Abstract
The critical involvement of GPCRs (G-protein-coupled receptors) in nearly all physiological processes, and the presence of these receptors at the interface between the extracellular and the intracellular milieu, has positioned these receptors as pivotal therapeutic targets. Although a large number of drugs targeting GPCRs are currently available, significant efforts have been directed towards understanding receptor properties, with the goal of identifying and designing improved receptor ligands. Recent advances in GPCR pharmacology have demonstrated that different ligands binding to the same receptor can activate discrete sets of downstream effectors, a phenomenon known as `ligand-directed signal specificity', which is currently being explored for drug development due to its potential therapeutic advantage. Emerging studies suggest that GPCR responses can also be modulated by contextual factors, such as interactions with other GPCRs. Association between different GPCR types leads to the formation of complexes, or GPCR heteromers, with distinct and unique signalling properties. Some of these heteromers activate discrete sets of signalling effectors upon activation by the same ligand, a phenomenon termed `heteromer-directed signalling specificity'. This has been shown to be involved in the physiological role of receptors and, in some cases, in disease-specific dysregulation of a receptor effect. Hence targeting GPCR heteromers constitutes an emerging strategy to select receptor-specific responses and is likely to be useful in achieving specific beneficial therapeutic effects.
Keywords: adrenergic receptor, β-arrestin, G-protein-coupled receptor (GPCR), heteromerization, mitogen-activated protein kinase (MAPK), signal specificity
INTRODUCTION
GPCRs (G-protein-coupled receptors), also called seven-transmembrane receptors or heptahelical receptors, are a large family of cell-surface proteins capable of binding a diverse array of molecules (i.e. photons, ions, nucleotides, amino acids, amines and peptides). At the interface between the extracellular and the intracellular milieu, they are involved in the regulation of nearly every physiological process by converting extracellular stimuli into intracellular responses. GPCRs account for up to 50 % of currently marketed drugs [1–4] and continue to be the focus of intense research in drug development. New concepts that serve as the basis for the search and design of improved ligands have challenged classical pharmacological theories such as that of ligand intrinsic efficacy. For example, it was classically thought that ligand binding to a receptor stimulates or inhibits all receptor functions equally and consistently for each ligand at a given receptor, irrespective of the environment. However, the lack of consistency between this theory and the more complex receptor signalling behaviour observed in experiments led to the formulation of new concepts, for example that drugs exert their effects in a system-dependent manner. One of the most widely explored ideas is that of functional selectivity: ligands induce or stabilize unique ligand-specific receptor conformations that can result in differential activation of signal transduction pathways (reviewed in [5]). Moreover, association between different GPCR types, or heteromerization, is emerging as a new mechanism that can regulate GPCR function. Receptor–receptor interaction is thought to result in the stabilization of specific receptor conformations, conducive to coupling to discrete effectors, a mechanism also called heteromer-directed signal specificity. In the present review, we will document these novel mechanisms which allow for the fine-tuning of GPCR responses and open new areas of research for drug discovery.
LIGAND-DIRECTED SIGNAL SPECIFICITY
Over the last two decades, a large amount of data has indicated that different ligands for one receptor are capable of activating distinct, yet overlapping, groups of downstream effectors. This has led to the new idea that a ligand's intrinsic efficacy does not adequately match experimental observations, and has led to the proposal of additional novel mechanisms that would more accurately reflect experimental data. According to this theory, a receptor can adopt different active conformations [6] induced and/or stabilized by ligands, resulting in activation of distinct effectors associated with that receptor [5,7–10]. The accumulating evidence for multiple signalling states of GPCRs has been reviewed recently [11–14].
Studies of the β2-AR (adrenergic receptor), a prototypical GPCR, have contributed greatly to the concept of ligand-directed signal specificity. Early studies tested the hypothesis that agonists with different properties (full or partial agonists) could lead to different ligand-induced receptor conformations, and that this could be reflected by the ability of these ligands to induce differential receptor phosphorylation. In vitro treatment of the β2-AR with different ligands, such as the full agonist isoprenaline (isoproterenol) and various partial agonists with different efficacies, revealed a linear correlation between adenylate cyclase activity and receptor phosphorylation [15]. These results were confirmed by studies that demonstrated in intact cells the phosphorylation of β2-AR at different sites in response to isoprenaline, adrenaline (epinephrine) and dopamine [16], suggesting agonist-dependent differential regulation of β2-AR phosphorylation. Adoption of different active conformations by the β2-AR was explored further using other approaches. Fluorescence lifetime analysis of a reporter fluorophore covalently attached to the G-protein-coupling domain of the β2-AR revealed that the conformation induced by a full agonist could be distinguished from that induced by a partial agonist [17]. Moreover, fluorescence quenching of fluorescein-tagged β2-AR, a technique that reports agonist-induced conformational changes in the receptor, demonstrated that a change in the fluorescence intensity of fluorescein–β2-AR was proportional to the biological efficacy of the agonist [18]. This confirmed the notion that GPCRs are capable of adopting different active conformations and that specific ligands can induce and/or stabilize such conformations.
Another type of ligand-directed signal specificity has been uncovered using the β2-AR. Drake and co-workers [19] identified several ligands that displayed a significant bias towards β-arrestin signalling. These compounds were able to stimulate β-arrestin translocation and signalling to a much greater extent relative to their efficacy for G-protein-dependent activity [19]. These β-arrestin-biased agonists harboured a common structural feature supporting the notion that the stabilization of a specific receptor conformation conducive to β-arrestin-dependent activity is conferred by specific structural motifs of these ligands (that could induce/stabilize a discrete receptor conformation). Another study comparing the β-arrestin bias between 16 β-AR antagonists (for classical G-protein-mediated signalling) revealed that carvedilol functions as an agonist by recruiting β-arrestin and signalling in a β-arrestin-dependent manner [20]. Considering that carvedilol is uniquely effective in the treatment of heart failure, it is likely that arrestin-biased β-AR ligands could be useful drugs for the treatment of heart failure. This suggests an important clinical role for ligand-directed signal specificity. Similarly, other compounds that have been shown to act as inverse agonists for one signalling pathway can behave as agonists for another signalling pathway. ICI 118,551, an inverse agonist for the β2-AR/Gs-coupled cAMP response, was shown to act as an agonist in the G-protein-independent β-arrestin-mediated ERK (extracellular-signal-regulated kinase) MAPK (mitogen-activated protein kinase) signalling pathway [21,22]. These ligands, by activating distinct pathways, support the concept of ligand-directed signal specificity. This underscores the importance of testing ligands for a variety of biological responses to assess their biological potential. Taken together, the large amount of data collected in studies focusing on the β2-AR have demonstrated that the notion of ligand-directed signal specificity can be observed and measured, and is likely to be useful in the design of ligands with targeted effects.
