Abstract
Targeting mTOR complex 1 (mTORC1), which regulates general protein translation, represents one of the most attractive approaches to treating cancer, since up-regulation of this pathway is a common hallmark in many tumors. Nevertheless, the use of rapamycin and its analogs in the clinic has revealed that mTORC1 pathway is embedded in a network of signaling cross-talks and feedbacks which might reduce its effectiveness in cancer. We have recently described a novel signaling feedback stemming from mTORC1 inhibition, which leads to the activation of ERK-MAPK (MAPK) pathway. The observation that MAPK is activated by rapamycin and its analogs in vitro, in mouse models, and cancer patient biopsies sets the rationale for the combined use of MAPK and mTORC1 inhibitors in cancer therapy. In this extra-view, we integrate our findings into the mTORC1 signaling network and discuss its relevance for the design of combinatorial therapies with mTORC1 inhibitors.
The mammalian target of rapamycin (mTOR) was originally described in yeast as the target of rapamycin, a macrolide with potent immunosuppressant activity1–4. It is currently accepted that this kinase functions within two multi-protein complexes, sharing mTOR itself and GβL: mTORC1 associates with RAPTOR (Regulatory associated protein of mTOR) and PRAS40 (Proline-rich AKT substrate of 40KDa) whereas mTORC2 includes RICTOR (Rapamycin-insensitive companion of mTOR), Protor and SIN-15, 6. Rapamycin and its analogs target preferentially mTORC1, while mTORC2 disassembly and therefore, loss-of-function by this family of compounds is observed in a limited number of cell types after chronic exposure to the drug5. mTOR is a key component of the highly oncogenic PI3K (Phosphoinositide 3-Kinase) pathway at two different levels (see below for a description of the PI3K pathway): mTORC1 regulates protein translation downstream of the PI3K pathway5, while mTORC2 functions upstream, in charge of the phosphorylation and activation of the AKT kinase7, 8.
The composition of the PI3K pathway underlines its critical role in cell physiology and therefore the relevance of its tight regulation. From PI3K to mTOR, this cascade contains numerous checkpoints that fine-tune the extracellular signals that are sensed, internalized and transmitted by membrane receptors. The canonical PI3K pathway starts at the plasma membrane where the receptor (i.e. Insulin receptor, IGF receptor 1, Tyrosine Kinase Receptor class I) binds its ligand, and through adaptor proteins (i.e. Insulin receptor substrate-1 - IRS-1) activates PI3K. This kinase in turn increases the production of phosphoinositides phosphorylated in position 3’, primarily PI-3,4,5P3 (PIP3), which recruits among others, PDK1 (Phosphoinositide-dependent Kinase 1) and AKT to the membrane through their PH (phosphoinositide binding) domain. At the plasma membrane, AKT is phosphorylated and fully activated by PDK1 and mTORC25. Active AKT can in turn phosphorylate its more than 100 targets, leading to the regulation of cell survival, proliferation, metabolism and protein translation9. Importantly, AKT activates mTORC1 pathway through the inhibition of two negative regulators: TSC210–12, which together with TSC1 inhibits the small GTPase Rheb (Ras homolog enriched in brain)13–16; and PRAS40, which competes with Rheb to negatively regulate mTORC117, 18. Rheb is considered the most proximal activator of mTORC1; and although the exact mechanism by which it activates the kinase is not clear, it may involve direct interaction with the complex as well as the inhibition of the mTORC1 inhibitor FKBP3819.
As mentioned above, along the PI3K pathway a high number of negative regulators ensure the correct intensity of the transmitted signal. Beside the TSC complex, PRAS40 and FKBP38, the PI3K pathway is regulated upstream by the lipid phosphatase PTEN (Phosphatase and Tensin homolog deleted in chromosome Ten)20. PTEN dephosphorylates phosphoinositides in position 3’, therefore promoting the termination of the signal elicited by PI3K21, 22.
The relevance of this pathway in cancer arises from two evidences. Firstly, there are several negative regulators within the PI3K pathway whose mutations lead to familiar syndromes characterized by abnormal tissue-specific proliferation and cancer susceptibility. Heterozygous PTEN mutations cause Cowden, Lhermitte–Duclos, Bannayan–Riley–Rubalca, Proteus, Proteus-like23 familiar syndromes, whereas TSC1/2 complex was named after the syndrome (Tuberous Sclerosis24) that is the result of mutations in these two proteins. LKB1 regulates a pathway that converges with PI3K in the regulation of mTORC1, and is responsible for Peutz-Jeghers syndrome25. Secondly, mTORC1 is hyperactive in many cancers due to sporadic mutations, amplifications or losses upstream regulators, including PI3K, PTEN, LKB1, PML, AKT, TSC2 and Rheb5, 26–30. In particular, loss of the tumor suppressor PML has been recently shown to deregulate the PI3K pathway at multiple levels. Pml-loss cooperates with Pten heterozygosity in mouse models through the decrease of PP2A-mediated nuclear Akt dephosphorylation30. Loss of this tumor suppressor also deregulates the PI3K pathway both upstream, through delocalization of PTEN29, and downstream through activation of mTOR and increase of its cytoplasmic partitioning28.
