Abstract
Isoform-specific signaling of Akt, a major signaling hub and a prominent therapeutic target, remained poorly defined until recently. Subcellular distribution, tissue-specific expression, substrate specificity, and posttranslational modifications are believed to underlie isoform-specific signaling of Akt. The studies reported here show inhibition of Akt2 activity under physiologically relevant conditions of oxidation created by PDGF-induced reactive oxygen species. Combined MS and functional assays identified Cys124 located in the linker region between the N-terminal pleckstrin homology domain and the catalytic kinase domain as one of the unique regulatory redox sites in Akt2 with functional consequence on PDGF-stimulated glucose uptake. A model is proposed describing the consequence of increased endogenous oxidation induced by extracellular cues such as PDGF on Akt2 activity.
Keywords: disulfide, receptor tyrosine kinase, DCF, DCP-Bio1
PKB/Akt is a major signaling hub between cytokine, growth factor, and integrin signaling pathways of consequence to many biological processes. Energy storage, protein synthesis, cell survival and growth, cell cycle progression, and cell death are differentially regulated by the three known isoforms of Akt kinase: Akt1/PKBα, Akt2/PKBβ, and Akt3/PKBγ (1). Although the molecular features underlying isoform-specific functional predominance are largely unknown, hypotheses to explain Akt isoform specificity include selective interactions with substrates and/or binding partners, tissue specificity, subcellular location, and temporal changes in activation profiles of Akt isoforms (2). Posttranslational modifications may contribute to this isoform-specific signaling, but reports of modifications are lacking.
Reactive oxygen species (ROS) are integral to cytokine and growth factor signaling; ROS generation in response to these extracellular cues is well documented (3). Earlier reports, in particular from Sundaresan et al. (4), showed ROS generation in response to PDGF stimulation of vascular smooth muscle cells and suggested that H2O2 can relay redox signals to regulate physiological signaling in response to growth factors. More recently, it has been shown that PrxI phosphorylation at Y194 by Src family tyrosine kinases inhibited PrxI and resulted in H2O2 accumulation at the cellular membrane where receptor tyrosine kinase activation occurs (5). Unanswered questions include which proteins are oxidized by receptor tyrosine kinase-induced ROS, which specific cysteine site(s) undergo oxidation, and the consequence of oxidation on activity of these proteins and propagation of receptor tyrosine kinase signaling. Recently, the synthesis of several dimedone-based chemoselective reagents capable of specific labeling of sulfenic acid oxidized proteins was reported; these reagents allow for specific enrichment and identification of oxidized proteins (6–10). In this study, we used a biotin-tagged 1,3-cyclohexadione derivative, DCP-Bio1 (Fig. 1A), to investigate isoform-specific effects of PDGF-induced ROS on Akt1, Akt2, and Akt3 kinase activities. We report selective down-regulation of Akt2 kinase activity by PDGF-induced oxidation, with implications for molecular and cellular functions controlled by PDGF. Specifically, we found that (i) PDGF-induced ROS inhibited Akt2 kinase activity (estimated extent of PDGF-induced oxidation of Akt2 is 45–66%); (ii) Cys124 in Akt2 is a cysteine residue targeted by oxidation and at least partly responsible for inhibition of Akt2 activity; (iii) adding H2O2 to NIH 3T3 cells induces indiscriminate activation of Akt1 and Akt2 through phosphorylation; however, intracellular accumulation of H2O2 results in isoform-specific down-regulation of Akt2 activity through oxidation; and (iv) a functional relationship exists between PDGF-dependent redox regulation of Akt2 and physiological outputs such as PDGF-induced glucose uptake.
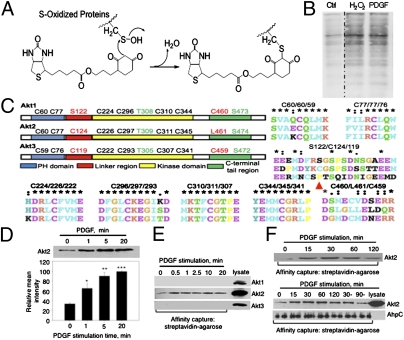
Fig. 1.
PDGF-dependent oxidation of Akt2 kinase in NIH 3T3 cells. (A) Labeling reaction of S-oxidized proteins with DCP-Bio1, a biotin-tagged, sulfenic acid-specific reagent. (B) Oxidation of cellular proteins in NIH 3T3 cells by H2O2 (100 μM, 10 min) treatment and by PDGF (20 ng/mL, 5 min) induced ROS. The DCP-Bio1–labeled proteins were affinity captured with streptavidin-agarose and probed by Western blot with HRP-linked streptavidin. (C) Amino acid sequence alignment of the Cys containing regions in Akt1 (Top), Akt2 (Middle), and Akt3 (Bottom). Domain-specific locations of all cysteines within the three Akt isoforms are represented (P121 in Akt3 is shown with red arrow). (D) PDGF-dependent Akt2 oxidation: NIH 3T3 cells were stimulated with PDGF (20 ng/mL) and lysed with DCP-Bio1 containing lysis buffer. The oxidized and DCP-Bio1–labeled proteins were affinity captured with streptavidin-agarose and probed by Western blot using an anti-Akt2 antibody. Corresponding P values for 1 min: 0.0300; 5 min: 0.002; and 20 min: <0.001. (E) Labeling of Akt2 and not Akt1 or Akt3 by DCP-Bio1 in NIH 3T3 cells. Lysates from PDGF-stimulated NIH 3T3 cells were used to capture DCP-Bio1–labeled proteins and probed for Akt1, Akt2, and Akt3 using isoform-specific antibodies (cell lysates are included as controls). (F) Akt2 oxidation is PDGF-dependent and reversible, decreasing with prolonged PDGF stimulation. Lysates from PDGF-stimulated NIH 3T3 cells were used to capture DCP-Bio1–labeled proteins and probed for Akt2. Lower: Two additional controls were used in which cells were treated with PDGF alone for the first 30 min and then incubated further for 30 and 90 min without PDGF. Additionally, biotinylated AhpC was added to the normalized lysates (1 μg AhpC/500 μg protein) as an internal control before affinity capture and probed for AhpC.
