Abstract
The direction and specificity of endolysosomal membrane trafficking is tightly regulated by various cytosolic and membrane-bound factors, including soluble NSF attachment protein receptors (SNAREs), Rab GTPases, and phosphoinositides. Another trafficking regulatory factor is juxtaorganellar Ca2+, which is hypothesized to be released from the lumen of endolysosomes and to be present at higher concentrations near fusion/fission sites. The recent identification and characterization of several Ca2+ channel proteins from endolysosomal membranes has provided a unique opportunity to examine the roles of Ca2+ and Ca2+ channels in the membrane trafficking of endolysosomes. SNAREs, Rab GTPases, and phosphoinositides have been reported to regulate plasma membrane ion channels, thereby suggesting that these trafficking regulators may also modulate endolysosomal dynamics by controlling Ca2+ flux across endolysosomal membranes. In this review, we will discuss about the roles of phosphoinositides, Ca2+, and potential interactions between endolysosomal Ca2+ channels and phosphoinositides in endolysosomal dynamics.
Keywords: Ca2+; lysosomes; TRPML channel; PI(3,5)P2; membrane trafficking
Overview of endolysosomal dynamics
Mammalian endosomes and lysosomes (collectively referred to as endolysosomes) frequently exchange their contents, or cargos, with each other or with other intracellular organelles/vesicles through membrane fusion and fission processes. These events are essential for many cellular functions including protein transport, signaling transduction, transmitter release, autophagy, and lysosomal degradation of macromolecules [1,2]. Based on their membrane composition and function, endosomes can be classified as early endosomes, recycling endosomes, or late endosomes [3]. Upon endocytosis, plasma membrane lipids and proteins are first delivered to early endosomes, where they are either sorted to recycling endosomes to be trafficked back to the plasma membrane, or are processed through the late endosome-lysosome (LEL) pathway for degradation [1,4].
Endolysosomal membrane trafficking is a spatiotemporally regulated process that ensures proper delivery of cargo via the endolysosomal pathway. Both early and late endosomes can bud inwardly from their limited membranes to form luminal vesicles, which are referred to as multi-vesicular bodies (MVB). These vesicles contain ubiquitinated membrane proteins which are eventually sorted into lysosomes that contain acidic hydrolases for macromolecular digestion [3]. However lysosomes can also fuse with late endosomes to acquire macromolecules that are sorted for lysosomal degradation, or to obtain newly synthesized hydrolytic enzymes from the trans-Golgi network (TGN) pathway [2,5]. When lysosomes fuse with autophagosomes or phagosomes, fusion events mediate the degradation of obsolete cytoplasmic components/organelles or invading pathogens, respectively [2,6]. In addition, lysosomes have also been shown to mediate plasma membrane repair via the exocytosis of lysosomal contents, i.e., lysosomal exocytosis [7]. On the other hand, lysosomes can be regenerated from late endosome-lysosome hybrids based on membrane fission events, which is a process commonly referred to as lysosome biogenesis [2,8,9]. Membrane fission also facilitates the recycling of materials obtained from late endosomes or lysosomes to the TGN via retrograde transport vesicles [2,10]. When lysosomal trafficking is interrupted or compromised, a variety of neurological or lysosomal storage diseases can develop [2,11–14].
Endolysosomal trafficking, i.e., membrane fusion/fission, is regulated by sequential and stepwise recruitment of a variety of cytosolic and membrane-bound proteins and factors [13,15–17]. Extensive studies of exocytosis, particularly neurotransmission, have elucidated many of the essential components of membrane fusion events that take place during exocytosis [16]. For example, soluble NSF attachment protein receptors (SNAREs) have been identified as the basic structural components needed for membrane fusion events [16,18]. For the regulation of pre-fusion steps (i.e., priming, tethering, and docking), Rab small GTPases, Rab effectors, tethering factors, and phosphoinositides (PIPs) have been found to be important factors [13,15,16,18,19]. Numerous in vitro and in vivo studies suggest that intracellular membrane fusion events in the endolysosomal pathway are regulated by mechanisms similar to those used in exocytosis [15,16,20]; loss-of-function studies in yeast and mammalian cells have identified a similar, yet unique, set of proteins mediating endolysosome trafficking, i.e., endolysosome-specific SNAREs/tethering factors/Rabs/PIPs. Likewise, while previous studies have also demonstrated that Ca2+ influx through voltage-gated Ca2+ channels triggers the final step in exocytotic membrane fusion events during neurotransmission [16], levels of juxtaorganellar Ca2+ are hypothesized to regulate endolysosomal dynamics [2,20–22]. With membrane fission events generally viewed as the reverse of membrane fusion events, fission and fusion events would be expected to share many common mechanisms, including regulation by Rabs, phosphoinositides, and Ca2+ levels near the fission site [2,13,20,23–26].
Currently, the most challenging question in the field of endolysosomal trafficking is how the aforementioned factors are coordinated to control the direction and specificity of trafficking processes. While many components of trafficking pathways have been readily assigned to one of the following steps, i.e., priming, tethering, docking, or fusion [15,18], there may be additional roles mediated by these factors that remain unknown. For example, recent evidence suggests that in addition to their roles as structural determinants of fusion, SNAREs can also regulate Rab effectors [13] and plasma membrane Ca2+ channels [27]. In yeast, trans-SNARE complex formation may directly induce Ca2+ release from the lumen of the vacuole [28]. Similarly, phosphoinositides have been shown to be important not only for the recruitment of Rabs and tethering factors, but also for interactions with SNAREs [29,30] and Ca2+ sensors [29]. In addition, recent results in our laboratory have suggested that phosphoinositides directly activate Ca2+ release channels [31]. In this review, the roles of phosphoinositides and Ca2+ in regulating endolysosomal dynamics will be discussed and analyzed.
Trans-SNARE complexes in endolysosomes: structural components of the fusion machinery
After tethering factors make contact with two endolysosomal membranes, a trans-SNARE complex must be formed prior to membrane fusion [32]. Tethering factors that have been identified include EEA1 for early endosomes [15], and HOPS for late endosomes [33]. The resulting SNARE complex that is formed, is a highly stable, four-helix bundle formed by three Q-SNAREs on one membrane, and one R-SNARE on the opposing membrane [15]. In lysosomes, Vamp7 has been identified as an R-SNARE, and Syntaxins −7 and −8 are Q-SNAREs present on LELs [2]. After a trans-SNARE complex is formed, a bilayer mixing reaction is initiated [2,15]. However, SNARE interactions alone are not sufficient for membrane fusion to efficiently occur [15]. Additional regulatory proteins are required, and these include Rab effectors and Ca2+ sensor synaptotagmins which facilitate interactions between the fusing membranes and SNAREs [15,18,29]. To read more on this topic, there are several excellent reviews that are available (see Refs. [15,18,19]).
Rab small GTPases: primitive organelle tags and coordinators of endolysosomal trafficking
Rab proteins are compartmentalized in specific organelle membranes, establishing the basic tags to define organelle identity [13]. Rab proteins can recruit additional effectors, and depending on the types of Rab proteins and effectors present, an organelle’s identity and transport specificity is further determined [13]. For example, while Rab5 is localized in the early endosome, Rab7 is localized to LELs. Rab effectors including sorting adaptors, tethering factors, lipid kinases, and lipid phosphatases [13]. Tethering factors mediate vesicle tethering by interacting with molecules on the acceptor membrane [19], while lipid kinases produce phosphoinositides (see below) to further define vesicular identity and the direction of membrane trafficking [17,25,34]. Importantly, it is the interactions between Rab proteins and lipid kinases/phosphatases that form a functional interaction loop to regulate membrane trafficking [34]. While phosphoinositides can recruit phosphoinositide-binding proteins to regulate the activity of GTPases, GTPases can in turn control the activity of PIP-metabolizing enzymes [34]. Rabs and Rab effectors can also interact with SNAREs and their accessory proteins to regulate the formation of SNARE complexes, thus modulating membrane fusion processes [13]. This topic has also been excellently reviewed elsewhere (see Ref. [13]).
