Abstract
Canonical Wnt-signalling has been implicated in mouse and human embryonic stem cell (ESC) maintenance, however its requirement is controversial. β-catenin is the key component in this highly conserved Wnt pathway, acting as a transcriptional transactivator. Yet, β-catenin has additional roles at the plasma membrane regulating cell-cell adhesion, complicating the analyses of cells/tissues lacking β-catenin. We report here the generation of a β-catenin deficient mouse ESC (mESC) line and show that self-renewal is maintained in absence of β-catenin. Cell-adhesion is partially rescued by plakoglobin up-regulation, but fails to be maintained during differentiation. When differentiated as aggregates, wild-type mESCs form descendents of all three germ layers, while mesendodermal germ layer formation and neuronal differentiation are defective in β-catenin deficient mESCs. A Tcf/Lef-signalling defective β-catenin variant, which re-establishes cadherin-mediated cell-adhesion, rescues definitive endoderm and neuroepithelial formation, suggesting that β-catenin cell-adhesion function is more important than its signalling function for these processes.
Keywords: beta-catenin, plakoglobin, stemness, endoderm differentiation, cell- adhesion
ESCs are self-renewing, fully pluripotent cells. Descendents from all three germ layers can be produced in vitro by allowing ESCs to differentiate as aggregates, known as embryoid bodies (EBs) 1–3. Various signalling pathways, including Leukemia inhibitory factor (LIF), bone morphogenetic protein 4, and Wnt/β-catenin signalling can maintain mouse ESCs self-renewal capacity 4–7. Although cytokines required for human and mESCs differ substantially 8–11, activation of Wnt/β-catenin signalling is beneficial for both 12. Wnt-ligand binding to its receptor complex, composed out of a serpentine receptor encoded by the Frizzled gene family and a co-receptor of the low-density lipoprotein related family encoded by the Lrp5 and Lrp6 genes, activates the pathway. Ligand-binding results in inhibition of the destruction complex composed out of Adenomatosis polyposis coli, Axin, Casein kinase 1α (CK1α) and the Glycogen synthase kinase 3β (GSK3β) 13, 14. This changes the fate of β-catenin, which in absence of Wnt-ligands would be phosphorylated by CK1α and GSK3β, subsequently ubiquitinated and degraded via the proteosome pathway. In response to Wnt-signalling, β-catenin accumulates, translocates to the nucleus and transactivates target genes, such as Axin2, in a complex with TCF/LEF transcription factors 14.
Wnt/β-catenin requirement in ESC self-renewal has been addressed yielding somewhat contradictory results 15–18. Inhibition of Wnt/β-catenin signalling in mESCs using a dominant-negative ΔNhLef1 did not affect self-renewal 16, while studies analyzing β-catenin deficient mESCs differed substantially. Two studies, using either a previously 19 or a newly established β-catenin deficient mESC line, reported defects in stemness 15, 18. A third study, using β-catenin null mESCs of unclear origin, reported no such defects 17. In two of the three studies the mESCs, according to their molecular characterization, were actually epiblast-like stem cells (EpiSCs) 15, 17. Thus, there is clearly need to establish additional independent β-catenin deficient mESCs in order to shed light on the requirement of Wnt/β-catenin in ESC self-renewal. Yet, β-catenin is not only an essential component of the canonical Wnt pathway, it is also an integral component of adherens junctions linking classical cadherins to α-catenin and the actin cytoskeleton 20. This second function of β-catenin can also be performed by the related family member plakoglobin, which is also an essential desmosomal component 19, 21–23. Given the dual functions of β-catenin it is difficult to unambiguously distinguish between β-catenin requirement in Wnt-signalling versus cell-adhesion in cellular and developmental processes.
Here we established new β-catenin deficient mESCs with a deletion of exons 3–6 to address the role of β-catenin in mESC self-renewal and differentiation and show thatβ-catenin is not required for self-renewal. Under self-renewal conditions, plakoglobin partially substitutes for the β-catenin cell-adhesion function, but fails to do so during differentiation. In order to address to what extent β-catenin signalling versus cell-adhesion functions are required during in vitro differentiation of mESCs, we generated β-catenin-rescued mESCs expressing either wild-type or a TCF/LEF-signalling defective β-catenin variant. Our findings show that the cell-adhesion function of β-catenin is more important than its signalling function for definitive endoderm formation and promotion of neuronal differentiation.
Results
Characterization of β-catenin deficient mESCs
mESCs were derived from β-catenin fl/fl (β-catfl/fl) 24 blastocysts and individual feeder-free β-catfl/fl mESCs were established using LIF and serum. Several β-catenin deficient mESC subclones (referred to as β-catΔ/Δ) were established from one β-catfl/fl mESC line (NLβ-12) using adenoviral mediated Cre-recombinase and two were used for further characterization (Fig. 1a). The parental β-catfl/fl mESC line, NLβ-12, was fully pluripotent as it gave rise to chimeric mice and transmitted through the germline (see Supplementary Information, Fig. S1 online).
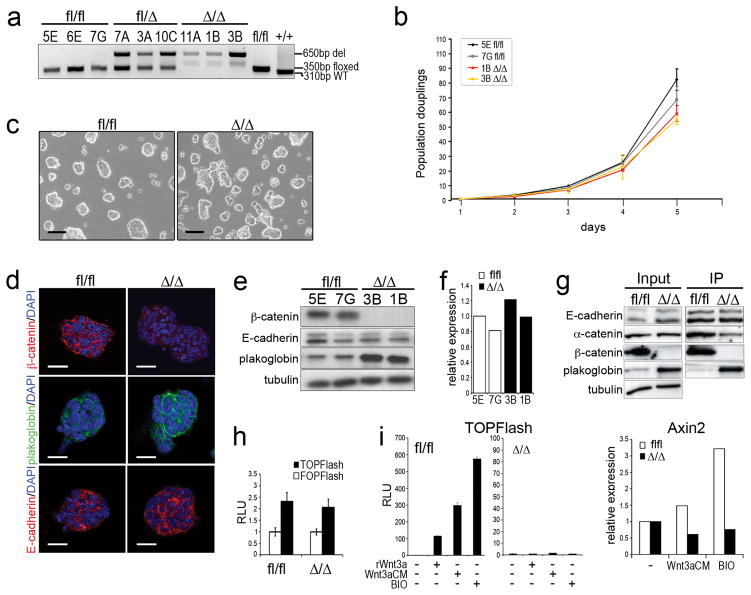
Figure 1.
Characterization of β-catfl/fl and β-catΔ/Δ mESCs.
(a) Genotyping PCR for β-catfl/fl, β-catfl/Δ and β-catΔ/Δ mESC clones.
(b) Population doublings of β-catfl/fl and β-catΔ/Δ mESC clones grown for 5 days. Cells were collected and counted each day (performed in triplicates).
(c) Morphological appearance of β-catfl/fl and β-catΔ/Δ mESCs. Scale bar: 100 μm.
(d) Confocal images of immunofluorescent stainings for β-catenin, plakoglobin and E-cadherin of β-catfl/fl and β-catΔ/Δ mESC colonies. Scale bar: 20 μm.
(e) Western blots for β-catenin, E-cadherin, plakoglobin and tubulin in mESCs.
(f) Relative plakoglobin expression levels in β-catfl/fl and β-catΔ/Δ mESCs (n=1).
(g) Western blot for E-cadherin, α-catenin, β-catenin and plakoglobin showing input and E-cadherin immunoprecipitats (IP) in β-catfl/fl and β-catΔ/Δ mESCs.
(h) Histogram showing representative example of BAR (TOPFlash) and fuBAR (FOPFlash) assays using stable β-catfl/fl and β-catΔ/Δ mESC lines.
(i) Histogram showing representative example of TOPFlash assay of stable BAR βcatfl/fl and β-catΔ/Δ mESCs stimulated with rWnt3a, Wnt3aCM or BIO. Error bars ± s.d in (h) and (i) are calculated on the basis of three measurements.
(j) Histogram showing relative Axin2 expression level in β-catfl/fl and β-catΔ/Δ mESCs stimulated with Wnt3aCM and BIO. Data are mean of two biological replicates.
β-catΔ/Δ and β-catfl/fl mESCs showed an overall similar proliferation behaviour and colony appearance (Fig. 1b, c). β-catΔ/Δ colonies stained negative for β-catenin by immunocytochemistry, while E-cadherin and plakoglobin staining and distribution were normal (Fig. 1d). Interestingly, staining intensity of the latter was increased, as were protein levels (Fig. 1d, e), but transcript levels were unchanged (Fig. 1f). Thus, similar to F9 teratocarcinoma stem cells, plakoglobin substitutes for β-catenin cell-adhesion function in mESCs 22. This was confirmed by immunoprecipitation experiments, showing increased plakoglobin levels bound to E-cadherin in β-catΔ/Δ versus β-catfl/fl mESCs (Fig. 1g).
