Abstract
We have generated a mouse strain carrying a Cre-ERT2 knock-in allele at the Pax7 locus, the Pax7CE allele (Lepper et al., 2009). Combining Pax7CE and the R26RLacZ Cre reporter allele, here we describe temporal-specific tamoxifen (tmx)-inducible lineage tracing of embryonic Pax7-expressing cells. In particular, we focus on the somitic lineage. Tmx-inducible Cre activity directed by the Pax7CE allele is similar to the endogenous Pax7 expression pattern. The somitic Pax7-expressing cells selectively marked at embryonic day 9.5 (E9.5) give rise to dorsal dermis and brown adipose tissue, in addition to dorsal aspects of trunk muscles and the diaphragm muscle. However, they do not contribute to ventral body wall and limb muscles. After E12.5, marked Pax7-expressing cells become lineage restricted to muscles. Descendants of these early marked Pax7-expressing cells begin to occupy sublaminal positions associated with the myofibers around E16.5, characteristic of embryonic satellite cells. Furthermore, they contribute to adult myofibers and regeneration competent satellite cells in the tibialis anterior muscle, providing evidence that some adult satellite cells are of embryonic origin.
Keywords: myogenesis, progenitors, stem cells, Pax7, inducible Cre, tamoxifen
Introduction
Somites are segmented paraxial mesodermal structures flanking both side of the spinal cord (Christ and Ordahl, 1995; Sanders, 2001). Elegant chick/quail chimeric studies have established that the vertebrate trunk musculoskeletal system and limb muscles are derived from the somite (Chavelier et al., 1977). In mammals, however, establishing the somite-to-musculature relationship has only become possible in recent years through recombination-based lineage tracing, typically using Cre recombinase in the mouse. This approach relies on Cre expression driven by the enhancer/promoter of a chosen gene, either as a transgene or a knock-in (KI) allele, and a reporter expressing a permanent tracer after Cre-mediated recombination (Soriano, 1999).
For somitic cell lineage tracing in the mouse, several Cre KI alleles have been used, including Myf5 and MyoD (Gensch et al., 2008; Kanisicak et al., 2009; Seale et al., 2008). Myf5 and MyoD encode basic-helix-loop-helix transcriptional determinants that initiate the myogenic differentiation program (reviewed in Cossu, 1996). They are expressed in a somite component called the myotome. Lineage studies using Myf5Cre or MyoDCre KI alleles have demonstrated that descendants of cells expressing either gene directly contribute to muscles (Gensch et al., 2008; Kanisicak et al., 2009; Seale et al., 2008). Unexpectedly, they were also found to occupy the position of satellite cells, the presumed muscle stem cells (Kanisicak et al., 2009; Kuang et al., 2007). In addition, Myf5-descendants give rise to brown adipose tissue (BAT), cartilage, and dermis (Gensch et al., 2008; Seale et al., 2008). These descriptive studies provide novel information and beg reconsideration of the relationship between cells that are “presumed” to be specified to a myogenic fate, and the fates of their descendants.
Expansion of the muscle anlagen throughout embryogenesis has been thought to rely on a population of progenitor cells that do not express Myf5 or MyoD. These “progenitors” are proposed to express Pax3 and/or Pax7, genes which encode closely related paired-homeodomain transcription factors of the Pax gene family (Horst et al., 2006). Pax3 and Pax7 are together essential for the myogenic potential, survival, and proliferation of myogenic progenitors (Relaix et al., 2005). Their expression patterns largely overlap within the somite but with temporal and spatial differences (Horst et al., 2006; Jostes et al., 1990). Pax3 is initially expressed in somitic precursors, i.e. the presomitic mesoderm (Fan and Tessier-Lavigne, 1994), and soon after somite formation Pax3 expression becomes restricted to the dermomyotome, with higher expression levels at the lateral lip. Pax7 expression starts in dermomyotome of more mature somites (Fan and Tessier-Lavigne, 1994), and is evenly distributed in the medial and central portions of the dermomyotome. Reporters knocked into each locus have shown that mixed populations of Pax3+/Pax7−, Pax3−/Pax7+, and Pax3+/Pax7+ cells can be found at E10.5 (Relaix et al., 2005). Pax3+/Pax7+ cells at the central dermomyotome appear to divide tangentially and form a “secondary myogenic domain” just beneath the dermomyotome and abutting the Myf5+ and MyoD+ myotome. This secondary domain is a likely source for embryonic muscle expansion (Relaix et al., 2005).
Pax3 and Pax7 expression patterns diverge after E13.5 (Horst et al., 2006). While lineage tracing via a Pax3Cre KI allele did show that Pax3 descendant cells contribute to muscles and satellite cells (Schienda et al., 2006), it remains controversial exactly what muscle groups contain Pax3+ cells in the trunk and limb after E13.5 (Horst et al., 2006; Kuang et al., 2006; Relaix et al., 2006). By contrast, it is generally agreed that Pax7 expression is detected in mono-nucleated cells associated with trunk and limb muscles throughout embryogenesis. When presumed muscle progenitor/stem cells become embedded in the sublaminal space of myofibers around E16.5, Pax7 expression is found in these cells (Relaix et al., 2005). Pax7 is also expressed in adult satellite cells (Seale et al., 2000). However, these static expression data fall short to establish a direct lineage relationship between early Pax7+ and later Pax7+ cells. While a Pax7Cre KI allele was used to document that Pax7 descendants occupy embryonic muscle anlagen (Hutcheson et al., 2009; Keller et al., 2004), any possible differences in the developmental potential of Pax7-expressing populations at distinct developmental time points could not be ascertained because the constitutive Cre cumulatively marks all descendants of cells that express Cre at one time. Lineage tracing by the use of Pax3Cre, Myf5Cre, or MyoDCre suffers from the same shortcoming.
Here, we focus on the lineage(s) of Pax7-expressing cells in embryonic myogenesis using a tamoxifen inducible Cre-ERT2 knocked into the Pax7 locus, the Pax7CE allele (Lepper et al., 2009). We are interested in the following questions. First, at what time do Pax7-expressing cells become myogenic fate-restricted? Second, do Pax7-expressing dermomyotome cells migrate into the limb to form limb muscles? And third, do embryonic Pax7-expressing cells directly contribute to myofibers and satellite cells in the adult? A Pax7Cre-ER KI allele, in which the IRES-Cre-ERT is inserted immediately after the stop codon of Pax7 for co-expression of Pax7 and Cre-ERT, was used to monitor adult muscle homeostasis via satellite cell incorporation (Nishijo et al., 2009). The Pax7CE allele was previously used for lineage tracing of satellite cells in the muscle injury/regeneration paradigm (Lepper et al., 2009). Below, we describe the use of the Pax7CE allele for inducible lineage tracing during embryogenesis to answer the questions posed above.