Phosphoinositides: organelle tags and regulators of endolysosomal trafficking
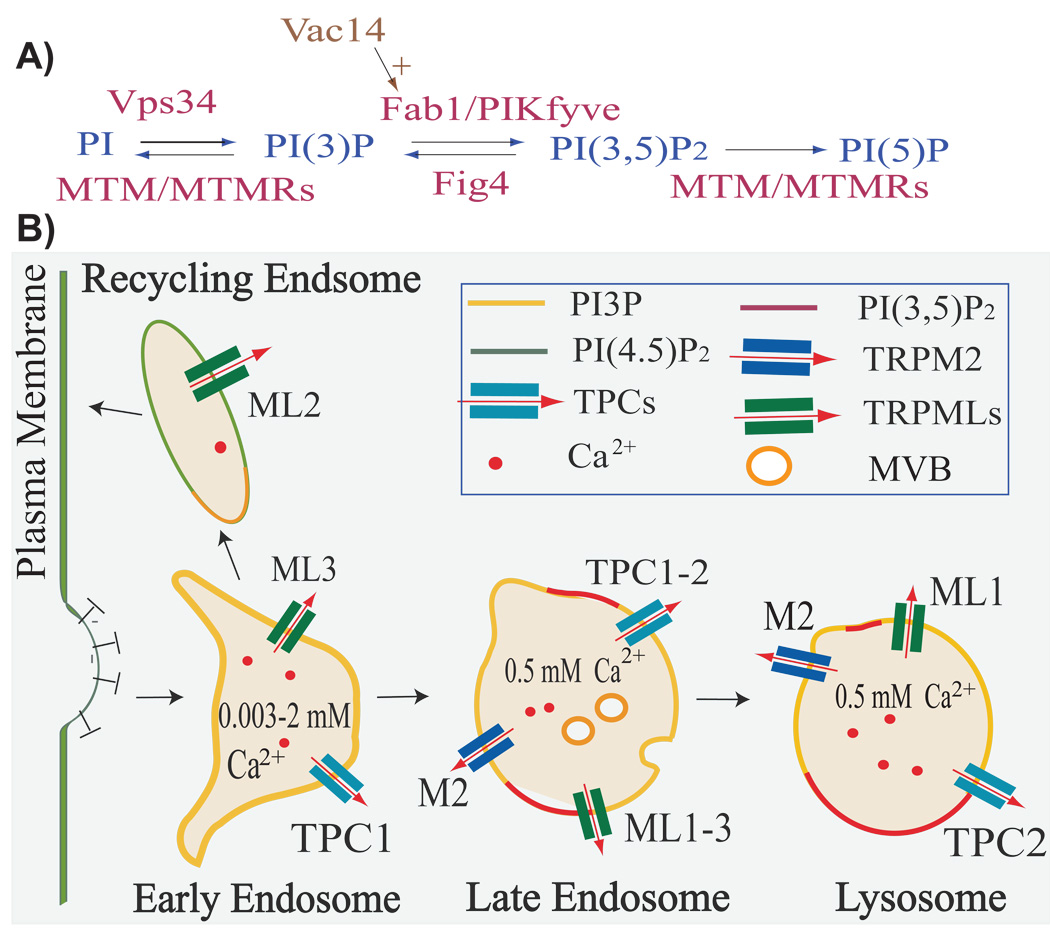
Phosphoinositides (PIPs) are another class of molecules that determine the vesicular identity and direction of membrane trafficking [12,17,25]. Of the seven PIP isoforms known, PI(3)P and PI(3,5)P2 are exclusively localized to the endolysosome system. PI(3)P is mainly localized on the cytoplasmic side of endosomes, while PI(3,5)P2 is believed to localize exclusively to LELs [25,35] (see Fig. 1). Overall, both PI(3)P and PI(3,5)P2 have multiple roles that are essential for endolysosomal trafficking.
Fig. 1. Phosphoinositides and Ca2+ channels in endolysosomes.
A: An overview of PI(P) synthesis in endolysosomes. PI(3)P is synthesized from PI via a PI 3-kinase (Vps34) present in endosomes, while PI(3,5)P2 is synthesized from PI(3)P via a PI 5-kinase (PIKfyve/Fab1). PIKfyve is a PI(3)P effector that is associated with late endosomes and lysosomes (LELs), and is positively regulated by Vac14. PI(3,5)P2 can be dephosphorylated by Fig4 or MTM/MTMRs to generate PI(3)P or PI5P, respectively.
B: The distribution of PI(3)P and PI(3,5)P2 associated with the membranes of early and late endosomes, as well as lysosomes, are represented by yellow and red outlines at the membrane of these vesicle types. respectively [35]. TRPML1–3, TRPM2, and TPC1–2 are putative Ca2+ release channels associated with endolysosomal membranes that are also labeled in this diagram [69,75,76]. TRPML1–3 channels are predominantly localized to LELs, while TRPML2 and TRPML3 are also associated with recycling and early endosomes, respectively. TPC2 is mainly localized in the LELs, and TPC1 is mainly associated with early and late endosomes. TRPM2 is expressed in specific cell types, and is associated with LELs as well as the plasma membrane. The concentration of Ca2+ stores associated with each vesicle type is also indicated, with luminal [Ca2+] estimated to be in the micro- to millimolar ranges.
PI(3)P in early endosomes
PI(3)P is essential for almost every single aspect of membrane trafficking of early endosome: fusion, fission, sorting, and maturation. PI(3)P is mainly synthesized from PI (see Fig. 1A) by PI 3-kinase Vps34 (Vps34p in yeast), which localizes to endosomes and is expressed in all eukaryotes [25]. Correspondingly, yeast cells lacking Vps34p are defective in endosomal sorting; homotypic fusion of early endosomes are sensitive to the broad spectrum PI 3-kinase inhibitor, wortmannin [36]. These defects cannot be rescued by constitutively active Rab5, suggesting that Vps34 functions downstream of Rab5, and is an effector of Rab5 [13]. PI(3)P has more than 70 effector proteins in mammalian cells, all of which contain PI(3)P binding motifs such as FYVE, PX, or PH domains [37]. Selective recruitment of these effectors by PI(3)P may provide a mechanism by which the directionality for incoming vesicles and endosomes is established. For example, PI(3)P is a critical recognition marker for many tethering/regulatory factors such as EEA1 [38]. Moreover, acute enzymatic depletion of PI(3)P from Rab5-positive endosomes has been shown to disrupt the maturation of endosomes [39]. Thus, PI(3)P is critical for the maturation of endosomes, and for fusion events with other intracellular organelles.
PI(3)P is also important for fission events of endosomes and essential for the formation of MVBs [40], a process facilitated by endosomal sorting complex required for transport (ESCRT) machinery. ESCRT sorts proteins that are intended to be degraded in lysosomes into the late endosome-lysosome pathway [4]. Since multiple components of the ESCRT complex contain PI(3)P binding modules, interactions with PI(3)P are essential for the recruitment of these proteins to endosomes [4]. Correspondingly, disruption of PI(3)P synthesis by wortmannin affects the formation of internal vesicles within MVBs [40].
PI(3)P in autophagosomes and phagosomes
PI(3)P is also important for the formation of autophagosomes, playing an essential role in autophagy [41]. For example, during amino acid starvation, autophagy-specific proteins are specifically recruited to PI(3)P-enriched structures [42]. Indeed, Vps34 is an essential component of the core complex involved in autophagy [43]. Inhibition of PI3K with wortmannin prevents fusion of autophagosomes with LELs [44].
PI(3)P also has a role in phagocytosis and is important for the formation and maturation of phagosomes [45–47]. Upon phagocytosis, invading microorganisms or latex beads are packaged into phagosomes, which then recruit various trafficking regulators to modify themselves and eventually fuse with lysosomes to form phago-lysosomes, a process referred to phagosome maturation [47]. Inhibition of Vps34 with wortmannin prevents fusion of the phagosomes with late endosomes [48]. Recruitment of PI(3)P effectors such as EEA1 is necessary for phagosomal maturation [49,50]. Pathogenic bacteria such as Mycobacterium Tuberculosis prevent phagosomal maturation (commonly referred to as phagosomes maturation arrest) by disrupting Rab, PtdIns(3)P, or Ca2+ signaling [46]. Although it is described in more detail below, PI(3)P is an essential substrate for the synthesis of PI(3,5)P2 (see Fig. 1A) [17], and therefore, the observed effects could be due to insufficient synthesis of PI(3,5)P2.
