Abstract
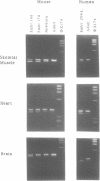
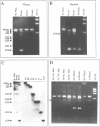
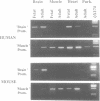
Dystrophin transcripts were shown to be alternatively spliced in a pattern characteristic of both tissue type and developmental stage. Multiple novel spliced forms of dystrophin mRNA were identified in murine brain tissue, skeletal and cardiac muscle, diaphragm, and human cardiac Purkinje fibers. The transcript diversity was greatest in adult, non-skeletal muscle tissues. Sequence analysis revealed that four tandem exons of the murine gene are differentially spliced in at least 11 separate patterns to generate distinct isoforms. Two of these forms were observed in all tissues examined, while several others were uniquely observed in cardiac muscle and brain. Cardiac Purkinje fibers express an isoform primarily observed in brain tissue. Several spliced transcripts were observed only in postnatal development. Differential utilization of a fifth exon results in two mRNA splice forms that encode separate embryonic and adult C-termini of dystrophin. Comparison of murine with human dystrophin mRNAs showed that similar isoform expression patterns exist across species. These observations suggest that functionally distinct isoforms of the dystrophin protein are expressed in separate tissues and at different stages of development. These isoforms may be of significance in understanding the various tissue-specific effects produced by dystrophin gene mutations in Duchenne and Becker muscular dystrophy patients.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arahata K., Ishiura S., Ishiguro T., Tsukahara T., Suhara Y., Eguchi C., Ishihara T., Nonaka I., Ozawa E., Sugita H. Immunostaining of skeletal and cardiac muscle surface membrane with antibody against Duchenne muscular dystrophy peptide. Nature. 1988 Jun 30;333(6176):861–863. doi: 10.1038/333861a0. [DOI] [PubMed] [Google Scholar]
- Bar S., Barnea E., Levy Z., Neuman S., Yaffe D., Nudel U. A novel product of the Duchenne muscular dystrophy gene which greatly differs from the known isoforms in its structure and tissue distribution. Biochem J. 1990 Dec 1;272(2):557–560. doi: 10.1042/bj2720557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett V., Lambert S. The spectrin skeleton: from red cells to brain. J Clin Invest. 1991 May;87(5):1483–1489. doi: 10.1172/JCI115157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieber F. R., Hoffman E. P., Amos J. A. Dystrophin analysis in duchenne muscular dystrophy: use in fetal diagnosis and in genetic counseling. Am J Hum Genet. 1989 Sep;45(3):362–367. [PMC free article] [PubMed] [Google Scholar]
- Bonilla E., Samitt C. E., Miranda A. F., Hays A. P., Salviati G., DiMauro S., Kunkel L. M., Hoffman E. P., Rowland L. P. Duchenne muscular dystrophy: deficiency of dystrophin at the muscle cell surface. Cell. 1988 Aug 12;54(4):447–452. doi: 10.1016/0092-8674(88)90065-7. [DOI] [PubMed] [Google Scholar]
- Breitbart R. E., Nguyen H. T., Medford R. M., Destree A. T., Mahdavi V., Nadal-Ginard B. Intricate combinatorial patterns of exon splicing generate multiple regulated troponin T isoforms from a single gene. Cell. 1985 May;41(1):67–82. doi: 10.1016/0092-8674(85)90062-5. [DOI] [PubMed] [Google Scholar]
- Briggs M. M., Lin J. J., Schachat F. H. The extent of amino-terminal heterogeneity in rabbit fast skeletal muscle troponin T. J Muscle Res Cell Motil. 1987 Feb;8(1):1–12. doi: 10.1007/BF01767259. [DOI] [PubMed] [Google Scholar]