With all these evidences, it is clear that the PI3K pathway represents a solid ground for the development of anticancer drugs. One of the most attractive possibilities lies in targeting mTORC131. The advantage of having available a natural and well-tolerated inhibitor of the complex (rapamycin, rapidly followed by the design of more potent analogs) promoted a number of studies and clinical trials aimed at testing the efficiency of these compounds in the treatment of cancer.
mTORC1 inhibition activates PI3K
Numerous studies had suggested the existence of a mTORC1-PI3K feedback loop in vitro (described below), but it was not until 2006 that the groups of Neil Rosen and Jose Baselga demonstrated that pharmacological mTORC1 inhibition leads to AKT activation in human cancer biopsies32, 33. Subsequent studies expanded this notion to include other types of tumors, such as glioblastomas34. This finding has important therapeutic implications, since it would imply that part of the unexpectedly poor results of these compounds in clinical trials might be due to this negative feedback, and that in order to improve the anticancer potency of mTORC1 inhibitors, the PI3K pathway should be concomitantly blocked upstream.
The idea of a negative feedback regulating PI3K was built on initial observations showing that chronic insulin treatment35–41 as well as Tsc2-loss42–45 led to a down-regulation of the upstream PI3K pathway. Further studies provided the mechanism responsible for this feedback. mTORC1 activates S6K1, which inhibits IRS-1 through phosphorylation in serine 30242. But beside its phosphorylation at this site, IRS-1 can be down-regulated through transcriptional repression42 and phosphorylated at different residues by S6K1 as well as by other members of the pathway46–49. Moreover, IRS-1-independent feedbacks have been proposed, involving the regulation of IGF1R, IGF1, PDGFR, eIF4E-NBS-1 and IRS-250–55 and the modulation of mTORC2 by the TSC complex56.
MAPK pathway is activated upon mTORC1 inhibition
A recent study from our group demonstrates that mTORC1, when inhibited, activates the MAPK pathway as well57. In this study we analyzed biopsies from patients enrolled in a clinical trial of the rapamycin analog Everolimus33, who received a weekly high dose or a daily lower dose of the compound. 4-week treatment showed an efficient inhibition of mTORC1 in both administration schedules. Analysis of these samples surprisingly showed a strong up-regulation of the MAPK pathway by means of ERK phosphorylation, particularly in patients enrolled in the weekly administration regimen.
The activation of MAPK by Everolimus in cancer patient biopsies led us to hypothesize that this feedback could also be a cause of the poor anticancer activity of mTORC1 inhibitors. First, we validated this observation in vitro and in mouse models and next we attempted to decipher the mechanism of activation. The phosphorylation of ERK under these conditions required proper Ras and MEK1/2 function. Moreover, abrogation of the PI3K feedback by expression of a rapamycin insensitive-constitutively active S6K, or by pharmacological inhibition of PI3K, reduced MAPK activation upon rapamycin treatment. Moreover, the connection between the PI3K and the MAPK pathway is corroborated by two additional evidences: i) insulin and IGF-1 treatment synergize with rapamycin in the activation of MAPK; and, ii) PI3K inhibition prevents the activation of MAPK induced by insulin treatment.
Our results suggest that MAPK activation by mTORC1 inhibitors is mediated by S6K-PI3K-Ras signaling. Yet it remains to be fully determined the exact mechanism that leads to MAPK activation upon mTORC1 inhibition, whether it is always accompanied by concomitant activation of AKT and in which circumstances PI3K directs its signals to Ras-MAPK.
If MAPK is activated in conditions of mTORC1 inhibition, can we improve the anticancer potency of this family of compounds by combining them with MEK1/2 inhibitors? MEK1/2 inhibitors are currently being tested in the clinic as anticancer compounds58. They are directed to cancers with hyperactive MAPK pathway, which is frequently due to amplification of membrane tyrosine Kinase Receptors (EGFR, ERBB2), and activating mutations in upstream regulators of MAPK (Ras, B-Raf)59. First, we determined in vitro the cellular response to combined MEK1/2 (utilizing UO126 and PD0325901) and mTORC1 (utilizing rapamycin) inhibition. Concomitant MAPK inhibition increased cell growth arrest induced by rapamycin, with no apparent induction of cell death (neither apoptosis or autophagy). Moreover, this combination in vivo resulted in enhanced antitumoral potential compared to either compound as single agent with no visible toxicity. Further histological analysis revealed that in vivo, PD0325901 exerted strong proapoptotic activity, while Everolimus remained cytostatic. Importantly, the combination led to lower proliferative rate and higher apoptosis in these tumors, thereby resulting in tumor regression.
Learning from the feedbacks to improve anticancer therapies
One of the characteristics of cancer cells is that they depend on their genetic and molecular alterations, and therefore they become, in the words of I. Bernard Weinstein, addicted to oncogenic events60. Hence targeting the pathway that drives the cancer cell may be the most efficient approach to selectively fighting cancer. In so doing, since the rest of the organism will have intact signaling balance may better tolerate the pharmacological manipulation of the pathway. However, our results together with other studies, suggest that cancer cells treated with mTORC1 inhibitors may utilize these feedbacks as “addiction bypass pathways”.
