Abstract
Exercise and weight loss are cornerstones in the treatment and prevention of type 2 diabetes, and both interventions function to increase insulin sensitivity and glucose uptake into skeletal muscle. Studies in rodents demonstrate that the underlying mechanism for glucose uptake in muscle involves site-specific phosphorylation of the Rab-GTPase-activating proteins AS160 (TBC1D4) and TBC1D1. Multiple kinases, including Akt and AMPK, phosphorylate TBC1D1 and AS160 on distinct residues, regulating their activity and allowing for GLUT4 translocation. In contrast to extensive rodent-based studies, the regulation of AS160 and TBC1D1 in human skeletal muscle is not well understood. In this study, we determined the effects of dietary intervention and a single bout of exercise on TBC1D1 and AS160 site-specific phosphorylation in human skeletal muscle. Ten obese (BMI 33.4 ± 2.4, M-value 4.3 ± 0.5) subjects were studied at baseline and after a 2-wk dietary intervention. Muscle biopsies were obtained from the subjects in the resting (basal) state and immediately following a 30-min exercise bout (70% V̇o2 max). Muscle lysates were analyzed for AMPK activity and Akt phosphorylation and for TBC1D1 and AS160 phosphorylation on known or putative AMPK and Akt sites as follows: AS160 Ser711 (AMPK), TBC1D1 Ser231 (AMPK), TBC1D1 Ser660 (AMPK), TBC1D1 Ser700 (AMPK), and TBC1D1 Thr590 (Akt). The diet intervention that consisted of a major shift in the macronutrient composition resulted in a 4.2 ± 0.4 kg weight loss (P < 0.001) and a significant increase in insulin sensitivity (M value 5.6 ± 0.6), but surprisingly, there was no effect on expression or phosphorylation of any of the muscle-signaling proteins. Exercise increased muscle AMPKα2 activity but did not increase Akt phosphorylation. Exercise increased phosphorylation on AS160 Ser711, TBC1D1 Ser231, and TBC1D1 Ser660 but had no effect on TBC1D1 Ser700. Exercise did not increase TBC1D1 Thr590 phosphorylation or TBC1D1/AS160 PAS phosphorylation, consistent with the lack of Akt activation. These data demonstrate that a single bout of exercise regulates TBC1D1 and AS160 phosphorylation on multiple sites in human skeletal muscle.
Keywords: glucose transport, adenosine 5′-monophosphate-activated protein kinase
with the dramatic increase in rates of type 2 diabetes throughout the world, there is a pressing need to understand the molecular mechanisms by which therapeutic interventions such as exercise and weight loss lead to improvements in glucose homeostasis and skeletal muscle glucose metabolism. Skeletal muscle is critical for glucose disposal, which is mediated by the transport of glucose into muscle cells through the glucose transporter protein GLUT4. In skeletal muscle, both contraction and insulin increase glucose transport via the stimulation of GLUT4 translocation from intracellular depots to the sarcolemma and transverse tubules (11, 17). GLUT4 translocation is regulated by complex signaling mechanisms (10, 13), and the Rab-GTPase-activating proteins (Rab-GAP) AS160 and TBC1D1 have recently been identified as distal components of this process (3, 8, 16, 26, 36). In the basal state, where glucose transport is low, AS160 and TBC1D1 are thought to retain GLUT4 in the intracellular vesicles by keeping target Rabs in an inactive, GDP-bound state (28). When the Rab-GAP activity is inhibited by phosphorylation, GLUT4 vesicles are released for translocation to the cell surface with increased transport of glucose across the cell membrane as a consequence (28).
The homology of AS160 and TBC1D1 is only ∼50%, but the Rab-GAP domains are 79% identical, and the proteins may have some overlapping function (26). Whereas AS160 is ubiquitously expressed, TBC1D1 is almost exclusively expressed in skeletal muscles, suggesting that there may be a specialized function of TBC1D1 in muscle (31). Using adult mouse skeletal muscle, we and others have identified several phosphorylation sites on TBC1D1 using mass spectrometry (5, 31). Some of these phosphorylation sites are important functionally, since combined mutation of several sites impaired contraction-stimulated glucose transport in mouse skeletal muscle (3, 36). In contrast, single mutations of these phosphorylation sites had no effect on glucose transport (3, 36). To study the regulation of these different TBC1D1 phosphorylation sites by various stimuli, we have raised phosphospecific antibodies against four of these sites (36). Using these antibodies, we have determined that Ser231, Ser660, and Ser700 on TBC1D1 can be phosphorylated by AMP-activated protein kinase (AMPK) and that Thr590 is regulated by Akt in mouse skeletal muscle (36). In human skeletal muscle, it has been shown recently that exercise rapidly increases phosphorylation at Ser237 (equivalent to the Ser231 in mouse TBC1D1) (9). Whether exercise increases phosphorylation of these additional sites on TBC1D1 in human skeletal muscle has not been investigated.
Similar to TBC1D1, AS160 contains multiple phosphorylation sites (28), and the regulation of these sites has been studied in both mouse (15, 16, 34) and human skeletal muscle (33, 37). In human vastus lateralis skeletal muscle, insulin stimulation was first shown to increase Thr642 phosphorylation (14), and more recently insulin has been reported to regulate Ser318, Ser341, Ser588, Ser666, and Ser751 (33, 37). Phosphorylation of AS160 on Ser711, a site regulated by both AMPK and insulin in mouse muscle (35), is also increased by exercise in human skeletal muscle (35).
Diet intervention and weight loss have beneficial effects on skeletal muscle insulin sensitivity (1) and can also increase contraction-stimulated glucose uptake (23). The underlying mechanisms are unknown but may involve alterations in glycogen concentrations (25). Reductions in carbohydrate intake are known to reduce muscle glycogen concentrations, which can lead to enhanced glucose uptake both in the basal state and after exercise (23, 25). This raises the possibility that the effects of diet intervention could be mediated through alterations in TBC1D1 and AS160 expression and/or phosphorylation in skeletal muscle.