Results and Discussion
PDGF-Stimulated ROS Induces Oxidation of PLCγ1 and Akt2.
NIH 3T3 cells stimulated with PDGF were lysed in the presence of DCP-Bio1 (Materials and Methods). DCP-Bio1 labeling of cellular proteins increased within 5 min of PDGF stimulation, comparable to labeling in H2O2-treated NIH 3T3 cells (Fig. 1B and Fig. S1A). In our preliminary MS experiments using DCP-Bio1 labeled cell lysates of NIH 3T3 cells stimulated with PDGF, we found several signaling proteins oxidized by PDGF stimulation. These included PLCγ1 and Akt2, both well-known mediators of PDGF signaling. Redox sensitivity of PLCγ1 was reported in PC12 cells stimulated with dopamine (11). Western blot analysis of streptavidin-agarose pull-down samples from PDGF-stimulated cells lysed in the presence of DCP-Bio1 showed increased PLCγ1 oxidation with PDGF stimulation compared with unstimulated control (Fig. S1B). For Akt2, the peptide identified by MS contained a cysteine site (Cys124) labeled by DCP-Bio1. Although the correlation factor for assignment of oxidation site in Akt2 was low (Xcorr 2.05, z +2), we decided to further examine the potential regulatory function of Akt2 oxidation on PDGF signaling.
The Akt isoforms share structural features: the N-terminal pleckstrin homology (PH) domain, a flexible linker region connecting the PH and catalytic kinase domains, and a C-terminal regulatory tail. The PH and kinase domains are largely conserved in the Akt isoforms while the linker region has the lowest percent identity among Akt isoforms (46% vs. 80% and 90% for PH and kinase domains, respectively) (12). Cys124 is located in this linker region and is conserved in mice and humans. It is not conserved in Akt1 and Akt3, although Akt3 contains a cysteine residue Cys119 in this region. Sequence alignment using BLAST (National Center for Biotechnology Information) of the linker regions suggests that Cys124 in Akt2 (Uniprot accession no. Q60823) aligns with Ser122 in Akt1 (Uniprot accession no. P31750) and Pro121 in Akt3 (Uniprot accession no. Q9Y243) (Fig. 1C). A recent large-scale phosphoproteomics study of mouse liver reported Akt1 phosphorylation at several serine sites (Ser124, Ser126, and Ser129) in the linker region of this Akt isoform, but the functional consequences on Akt1 signaling were not reported (13). These findings motivated new studies to elucidate how oxidation regulates Akt isoforms and the role of Cys124 in the redox response of Akt2. Confirmatory Western blot analysis was done to monitor labeling of oxidized Akt2 by DCP-Bio1 in NIH 3T3 cells stimulated with PDGF. Cells were lysed in the presence of DCP-Bio1 at different times of PDGF stimulation. DCP-Bio1–labeled proteins were enriched using streptavidin-agarose affinity capture and probed for Akt1, Akt2, or Akt3 proteins using isoform-specific antibodies. We found statistically significant increases in incorporation of DCP-Bio1 label in Akt2 in response to PDGF stimulation (Fig. 1D). Akt1 or Akt3 were not labeled by DCP-Bio1 (Fig. 1E).
One criterion for a posttranslational modification to function as a signaling event is reversibility (e.g., phosphorylation/dephosphorylation). Two experiments were performed to address reversibility of Akt2 oxidation in response to PDGF stimulation. First, PDGF stimulation time was extended to 120 min; Akt2 oxidation peaked at 30 min, then gradually decreased at 60 and 120 min (Fig. 1F, Upper). In the second experiment, fresh media without PDGF was introduced after 30 min of PDGF treatment, and Akt2 oxidation was monitored at 30 min and 90 min after PDGF removal. Oxidation returned to basal levels after 90 min incubation without PDGF (Fig. 1F, Lower). Pretreatment with ROS-reducing agents (N-acetyl cysteine and PEG-catalase) blocked both PDGF-induced oxidation and inhibition of Akt2 activity (Fig. S2A), supporting the reversibility of PDGF-induced oxidation of Akt2.
To avoid postlysis oxidation, we used lysis buffer supplemented with catalase (200 U/mL) (14). Evidence that oxidation was not due to postlysis events is depicted in Fig. 1D and Fig. S3 A and B, which show (i) significantly increased DCP-Bio1 incorporation with increasing PDGF stimulation time; (ii) a similar time course of Akt2 labeling by DCP-Bio1 in response to PDGF using degassed lysis buffer at pH 5.5 (Fig. S3A); and (iii) lack of Akt2 labeling by DCP-Bio1 when recombinant Akt2 was spiked in lysis buffer (Fig. S3B).
Redox Regulation of Akt2 Kinase Activity.
Oxidation of critical cysteines in various signaling proteins is considered an important posttranslational regulatory event of signaling. For example, oxidation of a cysteine residue in PKA decreased PKA activity by promoting dephosphorylation at the proximal Thr197 site. On the other hand, cysteine oxidation of another member of the AGC family of kinases, PKCδ, positively affected its activity (15). More recently, MKK6 oxidation was linked to disulfide bond formation and inhibition of ATP binding (16), whereas ataxia telangiectasia mutated (ATM) oxidation promoted the active dimer state and increased activity of this protein (17). Previous studies have also established Akt as a redox-sensitive protein and showed that adding oxidants such as H2O2 to cells activates Akt by enhancing phosphorylation at the Thr308/9 and Ser473/4 sites (18–20), leading to the typical perception of ROS as an activator of Akt. However, Akt activation in response to such oxidation could be the indirect result of several mechanisms, such as oxidative inactivation of phosphatase and tensin homolog (PTEN), ligand-independent dimerization of receptor tyrosine kinases, oxidative inactivation of protein tyrosine phosphatases, and loss of feedback inhibition via MAPKs (3).