How can this problem be solved? One of the most obvious approaches to improving the effectiveness of mTORC1 inhibitors is ‘combining to win’61. Although it had been initially proposed that drug combinations would be useful in delaying the appearance of resistance, our observations also suggest that drug combinations would break the “addiction bypass” and therefore render the cancer cells sensitive to the treatment. But, importantly, when drugs are combined, the undesired effects on non-transformed cells increase due to the profound alteration of cell homeostasis. Therefore, a careful analysis of the best combinatorial approach is required. In this sense, the first generation of PI3K inhibitors, which would in theory represent the most efficient approach in targeting the PI3K-mTOR pathway while avoiding undesired feedbacks, proved to have little specificity and therefore high toxicity in vivo62. This issue is likely to be solved with the design of second-generation –more specific-inhibitors and PI3K isoform-specific inhibitors63. On the other hand, MAPK pathway inhibitors have been the subject of intense investigation for the treatment of cancers with alterations within the pathway, from EGFR (e.g. Gelfitinib64), B-Raf (Sorafenib65, 66) and MEK1/2 (PD0325901, ARRY-14288658) inhibitors to compounds that prevent the farnesylation of small GTPases like Ras (FTIs67, 68).
MEK1/2 inhibitors are well-tolerated in patients, and are already being tested in the clinic for cancer treatment58. The first MEK1/2 inhibitor entering clinical trials, CI-1040, was well-tolerated but with low antitumoral activity in an unselected patient population69. A new generation of MEK1/2 inhibitors is now being tested with better results, including PD0325901, ARRY-14288658 and XL51870 (Fig. 1). In our study, we used heterotopic xenografts to test the antitumoral effect of the combination of Everolimus and PD0325901, and the undesired toxicity that may occur. Everolimus was set at the maximum tolerated dose (MTD), 10 mg/Kg/injection and co-administered daily with PD0325901 at 20 mg/Kg. This combination turned out to be toxic in the first week of treatment, reducing body weight in more than 20%. We therefore lowered the dose of PD0325901 to 15 mg/Kg in a day ON/day OFF schedule. This administration scheme resulted in no visible toxicity, yet increased the antitumoral potential of either compound as single agent. The fact that our preliminary in vivo data support a non-toxic combinability of these two group of compounds lead us to speculate that the combination of mTORC1 and MEK1/2 inhibitors will prove very effective in targeting molecularly selceted cancers, after a careful evaluation of the optimum dose and administration schedule. In line with the potential therapeutic benefit of combining mTORC1 and MEK1/2 inhibitors, other groups have demonstrated that in different cancer animal models (e.g. lung and prostate cancer) and cell lines, the combination has synergistic effects on the anticancer activity71, 72.
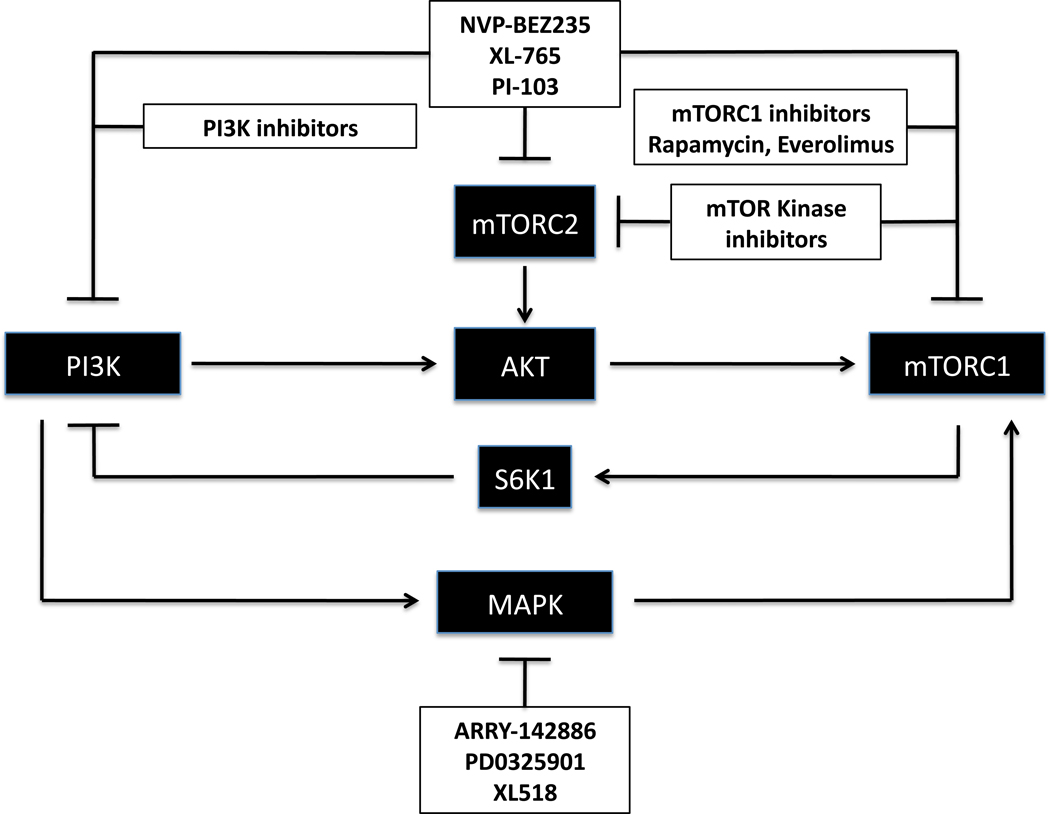
Figure 1. Therapeutic approaches to target mTORC1-feedback loops in human cancer.
Drug combinations that would target the mTORC1 pathway with the concomitant inhibition of mTORC1-feedback loops. Detailed information about each compound can be found in the text. Components of the different signaling cascades are represented in closed boxes and the different families of inhibitors in open boxes.
