Abstract
Regulation of the Rho switch has been typically centered on their main regulators, RhoGEFs and RhoGAPs. On the side, RhoGDI proteins have been considered mostly as passive regulators devoid of catalytic activity simply holding Rho proteins in the cytosol. In the May issue of Nature Cell Biology,1 we describe a novel evolutionary conserved function for RhoGDI1 as a chaperoning protein which prevents degradation of prenylated Rho GTPases. The limited amount of RhoGDI1 in cells generates a competitive balance between GTPases in order to prevent their degradation. Therefore, this creates a crosstalk regulatory mechanism of Rho proteins, whereby the level of one Rho protein can affect both the level and activity of the others. For example, overexpression of a single GTPase will promote the degradation and inactivation of all endogenous Rho proteins bound to GDI. These results suggest that some of the conclusions drawn from studies that manipulate Rho protein levels may need to be reevaluated. Here, we discuss some of the consequences of this mechanism on the regulation of Rho proteins, the dissociation of Rho-RhoGDI complexes by GDF and whether this regulation might be extended to other GTPases of the Ras superfamily.
Key words: RhoGDI, guanine nucleotide dissociation inhibitor, protein degradation, Rho GTPases, crosstalk
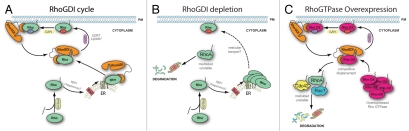
Rho proteins have traditionally been envisioned as molecular switches, turned on or off by various stimuli. In this binary view, the regulation of Rho proteins relies mostly on the molecules in charge of flipping the switch, RhoGEFs and RhoGAPs, while RhoGDI proteins have been relegated to a secondary passive role, simply holding the GTPases inactive in the cytosol.2 In a Letter published in the May issue of Nature Cell Biology, we uncover unexpected functions of RhoGDI1, which turns out to be critical for both homeostasis of Rho proteins and crosstalk between family members1 (Fig. 1). We report that in eukaryotic cells, including mammalian cells and the yeast Saccharomyces cerevisiae, depletion of RhoGDI1 or its yeast ortholog RDI1, results in an almost complete degradation of RhoA, Rac1 and Cdc42 by the proteasome (Rho1 and Cdc42 in yeast). The small fraction of Rho GTPases that are not lost to degradation are mostly active and associated with cellular membranes, most likely because they cannot be extracted efficiently from membranes in the absence of RhoGDI. The non-degraded Rho molecules accumulate at the Endoplasmic Reticulum (ER) membrane suggesting that RhoGDI is required to shuttle Rho GTPases from the ER, where post-translational processing occurs, to other membranes. Newly synthesized Rho proteins undergo C-terminal prenylation in the cytosol before transiting to the cytosolic face of the ER where their C-terminus is cleaved and carboxymethylated. This prenyl modification is absolutely required for the anchorage of Rho proteins to membranes and coordinated signaling. Paradoxically, we found that addition of this essential hydrophobic lipid moiety disturbs the proper folding and stability of Rho GTPases. Indeed, depletion of RhoGDI triggers recruitment of the molecular chaperone machinery to Rho proteins and leads to their degradation. Conversely, inhibition of geranylgeranylation favors the accumulation of Rho proteins and prevents their degradation.
Figure 1.
Regulation of RhoGTPases homeostasis by RhoGDI. (A) Following synthesis, RhoGTPases are geranylated in the cytosol and then transported to the ER where the C-terminal tail is cleaved and methylated. Rho proteins are then extracted from the ER membrane, most likely by RhoGDI. At steady state the majority of the RhoGTPases are associated with RhoGDI in the cytosol. Upon activation by GEFs, they translocate to the plasma membrane where they interact with effector proteins. Following inactivation by RhoGAPs, Rho-GTPases are extracted from the membrane by RhoGDI. (B) RhoGDI depletion promotes the rapid almost complete degradation of the major RhoGTPases, including RhoA, Rac1 and Cdc42. The remaining GTPases are mostly GTP-bound (active) and associated with membranes. Newly synthesized Rho proteins accumulate in the ER membrane. (C) Overexpression of a single RhoGTPase displaces the endogenous GTPases from their binding to RhoGDI and targets them to degradation. In contrast RhoGDI depletion, RhoGTPase overexpression results in complete inactivation of the displaced GTPases. Since RhoGDI is still able to extract the GTPases from the membrane, over time the membrane associated GTPases cycle back to RhoGDI where they are displaced by the excess of exogenously expressed proteins and are finally degraded.