To determine whether direct oxidation has activating or inhibitory consequences on Akt1, Akt2, or Akt3, we conducted experiments to monitor how oxidation affects their ability to phosphorylate GSK-3α/β, a known substrate of Akt proteins. Experiments using recombinant active isoforms showed markedly increased redox sensitivity of Akt2 vs. Akt1 and Akt3 (Fig. 2 A–C). We identified the redox-sensitive cysteine(s) in Akt2 by oxidizing recombinant active Akt2 with H2O2 in the presence of dimedone, another cysteine sulfenic acid specific reagent, and the parent compound of DCP-Bio1. The oxidized product was analyzed by MS to identify the site(s) of cysteine oxidation on the basis of dimedone labeling. We confirmed oxidation at Cys124 in the linker domain and identified oxidation at Cys297 and Cys311 sites in the kinase domain of Akt2 (Fig. 3 A–C). The cysteines corresponding to Cys297 and Cys311 in Akt2 are conserved in all three Akt isoforms. Experiments using recombinant Akt1 and Akt3 oxidized by H2O2 in the presence or absence of dimedone confirmed a disulfide bond between these sites in both proteins and dimedone labeling of Cys296 in Akt1 (Fig. S4 A–E). Cys119 in the linker domain of Akt3 was not labeled by dimedone and not found in the disulfide bond with either Cys293 or Cys307 in the kinase domain of Akt3. Carbamidomethylated Cys119 tryptic peptide (generated by iodoacetamide after H2O2 treatment) was highly abundant, suggesting a true lack of oxidation (Fig. S4F). These results along with the data above (Fig. 2A) showed that disulfide formation between the Cys296/293 and Cys310/307 sites in Akt1 and Akt3, respectively, does not significantly alter their kinase activity. Murata et al. (21) have shown that prolonged exposure of cardiac H9c2 cells to exogenous H2O2 leads to formation of an intramolecular disulfide bond between the conserved cysteines (Cys296/297 and Cys310/311) within the kinase domain of Akt (Fig. 1C). Using site-directed mutagenesis of these cysteine sites in Akt1, they showed that an intramolecular disulfide bond targets Akt proteins to ubiquitin-proteasome coordinated degradation. The functional consequence of oxidation on specific Akt isoforms was not reported, and additionally, protein degradation was observed at >30 min exposure to 100 μM H2O2 (21).
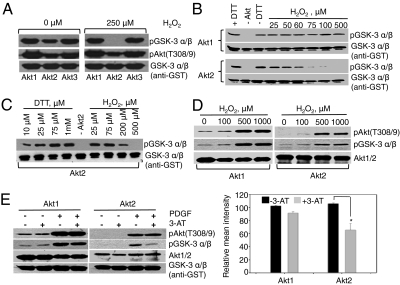
Fig. 2.
Isoform-specific inactivation of Akt2 kinase with oxidation and functional assessment of the effects of exogenous addition of H2O2 to NIH 3T3 cells vs. intracellular accumulation of H2O2 on Akt2 activity. (A) Kinase activities of recombinant purified active Akt1, Akt2, and Akt3 assayed after oxidation with 250 μM H2O2 (20 min, room temperature) using GSK-3α/β substrate. (B) Activity assay of recombinant purified active Akt1 and Akt2 proteins after oxidation with indicated concentrations of H2O2. Second blot in each pair is the substrate control (anti-GST). (C) Kinase assay with recombinant active Akt2 under oxidizing and reducing conditions of H2O2 and DTT with indicated concentrations. (D and E) Akt kinase assay was performed using Akt1 and Akt2 immunoprecipitated from NIH 3T3 cells after treatment by exogenous H2O2 for 30 min and endogenous accumulation of PDGF-stimulated H2O2 through catalase inhibition. Lower: Quantification of Akt2 activity (based on three replicate experiments; P value 0.016 for Akt2 ±3-AT data).
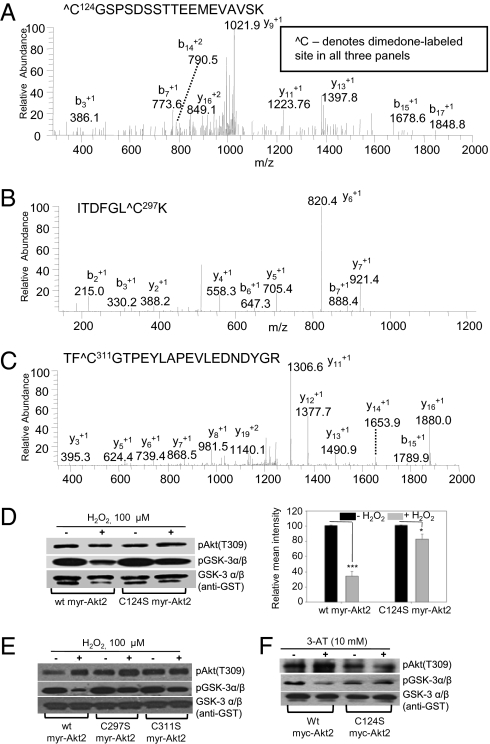
Fig. 3.
Identification of redox-sensitive cysteines in Akt2 by dimedone labeling and mass spectrometry and their effects on kinase activity. Recombinant active Akt2 was treated with H2O2 in the presence of 8 mM dimedone, then digested by trypsin and analyzed by MS as described in SI Materials and Methods. The ESI MS/MS spectra correspond to the following dimedone labeled peptides: (A) C124GSPSDSSTTEEMEVAVSK containing Cys124 site (Xcorr 1.33); (B) ITDFGLC297K containing Cys297 (Xcorr 2.0); and (C) TFC311GTPEYLAPEVLEDNDYGR containing Cys311 (Xcorr 3.57). (D) WT myr-Akt2 and Cys124Ser myr-Akt2 proteins immunoprecipitated from lysates of PDGF-stimulated (20 ng/mL, 10 min) NIH 3T3 transfectants were oxidized in vitro with 100 μM H2O2 before kinase activity assays. Right: Quantification of the kinase activity for untreated and H2O2-treated samples show a higher degree of inhibition of WT myr-Akt2 activity by oxidation (P < 0.001) relative to Cys124Ser myr-Akt2 (P = 0.029). (E) WT, Cys297Ser, and Cys311Ser myr-Akt2 proteins immunoprecipitated from lysates of PDGF-stimulated (20 ng/mL, 10 min) NIH 3T3 transfectants were oxidized in vitro with 100 μM H2O2 before kinase activity assays. (F) WT and Cys124Ser myc-Akt2 proteins immunoprecipitated from lysates of PDGF-stimulated (20 ng/mL, 10 min) and 3-AT–treated (10 mM overnight) Akt2KO MEF transfectants were assayed for their kinase activity.