Plakoglobin has reportedly a weak in vitro signalling activity 25, 26. Therefore, we analysed TCF/LEF-mediated gene expression using the sensitive lentiviral luciferase β-catenin activated reporter (BAR) system, with 12 multimerised TCF/LEF binding sites 27. Without external stimulation minimal reporter activity was detected in control and β-catΔ/Δ mESCs (Fig. 1h). Upon stimulation with the GSK3 inhibitor BIO, recombinant Wnt3a (rWnt3a), or Wnt3a conditioned medium (Wnt3aCM) BAR-reporter activity was increased in control, but not detectable above ground levels in βcatΔ/Δ mESCs (Fig. 1i, and data not shown). Likewise, Axin2 levels were increased in control, but not in β-catΔ/Δ mESCs (Fig. 1j). Stimulation had no effect on plakoglobin levels, nor did plakoglobin knock-down affect basal Axin2 levels (data not shown). Thus, our data suggest that the 2-fold increase in plakoglobin cannot substitute for β-catenin/TCF/LEF-mediated signalling activity.
Analysis of stem cell markers
No difference in Oct4, Nanog, Rex1 and Sox2 transcriptional (Fig. 2a), nor Oct4 and Nanog protein levels (Fig. 2b) were observed between β-catfl/fl and β-catΔ/Δ mESCs. FACS analysis showed no difference in SSEA-1 levels (data not shown). Furthermore, Stat3 phosphorylation kinetics was not altered (Fig. 2c). Late passages of β-catΔ/Δ and β-catfl/fl mESCs stained positive for the self-renewal marker alkaline phosphatase (AP) (Fig. 2d) and showed no differences in colony formation ability (data not shown). Concomitantly, transcriptomes analyses of β-catfl/fl and β-catΔ/ΔmESCs under standard conditions revealed significant differences (P<0.05) in only five genes besides β-catenin; with l7Rn6, H19, and Rhox5/Pem being elevated, and Trpv6 and Daam1 being decreased (see Supplementary Information, Table S1).
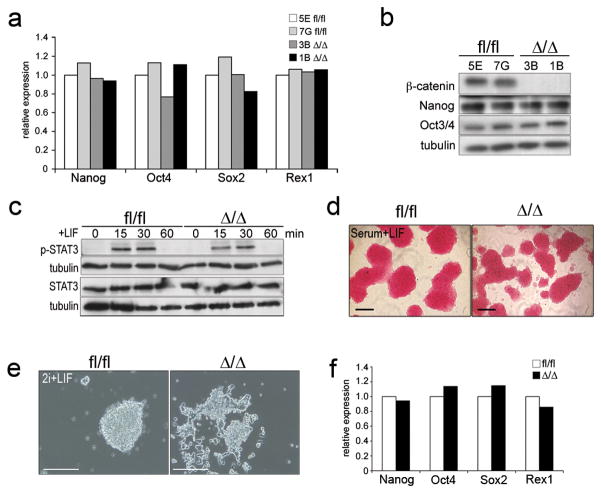
Figure 2.
Self-renewal markers are not affected in β-catfl/fl and β-catΔ/Δ mESCs under different culture conditions.
(a) Histogram showing relative expression levels of self-renewal markers Nanog, Oct4, Sox2 and Rex1 in β-catfl/fl and β-catΔ/Δ mESCs cultured in serum+LIF.
(b) Western blot of β-catenin, Nonog, Oct3/4 and tubulin in β-catfl/fl and β-catΔ/Δ mESCs cultured in serum+LIF.
(c) Western blots for Stat3 and p (Tyr705)-Stat3 levels in response to LIF stimulation.
(d) AP staining on passage 25 β-catfl/fl and β-catΔ/Δ mESCs cultured in serum+LIF.
(e) Morphology of β-catfl/fl and β-catΔ/Δ mESCs cultured in 2i+LIF without serum.
(f) Histogram showing relative expression levels of self-renewal markers Nanog, Oct4, Sox2 and Rex1 in β-catfl/fl and β-catΔ/Δ mESCs cultured in 2i+LIF.
Data in (a) and (f) are mean of two biological replicates. Scale bars in (d) and (e) are 100 μm.
Overall, our data suggest that β-catenin activity is not required for mESC self-renewal in presence of LIF and serum. To further establish that β-catenin is dispensable for mESC self-renewal, we tested β-catΔ/Δ mESCs under serum-free conditions using the 2i+LIF system, inhibiting mitogen-activated kinase kinase (MEK) using PD0325901, and GSK3 using CHIR99021 16. β-catΔ/Δ mESCs could readily be established in 2i+LIF from β-catfl/fl mESCs using adenovirally provided Cre-recombinase, but displayed a different morphology (Fig. 2e). Yet, Nanog, Oct4, Sox2 and Rex1 expression levels were indistinguishable from controls (Fig. 2f). β-catΔ/Δ mESCs formed colonies also under serum-free conditions in presence of PD0325901+LIF or CHIR99021+LIF, but not under PD0325901+CHIR99021 conditions (see Supplementary Information, Fig. S2 online). This establishes that β-catenin deficient mESCs are absolutely LIF dependent and that the GSK3 inhibitor, CHIR99021, has weak, β-catenin-independent effects. However, β-catΔ/Δ mESC propagation was only possible under serum-free conditions in presence of PD0325901+CHIR99021+LIF or PD0325901+LIF (data not shown).
Minor cell-adhesion defects in mESCs
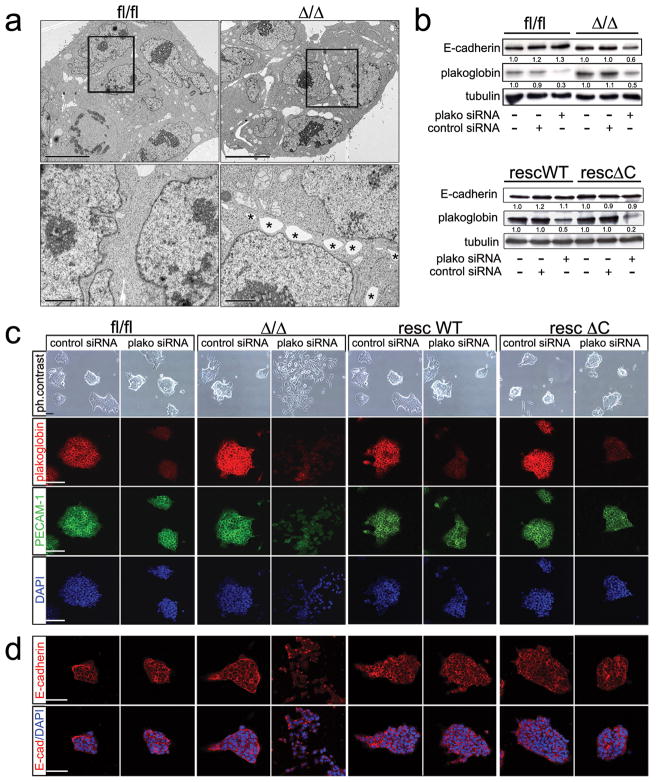
β-catΔ/Δ and control mESCs colonies looked similar in serum+LIF (Fig. 1c). However, we noticed subtle differences and analysed colonies at the ultrastructural level by transmission electron microscopy (TEM). This revealed cell-adhesion defects in β-catΔ/Δ mESC colonies, with apparent regions lacking cell-cell contacts (Fig. 3a), suggesting that plakoglobin cannot fully substitute for β-catenin. To show that plakoglobin indeed substitutes in part for β-catenin cell-adhesion, we performed siRNA knock-down (Fig. 3b). Corresponding with transfection efficiency about 85% of plakoglobin siRNA treated β-catΔ/Δ mESC colonies displayed a differentiated morphology, with cells being more dispersed and flattened. In β-catfl/fl only 5% had a different morphological appearance, while control, lamin A/C siRNA treated cultures, showed no alterations (Fig. 3c; see Supplementary Information, Fig. S3b online). Concomitant to the morphological changes, the cell-adhesion molecules PECAM-1 and E-cadherin were lost from the membrane and their protein levels reduced upon plakoglobin siRNA treatment (Fig. 3b–d). Expression of self-renewal markers, Nanog, Oct4 and Sox2, was, however not altered; only proliferation was slightly decreased (see Supplementary Information, Fig. S3c online). This shows that in absence of β-catenin plakoglobin is an essential component of cell-adhesion in mESCs, mediating adhesion via adherens junctions and desmosomes, and supports previous findings that cell-adhesion is not essential for self-renewal maintenance 16, 17. Furthermore, our data establish no requirement for plakoglobin in self-renewal maintenance. Next, we performed rescue experiments expressing different β-catenin versions, wild-type (βcatWT), C-terminal truncated (β-catΔC), and a point-mutated version (β-catM6) 28 from the Rosa26 locus in β-catΔ/Δ mESCs, referred to in the following as β-catrescWT, βcatrescΔC, and β-catrescM6. All three variants showed membrane localization (see Supplementary Information, Fig. S4a online) and restored cell-adhesion, as well as membranous localization of E-cadherin and PECAM-1 upon plakoglobin siRNA treatment (Fig. 3c, d). All three interacted with E-cadherin and their presence resulted in a slight reduction of plakoglobin (see Supplementary Information, Fig. S4b online). A slight further reduction was observed using mESCs stably expressing β-catenin isoforms under the CAG promoter yielding slightly increased levels of β-catenin variants (see Supplementary Information, Fig. S4c, d online), supporting the notion that the increased plakoglobin levels are due to protein stabilization via its recruitment to adherens junctions substituting for the loss of β-catenin.