Results
Making of the Pax7CE allele
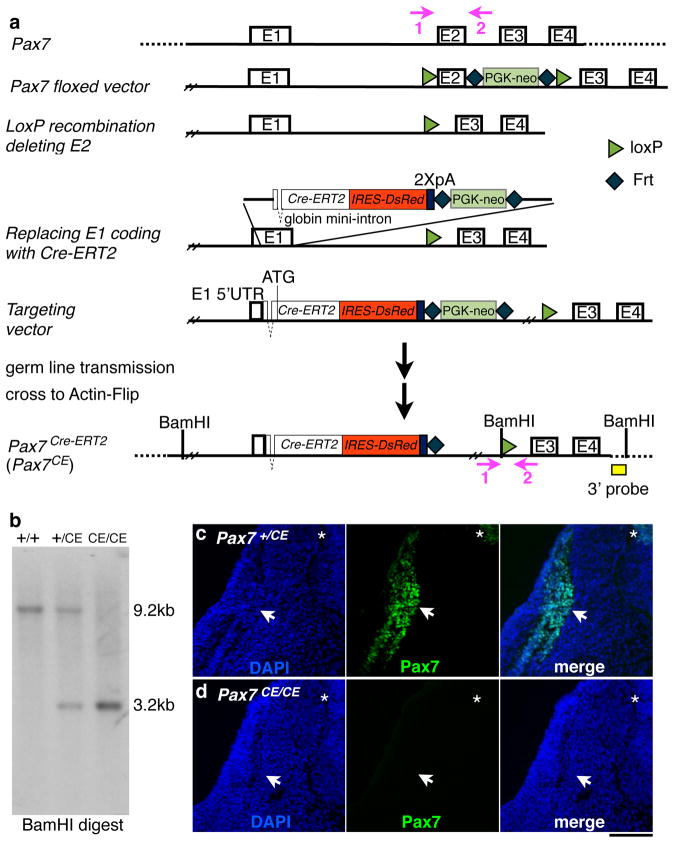
We previously described the Pax7CE allele briefly (Lepper et al., 2009). Here we provide detailed information (see Materials and Methods) for investigators who intend to use or characterize this allele further. A 20 Kb region of Pax7 was used for plasmid construction (Fig. 1a). A loxP site upstream and a Frt-Pgk-neo-Frt-loxP cassette (Liu et al., 2003) downstream of exon 2 (Fig. 1a) were inserted. This construct was used to generate a Pax7flox allele (Lepper et al., 2009). Exon 2 was then deleted to ensure creation of a nonfunctional allele even with a read-through transcript as exon 2 encodes part of the paired DNA binding domain of Pax7. Lastly, a Cre-ERT2-IRES-DsRed-2xpolyA-Frt-Pgk-neo-Frt cassette replaced the coding region of exon 1. The resulting construct was used for homologous recombination in R1 embryonic stem (ES) cells.
Figure 1. Pax7CE allele is a null allele.
a, schema for generating the Pax7CE allele via recombineering; modified from Lepper et al. (2009). Oligonucleotide primers, vectors, selection cassettes, 3′ probe, as well as locations of loxP sites and Cre-ERT2 cassette are detailed in Materials and Methods; E, exon; BamHI is used for Southern Blot analysis; pink arrows 1 and 2, location of genotyping PCR primers. b, Southern blot using the 3′ probe (yellow box in a). c and d, immunofluorescence of Pax7 on E10.5 Pax7+/CE and Pax7CE/CE embryos, respectively. From left to right for both panels: DAPI, Pax7, and merged image; transverse sections at the fore limb level; arrows, secondary myogenic domain; stars, dorsal spinal cord; scale bar = 100 μm.
Germ line transmitting animals were confirmed by Southern blot analysis (Fig. 1b), and mated to Actin-Flip mice to remove the Frt-Pgk-neo-Frt cassette. The Pax7CE allele is null as Pax7CE/CE embryos do not have detectable levels of Pax7 by Western blot (Lepper et al., 2009) or immunofluorescence (Fig. 1d) using a monoclonal antibody against Pax7 C-terminus (the sequence of which is not deleted). Pax7CE/CE mice display the previously described Pax7 null phenotype (Oustanina et al., 2004; Seale et al., 2000) in that they have poor post-natal muscle growth, high rate of lethality, and poor muscle regeneration after injury.
The Pax7CE allele directs tmx-inducible Cre activity in embryonic muscles
To test the tmx-inducible Cre activity derived from the Pax7CE allele, R26R+/LacZ Cre reporter mice (Soriano, 1999) were crossed to Pax7+/CE mice. Pregnant females were injected with a single dose of 1.5 mg tmx/40 gram body weight on one day between E6.5 and E15.5. Embryos were harvested one day after injection and subjected to whole mount X-gal histochemical reactions to visualize β-gal activity of the reporter (one day labeling/tracing). Higher tmx dosages caused high rate of embryo lethality and we have not been able to detect Cre or DsRed by immunostaining.
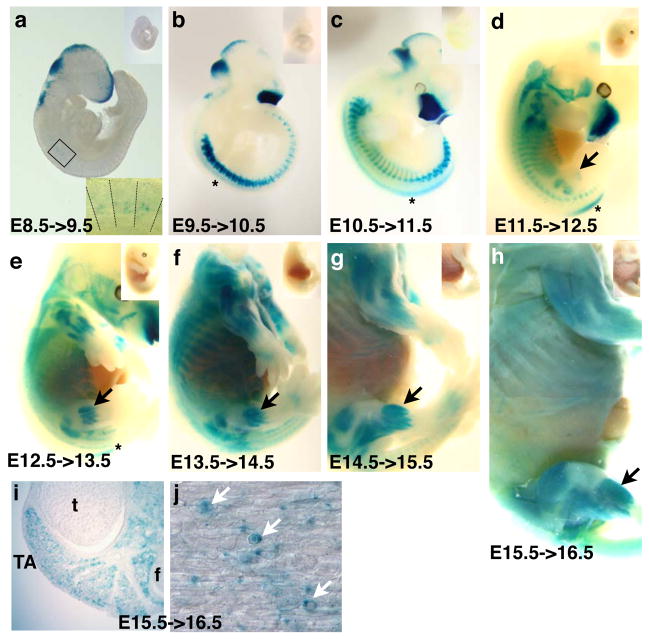
We did not find any β-gal+ cells in embryos injected at E6.5 and harvested at E7.5 (E6.5->7.5; not shown). For E7.5->8.5 embryos, a few scattered β-gal+ cells were found near the dorsal neural tube, presumably early neural crest cells (not shown; (Mansouri et al., 1996)). In E8.5->9.5 samples, staining in the craniofacial region, dorsal brain and spinal cord, cephalic neural crest cells, and anterior 3–7 somites was seen (Fig. 2a). For E9.5->10.5, E10.5->11.5, E11.5->12.5, E12.5->13.5, and E13.5->14.5 embryos, craniofacial staining persisted and the dorsal spinal cord staining pattern shifted in an anterior to posterior direction (Fig. 2c–e). β-gal+ somitic cells were readily detected in a segmented pattern (Fig. 2a–d), and a gradual increase of the β-gal+ population towards the ventral part of the body was also seen, following rib extensions. Staining in the abdominal muscles became apparent after E12.5 (Fig. 2e). The staining patterns in the later embryonic stages (after E13.5) corresponded to anatomical positions of the muscle anlagen in the trunk and limbs (Fig. 2g, h). In E15.5->16.5 labeled embryos, hind limb muscles contained many single β-gal+ cells, while myofibers were stained relatively weakly in intensity (Fig. 2i, j). At all stages, only embryos that were Pax7+/CE;R26R+/LacZ showed positive staining for β-gal activity; without tmx injection, no β-gal activity was observed (upper right insets in Fig. 2 panels). These data demonstrate that Cre activity is tightly regulated by tmx.
Figure 2. Pax7CE allele allows tightly controlled β-gal labeling by Tamoxifen administration.
A–H, whole mount X-gal histochemical staining of Pax7+/CE;R26R+/LacZ embryos one day after tmx administration; stages as indicated. The upper right insets are Pax7+/CE;R26R+/LacZ control embryos without tmx treatment, showing no X-gal signal. In a, the lower right inset is a magnified image of boxed cervical somites; lines demarcate somite boundaries. b–e, stars, staining in the spinal cord; d–h, black arrows, staining in hind limb muscles. i, cross section of E15.5->16 hind limb; t, tibia, f, fibula; TA, tibialis anterior muscles. j, longitudinal section of E15.5->16 TA muscle; white arrows, single round β-gal+ cells present in muscle fibers.