Mechanistically, oxidation of redox-sensitive kinases can alter activity by glutathionylation, inter- or intramolecular disulfide bond formation, formation of higher oxidation species (-SO2; -SO3), and structural modification promoting phosphorylation/dephosphorylation and/or protein degradation (15). To determine whether catalytic decline in Akt2 activity after oxidation is due to an intermolecular disulfide bond between the Akt2 monomers, the kinase assay was performed under oxidizing conditions and reaction components were separated on nonreducing SDS/PAGE (Fig. S5A). Inhibition of Akt2 activity by oxidation cannot be attributed to formation of intermolecular disulfides (dimer state) (Fig. S5A). Glutathionylation was not responsible for Akt2 inhibition, because glutathione was not in the reaction mixture. Glutathione may play a protective role in cells and protect Akt2 against oxidation at key cysteine sites; attempts to monitor Akt2 glutathionylation in NIH 3T3 cells stimulated with PDGF were inconclusive owing to the poor quality of the antiglutathione antibodies and inadequate signal in the MS analysis (perhaps due to low or lack of glutathionylation). Overoxidation to Cys-SO2/3H species or other oxidation events (Met/Trp oxidation, Tyr/Trp nitration) was ruled out by experiments described in Fig. S5B, which show recovery of Akt2 kinase activity by tris(2-carboxyethyl)phosphine (TCEP) reduction after adding H2O2 to recombinant active Akt2. PDGF-stimulated cells in the presence of 3-aminotriazole (3-AT) showed similar results (Fig. S6C). Hyperoxidation of cysteines, Met/Trp oxidation, or Tyr/Trp nitration are not TCEP-reversible events and so cannot account for reduced Akt2 kinase activity. These results cumulatively point to formation of intramolecular disulfide bond(s) as responsible for the effects of oxidation on Akt2 activity. MS data from dimedone labeling and Akt2 oxidation experiments (Fig. 3 A–C and Fig. S5D) pointed to Cys124-Cys297 and Cys124-Cys311 as potential intramolecular disulfides responsible for Akt2 inhibition by oxidation. These results are consistent with resistance to oxidation in the Cys124Ser Akt2 mutant (Fig. 3D), Cys297Ser, and Cys311Ser Akt2 mutants (Fig. 3E). Akt3, which contains a cysteine site in the linker region (Cys119), was not redox sensitive (Fig. 2A). To further investigate lack of redox sensitivity of Akt1, Ser122 in Akt1 was mutated to cysteine. WT and Ser122Cys Akt1 were transfected in NIH 3T3 cells and stimulated with PDGF in the absence or presence of 3-AT. Decreased kinase activity of these proteins was not observed (Fig. S5C). Together, these data showed that the presence of a cysteine in the linker region alone is not sufficient for inducing redox sensitivity in Akt isoforms. Other structural elements are needed to transform this cysteine into a hyperactive site.
Effects of Exogenous Treatment of H2O2 vs. Intracellular Accumulation of H2O2 on Akt2 Activity.
Because the studies above were done with recombinant Akt proteins, we next set out to determine whether redox inactivation of Akt2 kinase was reflected in NIH 3T3 cells. We used two different approaches to oxidize NIH 3T3 cells: exogenous treatment with increasing concentrations of H2O2 and endogenous accumulation of H2O2 by treatment with catalase inhibitor 3-AT. Control experiments showed that preincubation of NIH 3T3 cells with 3-AT before PDGF treatment substantially decreased catalase protein (Fig. S7A). Previous studies also showed that 3-AT treatment causes the catalase to form insoluble aggregates, thus decreasing soluble protein (22). The consequence of 3-AT treatment on the extent of protein oxidation in cells treated or not with PDGF was monitored using lysis buffer supplemented with DCP-Bio1 (Fig. S7B). As expected, decreased level/activity of catalase correlated with increased oxidized, DCP-Bio1–labeled proteins.
Cellular treatment with increasing concentrations of H2O2 resulted in increased phosphorylation of Akt1 and Akt2 at the Thr308/9 phosphosite reflected in increased catalysis of their substrate GSK-3α/β phosphorylation (Fig. 2D). [Phosphorylation at Thr308 site in Akt2 is a representative of Akt2 phosphorylation status. Phosphorylation at Ser473 was also monitored for a number of studies (Fig. S8). No notable differences in phosphorylation at the two sites under all tested conditions were observed.] This observation corroborates others showing that oxidation increases Akt phosphorylation because of other contributing events, not direct Akt2 oxidation (3). In contrast, inhibition of cellular catalase by 3-AT led to oxidative inactivation of Akt2 kinase in an isoform-specific manner without affecting PDGF-stimulated phosphorylation at the activating phosphosite residues (Fig. 2E). This observation mirrored previous in vitro kinase assays linking oxidation to inhibition (Fig. 2 A–C), consistent with the degree of oxidation induced by pretreatment with 3-AT (Fig. S7B). In contrast, pretreatment of cells with N-acetyl cysteine or PEG-catalase to reduce accumulation of ROS increased Akt2 activity 1.8- to 3.2-fold when cells were stimulated with PDGF (Fig. S2A). Additionally, chemical inhibition of two ROS sources activated by PDGF, NADPH oxidase, and mitochondrial electron transport chain (ETC), using VAS2870 and rotenone (mitochondrial complex I inhibitor), respectively, showed an activating effect on Akt2 activity (Fig. S2B). More recently, Katsuyama et al. (23) showed that PDGF-induced NOX expression depends on the mitochondrial ETC, and rotenone suppressed induction of NOX1 mRNA. The stronger effect of rotenone on Akt2 activity is consistent with this notion. To determine whether these observations stemmed from PDGF-induced oxidation at Cys124, this cysteine site was mutated to serine and the Cys124Ser myr-Akt2 mutant was expressed in NIH 3T3 fibroblasts alongside a control using the WT myr-Akt2 construct. The WT myr-Akt2 and Cys124Ser mutants were immunoprecipitated from cell lysates and subjected to in vitro oxidation with H2O2. H2O2 treatment caused significantly less Akt2 activity relative to the Cys124Ser Akt2 mutant, proving that the Cys124 site is a major redox-sensitive site involved in regulating Akt2 kinase activity (Fig. 3D) (Western blots of a biological replicate are shown in Fig. S6A). Control experiments (Fig. S6B) showed similar kinase activities and sensitivities to oxidation for endogenous WT Akt2 and WT myr-Akt2 constructs; PDGF- and 3-AT–dependent oxidation of both constructs was recovered by TCEP treatment (Fig. S6C).