Figure 3.
Cell-cell adhesion defects in β-catfl/fl, β-catΔ/Δ mESCs and upon knock- down of plakoglobin.
(a) TEM pictures of β-catfl/fl and β-catΔ/Δ mESC colonies at low magnification (scale bar 10 μm) and high magnification of the boxed areas (scale bar 2 μm), showing non-adherent spaces between adjacent β-catΔ/Δ mESCs (asterisks) compared to the tight cell-cell adhesion between β-catfl/fl mESCs.
(b) Western blot for E-cadherin, plakoglobin and tubulin on mESC lysates 48 hrs after treatment with control or plakoglobin RNAi. Quantified E-cadherin and plakoglobin protein levels normalized to tubulin are shown by the numbers below the corresponding lanes.
(c) Phase contrast and confocal images of immunofluorescent stainings (scale bars 50 μm) for plakoglobin, PECAM-1 and DAPI on control siRNA and plakoglobin siRNA treated mESCs 48 hrs after transfection.
(d) Confocal images (scale bar 50 μm) of immunofluorescent stainings for E-cadherin and E-cadherin/DAPI on control siRNA and plakoglobin siRNA treated mESCs 48 hrs after transfection.
Cell-adhesion defects during EB differentiation
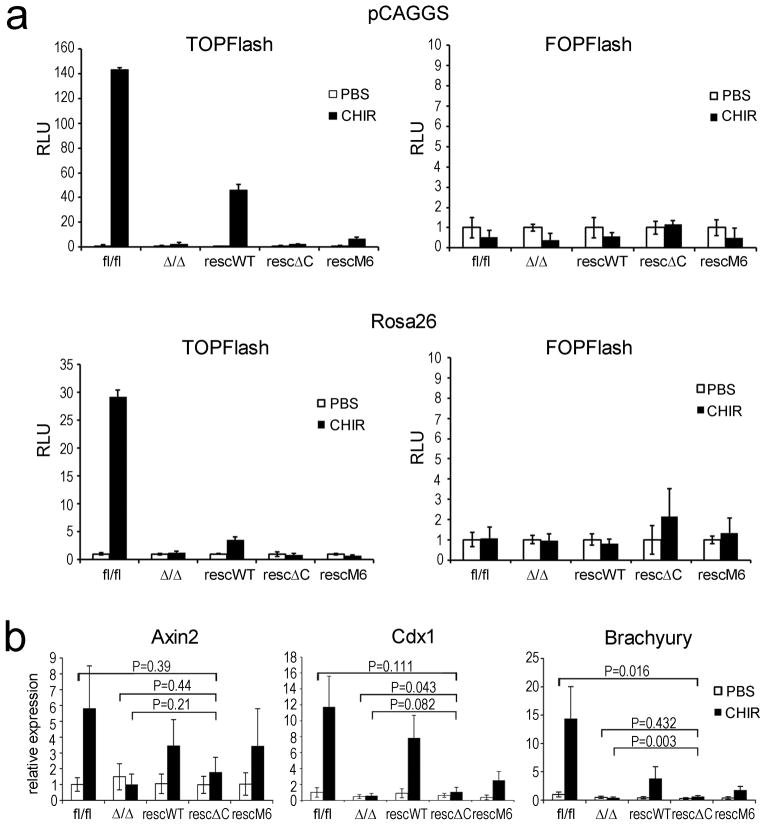
The in vitro differentiation potential of β-catΔ/Δ mESCs was analysed using EB differentiation. This revealed cell-adhesion defects at day 5, reflected in an increase of single cells and associated with a size decrease of β-catΔ/Δ compared to control EBs (Fig. 4a). Controls formed cyst-like structures at day 9, while this did not occur with β-catΔ/Δ cells (Fig. 4a, and data not shown). Concurrent with the appearance of cell-adhesion defects, plakoglobin levels decreased from day 5 onwards in β-catΔ/Δ, but also in control EBs (Fig. 4b). The reduced plakoglobin levels were probably insufficient to compensate for lack of β-catenin, while in control EBs β-catenin steadily increased. EB differentiation proceeded normally in the different Rosa26 rescued mESCs (Fig. 4a). To confirm previous observations suggesting that the βcatΔC and β-catM6 variants are compromised in TCF/LEF transcriptional activity 28, we generated the corresponding stable BAR reporter lines and activated canonical Wnt-signalling using CHIR99021. Surprisingly low reporter activity was observed in βcatrescWT and no activity in β-catrescΔC or β-catrescM6 mESCs (Fig. 5a). Since Rosa26-transgene expression was relatively low and given that residual TOPFlash reporter activity had been observed previously 28, we also assayed BAR reporter activity in the stable CAG-rescue mESCs. Here, the CAG-WTresc cells showed a more pronounced response and residual activity was observed in CAG-M6resc, but not in CAG-ΔCresc mESCs (Fig. 5a). Accordingly, expression of the endogenous Wnt/β-catenin/TCF regulated genes, Axin2, Cdx1 and Brachyury, was not up-regulated in β-catΔ/Δ and βcat-CAGrescΔC mESCs (Fig. 5b). Together, our data show that the β-catenin ΔC variant is defective in TCF/LEF mediated signalling.
Figure 4.
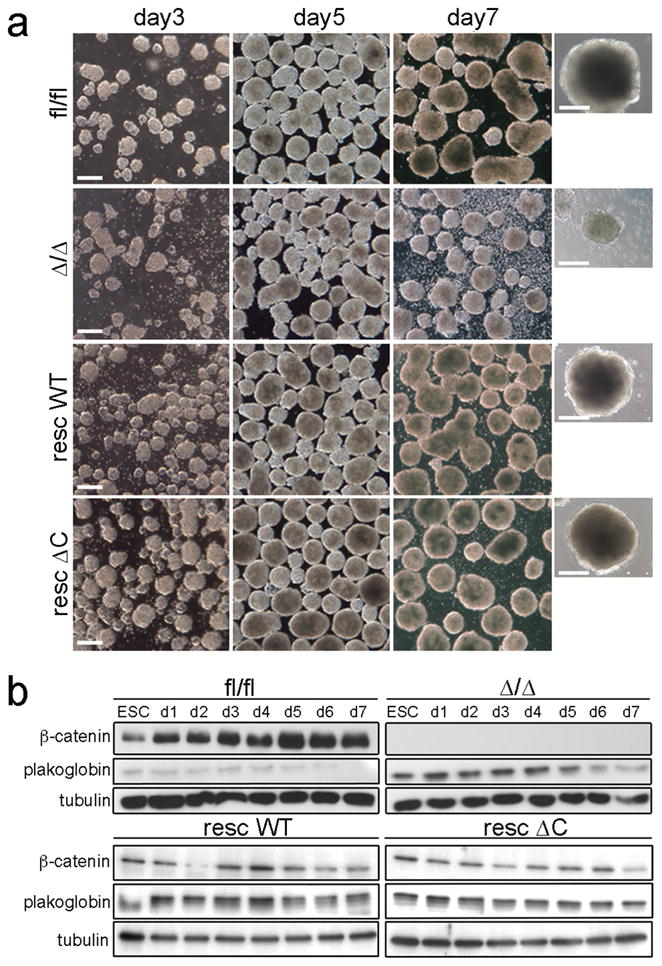
Morphological appearance and plakoglobin levels of β-catfl/fl, β-catΔ/Δ, β-catrescWT and β-catrescΔC EBs during differentiation.
(a) Phase contrast pictures of EBs at days 3, 5 and 7 of differentiation. Scale bar: 200μm. Last row: magnified view of single EBs of the different genotypes on day 7.
(b) Western blot for β-catenin, plakoglobin and tubulin from mESC and EB lysates analysed at indicated time points during differentiation. d: day.
Figure 5.
Analyses of TCF/LEF mediated transcriptional activity.
(a) Histogram showing representative example of TOPFlash (BAR reporter) and FOPFlash (fuBAR reporter) activity in stable mESCs expressing rescue constructs from pGAGGS or Rosa26 locus under unstimulated (PBS) and stimulated (3μM CHIR99021) conditions. Error bars ± s.d are calculated on the basis of three measurements.
(b) Histogram showing relative expression of endogenous β-catenin/Tcf regulated genes Axin2, Brachyury (T), and Cdx1 upon treatment with PBS or CHIR99021 (3μM). Data are mean ± s.d. of three biological replicates. P-values were calculated performing a two-tailed Student’s t-test.