Pax7CE-directed Cre activity in early somitic cells
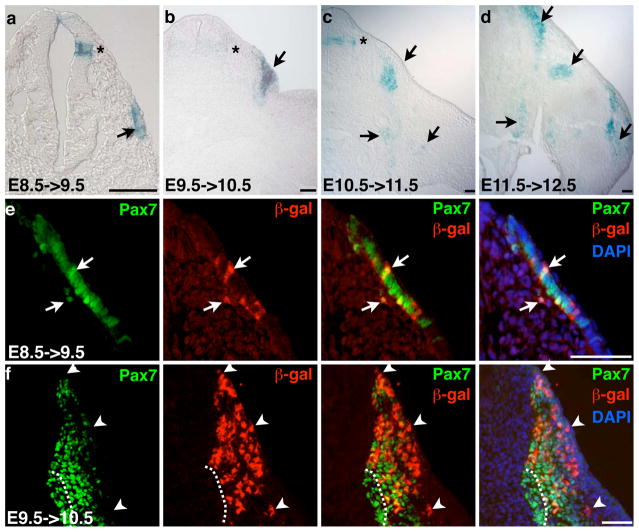
We further characterized younger tmx-marked embryos by sectioning. Transverse sections through stained embryos showed that β-gal+ cells were in the central dermomyotome of E8.5->9.5 tmx-labeled embryos at the branchial level (Fig. 3a). Staining in the dorsal neural tube was also seen. In E9.5->10.5 labeled somites, β-gal+ cells were in the dermomyotome as well as in a domain of cells just beneath it, consistent with the proposal that central dermomyotomal cells divide tangentially and migrate inward to form the secondary myogenic domain (Fig. 3b). In E10.5->11.5 samples, we noted very little staining in the dermomyotome. Rather, β-gal+ cells were mainly found in the secondary myogenic domain beneath the dermomyotome, as well as near the ventral body wall and around the shoulder girdle (Fig. 3c). In E11.5->12.5 labeled embryos, labeled cells were found along the trunk and limbs (Fig. 3d).
Figure 3. Pax7CE directs β-gal labeling in the dermomyotome and secondary myogenic domain.
a–d, transverse sections of X-gal stained Pax7+/CE;R26R+/LacZ embryos of stages indicated; a, cervical level; b–d, forelimb level; stages as indicated; arrows, β-gal+ cells in the trunk and limb; stars, β-gal+ cells in the dorsal spinal cord; scale bar = 50 μm. e and f, Pax7/β-gal double immunofluorescence on transverse sections of E8.5->9.5 (cervical level) and E9.5->10.5 (fore limb level) samples, respectively; white arrows, co-labeled cells; white arrowheads, β-gal+Pax7− cells at the dorsal somite; white dashed lines demarcate the ventro-medial Pax7+β-gal− population; scale bar = 50μm.
To determine the developmental timing of changes in Pax7 expression, we performed double immunofluorescence studies using anti-β-gal and anti-Pax7 antibodies on E8.5->9.5 and E9.5->10.5 samples. We found that E8.5->9.5 labeled β-gal+ cells in the dermomyotome were within the Pax7-expressing cell population (Fig. 3e). This indicates that E8.5->9.5 dermomyotomal cells are stable in Pax7 expression. For E9.5->10.5 labeling, most β-gal+Pax7+ cells were in the dermomyotome and dorsal portion of the secondary myogenic domain (Fig. 3f). There were β-gal+Pax7− cells intermingled within the dorsal Pax7+ domain, and in contrast, the most ventro-medial Pax7+ domain was devoid of β-gal+ cells. These data suggest that once dermomyotomal cells start to expand to form the secondary myogenic progenitor domain (E9.5->10.5), some cells maintain Pax7 expression (Pax7+β-gal+) and some cells lose Pax7 expression (Pax7−βgal+). In addition, some cells possibly express Pax7 de novo at the ventro-medial tip (Pax7+β-gal−) of the secondary myogenic domain.
Lineage tracing of Pax7-descendant cells in the myogenic lineage
The one-day lineage tracing data showed a progressive change in Pax7 expressing cell populations, similar to that of endogenous Pax7 expression (Horst et al., 2006; Mansouri and Gruss, 1998; Relaix et al., 2004). However, the lineage relationship between early Pax7+ dermomyotome precursors and embryonic muscles cannot be established by such a static survey. To directly test the lineage relationship, we carried out intermediate-term lineage tracing to E16.5 to better assign, based on anatomy, the embryonic contribution of Pax7-descendants marked at selective early time points.
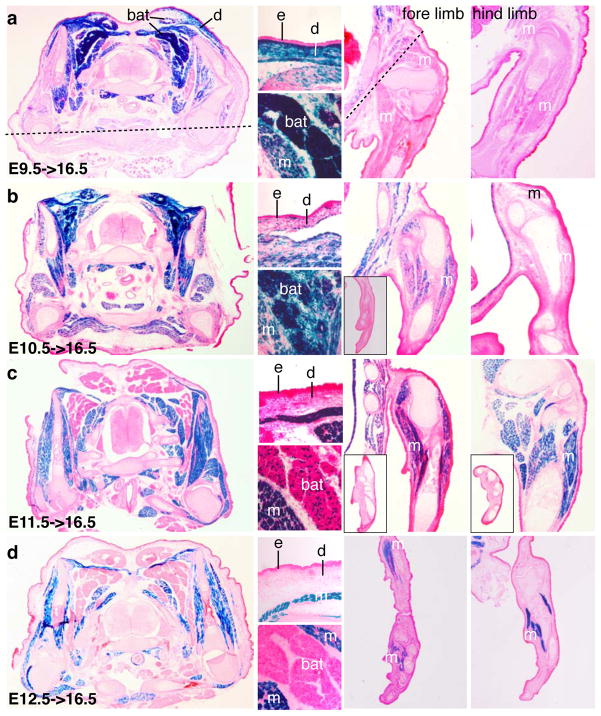
We marked Pax7-expressing cells at E9.5, E10.5, E11.5 and E12.5 by tmx, and examined the distribution of β-gal+ cells at E16.5 via X-gal histochemical reactions on sections (Fig. 4). Surprisingly, we found that E9.5 labeled cells contributed to the dorsal dermis, brown adipose tissue (BAT), and intercostal and intervertebral muscles, as assessed by the anatomical positions of β-gal+ cells (Fig. 4a). We did not find β-gal+ cells in the limb muscle, except for the fore limb shoulder. No ventral body wall muscles were β-gal+. Of E10.5->16.5 tracings, we found scattered labeling in the dermis but intense staining in BAT. Also, the dorsal muscles, ventral body wall muscles, and proximal fore limb muscles were now β-gal+ (Fig. 4b). In E11.5->16.5 embryos, there was no longer staining in the dorsal dermis, and a drastic reduction of staining in BAT was observed. Fore and hind limb muscles were now β-gal+ (Fig. 4c). Of E12.5->16.5 embryos, most if not all β-gal+ cells corresponded to the embryonic muscle anlagen; none were in the dorsal dermis or BAT. Staining of limb muscles now extended distally to the autopod. These data indicate that early Pax7-expressing cells have multiple developmental potentials and become restricted to a muscle-specific fate as development proceeds.
Figure 4. Inducible long-term lineage tracing reveals progressive changes of the developmental potential of Pax7-expressing cells.
a–d, lineage tracing as indicated. a, from left to right: First, transverse section at the branchial level; Top for dermis (d), bottom for brown adipose tissue (bat); upper fore limb; upper hind limb. Dashed line demarcates the ventral boundary of X-gal signal; m, muscles; e, epidermis. Panels b–d, organization of subfigures is the essentially the same as in panel a with following differences: 1) for Panel b fore limb figure, the mid level and toes (inset) are shown; 2) for c, mid levels of fore limb and hind limb are shown, as well as the toes (insets in each); 3) for d, toes are shown to demonstrate distal expansion of β-gal labeling.