PI(3,5)P2 in late endosomes and lysosomes
PI(3,5)P2 is a low-abundance phosphoinositide in the late endosome and lysosome
PI(3,5)P2 only represents ~0.05% of the total cellular phosphoinositides (PI(3)P, PI(4)P, PI(5)P, PI(3,4)P2, PI(4,5)P2, PI(3,5)P2, and PI3,4,5)P3) present in non-stimulated cells under basal conditions [35,51]. This is ~10-fold less than the levels of PI(3)P that are normally maintained in cells [52]. Due to its low abundance and lack of a specific detection probe [37], the majority of PI(3,5)P2 studies have focused on PI(3,5)P2-metabolizing enzymes, including PIKfyve/Fab1 and its regulators [52–55]. PI(3,5)P2 is generated from PI(3)P via PIKfyve/Fab1 (see Fig. 1), a PI 5-kinase that localizes to endolysosomes in both yeast and mammalian cells [17,35,51,56–58]. Therefore, PI(3,5)P2 is hypothesized to predominantly localize to the LELs [35,52,55,59]. The activity of PIKfyve/Fab1 has been shown to be positively regulated by several associated proteins such as Fig4 and Vac14 [35,52,54,55]. However, PI(3,5)P2 can be hydrolyzed to PI(5)P by the myotubularin (MTM/MTMR)-family of PI-3 phosphatase [17,35,60]. Furthermore, although the global concentration of PI(3,5)P2 is very low, the local concentration in PIKfyve-enriched microdomains is likely to increase in response to stimuli [58,61], and can reach levels similar to those of PI(4,5)P2 at the plasma membrane (~10 µM) [56,57,62]. Correspondingly, mutations in PI(3,5)P2-metabolizing enzymes have been shown to be responsible for a variety of neurodegenerative diseases including amyotrophic lateral sclerosis (ALS) and Charcot-Marie-Tooth (CMT) disease in humans [35,55,63].
What are cellular consequences of PI(3,5)P2-deficiency?
At the cellular level, PI(3,5)P2-deficient cells, such as fibroblasts from Fig4 or Vac14 KO mice, exhibit enlarged endolysosomes/vacuoles and trafficking defects in the late endocytic pathways, which include retrograde late endosome/lysosome-to-TGN trafficking, MVB formation, autophagosome-lysosome fusion, and exocytosis [52,54,55,64,65]. In studies of Vac14−/− cells, immunoreactivity of a cation-independent mannose-6-phosphate receptor (ciM6PR), a membrane protein that shuttles between TGN and the LEL, was detected in Lamp1-positive compartments rather than in the TGN [52] , suggesting that reformation of ciM6PR-positive transport vesicles from LELs was compromised [52]. PI(3,5)P2 has also been shown to have an essential role in the fusion of late endosomes or lysosomes with other organelles, including autophagosomes [65]. Consistent with a role for PI(3,5)P2 in exocytosis, PIKfyve has been implicated in the translocation of several ion channels and transporters to the plasma membrane [66].
What are the effectors of PI(3,5)P2?
Like PI(3)P, PI(3,5)P2 also exerts its effect through the recruitment of effector proteins that bind PI(3,5)P2, although the number of effectors associated with PI(3,5)P2 is much smaller than the number of PI(3)P effectors [35]. Atg18 is a protein that potentially mediates retrograde trafficking following its binding of PI(3,5)P2 in yeast [67]. Similarly, WIP1 has been shown to bind both PI(3)P and PI(3,5)P2 to affect ciM6PR recycling in mammalian cells, thereby suggesting that it is an effector of PI(3,5)P2 [68]. Finally, mucolipin subfamily of Transient Receptor Potential channels (TRPMLs) were recently identified as novel PI(3,5)P2 effectors that mediate the release of Ca2+ from endolysosomes [31] (see details below). Overall, the identification of multiple PI(3,5)P2 effectors is consistent with the multifaceted actions of this lipid in membrane trafficking.
Ca2+ and Ca2+ channels in endolysosomal trafficking
Ca2+-dependent fusion and fission
The transient release of Ca2+ from endosomes/lysosomes in close proximity to fusion/fission sites is hypothesized to be important for the trafficking of endolysosomal membranes [2,20,22,69]. In vitro membrane fusion assays using cell extracts from yeast or mammalian cells have shown that both homotypic and heterotypic fusion events between late endosomes and lysosomes are inhibited by BAPTA, but not by EGTA [21,23]. Although both BAPTA and EGTA are Ca2+ chelators, the former binds Ca2+ 10–100 times faster than the latter. The most plausible explanation for this distinct difference in sensitivity to BAPTA vs. EGTA, is that the putative fusion site is in close proximity to the Ca2+ release site [20,23,70]. By using membrane-permeable forms of Ca2+ chelators, i.e., BAPTA and EGTA labeled with acetoxymethyl ester (AM), additional studies of intact cells have further demonstrated that distinct BAPTA/EGTA sensitivities are also associated with multiple steps during endolysosomal transport, including endolysosomal fusion and retrograde trafficking from endolysosomes to the TGN [20,22,24,70]. Thus, both in vitro and in vivo evidence suggests that Ca2+ release from endolysosomes participates in most, if not all, fusion and fission events along the endolysosomal pathway [69].
Endolysosomes as Ca2+ release stores
Based on the ionic composition and electrical properties of endolysosomes, the capacity for endolysosomes to release Ca2+ during membrane trafficking has been considered [69] . The luminal Ca2+ concentration of lysosomes is estimated to be ~0.5 mM [11,71], while Ca2+ concentrations associated with endosomes have been shown to vary from 0.003 to 2 mM [72]. Thus, both endosomes and lysosomes may represent sources of Ca2+ release along the endolysosome trafficking pathway (Fig. 1B). Dysregulation of luminal Ca2+ concentration is known to cause a block of phagosome maturation in macrophages [73], and trafficking defects in neurons and subsequent neurodegenerative diseases [11,74].
Candidate Ca2+ release channels in endolysosomes
Recently, several putative endolysosome Ca2+ release channels have been identified using Ca2+ imaging and a lysosomal patch-clamp technique (for reviews see Refs. [69,75,76]; Fig. 1B). Previous studies have also functionally characterized TRPMLs to be inward rectifying Ca2+ permeable channels [75]. In addition, loss-of-function studies in C elegans, Drosophila, and mammalian cells suggest that TRPMLs are required for multiple steps in the fusion/fission processes of endolysosomes [75,77–81]. TRPML1 has been shown to be ubiquitously expressed, while TRPML2 and TRPML3 are associated with a more restricted expression profile : TRPML2 is expressed in thymus, kidney, spleen, and other tissues; TRPML3 is expressed in cochlea, kidney, skin, and other tissues [75]. Furthermore, while all three TRPMLs are primarily associated with the LELs, TRPML2 and TRPML3 have also been associated with recycling endosomes and early endosomes, respectively [75].
There are at least four more cation channels present in the endolysosome. Two-Pore channels (TPC1 and TPC2) are another type of Ca2+ channel that are ubiquitously expressed and are associated with endolysosomes. Recent studies have shown that TPCs may mediate nicotinic acid adenine dinucleotide phosphate (NAADP)-activated Ca2+ release from endolysosomes [76], while overexpression of TPCs has been shown to cause defective membrane trafficking in endolysosomes [82]. Finally, TRPV2 and TRPM2 were detected as cell-specific Ca2+ permeable channels present in endolysosomes [83,84]. However, characterization of these channels in the membrane trafficking of endolysosomes is ongoing.
PI(3)P, PI(3,5)P2, and Ca2+ in endolysosomal trafficking
Unlike neurotransmission where the stimulus to trigger Ca2+ flux (i.e., membrane depolarization) is known, the activation and regulation of vesicular Ca2+ channels remains largely unexplored. However, due to the specificity involved in endolysosomal trafficking processes, Ca2+ release needs to be closely regulated [20]. Given that plasma membrane channels are known to be regulated by Rab proteins [85], SNAREs [27], and phosphoinositides [86], one possible regulatory mechanism for endolysosomal Ca2+ release channels is modulation by these trafficking players. However, this hypothesis is difficult to test due to the inaccessibility of endolysosomal channels to conventional functional assays [87].
Based on the co-localization of endolysosomal channels and PI(3)P/PI(3,5)P2 in endolysosomes [69,75,76], it is possible that PI(3)P and PI(3,5)P2 have the capacity to modulate the function of endolysosomal channels such as TRPMLs. Interestingly, PI(3,5)P2-deficient cells and TRPML1−/− cells share a number of similarities in the trafficking defects exhibited by these two cell types [35,75]. For example, PI(3,5)P2-deficient cells and TRPML1−/− cells exhibit enlarged endolysosomes/vacuoles and trafficking defects in the late endocytic pathway, and these include defects in LEL-to-TGN retrograde trafficking, autophagosome-lysosome fusion, and exocytosis [31]. In TRPML1−/− cells, the processes of retrograde trafficking from LEL to TGN, and the reformation of lysosomes from LEL hybrid organelles, are both impaired [77,78,88]. Similarly, these same defects are also associated with PI(3,5)P2-deficient cells [52,55]. Moreover, fusion of LELs with other intracellular compartments has been shown to be compromised in both TRPML1−/− and PI(3,5)P2-deficient cells [35,65,75]. In combination, these observations suggest that PI(3,5)P2 and TRPML1 modulate multiple endolysosomal trafficking processes coordinately.