- Bulman D. E., Murphy E. G., Zubrzycka-Gaarn E. E., Worton R. G., Ray P. N. Differentiation of Duchenne and Becker muscular dystrophy phenotypes with amino- and carboxy-terminal antisera specific for dystrophin. Am J Hum Genet. 1991 Feb;48(2):295–304. [PMC free article] [PubMed] [Google Scholar]
- Chamberlain J. S., Gibbs R. A., Ranier J. E., Nguyen P. N., Caskey C. T. Deletion screening of the Duchenne muscular dystrophy locus via multiplex DNA amplification. Nucleic Acids Res. 1988 Dec 9;16(23):11141–11156. doi: 10.1093/nar/16.23.11141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain J. S., Pearlman J. A., Muzny D. M., Gibbs R. A., Ranier J. E., Caskey C. T., Reeves A. A. Expression of the murine Duchenne muscular dystrophy gene in muscle and brain. Science. 1988 Mar 18;239(4846):1416–1418. doi: 10.1126/science.3347839. [DOI] [PubMed] [Google Scholar]
- Chelly J., Hamard G., Koulakoff A., Kaplan J. C., Kahn A., Berwald-Netter Y. Dystrophin gene transcribed from different promoters in neuronal and glial cells. Nature. 1990 Mar 1;344(6261):64–65. doi: 10.1038/344064a0. [DOI] [PubMed] [Google Scholar]
- Coulombe A., Lefèvre I. A., Baro I., Coraboeuf E. Barium- and calcium-permeable channels open at negative membrane potentials in rat ventricular myocytes. J Membr Biol. 1989 Oct;111(1):57–67. doi: 10.1007/BF01869209. [DOI] [PubMed] [Google Scholar]
- Epstein H. F., Fischman D. A. Molecular analysis of protein assembly in muscle development. Science. 1991 Mar 1;251(4997):1039–1044. doi: 10.1126/science.1998120. [DOI] [PubMed] [Google Scholar]
- Ervasti J. M., Campbell K. P. Membrane organization of the dystrophin-glycoprotein complex. Cell. 1991 Sep 20;66(6):1121–1131. doi: 10.1016/0092-8674(91)90035-w. [DOI] [PubMed] [Google Scholar]
- Ervasti J. M., Ohlendieck K., Kahl S. D., Gaver M. G., Campbell K. P. Deficiency of a glycoprotein component of the dystrophin complex in dystrophic muscle. Nature. 1990 May 24;345(6273):315–319. doi: 10.1038/345315a0. [DOI] [PubMed] [Google Scholar]
- Feener C. A., Koenig M., Kunkel L. M. Alternative splicing of human dystrophin mRNA generates isoforms at the carboxy terminus. Nature. 1989 Apr 6;338(6215):509–511. doi: 10.1038/338509a0. [DOI] [PubMed] [Google Scholar]
- Fong P. Y., Turner P. R., Denetclaw W. F., Steinhardt R. A. Increased activity of calcium leak channels in myotubes of Duchenne human and mdx mouse origin. Science. 1990 Nov 2;250(4981):673–676. doi: 10.1126/science.2173137. [DOI] [PubMed] [Google Scholar]
- Franco A., Jr, Lansman J. B. Calcium entry through stretch-inactivated ion channels in mdx myotubes. Nature. 1990 Apr 12;344(6267):670–673. doi: 10.1038/344670a0. [DOI] [PubMed] [Google Scholar]
- Helfman D. M., Roscigno R. F., Mulligan G. J., Finn L. A., Weber K. S. Identification of two distinct intron elements involved in alternative splicing of beta-tropomyosin pre-mRNA. Genes Dev. 1990 Jan;4(1):98–110. doi: 10.1101/gad.4.1.98. [DOI] [PubMed] [Google Scholar]
- Hoffman E. P., Brown R. H., Jr, Kunkel L. M. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987 Dec 24;51(6):919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- Hoffman E. P., Garcia C. A., Chamberlain J. S., Angelini C., Lupski J. R., Fenwick R. Is the carboxyl-terminus of dystrophin required for membrane association? A novel, severe case of Duchenne muscular dystrophy. Ann Neurol. 1991 Oct;30(4):605–610. doi: 10.1002/ana.410300414. [DOI] [PubMed] [Google Scholar]