Agonist-specific signalling has been reported for a number of other receptors, indicating that this is a generalizable phenomenon among GPCRs. The large portfolio of adrenergic ligands has been helpful in defining ligand-directed signalling and changes in receptor conformation. For example, the β1-AR ligands isoprenaline, bucindolol and propranolol have full, partial and inverse agonist properties for the adenylate cyclase pathway respectively. However, they all behave as agonists for the MAPK pathway. These interesting properties were used to probe ligand-specific stabilization of receptor conformation using BRET (bioluminescence resonance energy transfer) between the receptor and the G-protein [23]. These studies demonstrated that discrete local conformational changes can selectively promote the recruitment of distinct downstream effectors involving either or both G-protein-dependent and -independent signalling [23].
Recruitment of distinct downstream effectors was also shown in the case of the bombesin and cannabinoid receptors. For example, the bombesin receptor has been shown to be able to couple to different G-proteins, depending on the agonist. Stimulation of the bombesin receptor with a substance P analogue was able to activate MAPK through a Gαi-mediated pathway, whereas stimulation with bombesin was able to activate JNK (c-Jun N-terminal kinase) signalling through a Gα12-mediated pathway [24]. Several studies have taken advantage of the large number of CB1R (cannabinoid 1 receptor) agonists and their structural diversity to examine ligand specificity of CB1R coupling. For example, Hu210 was found to couple to either Gαi and Gαo to elicit maximal activation [25]. In contrast, WIN 55212-2 and AEA (anandamide) induced a stronger coupling to Gαi as compared with Gαo, whereas THC (Δ9-tetrahydrocannabinol) produced submaximal responses for both Gαi and Gαo. Another study reported the ability of WIN 55212-2 to elicit CB1R coupling to Gαq, leading to an intracellular calcium response [26]. These examples underscore the fact that the nature of the agonist is critical for the promotion of receptor coupling to specific G-proteins, and that a given receptor can lead to an array of downstream signalling, depending on the nature of the activating ligand.
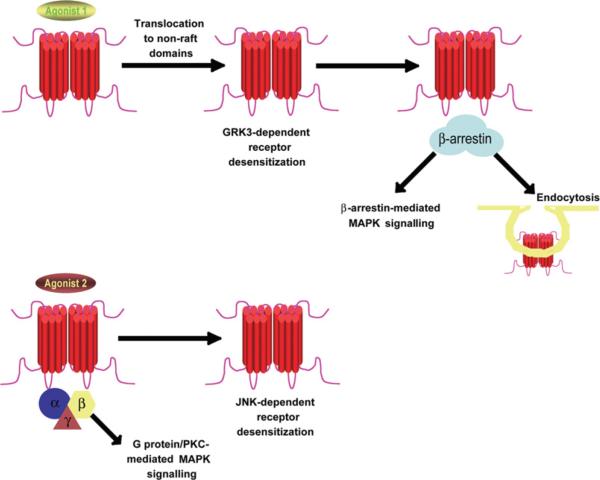
Studies with opioid receptors have also revealed agonist-directed signal specificity (summarized in Figure 1). MOR (μ-opioid receptor) agonists have been shown to have different properties in terms of their ability to induce receptor endocytosis, arrestin recruitment and membrane microdomain sequestration, indicating a marked potential for different opioid ligands to affect specific receptor responses. Although the endogenous ligands enkephalins and exogenous ligand ethorphine were shown to induce MOR internalization, morphine had no effect of MOR endocytosis in transfected HEK (human embryonic kidney)-293 cells [27] and in neurons [28,29]. This was later attributed to differences in a ligand's ability to induce β-arrestin translocation to activated MOR [30] (since β-arrestin binding to GPCRs contributes to the promotion of endocytosis [31]). In agreement with the poor propensity of morphine to recruit β-arrestin, morphine activates MAPK via the G-protein/PKC (protein kinase C)-dependent pathway, but not the β-arrestin-dependent pathway, whereas other ligands such as ethorphine and fentanyl activate MAPK in an arrestin-dependent manner [32]. In addition, ethorphine, but not morphine, was able to induce MOR translocation to non-raft domains, allowing arrestin-mediated MAPK phosphorylation [33].
Figure 1. Ligand-mediated signal specificity of MOR.
Abundant literature has characterized differential effects of distinct MOR ligands on receptor responses, as summarized in this Figure. Binding of one class of agonists (such as etorphine and enkephalins) to MOR leads to translocation of the receptor to non-raft domains [33], GRK3-dependent desensitization [37] and recruitment of β-arrestin, with the initiation of receptor endocytosis [27–29] and β-arrestin-mediated signalling [33]. In contrast, binding of another class of agonists (such as morphine) to MOR induces G-protein-mediated signalling and JNK-dependent desensitization [37], and does not lead to translocation to non-raft domains, β-arrestin recruitment or endocytosis [27–29,32,33].
Such information can be used to develop new agonists with selective properties. This has been the case with the discovery of a new MOR agonist, herkinorin, that, compared with morphine, exhibits a lesser propensity to recruit β-arrestin [34]. Since β-arrestin2 plays an important role in the development of morphine-induced tolerance, constipation and respiratory depression [35,36], identifying drugs that can activate the receptor without recruiting β-arrestin may be a promising step in the development of opiate analgesics providing pain relief without the adverse side effects.
In a recent study, ligand-directed signalling was extended to distinct mechanisms of receptor desensitization observed for two classes of MOR agonists [37]. Acute analgesic tolerance to morphine, morphine 6-glucuronide and buprenorphine was JNK-dependent, but not GRK3 (G-protein receptor kinase 3)-dependent. In contrast, analgesic tolerance to fentanyl, methadone and oxycodon was GRK3-dependent, but not JNK-dependent. Furthermore, acute MOR desensitization after morphine treatment, but not after DAMGO {[D-Ala2-MePhe4-Gly(ol)5]enkephalin} treatment, was also blocked by JNK inhibition. These suggest that some ligands, through specific activation of JNK signalling, play a role in the mechanism of GPCR desensitization, with physiological consequences on the regulation of analgesia [37].
DOR (δ-opioid receptor) properties have also been shown to be modulated by ligands. Ethorphine, a non-selective DOR agonist, was shown to activate DOR by coupling to Gαi1,Gαi2,Gαi3 and to pertussis toxin-insensitive Gα subunits. In contrast, the peptidic agonists DPDPE ([D-Pen2,5]-enkephalin) and deltorphin mainly led to signalling by coupling to Gαi2 and Gαo2 subunits [38]. A study comparing morphine and ethorphine found that only ethorphine, but not morphine, could induce DOR phosphorylation, β-arrestin recruitment and endocytosis [39]. These observations suggest that morphine and ethorphine have conserved properties with respect to stabilization of receptor active conformations, conducive or not to β-arrestin recruitment, irrespective of the receptor (MOR and DOR). In addition, an interesting study documenting differences between DPDPE- and TIPP (H-Tyr-Tic-Phe-Phe-OH)-mediated effects found that, although both ligands inhibited adenylate cyclase and activated MAPK, only DPDPE induced desensitization and internalization of DOR. The authors demonstrated further that DPDPE, but not TIPP, activated specific downstream effectors such as Src. The latter activated β-arrestin by promoting β-arrestin recruitment and by increasing β-arrestin dephosphorylation at the plasma membrane [40]. These examples highlight the number of mechanisms through which different ligands stimulating a given receptor can activate various sets of downstream effectors.