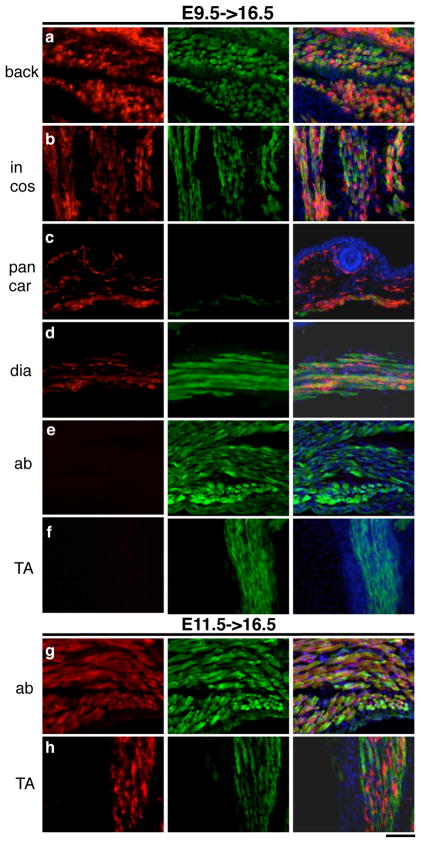
To further confirm the myogenic identity of β-gal+ cells in E9.5->16.5 and E11.5->16.5 embryos, we performed double immunofluorescence using MF20 (a monoclonal against myosin heavy chain) and anti-β-gal antibodies on sections. For E9.5->16.5 lineage tracing, β-gal+ lineages corresponded to the back, intercostal, diaphragm, and panniculus carnosus muscles as determined by MF20+/β-gal+ myofibers (Figs. 5a–d). We also detected β-gal+ cells in the dermis underneath the hair follicle. However, the ventral body wall and the hind limb TA muscles did not have any β-gal+ cells (Fig. 5e, f). Immuno-labeling of E11.5->16.5 sections again revealed the back, intercostal, diaphragm, and panniculus carnosus muscles as β-gal+MF20+ (not shown). In addition, the ventral body wall muscles (Fig. 5g) and the hind limb TA muscles (Fig. 5h) were β-gal+, while no β-gal+ cells were found in the dermis (not shown), consistent with data obtained by X-gal histochemistry. We noted that strongly stained β-gal+ cells were intermingled between the MF20+ myofibers, while β-gal signals within myofibers were weak and uneven. This is likely because, while labeled myogenic progenitors exhibit strong β-gal signal, incorporation of these cells into myofibers via focal fusion causes uneven staining within myofibers. In addition, a given myofiber likely contain only a fraction of β-gal producing cells, leading to weak staining signal by dilution. Importantly, these data indicate that the population of Pax7-expressing cells marked at E11.5, which constitutes limb muscles, is distinct from the one marked in the E9.5 dermomyotome.
Figure 5. β-gal+ cells contribute to myofibers.
Double immunofluorescence for β-gal (in red, first column) and myosin heavy chain (MF-20 in green, second column) were performed on E9.5->16.5 (top) and E11.5->16.5 (bottom) samples. Merged images with DAPI (blue) stain are in the third column. The muscle groups shown are: a, the back (back) muscles; b, the intercostal (in cos) muscles; c, the panniculus carnovous (pan car) or subcutaneous muscle; d, the diaphragm (dia) muscle; e and g, the abdominal (ab) muscles; f and h, the TA muscles. Scale bar = 50 μm.
Early Pax7+ cells directly contribute to trunk and limb embryonic satellite cells
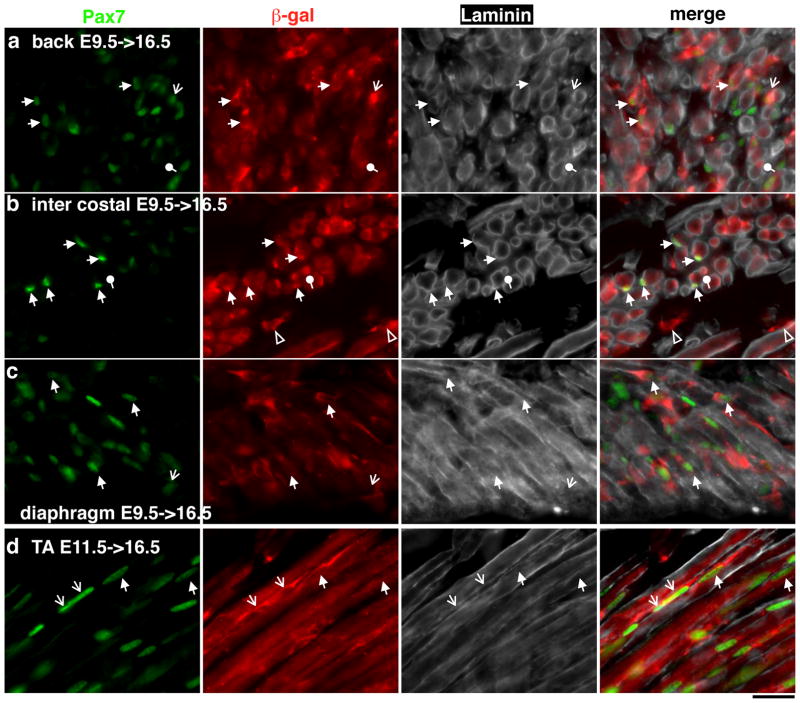
To test whether E9.5 and E11.5 marked β-gal+ cells can in fact contribute to embryonic satellite cells, we asked whether there are β-gal+Pax7+ cells occupying the sublaminal space of myofibers at E16.5 using an antibody against Laminin. From E9.5->16.5 samples, we found β-gal+Pax7+ cells within the Laminin boundaries of myofibers in several muscle groups including the back (Fig. 6a), intercostal (Fig. 6b), and diaphragm (Fig. 6c) muscles. Thus, the dermomyotomal cells labeled at E9.5 can ultimately give rise to trunk embryonic satellite cells, consistent with the chick-quail chimera data (Gros et al., 2005). Similarly, we found that E11.5->16.5 labeled β-gal+ cells retained Pax7 expression and occupied the sublaminal space in the same muscle groups (not shown) as well as in the TA muscle (Fig. 6d). In surveyed muscle groups, we found β-gal+Pax7+ cells outside the lamina, indicating that not all have entered their niche at E16.5. We also found localized β-gal+Pax7− staining within the lamina boundary of some myofibers, particularly in the intercostal muscle sections (Fig. 6b). These were likely Pax7-descendants that had just fuse with the myofiber, causing focal β-gal staining. However, we cannot exclude them as Pax7− satellite cells. If so, it implies that some Pax7− satellite cells are of Pax7 descend. Overall, the lamina integrity and observable sublaminal β-gal+Pax7+ cell fractions vary between muscle groups, likely reflecting differences in developmental tempo or in Pax7+ satellite cell density among muscle groups.
Figure 6. Marked β-gal+ cells occupy sub-laminal space at E16.5.
Triple immunofluorescence of Pax7 (in green, first column), β-gal (in red, second column), and Laminin (in white, third column). Merged images are in the fourth column. a, b, and c are E9.5->16.5 samples of the back muscle, intercostal muscle and diaphragm muscle, respectively. d, TA muscle of E11.5->16.5. Solid arrows, sub-laminal β-gal+Pax7+ cells; lined arrows, β-gal+Pax7+ cells outside of the Laminin boundary; round arrows, βgal+Pax7− staining within the Laminin boundary, likely recently fused myocytes. Scale bar = 20 μm.