The overall goal of the current study was to test the hypotheses that 1) a single bout of exercise increases TBC1D1 and AS160 phosphorylation in human skeletal muscle and 2) exercise-induced phosphorylation of TBC1D1 and AS160 is enhanced after a dietary intervention. For this purpose we studied obese nondiabetic subjects, a population at high risk for developing type 2 diabetes. We found that dietary intervention had no effect on TBC1D1 and AS160 expression or phosphorylation. However, a single bout of moderate-intensity exercise significantly increased site-specific phosphorylation of these Rab-GAP proteins, suggesting that TBC1D1 and AS160 may be important in the regulation of exercise-stimulated glucose transport in skeletal muscle.
MATERIALS AND METHODS
Subjects.
Ten obese but otherwise healthy subjects (7 women, 3 men) without any ongoing pharmacological treatment except for oral contraceptives were recruited for the study. Subject characteristics and prediet food intake are given in Table 1. All potential participants underwent a comprehensive medical examination as well as routine blood sample tests to exclude any unknown medical conditions, including type 2 diabetes. None of the subjects were performing any strenuous physical exercise on a regular basis. The study was approved by the regional ethics committee in Stockholm, Sweden, and all participants gave their written informed consent after being informed about the purpose and nature of the study.
Table 1.
Subject characteristics and diet composition before and at the end of the 2-wk diet intervention
| Prediet | Postdiet | Paired t-test | |
|---|---|---|---|
| Body weight, kg | 96.8 ± 2.5 | 92.6 ± 2.4 | P < 0.001 |
| Fat mass, kg | 43.6 ± 1.4 | 42.4 ± 1.6 | P < 0.05 |
| Lean body mass, kg | 52.4 ± 3.0 | 50.1 ± 2.8 | P < 0.001 |
| Food intake, kJ·kg−1·day−1 | 78.7 ± 4.7 | 72.5 ± 2.7 | P = 0.30 |
| Energy from fat, % | 31.4 ± 2.5 | 59.2 ± 1.5 | P < 0.001 |
| Energy from protein, % | 18.9 ± 1.7 | 35.9 ± 1.7 | P < 0.001 |
| Energy from carbohydrates, % | 47.8 ± 2.6 | 4.6 ± 0.3 | P < 0.001 |
| M value, mg·kg−1·min−1 | 4.3 ± 0.5 | 5.6 ± 0.6 | P < 0.001 |
| Total cholesterol, mM | 5.4 ± 0.3 | 5.1 ± 0.3 | P = 0.11 |
| HDL cholesterol, mM | 1.3 ± 0.1 | 1.3 ± 0.1 | P = 0.17 |
| LDL cholesterol, mM | 3.5 ± 0.2 | 3.5 ± 0.3 | P = 0.85 |
| Triglyceride, mM | 1.25 ± 0.1 | 0.92 ± 0.1 | P < 0.05 |
| Blood glucose, mM | 4.6 ± 0.2 | 4.3 ± 0.2 | P = 0.001 |
| Insulin, pM | 100 ± 33 | 83 ± 59 | P = 0.11 |
| Steady-state glucose, mM | 4.3 ± 0.2 | 4.4 ± 0.4 | P = 0.93 |
| Steady-state insulin, pM | 590 ± 142 | 511 ± 162 | P = 0.16 |
Data are presented as means ± SE; 10 subjects (7 women and 3 men) were studied before and at the end of the 2-wk diet intervention. All blood samples, except for steady-state samples, were drawn after an overnight fast. Steady-state glucose and insulin were drawn during the last 60 min of a hyperinsulinemic normoglycemic glucose clamp.
Study protocol.
All subjects underwent a 2-wk period of dietary intervention, as described below. Before and after the diet intervention, subjects underwent assessment of maximal oxygen uptake (V̇o2 max), body composition (dual-energy X-ray absorption), and insulin sensitivity (hyperinsulinemic clamp). On a separate occasion before and after the dietary intervention, a muscle specimen was obtained from the vastus lateralis muscle at basal and after a 30-min period of cycle ergometer exercise at a workload corresponding to 70% of V̇o2 max.
Exercise protocol and muscle biopsies.
Maximal oxygen consumption (V̇o2 max) was determined on a cycle ergometer, using a stepwise load increase protocol. O2 uptake, ventilatory volume, and CO2 production were measured on line (Sensormedics Vmax 229), and the leveling-off criterion was used to define V̇o2 max (38).
In the morning on the day of the exercise protocol, a basal muscle specimen was obtained after a 30-min period of rest. A 5- to 7-mm incision was made using local anesthesia and sterile conditions 12–15 cm proximal to the superior border of the patella, and a muscle specimen was obtained from the superficial border of the vastus lateralis muscle using the Bergstrom needle. This was followed by a 30-min period of exercise on the cycle ergometer at a workload corresponding to 70% of determined V̇o2 max, after which another muscle specimen was obtained from the contralateral leg following the same procedure. The muscle tissue was immediately dissected free from fat and connective tissue and placed in liquid nitrogen.
Hyperinsulinemic normoglycemic clamp.
The subjects reported to the laboratory after an overnight fast. After basal blood sampling, a hyperinsulinemic normoglycemic glucose clamp was started. Repeated blood sampling was performed during the steady-state period of the clamp, as described below.
Normoglycemic hyperinsulinemia was created by continuous infusion of insulin (Actrapid; NovoNordisk, Bagsvaerd, Denmark), which was given at a rate of 1.0 mU·kg−1·min−1, aiming to yield a standardized plasma insulin concentration. The blood glucose concentration was kept at normoglycemic levels (as close as possible to 4.5 mM) by controlling blood glucose levels at least every 10 min by adjusting the flow rate of a variable 200 mg/ml glucose infusion (Braun, Melsungen, Germany). After insulin had been infused for ∼60 min, steady-state glucose concentrations were achieved, and the amount of glucose infused during the following 60 min was used to calculate whole body insulin sensitivity (mg glucose·kg body wt−1·min−1), as described earlier (6).
Body composition, including body fat and fat-free mass, was measured using dual-energy X-ray absorptiometry (Prodigy Lunar; GE Lunar, Madison, WI).