Comparison of the involvement of β-arrestin2 in the response to two serotonin receptor [5-HT2AR (5-hydroxytryptamine 2A receptor)] agonists [serotonin and DOI (2,5-dimethoxy-4-iodoamphetamine)], demonstrated opposite behaviours [41]. β-Arrestin2 was shown to be required for endocytosis, MAPK signalling and in vivo behavioural responses to serotonin; in contrast, cellular and behavioural responses to DOI were not affected by the absence of β-arrestin2 [41]. These findings suggest that serotonin and DOI lead to distinct patterns of activation of downstream effectors.
Differences in a ligand's ability to induce receptor endocytosis has also been reported for the apelin receptor. The active peptide ligand K17F leads to inhibition of cAMP production and induces apelin receptor internalization. Although successive N-terminal amino-acid deletions of the K17F peptide do not alter binding or the inhibition of cAMP production, they lead to a progressive reduction of receptor internalization [42]. This implies that a structural determinant in the N-terminus of the K17F peptide is necessary for stabilizing receptor conformations conducive to signalling and endocytosis. Further characterization of K17F and shorter peptides could indicate whether receptor endocytosis is due to a bias of these different peptides towards β-arrestin.
Although the majority of the studies carried out thus far (as described above) have mostly used pharmacological approaches to assess multiple receptor active conformation states, recent studies using biophysical techniques, such as intramolecular FRET (Förster resonance energy transfer), have examined real-time changes in receptor active conformations. These studies provide direct evidence for the capacity of a receptor to adopt different active conformations. Using a receptor C-terminally labelled with CFP (cyan fluorescent protein) and upon introduction of a fluorescein arsenical hairpin binder at different sites in the third intracellular loop of the α2-AR, Zurn et al. [43] were able to measure differences in FRET efficiencies that reflect different rearrangements within the receptor structure upon binding of a variety of ligands. They showed that partial and full agonists induce different conformational signatures of the receptor [43].
Interestingly, using a similar intramolecular FRET approach, a recent study has shown that allosteric ligands (compounds that can selectively modulate the binding and activity of receptor agonists) were capable of altering the agonist-occupied receptor conformation [44]. Agonists of the M2 acetylcholine receptor, such as acetylcholine and carbachol, induced rapid changes in intramolecular FRET, supporting agonist-induced conformational changes in the M2 acetylcholine receptor. The allosteric ligands gallamine and dimethyl-W84, while not inducing changes in FRET when given alone, increased FRET when given in the presence of an agonist [44]. This suggests that allosteric ligands modulate receptor activity through conformational changes transmitted to the orthosteric site or directly to the effector coupling sites. Taken together, these studies demonstrate that receptors are capable of adopting several active conformations that are thought to activate a discrete set of downstream effectors. Ligands that induce and/or stabilize specific conformations direct receptor signalling to a specific pathway that has proven in some cases to be clinically relevant. Developing ligands capable of activating a given group of signalling effectors is likely to yield compounds with beneficial therapeutic effects.
HETEROMER-DIRECTED SIGNAL SPECIFICITY
The notion that ligand binding to an allosteric site is able to induce receptor conformational change suggests that protein interactions could have a similar effect on receptors' conformations. This is particularly relevant in the case of receptor–receptor interactions, or heteromerization, where one protomer is likely to induce changes in the active conformation of its interaction partner. Indeed, accumulating evidence indicates that, within GPCR heteromers, protomers influence one another, leading to novel pharmacological properties of the receptors. Many studies have examined the modulation of pharmacology by receptor–receptor association, and recent studies have focused on the modulation of receptor signalling by heteromerization, pointing to a role for heteromer-specific signalling in receptor function. Finally, studies using current biophysical techniques have been able to demonstrate changes in receptor conformation by heteromerization through ligand binding to the associated receptor. In this section, we will describe the main findings relevant to heteromer-directed signal specificity and discuss their importance for the development of therapeutic agents targeting GPCRs.
New pharmacological properties of GPCR heteromers
Many studies have reported that receptor heteromerization leads to new binding properties [45–50], suggesting that heteromerization induces an alteration in the conformation of the ligand-binding site. The identification of an agonist, 6′-GNTI [6′-guanidinyl-17-(cyclopropylmethyl)-6,7-dehydro-4,5a-epoxy-3,14-dihydroxy-6,7-2′,3′-indolomorphinan], that shows a relative selectivity for DOR–KOR (κ-opioid receptor) heteromers [51] supports the notion that receptors within a heteromer are capable of adopting active conformations that are absent in their homomeric counterparts.
G-protein activation
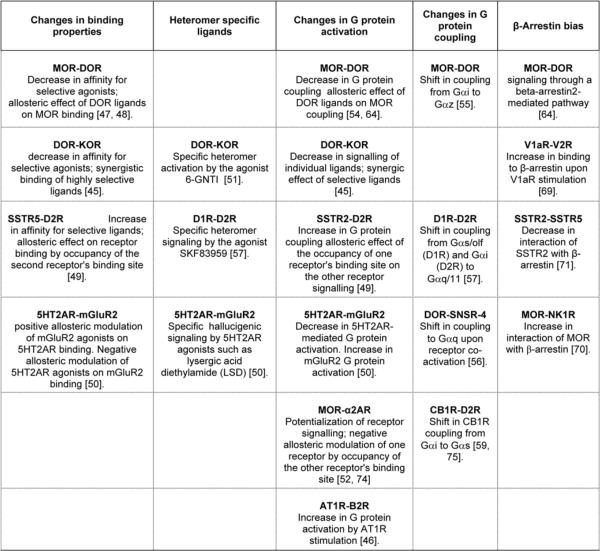
There are several examples showing that heteromerization alters receptor activity and G-protein activation (Figure 2). For instance, MOR association with DOR decreases MOR activity in response to selective agonists [48], MOR association with α2-AR increases its activity [52], and association with CB1R does not affect MOR activity [53]. Heteromerization between the AngII (angiotensin II) receptor AT1R (type 1 AngII receptor) and the bradykinin receptor B2 reportedly increases the G-protein coupling of AT1R in response to its ligand AngII [46]. Thus, as G-protein activation is typically mediated through stabilization of specific GPCR-active conformations, in the case of heteromers, interaction with the associated receptor probably contributes to this stabilization of active conformations.
Figure 2. Examples of the alteration of receptor properties by heteromerization.
A mechanism of allosteric modulation between two protomers has also been proposed since receptor occupancy can lead to an increased G-protein activation of the associated receptor within a heteromer. For example, G-protein activation by MOR ligands is increased by the occupancy of DOR in the context of the MOR–DOR heteromer [54]. Similarly, signalling by the SST (somatostatin) analogue SST-14 is increased as a result of receptor occupancy by the D2R (dopamine D2 receptor) agonist quinpirole in the context of the D2R–SSTR5 (SST receptor 5) heteromer [49]. Furthermore, activation of Gαq/11 by the serotonin receptor 5HT2AR is decreased, and activation of Gαi by 5HT2AR is markedly enhanced by heteromerization with mGluR2 (metabotropic glutamate receptor type 2) [50]. The specific conformation of 5HT2AR conferred by heteromerization with mGluR2 was shown to lead to a unique pattern of downstream effector activation (decreased Gαq/11 and increased Gαi coupling) upon stimulation with 5HT2AR agonists such as LSD (lysergic acid diethylamide); this was proposed to be responsible for the hallucinogenic effect of these compounds [50], suggesting that heteromer-specific active receptor conformations play a critical role in drug effects in vivo.