E11.5 marked Pax7-expressing cells contribute to functional adult satellite cells of the TA muscle
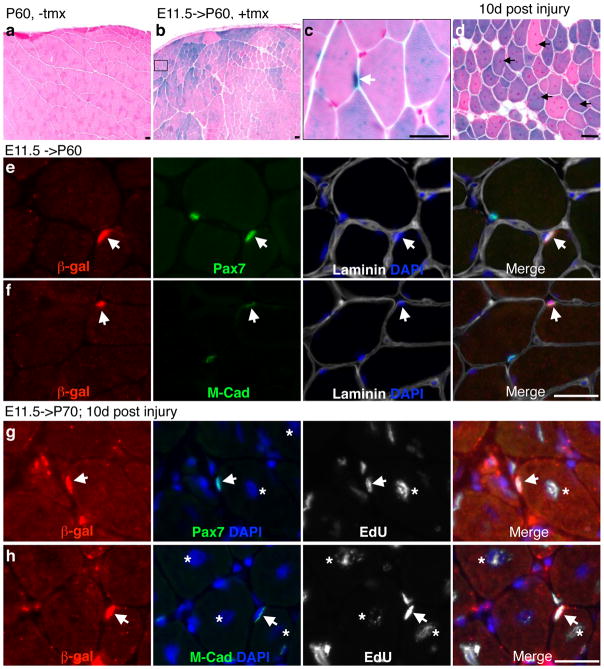
While we have shown that early Pax7+ cells can contribute to sublaminal progenitors and myofibers at E16.5, these Pax7+ progenitors and their descendant myofibers may be progressively replaced by de novo populations of myogenic progenitors after E16.5 up to adulthood. If so, early marked Pax7-descendants will not contribute to adult muscles and satellite cells. To test this, we used the hind limb TA muscle as a paradigm. We marked Pax7-expressing cells at E11.5, the earliest population that give rise to TA muscles at E16.5, and examined whether they were still present in sexually mature animals at 2 months of age. We found many β-gal+ myofibers in the adult TA muscle of E11.5 tmx-treated animals (Fig. 7b). The staining intensity varied amongst myofibers, presumably the result of clonal labeling of Pax7-expressing progenitors. At higher magnifications, punctuated and/or unevenly distributed blue staining patterns were also noted within myofibers (Fig. 7c), suggesting that when not all myonuclei are derived from β-gal marked progenitors, the distribution of β-gal protein varies within a myofiber syncytium. Stochastic exclusion of β-gal by myofibril structural proteins may also contribute to a punctuated appearance. Importantly, these data indicate that adult TA myofibers retain Pax7-descendants derived from E11.5.
Figure 7. Embryonically marked β-gal+ cells can become functional adult satellite cells.
a, postnatal day 60 (P60) TA muscle from a non-tmx (−tmx) treated Pax7+/CE;R26R+/LacZ animal (control). b, P60 TA sample from a tmx treated (+tmx) embryo at E11.5. c, a magnified image from boxed area in b to show strong X-gal staining at the periphery of the myofiber (white arrow) and uneven X-gal signal within myofibers. d, X-gal staining of TA muscle regenerates from an E11.5->P70 animal; arrows, central nuclei of regenerated myofibers. Injury was performed at P60 by CTX and the sample was harvested 10 days (10d) later at P70. e, E11.5->P60 TA muscles subjected to immunofluorescent staining for β-gal, Pax7, and Laminin; merged image with DAPI stain to the right. f, same as in e, except staining for M-cadherin (M-cad). Arrowheads indicate β-gal+ satellite cells. g and h are fluorescent images of β-gal/Pax7/EdU and β-gal/M-cad/EdU, respectively, of 10-day TA muscle regenerates. EdU was administered daily on days 2–5 post injury. Scale bars = 20 μm.
At the same time, we also found strong X-gal stained signals at the periphery of myofibers, reminiscent of satellite cells (Fig. 7c). To determine whether these β-gal+ cells were indeed adult satellite cells, we immuno-labeled for Pax7, Laminin, and β-gal. Indeed some Pax7+ cells were β-gal+ and embedded in the Laminin boundary (Fig. 7e). We next examined whether these myofiber associated β-gal+ cells are quiescent satellite cells by using M-cadherin for co-labeling; M-cadherin displays polarized localization at the muscle/quiescent satellite cell junction. As expected, β-gal+ cells did display polarized M-cadherin staining at the muscle junction within the sublaminal space, indicating that E11.5 labeled β-gal+ cells can become quiescent satellite cells in the adult.
We next tested whether these adult β-gal+ cells descended from E11.5 progenitors could function in muscle regeneration after acute injury. As many existing myofibers were already β-gal+, and not all satellite cells were labeled with β-gal, the presence of β-gal+ regenerative myofibers (as evidenced by their central nuclei) after injury alone would not be sufficient to determine the active participation of E11.5 marked β-gal+ satellite cells (Fig. 7d). Therefore, as an additional criterion, we reasoned that functional β-gal+ satellite cell should be able to proliferate and re-occupy their niche after injury-induced regeneration. We performed Cardiotoxin (CTX) mediated injury at 2 months of age, followed by daily 5-ethynyl-2′-deoxyuridine (EdU, a BrdU analog to assay for DNA replication (Salic and Mitchison, 2008)) administrations between days 2 and 5 after injury, and then harvested the muscles at day 10 post injury (experimental paradigm developed previously (Lepper et al., 2009)). We found that within the regenerative area, β-gal+ cells had proliferated and retained Pax7 expression after the regenerative process (Fig. 7g). We also found that the proliferated β-gal+ cells could re-establish their niche as evidenced by polarized M-cadherin staining at the muscle junction. All together, our data provide evidence that as early as E11.5, Pax7-expressing cells have been specified to become functional adult satellite cells in the TA muscle.
Discussion
Using the Pax7CE allele for inducible lineage tracing, we found that 1) between E9.5 and E10.5 Pax7-expressing cells are multi-potent; 2) Pax7-expressing cells only become restricted to the myogenic lineage after E12.5; 3) limb muscles and their satellite cells do not come from dermomyotomal cells which express Pax7 at E9.5; 4) the adult TA muscles and their associated satellite cells can be traced back to as early as E11.5 Pax7-expressing cells. Our results provide a foundation for future discussion of muscle development.
Early Pax7+ dermomyotomal cells are multi-potent
E9.5 β-gal marked Pax7+ dermomyotomal cells contribute to muscles, dorsal dermis, and interscapular BAT. No β-gal+ cells were found in the presomitic mesoderm or the sclerotomal compartment (Figs. 2 and 3), supporting that the aforementioned three cell types share a common progenitor in the dermomyotome. This is consistent with lineage data obtained by using the En1Cre and En1Cre-ER KI alleles (Atit et al., 2006); En1 is expressed in the central dermomyotome and En1-expressing descendant cells give rise to the same three cell types (Atit et al., 2006). Together, these data provide unequivocal evidence that the central dermomyotome contains multi-potent progenitors for muscles, dorsal dermis and interscapular BAT.
Pax7-expressing cells marked at E10.5 still contribute to dermis and BAT However, in E11.5 labeled/traced embryos, the dermis no longer has detectable β-gal activity while some BAT did. By Contrast, in E12.5->16.5 samples, labeling is no longer found in dermis and BAT. Thus, Pax7-expressing cells lose their potentials for dermis and BAT with a slightly different tempo. The progressive transition of Pax7-expressing cells from having multi-potential to becoming myogenically restricted between E9.5 and E12.5 corresponds to the time frame when the dermomyotome gradually loses its epithelial structure. We propose that during this period Pax7+ cells that delaminated from the dermomyotome to migrate inward are myogenically restricted, but those remaining in the dermomyotome maintain other developmental potentials.