PI(3,5)P2 binds to and activates TRPML1
Using direct patch-clamping of endolysosomal membranes, Dong et al. have reported that PI(3,5)P2 is able to robustly activate TRPML1 in both heterologous and endogenous systems [31]. In contrast, decreases in PI(3,5)P2 levels by chelation (i.e., using a PI(3,5)P2 antibody or poly-lysine), or by overexpression of PI-3 phosphatase (MTM1), has been shown to suppress the basal current associated with TRPML1 overexpression [31]. Based on these results, PI(3,5)P2 would appear to be the primary determinant of TRPML1’s channel activity in endolysosomes [31]. Correspondingly, none of the other PIPs, including PI(3)P and PI(5)P, have been shown to activate TRPML1 [31]. Therefore, TRPML1 activation is hypothesized to be restricted to PI(3,5)P2-enriched LEL membranes.
An additional consideration is whether PI(3,5)P2 induces TRPML1-mediated Ca2+ release from endolysosomes. While PI(3)P levels have been reported to increase in autophagosomes of autophagic cells [17], direct evidence to indicate that local PI(3,5)P2 levels are affected in endolysosomes of mammalian cells is still lacking. However, a transient increase in levels of PI(3,5)P2 level in mammalian endolysosomes has been associated with recruitment of PIKfyve in response to insulin and osmotic stress [66] . Furthermore, in yeast, hyper-osmotic stress has been shown to induce a dramatic increase (> 10-fold) in global PI(3,5)P2 levels [89], and to induce the release of Ca2+ from vacuoles [90]. Thus, it is possible that mammalian cells are also responsive to physiologically relevant acute stimuli, or constitutive signals, which result in the activation and recruitment of PIKfyve to induce Ca2+ release in TRPML1-endolysosomes.
Mechanisms associated with interactions between PI(3,5)P2 and TRPML1
PI(3,5)P2-dependent modulation of TRPML1 appears to be physiologically significant. For example, the enlarged endolysosome phenotype associated with Vac14−/− fibroblasts can be largely rescued by overexpressing wild-type TRPML1, but not by a PI(3,5)P2-insensitive mutant TRPML1 [31]. A possible reason for this observation is that overexpression of TRPML1 can increase PI(3,5)P2-dependent, TRPML1-mediated Ca2+ release, thereby rescuing trafficking defects of PI(3,5)P2-deficient cells [31]. Alternatively, the basal activity of TRPML1 resulting from overexpression may alter the ionic composition of the vacuoles, thereby affecting their morphology. A third possible explanation is that PI(3,5)P2-dependent fusion/fission processes are restored based on an increase in basal TRPML1 channel activity, which may compensate for the decrease in activation signals, including activation by PI(3,5)P2 [31]. It is anticipated that the TRPML chemical agonists recently identified may prove helpful in distinguishing these possibilities [91].
The exact concentration of local PI(3,5)P2 in the LEL under basal conditions, or upon cellular stimulation, is not known. Therefore, the mechanisms by which PI(3,5)P2 modulates TRPMLs under physiological conditions remains unclear. A key question is whether PI(3,5)P2 is the sole activator of TRPML1, or are there other factors involved? It is likely that constitutive signals, or acute stimuli, recruit/activate PIKfyve present in microdomains, thereby resulting in a rapid and transient increase in PI(3,5)P2 and TRPML1-mediated Ca2+ release (Fig. 2A). However, although the EC50 for recombinant TRPML1 is only ~50 nM [31], higher concentrations of PI(3,5)P2 are required to activate endogenous TRPML1 (Wang, Dong, and Xu; unpublished data). Thus, it remains unclear whether physiological levels of PI(3,5)P2, which are presumably in the low nanomolar range, are sufficient to activate endogenous TRPML1 in endolysosomes.
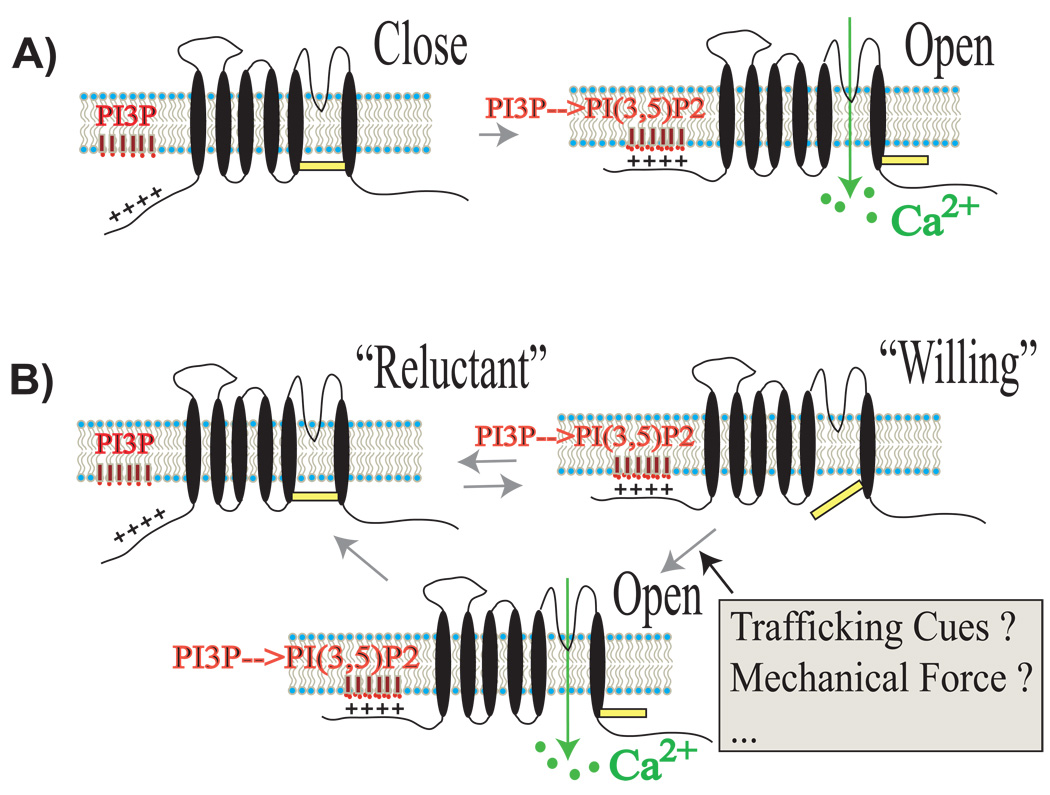
Fig. 2. Two working models for PI(3,5)P2-dependent TRPML1 activation in endolysosomes.
A: “Activation” model. Physiological levels of PI(3,5)P2 activate TRPML1 by binding to the poly-basic domain in the N- terminus of TRPML1, leading to the release of Ca2+.
B: “Sensitization” model. In this model, physiological levels of PI(3,5)P2 are not sufficient to activate TRPML1. Instead, PI(3,5)P2 is hypothesized to sensitize TRPML1 by converting the channel from a “reluctant” state to a “willing state” (see ref. [92]). Channel opening/activation and Ca2+ release are then induced by unidentified trafficking cues, one of which may be the mechanical force generated during pre-fusion membrane curvature/bending. This model confers multiple layers of regulation on TRPML1 activity. Under stress conditions that are analogous to hyperosmotic shock in yeast, acute stimuli may induce high levels of PI(3,5)P2 to sufficiently activate TRPML1 as shown in A.
