Abstract
The purpose of this work was to determine whether there are differences in PIK3CA mutation status and PTEN protein expression between primary and matched metastatic breast tumors as this could influence patient management. Fifty-micron paraffin sections were used for DNA extraction and 3-micron slides for immunohistochemistry (IHC) and fluorescent in-situ hybridization (FISH). ER, PR and HER2 IHC were repeated in a central laboratory for both primary and metastasis. PTEN levels were assessed by IHC and PI3K pathway mutations detected by a mass spectroscopy-based approach. Median age was 48 years (range, 30 to 83 years). Tumor subtype included 72% hormone receptor-positive/HER2-negative, 20% HER2-positive, and less than 7.8% triple receptor negative. Tissues were available for PTEN IHC in 46 primary tumors and 52 metastases. PTEN was lost in 14 (30%) primary tumors and 13 (25%) metastases. There were 5 cases of PTEN loss and eight cases of PTEN gain from primary to metastasis (26% discordance). Adequate DNA was obtained on 46 primary tumors and on 50 metastases for PIK3CA analysis. PIK3CA mutations were detected in 19 (40%) of primary tumors and 21 (42%) of metastases. There were five cases of PIK3CA mutation loss, and four cases of mutation gain (18% discordance). There was an increase of the level of PIK3CA mutations in four cases, and decrease in one from primary to metastasis. There is a high level of discordance in PTEN level, PIK3CA mutations, and receptor status between primary and metastatic disease that may influence patient selection and response to PI3K-targeted therapies.
Keywords: PIK3CA mutations, PTEN loss, Breast Cancer, Metastasis
Introduction
Given the ability of hormone receptors (HR) and HER2 levels to predict response to therapy, it is currently recommended that they should be determined on every primary invasive breast cancer. However, changes in receptor status over disease progression and treatment have been described that could alter response to therapy. Concordance rates between primary tumors and recurrence site of 71% and 56% have been reported for estrogen receptor (ER) and progesterone receptor (PR) expression, respectively, and discrepancy rates for HER2 expression between primary tumors and matched metastasis may be as high as 20% (1,2). Further, in a series of patients with HER2-positive breast cancer treated preoperatively with trastuzumab, we reported that the HER2 status assessed by gene amplification changed to negative in over 30% of the cases who had extensive residual disease at the time of surgery (3). We recently reported that discordance of receptor status between primary and metastatic disease correlates with survival in patients with breast cancer (4). This suggests that optimal patient outcomes and responses in clinical trials could potentially be achieved by obtaining and evaluating tissue from metastatic sites.
The phosphatidylinositol 3-kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) pathway mediates multiple cellular functions critical to tumor initiation, progression and outcomes, including growth and proliferation, metabolism, motility, migration, invasion, angiogenesis, survival and autophagy (5). Tight regulation of this pathway is paramount to ensure that multiple cellular inputs are integrated for appropriate cellular outcomes. Frequent deregulation and aberrations of this pathway have been implicated not only in breast cancer development and progression (6), but in breast cancer therapy resistance (7–10). Thus, multiple drugs targeting the PI3K/Akt/mTOR pathway are in development and in early clinical trials as mono and combination therapies in breast cancer (6). Our group has also shown significant changes from primary to metastasis on phosphorylation events that serve as biomarkers for response to PI3K/Akt/mTOR signaling targeted therapies (48% discordance on p-Akt and p-4E-BP1) (11). A discordance between the level of potential biomarkers in primary tumor and metastasis could occur due to alterations in gene expression, possibly due to different microenvironmental stimuli, from stochastic events during metastasis, from clonal selection during metastasis or from clonal evolution either at the primary site or in the metastasis. However, as new targeted therapies enter clinical trials in the metastatic setting using signatures from the primary tumor for patient selection may have profound outcome implications if biomarkers are discordant between the primary and metastatic site(s). PIK3CA mutation and loss of PTEN expression are being pursued as potential predictors of response to novel PI3K pathway inhibitors. Thus, to optimize patient selection for PI3K-targeted therapy, it is critical to determine whether mutation status of PI3K pathway components and PTEN loss in the primary tumor is concordant with the status of these markers in the metastases.
The objective of this study was to determine whether there are differences in mutation status of components of the PI3K pathway and in PTEN protein expression between primary and metastatic tumors.
Methods
Under a research collaboration between MD Anderson Cancer Center and the Hospital Clinico Universitario de Valencia, Spain, we located paraffin blocks and corresponding clinical information of 50 matched pairs of primary breast cancer and biopsies of their corresponding asynchronous metastasis (distant nodes, skin, liver, lung, and bone). Paraffin blocks were sectioned. Two 50-micron thick cuts were used for DNA extraction, and 3-micron slides were used for IHC and florescent in-situ hybridization (FISH). All cases were reviewed by dedicated breast pathologists. ER, PR and HER2 staining were repeated in a central lab in both primary and metastatic tumors. FISH analysis was done in all cases that had 2+ staining by IHC, or if there was a discordant result between the primary tumor and the metastasis. The institutional review board of both institutions approved the laboratory studies and chart reviews.
IHC analysis to determine ER (clone SP1) and PR (clone 1E2) status was performed on 3-μm sections of formalin-fixed, paraffin-embedded tissues, using a Benchmark XT instrument (Ventana, Tucson, AZ). Both, Allred score (12) and percentage of nuclear staining were determined. Tumors with moderate to intense nuclear staining of at least 1% or more (13) or an Allred score equal or greater than 3/8 (12) were considered ER or PR-positive. IHC analysis for HER2 was performed under similar conditions, using the Pathway anti-HER-2/neu [4B5] monoclonal antibody (Ventana, Tucson, AZ). FISH analysis was performed using HER2 FISH Pharm Dx (Dako, Carpinteria, CA) according to the manufacturer’s instructions. HER2-positive was defined as 3+ receptor over-expression on IHC staining (strong membranous staining in at least 30% of cells), and/or gene amplification found on FISH. A gene copy/CEP-17 ratio greater than 2.2 was considered amplified (14).