- Koenig M., Beggs A. H., Moyer M., Scherpf S., Heindrich K., Bettecken T., Meng G., Müller C. R., Lindlöf M., Kaariainen H. The molecular basis for Duchenne versus Becker muscular dystrophy: correlation of severity with type of deletion. Am J Hum Genet. 1989 Oct;45(4):498–506. [PMC free article] [PubMed] [Google Scholar]
- Koenig M., Monaco A. P., Kunkel L. M. The complete sequence of dystrophin predicts a rod-shaped cytoskeletal protein. Cell. 1988 Apr 22;53(2):219–228. doi: 10.1016/0092-8674(88)90383-2. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lambert S., Yu H., Prchal J. T., Lawler J., Ruff P., Speicher D., Cheung M. C., Kan Y. W., Palek J. cDNA sequence for human erythrocyte ankyrin. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1730–1734. doi: 10.1073/pnas.87.5.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. C., Pearlman J. A., Chamberlain J. S., Caskey C. T. Expression of recombinant dystrophin and its localization to the cell membrane. Nature. 1991 Jan 24;349(6307):334–336. doi: 10.1038/349334a0. [DOI] [PubMed] [Google Scholar]
- Lemaire C., Heilig R., Mandel J. L. The chicken dystrophin cDNA: striking conservation of the C-terminal coding and 3' untranslated regions between man and chicken. EMBO J. 1988 Dec 20;7(13):4157–4162. doi: 10.1002/j.1460-2075.1988.tb03311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libri D., Lemonnier M., Meinnel T., Fiszman M. Y. A single gene codes for the beta subunits of smooth and skeletal muscle tropomyosin in the chicken. J Biol Chem. 1989 Feb 15;264(5):2935–2944. [PubMed] [Google Scholar]
- Lidov H. G., Byers T. J., Watkins S. C., Kunkel L. M. Localization of dystrophin to postsynaptic regions of central nervous system cortical neurons. Nature. 1990 Dec 20;348(6303):725–728. doi: 10.1038/348725a0. [DOI] [PubMed] [Google Scholar]
- Lux S. E., John K. M., Bennett V. Analysis of cDNA for human erythrocyte ankyrin indicates a repeated structure with homology to tissue-differentiation and cell-cycle control proteins. Nature. 1990 Mar 1;344(6261):36–42. doi: 10.1038/344036a0. [DOI] [PubMed] [Google Scholar]
- Nomura H., Hizawa K. Histopathological study of the conduction system of the heart in Duchenne progressive muscular dystrophy. Acta Pathol Jpn. 1982 Nov;32(6):1027–1033. doi: 10.1111/j.1440-1827.1982.tb02082.x. [DOI] [PubMed] [Google Scholar]
- Nudel U., Zuk D., Einat P., Zeelon E., Levy Z., Neuman S., Yaffe D. Duchenne muscular dystrophy gene product is not identical in muscle and brain. Nature. 1989 Jan 5;337(6202):76–78. doi: 10.1038/337076a0. [DOI] [PubMed] [Google Scholar]
- Smith C. W., Patton J. G., Nadal-Ginard B. Alternative splicing in the control of gene expression. Annu Rev Genet. 1989;23:527–577. doi: 10.1146/annurev.ge.23.120189.002523. [DOI] [PubMed] [Google Scholar]
- Towbin J. A., Wu D. R., Chamberlain J., Larsen P. D., Seltzer W. K., McCabe E. R. Characterization of patients with glycerol kinase deficiency utilizing cDNA probes for the Duchenne muscular dystrophy locus. Hum Genet. 1989 Sep;83(2):122–126. doi: 10.1007/BF00286703. [DOI] [PubMed] [Google Scholar]
- Wessels A., Ginjaar I. B., Moorman A. F., van Ommen G. J. Different localization of dystrophin in developing and adult human skeletal muscle. Muscle Nerve. 1991 Jan;14(1):1–7. doi: 10.1002/mus.880140102. [DOI] [PubMed] [Google Scholar]
- Zubrzycka-Gaarn E. E., Bulman D. E., Karpati G., Burghes A. H., Belfall B., Klamut H. J., Talbot J., Hodges R. S., Ray P. N., Worton R. G. The Duchenne muscular dystrophy gene product is localized in sarcolemma of human skeletal muscle. Nature. 1988 Jun 2;333(6172):466–469. doi: 10.1038/333466a0. [DOI] [PubMed] [Google Scholar]