Switch in G-protein coupling
Accumulating evidence indicates that heteromerization can lead to a switch in G-protein coupling (Figure 2). In some cases, such as with the MOR–DOR heteromer, this has been shown to be a ligand-independent phenomenon (i.e. the receptors in the heteromer are coupled to distinct G-proteins without ligand stimulation). Although MOR and DOR both couple to Gαi when individually expressed, the heteromer has been shown in some cases to selectively couple to Gαz [55]. In other cases, ligand binding contributes to the coupling selectivity of the heteromer. An interesting study reported the behaviour of the DOR–SNSR-4 (sensory neuron-specific receptor-4) heteromer. Heteromerization of DOR–SNSR-4 did not affect the G-protein coupling of individual receptors (activation of Gαq for SNSR and of Gαi/o for DOR) when individual receptors were specifically stimulated. However, simultaneous activation of the two protomers by the mixed agonist BAM22, or by two receptor-specific agonists, led to a switch in signalling from a Gαi/o- to Gαq-mediated signalling pathway as shown by activation of PLC (phospholipase C) (Gαq-mediated) without inhibition of the adenylate cyclase (Gαi/o-mediated) [56]. Studies on D1R (dopamine D1 receptor)–D2R heteromers have demonstrated that heteromerization leads to a switch from a Gαs/olf (D1R) or Gαi (D2R) to a Gαq/11-mediated response [57,58]. In addition, these studies have provided evidence for the ability of the compound SKF83959, previously known as a D1R agonist, to bind the D1R–D2R heteromer and activate the Gαq pathway in the brain [57]. In addition, heteromerization between CB1R and D2R reportedly involves a switch in CB1R coupling from Gαi to Gαs upon co-activation of D2R [59]. CB1R represents an example of a receptor whose activity can be regulated by ligandand heteromer-directed signalling. It is likely that these different mechanisms contribute to the wide range of physiological effects reported for CB1R [60–63]. Taken together, these examples of switching in G-protein coupling suggest the exciting possibility of a diversification of receptor properties by heteromerization that could contribute to the regulation of receptor function in vivo.
β-Arrestin-biased heteromer signalling
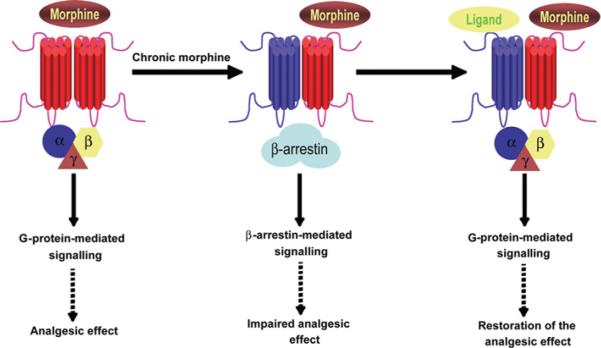
Although, as described above, ligands can stabilize receptor conformations specifically conducive to β-arrestin signalling, recent studies have also demonstrated a role for receptor heteromerization in the activation of β-arrestin-mediated signalling (summarized in Figure 3). Stimulation of the MOR–DOR heteromer with DAMGO (a MOR agonist) or deltorphin II (a DOR agonist), which each stimulates G-protein-mediated signalling when activating MOR or DOR in isolation, leads to a β-arrestin-dependent MAPK phosphorylation [64]. It was also found that this switch from G-protein- to β-arrestin-mediated signalling, in turn, results in the cytoplasmic retention of phosphorylated MAPK, leading to differential activation of transcription factors (as compared with the MOR homomers) [64]. Interestingly, mice lacking β-arrestin2 exhibit amplified and prolonged morphine-induced analgesia [65] and do not develop tolerance to morphine [35]; these observations are in contradiction with the lack of interaction between morphine and β-arrestin in vitro. This can be examined in light of MOR–DOR heteromer-biased arrestin signalling. Both β-arrestin2-knockout [35] and DOR-knockout mice [66] exhibit enhanced antinociceptive responses to morphine and impaired development of tolerance, suggesting that the role of β-arrestin2 and DOR in regulating morphine analgesia and tolerance could be the result of MOR–DOR heteromer-specific signalling that involves β-arrestin2. This mechanism has been recently substantiated by the enhanced detection of MOR–DOR heteromers after chronic morphine treatment, using a heteromer selective antibody [67]. Interestingly, co-stimulation of MOR–DOR with an MOR agonist together with a low concentration of a DOR antagonist leads to a dissociation of β-arrestin from the heteromer and to an increase in G-protein activation [54,64]. Moreover, the observation that low doses of DOR antagonists potentiate morphine-mediated analgesia in vivo [54,68] suggests that MOR–DOR heteromers play a pivotal role in the regulation of pain and that ligands targeting the heteromer could function as potent analgesics.
Figure 3. Heteromer-mediated signal specificity: the case of the MOR homomers and MOR–DOR heteromers.
Under normal physiological conditions, MOR is found mostly in the homomeric state. Morphine stimulation induces G-protein-mediated signalling and leads to analgesic effects. In contrast, upon chronic treatment with morphine the abundance of MOR–DOR heteromers increases [67]. MOR–DOR heteromers recruit β-arrestin, which alters MOR signalling [64]; this is thought to contribute to the impaired analgesic effect of morphine upon its chronic administration. Occupancy of the DOR-binding site allows for the restoration of MOR signalling and analgesic effects of morphine [54,64].
Interaction between GPCRs and β-arrestin has been shown to be regulated by heteromerization of other receptor pairs (Figure 2). In the case of the vasopressin receptors, while both V1AR (vasopressin 1A receptor) and V2R (vasopressin 2 receptor) can be endocytosed through a β-arrestin-mediated mechanism, β-arrestin dissociates rapidly from V1AR, allowing its rapid recycling to the plasma membrane while β-arrestin remains associated with V2R in endosomes. Heteromerization of V1ARand V2R alters β-arrestin recruitment and receptor endocytosis, such that the two receptors are endocytosed as stable heteromers upon activation. In this case, both receptors co-traffic with β-arrestin in endosomes where the stable interaction inhibits the recycling of V1AR to the plasma membrane, thus conferring a V2R-like endocytotic and recycling pattern to the V1AR–V2R heteromer [69]. Similarly, in the case of the MOR–NK1 (tachykinin receptor 1) heteromer, stimulation with an agonist to one of the receptors leads to cross-phosphorylation of the other receptor [70]. SP (an NK1 agonist) and DAMGO (a MOR agonist) induce co-internalization of NK1 and MOR into the same endosomal compartment in a stable complex with β-arrestin [70]. This suggests new properties of MOR within the MOR–NK1 heteromer, since MOR alone does not form stable complexes with β-arrestin. A report documenting heteromerization between SSTR2 and SSTR5 depicts an opposite scenario. Whereas activation of SSTR2 results in the recruitment and stable association with β-arrestin followed by receptor internalization and accumulation of receptor-arrestin complexes in intracellular vesicles, heteromerization with SSTR5 increases the recycling rate of internalized SSTR2 by destabilizing its interaction with β-arrestin [71]. This modulation of β-arrestin interaction with SSTR2 probably reduces receptor desensitization, as supported by the observation that L-779,976, a SSTR2-selective agonist, more efficiently inhibits adenylate cyclase, activates MAPK and induces the cyclin-dependent kinase inhibitor p27Kip1 in heteromer-expressing cells, compared with cells expressing SSTR2 alone [71]. These studies indicate a special role for β-arrestin in directing signals from interacting GPCRs and suggest that β-arrestin may play a pivotal role in orchestrating fine-tune signalling regulation of GPCR heteromers.