Subsequent EB differentiation studies were performed using the Rosa26 βcatrescWT and β-catrescΔC mESCs, since β-catM6 mESCs showed residual TCF/LEF- mediated activity and observed transgene-silencing in CAG-rescued mESCs during EB differentiation (data not shown),
Rescue of Endoderm and Neuronal Differentiation Defects
Genetic inactivation of β-catenin in mice results in an early developmental arrest around E7.0, with defects in anterior-posterior axis, mesoderm, definitive endoderm and ectoderm formation 19, 29. Maternal β-catenin is provided until E4.5 and might therefore compensate for loss of zygotic β-catenin activity obscuring its absolute requirement in early mouse development 29, 30. EB formation from mESCs allows differentiation of descendents of all three germ layers and resembles to a large degree normal postimplantation embryonic development 2, 3, 31, 32. Thus, enabling us to examine the in vitro differentiation potential of β-catfl/fl, β-catΔ/Δ, β-catrescWT, and βcatrescΔC mESCs. Expression profiles of the early ectodermal markers Nestin, Fgf5 and Pax6 were similar in β-catΔ/Δ and β-catfl/fl EBs during differentiation (Fig. 6a), suggesting that ectoderm induction occurs normally. Nevertheless, differentiation ofβ3-tubulin (TuJ) positive neurons was severely compromised in β-catΔ/Δ EBs (Fig. 6b). In contrast, TuJ positive neurons were readily detected in EBs derived from βcatrescWT and signalling-defective β-catrescΔC mESCs (Fig. 6b). Neuronal differentiation in monolayer cultures revealed that β-catΔ/Δ mESCs have, in principle, the potential to differentiate into neurons, but compared to β-catfl/fl mESCs this is strongly reduced (Fig. 6c). Again differentiation of TuJ positive neurons was restored in β-catrescWT and signalling-defective β-catrescΔC cells (Fig. 6c).
Figure 6.
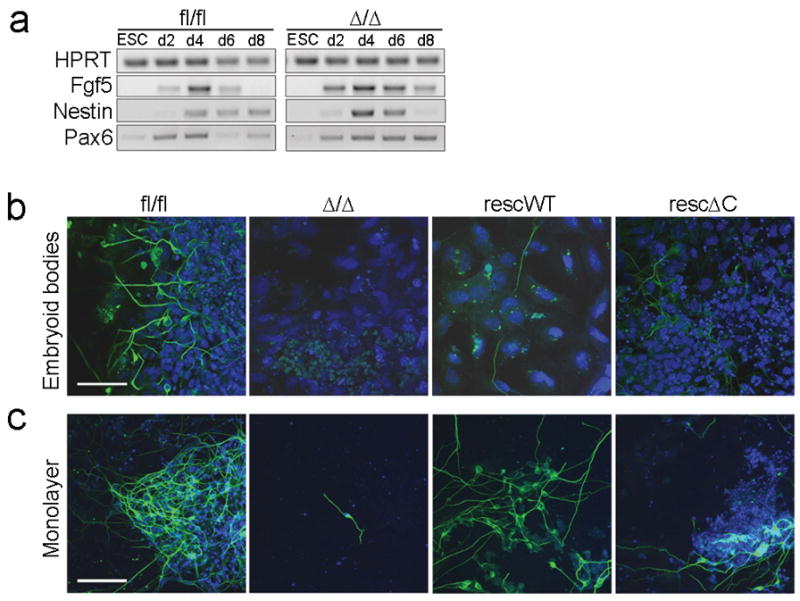
Neuro-ectodermal differentiation potential of β-catfl/fl, β-catΔ/Δ, β-catrescWT and β-catrescΔC EBs.
(a) RT-PCR analyses of ectodermal markers Fgf5, Nestin and Pax6 in mESCs and during EB differentiation time course of β-catfl/fl and β-catΔ/Δ mESCs.
(b, c) Immunofluorescent staining for TuJ positive neurons (b) in differentiated EBs at day 16; (c) in monolayer cultures of mESCs at day 14. Nuclei are stained for DAPI (blue). Scale bars 50 μm.
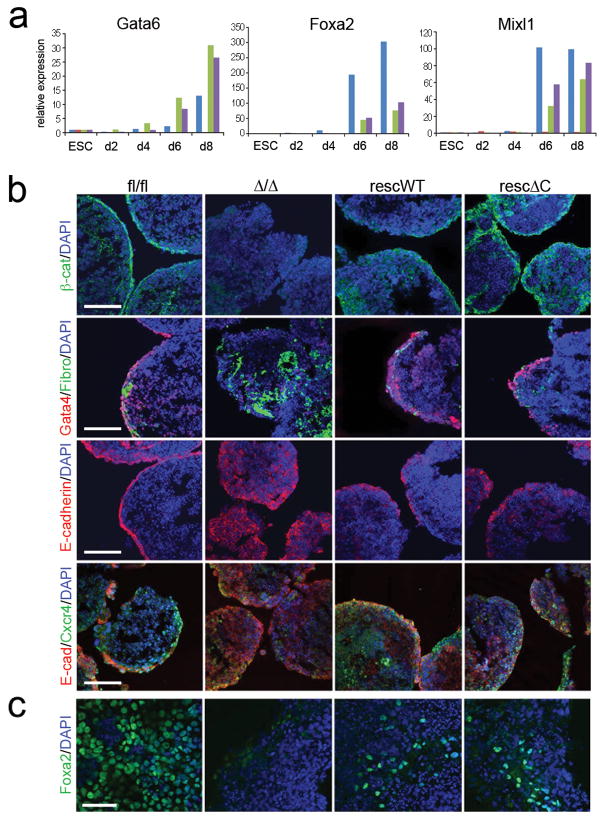
Mesendodermal marker, Mixl1, expression was absent in β-catΔ/Δ EBs, but restored in β-catrescWT and signalling-defective β-catrescΔC EBs (Fig. 7a). Similarly, expression of the endodermal markers, Foxa2 and Gata6, was absent in β-catΔ/Δ EBs (Fig. 7a), but restored in β-catrescWT and β-catrescΔC EBs (Fig. 7a, see also Supplementary Information, Fig. S5 online). Loss and rescue of endoderm formation in EBs derived from β-catΔ/Δ and β-catrescWT and β-catrescΔC mESCs, respectively, was confirmed by immunofluorescent staining for the endodermal markers Gata4, Foxa2 and Cxcr4 (Fig. 7b, c). In β-catfl/fl EBs endodermal cells stained positive for Gata4, E-cadherin, and Cxcr4 and located to the outer region associated with a fibronectin-positive basement membrane (Fig. 7b). In β-catΔ/Δ EBs, Gata4 staining was severely reduced, staining for fibronectin and E-cadherin was very dispersed, and the Cxcr4 positive cells did not localize to the outer layer (Fig. 7b). In contrast, an outer layer with localized Gata4, E-cadherin and Cxcr4 staining was restored in β-catrescWT and β-catrescΔC EBs (Fig. 7b). The presence of Cxcr4 suggests that definitive endoderm is being formed 33. Foxa2 positive cells were detected in dissociated EBs derived from β-catfl/fl, β-catrescWT and β-catrescΔC mESCs, but not in EBs derived from β-catΔ/Δ mESCs (Fig. 7c). Thus, our data suggest that TCF/LEF-mediated signalling activity is not absolutely required for endoderm formation. In addition, our data suggest that the cell-adhesion function of β-catenin promotes neuron formation in vitro. In agreement with the in vitro data, we found that in vivo β-catrescWT and β-catrescΔC mESCs show a higher chimeric embryonic contribution compared to β-catΔ/Δ mESCs and that they can in principle contribute to all three germ layers, but were preferentially found in endodermal and ectodermal layers (see Supplementary Information, Fig. S6 online).
Figure 7.
Mesendodermal differentiation potential of β-catfl/fl, β-catΔ/Δ, β-catrescWT and β-catrescΔC EBs.
(a) Histograms showing relative expression of endodermal markers Gata6, Foxa2 and Mixl1 in mESCs and during EB differentiation. Data are mean of two biological replicates.
(b) Immunofluorescent staining for β-catenin/DAPI, Gata4/Fibronectin (Fibro)/DAPI, E-cadherin/DAPI, and E-cad/Cxcr4/DAPI in differentiated EBs at day 8. Arrowheads point at Gata4 positive cells in β-catΔ/Δ EBs. Scale bar 100 μm.
(c) Immunofluorescent staining for Foxa2/DAPI on plated EBs at day 16. Scale bar 50 μm.