An additional approach that is being explored concerns the use of dirty drugs, defined as compounds that target multiple protein kinases at once. These compounds might prove useful since they would abrogate undesired feedbacks (Fig. 1). Indeed, different pharmaceutical companies are currently developing compounds that inhibit both mTOR and PI3K (NVP-BEZ235 from Novartis73, XL765 from Exelixis and PI-103 from Piramed74). These drugs have already been shown to display a potent anticancer activity in cell lines and breast cancer tumor xenografts75 and mouse models of CML (PI-10376), as well as to inhibit vessel integrity during development (NVP-BEZ23577). In the same line, non-specific drugs targeting MAPK pathway have turned out to be useful in the treatment of certain cancers. The most evident example is Sorafenib, which was produced originally as a B-Raf inhibitor and was later discovered to inhibit as well PDGFR and VEGFR2, among other proteins58.
It must be noted, however, that while dirty drugs may provide a therapeutic benefit through the targeting of multiple kinases, this approach could at the same time disclose a strong handicap due to toxicity-related side effects. Therefore, a careful, individualized evaluation of the benefit versus toxicity of dirty drugs -as well as patient stratification- would be required in order to optimize their results in cancer therapy.
Lastly, it is attractive to hypothesize that mTOR kinase inhibitors will have an increased anticancer activity, since they would inhibit both translation (mTORC1) as well as AKT (mTORC2), thereby resulting in reduced mTORC1-PI3K-AKT feedback signaling (Fig. 1). Although additional studies are required to determine the level of toxicity of these compounds, novel drugs with these characteristics are entering, or might soon enter, clinical trials for the treatment of human cancers.
Outlook
mTORC1 is not only a critical complex where signaling pathways converge to regulate protein translation, it also acts as a homeostatic sensor that integrates the pro-survival, proliferative and metabolic signals and in turn emits messages to upstream regulators to tune the amount of signal that can be sensed from the extracellular environment. The multiple feedbacks and cross-talks stemming from mTORC1 underline that a full understanding of the signaling consequences of specific pathway inhibition is required for an efficient anticancer therapy design. Lastly, the discovery of this novel mTORC1-MAPK feedback opens new immediate routes in understanding the function of this regulation in cell physiology and homeostasis and how it can be utilized to improve anticancer therapies.
Acknowledgements
We thank the members of the Pandolfi laboratory for discussion and comments. In particular, we would like to thank Elisabeth Mack and Caterina Nardella for critical suggestions. This work is supported by an NIH/NCI grant (CA84292, to P.P. Pandolfi). Arkaitz Carracedo is supported by the European Molecular Biology Organization.
References
- 1.Heitman J, Movva NR, Hall MN. Targets for cell cycle arrest by the immunosuppressant rapamycin in yeast. Science. 1991;253:905–909. doi: 10.1126/science.1715094. [DOI] [PubMed] [Google Scholar]
- 2.Kunz J, Henriquez R, Schneider U, Deuter-Reinhard M, Movva NR, Hall MN. Target of rapamycin in yeast, TOR2, is an essential phosphatidylinositol kinase homolog required for G1 progression. Cell. 1993;73:585–596. doi: 10.1016/0092-8674(93)90144-f. [DOI] [PubMed] [Google Scholar]
- 3.Brown EJ, Albers MW, Shin TB, Ichikawa K, Keith CT, Lane WS, Schreiber SL. A mammalian protein targeted by G1-arresting rapamycin-receptor complex. Nature. 1994;369:756–758. doi: 10.1038/369756a0. [DOI] [PubMed] [Google Scholar]
- 4.Sabatini DM, Erdjument-Bromage H, Lui M, Tempst P, Snyder SH. RAFT1: a mammalian protein that binds to FKBP12 in a rapamycin-dependent fashion and is homologous to yeast TORs. Cell. 1994;78:35–43. doi: 10.1016/0092-8674(94)90570-3. [DOI] [PubMed] [Google Scholar]
- 5.Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell. 2007;12:9–22. doi: 10.1016/j.ccr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 6.Pearce LR, Huang X, Boudeau J, Pawlowski R, Wullschleger S, Deak M, Ibrahim AF, Gourlay R, Magnuson MA, Alessi DR. Identification of Protor as a novel Rictor-binding component of mTOR complex-2. Biochem J. 2007;405:513–522. doi: 10.1042/BJ20070540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sarbassov DD, Ali SM, Kim DH, Guertin DA, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol. 2004;14:1296–1302. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 8.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 9.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dan HC, Sun M, Yang L, Feldman RI, Sui XM, Ou CC, Nellist M, Yeung RS, Halley DJ, Nicosia SV, Pledger WJ, Cheng JQ. Phosphatidylinositol 3-kinase/Akt pathway regulates tuberous sclerosis tumor suppressor complex by phosphorylation of tuberin. J Biol Chem. 2002;277:35364–35370. doi: 10.1074/jbc.M205838200. [DOI] [PubMed] [Google Scholar]