These findings, while providing compelling evidence for heteromer-directed signalling specificity, except in the case of the detection of MOR–DOR heteromer with a selective antibody [67], are indirect and do not rule out the possibility that some of the observed effects are not via a direct receptor–receptor interaction.
Direct evidence for heteromerization-induced conformational change
Using modern biophysical technology, a few studies have been able to directly show the impact of heteromerization on receptor conformation, supporting results from other approaches that suggest that receptor–receptor interaction and allosteric modulation could contribute to the stabilization or induction of unique receptor conformations. One set of studies utilized a heteromeric complex in which each of the protomers can be selectively activated by a structurally different ligand, such as heteromers between the wild-type BLT1 (leukotriene B4 receptor) and a BLT1-ALXR (lipoxin A4 receptor) chimaera (which is not activatable by leukotriene B4 but can be activated by ALXR agonists). Labelling the BLT1 protomer with a single 5-hydroxytryptophan allowed detection of changes in receptor conformation, since a change in fluorescence emission indicates receptor conformational changes. This approach demonstrated that agonist binding to one of the protomers leads to conformational changes on the other protomer, in agreement with the model of a cross-conformational change transmitted between the two protomers within a receptor dimer [72,73]. However, agonist binding did not trigger full activation of the ligand-free protomer, suggesting the absence of a transactivation mechanism, where the signal would be transmitted from the ligand-activated protomer to the neighbouring protomer in the receptor dimer [72,73]. Another study has used FRET microscopy to examine early molecular events underlying the interaction between MOR and α2A-AR [74]. Changes in α2A-AR conformation was measured by changes in intramolecular FRET (as described above), and was examined in the context of the co-expression with MOR and treatment with noradrenaline (norepinephrine) alone, or in combination with morphine. This study showed that morphine binding to MOR affects the noradrenaline-bound α2A-AR conformation. The authors demonstrate a conformational change that propagated from one receptor to the other, leading to a rapid inactivation of the second receptor. This is in agreement with the decrease in signalling by Gαi and the downstream MAPK cascade observed upon co-stimulation of the two protomers [52]. Biophysical approaches confirm that heteromerization can induce conformational changes in interacting receptors, supporting heteromer-directed signal specificity as the basis for the new pharmacological properties of the receptors within heteromeric complexes.
CONCLUSIONS
The studies documented in the present review underscore the complexity of the regulation of GPCR signalling. The nature of the ligand as well as receptor–receptor interaction can both lead to selective activation of downstream effectors, with emerging roles in pathophysiology. Characterization of specific signalling pathways responsible for a given physiological effect and identification of the select signalling pathways activated by a given ligand or in the context of a specific receptor heteromer will be helpful in designing new pathway-selective therapeutic agents.
ACKNOWLEDGEMENTS
We thank Ittai Bushlin and Maribel Lim for careful reading of the manuscript prior to submission.
FUNDING The authors' work was supported by the National Institutes of Health [grant numbers DA08863, DA01952, GM071558 (to L.A.D.), AA017067 (to R.R.)].
Abbreviations used
- ALXR
lipoxin A4 receptor
- AngII
angiotensin II
- AR
adrenergic receptor
- AT1R
type 1 AngII receptor
- BLT1
leukotriene B4 receptor
- CB1R
cannabinoid 1 receptor
- D1R
dopamine D1 receptor
- D2R
dopamine D2 receptor
- DAMGO
[D-Ala2-MePhe4-Gly(ol)5]enkephalin
- DOI
2,5-dimethoxy-4-iodoamphetamine
- DOR
δ-opioid receptor
- DPDPE
[D-Pen2,5]-enkephalin
- ERK
extracellular-signal-regulated kinase
- FRET
Förster resonance energy transfer
- GPCR
G-protein-coupled receptor
- GRK3
G-protein receptor kinase 3
- 5-HT2AR
5-hydroxytryptamine 2A receptor
- JNK
c-Jun N-terminal kinase
- KOR
κ-opioid receptor
- MAPK
mitogen-activated protein kinase
- mGluR2
metabotropic glutamate receptor type 2
- MOR
μ-opioid receptor
- NK1
tachykinin receptor 1
- PKC
protein kinase C
- SNSR-4
sensory neuron-specific receptor-4
- SST
somatostatin
- SSTR
SST receptor
- TIPP
H-Tyr-Tic-Phe-Phe-OH
- V1AR
vasopressin 1A receptor
- V2R
vasopressin 2 receptor
REFERENCES
- 1.Ma P, Zemmel R. Value of novelty? Nat. Rev. Drug Discovery. 2002;1:571–572. doi: 10.1038/nrd884. [DOI] [PubMed] [Google Scholar]
- 2.Drews J. Genomic sciences and the medicine of tomorrow. Nat. Biotechnol. 1996;14:1516–1518. doi: 10.1038/nbt1196-1516. [DOI] [PubMed] [Google Scholar]
- 3.Hill SJ. G-protein-coupled receptors: past, present and future. Br. J. Pharmacol. 2006;147(Suppl. 1):S27–S37. doi: 10.1038/sj.bjp.0706455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pierce KL, Premont RT, Lefkowitz RJ. Seven-transmembrane receptors. Nat. Rev. Mol. Cell Biol. 2002;3:639–650. doi: 10.1038/nrm908. [DOI] [PubMed] [Google Scholar]
- 5.Urban JD, Clarke WP, von Zastrow M, Nichols DE, Kobilka B, Weinstein H, Javitch JA, Roth BL, Christopoulos A, Sexton PM, et al. Functional selectivity and classical concepts of quantitative pharmacology. J. Pharmacol. Exp. Ther. 2007;320:1–13. doi: 10.1124/jpet.106.104463. [DOI] [PubMed] [Google Scholar]
- 6.Kenakin T. Functional selectivity through protean and biased agonism: who steers the ship? Mol. Pharmacol. 2007;72:1393–1401. doi: 10.1124/mol.107.040352. [DOI] [PubMed] [Google Scholar]
- 7.Kenakin T. Agonist-receptor efficacy. I. Mechanisms of efficacy and receptor promiscuity. Trends Pharmacol. Sci. 1995;16:188–192. doi: 10.1016/s0165-6147(00)89020-3. [DOI] [PubMed] [Google Scholar]