Discussion
Canonical Wnt-signalling activation has been implicated in pluripotency and self-renewal maintenance in human and mouse ESCs 7, 34–36. Nevertheless, its absolute requirement for mESCs self-renewal is still unclear. While nuclear localization of β-catenin has been reported in wild-type mESCs under standard conditions 15, 37, we and others failed to observe nuclear β-catenin or significant TCF/LEF mediated transcriptional activity in wild-type ESCs 7, 15, 34, 38. Still, the possibility of β-catenin having positive effects on ESC self-renewal in a TCF/LEF-independent manner remains. This possibility was favoured in a recent study reporting that β-cat−/− mESCs, generated by Huelsken and colleagues, displayed defects in stemness marker expression 15, 19. The overall changes in this β-cat−/− mESC line, however, exhibit an epiblast-like gene expression profile 15. In contrast, the β-catΔ/Δ mESCs described here, generated from a fully pluripotent β-catfl/fl mESCs in vitro by Cre-mediated recombination, showed no alteration of self-renewal markers under serum and serum-free conditions and only few differences by transcriptomes analysis. Our data are in accordance to data in the accompanying study by Wray et al 39, which independently established β-catenin deficient mESCs. Collectively, our data show that mESC self-renewal does not absolutely require endogenous β-catenin activity and as we have shown does also not depend on plakoglobin in the absence of β-catenin. Furthermore, our two studies demonstrate that β-catenin deficient cultures are absolutely LIF-dependent. This is in contrast to a recent report showing that β-cat−/− mESCs maintain self-renewal in absence of LIF, but depend on activin 17. Activin-dependence is one of the hallmarks of EpiSCs, which express Oct4, but are LIF independent 40. Thus, the β-cat−/− mESCs used in the study by Soncin and colleagues are probably EpiSCs 17, explaining the differences in LIF dependency.
In addition to its central role in Wnt-signalling, β-catenin is a component of adherens junctions connecting classical cadherins via α-catenin to the actin cytoskeleton 20. Plakoglobin can also bind to cadherins and participate in adherens junctions 20, 41, 42 and has been shown to substitute there for β-catenin 19, 22, 23, 29. Similar to F9 teratocarcinoma cells 22, membranous plakoglobin levels were increased in β-catenin deficient mESCs. This was not associated with altered transcription, ruling out a transcriptional feed-back and suggesting a post-transcriptional mechanism via prolonged protein stability due to augmented recruitment of plakoglobin to adherens junctions in absence of β-catenin. Posttranscriptional regulation of protein levels seems a common mechanism in cell-adhesion; e.g. α-catenin regulation by E-cadherin43. However, our study shows that plakoglobin cannot fully compensate for lack of β-catenin. In agreement with the observation that α-catenin levels influence the strength of E-cadherin bonds between cells 44–46, we propose that the α-catenin reduction in the E-cadherin-plakoglobin complexes weakens mESC cell-adhesion and furthermore our data suggest that plakoglobin and β-catenin might bind α-catenin differentially. In accordance with other in vivo finding19, 47–50, but contrary to in vitro findings25, 26, 51, we found no evidence that plakoglobin participates in TCF/LEF-mediated signalling activity.
Embryos lacking zygotic β-catenin activity show defects in A/P patterning, mesoderm and definitive endoderm formation 19 and increased ectodermal apoptosis 29. Blastomere adhesion defects become apparent upon removal of maternal β-catenin 52, which are not substituted for by plakoglobin, as it is not present at early preimplantation stages, and associates with E-cadherin and α-catenin only from the early morula stage onwards 30. Wnt/β-catenin pathway activation occurs in the embryo around E6.5 and in EBs around day 3–4 32, 53. Thus, maternal β-catenin may participate in cell-adhesion in embryos lacking zygotic β-catenin, but not in canonical Wnt-signalling. β-catenin deficient EBs displayed adhesion defects during differentiation, which were associated with plakoglobin down-regulation. Furthermore, mutant EBs showed, similar to what has been observed in β-catenin mutant embryos, defects in endoderm induction, while ectoderm induction occurred normally. The rescue of induction and formation of definitive endoderm in EBs derived from β-catrescΔC mESCs suggests that the cell-adhesion function of β-catenin is required at least for endoderm formation. Mesoderm formation was neither rescued in β-catrescΔC nor control β-catrescWT mESCs, as judged by the absence of Brachyury expression (data not shown). This is likely to be due to the moderate Rosa26 transgene levels, which are probably too low to allow participation in signalling. By addition of the CAG promoter 54 we augmented transgene expression from the Rosa26 locus and rescued Brachyury expression in EBs derived from Rosa26-CAG βcatrescWT, but not from Rosa26-CAG β-catrescΔC mESCs (see Supplementary Information, Fig. S5 online). This is in agreement with the notion that Brachyury expression is under transcriptional β-catenin control 55, 56. Thus, mesoderm induction may not require β-catenin cell-adhesion function to the same extend as its transcriptional activity.
The fact that loss of β-catenin affected neuronal differentiation was unexpected, given that ectoderm induction occurred normally in β-catenin deficient EBs and accumulating in vivo and in vitro evidence suggests that active canonical Wnt- signalling inhibits neuronal differentiation 16, 53, 57–59. Nevertheless, our finding coincide with other in vitro data, showing that Wnt/β-catenin signalling might actually promote neuronal differentiation 60, 61. But, most excitingly our data from the Rosa26 β-catrescΔC EBs, support in vivo findings that β-catenin participating in cell-adhesion plays a permissive role in neuronal differentiation by supporting neuroepithelial formation 62, 63. Given that α-catenin has been implicated in brain development 64, we can however, not completely rule out that neuronal differentiation defects are not in part due to the observed altered α-catenin distribution (Fig. 1g and see also Supplementary Information, Fig. S7 online). However, as nuclear levels were not altered (see Supplementary Information, Fig. S7a,b online), we exclude a potential role in the nucleus 65. The previously proposed model implies, in agreement with our findings, that the number of functional adherens junctions controls brain development, and that this may involve a Hh-signalling feed-back loop 64. Whether the latter is also the case in our system remains to be seen.
Cell adhesion is an important aspect of embryonic development. For bi-functional molecules such as β-catenin it is utterly important to decipher its individual functional contributions in development. Our data suggest, that β-catenin cell-adhesion and not its TCF/LEF-mediated signalling function is important for neuroepithelial and endoderm formation in EBs. Thus, despite plakoglobin up-regulation in β-catenin mutants at E7.0 19, cell-adhesion defects may contribute to some phenotypic aspects, as others have recently observed63. In this context it is interesting that Wnt3 mutants and double-mutants for Lrp5/6 phenocopy β-catenin mutants, with the exception that anterior visceral endoderm (AVE) movement towards anterior still occurs 19, 66, 67. Thus, the failure to proper position the AVE in β-catenin mutants might be associated with defects in cell-adhesion.
Methods
mESC Establishment, Culture Conditions, Blastocyst injections
Individual day 3.5 old blastocysts isolated from β-catfl/fl females intercrossed with β-catfl/fl males were seeded on irradiated MEFs in 4-well plates in ESC medium (high glucose DMEM, 15% FBS, 2mM L-glutamine, 2mM penicillin/streptomycin, 1mM sodium pyruvate, 1x MEM non-essential amino acids, 0.1mM β-mercaptoethanol, 1000 U/ml LIF). After 5–7 days the inner cell mass was dissociated using 0.05% trypsin-EDTA and plated on MEFs. From these cultures colonies with ESC-like morphology were selected, eventually adapted for feeder-free conditions and further characterized. β-catΔ/Δ mESCs were established from the fully pluripotent NLβ-12 β-catfl/fl mESC line through infection with an Adeno-Cre virus (University of Iowa) at MOI 150 for 5×104 mESCs. 24 hrs later individual mESCs were seeded in 96-wells and PCR genotyped (primers: 5′-AGAATCACGGTGACCTGGGTTAAA-3′, 5′-CAGACAGACAGCACCTTCAGCACTC-3′, 5′-CAGCCAAGGAGAGCAGGTGAGG-3′). Two mESC clones of each genotype, β-catfl/fl and β-catΔ/Δ, were further characterized. For serum-free cultures mESCs were cultured in N2B27 medium (StemCellSciences) supplemented with 1000 U/ml EsgroLIF (Chemicon), 3μM CHIR99021 and 1μM PD0325901 (both StemGent). Blastocyst injections were performed with the mESC clone NLβ-12 (on feeder, passage 18). The resulting high-grade chimera was crossed to C57Bl/6 and the offspring was analysed by PCR for germ line transmission of the β-catenin floxed allele. Blastocyst injections were also performed for β-catrescWT and β-catrescΔC mESCs clones stably marked with H2B-Gfp (Addgene plasmid 11680) 68 injecting 10–15 mESCs per blastocyst. Embryos were recovered 6 days later, staged and visually examined for their grade of chimerism using a LSM 710 Spectral Confocal microscope (Zeiss).