Dietary intervention.
The subjects were instructed to consume a low-carbohydrate, high-protein, high-fat diet. Each subject met individually before, after 1 wk, and after the dietary intervention period with a registered dietitian to perform a 24-h dietary recall and to review the central features of the diet. The macronutrient content of the diet was calculated using Dietist XP (Bromma, Sweden) software. The diet consisted of limiting carbohydrate intake without restricting consumption of fat and protein. In brief, subjects were instructed to reduce carbohydrate intake to 21 g/day but were allowed to eat protein and fat as much and as often as they wanted. This included fresh fish, eggs, beef, turkey, chicken breasts without skin, fresh ham slices, raw or steamed vegetables, and butter. Only limited amounts of cheese and cream cheese were allowed. In addition, specific brands of salad dressings and snack foods with limited carbohydrate content were allowed, whereas sauces, gravies, or other ingredients that contain carbohydrates were not recommended. The complete diet instructions can be found in the supplemental data. Subjects were instructed to maintain their regular degree of physical exercise during the intervention period.
Polymerase chain reaction.
Total RNA was isolated from human muscle and was reverse-transcribed to cDNA. TBC1D1 was amplified by PCR with splice exon-flanking primers. Primers utilized were as follows: first set, forward 5′-CCATCAGTGTGGATCTGGA-3′ and reverse 5′-GCCCATCTTCACAAACTGGT-3′; second set, forward 5′-TCAGGAGCAGGCGACTATTT-3′ and reverse 5′-CACAGGAGTCCCACCAGAAT-3′. Amplicons were separated by agarose gel electrophoresis and imaged with ethidium bromide staining under UV light.
Measurements of intracellular signaling.
Muscle biopsies were homogenized in a buffer containing 20 mM Tris, 50 mM NaCl, 5 mM Na4P2O7, 50 mM NaF, 250 mM sucrose, 2 mM DTT, 1% Triton-X 100, 2 μg/ml aprotinin, 5 μg/ml leupeptin, 0.5 μg/ml pepstatin, 10 μg/ml antipain, 1.5 mg/ml benzamidine, and 500 μmol/l phenylmethanesulfonylfluoride, pH 7.4. Muscle lysates (200 μg) were immunoprecipitated with specific antibodies to the α1 and α2 catalytic subunits of AMPK and protein A beads (Sigma). The reaction was performed using the synthetic SAMS peptide as substrate, as described previously (20, 39).
Western blot analyses were used to assess protein and phosphorylation levels of various proteins. Antibodies to AMPKα1 and phosphospecific (Ser79)-acetyl-CoA carboxylase (ACC) antibody were from Millipore (Billerica, MA). GLUT4 antibody was from Chemicon (Temecula, CA). Phosphospecific AMPK (Thr172), Akt, phosphospecific Akt (Ser473 and Thr308), TCB1D1, and AS160 antibodies were from Cell Signaling Technology (Beverly, CA). ACC expression was assessed using HRP-conjugated streptavidin (Pierce Chemical, Rockford, IL). Antibody to AMPKα2 was generated as described previously (20). Phosphospecific TBC1D1 (Ser231, Ser660, Ser700, Thr591) and TCB1D4 Ser711 were generated as described previously (35, 36). Blots were developed using ECL reagents (Amersham Pharmacia Biotech, Piscataway, NJ) and quantified using FluorChem version 2.01 (Alpha Innotech, San Leandro, CA).
Glycogen concentrations.
Muscle samples were hydrolyzed in 2 N HCl at 100°C for 2 h followed by neutralization with 2 N NaOH, and glycogen content was measured by the hexokinase enzymatic method using a glucose HK reagent (Eagle Diagnostics, Desoto, TX).
Statistical analysis.
Data are expressed as means ± SE. Normality of the data was tested with the Kolmogorov-Smirnov test of normal distribution. Where P > 0.20, the data was considered to be normally distributed. All normally distributed data were compared using Student's t-test or one- or two-way ANOVA. The differences between groups were considered significant when P < 0.05.
RESULTS
Clinical and metabolic characteristics of the subjects.
The effects of the diet intervention on subject characteristics and food intake are summarized in Table 1. There was a major shift in macronutrient intake during the diet intervention. The percent energy intake from carbohydrates was reduced from 48 ± 8 to 5 ± 1, a 90% reduction. Fat and protein increased from 32 ± 8 and 19 ± 5 to 59 ± 5 and 36 ± 5, respectively (P < 0.05 for all). The caloric intake during the diet intervention was not statistically significant from the prediet. As has been shown in previous studies, this diet causes a rapid weight loss (2). The average weight loss after diet intervention was 4% (4.2 ± 0.4 kg), and this was associated with an increase in insulin sensitivity as indicated by an ∼30% increase in the M value and a small but significant decrease in fasting blood glucose concentrations. The weight loss was composed of a 4% loss of lean body mass and 3% loss of fat mass. There was also a significant decrease in plasma triglyceride concentrations, whereas cholesterol concentrations were unaffected.
Human skeletal muscle expresses multiple splice isoforms of TBC1D1.