It is also possible that, in vivo, additional regulatory mechanisms for TRPML1 include factors other than PI(3,5)P2. For example, PI(4,5)P2 has been shown to be a “permissive” factor in the activation of voltage gated Ca2+ channels in response to depolarization [92]. Similarly, PI(3,5)P2 may “sensitize”, yet not fully “activate” TRPML1. Increases in levels of PI(3,5)P2 may also promote Ca2+ channels to be maintained in a “willing state”, thereby facilitating channel activation in the absence of secondary activation factors (see Fig. 2B). Conversely, in the absence of PI(3,5)P2, activation of Ca2+ channels may require much higher concentrations or stronger binding interactions by activation factors that exceed the physiologically permitted range. Another activation factor to consider is that of mechanical force, which may be generated by the membrane curvature achieved prior to fusion [15]. For example, since the Ca2+ release site is hypothesized to be in close proximity to the fusion/fission sites [20,23,70], such mechanical force may be sensed by Ca2+ release channels. Correspondingly, YVC1/TRPY1, the TRPML homolog in yeast, has been shown to be directly gated by mechanical force [93]. Further studies will be needed to test whether TRPML1 is synergistically gated by PI(3,5)P2 and mechanical force. However, other activation factors should be evaluated as well, including proteins that are recruited to interact with TRPML1 following transient elevations in levels of PI(3,5)P2. These studies would also test the hypothesis that PI(3,5)P2, in coordination with these ‘cofactors’, can achieve specific activation of endolysosomal Ca2+ release channels.
Local and transient increases in levels of PI(3,5)P2 and Ca2+
The ability of PI(3,5)P2 to specifically activate TRPML1 suggests that acute regulation of Ca2+ release via TRPMLs can be achieved by regulating PI metabolizing enzymes, such as PIKfyve, or Fig4 and MTM/MTMR phosphatases. These PI-metabolizing enzymes are usually localized to the cytosol [58]. However, these enzymes can be recruited to endolysosomal membranes based on the binding of phosphoinositides to their PI-binding domains [37], or by interacting with key components of the trafficking pathways [58]. For example, PI(3)P can recruit Rab5, and activation of Rab5 can further stimulate the activity of Vps34 PI-3 kinase [94]. Similar mechanisms may also be employed by PI(3,5)P2, Rab7, and PI(3,5)P2-metabolising enzymes [58]. For TRPML1, it is not inactivated by voltage, or desensitized by Ca2+ [31]. Therefore, to avoid excessive Ca2+ release from endolysosomes, activation of TRPML1 needs to be transient. It has been hypothesized that this can be achieved if the synthesis and turnover of PI(3,5)P2 is active and tightly regulated [58]. This has been shown to be the case in yeast where Vac14 physically associates with Fig4, Vac7, and Fab1 [54,58,95], and the formation of the Vac14-Fig4 complex is required for rapid increases in the synthesis and turnover of PI(3,5)P2 in response to hyperosmotic stress [56]. The proximity of PI(3,5)P2-metabolizing enzymes to each other further supports this hypothesis, and has been shown to facilitate the ability of complexes to form near fusion/fission sites [96].
PI(3)P and endolysosomal Ca2+ channels
Like PI(3,5)P2 activation of TRPML1 in the LEL, PI(3)P in the early endosome may also modulate Ca2+ channels in the early endosome, for example, TRPV2, TPC1, and TRPML3. However, because the concentration of PI(3)P in the early endosome under resting conditions might be already high [52], it is likely that PI(3)P plays a “permissive” role in channel activation, similar to the effect of PI(4,5)P2 on plasma membrane channels [86]. These possibilities remain to be tested. It is worth mentioning that both PI(3)P production and intracellular Ca2+ release are inhibited during phagosomes maturation arrest upon phagocytosis of pathogenic bacteria [45–47].
Cross-talk between Ca2+ signaling and phosphoinositide effectors
Are there synergistic actions of trafficking regulators?
Activation of more than one trafficking factor simultaneously appears to provide specificity for the membrane trafficking that takes place in a cell [15]. For example, the functional interplay between phosphoinositides and small GTPases has been well characterized, with phosphoinositides shown to be important for recruiting GTPases, and GTPases being able to activate phosphoinositide-metabolizing enzymes [34]. Therefore, although both phosphoinositides and GTPases can recruit effectors to specific organelles, the binding affinity for each class of interactions is low. To compensate for this, a combination of phosphoinositides and GTPases have high affinity interactions with their effectors, which facilitates a coincidence detection mechanism [34].
Are there interactions between endolysosomal Ca2+ channels and other PI(3,5)P2 effectors?
PI(3,5)P2-deficient cells have been observed to have more defects in their membrane trafficking processes than TRPML1−/− cells [31], suggesting that Ca2+ is not the only downstream effector of PI(3,5)P2. PI(3,5)P2 has been shown to recruit the necessary cytosolic proteins for membrane fusion and fission, to activate TRPML1, and may regulate the functions of other endolysosomal membrane proteins to determine the physical properties and fusogenic potential of endolysosomal membranes [17,31,35]. It is also possible that Ca2+ and its effectors (i.e., Ca2+ sensors) may in turn regulate the activities of PI(3,5)P2-metabolizing enzymes and functions of other PI(3,5)P2 effectors.
Pre-fusion mechanisms have been established for phosphoinositides such as PI(4,5)P2 and PI(3)P [17,18,30]. Therefore, it has also been hypothesized that PI(3,5)P2 participates in endolysosomal pre-fusion steps. For exocytosis, PI(4,5)P2 has been shown to be a critical cofactor that recruits protein priming factors to facilitate SNARE-dependent fusion [97], and is required for generating the membrane curvature necessary for membrane fusion [29]. In reconstituted proteoliposome studies, it has been demonstrated that the yeast vacuole Q-SNARE protein, Vam7p, binds to PI(3)P and promotes the formation of a SNARE complex [18,30]. Studies of pre-fusion steps (i.e., tethering and docking) have led a testable hypothesis that increases in levels of PI(3,5)P2 may lead to the direct activation of TRPML1 to elevate levels of juxtaorganellar Ca2+, thereby triggering fusion events (Fig. 3). Moreover, regarding the hypothesis that PI(3,5)P2 configures TRPML1 in a “willing” state, the assembly of SNARE complexes and the membrane curvature involved in this process, may provide a necessary “activation factor” for TRPML1-mediated Ca2+ release (Fig. 2B). Indeed, trans-SNARE complex formation is shown to promote Ca2+ release from yeast vacuoles [28]. In the vertex of fusion-ready vesicles, there are rapid changes in the concentrations of both PI(3,5)P2 and proteins involved in pre-fusion and fusion steps. If these proteins can affect the gating of TRPML1 through protein-protein interaction, TRPML1-mediated Ca2+ release could be readily triggered in the presence of high PI(3,5)P2 . In combination, these roles for PI(3,5)P2 indicate that PI(3,5)P2 may function as a phosphoinositide switch that controls the function and maturation of organelles enriched with PI(3,5)P2, i.e., LELs. For endolysosomes, membrane fusion/fission events may be triggered by Ca2+ released from TRPML1-containing LELs following PIKfyve–mediated synthesis of PI(3,5)P2 from PI(3)P, and subsequent recruitment of trafficking factors (see Fig. 3). Moreover, regulation of this process could be provided by dephosphorylation of PI(3,5)P2 by Fig4 or MTMR phosphatases.
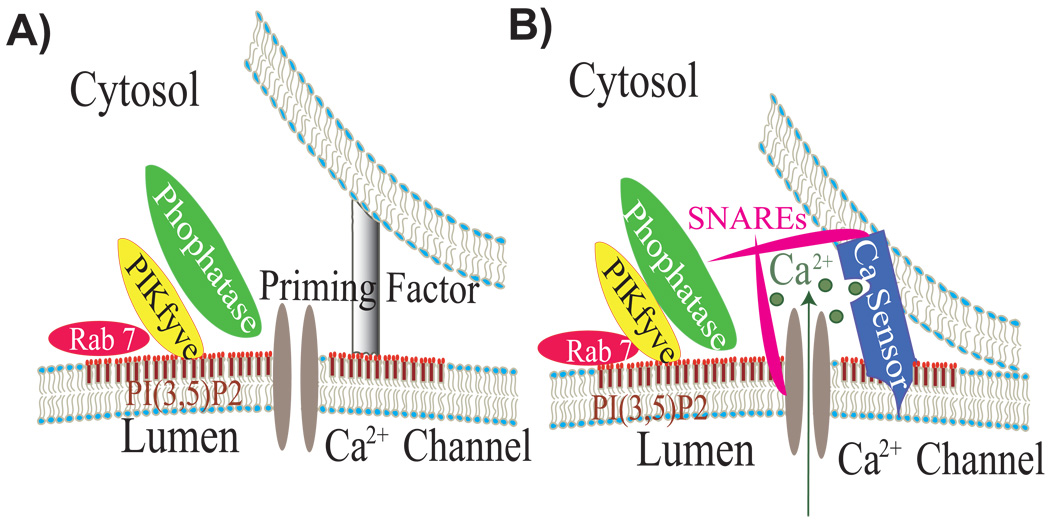
Fig. 3. A proposed model for PI(3,5)P2- and Ca2+-dependent endolysosomal membrane fusion.