PTEN IHC was performed using monoclonal mouse anti-Human PTEN antibody Clone 6H2.1 from Dako at 1:100 dilution. Negative control slides without primary antibody were included for each staining. Both cytoplasmic and nuclear PTEN staining in the tumor and non-neoplastic ductal epithelium and stroma were quantified. PTEN staining in the non-neoplastic normal epithelium, intratumoral and extratumoral stromal cells served as the internal positive control. Cases in which stromal staining was not observed were considered inevaluable. PTEN expression level was scored semiquantitatively based on staining intensity and distribution using the immunoreactive score (IRS) from as following: IRS = SI (staining intensity) × PP (percentage of positive cells). SI was determined as 0 = negative; 1 = weak; 2 = moderate; and 3 = strong. PP was defined as 0, <1%; 1, 1%–10%; 2, 11%–50%; 3, 51%–80%; and 4, >80% positive cells. Ten visual fields from different areas of each tumor were used for the IRS evaluation. Tumors with IRS of 0 were considered to have PTEN loss.
At least 70% tumor nuclear cellularity was confirmed in the samples used for DNA extraction. DNA was extracted using the QiaAMP microkit (Qiagen Inc. Valencia, CA) according to manufacture’s instructions. A mass spectroscopy-based approach evaluating single nucleotide polymorphisms (SNPs) was used to detect known mutations in PIK3CA (PIK3CA_A1046V, PIK3CA_C420R, PIK3CA_E110K, PIK3CA_E418K, PIK3CA_E453K, PIK3CA_E542K, PIK3CA_E545K, PIK3CA_F909L, PIK3CA_G1049R, PIK3CA_G451L456_V, PIK3CA_H1047L, PIK3CA_H1047R, PIK3CA_H1047Y, PIK3CA_H701P, PIK3CA_K111N, PIK3CA_M1043V, PIK3CA_N345K, PIK3CA_P539R, PIK3CA_Q060K, PIK3CA_Q546E, PIK3CA_R088Q, PIK3CA_S405F and PIK3CA_T1025S), AKT1 (AKT1_E17K_G49A, AKT1_G173R_G517C, AKT1_G173R_G517C), AKT2 (AKT2_E17K_G49A, AKT2_G175R_G523C), and AKT3 (AKT3_E17K_G49K, AKT3_G171R_G511A). Polymerase chain reaction (PCR) and extension primers for each gene were designed using Sequenom, Inc. (San Diego, CA) Assay Design. PCR-amplified DNA was cleaned using EXO-SAP (Sequenom) primer extended by IPLEX chemistry, desalted using Clean Resin (Sequenom) and spotted onto Spectrochip matrix chips using a nanodispenser (Samsung). Chips were run in duplicate on a Sequenom MassArray MALDI-TOF MassArray system. Sequenom Typer Software and visual inspection were used to interpret mass spectra. Reactions where equal or more than 8% of the resultant mass run in the mutant site in both directions were scored as positive. The MassArray approach allows quantification of the percent of the DNA present that demonstrates the PIK3CA mutation, which reflects the fraction of cells with the mutations. Reactions in which there was more than a 50% increase or decrease in the value of DNA present that demonstrates the PIK3CA mutation, the mutation score were also reported. In preliminary work, we directly compared standard Sanger sequencing and the Sequenom MassARRAY detection on 100 tumor samples. Sanger sequencing showed 11 out of the 100 samples to have PIK3CA mutations. Using the Sequenom method, we detected all of the PIK3CA somatic mutations expected from Sanger sequencing analysis plus an additional 11 putative mutations. The utility and accuracy of the probes as well as the ability to quantify aberrations were confirmed with analysis of breast cancer cell lines with known PIK3CA mutations prior to analyzing patient samples. As demonstrated by mixing experiments using cells with known mutations, the Sequenom approach can detect a mutation even if it is present in only 5% of the cell population and can give quantitative information on each mutation.
Patients were categorized according to their PIK3CA mutation and or PTEN status. Patient and tumor characteristics were tabulated and compared between the chi-square and the Fisher test as appropriate. Time to recurrence (TTR) was measured from the date of diagnosis to the date of first documented local or distant recurrence (metastasis biopsy). The Kaplan-Meier product limit method was used to estimate the TTR of all patients by concordant and discordant marker (PIK3CA and/or PTEN) status, and groups were compared with the log-rank statistic. P-values less than 0.05 were considered statistically significant; all tests were two-sided. Statistical analyses were carried out using SAS 9.2 (SAS Institute Inc., Cary, NC) and S-Plus 7.0 (Insightful Corporation, Seattle, WA).