Myf5Cre marked cells have also been shown to contribute to muscle and BAT (Seale et al., 2008), implying that Myf5-expressing cells are not restricted to the muscle lineage. Because embryos carrying a Myf5LacZ KI allele show β-gal+ cells in the presomitic mesoderm (Cossu G., 1996; Teboul et al., 2003), Myf5Cre likely also directs Cre activity there (Gensch et al., 2008). If so, results from these studies are not surprising as presomitic mesoderm is the precursor to somites, explaining pleiotropic labeling of other somitic derivatives by Myf5Cre (Gensch et al., 2008). The constitutive Cre also cannot be used to determine temporal changes of non-muscle potentials of Myf5-expressing cells. Our results therefore demonstrate the advantage of temporal-specific lineage tracing.
Pax7-expressing cells contribute to progressively more ventral muscle groups
Long term lineage tracing data indicate that muscle groups derived from cell marking at later time points (e.g. E12.5) include those derived from marking at earlier time points (e.g. E9.5). Although this suggests that E9.5 Pax7-expressing cells continue to express Pax7 for later cell marking/tracing to the same muscle groups, we cannot exclude that there are de novo Pax7-expressing cells which also contribute to the same muscle groups. On the other hand, ventral body wall muscles are only labeled by lineage marking after E10.5, indicating a new group of Pax7-expressing cells emerging after E9.5 labeling. We suggest that Pax7+β-gal− cells present at the most ventro-medial edge of E9.5->10.5 somites (Fig. 3f) are likely de novo Pax7-expressing cells for ventral body wall muscles.
Hind limb muscles are labeled only at E11.5 or later, indicating that yet another set of cells activates Pax7 after E10.5. We note a proximal to distal expansion of labeled limb muscle groups from E10.5 to E12.5. This suggests that migrating myoblasts activate Pax7 expression in a proximal to distal sequence after their arrival in the limb bud, consistent with the static expression survey of Pax7 (Relaix et al., 2004). Thus, limb muscles must originate from a population of cells other than those Pax7+/β-gal+ cells labeled at E9.5. Lineage tracing using the M-Cre transgene (Brown et al., 2005) has shown that the lateral-most Pax3-expressing dermomyotomal cells give rise to limb muscles and satellite cells (Schienda et al., 2006). Chick-quail chimera data also indicate that trunk and limb muscles are derived from distinct somitic compartments (Ordahl and Le Douarin, 1992). Our data therefore support that Pax7 is likely activated in Pax3+ limb myoblasts after they enter the limb.
Embryonic Pax7+ cells directly contribute to functional satellite cells in the adult
We have demonstrated that embryonic and adult Pax7+ satellite cells in the TA muscle are, at least in part, derived from E11.5 Pax7-expressing cells. Pax7-expressing cells marked at E12.5, E13.5 and E16.5, also give rise to TA muscle satellite cells in the adult (not shown). Furthermore, these embryonically marked cells can proliferate after injury (β-gal+EdU+ cells in Fig. 7). Since these cells retain EdU at day 10 (time of assay) and yet EdU is administered between days 2 and 5 post injury, we take this as evidence for self-renewal. Because of incomplete labeling by a single tmx injection, we cannot determine whether or not there are newly emerging Pax7-expressing cells after birth that also contribute to adult satellite cells.
Lineage tracing using Pax3Cre, Myf5Cre or MyoDCre KI alleles demonstrated that descendants of cells expressing any of these genes could become satellite cells postnatally (Kanisicak et al., 2009; Kuang et al., 2007; Schienda et al., 2006). Because both the Pax3 and Myf5 loci are transcribed early in presomitic cells and both studies used a constitutive Cre, it is difficult to tease out when and where Pax3Cre and Myf5Cre start to mark future satellite cells. Satellite cell labeling by MyoDCre is less easy to explain, as the onset of MyoD expression is typically thought to control the transition into terminal differentiation. One possibility is that active myoblasts co-express Pax7 and MyoD transiently, a fraction of which turn off MyoD and revert to Pax7+ satellite cells. During this phase of co-expression, MyoD-directed Cre activity marks “future reverted” satellite cells. Such “reversion” is not without precedent as differentiating cells are able to back track to stem cell status under defined conditions (Kai and Spradling, 2004).
The limitations of using the Pax7CE allele during embryogenesis
While the Pax7CE allele allows us to discover the dynamic developmental potentials of Pax7-expressing cells, the frequency of reporter activation by a single administration of tmx is not high: 12% (β-gal+Pax7+/Pax7+; 185 Pax7+ cells counted) in E8.5->9.5 β-gal+ cervical somites, and 38% (β-gal+Pax7+/Pax7+; 546 Pax7+ cells counted) in E9.5–E10.5 fore limb somites. Cre expression levels, allowable tmx dosage, and developmental stages are likely contributors to labeling efficiency. Low efficiency labeling has the advantage for tracing small population of cells, but also the disadvantage with the need to survey multiple embryos for accurate interpretation. Our interpretations are based on more than 5 independent samples per stage, which should be sufficient for conclusion. Another caveat of the tmx administration is that tmx is presumed to be active over a 12 hr period (Nakamura et al., 2006). Changes of developmental potentials that occur in a smaller time window therefore cannot be ascertained. Thus, Pax7CE is not ideal for efficient conditional gene inactivation in early embryos, but may be useful for making genetic mosaics and analyzing marked mutant cells. The coincidence of recombination at the to-be-inactivated gene locus and the lineage tracer locus will need to be determined by each investigator who plans to use the Pax7CE allele.
Materials and Methods
Constructing the Pax7CE allele by recombineering (Fig. 1a)
Step 1
The pL253 vector (Liu et al., 2003) was modified by inserting a PacI site in front of the NotI site. Transfer of a 129sv BAC containing the Pax7 genomic fragment (purchased from Invitrogen) into pL253 (containing a tk negative selection cassette) was accomplished by recombineering in DY380 bacteria (Liu et al., 2003). Two annealed double stranded oligonucleotides were ligated to PL253 as 5′ and 3′ arms for recombineering: NotI oligos (~18 kb upstream of exon 2): GGCCGCAGTGGCTTTAACCTCTCTGACCTTCGGTCTTCCCATCTGTGAAATGG GGTCATTGGGATTCTA (sense); TAGAATCCCAATGACCCCATTTCACAGATGG GAAGACCGAAGGTCAGAGAGGTTAAAGCCACTGC (anti-sense). BamH1 oligos (~2.1 kb downstream of exon 2): GATCCGACCCTTGGCATCTGGGAACTTGGCCTC CAGCCTCTGACACATAGTGCTTGCCAAGAGGAAAGCA (sense); TGCTTTCCT CTTGGCAAGCACTATGTGTCAGAGGCTGGAGGCCAAGTTCCCAGATGCCAAG GGTCG (anti-sense).
Step 2
The loxP-pgk-neo-loxP cassette of pL452 (Liu et al., 2003) was inserted 135 bp upstream of exon 2 by recombineering using PCR amplified 5′ and 3′ homology arms. PCR 5′ homology fragment (300 bp): TAGGAATTCAAAGTCCCTCTACACTGCA (5′ primer); CACAGTCCTGGGTGCTCAAG (3′ primer). PCR 3′ homology fragment (340 bp): GTCTCCTGATATCGGCACAG (5′ primer); TAGGGATCCACAATCCAAGTCC CAGAAGCCAG (3′ primer).
Step 3
Removal of pgk-neo cassette by arabinose-induced Cre in EL350, leaving one loxP site upstream of exon 2.
Step 4
Insertion of the Frt-pgk-neo-Frt-loxP cassette of pL451 (Liu et al., 2003) at 153 bp downstream of exon 2 by recombineering using PCR amplified 5′ and 3′ homology arms. PCR 5′ homology fragment (320bp): TAGGAATTCTAGGGACTGTATCTC AGAGCT (5′ primer); GAACCACATCCGTCACAAGA (3′ primer). PCR 3′ homology fragment (346bp): ACCTGCTTCAGAGGTGGAGGT (5′ primer); TAGGGATCCGTT AGCTGAGCTCTAGATTG (3′ primer). This step created the Pax7flox construct. A BamHI site was introduced next to the second loxP site for Southern.