- 8.Kenakin T. Agonist-receptor efficacy. II. Agonist trafficking of receptor signals. Trends Pharmacol. Sci. 1995;16:232–238. doi: 10.1016/s0165-6147(00)89032-x. [DOI] [PubMed] [Google Scholar]
- 9.Roth BL, Chuang DM. Multiple mechanisms of serotonergic signal transduction. Life Sci. 1987;41:1051–1064. doi: 10.1016/0024-3205(87)90621-7. [DOI] [PubMed] [Google Scholar]
- 10.Violin JD, Lefkowitz RJ. β-Arrestin-biased ligands at seven-transmembrane receptors. Trends Pharmacol. Sci. 2007;28:416–422. doi: 10.1016/j.tips.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 11.Perez DM, Karnik SS. Multiple signaling states of G-protein-coupled receptors. Pharmacol. Rev. 2005;57:147–161. doi: 10.1124/pr.57.2.2. [DOI] [PubMed] [Google Scholar]
- 12.Kenakin T. Allosteric theory: taking therapeutic advantage of the malleable nature of GPCRs. Curr. Neuropharmacol. 2007;5:149–156. doi: 10.2174/157015907781695973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kobilka BK, Deupi X. Conformational complexity of G-protein-coupled receptors. Trends Pharmacol. Sci. 2007;28:397–406. doi: 10.1016/j.tips.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 14.Hoffmann C, Zurn A, Bunemann M, Lohse MJ. Conformational changes in G-protein-coupled receptors: the quest for functionally selective conformations is open. Br. J. Pharmacol. 2008;153(Suppl. 1):S358–S366. doi: 10.1038/sj.bjp.0707615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benovic JL, Staniszewski C, Mayor F, Jr, Caron MG, Lefkowitz RJ. β-Adrenergic receptor kinase. Activity of partial agonists for stimulation of adenylate cyclase correlates with ability to promote receptor phosphorylation. J. Biol. Chem. 1988;263:3893–3897. [PubMed] [Google Scholar]
- 16.Trester-Zedlitz M, Burlingame A, Kobilka B, von Zastrow M. Mass spectrometric analysis of agonist effects on posttranslational modifications of the β-2 adrenoceptor in mammalian cells. Biochemistry. 2005;44:6133–6143. doi: 10.1021/bi0475469. [DOI] [PubMed] [Google Scholar]
- 17.Ghanouni P, Gryczynski Z, Steenhuis JJ, Lee TW, Farrens DL, Lakowicz JR, Kobilka BK. Functionally different agonists induce distinct conformations in the G protein coupling domain of the β2 adrenergic receptor. J. Biol. Chem. 2001;276:24433–24436. doi: 10.1074/jbc.C100162200. [DOI] [PubMed] [Google Scholar]
- 18.Ghanouni P, Steenhuis JJ, Farrens DL, Kobilka BK. Agonist-induced conformational changes in the G-protein-coupling domain of the β2 adrenergic receptor. Proc. Natl. Acad. Sci. U.S.A. 2001;98:5997–6002. doi: 10.1073/pnas.101126198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Drake MT, Violin JD, Whalen EJ, Wisler JW, Shenoy SK, Lefkowitz RJ. β-Arrestin-biased agonism at the β2-adrenergic receptor. J. Biol. Chem. 2008;283:5669–5676. doi: 10.1074/jbc.M708118200. [DOI] [PubMed] [Google Scholar]
- 20.Wisler JW, DeWire SM, Whalen EJ, Violin JD, Drake MT, Ahn S, Shenoy SK, Lefkowitz RJ. A unique mechanism of β-blocker action: carvedilol stimulates β-arrestin signaling. Proc. Natl. Acad. Sci. U.S.A. 2007;104:16657–16662. doi: 10.1073/pnas.0707936104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Azzi M, Charest PG, Angers S, Rousseau G, Kohout T, Bouvier M, Pineyro G. β-arrestin-mediated activation of MAPK by inverse agonists reveals distinct active conformations for G protein-coupled receptors. Proc. Natl. Acad. Sci. U.S.A. 2003;100:11406–11411. doi: 10.1073/pnas.1936664100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baker JG, Hall IP, Hill SJ. Agonist and inverse agonist actions of β-blockers at the human β2-adrenoceptor provide evidence for agonist-directed signaling. Mol. Pharmacol. 2003;64:1357–1369. doi: 10.1124/mol.64.6.1357. [DOI] [PubMed] [Google Scholar]
- 23.Galandrin S, Oligny-Longpre G, Bonin H, Ogawa K, Gales C, Bouvier M. Conformational rearrangements and signaling cascades involved in ligand-biased mitogen-activated protein kinase signaling through the β1-adrenergic receptor. Mol. Pharmacol. 2008;74:162–172. doi: 10.1124/mol.107.043893. [DOI] [PubMed] [Google Scholar]
- 24.MacKinnon AC, Waters C, Jodrell D, Haslett C, Sethi T. Bombesin and substance P analogues differentially regulate G-protein coupling to the bombesin receptor. Direct evidence for biased agonism. J. Biol. Chem. 2001;276:28083–28091. doi: 10.1074/jbc.M009772200. [DOI] [PubMed] [Google Scholar]
- 25.Glass M, Northup JK. Agonist selective regulation of G proteins by cannabinoid CB1 and CB2 receptors. Mol. Pharmacol. 1999;56:1362–1369. doi: 10.1124/mol.56.6.1362. [DOI] [PubMed] [Google Scholar]
- 26.Lauckner JE, Hille B, Mackie K. The cannabinoid agonist WIN55, 212-212 increases intracellular calcium via CB1 receptor coupling to Gq/11 G proteins. Proc. Natl. Acad. Sci. U.S.A. 2005;102:19144–19149. doi: 10.1073/pnas.0509588102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keith DE, Murray SR, Zaki PA, Chu PC, Lissin DV, Kang L, Evans CJ, von Zastrow M. Morphine activates opioid receptors without causing their rapid internalization. J. Biol. Chem. 1996;271:19021–19024. doi: 10.1074/jbc.271.32.19021. [DOI] [PubMed] [Google Scholar]
- 28.Keith DE, Anton B, Murray SR, Zaki PA, Chu PC, Lissin DV, Monteillet-Agius G, Stewart PL, Evans CJ, von Zastrow M. μ-Opioid receptor internalization: opiate drugs have differential effects on a conserved endocytic mechanism in vitro and in the mammalian brain. Mol. Pharmacol. 1998;53:377–384. [PubMed] [Google Scholar]
- 29.Sternini C, Spann M, Anton B, Keith DE, Jr, Bunnett NW, von Zastrow M, Evans C, Brecha NC. Agonist-selective endocytosis of μ opioid receptor by neurons in vivo. Proc. Natl. Acad. Sci. U.S.A. 1996;93:9241–9246. doi: 10.1073/pnas.93.17.9241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang J, Ferguson SS, Barak LS, Bodduluri SR, Laporte SA, Law PY, Caron MG. Role for G protein-coupled receptor kinase in agonist-specific regulation of μ-opioid receptor responsiveness. Proc. Natl. Acad. Sci. U.S.A. 1998;95:7157–7162. doi: 10.1073/pnas.95.12.7157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wolfe BL, Trejo J. Clathrin-dependent mechanisms of G protein-coupled receptor endocytosis. Traffic. 2007;8:462–470. doi: 10.1111/j.1600-0854.2007.00551.x. [DOI] [PubMed] [Google Scholar]