PDGF-Induced Oxidation Allows Distinct Regulation of Akt Kinase Isoforms.
Regulation of Akt2 activity by oxidation under physiological conditions of PDGF-stimulated ROS indicates that PDGF, and perhaps other ROS-inducing growth factors, use oxidation to adjust timing and/or amplitude of signaling events relayed through redox-sensitive signaling proteins. Indeed, when TCEP recovery assay was performed for Akt2 immunoprecipitates obtained from differentiated C2C12 myotubes that were stimulated with PDGF, insulin, or TNF-α for 30 min, PDGF- and TNF-α–treated samples both showed increased Akt2 activity, whereas activity was unchanged for the insulin-treated samples with or without TCEP (Fig. S9A). Time course data for TNF-α−induced DCP-Bio1 labeling of Akt2 is included in Fig. S9A (Lower). Insulin-induced ROS was shown to be important for differentiation of myoblasts into myotubes (24–26). In accordance with these studies, insulin treatment induced Akt2 oxidation and decreased Akt2 activity in myoblasts, unlike the results in myotubes (Fig. S9B).
To quantify the effect of PDGF-induced oxidation on Akt2 activity, the catalytic activity of immunoprecipitated Akt2 from lysates of PDGF-stimulated cells was determined with or without TCEP. A kinetic lag was observed in GSK-3α/β phosphorylation by Akt2 without TCEP, which was reversed by addition of TCEP to reaction mixture (Fig. 4 A and B). A side-by-side comparison of the effect of TCEP on Akt2 and Akt1 activities showed a significantly greater Akt2 activation (3.1-fold) than Akt1 (1.3-fold) (Fig. 4B, Right). The small effect of TCEP on Akt1 activity could be due to the reduction of the Cys296/310 disulfide bond, as reported earlier (21). These data suggest that PDGF-induced ROS decreases Akt2 activity by ≈66% on the basis of initial rates of GSK-3α/β phosphorylation (−TCEP: 9.7 min−1; +TCEP: 28.25 min−1). Correcting for non–PDGF-induced oxidation which, according to the data in Fig. 1D, has an upper limit of ≈30% of total oxidized Akt2, PDGF-driven Akt2 oxidation comprises ≈45% of total active phosphorylated Akt2.
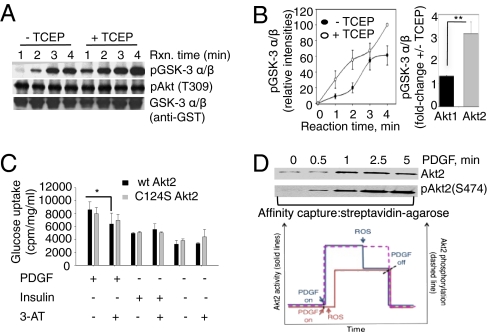
Fig. 4.
Regulation of Akt2 kinase activity in response to PDGF-induced oxidation in NIH 3T3 cells. (A) Akt2 was immunoprecipitated from lysates of PDGF-stimulated NIH 3T3 cells, and kinase assays were performed by incubation with GSK-3α/β substrate for indicated time points with and without TCEP (1 mM). (B) Time course of GSK-3α/β phosphorylation catalyzed by Akt2 kinase in the absence (filled circles) or presence (open circles) of TCEP is shown as means of three replicates. Right: Fold-activation by TCEP of Akt1 and Akt2 immunoprecipitated from PDGF-stimulated NIH 3T3 cells (10-min stimulation). Phosphorylation of GSK-3α/β by Akt1 and Akt2 was monitored in the absence and presence of TCEP, and the reaction was quenched by 4× SDS sample buffer at 2 min. The 2-min time point was chosen to ensure conditions under which there is linear increase in substrate phosphorylation for both Akt isoforms. (C) Effect of PDGF and insulin stimulation on glucose uptake by Akt2 knockout MEFs transduced with WT myc-Akt2 and Cys124Ser myc-Akt2. MEFs infected with WT and Cys124Ser Akt2 constructs were subjected to overnight serum starvation with and without 3-AT (10 mM) treatment. Glucose uptake was measured the following day with PDGF (20 ng/mL) or insulin (200 nM) alone for 30 min along with the unstimulated control for basal uptake. 3-AT inhibited the PDGF-induced glucose uptake of WT myc-Akt2 MEFs significantly. *P < 0.05, one-way ANOVA. (D) PDGF-stimulated NIH 3T3 cell lysates labeled with sulfenic acid-specific reagent DCP-Bio1 were affinity captured using streptavidin-agarose conjugate and probed for Akt2 and pS474 Akt2 to assess oxidation and phosphorylation status of Akt2, respectively. Lower: Proposed two-state (on/off) model for regulating Akt2 activity with PDGF induced oxidation. Blue solid path, phosphorylation of Akt2 kinase precedes oxidation, and an activity burst is observed. Red solid path, Akt2 phosphorylation and oxidation in response to PDGF addition occur in parallel, and the enzyme works at an overall lower but constant activity. The phosphorylation status of Akt2 is represented by the dashed magenta line.