Microdomain specific recruitment of an array of tethering and priming factors has the potential to bring into close proximity these factors between an endolysosomal membrane (lower membrane illustrated) and a membrane of another vesicular compartment (upper membrane illustrated).
A: Recruited proteins that may be involved include Rab GTPases (Rab7 for LEL), lipid kinases (PIKfyve for LEL), and several phosphatases (Fig4 and MTM/MTMRs). Activation of the assembled PI(3,5)P2-metabolizing complex would ensure a transient and local increase in the level of PI(3,5)P2. PI(3,5)P2 effectors would also be subsequently recruited and/or activated.
B: The release of Ca2+ from the lumen of endolysosomes would transiently elevate the levels of juxtaorganellar Ca2+ to activate a putative Ca2+ sensor protein, such as ALG-2/Synaptotagmin/CaM, to promote the fusion of the lipid bilayers. Both SNARE complex formation and PI(3,5)P2 may facilitate Ca2+ release. An increase in PI(3,5)P2 levels could also alter the physical properties of the membranes involved, and regulate the interactions between SNARE complexes and Ca2+ sensor proteins.
What are the Ca2+ sensors in the endolysosome system?
Given the specificity of endolysosomal trafficking processes, compartment-specific Ca2+ sensors might also be involved. The best candidate is ALG-2, a penta-EF-hand protein that physically associates with TRPML1 in a Ca2+-dependent manner [98]. Calmodulin has also been implicated in endolysosomal trafficking processes [21–23], with vacuolar fusion being found to be defective in loss-of-function calmodulin mutants in yeast [21]. Synaptotagmins are another type of Ca2+ sensor that have been shown to be essential for exocytosis [86]. Since lysosomes also undergo Ca2+-regulated exocytosis for plasma membrane repair [7], synaptotagmins may play a role in the fusion of lysosomes with other membranes. In addition, recent studies have demonstrated that Doc2b, a high-affinity Ca2+ sensor, is important for the spontaneous release of neurotransmitters, and may play a role in endolysosomal trafficking [99].
How do Ca2+ sensors transduce an increase in levels of juxtaorganellar Ca2+ to affect fusion/fission events?
One possibility is that upon binding of Ca2+, a putative Ca2+ sensor may exhibit high-affinity associations with phospholipids such as PI(3,5)P2, thus bringing two membranes into closer proximity. This has been observed during the release of neurotransmitters using functional assays of synaptotagmins and PI(4,5)P2 [100]. Another possibility is that a putative Ca2+ sensor may interact with SNARE proteins to facilitate the formation of a SNARE complex, thus promoting membrane fusion. Correspondingly, calmodulin has been shown to interact with SNAREs and promote intracellular membrane fusion [22]. A third possibility is that Ca2+ signaling may regulate other trafficking factors, including phosphoinositide effectors. For example, TRPML2 and Ca2+ have been shown to promote the activity of Arf6 GTPase and other ArfGAPs, respectively [101]. Ca2+/calmodulin is shown to be a positive regulator of Vps34 in phagosomes [47]. Thus, functional cross-talk between endolysosomal Ca2+ channels, phosphoinositides, and other phosphoinositide effectors may regulate the release of Ca2+ in a spatiotemporal manner, thus ensuring the specificity of endolysosomal membrane trafficking.
Conclusions and Prospects
Studies of PI(3,5)P2 and TRPMLs have provided valuable insight into the gating mechanisms that regulate Ca2+ channels along the endolysosomal pathway. Endolysosomal Ca2+ channels may also be modulated by lipids enriched in endolysosomes, similar to various channels at the plasma membrane whose functions are modulated by phospholipids. Correspondingly, phosphoinositides such as PI(3)P and PI(3,5)P2 are hypothesized to modulate other Ca2+ channels, such as TPCs and TRPM2, in the endolysosomal system. Many neurodegenerative diseases, including Alzheimer’s and Parkinson’s disease, as well as lysosomal storage diseases, exhibit abnormal lipid accumulation and defective endolysosomal trafficking and autophagy [102]. While several lysosomal storage diseases have been characterized by a progressive accumulation of sphingolipids, mucopolysaccharides, and oligosaccharides in endolysosomes [103], it is an intriguing possibility that abnormal lipid accumulation may lead to trafficking defects and the compromise of phosphoinositide-regulated Ca2+ channels.
It is anticipated that future studies would address the following areas of interest:
Identification of other intracellular Ca2+ channels that are involved in endolysosomal trafficking, and their possible activation mechanisms. These studies will help further characterize the roles of endolysosomal Ca2+ channels in membrane trafficking.
The use of high resolution imaging to monitor Ca2+ transients during both constitutive and regulated membrane trafficking, including membrane fusion and fission events.
An examination of downstream events following the release of Ca2+ which facilitate fusion and fission events involving endolysosomal membranes.
Acknowledgements
We apologize to colleagues whose works are not cited due to space limitations; and in many cases, review articles were referenced at the expense of original contributions. Work in the author’s laboratory is supported by a NIH RO1 grant (NS062792 to H.X.) and a Sloan Research Fellowship (to H.X). We appreciate the encouragement and helpful comments from other members of the Xu laboratory.
Abbreviations
- TRP
transient receptor potential
- TRPML
Mucolipin TRP
- SNARE
soluble NSF attachment protein receptors
- LEL
late endosome and lysosome
- MVB
multi-vesicular body
- TGN
trans-Golgi network
- PIP
phosphoinositide
- NAADP
nicotinic acid adenine dinucleotide phosphate
References
- 1.Maxfield FR, McGraw TE. Endocytic recycling. Nat Rev Mol Cell Biol. 2004;5:121–132. doi: 10.1038/nrm1315. [DOI] [PubMed] [Google Scholar]
- 2.Luzio JP, Pryor PR, Bright NA. Lysosomes: fusion and function. Nat Rev Mol Cell Biol. 2007;8:622–632. doi: 10.1038/nrm2217. [DOI] [PubMed] [Google Scholar]
- 3.de Duve C. The lysosome turns fifty. Nat Cell Biol. 2005;7:847–849. doi: 10.1038/ncb0905-847. [DOI] [PubMed] [Google Scholar]
- 4.Williams RL, Urbe S. The emerging shape of the ESCRT machinery. Nat Rev Mol Cell Biol. 2007;8:355–368. doi: 10.1038/nrm2162. [DOI] [PubMed] [Google Scholar]
- 5.Bright NA, Gratian MJ, Luzio JP. Endocytic delivery to lysosomes mediated by concurrent fusion and kissing events in living cells. Curr Biol. 2005;15:360–365. doi: 10.1016/j.cub.2005.01.049. [DOI] [PubMed] [Google Scholar]
- 6.He C, Klionsky DJ. Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet. 2009;43:67–93. doi: 10.1146/annurev-genet-102808-114910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reddy A, Caler EV, Andrews NW. Plasma membrane repair is mediated by Ca(2+)-regulated exocytosis of lysosomes. Cell. 2001;106:157–169. doi: 10.1016/s0092-8674(01)00421-4. [DOI] [PubMed] [Google Scholar]
- 8.Mullock BM, Bright NA, Fearon CW, Gray SR, Luzio JP. Fusion of lysosomes with late endosomes produces a hybrid organelle of intermediate density and is NSF dependent. J Cell Biol. 1998;140:591–601. doi: 10.1083/jcb.140.3.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luzio JP, Rous BA, Bright NA, Pryor PR, Mullock BM, et al. Lysosome-endosome fusion and lysosome biogenesis. J Cell Sci. 2000;113(Pt 9):1515–1524. doi: 10.1242/jcs.113.9.1515. [DOI] [PubMed] [Google Scholar]