- 32.Zheng H, Loh HH, Law PY. β-Arrestin-dependent μ-opioid receptor-activated extracellular signal-regulated kinases (ERKs) translocate to nucleus in contrast to G protein-dependent ERK activation. Mol. Pharmacol. 2008;73:178–190. doi: 10.1124/mol.107.039842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zheng H, Chu J, Qiu Y, Loh HH, Law PY. Agonist-selective signaling is determined by the receptor location within the membrane domains. Proc. Natl. Acad. Sci. U.S.A. 2008;105:9421–9426. doi: 10.1073/pnas.0802253105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Groer CE, Tidgewell K, Moyer RA, Harding WW, Rothman RB, Prisinzano TE, Bohn LM. An opioid agonist that does not induce μ-opioid receptor–arrestin interactions or receptor internalization. Mol. Pharmacol. 2007;71:549–557. doi: 10.1124/mol.106.028258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bohn LM, Gainetdinov RR, Lin FT, Lefkowitz RJ, Caron MG. μ-Opioid receptor desensitization by β-arrestin-2 determines morphine tolerance but not dependence. Nature. 2000;408:720–723. doi: 10.1038/35047086. [DOI] [PubMed] [Google Scholar]
- 36.Raehal KM, Walker JK, Bohn LM. Morphine side effects in β-arrestin 2 knockout mice. J. Pharmacol. Exp. Ther. 2005;314:1195–1201. doi: 10.1124/jpet.105.087254. [DOI] [PubMed] [Google Scholar]
- 37.Melief EJ, Miyatake M, Bruchas MR, Chavkin C. Ligand-directed c-Jun N-terminal kinase activation disrupts opioid receptor signaling. Proc. Natl. Acad. Sci. U.S.A. 2010;107:11608–11613. doi: 10.1073/pnas.1000751107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Allouche S, Polastron J, Hasbi A, Homburger V, Jauzac P. Differential G-protein activation by alkaloid and peptide opioid agonists in the human neuroblastoma cell line SK-N-BE. Biochem. J. 1999;342:71–78. [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang J, Ferguson SS, Law PY, Barak LS, Caron MG. Agonistspecific regulation of δ-opioid receptor trafficking by G protein-coupled receptor kinase and β-arrestin. J. Recept. Signal Transduct. Res. 1999;19:301–313. doi: 10.3109/10799899909036653. [DOI] [PubMed] [Google Scholar]
- 40.Hong MH, Xu C, Wang YJ, Ji JL, Tao YM, Xu XJ, Chen J, Xie X, Chi ZQ, Liu JG. Role of Src in ligand-specific regulation of δ-opioid receptor desensitization and internalization. J. Neurochem. 2009;108:102–114. doi: 10.1111/j.1471-4159.2008.05740.x. [DOI] [PubMed] [Google Scholar]
- 41.Schmid CL, Raehal KM, Bohn LM. Agonist-directed signaling of the serotonin 2A receptor depends on β-arrestin-2 interactions in vivo. Proc. Natl. Acad. Sci. U.S.A. 2008;105:1079–1084. doi: 10.1073/pnas.0708862105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.El Messari S, Iturrioz X, Fassot C, De Mota N, Roesch D, Llorens-Cortes C. Functional dissociation of apelin receptor signaling and endocytosis: implications for the effects of apelin on arterial blood pressure. J. Neurochem. 2004;90:1290–1301. doi: 10.1111/j.1471-4159.2004.02591.x. [DOI] [PubMed] [Google Scholar]
- 43.Zurn A, Zabel U, Vilardaga JP, Schindelin H, Lohse MJ, Hoffmann C. Fluorescence resonance energy transfer analysis of α2a-adrenergic receptor activation reveals distinct agonist-specific conformational changes. Mol. Pharmacol. 2009;75:534–541. doi: 10.1124/mol.108.052399. [DOI] [PubMed] [Google Scholar]
- 44.Maier-Peuschel M, Frolich N, Dees C, Hommers LG, Hoffmann C, Nikolaev VO, Lohse MJ. A FRET-based M2 muscarinic receptor sensor reveals rapid kinetics of allosteric modulation. J. Biol. Chem. 2010;285:8793–8800. doi: 10.1074/jbc.M109.098517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jordan BA, Devi LA. G-protein-coupled receptor heterodimerization modulates receptor function. Nature. 1999;399:697–700. doi: 10.1038/21441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.AbdAlla S, Lother H, Quitterer U. AT1-receptor heterodimers show enhanced G-protein activation and altered receptor sequestration. Nature. 2000;407:94–98. doi: 10.1038/35024095. [DOI] [PubMed] [Google Scholar]
- 47.George SR, Fan T, Xie Z, Tse R, Tam V, Varghese G, O'Dowd BF. Oligomerization of μ-and δ-opioid receptors. Generation of novel functional properties. J. Biol. Chem. 2000;275:26128–26135. doi: 10.1074/jbc.M000345200. [DOI] [PubMed] [Google Scholar]
- 48.Gomes I, Jordan BA, Gupta A, Trapaidze N, Nagy V, Devi LA. Heterodimerization of μ and δ opioid receptors: a role in opiate synergy. J. Neurosci. 2000;20:RC110. doi: 10.1523/JNEUROSCI.20-22-j0007.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rocheville M, Lange DC, Kumar U, Patel SC, Patel RC, Patel YC. Receptors for dopamine and somatostatin: formation of hetero-oligomers with enhanced functional activity. Science. 2000;288:154–157. doi: 10.1126/science.288.5463.154. [DOI] [PubMed] [Google Scholar]
- 50.Gonzalez-Maeso J, Ang RL, Yuen T, Chan P, Weisstaub NV, Lopez-Gimenez JF, Zhou M, Okawa Y, Callado LF, Milligan G, et al. Identification of a serotonin/glutamate receptor complex implicated in psychosis. Nature. 2008;452:93–97. doi: 10.1038/nature06612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Waldhoer M, Fong J, Jones RM, Lunzer MM, Sharma SK, Kostenis E, Portoghese PS, Whistler JL. A heterodimer-selective agonist shows in vivo relevance of G protein-coupled receptor dimers. Proc. Natl. Acad. Sci. U.S.A. 2005;102:9050–9055. doi: 10.1073/pnas.0501112102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jordan BA, Gomes I, Rios C, Filipovska J, Devi LA. Functional interactions between μ opioid and α 2A-adrenergic receptors. Mol. Pharmacol. 2003;64:1317–1324. doi: 10.1124/mol.64.6.1317. [DOI] [PubMed] [Google Scholar]