Results
Patients were categorized according to breast cancer subtype and their tumor characteristics are presented in Table 1. Median age was 48 years (range, 30 to 83 years), 58.8% of the patients were postmenopausal. Seventy-eight percent of the tumors had a ductal histology; more than 50% had a high nuclear grade and lymphovascular space invasion. Distribution by tumor subtype included 72.5% hormone receptor-positive and HER2-negative (HR), 19.6% HER2-positive (HER2), and less than 10% triple receptor negative (TNBC). Ninety-eight percent of patients received systemic chemotherapy with an anthracycline or an anthracycline/taxane-based regimen (53%). All patients with HR tumors received adjuvant endocrine therapy. Distant disease free survival was 90 months, (range: 5–210 months). Initial distribution of the metastatic sites included bone only in 6 (11.8%) patients, visceral only in 6 (11.8%) patients and bone and visceral in 39 (76.4%) patients. Metastasis tissues were obtained from at least one metastatic site in all cases, two metastatic sites in seven cases, and three metastatic sites in one case. The time between obtaining samples of primary tumor and metastases was 46.5 months, (range from 10 to 229 months). Metastatic samples were obtained from soft tissue: 17 (28.8%), lymph nodes: nine (15.2%), lung: eight (11.8%), bone: six (10.2%), liver: three (5.1%), brain: two (3.4%), pleura: two (3.4%), ovary: two (3.4%), peritoneum: one (1.7%), and bowel: one (1.7%).
Table 1.
Patients and Primary Breast Cancer Characteristics
| Characteristic | Total | PIK3CA Mutations | PTEN Loss | |||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| 51 | 100% | 19/47 | 40.4% | 14/46 | 30.4% | |
| Age at Diagnosis | ||||||
| Median | 48 | (30–83) | 50 | (30–83) | 45 | (31–70) |
| Menopausal Status | ||||||
| Pre-menopausal | 30 | 58.8% | 8/26 | 30.8% | 9/29 | 31% |
| Post-menopausal | 21 | 41.2% | 11/21 | 52.4% | 5/17 | 29.4% |
| Histology | ||||||
| Ductal | 40 | 78.4% | 12/36 | 33.3% | 12/37 | 32.4% |
| Lobular | 8 | 15.7% | 4/8 | 50% | 1/6 | 16.7% |
| Other | 3 | 5.9% | 3/3 | 100% | 1/3 | 33.3% |
| Nuclear Grade | ||||||
| 1 | 2 | 3.9% | 1/2 | 50% | 0/0 | 0% |
| 2 | 21 | 41.2% | 8/20 | 40% | 6/20 | 30% |
| 3 | 28 | 54.9 | 10/25 | 40% | 8/26 | 37.8% |
| Lymphovascular Invasion | ||||||
| Positive | 45 | 88.2% | 17/41 | 41.5% | 11/41 | 26.8% |
| Negative | 6 | 11.8% | 2/6 | 33.3% | 3/5 | 60% |
| Breast cancer Subtype | ||||||
| Hormone Receptor-Positive | 37 | 72.5% | 15/34 | 44.1% | 10/35 | 28.6% |
| HER2-Positive | 10 | 19.6% | 3/9 | 33.3% | 2/9 | 22.2% |
| Triple Receptor-Negative | 4 | 7.8% | 1/4 | 25% | 2/3 | 66.7% |
| Systemic Chemotherapy | ||||||
| Anthracycline-based | 23 | 45.1% | 9/18 | 50% | 7/21 | 30% |
| Anthracycline and Taxane-based | 27 | 52.9% | 9/27 | 33.3% | 6/25 | 24% |
| No chemotherapy | 2 | 3.9% | 1/2 | 50% | 1/2 | 50% |
| Systemic Endocrine Therapy | ||||||
| Tamoxifen | 35 | 68.6% | 13/32 | 40.6% | 8/33 | 24.2% |
| Aromatase Inhibitor | 2 | 3.9% | 2/2 | 100% | 1/2 | 50% |
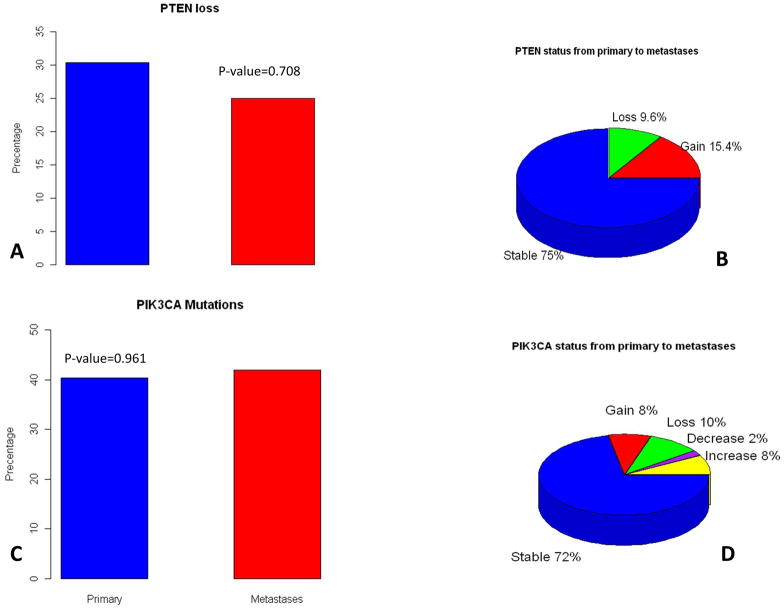
Tissues were available for PTEN IHC in 46 primary tumors and 52 metastases. PTEN loss was found in 14 (30.4%) of the primary tumors and 13 (25%) of the metastases. There were no significant differences in the proportion of tumors with PTEN loss between primary tumors and metastasis, (P=0.708), (Figure 1). Adequate DNA was obtained on all 46 primary tumors and on 50 of the 59 metastases. Mutations in AKT1, AKT2 or AKT3 were not detected. Mutations in PIK3CA_H1047_A3140 and PIK3CA_E545_G1633 were found in this sample set. All others tested were not detected. A mutation in PIK3CA was found in 19 (40.4%) of the primary tumors and 21 (42%) of the metastases. Mutations in PIK3CA_E545_G1633 occurred in only one primary tumor and in one of its corresponding metastasis. All other PIK3CA mutations occurred at PIK3CA_H1047_A3140. There were no significant differences in the proportion of tumors with PIK3CA mutations between primary tumors and metastasis, (P=0.961), (Figure 1).