- 10.Bonifacino JS, Rojas R. Retrograde transport from endosomes to the trans-Golgi network. Nat Rev Mol Cell Biol. 2006;7:568–579. doi: 10.1038/nrm1985. [DOI] [PubMed] [Google Scholar]
- 11.Lloyd-Evans E, Morgan AJ, He X, Smith DA, Elliot-Smith E, et al. Niemann-Pick disease type C1 is a sphingosine storage disease that causes deregulation of lysosomal calcium. Nat Med. 2008;14:1247–1255. doi: 10.1038/nm.1876. [DOI] [PubMed] [Google Scholar]
- 12.McCrea HJ, De Camilli P. Mutations in phosphoinositide metabolizing enzymes and human disease. Physiology (Bethesda) 2009;24:8–16. doi: 10.1152/physiol.00035.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol. 2009;10:513–525. doi: 10.1038/nrm2728. [DOI] [PubMed] [Google Scholar]
- 14.Parkinson-Lawrence EJ, Shandala T, Prodoehl M, Plew R, Borlace GN, et al. Lysosomal storage disease: revealing lysosomal function and physiology. Physiology (Bethesda) 2010;25:102–115. doi: 10.1152/physiol.00041.2009. [DOI] [PubMed] [Google Scholar]
- 15.Martens S, McMahon HT. Mechanisms of membrane fusion: disparate players and common principles. Nat Rev Mol Cell Biol. 2008;9:543–556. doi: 10.1038/nrm2417. [DOI] [PubMed] [Google Scholar]
- 16.Suudhof TC. Neurotransmitter release. Handb Exp Pharmacol. 2008:1–21. doi: 10.1007/978-3-540-74805-2_1. [DOI] [PubMed] [Google Scholar]
- 17.Poccia D, Larijani B. Phosphatidylinositol metabolism and membrane fusion. Biochem J. 2009;418:233–246. doi: 10.1042/BJ20082105. [DOI] [PubMed] [Google Scholar]
- 18.Wickner W. Membrane fusion: five lipids, four SNAREs, three chaperones, two nucleotides, and a Rab, all dancing in a ring on yeast vacuoles. Annu Rev Cell Dev Biol. 2010;26:115–136. doi: 10.1146/annurev-cellbio-100109-104131. [DOI] [PubMed] [Google Scholar]
- 19.Yu IM, Hughson FM. Tethering factors as organizers of intracellular vesicular traffic. Annu Rev Cell Dev Biol. 2010;26:137–156. doi: 10.1146/annurev.cellbio.042308.113327. [DOI] [PubMed] [Google Scholar]
- 20.Hay JC. Calcium: a fundamental regulator of intracellular membrane fusion? EMBO Rep. 2007;8:236–240. doi: 10.1038/sj.embor.7400921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peters C, Mayer A. Ca2+/calmodulin signals the completion of docking and triggers a late step of vacuole fusion. Nature. 1998;396:575–580. doi: 10.1038/25133. [DOI] [PubMed] [Google Scholar]
- 22.Burgoyne RD, Clague MJ. Calcium and calmodulin in membrane fusion. Biochim Biophys Acta. 2003;1641:137–143. doi: 10.1016/s0167-4889(03)00089-2. [DOI] [PubMed] [Google Scholar]
- 23.Pryor PR, Mullock BM, Bright NA, Gray SR, Luzio JP. The role of intraorganellar Ca(2+) in late endosome-lysosome heterotypic fusion and in the reformation of lysosomes from hybrid organelles. J Cell Biol. 2000;149:1053–1062. doi: 10.1083/jcb.149.5.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahluwalia JP, Topp JD, Weirather K, Zimmerman M, Stamnes M. A role for calcium in stabilizing transport vesicle coats. J Biol Chem. 2001;276:34148–34155. doi: 10.1074/jbc.M105398200. [DOI] [PubMed] [Google Scholar]
- 25.Roth MG. Phosphoinositides in constitutive membrane traffic. Physiol Rev. 2004;84:699–730. doi: 10.1152/physrev.00033.2003. [DOI] [PubMed] [Google Scholar]
- 26.Luzio JP, Gray SR, Bright NA. Endosome-lysosome fusion. Biochem Soc Trans. 2010;38:1413–1416. doi: 10.1042/BST0381413. [DOI] [PubMed] [Google Scholar]
- 27.Zamponi GW. Regulation of presynaptic calcium channels by synaptic proteins. J Pharmacol Sci. 2003;92:79–83. doi: 10.1254/jphs.92.79. [DOI] [PubMed] [Google Scholar]
- 28.Merz AJ, Wickner WT. Trans-SNARE interactions elicit Ca2+ efflux from the yeast vacuole lumen. J Cell Biol. 2004;164:195–206. doi: 10.1083/jcb.200310105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bai J, Tucker WC, Chapman ER. PIP2 increases the speed of response of synaptotagmin and steers its membrane-penetration activity toward the plasma membrane. Nat Struct Mol Biol. 2004;11:36–44. doi: 10.1038/nsmb709. [DOI] [PubMed] [Google Scholar]
- 30.Xu H, Wickner W. Phosphoinositides function asymmetrically for membrane fusion, promoting tethering and 3Q-SNARE subcomplex assembly. J Biol Chem. 2010;285:39359–39365. doi: 10.1074/jbc.M110.183111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dong XP, Shen D, Wang X, Dawson T, Li X, et al. PI(3,5)P(2) Controls Membrane Traffic by Direct Activation of Mucolipin Ca Release Channels in the Endolysosome. Nat Commun. 2010;1 doi: 10.1038/ncomms1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weber T, Zemelman BV, McNew JA, Westermann B, Gmachl M, et al. SNAREpins: minimal machinery for membrane fusion. Cell. 1998;92:759–772. doi: 10.1016/s0092-8674(00)81404-x. [DOI] [PubMed] [Google Scholar]
- 33.Hickey CM, Wickner W. HOPS initiates vacuole docking by tethering membranes before trans-SNARE complex assembly. Mol Biol Cell. 2010;21:2297–2305. doi: 10.1091/mbc.E10-01-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Di Paolo G, De Camilli P. Phosphoinositides in cell regulation and membrane dynamics. Nature. 2006;443:651–657. doi: 10.1038/nature05185. [DOI] [PubMed] [Google Scholar]
- 35.Dove SK, Dong K, Kobayashi T, Williams FK, Michell RH. Phosphatidylinositol 3,5-bisphosphate and Fab1p/PIKfyve underPPIn endo-lysosome function. Biochem J. 2009;419:1–13. doi: 10.1042/BJ20081950. [DOI] [PubMed] [Google Scholar]
- 36.Li G, D'Souza-Schorey C, Barbieri MA, Roberts RL, Klippel A, et al. Evidence for phosphatidylinositol 3-kinase as a regulator of endocytosis via activation of Rab5. Proc Natl Acad Sci U S A. 1995;92:10207–10211. doi: 10.1073/pnas.92.22.10207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kutateladze TG. Translation of the phosphoinositide code by PI effectors. Nat Chem Biol. 2010;6:507–513. doi: 10.1038/nchembio.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Simonsen A, Lippe R, Christoforidis S, Gaullier JM, Brech A, et al. EEA1 links PI(3)K function to Rab5 regulation of endosome fusion. Nature. 1998;394:494–498. doi: 10.1038/28879. [DOI] [PubMed] [Google Scholar]
- 39.Fili N, Calleja V, Woscholski R, Parker PJ, Larijani B. Compartmental signal modulation: Endosomal phosphatidylinositol 3-phosphate controls endosome morphology and selective cargo sorting. Proc Natl Acad Sci U S A. 2006;103:15473–15478. doi: 10.1073/pnas.0607040103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fernandez-Borja M, Wubbolts R, Calafat J, Janssen H, Divecha N, et al. Multivesicular body morphogenesis requires phosphatidyl-inositol 3-kinase activity. Curr Biol. 1999;9:55–58. doi: 10.1016/s0960-9822(99)80048-7. [DOI] [PubMed] [Google Scholar]
- 41.Simonsen A, Tooze SA. Coordination of membrane events during autophagy by multiple class III PI3-kinase complexes. J Cell Biol. 2009;186:773–782. doi: 10.1083/jcb.200907014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Axe EL, Walker SA, Manifava M, Chandra P, Roderick HL, et al. Autophagosome formation from membrane compartments enriched in phosphatidylinositol 3-phosphate and dynamically connected to the endoplasmic reticulum. J Cell Biol. 2008;182:685–701. doi: 10.1083/jcb.200803137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tassa A, Roux MP, Attaix D, Bechet DM. Class III phosphoinositide 3-kinase--Beclin1 complex mediates the amino acid-dependent regulation of autophagy in C2C12 myotubes. Biochem J. 2003;376:577–586. doi: 10.1042/BJ20030826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Munafo DB, Colombo MI. A novel assay to study autophagy: regulation of autophagosome vacuole size by amino acid deprivation. J Cell Sci. 2001;114:3619–3629. doi: 10.1242/jcs.114.20.3619. [DOI] [PubMed] [Google Scholar]