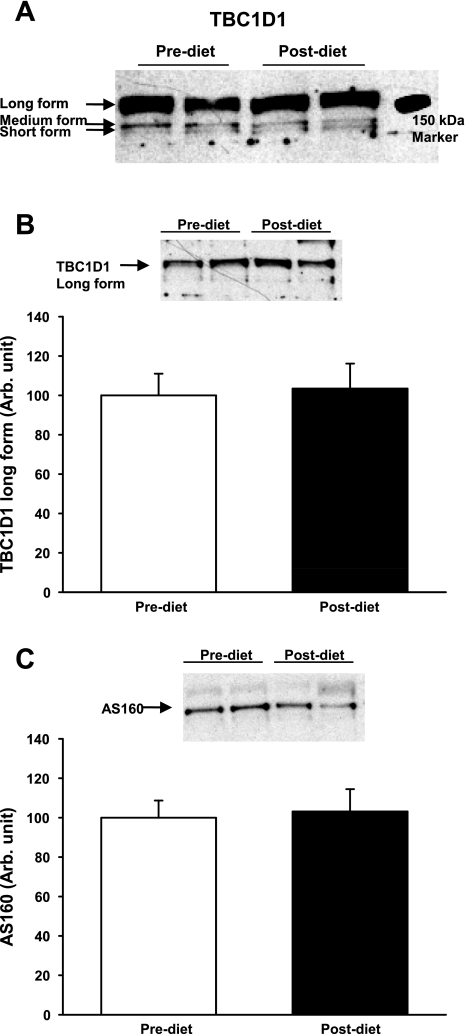
Mouse skeletal muscles express the short and long splice isoform of TBC1D1, with the long form predominant (31). Interestingly, only the long form contains the Ser660 and Ser700 phosphorylation sites, whereas the Ser231 and Thr590 sites are expressed in both splice variants (3). It is not known whether multiple splice variants of TBC1D1 are expressed in human skeletal muscle, and therefore, we determined the relative expression of the long and short TBC1D1 splice variants in human skeletal muscle by amplifying TBC1D1 by PCR with splice exon-flanking primers. The amplicons were separated by agarose gel electrophoresis. Two sets of primers each yielded three products (Amplicon DNA level: 1:0.41:0.28) (Supplemental Fig. S1; Supplemental Material for this article is available online at the AJP-Endocrinology and Metabolism website). Sequencing results confirmed that all three amplicons are splice variants of TBC1D1. The short-form TBC1D1 is missing the entire splice exon (SE) domain, whereas the medium form lacks only the NH2-terminal part of the SE domain in TBC1D1. Our results suggest that three splice variants of TBC1D1 are expressed in human skeletal muscle. The weight of the short form is predicted to be ∼140 kDa, the medium form 146–148 kDa, and the long form ∼155 kDa. As shown in Fig. 1A, TBC1D1 could be detected by a clear band just above the 150-kDa marker, and at longer exposures two faint bands below the 150-kDa marker could be observed. These data suggest that the long form of TBC1D1 is the predominant splice variant expressed in human skeletal muscle. We quantified the predominant TBC1D1 band (Fig. 1B) and AS160 (Fig. 1C) and did not see an effect of diet intervention on protein expression of TBC1D1 and AS160.
Fig. 1.
Muscle biopsies were taken before (open bars) and after a 2-wk intervention with an isocaloric diet with a very low carbohydrate content (closed bars). The protein expression of TBC1D1 in these biopsies was analyzed by Western blotting. A: a long exposure of TBC1D1 immunoblots revealed 3 splice variants of TBC1D1, with 1 prominent band that migrated above the 150-kDa marker (long form) and 2 additional bands below 150 kDa. (medium and short form). B and C: there were no differences in the protein expression of the long form of TBC1D1 and AS160 after diet intervention.
Exercise increases site-specific TBC1D1 phosphorylation in human skeletal muscle.
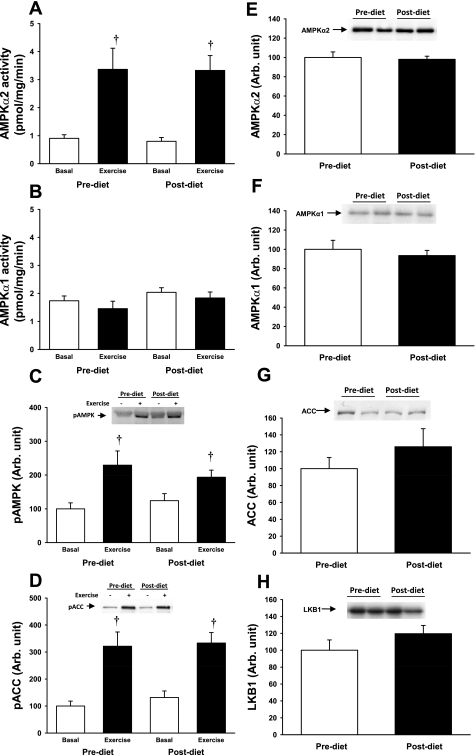
AMPK and Akt are established upstream kinases of TBC1D1 and AS160 in skeletal muscle (15, 31, 34). Both before and after the dietary intervention, 30 min of moderate-intensity exercise significantly increased activity of AMPKα2 (Fig. 2A), the predominant catalytic isoform expressed in human skeletal muscle, but had no effect on AMPKα1 activity (Fig. 2B). The 2-wk diet intervention had no effect on AMPK activity in the basal state or after exercise stimulation (Fig. 2, A and B). Consistent with these data, AMPK Thr172 phosphorylation (Fig. 2C) and phosphorylation of the AMPK substrate ACC (Fig. 2D) were increased with exercise, with no effect of the dietary intervention. Exercise did not lead to detectable increases in Akt Ser473 and Akt Thr308 phosphorylation before or after the dietary intervention (Supplemental Fig. S2). Thus, 30 min of moderate-intensity exercise in obese individuals increases AMPKα2 activity but has no effect on Akt or AMPKα1 activity. Skeletal muscle protein expression of AMPK (Fig. 2E), ACC (Fig. 2F), Akt, and the AMPK upstream kinase LKB1 (Fig. 2G) was not altered following the 2 wk on the low-carbohydrate diet.
Fig. 2.
A and B: AMP-activated protein kinase (AMPK)α2 and -α1 activity were measured in muscle biopsies taken before (open bars) and after 30 min of exercise on a cycle ergometer at 70% of maximal oxygen uptake (V̇o2 max; closed bars). Exercise increased AMPKα2 activity 3-fold before and after diet intervention, whereas AMPKα1 activity remained at basal level after exercise. C and D: phosphorylation of AMPK (pAMPK) and the downstream target acetyl-CoA carboxylase (ACC) was assessed by Western blotting, and the increase in AMPKα2 activity was associated with a significant increase in AMPK phosphorylation and phosphorylation of ACC. E–H: there were no differences in expression of the AMPKα2 and -α1 isoforms, ACC, or the AMPK kinase LKB1 before (open bars) and after diet intervention (closed bars). †P < 0.01.