- 11.Inoki K, Li Y, Zhu T, Wu J, Guan KL. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol. 2002;4:648–657. doi: 10.1038/ncb839. [DOI] [PubMed] [Google Scholar]
- 12.Potter CJ, Pedraza LG, Xu T. Akt regulates growth by directly phosphorylating Tsc2. Nat Cell Biol. 2002;4:658–665. doi: 10.1038/ncb840. [DOI] [PubMed] [Google Scholar]
- 13.Garami A, Zwartkruis FJ, Nobukuni T, Joaquin M, Roccio M, Stocker H, Kozma SC, Hafen E, Bos JL, Thomas G. Insulin activation of Rheb, a mediator of mTOR/S6K/4E-BP signaling, is inhibited by TSC1 and 2. Mol Cell. 2003;11:1457–1466. doi: 10.1016/s1097-2765(03)00220-x. [DOI] [PubMed] [Google Scholar]
- 14.Inoki K, Li Y, Xu T, Guan KL. Rheb GTPase is a direct target of TSC2 GAP activity and regulates mTOR signaling. Genes Dev. 2003;17:1829–1834. doi: 10.1101/gad.1110003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tee AR, Manning BD, Roux PP, Cantley LC, Blenis J. Tuberous sclerosis complex gene products, Tuberin and Hamartin, control mTOR signaling by acting as a GTPase-activating protein complex toward Rheb. Curr Biol. 2003;13:1259–1268. doi: 10.1016/s0960-9822(03)00506-2. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y, Gao X, Saucedo LJ, Ru B, Edgar BA, Pan D. Rheb is a direct target of the tuberous sclerosis tumour suppressor proteins. Nat Cell Biol. 2003;5:578–581. doi: 10.1038/ncb999. [DOI] [PubMed] [Google Scholar]
- 17.Sancak Y, Thoreen CC, Peterson TR, Lindquist RA, Kang SA, Spooner E, Carr SA, Sabatini DM. PRAS40 is an insulin-regulated inhibitor of the mTORC1 protein kinase. Mol Cell. 2007;25:903–915. doi: 10.1016/j.molcel.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 18.Vander Haar E, Lee SI, Bandhakavi S, Griffin TJ, Kim DH. Insulin signalling to mTOR mediated by the Akt/PKB substrate PRAS40. Nat Cell Biol. 2007;9:316–323. doi: 10.1038/ncb1547. [DOI] [PubMed] [Google Scholar]
- 19.Bai X, Ma D, Liu A, Shen X, Wang QJ, Liu Y, Jiang Y. Rheb activates mTOR by antagonizing its endogenous inhibitor, FKBP38. Science. 2007;318:977–980. doi: 10.1126/science.1147379. [DOI] [PubMed] [Google Scholar]
- 20.Carracedo A, Salmena L, Pandolfi PP. SnapShot: PTEN signaling pathways. Cell. 2008;133:550 e1. doi: 10.1016/j.cell.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 21.Maehama T, Dixon JE. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273:13375–13378. doi: 10.1074/jbc.273.22.13375. [DOI] [PubMed] [Google Scholar]
- 22.Stambolic V, Suzuki A, de la Pompa JL, Brothers GM, Mirtsos C, Sasaki T, Ruland J, Penninger JM, Siderovski DP, Mak TW. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell. 1998;95:29–39. doi: 10.1016/s0092-8674(00)81780-8. [DOI] [PubMed] [Google Scholar]
- 23.Orloff MS, Eng C. Genetic and phenotypic heterogeneity in the PTEN hamartoma tumour syndrome. Oncogene. 2008;27:5387–5397. doi: 10.1038/onc.2008.237. [DOI] [PubMed] [Google Scholar]
- 24.Jozwiak J, Jozwiak S, Wlodarski P. Possible mechanisms of disease development in tuberous sclerosis. Lancet Oncol. 2008;9:73–79. doi: 10.1016/S1470-2045(07)70411-4. [DOI] [PubMed] [Google Scholar]
- 25.Sanchez-Cespedes M. A role for LKB1 gene in human cancer beyond the Peutz-Jeghers syndrome. Oncogene. 2007;26:7825–7832. doi: 10.1038/sj.onc.1210594. [DOI] [PubMed] [Google Scholar]
- 26.Nardella C, Chen Z, Salmena L, Carracedo A, Alimonti A, Egia A, Carver B, Gerald W, Cordon-Cardo C, Pandolfi PP. Aberrant Rheb-mediated mTORC1 activation and Pten haploinsufficiency are cooperative oncogenic events. Genes Dev. 2008;22:2172–2177. doi: 10.1101/gad.1699608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mavrakis KJ, Zhu H, Silva RL, Mills JR, Teruya-Feldstein J, Lowe SW, Tam W, Pelletier J, Wendel HG. Tumorigenic activity and therapeutic inhibition of Rheb GTPase. Genes Dev. 2008;22:2178–2188. doi: 10.1101/gad.1690808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bernardi R, Guernah I, Jin D, Grisendi S, Alimonti A, Teruya-Feldstein J, Cordon-Cardo C, Simon MC, Rafii S, Pandolfi PP. PML inhibits HIF-1alpha translation and neoangiogenesis through repression of mTOR. Nature. 2006;442:779–785. doi: 10.1038/nature05029. [DOI] [PubMed] [Google Scholar]