Michaelson et al. previously reported that the amount of RhoGDI1 in cells is roughly equivalent to the sum of RhoA, Rac1 and Cdc42 suggesting that these GTPases compete for binding to the limited pool of RhoGDI.3 Since GDI-free GTPases are targeted for degradation, this implies competition between Rho proteins for binding to the limited pool of RhoGDI in order to avoid degradation. This competitive equilibrium predicts a previously unnoticed crosstalk between Rho proteins in which any physiological or experimental situation altering the level of a given GTPase should affect the level and activity of the other members binding to RhoGDI. We found that overexpression of any wildtype or mutant form of RhoA, Rac1 or Cdc42 proportionally displaces the other members from binding to RhoGDI leading to their degradation. In parallel, the activity of the displaced GTPases is reduced in these conditions (Fig. 1). This decrease in Rho GTPase activity is similar to that observed when Rho GTPases expression is silenced using siRNA, and contrasts with the activation of Rho GTPases caused by RhoGDI1 depletion. While in all these experimental conditions the protein levels of Rho GTPases decrease through proteasomal-mediated degradation, the differences observed in the activity can be explained by the presence or absence of RhoGDI, which can extract and inactivate the Rho proteins at the membrane.
The implications derived from this discovery are of great significance. Overexpression of Rho GTPases, in particular the constitutively active and dominant negative mutants, has been used systematically for the past 20 years and, despite the increase in the use of RNAi technology, it is still being used quite abundantly. Our results suggest that many of conclusions drawn from studies that relied exclusively in the overexpression of Rho GTPases should be taken with caution and may need to be revisited.
Overall, our study uncovered (i) a new function for RhoGDI as an essential regulator of Rho protein stability, (ii) a novel crosstalk between Rho proteins at the level of RhoGDI and (iii) that RhoGDI shuttles prenylated Rho proteins from the ER to the plasma membrane. However, although our work sheds new light on the role of RhoGDI, it also draws attention to the many unanswered questions concerning the functions and regulation of RhoGDI.
The physiological function of RhoGDI and its importance remain elusive. Is it a passive secondary regulator as originally thought, or is it a key player in the regulation of Rho proteins? So far, genetic models have provided ambiguous information. Although our study, restricted to cellular models, suggests an important function of RhoGDI, loss of RhoGDI1 in mouse and yeast generates a rather mild phenotype. In mice, disruption of the arhgdia gene results in viable animals displaying proteinuria similar to nephrotic syndrome, which progresses to renal failure, and several other defects in the reproductive system and function.4 This renal dysfunction has been associated with increased Rac1 activation in the kidney,5 which is consistent with our findings. This phenotype is fairly mild as compared to those of mice lacking Rac1 or Cdc42 for instance, which suffer embryonic lethality.6,7 Similarly, RDI1 is not needed for yeast survival or growth.8,9 However, disruption of RDI1 revealed a dual mode of recycling Cdc42: a fast recycling pathway mediated by Rdi1 and a slow recycling pathway mediated by endocytosis.8 In our study, although the level and activity of GTPases were deeply affected by loss of RhoGDI1, cells were able to proliferate and survive, and only migration of fast migrating cells was affected by loss of RhoGDI1. How can we explain these apparent discrepancies? Is there an alternative recycling pathway for Rho proteins that is independent of RhoGDI? Can other proteins such as chaperones provide enough of a GDI-like function that allows the organisms to survive? Answering these questions will help clarify the real role of RhoGDI in the Rho GTPase cycle.
