Scholar]
- 29. Janssen R., et al. 2004. The same IkappaBalpha mutation in two related individuals leads to completely different clinical syndromes. J. Exp. Med. 200:559–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kawai T., Akira S. 2010. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat. Immunol. 11:373–384 [DOI] [PubMed] [Google Scholar]
- 31. Kenny E. F., O'Neill L. A. 2008. Signalling adaptors used by Toll-like receptors: an update. Cytokine 43:342–349 [DOI] [PubMed] [Google Scholar]
- 32. Krause J. C., et al. 2009. Very late-onset group B Streptococcus meningitis, sepsis, and systemic shigellosis due to interleukin-1 receptor-associated kinase-4 deficiency. Clin. Infect. Dis. 49:1393–1396 [DOI] [PubMed] [Google Scholar]
- 33. Ku C. L., et al. 2005. NEMO mutations in 2 unrelated boys with severe infections and conical teeth. Pediatrics 115:e615–e619 [DOI] [PubMed] [Google Scholar]
- 34. Ku C. L., et al. 2007. IRAK4 and NEMO mutations in otherwise healthy children with recurrent invasive pneumococcal disease. J. Med. Genet. 44:16–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ku C. L., et al. 2007. Selective predisposition to bacterial infections in IRAK-4-deficient children: IRAK-4-dependent TLRs are otherwise redundant in protective immunity. J. Exp. Med. 204:2407–2422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lavine E., et al. 2007. Cellular and humoral aberrations in a kindred with IL-1 receptor-associated kinase-4 deficiency. J. Allergy Clin. Immunol. 120:948–950 [DOI] [PubMed] [Google Scholar]
- 37. Lee W. I., et al. 2005. Molecular analysis of a large cohort of patients with the hyper immunoglobulin M (IgM) syndrome. Blood 105:1881–1890 [DOI] [PubMed] [Google Scholar]
- 38. Lin S. C., Lo Y. C., Wu H. 2010. Helical assembly in the MyD88-IRAK4-IRAK2 complex in TLR/IL-1R signalling. Nature 465:885–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lopez-Granados E., et al. 2008. A novel mutation in NFKBIA/IKBA results in a degradation-resistant N-truncated protein and is associated with ectodermal dysplasia with immunodeficiency. Hum. Mutat. 29:861–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Martinez-Pomar N., et al. 2005. A new mutation in exon 7 of NEMO gene: late skewed X-chromosome inactivation in an incontinentia pigmenti female patient with immunodeficiency. Hum. Genet. 118:458–465 [DOI] [PubMed] [Google Scholar]
- 41. McDonald D. R., Brown D., Bonilla F. A., Geha R. S. 2006. Interleukin receptor-associated kinase-4 deficiency impairs Toll-like receptor-dependent innate antiviral immune responses. J. Allergy Clin. Immunol. 118:1357–1362 [DOI] [PubMed] [Google Scholar]
- 42. McDonald D. R., et al. 2007. Heterozygous N-terminal deletion of IkappaBalpha results in functional nuclear factor kappaB haploinsufficiency, ectodermal dysplasia, and immune deficiency. J. Allergy Clin. Immunol. 120:900–907 [DOI] [PubMed] [Google Scholar]
- 43. Medvedev A. E., et al. 2003. Distinct mutations in IRAK-4 confer hyporesponsiveness to lipopolysaccharide and interleukin-1 in a patient with recurrent bacterial infections. J. Exp. Med. 198:521–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Minakawa S., et al. 2009. Successful umbilical cord blood transplantation for intractable eczematous eruption in hypohidrotic ectodermal dysplasia with immunodeficiency. Clin. Exp. Dermatol. 34:e441–e442 [DOI] [PubMed] [Google Scholar]
- 45. Mooster J. L., et al. 2010. Immune deficiency caused by impaired expression of nuclear factor-kappaB essential modifier (NEMO) because of a mutation in the 5′ untranslated region of the NEMO gene. J. Allergy Clin. Immunol. 126:127–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Niehues T., et al. 2004. Nuclear factor kappaB essential modulator-deficient child with immunodeficiency yet without anhidrotic ectodermal dysplasia. J. Allergy Clin. Immunol. 114:1456–1462 [DOI] [PubMed] [Google Scholar]
- 47. O'Neill L. A., Bowie A. G. 2007. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat. Rev. Immunol. 7:353–364 [DOI] [PubMed] [Google Scholar]
- 48. Orange J. S., et al. 2002. Deficient natural killer cell cytotoxicity in patients with IKK-gamma/NEMO mutations. J. Clin. Invest. 109:1501–1509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Orange J. S., et al. 2004. The presentation and natural history of immunodeficiency caused by nuclear factor kappaB essential modulator mutation. J. Allergy Clin. Immunol. 113:725–733 [DOI] [PubMed] [Google Scholar]
- 50. Orange J. S., et al. 2004. Human nuclear factor kappa B essential modulator mutation can result in immunodeficiency without ectodermal dysplasia. J. Allergy Clin. Immunol. 114:650–656 [DOI] [PubMed] [Google Scholar]
- 51. Orange J. S., Levy O., Geha R. S. 2005. Human disease resulting from gene mutations that interfere with appropriate nuclear factor-kappaB activation. Immunol. Rev. 203:21–37 [DOI] [PubMed] [Google Scholar]