Figure 1.
Proportion of primary tumor and metastases with PTEN loss and PIK3CA mutations (A and C). Distribution of the changes in PTEN levels and PIK3CA mutation status from primary tumor to metastases (B and C).
The results of the frequency of PTEN loss and PIK3CA mutations by tumor subtype are summarized in Table 2.
Table 2.
PTEN Loss and PIK3CA Mutations by Breast Cancer Subtype
| Primary Tumor | Hormone Receptor- Positive (n=37) | HER2-Positive (n=10) | Triple Negative (n=4) | Total (51) |
|---|---|---|---|---|
| PTEN Loss: | 10/32 | 2/10 | 2/4 | 14/46 (30.4%) |
| PIK3CA Mutations: | 15/34 | 3/9 | 1/4 | 19/47 (40.4%) |
| Both: | 4/32 | 1/10 | 1/4 | 6/46 (13%) |
| Either or Both: | 19/34 | 4/9 | 2/4 | 25/47 (53.2%) |
|
| ||||
| Metastasis | Hormone Receptor- positive (n=38) | HER2-positive (n=12) | Triple Negative (n=6) | Total (56) |
|
| ||||
| PTEN Loss: | 10/34 | 2/12 | 1/6 | 13/52 (25%) |
| PIK3CA Mutations | 15/34 | 4/11 | 2/5 | 21/50 (42%) |
| Both: | 2/34 | 1/12 | 1/6 | 4/50 (8%) |
| Either or Both: | 17/34 | 5/12 | 3/6 | 25/50 (50%) |
When looking at standard of care breast cancer markers (ER, PR and HER2), there were 51 pairs of primary tumors and metastases, samples for two metastatic sites in seven cases, and three metastatic sites in one case for a total of 59 comparisons. There was a 25.4% discordance rate from primary to metastasis: four tumors lost ER, seven tumors lost PR and one tumor lost HER2. Two tumors became PR-positive and one tumor became HER2-positive in their metastatic sites.
For PTEN analysis, there were 51 pairs of primary tumors and metastases, samples for two metastatic sites in seven cases, and three metastatic sites in one case for a total of 59 comparisons. Ten slides (9 comparisons) were considered inevaluable due to lack of adequate tissue. There were five cases of PTEN loss and eight cases of PTEN gain from primary to metastasis for a 26% discordance rate. When looking at PTEN loss, three of the five primary tumors were HR-positive and stayed HR-positive in their metastatic sites. One tumor was HER2-positive and had a PIK3CA mutation in detected in both primary and metastasis. Interestingly one tumor was a primary TNBC at diagnosis, was found to be HR-positive at the metastatic site, and also gained a PIK3CA mutation. All these patients received chemotherapy (anthracycline and taxane-based), and all but the TNBC patient received adjuvant tamoxifen. When looking at the cases that became PTEN positive, seven of the eight tumors were HR-positive in both primary and metastases; three of them had a PIK3CA mutation in both primary and metastases. However, one tumor demonstrated a significant increase in the number of cells with PIK3CA mutations in the metastatic site. One tumor classified as HER2-positive in the primary site, became a TNBC at the metastatic site. All these patients received chemotherapy (anthracycline and taxane-based), and adjuvant tamoxifen. The patient with the HER2-positive primary tumor that changed to TNBC in the metastasis did not receive trastuzumab.
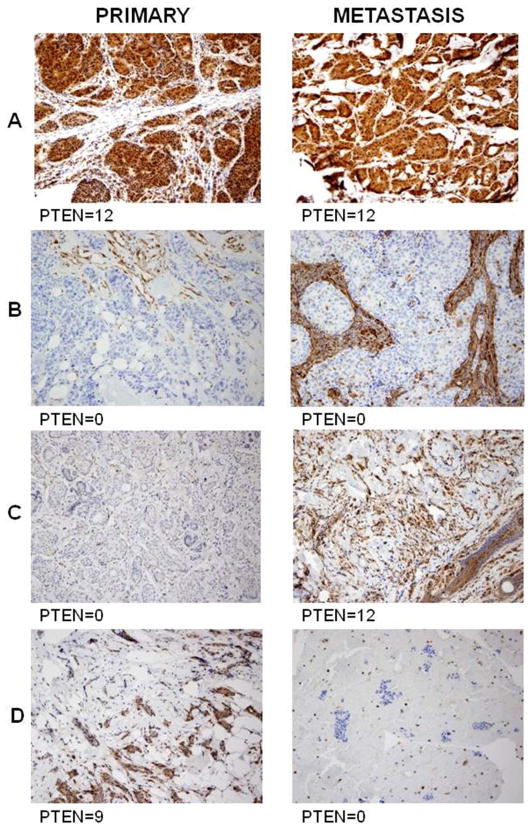
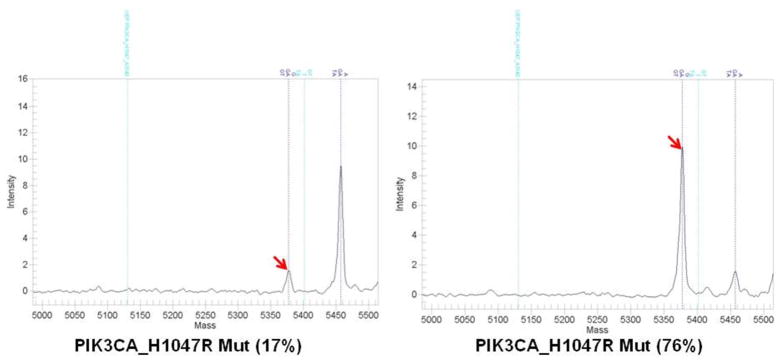
In two cases in which there were more than one metastases, there were discordant findings between the metastases. One primary tumor in which PTEN was lost, PTEN continued to be lost in the bone biopsy, however, there was PTEN gain (score of 8) in the mediastinal node metastasis. In a second primary tumor in which PTEN was positive, PTEN continued to be positive in the liver biopsy, however, there was PTEN loss in the ovary biopsy. Both tumors had PIK3CA mutations in the primary and all metastatic sites. Figure 2 shows representative matched cases of primary and metastatic disease including stable positive and negative staining, and show changes in PTEN levels from absent to gain, and from present to loss. For PIK3CA mutation analysis, there were 51 pairs of primary tumors and metastases, samples for two metastatic sites in seven cases, and three metastatic sites in one case for a total of 59 comparisons. There was insufficient DNA in 13 samples (11 comparisons). There were five cases were PIK3CA mutations were detected in the primary tumor but not in the metastasis, and four cases where PIK3CA mutation was not detected in the primary but was detected in the metastasis, for an 18% discordance rate. Figure 3 shows a representative case of a marked increase levels of PIK3CA mutation. There was increase in the fraction of DNA with the PIK3CA mutation by more than 50% in four cases between the primary and the metastasis, and a decrease in the percentage of cells with the PIK3CA mutation by more than 50% in one case from primary to metastasis.