The comparison between Rho and Rab GTPases may provide valuable hints on the function and regulation of RhoGDI. Similar to Rho proteins, Rab GTPases are prenylated and regulated by GDIs (RabGDIs). However, in contrast to Rho proteins, the molecular mechanisms regulating the interactions between Rabs and RabGDIs are better understood (reviewed in ref. 10 and 11). Newly synthesized Rab proteins associate with Rab escort proteins (REPs). This complex can then bind to the Rab geranylgeranyl transferases (GGTs) which prenylate the Rab proteins and release them to RabGDIs. RabGDIs then shuttle prenylated Rabs to/from various membranes. The parallel with Rabs prompts us to wonder, for instance, if there are escort proteins that shuttle Rho GTPases or whether RhoGDIs can assume this function. Alternatively, molecular chaperones such as Hsp70 or Hsp90 may convey unprenylated Rho GTPases to the ER. Loss of RhoGDI1 results in retention of Rho proteins in the ER, suggesting that GDI is required to shuttle Rho proteins from the ER but not to the ER.1 Chaperones, such as Hsp90, bind Rho GTPases in the absence of GDI, suggesting that they may interact before Rho proteins enter the ER.1
Finally, the concept and identity of GDI Dissociation Factors (GDF), which has been largely documented for Rabs, still remains a matter of debate for Rho proteins. Fundamentally, a GDF at physiological concentration can release a prenylated GTPase from its GDI, an interaction that is usually very tight (affinity of Rab9 for RabGDI, Kd = 20 nM). Several proteins such as the ERM (ezrin, radixin, moesin) proteins, the neurotropin receptor (p75NTR) and the tyrosine kinase Etk have been reported to displace Rho proteins from RhoGDI.12–14 However, none of these diverse proteins constituted an archetypal model of GDF for Rho proteins. In contrast, the evolutionary conserved Ypt—interacting protein family (Yip), constitutes a model of GDI release for Rab proteins.11 Our recent findings strongly argue that displacement of Rho proteins from RhoGDI has to be somehow coupled to their association with some other cellular entity in order to prevent their degradation. Recent studies in the Rho and the Rab fields explore the exciting possibility that GDI displacement could be coupled to nucleotide exchange, i.e., that GEFs may behave as GDFs. For instance, the DrrA/SidM protein from Legionella pneumophila possesses a GEF domain and can simultaneously trigger GDP for GTP nucleotide exchange and GDI displacement from Rab1-GDI complexes.15,16 As for Rho proteins, the Cerione lab showed that, in the presence of lipid membranes, RhoGDI has a higher affinity for GDP-Cdc42 than for GTP-Cdc42.17 Therefore, Rho protein activation at membranes would decrease the affinity of GTP-bound Rho proteins for RhoGDI while increasing their affinity for the effector proteins. This would slow down the release of GTPases from membranes and favor the accumulation of Rho GTPases at the membrane. Although not the active displacement model originally envisioned, this mechanism would have the advantage of directly coupling Rho proteins to the membrane, preventing their degradation.
The degradation of free Rho proteins could also be a regulatory pathway in the case of GDF-independent dissociation of Rho-RhoGDI complexes. In our model (Fig. 1) we have arbitrarily assumed a constant similar affinity of all the Rho GTPases for RhoGDI, but several groups have shown that posttranslational modifications on RhoGDI can modulate its affinity for specific GTPases. For example, phosphorylation of RhoGDI1 by Pak1 reduced its affinity for Rac1 but not RhoA, promoting its release and subsequent activation.18 Similarly, Ser34 or Ser96 phosphorylation selectively releases and activates RhoA but not Rac1 or Cdc42.19,20 Ser96 phosphorylation also induces the selective release and activation of RhoG which promotes Rac1 activation downstream.21 None of these studies has analyzed the total levels of the Rho GTPases after RhoGDI1 phosphorylation, but based on our results, it is possible that the phosphorylation-mediated release of individual GTPases targets them to degradation and serves as a mechanism to regulate their levels.
Is this type of regulation by a GDI or a GDI-like molecule relevant for other GTPases? Many GTPases are post-translationally modified by lipid moieties, including Rab, Ras, Arf and Rho proteins. However, GDI proteins have only been described for Rab and Rho GTPases. It has been proposed by the Kloog lab that galectin 1 and 3 may behave as GDI-like proteins for Ras and have structural features similar to RhoGDI.22–25 Indeed, chemical compounds preventing protein prenylation such as statins or farnesyl thiosalicylate (FTS) induce accumulation of Rho or Ras proteins suggesting that preventing prenylation is sufficient to bypass GTPase degradation.1,26 Therefore, it is tempting to speculate that other GTPases of the Ras superfamily may be regulated similarly by GDI-like molecules. Such a general regulatory mechanism would be particularly interesting for Ras since galectin 1 and 3 bind preferentially activated Ras rather than GDP-Ras. Future investigations will certainly answer these questions.
Finally, in the era of genomics, proteomics and transcriptomics, it looks like these molecular switches of the Rho family still require a lot of unexpected wiring to illuminate their signaling pathways.