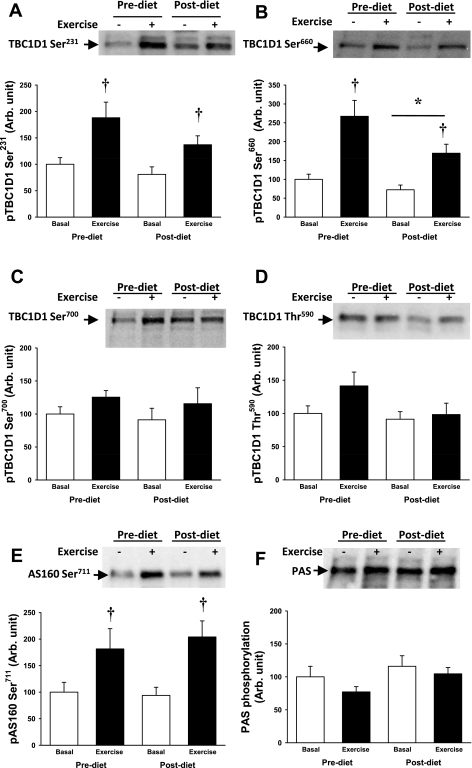
Using phosphoproteomics and immunoblotting, we determined previously that Ser231, Ser660, and Ser700 on TBC1D1 are phosphorylated in mouse skeletal muscle after contraction and during stimulation with the AMPK activator 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR) (31, 36). We also identified Thr590 as an Akt site that is phosphorylated with insulin stimulation (31, 36). Using site-specific antibodies that recognize TBC1D1 phosphorylation on these residues, we determined regulation of TBC1D1 phosphorylation in human skeletal muscle. Exercise significantly increased Ser231and Ser660 phosphorylation, whereas there was only a slight tendency for exercise to increase TBC1D1 Ser700 phosphorylation (Fig. 3, A–C). The insulin-responsive TBC1D1 Thr591 site (Fig. 3D) was not affected by the acute bout of exercise, consistent with the absence of Akt phosphorylation. Diet intervention reduced the basal level of Ser660 phosphorylation but did not modify the response to exercise. There was no effect of the low-carbohydrate diet intervention on any of the other TBC1D1 phosphorylation sites under either the basal or exercise condition (Fig. 3, A, C, and D).
Fig. 3.
TBC1D1 phosphorylation at sites Ser231, Ser660, Ser700, and Thr590, phosphorylation of AS160 at Ser711, and phospho-Akt substrate (PAS) phosphorylation were measured by Western blot. Open bars are before exercise and closed bars after 30 min of exercise on a cycle ergometer at 70% of V̇o2 max. A: exercise increased phosphorylation of TBC1D1 at Ser231, and there was no diet effect. B: exercise increased the phosphorylation of TBC1D1 at Ser660. There was a main effect of diet intervention on the phosphorylation level of Ser660. C and D: TBC1D1 phosphorylation at Ser700 and Thr590 was not changed after exercise or diet intervention. E: AS160 phosphorylation at Ser711 increased after exercise, and this exercise response was not affected by diet intervention. F: both AS160 and TBC1D1 contain phosphorylation sites that can be expected to be detected using the PAS antibody, but we did not see changes in the PAS signal after exercise or diet intervention. †P < 0.01; *P < 0.05.
We recently identified AS160 Ser711 as an AMPK consensus sequence in mouse skeletal muscle (35). In the current study, 30 min of exercise robustly increased AS160 Ser711 phosphorylation in human skeletal muscle (Fig. 3E). Consistent with the absence of Akt phosphorylation, exercise did not increase AS160 phospho-Akt substrate (PAS) phosphorylation (Fig. 3F). There was no effect of the low-carbohydrate diet intervention on AS160 Ser711 or PAS phosphorylation (Fig. 3, E and F).
Muscle glycogen and GLUT4 concentrations.
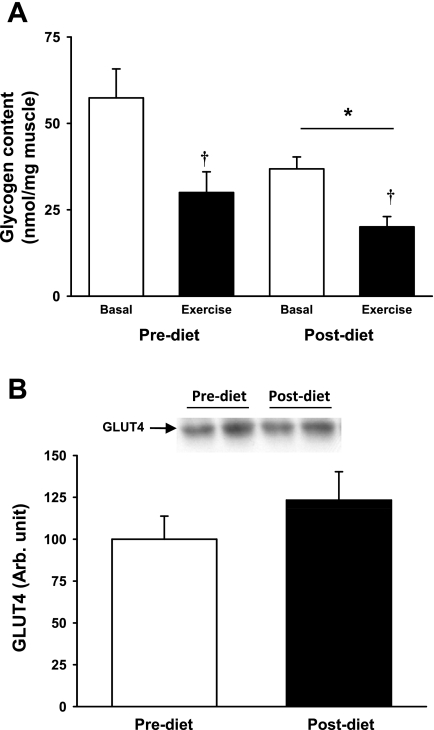
Muscle glycogen concentration is a major regulator of skeletal muscle glucose transport (10, 13). The 2-wk diet intervention, which was composed of low carbohydrate consumption, significantly reduced muscle glycogen concentrations (Fig. 4A). The single bout of acute exercise significantly decreased muscle glycogen concentrations both before and after the diet intervention (Fig. 4A). GLUT4 expression was not altered by the dietary intervention (Fig. 4B).
Fig. 4.
A: glycogen content was measured in biopsies taken before (open bars) and after 30 min exercise on a cycle ergometer at 70% of V̇o2 max (closed bars) in the pre- and postdiet conditions. Postdiet glycogen levels were reduced compared with the prediet levels (*P = 0.01 for main effect of diet), and 30 min of exercise reduced muscle glycogen content (†P < 0.01). B: expression of the glucose transporter GLUT4 in skeletal muscle was not changed by diet intervention.