Step 5
Removal of exon 2 by arabinose-induced Cre in EL350, leaving a loxP site.
Step 6
Replacement of exon 1 with the Cre-ERT2-IRES-DsRed-Frt-pgk-neo-Frt cassette:
Cloning of the cassette: 5′ to the β-globin intron linked to Cre-ERT2 (Feil et al., 1997) for efficient expression, a SpeI site was inserted. An IRES-DsRed-polyA cassette was joined by PCR amplified fragments from pIRES and pDsRed-express (Clontech) and inserted 3′ to Cre-ERT2 at the SacI site (creating 2 polyA signals). The SpeI-SalI fragment containing Cre-ERT2-IRES-DsRed-2XpolyA was then transferred to Bluescript SK+ vector. The Frt-pgk-neo-Frt cassette was PCR amplified from PL451 as an XhoI-SalI fragment and inserted at the 3′ SalI site. The entire cassette was contained in a SpeI-SalI fragment.
5′ and 3′ homology arms for recombineering were PCR fragments. PCR of the 5′ homology fragment used TAGACTAGTGCGCACGCTGGAGACGAATCCAG (5′ primer) and GAGGAGGGAGTCCGAGCCAGAGA (3′ primer). They amplified 4 bp to 470 bp upstream of ATG in exon 1, which was cut with SpeI and ligated to the 5′ end of the cassette. PCR of the 3′ homology fragment used TAGGTCGACACGGGAAGTTTC TAGAGCGGAC (5′ primer) and GTGGAAATAGAGTGTCGCTTGTTG (3′ primer). They amplified 120 bp to 400 bp downstream of exon 1, which was cut with SalI and ligated to 3′ end of the cassette.
The cassette ligated to 5 and 3′ homology arms were recombined into the plasmid generated in step 5 to obtain the Pax7CE construct. Thus, the coding sequence and the 5′ splice site of exon 1 were deleted. PacI site introduced in step 1 was used for linearization.
Homologous recombination in ES cells and germ line transmission
R1 ES cells were used per instruction (provided by Drs. Rossant and Nagy). Electroporated ES cells were subjected to positive (G418) and negative (gancyclovir) selections. Colonies were screened by PCR to identify homologous recombination using a primer 3′ to the loxP site (unique junction sequence: GCTATACGAAGTTATTAG GTGGATCCGT) and a primer 3′ to the construct (GTTGGACAGCGGTGTTGGGTG AATC). Positive clones were confirmed by Southern Blot analysis using BamHI digest and a 3′ probe (Fig. 1) generated by PCR as a 594 bp genomic fragment 3′ to the construct (CAACACCGCTGTCCAACCTTAAC; TGTCCCTGAAGGGATAGCAGT CCT). Three confirmed clones were used for CD1 morula aggregation to generate chimeras. A 100% germ line transmitting male was crossed to C57/BL6 Actin-FLIP mice (gift from Dr. Dymecki) to remove the Frt-pgk-neo-Frt cassette. Subsequent sibling intercrosses were used to maintain the colony. Pax7flox (Pax7tm1.1Fan/J) and Pax7CE (Pax7tm2.1(cre/ERT2)Fan/J) mice have been donated to the Jackson Laboratory.
Mice and embryos
Pax7CE males were crossed to R26RLacZ Cre reporter (Gt(ROSA)26Sortm1Sor/J, from the Jackson laboratory) female mice to obtain embryos. Vaginal plug date is designated as embryonic day 0.5 (E0.5). Tmx was administered to pregnant mice at 1.5 mg/40 gram body weight intraperitoneally. Injection and embryo harvesting time points are specified in the text. Extra-embryonic sacs were used for PCR genotyping. For the Pax7CE allele, primer 1 (ACTAGGCTCCACTCTGTCCTTC) and primer 2 (GCAGATGTAGGGACA TTCCAGTG) in Fig. 1a were used; wt, 724 bp; Pax7CE, 231 bp. For the R26RLacZ allele, 3 primers (Soriano, 1999) were used: AAAGTCGCTCTGAGTTGTTAT, GCGAAGAGTTTGTCCTCAACC, and GGAGCGGGAGAAATGGATATG; wt 603 bp; reporter ~250 bp.
Histochemical and immunofluorescent staining
For whole mount X-gal staining, embryos were fixed in 4%PFA/0.1M phosphate buffer (pH7.2) for 1–2 hrs, rinsed in phosphate buffer, stained by X-gal reactions overnight, and mounted, following a standard whole mount protocol (Hogan B, 1994). They were then cryo-sectioned for examination. For E16.5, X-gal staining of cryo-sections was performed according to Atit et al. (2006). For immunofluorescent studies, the same fixation and cryo-sectioning conditions were used without X-gal reactions. Primary antibodies used are: Anti-Pax7 (mouse, 1:20, DSHB), Anti-β-gal (chicken, 1:1000, Chemicon; rabbit, 1:2000, Cappel), Anti-Laminin (rabbit, 1:2000, Sigma), Anti-M-cadherin (mouse, 1:250, Santa Cruz). Secondaries are anti-mouse IgG1-Alexa 488, anti-rabbit Alexa 568, anti-rabbit Cy5, and anti-chicken Alexa 568; all used at 1:1000.
Muscle injury and EdU labeling
For injury, postnatal day 30 (P30) animals were used. Fifty μl of Cardiotoxin (CTX, 10μM) was injected into right TA muscles. 5-ethynyl-2′-deoxyuridine (EdU) was administered at 5 μg/gram body weight at days 2–5 post injury. TA muscles were harvested for fresh frozen sectioning, followed by immunofluorescence. EdU incorporation was detected using the Click-it kit (Invitrogen) using Azide-Alexa 647.
Microscopy and Imaging
To visualize whole mount embryos and cross sections of E16.5 mice, images were taken with a Cannon 3000 camera mounted on a SV11 (Zeiss) dissection scope. For sections of smaller embryos, a Nikon scope mounted with a Spot camera was used. For immunofluorescent images, an Axioskop mounted with an Axiocam was used. Fluorescent images were pseudo colored (specified in figures and legends) for overlapping purposes. Images were overlaid in the Photoshop Element program.