Acknowledgements
The authors would like to thank Keith Burridge for comments, the city of Nice (France) and Chloé Féral for financial support. The authors were supported by National Institutes of Health Grant #GM029860 (to K.B.), a Susan Komen Foundation Postdoctoral Fellowship and a AHA Beginning Grant in Aid (#5-40078 to R.G.M.) and a Fellowship from the city of Nice (to E.B.) and an Allocation INSERM InCa/AVENIR (#R08227AS to C.F.).
Extra View to: Boulter E, Garcia-Mata R, Guilluy C, Dubash A, Rossi G, Brennwald PJ, et al. Regulation of Rho GTPase crosstalk, degradation and activity by RhoGDI1. Nat Cell Biol. 2010;12:477–483. doi: 10.1038/ncb2049.
Footnotes
Previously published online: www.landesbioscience.com/journals/smallgtpases/article/12990
References
- 1.Boulter E, Garcia-Mata R, Guilluy C, Dubash A, Rossi G, Brennwald PJ, et al. Regulation of Rho GTPase crosstalk, degradation and activity by RhoGDI1. Nat Cell Biol. 2010;12:477–483. doi: 10.1038/ncb2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bos JL, Rehmann H, Wittinghofer A. GEFs and GAPs: critical elements in the control of small G proteins. Cell. 2007;129:865–877. doi: 10.1016/j.cell.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 3.Michaelson D, Silletti J, Murphy G, D'Eustachio P, Rush M, Philips MR. Differential localization of Rho GTPases in live cells: regulation by hypervariable regions and RhoGDI binding. J Cell Biol. 2001;152:111–126. doi: 10.1083/jcb.152.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Togawa A, Miyoshi J, Ishizaki H, Tanaka M, Takakura A, Nishioka H, et al. Progressive impairment of kidneys and reproductive organs in mice lacking Rho GDIalpha. Oncogene. 1999;18:5373–5380. doi: 10.1038/sj.onc.1202921. [DOI] [PubMed] [Google Scholar]
- 5.Shibata S, Nagase M, Yoshida S, Kawarazaki W, Kurihara H, Tanaka H, et al. Modification of mineralocorticoid receptor function by Rac1 GTPase: implication in proteinuric kidney disease. Nat Med. 2008;14:1370–1376. doi: 10.1038/nm.1879. [DOI] [PubMed] [Google Scholar]
- 6.Sugihara K, Nakatsuji N, Nakamura K, Nakao K, Hashimoto R, Otani H, et al. Rac1 is required for the formation of three germ layers during gastrulation. Oncogene. 1998;17:3427–3433. doi: 10.1038/sj.onc.1202595. [DOI] [PubMed] [Google Scholar]
- 7.Chen F, Ma L, Parrini MC, Mao X, Lopez M, Wu C, et al. Cdc42 is required for PIP(2)-induced actin polymerization and early development but not for cell viability. Curr Biol. 2000;10:758–765. doi: 10.1016/s0960-9822(00)00571-6. [DOI] [PubMed] [Google Scholar]
- 8.Slaughter BD, Das A, Schwartz JW, Rubinstein B, Li R. Dual modes of cdc42 recycling fine-tune polarized morphogenesis. Dev Cell. 2009;17:823–835. doi: 10.1016/j.devcel.2009.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tiedje C, Sakwa I, Just U, Hofken T. The Rho GDI Rdi1 regulates Rho GTPases by distinct mechanisms. Mol Biol Cell. 2008;19:2885–2896. doi: 10.1091/mbc.E07-11-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pfeffer S, Aivazian D. Targeting Rab GTPases to distinct membrane compartments. Nat Rev Mol Cell Biol. 2004;5:886–896. doi: 10.1038/nrm1500. [DOI] [PubMed] [Google Scholar]
- 11.Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol. 2009;10:513–525. doi: 10.1038/nrm2728. [DOI] [PubMed] [Google Scholar]
- 12.Kim O, Yang J, Qiu Y. Selective activation of small GTPase RhoA by tyrosine kinase Etk through its pleckstrin homology domain. J Biol Chem. 2002;277:30066–30071. doi: 10.1074/jbc.M201713200. [DOI] [PubMed] [Google Scholar]