Figure 2.
Immunohistochemical staining for PTEN in different cases of primary and metastatic disease. A and B show stable positive and negative staining respectively. C and D show changes in PTEN levels from absent to gain, and from present to loss respectively.
Figure 3.
A representative case of a marked increase levels of PIK3CA mutation at PIK3CA_H1047R from A primary tumor (17%) to B metastasis (76%).
When looking at cases where PIK3CA mutation was detected in the primary and not the metastasis, four tumors were HR-positive, and one was a TNBC. Interestingly from the four HR-positive tumors, one tumor was found to be HER2-positive at the metastatic site, and one tumor was found to be a TNBC in the metastatic site. There were no changes in PTEN IHC. Four these patients received chemotherapy (anthracycline and taxane-based), and adjuvant tamoxifen, and one patient did not received chemotherapy, but adjuvant letrozole. There was one case in which the primary tumor had a PIK3CA mutation (69%), the mutation was detected in a mediastinal metastasis, but with a significant decrease (24%), and where the mutation was not detected in a liver metastasis. PTEN was lost in the primary and metastatic samples. This case was a HR-positive tumor that became TNBC in both metastatic sites and received chemotherapy (anthracycline and taxane-based), and adjuvant tamoxifen.
When looking at cases where PIK3CA mutations were detected in the metastasis but not the primary, two of the four tumors were HR-positive; one was HER2-positive and one was a TNBC. One of the HR-positive tumors became PTEN-positive in the metastasis. The TNBC became HR-positive in the metastatic site and lost PTEN. Patients with HR-positive tumors received chemotherapy (anthracycline and taxane-based), and adjuvant tamoxifen. The patient with the HER2-positive tumor received adjuvat tamoxifen (tumor was ER-positive), and the patient with TNBC did not receive adjuvant systemic chemotherapy.
When looking at cases with more than 50% changes in the fraction of DNA with the PIK3CA mutation, all four tumors with increased fractions were HR-positive. One gained PTEN levels from a score of 0 to 4. All four received chemotherapy (three anthracycline-based, and one anthracycline and taxane-based), and adjuvant tamoxifen.
Table 3 and Figure 1 summarize the changes in markers between primary and metastatic breast cancer. Table 4 shows the significant changes in the percentage of the DNA fraction with PIK3CA mutations in the primary and metastatic tumors.
Table 3.
Changes in Marker Status from Primary to Metastatic Breast Cancer
| PTEN and PIK3CA Mutations Status
| |||||
|---|---|---|---|---|---|
| Marker | Changes from Primary Tumor to Metastases | ||||
| PTEN | = or >1 to Loss | 0 to Gain | Stable | ||
|
| |||||
| N= 46 Primary tumors | |||||
| 52 Metastases | 5 (9.6%) | 8 (15.4%) | 39 (75%) | ||
|
| |||||
| PIK3CA Mutation | Detected to Absent | Absent to Mutation | Increase in PIK3CA | Decrease in PIK3CA | Stable |
|
| |||||
| N= 47 Primary tumors | |||||
| 50 Metastases | 5 (10%) | 4 (8%) | 4 (8%) | 1 (2%) | 36 (72%) |
|
| |||||
|
Standard of Care Breast Cancer Markers
| |||||
| Marker | Changes from Primary Tumor to Metastases | ||||
|
| |||||
| Estrogen Receptor | Positive to Negative | Negative to Positive | Stable | ||
|
| |||||
| N= 51 Primary tumors | |||||
| 59 Metastases | 4 (6.8%) | 0(0%) | 55 (93.2%) | ||
|
| |||||
| Progesterone Receptor | Positive to Negative | Negative to Positive | Stable | ||
|
| |||||
| N= 51 Primary tumors | |||||
| 59 Metastases | 7 (11.9%) | 2 (3.4%) | 50 (84.7%) | ||
|
| |||||
| HER2 | Positive to Negative | Negative to Positive | Stable | ||
|
| |||||
| N= 51 Primary tumors | |||||
| 59 Metastases | 1 (1.7%) | 1 (1.7%) | 57 (96.6%) | ||
Table 4.