DISCUSSION
Understanding the mechanisms by which exercise and dietary interventions stimulate glucose transport could lead to novel treatments for metabolic diseases such as type 2 diabetes. Here, we studied a group of obese subjects at a high risk for type 2 diabetes (21) and examined the regulation of the AS160 and TBC1D1, Rab-GAP proteins known to be critical for glucose transport in skeletal muscle. We demonstrate that human muscle expresses multiple splice variants of TBC1D1 and that 30 min of moderate-intensity exercise in obese subjects increases site-specific TBC1D1 and AS160 phosphorylation. However, a diet intervention that resulted in significant weight loss and glycogen depletion did not alter basal or exercise-stimulated TBC1D1 and AS160 phosphorylation in muscle.
Most of our understanding of TBC1D1 phosphorylation comes from rodent models, where it has been shown that TBC1D1 is a substrate for multiple kinases (4, 22, 31, 36). In the current study, we found that exercise in human skeletal muscle robustly increased TBC1D1 Ser231 and Ser660 phosphorylation and that this increase mirrored the increase in AMPKα2 activity. Unlike Ser231 and Ser660, there was only a tendency to increase TBC1D1 Ser700 phosphorylation in response to 30 min of exercise. In mouse skeletal muscle, phosphorylation on the TBC1D1 Ser700 site during contractions is modest (36), whereas stimulation with the AMPK activator AICAR leads to potent Ser700 phosphorylation (36). The lower Ser700 phosphorylation compared with Ser231and Ser660 in response to contraction and exercise could be due to numerous factors, including activation of specific phosphatases or a higher level of Ser700 phosphorylation in the basal state. In mouse skeletal muscle, mutation of multiple phosphorylation sites impairs contraction-stimulated glucose transport, but there is no effect of single site mutation (3, 36). This indicates that TBC1D1 is regulated by a combination of phosphorylation events rather than by phosphorylation on a single site. Given the pattern of TBC1D1 phosphorylation observed in the current study, it is likely that glucose transport in human skeletal muscle also entails phosphorylation on multiple TBC1D1 sites.
Our results demonstrate that human skeletal muscles express three different splice variants of TBC1D1. The long form of TBC1D1 expresses all three AMPK consensus sites (Ser231, Ser660, Ser700), whereas the medium form contains the Ser231 and Ser700 sites, and the short form contains only Ser231. Therefore, the three splice variants may react differently to exercise. Our Western blot analysis indicates that the long form is the predominant splice variant expressed in the obese subjects recruited to this study. However, it is possible that alternative expression patterns may exist in other populations. Mutations in TBC1D1 are associated with an increased risk of obesity (19, 30), and in future studies it will be important to investigate whether variations in expression of the splice forms are associated with an increased risk of developing obesity and diabetes.
The exercise protocol used in this study did not activate Akt, indicating that the site-specific TBC1D1 and AS160 phosphorylation were not mediated through this kinase. Previous data show that only prolonged exercise for 60 min or more increases Akt and AS160 PAS phosphorylation in human skeletal muscle (7, 32). This is in agreement with the lack of a role of Akt in exercise-stimulated glucose transport because, whereas Akt plays a key role in the insulin signal to glucose transport, studies in Akt2-knockout mice and using the phosphatidylinositol 3-kinase inhibitor wortmannin clearly demonstrate that contraction-induced glucose transport is independent of Akt activation (13). We have shown previously that insulin regulates TBC1D1 phosphorylation on Thr590 through Akt2 in mouse skeletal muscle (36). In the current study, we did not obtain muscle biopsies during insulin stimulation, and therefore, we cannot determine whether increased Akt2 phosphorylation is associated with increased TBC1D1 Thr590 phosphorylation in human skeletal muscle.
The subjects investigated in the current study were obese individuals with a BMI >30 kg/m2. Several groups have found AMPK activity to be normal in insulin-resistant individuals after exercise (12, 20), but a recent report has found exercise-induced AMPK activity to be reduced in obese people and type 2 diabetic patients (29). Lean subjects were not the focus of this study, and therefore, we cannot conclude whether the activation of AMPK and phosphorylation of TBC1D1 and AS160 were normal in the obese subjects. However, the approximately threefold increase in AMPKα2 activity we observed is greater than a recent report on AMPKα2 activity in obese and type 2 diabetes groups (29). A likely explanation for this may be differences in fitness levels and the subsequent absolute intensity at which the exercise was performed. The obese subjects in our study exercised at an ∼150-W workload, whereas the exercise workload in the previous study was ∼50 W for the low-intensity protocol and ∼75 W for the moderate-intensity protocol. Exercise at such a low intensity is apparently not sufficient to activate AMPK. It would be interesting to examine TBC1D1 and AS160 under these conditions to determine whether low-intensity exercise can regulate the proteins' phosphorylation.
The low-carbohydrate diet used in the current study has been shown to cause significant weight loss over a short time period, but similarly to many other diet interventions the effect erodes over time (27). Our goal was to determine whether modulations of TBC1D1 and AS160 were immediate adaptations to weight loss, and therefore, subjects were examined after 2 wk of dietary intervention. At this time point the subjects had lost weight and had improved insulin sensitivity, suggesting satisfactory adherence to the diet protocol. The reduction in skeletal muscle glycogen content after diet intervention may at least partly explain the increased insulin sensitivity (25). The diet intervention also lowered the phosphorylation of TBC1D1 Ser660, but importantly, this did not affect exercise-induced phosphorylation of Ser660. The underlying mechanism for the reduced Ser660 phosphorylation is not known. One possibility is increased activity of a specific phosphatase or reduced activity of an upstream kinase. However, diet intervention did not affect AMPK activity despite the reduction of glycogen levels. In vitro, glycogen inhibits AMPK activity by a direct interaction between the AMPK β-subunit and glycogen molecules (18), but in vivo, a more complex relationship between glycogen levels and AMPK activity in skeletal muscle may exist. Similar to AMPK, protein expression of TBC1D1 and AS160 was not affected by dietary intervention, and modulations of TBC1D1 and AS160 do not appear to be early components of the adaptation to weight loss. However, we did not measure insulin signaling through TBC1D1 and AS160 in this study. Therefore, we do not know whether the improved insulin sensitivity after dietary intervention was associated with increased insulin-stimulated phosphorylation of AS160 and/or TBC1D1. However, this is clearly an important topic for future investigations.