Rosa26 and stable pCAGGS rescue mESCs and siRNA experiments
Targeting into the Rosa26 locus was performed using a modified Rosa26 targeting vector, pR26hygro (5′homology arm-SA-XbaI-SV40polyA-loxP-PGKhygro-loxP-3′ homology arm-PGKDTA) (kindly provided by A. Wutz) into which either wild-type full-length mouse β-catenin or N-terminal myc-tagged human versions of C-terminal truncated (ΔC) or M6 β-catenin (expression plasmids kindly provided by R. Grosschedl) had been inserted into the XbaI site by blunt-end cloning. For the Rosa26-CAG rescue lines myc-tagged wild-type and ΔC β-catenin were first cloned behind the CAG-promoter, fragments containing the CAG promoter and the β-catenin variants were then isolated and inserted into the XbaI site of pR26hygro by blunt-end cloning. Correctly targeted mESCs were identified by Southern blotting using Rosa26 5′ and 3′ external probes. For stable CAG-mESC lines, open reading frames were inserted by blunt-end cloning into the XhoI site of a modified pCAGGS vector containing a PGK neomycin selection cassette. Individual neomycin-resistant clones were established and tested by western for their β-catenin levels. For transient siRNA transfections mESCs were seeded onto 6-well plates at 2.5 × 104 cells/well the day before Lipofectamin2000 (Invitrogen) mediated transfection with 150 nM plakoglobin (5′-ACACCUACGACUCGGGCAU-3′, 5′-CCAAGCUGCUCAACGAUGA-3′, 5 ′-GGAACUACAGCUACGAGAA-3′, 5 ′-UGAGUGUAGAUGACGUCAA-3′) o r LaminA/C (5′-UGACCAUGGUUGAGGACAA-3′) OnTarget siRNA mix (Dharmacon).
Generation of Stable BAR Reporter Lines and Luciferase Assays
Stable BAR (beta-catenin activated reporter) and fuBAR (found-unresponsive beta-catenin activated reporter) mESCs were established by infecting mESCs with pBARLS and pFuBARLS lentiviruses followed by puromycin selection for 7 days. For TOPFLASH/FOPFLASH luciferase reporter assays BAR and fuBAR mESCs were seeded onto 12-well plates at a concentration of 5×104 cells/well. The Wnt pathway was activated the next day by treatment with 200 ng/ml rWnt3a (R&D Systems), 50% Wnt3aCM from stable Wnt3a producing L-cells, 2 μM BIO (Calbiochem) or 3 μM CHIR99021 respectively. Luciferase assays were performed in triplicates after 24 hrs using Dual-Glo luciferase assay (Promega) and the Synergy Fluorescence Plate Reader using at least two biological samples.
EB and neuron differentiation cultures
For EB differentiation, 1×106 feeder independent mESCs were plated onto 15 cm low attachment plates (Corning) and cultured in mESC medium without LIF (differentiation medium). For the hanging drop method 1×103 cells were plated per drop of differentiation medium and transferred after 60 hrs to low attachment plates (Corning). EBs were collected at indicated time points fixed in 4% paraformaldehyde and processed for immunocytochemical stainings or embedded in OCT (TissueTek) for cryosectioning. For high-density monolayer differentiation, 4×104 cells/cm2 mESCs were plated on laminin-coated coverslips in N2B27 medium (StemCellSciences) and cultured for 14 days. Medium was renewed every second day. TuJ-positive cells were counted in random fields.
AP staining, Immunohistochemistry and TEM
AP staining was performed using the alkaline phosphatase kit according to manufactures instructions (Sigma Aldrich). Immunohistochemistry was performed upon mESCs or EBs seeded on gelatin covered 12 mm glass coverslips (Novodirect) after 20 min fixation with 4% paraformaldehyde, followed by 10 min permeabilization in PBS with 0.5% Triton-X and a 1 hr blocking step with PBS/2% BSA. For immunohistochemistry on cryosection, EBs from hanging drop cultures were fixed for 30 min in 4% paraformaldehyde, embedded in OCT and sectioned at 8 μm. Incubation with primary antibodies against Nanog, CXCR4 (both 1:100; Abcam), β-catenin (anti-C-terminus 1:250; BD Transduction; anti-N-terminus 1:200; Alexis Biochemicals), E-cadherin, plakoglobin, PECAM-1 (all 1:200; BD-Transduction), Oct3/4 (1:100), Foxa2 (1:100), TuJ (1:200), GATA-4 (1:500) (all from Santa Cruz), Fibronectin (1:200; Sigma) was performed for 1 hr at RT or oN at 4°C in PBS/2% BSA. Incubation with corresponding secondary anti-mouse, anti-rabbit or anti-goat antibodies coupled with Alexa488 or Alexa674 (1:1000; Molecular Probes) were performed for 1 hr at RT in the dark in PBS/2% BSA. To stain nuclei, samples were incubated for 5 min in 10 μg/ml DAPI (Sigma) and mounted with ProLong® Gold antifade reagent (Molecular Probes). Pictures were taken using the LSM 510 Meta/Axiovert200M confocal microscope. For TEM, mESCs were grown for 2 days on gelatinized 12 mm UV-sterilized Aclar coverslips, fixed in 2% glutaraldehyde for 1 hr, rinsed 3 times with PBS, treated with 2% OsO4, rinsed with PBS, dehydrated 10 min each in a graded series of EtOH (40, 60, 80, 95, 100%). For embedding coverslips were incubated in a 100% EtOH/epoxy resin mixture (1:1) for 30 min at RT, followed by two 30 min incubations of the coverslips on 100% epoxy resin after which the coverslips were placed onto resin filled lids of Beem capsules and polymerized for 48 hrs at 60 °C. Resin blocks were sectioned on a Leica Ultracut UCT microtome. Sections were collected on metal grids and post-stained with Reynolds lead citrate (PbNO3)2 and uranyl acetate. Images were taken on a FEI Morgagni 268 electron microscope.
PCR analyses, Microarrays, Western blots and Co-Immunoprecipitation
RNA was isolated using RNeasy Kit (Qiagen). 1–2 μg RNA was used to prepare cDNAs using the SuperscriptII first strand cDNA synthesis system (Invitrogen). Amounts equivalent to 10–20 ng of the initial total RNA input were used as templates for real-time and RT-PCR analyses using gene primer pairs spanning exon-intron boundaries. Real-time PCR was performed using SYBR greenI nucleic acid gel stain (Roche) and TAKARA rTaq at least in duplicates. Products were analysed by agarose gel electrophoresis and melting curves. Values were calculated using the comparative ΔC(t) method and normalized to HPRT values, for relative expression levels displayed in histograms the values of control fl/fl mESCs were always set to 1. For semiquantitative RT-PCR mGAPDH and mHPRT were used as controls. All primers are listed in Supplementary Information, Table S2 online. In-house microarray platforms were used to compare total RNA from β-catfl/fl and β-catΔ/Δ mESCs. Cy3 and Cy5-labeled cRNA was prepared from 3–5 μg of total RNA by oligo-dT-primed reverse transcription. Activation/repression ratios for individual spots were calculated using GenePixPro5-chip software. Two biological replicas were analysed.
For western blots and immunoprecipitation (IP) analyses, protein was extracted from cultured mESCs or EBs. Protein concentrations were determined after Bradford (Biorad). For westerns, if not otherwise noted 10–20 μg extract was loaded per lane. For nuclear extracts cells were fractionated after 69 or the NE-PER Kit (Pierce) was used for nuclear and cytoplasmic protein extractions. For IPs Gammabind Plus Sepharose Beads (Amersham) and 1–2 mg of pre-cleared lysate were used. Incubations with 2 μg and 10 μg antibodies against E-cadherin and α-catenin, respectively, were performed at 4°C oN. Primary antibodies were directed against β-catenin (1:2000; anti-C-terminus, BD Transduction; anti-N-terminus, Alexis Biochemicals), plakoglobin (1:6000; (BD-Transduction), E-cadherin (BD- Transduction), α-catenin (Chemicon), tubulin (1:6000; Sigma), Stat3, phospho (705Tyr)-Stat3, myc-tag (Cell Signaling) and proteasome 20S-C2 (Abcam), HP1α (Cell Signaling), and used at dilution 1:1000, if not otherwise noted.
Supplementary Material
Acknowledgments
We thank Walter Birchmeier, Rudolf Grosschedl, Anton Wutz for reagents, Richard Latham for Wnt3aCM, Rodina Peachey, Guenter Resch, Pawel Pasierbeck, Gabi Stengl, Vuk Komnenovic and Mihaela Zeba for help and technical advice, Martin Radolf and Harald Scheuch for help with microarray studies, Christian Theussl and Jacek Wojciechowski for blastocyst injections, and Austin Smith for helpful comments. Research in the laboratory of CH has been supported by Boehringer Ingelheim, the project has been funded in part by the NoE Cells into Organs (LSHM-CT-2003-504468), NL was supported by the Austrian Science Found (FWF Grant No. P19281-B16) and MW is supported by a grant from the Arthritis Research Campaign (ARC Grant No. 18075). RTM is an investigator of the HHMI and TB was supported by NIH grant R01 GM081619-01.
Footnotes
Competing financial interest
The authors declare no competing financial interest.