References
- Atit R, Sgaier SK, Mohamed OA, Taketo MM, Dufort D, Joyner AL, Niswander L, Conlon RA. Beta-catenin activation is necessary and sufficient to specify the dorsal dermal fate in the mouse. Dev Biol. 2006;296:164–176. doi: 10.1016/j.ydbio.2006.04.449. [DOI] [PubMed] [Google Scholar]
- Brown CB, Engleka KA, Wenning J, Min Lu M, Epstein JA. Identification of a hypaxial somite enhancer element regulating Pax3 expression in migrating myoblasts and characterization of hypaxial muscle Cre transgenic mice. Genesis. 2005;41:202–209. doi: 10.1002/gene.20116. [DOI] [PubMed] [Google Scholar]
- Chavelier AKM, Mauger A. Limb-somite relationship: origin of the limb musculature. J Embryol exp Morph. 1977;41:245–258. [PubMed] [Google Scholar]
- Christ B, Ordahl CP. Early stages of chick somite development. Anat Embryol (Berl) 1995;191:381–396. doi: 10.1007/BF00304424. [DOI] [PubMed] [Google Scholar]
- Cossu GTS, Buckingham M. How is myogenesis initiated in the embryo. Trends Genet. 1996;12:218–223. doi: 10.1016/0168-9525(96)10025-1. [DOI] [PubMed] [Google Scholar]
- Fan CM, Tessier-Lavigne M. Patterning of mammalian somites by surface ectoderm and notochord: evidence for sclerotome induction by a hedgehog homolog. Cell. 1994;79:1175–1186. doi: 10.1016/0092-8674(94)90009-4. [DOI] [PubMed] [Google Scholar]
- Feil R, Wagner J, Metzger D, Chambon P. Regulation of Cre recombinase activity by mutated estrogen receptor ligand-binding domains. Biochem Biophys Res Commun. 1997;237:752–757. doi: 10.1006/bbrc.1997.7124. [DOI] [PubMed] [Google Scholar]
- Gensch N, Borchardt T, Schneider A, Riethmacher D, Braun T. Different autonomous myogenic cell populations revealed by ablation of Myf5-expressing cells during mouse embryogenesis. Development. 2008;135:1597–1604. doi: 10.1242/dev.019331. [DOI] [PubMed] [Google Scholar]
- Gros J, Manceau M, Thome V, Marcelle C. A common somitic origin for embryonic muscle progenitors and satellite cells. Nature. 2005;435:954–958. doi: 10.1038/nature03572. [DOI] [PubMed] [Google Scholar]
- Hogan BBR, Constantini F, Lacy E. Manipulating Mouse Embryos: a Laboratory Manual. 2. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press; 1994. [Google Scholar]
- Horst D, Ustanina S, Sergi C, Mikuz G, Juergens H, Braun T, Vorobyov E. Comparative expression analysis of Pax3 and Pax7 during mouse myogenesis. Int J Dev Biol. 2006;50:47–54. doi: 10.1387/ijdb.052111dh. [DOI] [PubMed] [Google Scholar]
- Hutcheson DA, Zhao J, Merrell A, Haldar M, Kardon G. Embryonic and fetal limb myogenic cells are derived from developmentally distinct progenitors and have different requirements for beta-catenin. Genes Dev. 2009;23:997–1013. doi: 10.1101/gad.1769009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jostes B, Walther C, Gruss P. The murine paired box gene, Pax7, is expressed specifically during the development of the nervous and muscular system. Mech Dev. 1990;33:27–37. doi: 10.1016/0925-4773(90)90132-6. [DOI] [PubMed] [Google Scholar]
- Kai T, Spradling A. Differentiating germ cells can revert into functional stem cells in Drosophila melanogaster ovaries. Nature. 2004;428:564–569. doi: 10.1038/nature02436. [DOI] [PubMed] [Google Scholar]
- Kanisicak O, Mendez JJ, Yamamoto S, Yamamoto M, Goldhamer DJ. Progenitors of skeletal muscle satellite cells express the muscle determination gene, MyoD. Dev Biol. 2009;332:131–141. doi: 10.1016/j.ydbio.2009.05.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller C, Hansen MS, Coffin CM, Capecchi MR. Pax3:Fkhr interferes with embryonic Pax3 and Pax7 function: implications for alveolar rhabdomyosarcoma cell of origin. Genes Dev. 2004;18:2608–2613. doi: 10.1101/gad.1243904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang S, Charge SB, Seale P, Huh M, Rudnicki MA. Distinct roles for Pax7 and Pax3 in adult regenerative myogenesis. J Cell Biol. 2006;172:103–113. doi: 10.1083/jcb.200508001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang S, Kuroda K, Le Grand F, Rudnicki MA. Asymmetric self-renewal and commitment of satellite stem cells in muscle. Cell. 2007;129:999–1010. doi: 10.1016/j.cell.2007.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepper C, Conway SJ, Fan CM. Adult satellite cells and embryonic muscle progenitors have distinct genetic requirements. Nature. 2009;460:627–631. doi: 10.1038/nature08209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Jenkins NA, Copeland NG. A highly efficient recombineering-based method for generating conditional knockout mutations. Genome Res. 2003;13:476–484. doi: 10.1101/gr.749203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansouri A, Gruss P. Pax3 and Pax7 are expressed in commissural neurons and restrict ventral neuronal identity in the spinal cord. Mech Dev. 1998;78:171–178. doi: 10.1016/s0925-4773(98)00168-3. [DOI] [PubMed] [Google Scholar]
- Mansouri A, Stoykova A, Torres M, Gruss P. Dysgenesis of cephalic neural crest derivatives in Pax7−/− mutant mice. Development. 1996;122:831–838. doi: 10.1242/dev.122.3.831. [DOI] [PubMed] [Google Scholar]
- Nakamura E, Nguyen MT, Mackem S. Kinetics of tamoxifen-regulated Cre activity in mice using a cartilage-specific CreER(T) to assay temporal activity windows along the proximodistal limb skeleton. Dev Dyn. 2006;235:2603–2612. doi: 10.1002/dvdy.20892. [DOI] [PubMed] [Google Scholar]
- Nishijo K, Hosoyama T, Bjornson CR, Schaffer BS, Prajapati SI, Bahadur AN, Hansen MS, Blandford MC, McCleish AT, Rubin BP, et al. Biomarker system for studying muscle, stem cells, and cancer in vivo. FASEB J. 2009;23:2681–2690. doi: 10.1096/fj.08-128116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordahl CP, Le Douarin NM. Two myogenic lineages within the developing somite. Development. 1992;114:339–353. doi: 10.1242/dev.114.2.339. [DOI] [PubMed] [Google Scholar]
- Oustanina S, Hause G, Braun T. Pax7 directs postnatal renewal and propagation of myogenic satellite cells but not their specification. Embo J. 2004;23:3430–3439. doi: 10.1038/sj.emboj.7600346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relaix F, Montarras D, Zaffran S, Gayraud-Morel B, Rocancourt D, Tajbakhsh S, Mansouri A, Cumano A, Buckingham M. Pax3 and Pax7 have distinct and overlapping functions in adult muscle progenitor cells. J Cell Biol. 2006;172:91–102. doi: 10.1083/jcb.200508044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relaix F, Rocancourt D, Mansouri A, Buckingham M. Divergent functions of murine Pax3 and Pax7 in limb muscle development. Genes Dev. 2004;18:1088–1105. doi: 10.1101/gad.301004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relaix F, Rocancourt D, Mansouri A, Buckingham M. A Pax3/Pax7-dependent population of skeletal muscle progenitor cells. Nature. 2005;435:948–953. doi: 10.1038/nature03594. [DOI] [PubMed] [Google Scholar]
- Salic A, Mitchison TJ. A chemical method for fast and sensitive detection of DNA synthesis in vivo. Proc Natl Acad Sci U S A. 2008;105:2415–2420. doi: 10.1073/pnas.0712168105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders EJ, Lash JW, Ordhal CP. The Origin and Fate of Somites. NATA Science Series,: I: Life and Behabioural Science. 2001:329. [Google Scholar]
- Schienda J, Engleka KA, Jun S, Hansen MS, Epstein JA, Tabin CJ, Kunkel LM, Kardon G. Somitic origin of limb muscle satellite and side population cells. Proc Natl Acad Sci U S A. 2006;103:945–950. doi: 10.1073/pnas.0510164103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale P, Bjork B, Yang W, Kajimura S, Chin S, Kuang S, Scime A, Devarakonda S, Conroe HM, Erdjument-Bromage H, et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature. 2008;454:961–967. doi: 10.1038/nature07182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale P, Sabourin LA, Girgis-Gabardo A, Mansouri A, Gruss P, Rudnicki MA. Pax7 is required for the specification of myogenic satellite cells. Cell. 2000;102:777–786. doi: 10.1016/s0092-8674(00)00066-0. [DOI] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Teboul L, Summerbell D, Rigby PW. The initial somitic phase of Myf5 expression requires neither Shh signaling nor Gli regulation. Genes Dev. 2003;17:2870–2874. doi: 10.1101/gad.1117603. [DOI] [PMC free article] [PubMed] [Google Scholar]