In conclusion, human skeletal muscle expresses three splice variants of TBC1D1, and the long form of the protein, which contains multiple regulated phosphorylation sites, is the predominant form expressed. These phosphorylation sites are known to regulate contraction-stimulated glucose transport in mouse muscle, and two of these sites, Ser231 and Ser660, are phosphorylated in response to exercise in obese humans. In addition, we show that exercise regulates AS160 phosphorylation of Ser711 in obese individuals but that the expression of TBC1D1 and AS160 is independent of dietary intervention and muscle glycogen levels. Thus, the regulation of TBC1D1 in human skeletal muscle may resemble what has been observed in mouse muscle, suggesting an important role of TBC1D1 in modulating glucose transport in human skeletal muscle.
GRANTS
This work was supported by funding from National Institute of Arthritis and Musculoskeletal and Skin Diseases Grants R01-AR-42238 and R01-AR-45670 (L. J. Goodyear), the Graetz Challenge Grant from the Joslin Diabetes Center (L. J. Goodyear), the American Diabetes Association Mentor-based Award (L. J. Goodyear), and Diabetes and Endocrinology Research Center Grant P30-DK-36836 (Joslin Diabetes Center). Additional funds to support this work were provided by the Danish Agency for Science Technology and Innovation to N. Jessen (grant no. 271-07-0719).
DISCLOSURES
No conflicts of interest, financial or otherwise, are decalred by the authors.
Supplementary Material
REFERENCES
- 1.No authors listed Overweight, obesity, and health risk. National Task Force on the Prevention and Treatment of Obesity. Arch Intern Med 160: 898–904, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Adam-Perrot A, Clifton P, Brouns F. Low-carbohydrate diets: nutritional and physiological aspects. Obes Rev 7: 49–58, 2006 [DOI] [PubMed] [Google Scholar]
- 3.An D, Toyoda T, Taylor EB, Yu H, Fujii N, Hirshman MF, Goodyear LJ. TBC1D1 regulates insulin- and contraction-induced glucose transport in mouse skeletal muscle. Diabetes 59: 1358–1365, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.An D, Vichaiwong K, Toyoda T, Taylor EB, Yu H, Hirshman MF, Fujii N, Goodyear LJ. Contraction and insulin differentially regulate TBC1D1 phosphorylation and function in skeletal muscle (Abstract). Diabetes 58: A52, 2009. [Google Scholar]
- 5.Chen S, Murphy J, Toth R, Campbell DG, Morrice NA, MacKintosh C. Complementary regulation of TBC1D1 and AS160 by growth factors, insulin and AMPK activators. Biochem J 409: 449–459, 2008 [DOI] [PubMed] [Google Scholar]
- 6.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol Endocrinol Metab Gastrointest Physiol 237: E214–E223, 1979 [DOI] [PubMed] [Google Scholar]
- 7.Deshmukh A, Coffey VG, Zhong Z, Chibalin AV, Hawley JA, Zierath JR. Exercise-induced phosphorylation of the novel Akt substrates AS160 and filamin A in human skeletal muscle. Diabetes 55: 1776–1782, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Eguez L, Lee A, Chavez JA, Miinea CP, Kane S, Lienhard GE, McGraw TE. Full intracellular retention of GLUT4 requires AS160 Rab GTPase activating protein. Cell Metab 2: 263–272, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Frosig C, Pehmoller C, Birk JB, Richter EA, Wojtaszewski JF. Exercise-induced TBC1D1 Ser237 phosphorylation and 14-3-3 protein binding capacity in human skeletal muscle. J Physiol 588: 4539–4548, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goodyear LJ, Kahn BB. Exercise, glucose transport, and insulin sensitivity. Annu Rev Med 49: 235–261, 1998 [DOI] [PubMed] [Google Scholar]
- 11.Hirshman MF, Goodyear LJ, Wardzala LJ, Horton ED, Horton ES. Identification of an intracelular pool of glucose transporters from basal and insulin-stimulated rat skeletal muscle. J Biol Chem 265: 987–991, 1990 [PubMed] [Google Scholar]
- 12.Hojlund K, Mustard KJ, Staehr P, Hardie DG, Beck-Nielsen H, Richter EA, Wojtaszewski JF. AMPK activity and isoform protein expression are similar in muscle of obese subjects with and without type 2 diabetes. Am J Physiol Endocrinol Metab 286: E239–E244, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Jessen N, Goodyear LJ. Contraction signaling to glucose transport in skeletal muscle. J Appl Physiol 99: 330–337, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Kane S, Sano H, Liu SC, Asara JM, Lane WS, Garner CC, Lienhard GE. A method to identify serine kinase substrates. Akt phosphorylates a novel adipocyte protein with a Rab GTPase-activating protein (GAP) domain. J Biol Chem 277: 22115–22118, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Kramer HF, Witczak CA, Fujii N, Jessen N, Taylor EB, Arnolds DE, Sakamoto K, Hirshman MF, Goodyear LJ. Distinct signals regulate AS160 phosphorylation in response to insulin, AICAR, and contraction in mouse skeletal muscle. Diabetes 55: 2067–2076, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Kramer HF, Witczak CA, Taylor EB, Fujii N, Hirshman MF, Goodyear LJ. AS160 regulates insulin- and contraction-stimulated glucose uptake in mouse skeletal muscle. J Biol Chem 281: 31478–31485, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Lauritzen HP, Galbo H, Toyoda T, Goodyear LJ. Kinetics of contraction-induced GLUT4 translocation in skeletal muscle fibers from living mice. Diabetes 59: 2134–2144, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McBride A, Ghilagaber S, Nikolaev A, Hardie DG. The glycogen-binding domain on the AMPK beta subunit allows the kinase to act as a glycogen sensor. Cell Metab 9: 23–34, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meyre D, Farge M, Lecoeur C, Proenca C, Durand E, Allegaert F, Tichet J, Marre