- 29.Song MS, Salmena L, Carracedo A, Egia A, Lo-Coco F, Teruya-Feldstein J, Pandolfi PP. The deubiquitinylation and localization of PTEN are regulated by a HAUSP-PML network. Nature. 2008 doi: 10.1038/nature07290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trotman LC, Alimonti A, Scaglioni PP, Koutcher JA, Cordon-Cardo C, Pandolfi PP. Identification of a tumour suppressor network opposing nuclear Akt function. Nature. 2006;441:523–527. doi: 10.1038/nature04809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garcia-Echeverria C, Sellers WR. Drug discovery approaches targeting the PI3K/Akt pathway in cancer. Oncogene. 2008;27:5511–5526. doi: 10.1038/onc.2008.246. [DOI] [PubMed] [Google Scholar]
- 32.O'Reilly KE, Rojo F, She QB, Solit D, Mills GB, Smith D, Lane H, Hofmann F, Hicklin DJ, Ludwig DL, Baselga J, Rosen N. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer research. 2006;66:1500–1508. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tabernero J, Rojo F, Calvo E, Burris H, Judson I, Hazell K, Martinelli E, Ramon y, Cajal S, Jones S, Vidal L, Shand N, Macarulla T, Ramos FJ, Dimitrijevic S, Zoellner U, Tang P, Stumm M, Lane HA, Lebwohl D, Baselga J. Dose- and schedule-dependent inhibition of the mammalian target of rapamycin pathway with everolimus: a phase I tumor pharmacodynamic study in patients with advanced solid tumors. J Clin Oncol. 2008;26:1603–1610. doi: 10.1200/JCO.2007.14.5482. [DOI] [PubMed] [Google Scholar]
- 34.Cloughesy TF, Yoshimoto K, Nghiemphu P, Brown K, Dang J, Zhu S, Hsueh T, Chen Y, Wang W, Youngkin D, Liau L, Martin N, Becker D, Bergsneider M, Lai A, Green R, Oglesby T, Koleto M, Trent J, Horvath S, Mischel PS, Mellinghoff IK, Sawyers CL. Antitumor activity of rapamycin in a Phase I trial for patients with recurrent PTEN-deficient glioblastoma. PLoS Med. 2008;5:e8. doi: 10.1371/journal.pmed.0050008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berg CE, Lavan BE, Rondinone CM. Rapamycin partially prevents insulin resistance induced by chronic insulin treatment. Biochem Biophys Res Commun. 2002;293:1021–1027. doi: 10.1016/S0006-291X(02)00333-9. [DOI] [PubMed] [Google Scholar]
- 36.Greene MW, Sakaue H, Wang L, Alessi DR, Roth RA. Modulation of insulin-stimulated degradation of human insulin receptor substrate-1 by Serine 312 phosphorylation. J Biol Chem. 2003;278:8199–8211. doi: 10.1074/jbc.M209153200. [DOI] [PubMed] [Google Scholar]
- 37.Hartley D, Cooper GM. Role of mTOR in the degradation of IRS-1: regulation of PP2A activity. J Cell Biochem. 2002;85:304–314. doi: 10.1002/jcb.10135. [DOI] [PubMed] [Google Scholar]
- 38.Haruta T, Uno T, Kawahara J, Takano A, Egawa K, Sharma PM, Olefsky JM, Kobayashi M. A rapamycin-sensitive pathway down-regulates insulin signaling via phosphorylation and proteasomal degradation of insulin receptor substrate-1. Mol Endocrinol. 2000;14:783–794. doi: 10.1210/mend.14.6.0446. [DOI] [PubMed] [Google Scholar]
- 39.Pirola L, Bonnafous S, Johnston AM, Chaussade C, Portis F, Van Obberghen E. Phosphoinositide 3-kinase-mediated reduction of insulin receptor substrate-1/2 protein expression via different mechanisms contributes to the insulin-induced desensitization of its signaling pathways in L6 muscle cells. J Biol Chem. 2003;278:15641–15651. doi: 10.1074/jbc.M208984200. [DOI] [PubMed] [Google Scholar]
- 40.Tremblay F, Marette A. Amino acid and insulin signaling via the mTOR/p70 S6 kinase pathway. A negative feedback mechanism leading to insulin resistance in skeletal muscle cells. J Biol Chem. 2001;276:38052–38060. doi: 10.1074/jbc.M106703200. [DOI] [PubMed] [Google Scholar]
- 41.Zhande R, Mitchell JJ, Wu J, Sun XJ. Molecular mechanism of insulin-induced degradation of insulin receptor substrate 1. Mol Cell Biol. 2002;22:1016–1026. doi: 10.1128/MCB.22.4.1016-1026.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harrington LS, Findlay GM, Gray A, Tolkacheva T, Wigfield S, Rebholz H, Barnett J, Leslie NR, Cheng S, Shepherd PR, Gout I, Downes CP, Lamb RF. The TSC1–2 tumor suppressor controls insulin-PI3K signaling via regulation of IRS proteins. J Cell Biol. 2004;166:213–223. doi: 10.1083/jcb.200403069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ma L, Teruya-Feldstein J, Behrendt N, Chen Z, Noda T, Hino O, Cordon-Cardo C, Pandolfi PP. Genetic analysis of Pten and Tsc2 functional interactions in the mouse reveals asymmetrical haploinsufficiency in tumor suppression. Genes Dev. 2005;19:1779–1786. doi: 10.1101/gad.1314405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Manning BD, Logsdon MN, Lipovsky AI, Abbott D, Kwiatkowski DJ, Cantley LC. Feedback inhibition of Akt signaling limits the growth of tumors lacking Tsc2. Genes Dev. 2005;19:1773–1778. doi: 10.1101/gad.1314605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shah OJ, Wang Z, Hunter T. Inappropriate activation of the TSC/Rheb/mTOR/S6K cassette induces IRS1/2 depletion, insulin resistance, and cell survival deficiencies. Curr Biol. 2004;14:1650–1656. doi: 10.1016/j.cub.