Significant changes in the levels of PIK3CA mutations in the primary and metastatic tumors
| Direction | PIK3CA Mutation Level | PIK3CA Mutation Level |
|---|---|---|
| Primary Tumor | Metastases | |
| Increase | 12% | 30% |
| Increase | 17% | 27% |
| Increase | 17% | 76% |
| Increase | 9% | 47% |
| Decrease | 69% | 24% |
There were no differences in median TTR in between patients that had discordant tumors compared to patients that had concordant tumors by PTEN levels and PIK3CA mutation status: 37 months vs. 40 months, P= 0.922.
The therapy for breast cancer patients is currently different based on hormone receptor and HER2 expression. The breast cancer subtype was different between the primary and the metastasis in 4 patients, which would result in a change in therapy based on current guidelines. For two HR-positive primary tumors: one was a TNBC and lost PTEN in both metastases, while maintaining a PIK3CA mutation in the primary in one metastatic site, but with the PIK3CA mutation not being detected in a second metastatic site. One HER2-positive cancer became TNBC and gained PTEN; and one TNBC became HER2-positive, which was associated with the detection of a PIK3CA mutation in the metastatic but not the primary site.
Discussion
The PI3K/Akt/mTOR signaling pathway is an emerging therapeutic target for cancer therapy. As a result there is an urgent need to identify robust markers that can determine pathway activity and likelihood of benefit from pathway targeting therapy. Mutations at the PIK3CA gene and the levels of PTEN are being studied as possible predictive markers for PI3K pathway inhibitors. However, it is unknown, if the assessment of these markers in the primary tumor accurately reflects the status of these markers in metastases arising from the same tumor. This is particularly important in breast cancer, where the primary tumor is removed surgically and the target for therapy is usually occult micro-metastases. We report the results of a systematic evaluation of concordance in PTEN levels and PI3K pathway mutations in matched primary tumor and metastases. We found that overall, PI3K pathway aberrations are common, and that half of primary tumors as well as metastases have either PIK3CA mutations, PTEN loss or both. The overall rates of PIK3CA mutation and PTEN loss were similar in the primary and matched metastasis. Further PIK3CA and PTEN loss were found together with approximately the expected frequency indicating that there was neither co-selection nor mutual exclusion for these events. However, we found marked discordances in PTEN levels (26%), PIK3CA mutations (18%), and receptor status (25%) between the primary tumor and metastases. Strikingly, there was both gain and loss of ability to detect PTEN and PIK3CA mutations between the primary and metastasis suggesting that at least in terms of PIK3CA and PTEN, metastasis is likely a stochastic event with metastatic competence not being dependent on aberrations in the PI3K/Akt/mTOR pathway.
Several studies have addressed the issue of discordance in expression of individual breast cancer markers (ER, PR and HER2) between primary tumor and recurrence/metastasis, but discordance rates varied substantially from study to study (1,2,11,15–17). Others have studied different markers with similar conflicting results. Lacroix et al, looked at the membrane protein levels of HER2 and epidermal growth factor (EGF) receptor as well as gene aberrations affecting these oncogenes in human primary and metastatic lesions. Among 57 patients, expression level and gene copy numbers of HER2 or EGF receptor were similarly altered in the primary tumor and metastatic lesions derived from the same patient (18). Using IHC or sandwich enzyme immunoassay investigators determine expression levels of HER2 and p53 proteins in 42 breast cancer samples from 21 patients who underwent surgery for primary tumors and surgical resection of asynchronous metastases. ER and PR were also measured by enzyme immunoassay in each case. Although, there was no difference in the positivity rate of HER2 and p53 expression between the primary tumors and the metastases, there was 50% discordance in ER and PR expression (19). Using IHC, Cardoso et al., reported the percentage of discordant biomarker status in the primary tumor and its metastatic lymph nodes to be 2% for HER2, 6% for p53, 15% for bcl-2, 19% for topoisomerase II-α, 24% for HSP27 and 30% for HSP70. For the subgroup of patients with positive biomarkers in the primary tumor, the percentage of discordance was 6% for HER2, 7% for p53, 14% for bcl-2, 19% for HSP70, 21% for topoisomerase II-α and 36% for HSP27. For the subgroup of patients with positive biomarkers in the lymph nodes, the percentage of discordance was 9% for bcl-2, 15% for HER2 and p53, 21% for topoisomerase II-α, 22% for HSP27 and 25% for HSP70 (20).
Previous reports have not compared PI3K pathway mutational status and PTEN levels in primary tumors and their corresponding metastases. However investigators recently reported the frequency of PIK3CA mutations in in-situ and invasive breast cancer. They sequenced exons 9 and 20 of PIK3CA in pure ductal carcinoma in situ (DCIS), DCIS adjacent to invasive carcinoma, and invasive ductal breast carcinomas. In a subset of cases, both in situ and invasive areas were analyzed from the same tumor. They found that the frequency of PIK3CA mutations was approximately 30% in all three histologic groups, consistent with previous reports for invasive cancer (21). Interestingly in a third of the cases, in situ and invasive areas of the same tumor were discordant for PIK3CA status, and in two cases in which multiple invasive and adjacent in situ areas within the same tumor were analyzed independently, they detected intratumor heterogeneity for PIK3CA mutations (22). Thus there appear to be multiple tumor subclones with different mutation status present in primary tumors. Using IHC, we previously looked at the discordance in the expression of pAkt (Ser473) and p4E-BP1 (Ser65) between primary breast cancer and matched surgically resected metastases. We found that most primary breast tumors and metastases expressed pAkt (76% of each). However of the 23 matched evaluable pairs, 11 (47.8%) had discordant IHC results. Similarly, although most of the primary and metastatic tumors were positive for p4E-BP1 (75% and 74%), of the 23 matched evaluable pairs, 8 (47.8%) were discordant (11).