- 53.Rios C, Gomes I, Devi LA. μ-Opioid and CB1 cannabinoid receptor interactions: reciprocal inhibition of receptor signaling and neuritogenesis. Br. J. Pharmacol. 2006;148:387–395. doi: 10.1038/sj.bjp.0706757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gomes I, Gupta A, Filipovska J, Szeto HH, Pintar JE, Devi LA. A role for heterodimerization of μ and δ opiate receptors in enhancing morphine analgesia. Proc. Natl. Acad. Sci. U.S.A. 2004;101:5135–5139. doi: 10.1073/pnas.0307601101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hasbi A, Nguyen T, Fan T, Cheng R, Rashid A, Alijaniaram M, Rasenick MM, O'Dowd BF, George SR. Trafficking of preassembled opioid μ-δ heterooligomer-Gz signaling complexes to the plasma membrane: coregulation by agonists. Biochemistry. 2007;46:12997–13009. doi: 10.1021/bi701436w. [DOI] [PubMed] [Google Scholar]
- 56.Breit A, Gagnidze K, Devi LA, Lagace M, Bouvier M. Simultaneous activation of the δ opioid receptor (δOR)/sensory neuron-specific receptor-4 (SNSR-4) hetero-oligomer by the mixed bivalent agonist bovine adrenal medulla peptide 22 activates SNSR-4 but inhibits δOR signaling. Mol. Pharmacol. 2006;70:686–696. doi: 10.1124/mol.106.022897. [DOI] [PubMed] [Google Scholar]
- 57.Rashid AJ, So CH, Kong MM, Furtak T, El-Ghundi M, Cheng R, O'Dowd BF, George SR. D1-D2 dopamine receptor heterooligomers with unique pharmacology are coupled to rapid activation of Gq/11 in the striatum. Proc. Natl. Acad. Sci. U.S.A. 2007;104:654–659. doi: 10.1073/pnas.0604049104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.So CH, Verma V, O'Dowd BF, George SR. Desensitization of the dopamine D1 and D2 receptor hetero-oligomer mediated calcium signal by agonist occupancy of either receptor. Mol. Pharmacol. 2007;72:450–462. doi: 10.1124/mol.107.034884. [DOI] [PubMed] [Google Scholar]
- 59.Glass M, Felder CC. Concurrent stimulation of cannabinoid CB1 and dopamine D2 receptors augments cAMP accumulation in striatal neurons: evidence for a Gs linkage to the CB1 receptor. J. Neurosci. 1997;17:5327–5333. doi: 10.1523/JNEUROSCI.17-14-05327.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Berghuis P, Rajnicek AM, Morozov YM, Ross RA, Mulder J, Urban GM, Monory K, Marsicano G, Matteoli M, Canty A, et al. Hardwiring the brain: endocannabinoids shape neuronal connectivity. Science. 2007;316:1212–1216. doi: 10.1126/science.1137406. [DOI] [PubMed] [Google Scholar]
- 61.Bromberg KD, Ma'ayan A, Neves SR, Iyengar R. Design logic of a cannabinoid receptor signaling network that triggers neurite outgrowth. Science. 2008;320:903–909. doi: 10.1126/science.1152662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guzman M, Sanchez C, Galve-Roperh I. Cannabinoids and cell fate. Pharmacol. Ther. 2002;95:175–184. doi: 10.1016/s0163-7258(02)00256-5. [DOI] [PubMed] [Google Scholar]
- 63.Harkany T, Guzman M, Galve-Roperh I, Berghuis P, Devi LA, Mackie K. The emerging functions of endocannabinoid signaling during CNS development. Trends Pharmacol. Sci. 2007;28:83–92. doi: 10.1016/j.tips.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 64.Rozenfeld R, Devi LA. Receptor heterodimerization leads to a switch in signaling: β-arrestin2-mediated ERK activation by μ-δ opioid receptor heterodimers. FASEB J. 2007;21:2455–2465. doi: 10.1096/fj.06-7793com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bohn LM, Lefkowitz RJ, Gainetdinov RR, Peppel K, Caron MG, Lin FT. Enhanced morphine analgesia in mice lacking β-arrestin 2. Science. 1999;286:2495–2498. doi: 10.1126/science.286.5449.2495. [DOI] [PubMed] [Google Scholar]
- 66.Zhu Y, King MA, Schuller AG, Nitsche JF, Reidl M, Elde RP, Unterwald E, Pasternak GW, Pintar JE. Retention of supraspinal δ-like analgesia and loss of morphine tolerance in δ opioid receptor knockout mice. Neuron. 1999;24:243–252. doi: 10.1016/s0896-6273(00)80836-3. [DOI] [PubMed] [Google Scholar]
- 67.Gupta A, Mulder J, Gomes I, Rozenfeld R, Bushlin I, Ong E, Lim M, Maillet E, Junek M, Cahill CM, Harkany T, Devi LA. Increased abundance of opioid receptor heteromers after chronic morphine administration. Sci. Signaling. 2010;3:ra54. doi: 10.1126/scisignal.2000807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Abul-Husn NS, Sutak M, Milne B, Jhamandas K. Augmentation of spinal morphine analgesia and inhibition of tolerance by low doses of μ-and δ-opioid receptor antagonists. Br. J. Pharmacol. 2007;151:877–887. doi: 10.1038/sj.bjp.0707277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Terrillon S, Barberis C, Bouvier M. Heterodimerization of V1a and V2 vasopressin receptors determines the interaction with β-arrestin and their trafficking patterns. Proc. Natl. Acad. Sci. U.S.A. 2004;101:1548–1553. doi: 10.1073/pnas.0305322101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pfeiffer M, Kirscht S, Stumm R, Koch T, Wu D, Laugsch M, Schroder H, Hollt V, Schulz S. Heterodimerization of substance P and μ-opioid receptors regulates receptor trafficking and resensitization. J. Biol. Chem. 2003;278:51630–51637. doi: 10.1074/jbc.M307095200. [DOI] [PubMed] [Google Scholar]
- 71.Grant M, Alturaihi H, Jaquet P, Collier B, Kumar U. Cell growth inhibition and functioning of human somatostatin receptor type 2 are modulated by receptor heterodimerization. Mol. Endocrinol. 2008;22:2278–2292. doi: 10.1210/me.2007-0334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Damian M, Martin A, Mesnier D, Pin JP, Baneres JL. Asymmetric conformational changes in a GPCR dimer controlled by G-proteins. EMBO J. 2006;25:5693–5702. doi: 10.1038/sj.emboj.7601449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Damian M, Mary S, Martin A, Pin JP, Baneres JL. G protein activation by the leukotriene B4 receptor dimer. Evidence for an absence of trans-activation. J. Biol. Chem. 2008;283:21084–21092. doi: 10.1074/jbc.M710419200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vilardaga JP, Nikolaev VO, Lorenz K, Ferrandon S, Zhuang Z, Lohse MJ. Conformational cross-talk between α2A-adrenergic and μ-opioid receptors controls cell signaling. Nat. Chem. Biol. 2008;4:126–131. doi: 10.1038/nchembio.64. [DOI] [PubMed] [Google Scholar]
- 75.Jarrahian A, Watts VJ, Barker EL. D2 dopamine receptors modulate Gα-subunit coupling of the CB1 cannabinoid receptor. J. Pharmacol. Exp. Ther. 2004;308:880–886. doi: 10.1124/jpet.103.057620. [DOI] [PubMed] [Google Scholar]