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 46.Jakobsen SN, Hardie DG, Morrice N, Tornqvist HE. 5'-AMP-activated protein kinase phosphorylates IRS-1 on Ser-789 in mouse C2C12 myotubes in response to 5-aminoimidazole-4-carboxamide riboside. J Biol Chem. 2001;276:46912–46916. doi: 10.1074/jbc.C100483200. [DOI] [PubMed] [Google Scholar]
- 47.Ozes ON, Akca H, Mayo LD, Gustin JA, Maehama T, Dixon JE, Donner DB. A phosphatidylinositol 3-kinase/Akt/mTOR pathway mediates and PTEN antagonizes tumor necrosis factor inhibition of insulin signaling through insulin receptor substrate-1. Proc Natl Acad Sci U S A. 2001;98:4640–4645. doi: 10.1073/pnas.051042298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tremblay F, Brule S, Hee Um S, Li Y, Masuda K, Roden M, Sun XJ, Krebs M, Polakiewicz RD, Thomas G, Marette A. Identification of IRS-1 Ser-1101 as a target of S6K1 in nutrient- and obesity-induced insulin resistance. Proc Natl Acad Sci U S A. 2007;104:14056–14061. doi: 10.1073/pnas.0706517104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tzatsos A, Kandror KV. Nutrients suppress phosphatidylinositol 3-kinase/Akt signaling via raptor-dependent mTOR-mediated insulin receptor substrate 1 phosphorylation. Mol Cell Biol. 2006;26:63–76. doi: 10.1128/MCB.26.1.63-76.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Culjkovic B, Tan K, Orolicki S, Amri A, Meloche S, Borden KL. The eIF4E RNA regulon promotes the Akt signaling pathway. J Cell Biol. 2008;181:51–63. doi: 10.1083/jcb.200707018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tamburini J, Chapuis N, Bardet V, Park S, Sujobert P, Willems L, Ifrah N, Dreyfus F, Mayeux P, Lacombe C, Bouscary D. Mammalian target of rapamycin (mTOR) inhibition activates phosphatidylinositol 3-kinase/Akt by up-regulating insulin-like growth factor-1 receptor signaling in acute myeloid leukemia: rationale for therapeutic inhibition of both pathways. Blood. 2008;111:379–382. doi: 10.1182/blood-2007-03-080796. [DOI] [PubMed] [Google Scholar]
- 52.Wan X, Harkavy B, Shen N, Grohar P, Helman LJ. Rapamycin induces feedback activation of Akt signaling through an IGF-1R-dependent mechanism. Oncogene. 2007;26:1932–1940. doi: 10.1038/sj.onc.1209990. [DOI] [PubMed] [Google Scholar]
- 53.Zhang H, Bajraszewski N, Wu E, Wang H, Moseman AP, Dabora SL, Griffin JD, Kwiatkowski DJ. PDGFRs are critical for PI3K/Akt activation and negatively regulated by mTOR. J Clin Invest. 2007;117:730–738. doi: 10.1172/JCI28984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang H, Cicchetti G, Onda H, Koon HB, Asrican K, Bajraszewski N, Vazquez F, Carpenter CL, Kwiatkowski DJ. Loss of Tsc1/Tsc2 activates mTOR and disrupts PI3K-Akt signaling through downregulation of PDGFR. J Clin Invest. 2003;112:1223–1233. doi: 10.1172/JCI17222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Carracedo A, Pandolfi PP. The PTEN-PI3K pathway: of feedbacks and cross-talks. Oncogene. 2008;27:5527–5541. doi: 10.1038/onc.2008.247. [DOI] [PubMed] [Google Scholar]
- 56.Huang J, Dibble CC, Matsuzaki M, Manning BD. The TSC1–TSC2 complex is required for proper activation of mTOR complex 2. Mol Cell Biol. 2008 doi: 10.1128/MCB.00289-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Carracedo A, Ma L, Teruya-Feldstein J, Rojo F, Salmena L, Alimonti A, Egia A, Sasaki AT, Thomas G, Kozma SC, Papa A, Nardella C, Cantley LC, Baselga J, Pandolfi PP. Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer. J Clin Invest. 2008;118:3065–3074. doi: 10.1172/JCI34739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kohno M, Pouyssegur J. Targeting the ERK signaling pathway in cancer therapy. Ann Med. 2006;38:200–211. doi: 10.1080/07853890600551037. [DOI] [PubMed] [Google Scholar]
- 59.Dhillon AS, Hagan S, Rath O, Kolch W. MAP kinase signalling pathways in cancer. Oncogene. 2007;26:3279–3290. doi: 10.1038/sj.onc.1210421. [DOI] [PubMed] [Google Scholar]
- 60.Weinstein IB. Cancer. Addiction to oncogenes--the Achilles heal of cancer. Science. 2002;297:63–64. doi: 10.1126/science.1073096. [DOI] [PubMed] [Google Scholar]