Author Contributions
NL performed, analysed and interpreted experiments. MW and DM performed pull-down studies, TB and RM generated and provided BAR/fuBAR lentiviruses, and commented on the paper. CH supervised the study and wrote the paper together with NL.
References
- 1.Murry CE, Keller G. Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell. 2008;132:661–680. doi: 10.1016/j.cell.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 2.Doetschman TC, Eistetter H, Katz M, Schmidt W, Kemler R. The in vitro development of blastocyst-derived embryonic stem cell lines: formation of visceral yolk sac, blood islands and myocardium. J Embryol Exp Morphol. 1985;87:27–45. [PubMed] [Google Scholar]
- 3.Burkert U, von Ruden T, Wagner EF. Early fetal hematopoietic development from in vitro differentiated embryonic stem cells. New Biol. 1991;3:698–708. [PubMed] [Google Scholar]
- 4.Smith AG, et al. Inhibition of pluripotential embryonic stem cell differentiation by purified polypeptides. Nature. 1988;336:688–690. doi: 10.1038/336688a0. [DOI] [PubMed] [Google Scholar]
- 5.Williams RL, et al. Myeloid leukaemia inhibitory factor maintains the developmental potential of embryonic stem cells. Nature. 1988;336:684–687. doi: 10.1038/336684a0. [DOI] [PubMed] [Google Scholar]
- 6.Ying QL, Nichols J, Chambers I, Smith A. BMP induction of Id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3. Cell. 2003;115:281–292. doi: 10.1016/s0092-8674(03)00847-x. [DOI] [PubMed] [Google Scholar]
- 7.Sato N, Meijer L, Skaltsounis L, Greengard P, Brivanlou AH. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat Med. 2004;10:55–63. doi: 10.1038/nm979. [DOI] [PubMed] [Google Scholar]
- 8.Xu RH, et al. Basic FGF and suppression of BMP signaling sustain undifferentiated proliferation of human ES cells. Nat Methods. 2005;2:185–190. doi: 10.1038/nmeth744. [DOI] [PubMed] [Google Scholar]
- 9.Daheron L, et al. LIF/STAT3 signaling fails to maintain self-renewal of human embryonic stem cells. Stem Cells. 2004;22:770–778. doi: 10.1634/stemcells.22-5-770. [DOI] [PubMed] [Google Scholar]
- 10.Reubinoff BE, Pera MF, Fong CY, Trounson A, Bongso A. Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro. Nat Biotechnol. 2000;18:399–404. doi: 10.1038/74447. [DOI] [PubMed] [Google Scholar]
- 11.Thomson JA, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 12.Umehara H, et al. Efficient derivation of embryonic stem cells by inhibition of glycogen synthase kinase-3. Stem Cells. 2007;25:2705–2711. doi: 10.1634/stemcells.2007-0086. [DOI] [PubMed] [Google Scholar]
- 13.Mao J, et al. Low-density lipoprotein receptor-related protein-5 binds to Axin and regulates the canonical Wnt signaling pathway. Mol Cell. 2001;7:801–809. doi: 10.1016/s1097-2765(01)00224-6. [DOI] [PubMed] [Google Scholar]
- 14.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 15.Anton R, Kestler HA, Kuhl M. Beta-catenin signaling contributes to stemness and regulates early differentiation in murine embryonic stem cells. FEBS Lett. 2007;581:5247–5254. doi: 10.1016/j.febslet.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 16.Ying QL, et al. The ground state of embryonic stem cell self-renewal. Nature. 2008;453:519–523. doi: 10.1038/nature06968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soncin F, et al. Abrogation of E-cadherin-mediated cell-cell contact in mouse embryonic stem cells results in reversible LIF-independent self-renewal. Stem Cells. 2009;27:2069–2080. doi: 10.1002/stem.134. [DOI] [PubMed] [Google Scholar]
- 18.Wagner RT, Xu X, Yi F, Merrill BJ, Cooney AJ. Canonical Wnt/beta-catenin regulation of liver receptor homolog-1 mediates pluripotency gene expression. Stem Cells. 2010;28:1794–1804. doi: 10.1002/stem.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huelsken J, et al. Requirement for beta-catenin in anterior-posterior axis formation in mice. J Cell Biol. 2000;148:567–578. doi: 10.1083/jcb.148.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kemler R. From cadherins to catenins: cytoplasmic protein interactions and regulation of cell adhesion. Trends Genet. 1993;9:317–321. doi: 10.1016/0168-9525(93)90250-l. [DOI] [PubMed] [Google Scholar]
- 21.Buxton RS, Magee AI. Structure and interactions of desmosomal and other cadherins. Semin Cell Biol. 1992;3:157–167. doi: 10.1016/s1043-4682(10)80012-1. [DOI] [PubMed] [Google Scholar]
- 22.Fukunaga Y, et al. Defining the roles of beta-catenin and plakoglobin in cell-cell adhesion: isolation of beta-catenin/plakoglobin-deficient F9 cells. Cell Struct Funct. 2005;30:25–34. doi: 10.1247/csf.30.25. [DOI] [PubMed] [Google Scholar]
- 23.Zhou J, et al. Upregulation of gamma-catenin compensates for the loss of beta-catenin in adult cardiomyocytes. Am J Physiol Heart Circ Physiol. 2007;292:H270–276. doi: 10.1152/ajpheart.00576.2006. [DOI] [PubMed] [Google Scholar]
- 24.Huelsken J, Vogel R, Erdmann B, Cotsarelis G, Birchmeier W. beta-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell. 2001;105:533–545. doi: 10.1016/s0092-8674(01)00336-1. [DOI] [PubMed] [Google Scholar]
- 25.Williams BO, Barish GD, Klymkowsky MW, Varmus HE. A comparative evaluation of beta-catenin and plakoglobin signaling activity. Oncogene. 2000;19:5720–5728. doi: 10.1038/sj.onc.1203921. [DOI] [PubMed] [Google Scholar]
- 26.Conacci-Sorrell ME, et al. Nr-CAM is a target gene of the beta-catenin/LEF-1 pathway in melanoma and colon cancer and its expression enhances motility and confers tumorigenesis. Genes Dev. 2002;16:2058–2072. doi: 10.1101/gad.227502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Biechele TL, Moon RT. Assaying beta-catenin/TCF transcription with beta-catenin/TCF transcription-based reporter constructs. Methods Mol Biol. 2008;468:99–110. doi: 10.1007/978-1-59745-249-6_8. [DOI] [PubMed] [Google Scholar]
- 28.Sustmann C, Flach H, Ebert H, Eastman Q, Grosschedl R. Cell-type-specific function of BCL9 involves a transcriptional activation domain that synergizes with beta-catenin. Mol Cell Biol. 2008;28:3526–3537. doi: 10.1128/MCB.01986-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haegel H, et al. Lack of beta-catenin affects mouse development at gastrulation. Development. 1995;121:3529–3537. doi: 10.1242/dev.121.11.3529. [DOI] [PubMed] [Google Scholar]
- 30.Ohsugi M, et al. Expression and cell membrane localization of catenins during mouse preimplantation development. Dev Dyn. 1996;206:391–402. doi: 10.1002/(SICI)1097-0177(199608)206:4<391::AID-AJA5>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 31.Keller GM. In vitro differentiation of embryonic stem cells. Curr Opin Cell Biol. 1995;7:862–869. doi: 10.1016/0955-0674(95)80071-9. [DOI] [PubMed] [Google Scholar]
- 32.ten Berge D, et al. Wnt signaling mediates self-organization and axis formation in embryoid bodies. Cell Stem Cell. 2008;3:508–518. doi: 10.1016/j.stem.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yasunaga M, et al. Induction and monitoring of definitive and visceral endoderm differentiation of mouse ES cells. Nat Biotechnol. 2005;23:1542–1550. doi: 10.1038/nbt1167. [DOI] [PubMed] [Google Scholar]
- 34.Ogawa K, Nishinakamura R, Iwamatsu Y, Shimosato D, Niwa H. Synergistic action of Wnt and LIF in maintaining pluripotency of mouse ES cells. Biochem Biophys Res Commun. 2006;343:159–166. doi: 10.1016/j.bbrc.2006.02.127. [DOI] [PubMed] [Google Scholar]
- 35.Hao J, Li TG, Qi X, Zhao DF, Zhao GQ. WNT/beta-catenin pathway up-regulates Stat3 and converges on LIF to prevent differentiation of mouse embryonic stem cells. Dev Biol. 2006;290:81–91. doi: 10.1016/j.ydbio.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 36.Singla DK, Schneider DJ, LeWinter MM, Sobel BE. wnt3a but not wnt11 supports self-renewal of embryonic stem cells. Biochem Biophys Res Commun. 2006;345:789–795. doi: 10.1016/j.bbrc.2006.04.125. [DOI] [PubMed] [Google Scholar]