M, Balkau B, Weill J, Delplanque J, Froguel P. R125W coding variant in TBC1D1 confers risk for familial obesity and contributes to linkage on chromosome 4p14 in the French population. Hum Mol Genet 17: 1798–1802, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Musi N, Fujii N, Hirshman MF, Ekberg I, Froberg S, Ljungqvist O, Thorell A, Goodyear LJ. AMP-activated protein kinase (AMPK) is activated in muscle of subjects with type 2 diabetes during exercise. Diabetes 50: 921–927, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Narayan KM, Boyle JP, Thompson TJ, Gregg EW, Williamson DF. Effect of BMI on lifetime risk for diabetes in the U.S. Diabetes Care 30: 1562–1566, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Pehmoller C, Treebak JT, Birk JB, Chen S, MacKintosh C, Hardie DG, Richter EA, Wojtaszewski JF. Genetic disruption of AMPK signaling abolishes both contraction- and insulin-stimulated TBC1D1 phosphorylation and 14-3-3 binding in mouse skeletal muscle. Am J Physiol Endocrinol Metab 297: E665–E675, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Phinney SD, Bistrian BR, Evans WJ, Gervino E, Blackburn GL. The human metabolic response to chronic ketosis without caloric restriction: preservation of submaximal exercise capability with reduced carbohydrate oxidation. Metabolism 32: 769–776, 1983 [DOI] [PubMed] [Google Scholar]
- 25.Richter EA, Derave W, Wojtaszewski JF. Glucose, exercise and insulin: emerging concepts. J Physiol 535: 313–322, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roach WG, Chavez JA, Miinea CP, Lienhard GE. Substrate specificity and effect on GLUT4 translocation of the Rab GTPase-activating protein Tbc1d1. Biochem J 403: 353–358, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sacks FM, Bray GA, Carey VJ, Smith SR, Ryan DH, Anton SD, McManus K, Champagne CM, Bishop LM, Laranjo N, Leboff MS, Rood JC, de Jonge L, Greenway FL, Loria CM, Obarzanek E, Williamson DA. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med 360: 859–873, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sakamoto K, Holman GD. Emerging role for AS160/TBC1D4 and TBC1D1 in the regulation of GLUT4 traffic. Am J Physiol Endocrinol Metab 295: E29–E37, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sriwijitkamol A, Coletta DK, Wajcberg E, Balbontin GB, Reyna SM, Barrientes J, Eagan PA, Jenkinson CP, Cersosimo E, DeFronzo RA, Sakamoto K, Musi N. Effect of acute exercise on AMPK signaling in skeletal muscle of subjects with type 2 diabetes: a time-course and dose-response study. Diabetes 56: 836–848, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stone S, Abkevich V, Russell DL, Riley R, Timms K, Tran T, Trem D, Frank D, Jammulapati S, Neff CD, Iliev D, Gress R, He G, Frech GC, Adams TD, Skolnick MH, Lanchbury JS, Gutin A, Hunt SC, Shattuck D. TBC1D1 is a candidate for a severe obesity gene and evidence for a gene/gene interaction in obesity predisposition. Hum Mol Genet 15: 2709–2720, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Taylor EB, An D, Kramer HF, Yu H, Fujii NL, Roeckl KS, Bowles N, Hirshman MF, Xie J, Feener EP, Goodyear LJ. Discovery of TBC1D1 as an insulin-, AICAR-, and contraction-stimulated signaling nexus in mouse skeletal muscle. J Biol Chem 283: 9787–9796, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Treebak JT, Birk JB, Rose AJ, Kiens B, Richter EA, Wojtaszewski JF. AS160 phosphorylation is associated with activation of α2β2γ1- but not α2β2γ3-AMPK trimeric complex in skeletal muscle during exercise in humans. Am J Physiol Endocrinol Metab 292: E715–E722, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Treebak JT, Frøsig C, Pehmøller C, Chen S, Maarbjerg SJ, Brandt N, MacKintosh C, Zierath JR, Hardie DG, Kiens B, Richter EA, Pilegaard H, Wojtaszewski JF. Potential role of TBC1D4 in enhanced post-exercise insulin action in human skeletal muscle. Diabetologia 52: 891–900, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Treebak JT, Glund S, Deshmukh A, Klein DK, Long YC, Jensen TE, Jørgensen SB, Viollet B, Andersson L, Neumann D, Wallimann T, Richter EA, Chibalin AV, Zierath JR, Wojtaszewski JF. AMPK-mediated AS160 phosphorylation in skeletal muscle is dependent on AMPK catalytic and regulatory subunits. Diabetes 55: 2051–2058, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Treebak JT, Taylor EB, Witczak CA, An D, Toyoda T, Koh HJ, Xie J, Feener EP, Wojtaszewski JF, Hirshman MF, Goodyear LJ. Identification of a novel phosphorylation site on TBC1D4 regulated by AMP-activated protein kinase in skeletal muscle. Am J Physiol Cell Physiol 298: C377–C385, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vichaiwong K, Purohit S, An D, Toyoda T, Jessen N, Hirshman MF, Goodyear LJ. Contraction regulates site-specific phosphorylation of TBC1D1 in skeletal muscle. Biochem J 431: 311–320, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vind BF, Pehmøller C, Treebak JT, Birk JB, Hey-Mogensen M, Beck-Nielsen H, Zierath JR, Wojtaszewski JF, Højlund K. Impaired insulin-induced site-specific phosphorylation of TBC1 domain family, member 4 (TBC1D4) in skeletal muscle of type 2 diabetes patients is restored by endurance exercise-training. Diabetologia 54: 157–167, 2011 [DOI] [PubMed] [Google Scholar]
- 38.Wallin L, Brudin LH. Physical working capacity determined by different types of bicycle exercise tests. Clin Physiol 8: 529–537, 1988 [DOI] [PubMed] [Google Scholar]
- 39.Yu H, Fujii N, Hirshman MF, Pomerleau JM, Goodyear LJ. Cloning and characterization of mouse 5′-AMP-activated protein kinase γ3 subunit. Am J Physiol Cell Physiol 286: C283–C292, 2004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.