Our study suggests that PIK3CA mutation status and PTEN levels may differ between primary tumors and their metastases. We had anticipated that if there were discordances, these would be consistent with higher PI3K/Akt/mTOR signaling in metastases because of molecular evolution, therapy exposure/selection and increased metastatic potential of active pathway clones. Unexpectedly, we found that almost the same proportion of patients had activating PIK3CA mutations in their primary tumor and not in the metastasis as had activating mutations in their metastasis but not in the primary. We found similar results when looking at PTEN levels. The gain and loss frequencies between primary and metastatic sites indicates that the aberrations in the PI3K pathway assayed are not obligatory for the metastatic process, did not drive the metastatic process and are not selected during the metastatic process. There were also some cases in which we detected marked differences in the percent of cells with PIK3CA mutations between primary and metastases indicative of dominance of different clones in the primary and metastasis. The underlying mechanism and clinical significance of these changes should be formally studied. However, it is clear that this should be considered in the design and implementation of trials of PI3K pathway targeted therapy.
Changes in receptor expression may either account for a true biological phenomenon or may result from inconsistent measurement. It has been argued that changes in receptor expression may occur at different time points of disease (molecular evolution), and possible reasons include: change during tumor progression as a consequence of genetic instabiilty or clonal selection (23), intratumoral heterogeneity (24,25), selection by therapy or metastatic process, and mutations induced by cellular exposure to systemic agents such as chemotherapy or hormonal therapy, or targeted therapy. However, evidence derived from transcriptional profiling suggests that the hard-wired characteristics of individual tumors do not change by chance throughout the course of the disease. Weigelt et al. (26) showed that gene expression profiles of metastases clustered closely to their corresponding primary tumors, which may indicate that the metastatic capability may be an intrinsic feature of the primary tumor. As an alternative hypothesis for this observation, the particular genomic aberrations driving the tumor may only allow the tumor to explore a limited transcriptional space and that processes regulating metastasis are not able to dominate the underlying transcriptional pattern.
Further studies are needed to confirm our findings, and if they represent a true biological discordance as discussed before or the limitations of marker development such as the poor reproducibility of IHC, or preanalytic variables including variation in processing and fixation of tumors, tumor hypoxia times, and tumor cellularity. However, it is important to emphasize that the MassArray approach applied to assess DNA mutations is highly robust and unlikely to demonstrated a significant false positive or negative rate and also to be able to detect relatively rare events in the tumor mass. As new targeted therapies are brought into clinical trials using primary tumor signatures for patient selection may not represent the signature status in the metastatic disease that is being treated and this may have significant outcome implications. Indeed, it may be necessary to develop approaches to assess predictive signatures present in metastatic disease to engender optimal outcomes. This could be through repeat biopsies, novel molecular imaging technologies or potentially through analysis of circulating DNA, microRNA or tumor cells. Identification of robust biomarkers that can accurately assess driver aberrations and predict response to therapies, and that can be used widely with low inter-laboratory variability is critical to successful delivery personalized cancer therapy.
Acknowledgments
Funding: This work was supported in part by SUC2-AACR-DT0209 01(AMG, GBM, FMB), National Cancer Institute 1K23CA121994-01 (AMG), ASCO Career Development Award (AMG), KGKG081099 (AMG, KSH, GBM), and National Cancer Institute through The University of Texas M. D. Anderson’s Cancer Center Support Grant (P30 CA016672).
References
- 1.Li BD, Byskosh A, Molteni A, Duda RB. Estrogen and progesterone receptor concordance between primary and recurrent breast cancer. J Surg Oncol. 1994;57:71–77. doi: 10.1002/jso.2930570202. [DOI] [PubMed] [Google Scholar]
- 2.Simon R, Nocito A, Hübscher T, Torhorst J, Schraml P, Bubendorf L, et al. Patterns of her-2/neu amplification and overexpression in primary and metastatic breast cancer. J Natl Cancer Inst. 2001;93:1141–1146. doi: 10.1093/jnci/93.15.1141. [DOI] [PubMed] [Google Scholar]
- 3.Mittendorf EA, Wu Y, Scaltriti M, Meric-Bernstam F, Hunt KK, Dawood S, et al. Loss of HER2 amplification following trastuzumab-based neoadjuvant systemic therapy and survival outcomes. Clin Cancer Res. 2009;15:7381–8. doi: 10.1158/1078-0432.CCR-09-1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liedtke C, Broglio K, Moulder S, Hsu L, Kau SW, Symmans WF, et al. Prognostic impact of discordance between triple receptor measurements in primary and recurrent breast cancer. Ann Oncol. 2009;20:1953–8. doi: 10.1093/annonc/mdp263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meric Berstam F, Gonzalez-Angulo AM. Targeting the mTOR signaling network for cancer therapy. J Clin Oncol. 2009;27:2278–87. doi: 10.1200/JCO.2008.20.0766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agarwal R, Carey M, Hennessy B, Mills GB. PI3K pathway-directed therapeutic strategies in cancer. Curr Opin Investig Drugs. 2010;11:615–28. [PubMed] [Google Scholar]