- 37.Takao Y, Yokota T, Koide H. Beta-catenin up-regulates Nanog expression through interaction with Oct-3/4 in embryonic stem cells. Biochem Biophys Res Commun. 2007;353:699–705. doi: 10.1016/j.bbrc.2006.12.072. [DOI] [PubMed] [Google Scholar]
- 38.Kielman MF, et al. Apc modulates embryonic stem-cell differentiation by controlling the dosage of beta-catenin signaling. Nat Genet. 2002;32:594–605. doi: 10.1038/ng1045. [DOI] [PubMed] [Google Scholar]
- 39.Wray J, Kalkan T, Gomez-Lopez S, Eckardt D, Cook A, Kemler R, Smith A. Inhibition of glycogen synthase kinase-3 alleviates Tcf3 repression of the pluripotency network and increases embryonic stem cell resistance to differentiation. Nat Cell Biol. 2011 doi: 10.1038/ncb2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brons IG, et al. Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature. 2007;448:191–195. doi: 10.1038/nature05950. [DOI] [PubMed] [Google Scholar]
- 41.Cowin P, Kapprell HP, Franke WW, Tamkun J, Hynes RO. Plakoglobin: a protein common to different kinds of intercellular adhering junctions. Cell. 1986;46:1063–1073. doi: 10.1016/0092-8674(86)90706-3. [DOI] [PubMed] [Google Scholar]
- 42.Peifer M, McCrea PD, Green KJ, Wieschaus E, Gumbiner BM. The vertebrate adhesive junction proteins beta-catenin and plakoglobin and the Drosophila segment polarity gene armadillo form a multigene family with similar properties. J Cell Biol. 1992;118:681–691. doi: 10.1083/jcb.118.3.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nagafuchi A, Takeichi M, Tsukita S. The 102 kd cadherin-associated protein: similarity to vinculin and posttranscriptional regulation of expression. Cell. 1991;65:849–857. doi: 10.1016/0092-8674(91)90392-c. [DOI] [PubMed] [Google Scholar]
- 44.Breen E, Clarke A, Steele G, Jr, Mercurio AM. Poorly differentiated colon carcinoma cell lines deficient in alpha-catenin expression express high levels of surface E-cadherin but lack Ca(2+)-dependent cell-cell adhesion. Cell Adhes Commun. 1993;1:239–250. doi: 10.3109/15419069309097257. [DOI] [PubMed] [Google Scholar]
- 45.Bajpai S, Feng Y, Krishnamurthy R, Longmore GD, Wirtz D. Loss of alpha-catenin decreases the strength of single E-cadherin bonds between human cancer cells. J Biol Chem. 2009;284:18252–18259. doi: 10.1074/jbc.M109.000661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bajpai S, et al. {alpha}-Catenin mediates initial E-cadherin-dependent cell-cell recognition and subsequent bond strengthening. Proc Natl Acad Sci U S A. 2008;105:18331–18336. doi: 10.1073/pnas.0806783105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kofron M, Spagnuolo A, Klymkowsky M, Wylie C, Heasman J. The roles of maternal alpha-catenin and plakoglobin in the early Xenopus embryo. Development. 1997;124:1553–1560. doi: 10.1242/dev.124.8.1553. [DOI] [PubMed] [Google Scholar]
- 48.Ben-Ze’ev A, Geiger B. Differential molecular interactions of beta-catenin and plakoglobin in adhesion, signaling and cancer. Curr Opin Cell Biol. 1998;10:629–639. doi: 10.1016/s0955-0674(98)80039-2. [DOI] [PubMed] [Google Scholar]
- 49.Miller JR, Moon RT. Analysis of the signaling activities of localization mutants of beta-catenin during axis specification in Xenopus. J Cell Biol. 1997;139:229–243. doi: 10.1083/jcb.139.1.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Teuliere J, et al. beta-catenin-dependent and -independent effects of DeltaN-plakoglobin on epidermal growth and differentiation. Mol Cell Biol. 2004;24:8649–8661. doi: 10.1128/MCB.24.19.8649-8661.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maeda O, et al. Plakoglobin (gamma-catenin) has TCF/LEF family-dependent transcriptional activity in beta-catenin-deficient cell line. Oncogene. 2004;23:964–972. doi: 10.1038/sj.onc.1207254. [DOI] [PubMed] [Google Scholar]
- 52.De Vries WN, et al. Maternal beta-catenin and E-cadherin in mouse development. Development. 2004;131:4435–4445. doi: 10.1242/dev.01316. [DOI] [PubMed] [Google Scholar]
- 53.Lindsley RC, Gill JG, Kyba M, Murphy TL, Murphy KM. Canonical Wnt signaling is required for development of embryonic stem cell-derived mesoderm. Development. 2006;133:3787–3796. doi: 10.1242/dev.02551. [DOI] [PubMed] [Google Scholar]
- 54.Niwa H, Yamamura K, Miyazaki J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene. 1991;108:193–199. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- 55.Arnold SJ, et al. Brachyury is a target gene of the Wnt/beta-catenin signaling pathway. Mech Dev. 2000;91:249–258. doi: 10.1016/s0925-4773(99)00309-3. [DOI] [PubMed] [Google Scholar]
- 56.Yamaguchi TP, Takada S, Yoshikawa Y, Wu N, McMahon AP. T (Brachyury) is a direct target of Wnt3a during paraxial mesoderm specification. Genes Dev. 1999;13:3185–3190. doi: 10.1101/gad.13.24.3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aubert J, Dunstan H, Chambers I, Smith A. Functional gene screening in embryonic stem cells implicates Wnt antagonism in neural differentiation. Nat Biotechnol. 2002;20:1240–1245. doi: 10.1038/nbt763. [DOI] [PubMed] [Google Scholar]
- 58.Yoshikawa Y, Fujimori T, McMahon AP, Takada S. Evidence that absence of Wnt-3a signaling promotes neuralization instead of paraxial mesoderm development in the mouse. Dev Biol. 1997;183:234–242. doi: 10.1006/dbio.1997.8502. [DOI] [PubMed] [Google Scholar]
- 59.Verani R, et al. Expression of the Wnt inhibitor Dickkopf-1 is required for the induction of neural markers in mouse embryonic stem cells differentiating in response to retinoic acid. J Neurochem. 2007;100:242–250. doi: 10.1111/j.1471-4159.2006.04207.x. [DOI] [PubMed] [Google Scholar]
- 60.Murashov AK, et al. Directed differentiation of embryonic stem cells into dorsal interneurons. Faseb J. 2005;19:252–254. doi: 10.1096/fj.04-2251fje. [DOI] [PubMed] [Google Scholar]
- 61.Otero JJ, Fu W, Kan L, Cuadra AE, Kessler JA. Beta-catenin signaling is required for neural differentiation of embryonic stem cells. Development. 2004;131:3545–3557. doi: 10.1242/dev.01218. [DOI] [PubMed] [Google Scholar]
- 62.Junghans D, Hack I, Frotscher M, Taylor V, Kemler R. Beta-catenin-mediated cell-adhesion is vital for embryonic forebrain development. Dev Dyn. 2005;233:528–539. doi: 10.1002/dvdy.20365. [DOI] [PubMed] [Google Scholar]
- 63.Hierholzer A, Kemler R. Beta-catenin-mediated signaling and cell adhesion in postgastrulation mouse embryos. Dev Dyn. 2010;239:191–199. doi: 10.1002/dvdy.22075. [DOI] [PubMed] [Google Scholar]
- 64.Lien WH, Klezovitch O, Fernandez TE, Delrow J, Vasioukhin V. alphaE-catenin controls cerebral cortical size by regulating the hedgehog signaling pathway. Science. 2006;311:1609–1612. doi: 10.1126/science.1121449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Giannini AL, Vivanco M, Kypta RM. alpha-catenin inhibits beta-catenin signaling by preventing formation of a beta-catenin*T-cell factor*DNA complex. J Biol Chem. 2000;275:21883–21888. doi: 10.1074/jbc.M001929200. [DOI] [PubMed] [Google Scholar]
- 66.Kelly OG, Pinson KI, Skarnes WC. The Wnt co-receptors Lrp5 and Lrp6 are essential for gastrulation in mice. Development. 2004;131:2803–2815. doi: 10.1242/dev.01137. [DOI] [PubMed] [Google Scholar]
- 67.Liu P, et al. Requirement for Wnt3 in vertebrate axis formation. Nat Genet. 1999;22:361–365. doi: 10.1038/11932. [DOI] [PubMed] [Google Scholar]
- 68.Kanda T, Sullivan KF, Wahl GM. Histone-GFP fusion protein enables sensitive analysis of chromosome dynamics in living mammalian cells. Curr Biol. 1998;8:377–385. doi: 10.1016/s0960-9822(98)70156-3. [DOI] [PubMed] [Google Scholar]
- 69.Neumann S, et al. Nesprin-2 interacts with {alpha}-catenin and regulates Wnt signaling at the nuclear envelope. J Biol Chem. 2010;285:34932–34938. doi: 10.1074/jbc.M110.119651. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.