To further investigate the functional consequence of the Cys124Ser mutation in Akt2, we monitored its effect on substrate phosphorylation and PDGF-induced glucose uptake. Akt2 knockout mouse embryonic fibroblasts (Akt2KO MEFs) were transduced with WT or Cys124Ser myc-Akt2 using retroviral infection as described earlier and stimulated with PDGF with or without 3-AT (27). 3-AT treatment of Akt2KO MEFs transduced with WT and Cys124Ser Akt2 mutant resulted in decreased WT Akt2 activity (Fig. 3F), whereas Cys124Ser Akt2 activity was unperturbed by oxidation. These data further confirm that Cys124 is involved in regulating redox sensitivity of Akt2 in cells. These results are consistent with the glucose uptake studies in Akt2KO MEFs, performed under the same experimental conditions (Fig. 4C). Glucose uptake was significantly inhibited by 3-AT in Akt2KO MEFs infected with WT myc-Akt2 but not Cys124Ser myc-Akt2 retroviral constructs. The effect of 3-AT on insulin-stimulated glucose uptake in WT or Cys124Ser Akt2KO MEFs was not statistically significant. There was no detectable difference in AS160, FOXO 1/3a, and GSK-3α/β phosphorylation between Akt2KO MEFs and cells transduced with WT or Cys124Ser Akt2 (Fig. S9C). These results emphasize that signaling events that link Akt2 activation and glucose uptake are incompletely understood. AS160, originally identified as an Akt2-specific substrate that regulates trafficking of glucose transporters, is also the target of kinases such as AMP-activated protein kinase (AMPK) among several other unknown enzymes (28, 29), thereby adding to the ambiguity of the Akt2-specific targets in glucose uptake. GSK-3α/β and FOXO 1/3a proteins are non–isoform-specific Akt substrates (and substrates for other kinases).
Based on the cumulative results included here, a model is proposed to describe effects of PDGF-induced ROS on Akt2 signaling. Depending on the relative timing of oxidation and phosphorylation, the following simplified scenarios may occur with consequences on the amplitude/timing of signaling through Akt2: (i) Akt2 could undergo phosphorylation by upstream kinases before its activity is muted by oxidation (Fig. 4D, blue path), and (ii) phosphorylation of Akt2 by upstream kinases and Akt2 oxidation by PDGF-induced ROS occur in parallel (Fig. 4D, red path). Akt2 activity would be the net result of oxidation and phosphorylation. In the first case, a burst in activity will be observed before the oxidation event attenuates or completely reduces Akt2 activity. In the second scenario, an activity burst would not occur and Akt2 activity would be lower, resulting in lower amplitude of signaling events mediated by this kinase. Western blot data (Fig. 4D and Fig. S9D) show parallel increases in oxidation and phosphorylation of signaling proteins, including Akt2, in response to PDGF stimulation. These findings support a two-state (on/off) model of Akt2 activation by phosphorylation, with oxidation determining the amplitude of signaling event that occurs at Akt2. On the other hand, PDGF-induced oxidation is not expected to regulate Akt1 activity, suggesting that spatial distribution, tissue-specific expression, and other components are more critical in regulating Akt1 signaling.
Summary.
These studies indicate that PDGF and other ROS-inducing stimuli inhibit Akt2 signaling under physiological conditions, with functional consequence on molecular and cellular processes induced by these stimuli. In addition, these studies highlight the importance of the ROS source and localization when oxidative effects on a biological system are investigated. Phosphorylation of a signaling protein does not necessarily translate into protein activity, although it is often equated with activity. Rather, other “silent” or unrecognized posttranslational modifications can modulate the activity or substrate specificity of signaling proteins in complex ways.
Materials and Methods
See SI Materials and Methods for detailed protocols.
Cell Culture, Cloning Procedures for WT and Cys124Ser Akt2, Transfection of NIH 3T3 Cells, and Activity Assays.
NIH 3T3 cells were cultured in the complete DMEM High Glucose (Invitrogen) medium. Confluent cells were serum starved for 16 h and stimulated the next day with PDGF-BB (20 ng/mL) at 37 °C for the indicated time points. The collected lysates were normalized, and Akt2 was immunoprecipitated for assaying activity. pEF6/V5-His TOPO construct of WT myr-Akt2 created by subcloning was used for generating Cys124Ser mutation by site-directed mutagenesis (SDM kit; Stratagene) with the following primers: forward 5′-CATGGACTACAAGTCTGGCTCCCCCAGTG-3′ and reverse 5′-CACTGGGGGAGCCAGACTTGTAGTCCATG-3′. These constructs were transfected in NIH 3T3 cells using lipofectamine 2000 (Invitrogen) and selected using blasticidin (6 μg/mL). After PDGF stimulation and lysis, normalized lysates were used to pull down WT and Cys124Ser myr-Akt2 proteins with V5 antibody for in vitro oxidation and activity assays.
Transfection of 293-T and Retroviral Infection of Akt2 Knockout MEFs.
Briefly 293-T cells were transfected when 30–40% confluent with 6 μg DNA of WT or Cys124Ser Akt2 cloned in pMIGR2 using FuGENE-6. Akt2 knockout MEFs were infected twice with viral supernatant harvested and filtered from 24 and 48 h of 293-T transfection. Infection efficiencies were assessed by Western blot detection of Akt2 expression.
Affinity Capture of DCP-Bio1 Labeled Proteins.
PDGF-stimulated NIH 3T3 lysates were incubated overnight at 4 °C with 20–100 μL streptavidin–agarose conjugate (Invitrogen). The next day, after washes, the DCP-Bio1–labeled proteins were eluted by heating at 100 °C for 10 min. The samples were then probed for Akt2 by Western blotting.