- 61.Baselga J. Targeting tyrosine kinases in cancer: the second wave. Science. 2006;312:1175–1178. doi: 10.1126/science.1125951. [DOI] [PubMed] [Google Scholar]
- 62.Crabbe T, Welham MJ, Ward SG. The PI3K inhibitor arsenal: choose your weapon! Trends Biochem Sci. 2007;32:450–456. doi: 10.1016/j.tibs.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 63.Yuan TL, Cantley LC. PI3K pathway alterations in cancer: variations on a theme. Oncogene. 2008;27:5497–5510. doi: 10.1038/onc.2008.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Herbst RS, Fukuoka M, Baselga J. Gefitinib--a novel targeted approach to treating cancer. Nat Rev Cancer. 2004;4:956–965. doi: 10.1038/nrc1506. [DOI] [PubMed] [Google Scholar]
- 65.Li N, Batt D, Warmuth M. B-Raf kinase inhibitors for cancer treatment. Curr Opin Investig Drugs. 2007;8:452–456. [PubMed] [Google Scholar]
- 66.Schreck R, Rapp UR. Raf kinases: oncogenesis and drug discovery. Int J Cancer. 2006;119:2261–2271. doi: 10.1002/ijc.22144. [DOI] [PubMed] [Google Scholar]
- 67.Karp JE, Lancet JE. Development of farnesyltransferase inhibitors for clinical cancer therapy: focus on hematologic malignancies. Cancer Invest. 2007;25:484–494. doi: 10.1080/07357900701359437. [DOI] [PubMed] [Google Scholar]
- 68.Zhu K, Hamilton AD, Sebti SM. Farnesyltransferase inhibitors as anticancer agents: current status. Curr Opin Investig Drugs. 2003;4:1428–1435. [PubMed] [Google Scholar]
- 69.Rinehart J, Adjei AA, Lorusso PM, Waterhouse D, Hecht JR, Natale RB, Hamid O, Varterasian M, Asbury P, Kaldjian EP, Gulyas S, Mitchell DY, Herrera R, Sebolt-Leopold JS, Meyer MB. Multicenter phase II study of the oral MEK inhibitor, CI-1040, in patients with advanced non-small-cell lung, breast, colon, and pancreatic cancer. J Clin Oncol. 2004;22:4456–4462. doi: 10.1200/JCO.2004.01.185. [DOI] [PubMed] [Google Scholar]
- 70.Rosen L, Galatin P, Fehling J, Laux I, Dinolfo M, Frye J, Laird D, Sikic B. A phase 1 dose-escalation study of XL518, a potent MEK inhibitor administered orally daily to subjects with solid tumors. J Clin Oncol. 2008;26 [Google Scholar]
- 71.Kinkade CW, Castillo-Martin M, Puzio-Kuter A, Yan J, Foster TH, Gao H, Sun Y, Ouyang X, Gerald WL, Cordon-Cardo C, Abate-Shen C. Targeting AKT/mTOR and ERK MAPK signaling inhibits hormone-refractory prostate cancer in a preclinical mouse model. J Clin Invest. 2008;118:3051–3064. doi: 10.1172/JCI34764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Legrier ME, Yang CP, Yan HG, Lopez-Barcons L, Keller SM, Perez-Soler R, Horwitz SB, McDaid HM. Targeting protein translation in human non small cell lung cancer via combined MEK and mammalian target of rapamycin suppression. Cancer research. 2007;67:11300–11308. doi: 10.1158/0008-5472.CAN-07-0702. [DOI] [PubMed] [Google Scholar]
- 73.Maira SM, Stauffer F, Brueggen J, Furet P, Schnell C, Fritsch C, Brachmann S, Chene P, De Pover A, Schoemaker K, Fabbro D, Gabriel D, Simonen M, Murphy L, Finan P, Sellers W, Garcia-Echeverria C. Identification and characterization of NVP-BEZ235, a new orally available dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor with potent in vivo antitumor activity. Mol Cancer Ther. 2008;7:1851–1863. doi: 10.1158/1535-7163.MCT-08-0017. [DOI] [PubMed] [Google Scholar]
- 74.Fan QW, Knight ZA, Goldenberg DD, Yu W, Mostov KE, Stokoe D, Shokat KM, Weiss WA. A dual PI3 kinase/mTOR inhibitor reveals emergent efficacy in glioma. Cancer Cell. 2006;9:341–349. doi: 10.1016/j.ccr.2006.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Serra V, Markman B, Scaltriti M, Eichhorn PJ, Valero V, Guzman M, Botero ML, Llonch E, Atzori F, Di Cosimo S, Maira M, Garcia-Echeverria C, Parra JL, Arribas J, Baselga J. NVP-BEZ235, a dual PI3K/mTOR inhibitor, prevents PI3K signaling and inhibits the growth of cancer cells with activating PI3K mutations. Cancer research. 2008;68:8022–8030. doi: 10.1158/0008-5472.CAN-08-1385. [DOI] [PubMed] [Google Scholar]
- 76.Kharas MG, Janes MR, Scarfone VM, Lilly MB, Knight ZA, Shokat KM, Fruman DA. Ablation of PI3K blocks BCR-ABL leukemogenesis in mice, and a dual PI3K/mTOR inhibitor prevents expansion of human BCR-ABL+ leukemia cells. J Clin Invest. 2008;118:3038–3050. doi: 10.1172/JCI33337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yuan TL, Choi HS, Matsui A, Benes C, Lifshits E, Luo J, Frangioni JV, Cantley LC. Class 1A PI3K regulates vessel integrity during development and tumorigenesis. Proc Natl Acad Sci U S A. 2008;105:9739–9744. doi: 10.1073/pnas.0804123105. [DOI] [PMC free article] [PubMed] [Google Scholar]