- 7.Perez-Tenorio G, Stal O. Activation of AKT/PKB in breast cancer predicts a worse outcome among endocrine treated patients. Br J Cancer. 2002;86:540–5. doi: 10.1038/sj.bjc.6600126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller TW, Perez-Torres M, Narasanna A, Guix M, Stål O, Pérez-Tenorio G, et al. Loss of Phosphatase and Tensin homologue deleted on chromosome 10 engages ErbB3 and insulin-like growth factor-I receptor signaling to promote antiestrogen resistance in breast cancer. Cancer Res. 2009;69:4192–201. doi: 10.1158/0008-5472.CAN-09-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nagata Y, Lan KH, Zhou X, Tan M, Esteva FJ, Sahin AA, et al. PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell. 2004;6:117–27. doi: 10.1016/j.ccr.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 10.Berns K, Horlings HM, Hennessy BT, Madiredjo M, Hijmans EM, Beelen K, et al. A functional genetic approach identifies the PI3K pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer Cell. 2007;12:395–402. doi: 10.1016/j.ccr.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 11.Akcakanat A, Sahin A, Shaye AN, Velasco MA, Meric-Bernstam F. Comparison of Akt/mTOR signaling in primary breast tumors and matched distant metastases. Cancer. 2008;112:2352–58. doi: 10.1002/cncr.23456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allred DC, Harvey JM, Berardo M, Clark GM. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol. 1998;11:155–68. [PubMed] [Google Scholar]
- 13.Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, et al. American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010;28:2784–95. doi: 10.1200/JCO.2009.25.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25:118–45. doi: 10.1200/JCO.2006.09.2775. [DOI] [PubMed] [Google Scholar]
- 15.Vincent-Salomon A, Jouve M, Genin P, Fréneaux P, Sigal-Zafrani B, Caly M, et al. HER2 status in patients with breast carcinoma is not modified selectively by preoperative chemotherapy and is stable during the metastatic process. Cancer. 2002;94:2169–73. doi: 10.1002/cncr.10456. [DOI] [PubMed] [Google Scholar]
- 16.Simon R, Nocito A, Hübscher T, Bucher C, Torhorst J, Schraml P, et al. Patterns of her-2/neu amplification and overexpression in primary and metastatic breast cancer. J Natl Cancer Inst. 2001;93:1141–46. doi: 10.1093/jnci/93.15.1141. [DOI] [PubMed] [Google Scholar]
- 17.Gancberg D, Di Leo A, Cardoso F, Rouas G, Pedrocchi M, Paesmans M, et al. Comparison of HER-2 status between primary breast cancer and corresponding distant metastatic sites. Ann Oncol. 2002;13:1036–43. doi: 10.1093/annonc/mdf252. [DOI] [PubMed] [Google Scholar]
- 18.Lacroix H, Iglehart JD, Skinner MA, Kraus MH. Overexpression of erbB-2 or EGF receptor proteins present in early stage mammary carcinoma is detected simultaneously in matched primary tumors and regional metastases. Oncogene. 1989;4:145–51. [PubMed] [Google Scholar]
- 19.Shimizu C, Fukutomi T, Tsuda H, Akashi-Tanaka S, Watanabe T, Nanasawa, et al. c-erbB-2 protein overexpression and p53 immunoreaction in primary and recurrent breast cancer tissues. J Surg Oncol. 2000;73:17–20. doi: 10.1002/(sici)1096-9098(200001)73:1<17::aid-jso5>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 20.Cardoso F, Di Leo A, Larsimont D, Gancberg D, Rouas G, Dolci S, et al. Evaluation of HER2, p53, bcl-2, topoisomerase II-alpha, heat shock proteins 27 and 70 in primary breast cancer and metastatic ipsilateral axillary lymph nodes. Ann Oncol. 2001;12:6415–20. doi: 10.1023/a:1011182524684. [DOI] [PubMed] [Google Scholar]
- 21.Stemke-Hale K, Gonzalez-Angulo AM, Lluch A, Neve RM, Kuo WL, Davies M, et al. An integrative genomic and proteomic analysis of PIK3CA, PTEN, and AKT mutations in breast cancer. Cancer Res. 2008;68:6084–91. doi: 10.1158/0008-5472.CAN-07-6854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miron A, Varadi M, Carrasco D, Li H, Luongo L, Kim HJ, et al. PIK3CA mutations in in situ and invasive breast carcinomas. Cancer Res. 2010;70:5674–8. doi: 10.1158/0008-5472.CAN-08-2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edgerton SM, Moore D, II, Merkel D, Thor AD. erbB-2 (HER-2) and breast cancer progression. Appl Immunohistochem Mol Morphol. 2003;11:214–21. doi: 10.1097/00129039-200309000-00003. [DOI] [PubMed] [Google Scholar]
- 24.Pertschuk LP, Axiotis CA, Feldman JG, Kim YD, Karavattayhayyil SJ, Braithwaite L. Marked intratumoral heterogeneity of the proto-oncogene Her-2/neu determined by three different detection systems. Breast J. 1999;5:369–74. doi: 10.1046/j.1524-4741.1999.97088.x. [DOI] [PubMed] [Google Scholar]
- 25.Kerbel RS. Growth dominance of the metastatic cancer cell: cellular and molecular aspects. Adv Cancer Res. 1990;55:87–132. doi: 10.1016/s0065-230x(08)60469-8. [DOI] [PubMed] [Google Scholar]
- 26.Weigelt B, Glas AM, Wessels LF, Witteveen AT, Peterse JL, van’t Veer LJ, et al. Gene expression profiles of primary breast tumors maintained in distant metastases. Proc Natl Acad Sci U S A. 2003;100:15901–5. doi: 10.1073/pnas.2634067100. [DOI] [PMC free article] [PubMed] [Google Scholar]