Induction of Oxidation in NIH 3T3 Cells.
Exogenous treatment with H2O2.
NIH 3T3 cells were serum starved overnight and treated next day with H2O2 (100 μM, 500 μM, and 1,000 μM) for 30 min at 37 °C before PDGF-BB stimulation (20 ng/mL, 10 min).
Endogenous increase in H2O2 through catalase inhibition.
NIH 3T3 cells were serum starved overnight in the presence of 10 mM 3-AT and stimulated similarly. Lysates from both experiments were normalized and Akt1 and Akt2 proteins were immunoprecipitated for assaying kinase activity.
Kinase Assay Using Immunoprecipitated Akt and Recombinant Active Akt Proteins.
Akt2 kinase activity was assayed by nonradioactive Akt kinase assay kit (Cell Signaling) using GSK-3α/β (1 μg, GST-linked) substrate in the presence of 0.4 mM each of ATP and MgCl2 at 30 °C for 30 min. For experiments comparing reaction kinetics under native and reducing conditions, TCEP (1 mM) was added before GSK-3α/β substrate. Recombinant active Akt1 and Akt2 proteins (Active Motif) were oxidized or reduced with increasing concentrations of H2O2 (25–500 μM) and DTT (10 μM to 1 mM), respectively, for 20 min at room temperature before incubation with substrates for 10 min at room temperature. The reaction mixture was separated by reducing SDS/PAGE and probed for pGSK-3α/β(Ser9/12), pAktT308/9, GST, and Akt1/Akt2 proteins using Western blotting.
Labeling of Oxidized Cysteines in Akt2 with Dimedone and Identification of Labeled Cysteines by MS.
Recombinant active Akt2 (21 μM) was incubated with H2O2 (1 mM) in the presence of dimedone (8 mM) for 1 h and quenched with 4× SDS sample buffer. The reaction mixture was separated and stained on SDS/PAGE gel with Gel Code Blue (Pierce). Excision and in-gel trypsin digestion of protein bands was performed according to standard protocols. Tryptic peptides were separated and analyzed by nano-LC (Dionex Ultimate 3000 System) coupled to a Thermo ESI LTQ mass spectrometer (SIM mode) for precursor ions containing dimedone-labeled cysteine. Peptides were identified with Bioworks 3.3 software. Methods for MS analysis of Akt1 and Akt3 are described in the SI Materials and Methods.
Glucose Uptake.
Akt2 knockout MEFs transduced with WT and Cys124Ser myc-Akt2 were serum starved overnight in the presence or absence of 3-AT (10 mM) and stimulated the next day with PDGF (20 ng/mL) or insulin (200 nM) for 30 min. The assay was carried out using 2-deoxyglucose (0.1 mM) and 2-deoxy-d-[3H] glucose (0.5 μCi/mL; Perkin-Elmer). The uptake was detected in Scintiverse BD scintillation mixture (Fisher Scientific) by a Beckman LS6000SC scintillation counter and normalized by the protein concentration.
Statistical Analysis (Common to All Figures).
Quantification of Western blots was performed using ImageJ. Statistical analysis (t test, one-way ANOVA) was based on a minimum of three biological replicates using SigmaPlot 11.0. Means and SEs are shown; asterisks indicate statistical significant changes compared with untreated controls [α = 0.05, P values of 0.01–0.05 (*), 0.001–0.01 (**), or <0.001 (***)].
Supplementary Material
Acknowledgments
We thank Dr. Jeffrey Field's laboratory (University of Pennsylvania School of Medicine) for providing Akt2 knockout mouse embryonic fibroblasts, pMIGR construct of WT myc-Akt2, and infection protocol; Dr. Jeffrey Rathmell's laboratory (Duke University) for providing pEF6/TOPO construct of WT myr-Akt1; and Dr. Osvaldo Delbono's laboratory (Wake Forest University School of Medicine) for their help on providing C2C12 cells and differentiation protocols. Financial support was provided by American Heart Association Scientist Development Program Grant SDG 0730069N (to C.M.F.) and National Institutes of Health Grants R01 CA136810 (to C.M.F.) and R33 CA126659 (to L.B.P.). Financial support for the development of DBond software came from the 21C Frontier Functional Proteomics Project, Korean Ministry of Education, Science and Technology (Grant FPR08-A1-020) and the National Core Research Center program through the Center for Cell Signaling Research and Drug Discovery Research (Grant R15-2006-020) to E.P.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1011665108/-/DCSupplemental.