- 13.Takahashi K, Sasaki T, Mammoto A, Takaishi K, Kameyama T, Tsukita S, et al. Direct interaction of the Rho GDP dissociation inhibitor with ezrin/radixin/moesin initiates the activation of the Rho small G protein. J Biol Chem. 1997;272:23371–23375. doi: 10.1074/jbc.272.37.23371. [DOI] [PubMed] [Google Scholar]
- 14.Yamashita T, Tohyama M. The p75 receptor acts as a displacement factor that releases Rho from Rho-GDI. Nat Neurosci. 2003;6:461–467. doi: 10.1038/nn1045. [DOI] [PubMed] [Google Scholar]
- 15.Machner MP, Isberg RR. A bifunctional bacterial protein links GDI displacement to Rab1 activation. Science. 2007;318:974–977. doi: 10.1126/science.1149121. [DOI] [PubMed] [Google Scholar]
- 16.Schoebel S, Oesterlin LK, Blankenfeldt W, Goody RS, Itzen A. RabGDI displacement by DrrA from Legionella is a consequence of its guanine nucleotide exchange activity. Mol Cell. 2009;36:1060–1072. doi: 10.1016/j.molcel.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 17.Johnson JL, Erickson JW, Cerione RA. New insights into how the Rho guanine nucleotide dissociation inhibitor regulates the interaction of Cdc42 with membranes. J Biol Chem. 2009;284:23860–23871. doi: 10.1074/jbc.M109.031815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DerMardirossian C, Schnelzer A, Bokoch GM. Phosphorylation of RhoGDI by Pak1 mediates dissociation of Rac GTPase. Mol Cell. 2004;15:117–127. doi: 10.1016/j.molcel.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 19.Dovas A, Choi Y, Yoneda A, Multhaupt HA, Kwon SH, Kang D, et al. Serine34 phosphorylation of RHO guanine dissociation inhibitor (RHOGDI{alpha}) links signaling from conventional protein kinase C to RHO GTPase in cell adhesion. J Biol Chem. 2010 doi: 10.1074/jbc.M109.098129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knezevic N, Roy A, Timblin B, Konstantoulaki M, Sharma T, Malik AB, et al. GDI-1 phosphorylation switch at serine 96 induces RhoA activation and increased endothelial permeability. Mol Cell Biol. 2007;27:6323–6333. doi: 10.1128/MCB.00523-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elfenbein A, Rhodes JM, Meller J, Schwartz MA, Matsuda M, Simons M. Suppression of RhoG activity is mediated by a syndecan 4-synectin-RhoGDI1 complex and is reversed by PKCalpha in a Rac1 activation pathway. J Cell Biol. 2009;186:75–83. doi: 10.1083/jcb.200810179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elad-Sfadia G, Haklai R, Balan E, Kloog Y. Galectin-3 augments K-Ras activation and triggers a Ras signal that attenuates ERK but not phosphoinositide 3-kinase activity. J Biol Chem. 2004;279:34922–34930. doi: 10.1074/jbc.M312697200. [DOI] [PubMed] [Google Scholar]
- 23.Elad-Sfadia G, Haklai R, Ballan E, Gabius HJ, Kloog Y. Galectin-1 augments Ras activation and diverts Ras signals to Raf-1 at the expense of phosphoinositide 3-kinase. J Biol Chem. 2002;277:37169–37175. doi: 10.1074/jbc.M205698200. [DOI] [PubMed] [Google Scholar]
- 24.Paz A, Haklai R, Elad-Sfadia G, Ballan E, Kloog Y. Galectin-1 binds oncogenic H-Ras to mediate Ras membrane anchorage and cell transformation. Oncogene. 2001;20:7486–7493. doi: 10.1038/sj.onc.1204950. [DOI] [PubMed] [Google Scholar]
- 25.Rotblat B, Niv H, Andre S, Kaltner H, Gabius HJ, Kloog Y. Galectin-1(L11A) predicted from a computed galectin-1 farnesyl-binding pocket selectively inhibits Ras-GTP. Cancer Res. 2004;64:3112–3118. doi: 10.1158/0008-5472.can-04-0026. [DOI] [PubMed] [Google Scholar]
- 26.Haklai R, Weisz MG, Elad G, Paz A, Marciano D, Egozi Y, et al. Dislodgment and accelerated degradation of Ras. Biochemistry. 1998;37:1306–1314. doi: 10.1021/bi972032d. [DOI] [PubMed] [Google Scholar]