- 52. Orstavik K. H., et al. 2006. Novel splicing mutation in the NEMO (IKK-gamma) gene with severe immunodeficiency and heterogeneity of X-chromosome inactivation. Am. J. Med. Genet. A 140:31–39 [DOI] [PubMed] [Google Scholar]
- 53. Pachlopnik Schmid J. M., et al. 2006. Transient hemophagocytosis with deficient cellular cytotoxicity, monoclonal immunoglobulin M gammopathy, increased T-cell numbers, and hypomorphic NEMO mutation. Pediatrics 117:e1049–e1056 [DOI] [PubMed] [Google Scholar]
- 54. Pai S. Y., et al. 2008. Allogeneic transplantation successfully corrects immune defects, but not susceptibility to colitis, in a patient with nuclear factor-kappaB essential modulator deficiency. J. Allergy Clin. Immunol. 122:1113–1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Perez de Diego R., et al. 2010. Human TRAF3 adaptor molecule deficiency leads to impaired Toll-like receptor 3 response and susceptibility to herpes simplex encephalitis. Immunity 33:400–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Permaul P., Narla A., Hornick J. L., Pai S. Y. 2009. Allogeneic hematopoietic stem cell transplantation for X-linked ectodermal dysplasia and immunodeficiency: case report and review of outcomes. Immunol. Res. 44:89–98 [DOI] [PubMed] [Google Scholar]
- 57. Picard C., et al. 2003. Pyogenic bacterial infections in humans with IRAK-4 deficiency. Science 299:2076–2079 [DOI] [PubMed] [Google Scholar]
- 58. Picard C., et al. 2010. Clinical features and outcome of patients with IRAK-4 and MyD88 deficiency. Medicine (Baltimore) 89:403–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Puel A., Picard C., Ku C. L., Smahi A., Casanova J. L. 2004. Inherited disorders of NF-kappaB-mediated immunity in man. Curr. Opin. Immunol. 16:34–41 [DOI] [PubMed] [Google Scholar]
- 60. Puel A., et al. 2006. The NEMO mutation creating the most-upstream premature stop codon is hypomorphic because of a reinitiation of translation. Am. J. Hum. Genet. 78:691–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ringwood L., Li L. 2008. The involvement of the interleukin-1 receptor-associated kinases (IRAKs) in cellular signaling networks controlling inflammation. Cytokine 42:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Salt B. H., et al. 2008. IKBKG (nuclear factor-kappa B essential modulator) mutation can be associated with opportunistic infection without impairing Toll-like receptor function. J. Allergy Clin. Immunol. 121:976–982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Smahi A., et al. 2000. Genomic rearrangement in NEMO impairs NF-kappaB activation and is a cause of incontinentia pigmenti. The International Incontinentia Pigmenti (IP) Consortium. Nature 405:466–472 [DOI] [PubMed] [Google Scholar]
- 64. Suzuki N., Saito T. 2006. IRAK-4—a shared NF-kappaB activator in innate and acquired immunity. Trends Immunol. 27:566–572 [DOI] [PubMed] [Google Scholar]
- 65. Suzuki N., Suzuki S., Yeh W. C. 2002. IRAK-4 as the central TIR signaling mediator in innate immunity. Trends Immunol. 23:503–506 [DOI] [PubMed] [Google Scholar]
- 66. Takada H., et al. 2006. Delayed separation of the umbilical cord in two siblings with interleukin-1 receptor-associated kinase 4 deficiency: rapid screening by flow cytometer. J. Pediatr. 148:546–548 [DOI] [PubMed] [Google Scholar]
- 67. Tono C., et al. 2007. Correction of immunodeficiency associated with NEMO mutation by umbilical cord blood transplantation using a reduced-intensity conditioning regimen. Bone Marrow Transplant. 39:801–804 [DOI] [PubMed] [Google Scholar]
- 68. van Bruggen, et al. 2010. Toll-like receptor responses in IRAK-4-deficient neutrophils. J. Innate Immun. 2:280–287 [DOI] [PubMed] [Google Scholar]
- 69. von Bernuth H., et al. 2006. A fast procedure for the detection of defects in Toll-like receptor signaling. Pediatrics 118:2498–2503 [DOI] [PubMed] [Google Scholar]
- 70. von Bernuth H., et al. 2008. Pyogenic bacterial infections in humans with MyD88 deficiency. Science 321:691–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Yang K., et al. 2005. Human TLR-7-, -8-, and -9-mediated induction of IFN-alpha/beta and -lambda is IRAK-4 dependent and redundant for protective immunity to viruses. Immunity 23:465–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Zhang S. Y., et al. 2007. Human Toll-like receptor-dependent induction of interferons in protective immunity to viruses. Immunol. Rev. 220:225–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Zhang S. Y., et al. 2007. TLR3 deficiency in patients with herpes simplex encephalitis. Science 317:1522–1527 [DOI] [PubMed] [Google Scholar]
- 74. Zonana J., et al. 2000. A novel X-linked disorder of immune deficiency and hypohidrotic ectodermal dysplasia is allelic to incontinentia pigmenti and due to mutations in IKK-gamma (NEMO). Am. J. Hum. Genet. 67:1555–1562 [DOI] [PMC free article] [PubMed] [Google Scholar]