References
- 1.Gonzalez E, McGraw TE. The Akt kinases: Isoform specificity in metabolism and cancer. Cell Cycle. 2009;8:2502–2508. doi: 10.4161/cc.8.16.9335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gonzalez E, McGraw TE. Insulin-modulated Akt subcellular localization determines Akt isoform-specific signaling. Proc Natl Acad Sci USA. 2009;106:7004–7009. doi: 10.1073/pnas.0901933106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Genestra M. Oxyl radicals, redox-sensitive signalling cascades and antioxidants. Cell Signal. 2007;19:1807–1819. doi: 10.1016/j.cellsig.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 4.Sundaresan M, Yu ZX, Ferrans VJ, Irani K, Finkel T. Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science. 1995;270:296–299. doi: 10.1126/science.270.5234.296. [DOI] [PubMed] [Google Scholar]
- 5.Woo HA, et al. Inactivation of peroxiredoxin I by phosphorylation allows localized H(2)O(2) accumulation for cell signaling. Cell. 2010;140:517–528. doi: 10.1016/j.cell.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 6.Charles RL, et al. Protein sulfenation as a redox sensor: Proteomics studies using a novel biotinylated dimedone analogue. Mol Cell Proteomics. 2007;6:1473–1484. doi: 10.1074/mcp.M700065-MCP200. [DOI] [PubMed] [Google Scholar]
- 7.Poole LB, et al. Fluorescent and affinity-based tools to detect cysteine sulfenic acid formation in proteins. Bioconjug Chem. 2007;18:2004–2017. doi: 10.1021/bc700257a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reddie KG, Seo YH, Muse Iii WB, Leonard SE, Carroll KS. A chemical approach for detecting sulfenic acid-modified proteins in living cells. Mol Biosyst. 2008;4:521–531. doi: 10.1039/b719986d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seo YH, Carroll KS. Facile synthesis and biological evaluation of a cell-permeable probe to detect redox-regulated proteins. Bioorg Med Chem Lett. 2009;19:356–359. doi: 10.1016/j.bmcl.2008.11.073. [DOI] [PubMed] [Google Scholar]
- 10.Seo YH, Carroll KS. Profiling protein thiol oxidation in tumor cells using sulfenic acid-specific antibodies. Proc Natl Acad Sci USA. 2009;106:16163–16168. doi: 10.1073/pnas.0903015106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim JR, Kwon KS, Yoon HW, Lee SR, Rhee SG. Oxidation of proteinaceous cysteine residues by dopamine-derived H2O2 in PC12 cells. Arch Biochem Biophys. 2002;397:414–423. doi: 10.1006/abbi.2001.2691. [DOI] [PubMed] [Google Scholar]
- 12.McKenna LB, Zhou GL, Field J. Isoform-specific functions of Akt in cell motility. Cell Mol Life Sci. 2007;64:2723–2725. doi: 10.1007/s00018-007-7247-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Villén J, Beausoleil SA, Gerber SA, Gygi SP. Large-scale phosphorylation analysis of mouse liver. Proc Natl Acad Sci USA. 2007;104:1488–1493. doi: 10.1073/pnas.0609836104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klomsiri C, et al. Use of dimedone-based chemical probes for sulfenic acid detection evaluation of conditions affecting probe incorporation into redox-sensitive proteins. Methods Enzymol. 2010;473:77–94. doi: 10.1016/S0076-6879(10)73003-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cross JV, Templeton DJ. Regulation of signal transduction through protein cysteine oxidation. Antioxid Redox Signal. 2006;8:1819–1827. doi: 10.1089/ars.2006.8.1819. [DOI] [PubMed] [Google Scholar]
- 16.Diao Y, et al. Oxidation-induced intramolecular disulfide bond inactivates mitogen-activated protein kinase kinase 6 by inhibiting ATP binding. Proc Natl Acad Sci USA. 2010;107:20974–20979. doi: 10.1073/pnas.1007225107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo Z, Kozlov S, Lavin MF, Person MD, Paull TT. ATM activation by oxidative stress. Science. 2010;330:517–521. doi: 10.1126/science.1192912. [DOI] [PubMed] [Google Scholar]
- 18.Dossumbekova A, et al. Akt activates NOS3 and separately restores barrier integrity in H2O2-stressed human cardiac microvascular endothelium. Am J Physiol Heart Circ Physiol. 2008;295:H2417–H2426. doi: 10.1152/ajpheart.00501.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin D, Salinas M, Fujita N, Tsuruo T, Cuadrado A. Ceramide and reactive oxygen species generated by H2O2 induce caspase-3-independent degradation of Akt/protein kinase B. J Biol Chem. 2002;277:42943–42952. doi: 10.1074/jbc.M201070200. [DOI] [PubMed] [Google Scholar]
- 20.Ushio-Fukai M, et al. Reactive oxygen species mediate the activation of Akt/protein kinase B by angiotensin II in vascular smooth muscle cells. J Biol Chem. 1999;274:22699–22704. doi: 10.1074/jbc.274.32.22699. [DOI] [PubMed] [Google Scholar]
- 21.Murata H, et al. Glutaredoxin exerts an antiapoptotic effect by regulating the redox state of Akt. J Biol Chem. 2003;278:50226–50233. doi: 10.1074/jbc.M310171200. [DOI] [PubMed] [Google Scholar]
- 22.Ueda M, et al. Effect of catalase-specific inhibitor 3-amino-1,2,4-triazole on yeast peroxisomal catalase in vivo. FEMS Microbiol Lett. 2003;219:93–98. doi: 10.1016/S0378-1097(02)01201-6. [DOI] [PubMed] [Google Scholar]
- 23.Katsuyama M, et al. Essential role of ATF-1 in induction of NOX1, a catalytic subunit of NADPH oxidase: involvement of mitochondrial respiratory chain. Biochem J. 2005;386:255–261. doi: 10.1042/BJ20041180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ardite E, Barbera JA, Roca J, Fernández-Checa JC. Glutathione depletion impairs myogenic differentiation of murine skeletal muscle C2C12 cells through sustained NF-kappaB activation. Am J Pathol. 2004;165:719–728. doi: 10.1016/s0002-9440(10)63335-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hansen JM, Klass M, Harris C, Csete M. A reducing redox environment promotes C2C12 myogenesis: Implications for regeneration in aged muscle. Cell Biol Int. 2007;31:546–553. doi: 10.1016/j.cellbi.2006.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lyons CN, Leary SC, Moyes CD. Bioenergetic remodeling during cellular differentiation: Changes in cytochrome c oxidase regulation do not affect the metabolic phenotype. Biochem Cell Biol. 2004;82:391–399. doi: 10.1139/o04-040. [DOI] [PubMed] [Google Scholar]
- 27.Zhou GL, et al. Opposing roles for Akt1 and Akt2 in Rac/Pak signaling and cell migration. J Biol Chem. 2006;281:36443–36453. doi: 10.1074/jbc.M600788200. [DOI] [PubMed] [Google Scholar]
- 28.Treebak JT, et al. AMPK-mediated AS160 phosphorylation in skeletal muscle is dependent on AMPK catalytic and regulatory subunits. Diabetes. 2006;55:2051–2058. doi: 10.2337/db06-0175. [DOI] [PubMed] [Google Scholar]
- 29.Kramer HF, et al. Distinct signals regulate AS160 phosphorylation in response to insulin, AICAR, and contraction in mouse skeletal muscle. Diabetes. 2006;55:2067–2076. doi: 10.2337/db06-0150. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.