- 45.Hernandez LD, Hueffer K, Wenk MR, Galan JE. Salmonella modulates vesicular traffic by altering phosphoinositide metabolism. Science. 2004;304:1805–1807. doi: 10.1126/science.1098188. [DOI] [PubMed] [Google Scholar]
- 46.Vergne I, Chua J, Lee HH, Lucas M, Belisle J, et al. Mechanism of phagolysosome biogenesis block by viable Mycobacterium tuberculosis. Proc Natl Acad Sci U S A. 2005;102:4033–4038. doi: 10.1073/pnas.0409716102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vergne I, Chua J, Singh SB, Deretic V. Cell biology of mycobacterium tuberculosis phagosome. Annu Rev Cell Dev Biol. 2004;20:367–394. doi: 10.1146/annurev.cellbio.20.010403.114015. [DOI] [PubMed] [Google Scholar]
- 48.Vieira OV, Botelho RJ, Grinstein S. Phagosome maturation: aging gracefully. Biochem J. 2002;366:689–704. doi: 10.1042/BJ20020691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fratti RA, Backer JM, Gruenberg J, Corvera S, Deretic V. Role of phosphatidylinositol 3-kinase and Rab5 effectors in phagosomal biogenesis and mycobacterial phagosome maturation arrest. J Cell Biol. 2001;154:631–644. doi: 10.1083/jcb.200106049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vieira OV, Harrison RE, Scott CC, Stenmark H, Alexander D, et al. Acquisition of Hrs, an essential component of phagosomal maturation, is impaired by mycobacteria. Mol Cell Biol. 2004;24:4593–4604. doi: 10.1128/MCB.24.10.4593-4604.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ikonomov OC, Sbrissa D, Delvecchio K, Xie Y, Jin JP, et al. The phosphoinositide kinase PIKfyve is vital in early embryonic development: Preimplantation lethality of PIKfyve−/− embryos but normality of PIKfyve+/− mice. J Biol Chem. 2011 doi: 10.1074/jbc.M111.222364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang Y, Zolov SN, Chow CY, Slutsky SG, Richardson SC, et al. Loss of Vac14, a regulator of the signaling lipid phosphatidylinositol 3,5-bisphosphate, results in neurodegeneration in mice. Proc Natl Acad Sci U S A. 2007;104:17518–17523. doi: 10.1073/pnas.0702275104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shisheva A, Rusin B, Ikonomov OC, DeMarco C, Sbrissa D. Localization and insulin-regulated relocation of phosphoinositide 5-kinase PIKfyve in 3T3-L1 adipocytes. J Biol Chem. 2001;276:11859–11869. doi: 10.1074/jbc.M008437200. [DOI] [PubMed] [Google Scholar]
- 54.Jin N, Chow CY, Liu L, Zolov SN, Bronson R, et al. VAC14 nucleates a protein complex essential for the acute interconversion of PI3P and PI(3,5)P(2) in yeast and mouse. EMBO J. 2008;27:3221–3234. doi: 10.1038/emboj.2008.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chow CY, Zhang Y, Dowling JJ, Jin N, Adamska M, et al. Mutation of Fig4 causes neurodegeneration in the pale tremor mouse and patients with CMT4J. Nature. 2007;448:68–72. doi: 10.1038/nature05876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Duex JE, Tang F, Weisman LS. The Vac14p–Fig4p complex acts independently of Vac7p and couples PI3,5P2 synthesis and turnover. J Cell Biol. 2006;172:693–704. doi: 10.1083/jcb.200512105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Duex JE, Nau JJ, Kauffman EJ, Weisman LS. Phosphoinositide 5-phosphatase Fig 4p is required for both acute rise and subsequent fall in stress-induced phosphatidylinositol 3,5-bisphosphate levels. Eukaryot Cell. 2006;5:723–731. doi: 10.1128/EC.5.4.723-731.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Botelho RJ, Efe JA, Teis D, Emr SD. Assembly of a Fab1 phosphoinositide kinase signaling complex requires the Fig4 phosphoinositide phosphatase. Mol Biol Cell. 2008;19:4273–4286. doi: 10.1091/mbc.E08-04-0405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bonangelino CJ, Nau JJ, Duex JE, Brinkman M, Wurmser AE, et al. Osmotic stress-induced increase of phosphatidylinositol 3,5-bisphosphate requires Vac14p, an activator of the lipid kinase Fab1p. J Cell Biol. 2002;156:1015–1028. doi: 10.1083/jcb.200201002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shen J, Yu WM, Brotto M, Scherman JA, Guo C, et al. Deficiency of MIP/MTMR14 phosphatase induces a muscle disorder by disrupting Ca(2+) homeostasis. Nat Cell Biol. 2009;11:769–776. doi: 10.1038/ncb1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ikonomov OC, Sbrissa D, Ijuin T, Takenawa T, Shisheva A. Sac3 is an insulin-regulated phosphatidylinositol 3,5-bisphosphate phosphatase: gain in insulin responsiveness through Sac3 down-regulation in adipocytes. J Biol Chem. 2009;284:23961–23971. doi: 10.1074/jbc.M109.025361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Whiteford CC, Brearley CA, Ulug ET. Phosphatidylinositol 3,5-bisphosphate defines a novel PI 3-kinase pathway in resting mouse fibroblasts. Biochem J. 1997;323(Pt 3):597–601. doi: 10.1042/bj3230597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chow CY, Landers JE, Bergren SK, Sapp PC, Grant AE, et al. Deleterious variants of Fig4, a phosphoinositide phosphatase, in patients with ALS. Am J Hum Genet. 2009;84:85–88. doi: 10.1016/j.ajhg.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Berwick DC, Dell GC, Welsh GI, Heesom KJ, Hers I, et al. Protein kinase B phosphorylation of PIKfyve regulates the trafficking of GLUT4 vesicles. J Cell Sci. 2004;117:5985–5993. doi: 10.1242/jcs.01517. [DOI] [PubMed] [Google Scholar]
- 65.Ferguson CJ, Lenk GM, Meisler MH. Defective autophagy in neurons and astrocytes from mice deficient in PI(3,5)P2. Hum Mol Genet. 2009;18:4868–4878. doi: 10.1093/hmg/ddp460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hill EV, Hudson CA, Vertommen D, Rider MH, Tavare JM. Regulation of PIKfyve phosphorylation by insulin and osmotic stress. Biochem Biophys Res Commun. 2010;397:650–655. doi: 10.1016/j.bbrc.2010.05.134. [DOI] [PubMed] [Google Scholar]
- 67.Dove SK, Piper RC, McEwen RK, Yu JW, King MC, et al. Svp1p defines a family of phosphatidylinositol 3,5-bisphosphate effectors. EMBO J. 2004;23:1922–1933. doi: 10.1038/sj.emboj.7600203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Proikas-Cezanne T, Ruckerbauer S, Stierhof YD, Berg C, Nordheim A. Human WIPI-1 puncta-formation: a novel assay to assess mammalian autophagy. FEBS Lett. 2007;581:3396–3404. doi: 10.1016/j.febslet.2007.06.040. [DOI] [PubMed] [Google Scholar]
- 69.Dong XP, Wang X, Xu H. TRP channels of intracellular membranes. J Neurochem. 2010;113:313–328. doi: 10.1111/j.1471-4159.2010.06626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen JL, Ahluwalia JP, Stamnes M. Selective effects of calcium chelators on anterograde and retrograde protein transport in the cell. J Biol Chem. 2002;277:35682–35687. doi: 10.1074/jbc.M204157200. [DOI] [PubMed] [Google Scholar]
- 71.Christensen KA, Myers JT, Swanson JA. pH-dependent regulation of lysosomal calcium in macrophages. J Cell Sci. 2002;115:599–607. doi: 10.1242/jcs.115.3.599. [DOI] [PubMed] [Google Scholar]
- 72.Gerasimenko JV, Tepikin AV, Petersen OH, Gerasimenko OV. Calcium uptake via endocytosis with rapid release from acidifying endosomes. Curr Biol. 1998;8:1335–1338. doi: 10.1016/s0960-9822(07)00565-9. [DOI] [PubMed] [Google Scholar]
- 73.Shaughnessy LM, Hoppe AD, Christensen KA, Swanson JA. Membrane perforations inhibit lysosome fusion by altering pH and calcium in Listeria monocytogenes vacuoles. Cell Microbiol. 2006;8:781–792. doi: 10.1111/j.1462-5822.2005.00665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lloyd-Evans E, Waller-Evans H, Peterneva K, Platt FM. Endolysosomal calcium regulation and disease. Biochem Soc Trans. 2010;38:1458–1464. doi: 10.1042/BST0381458. [DOI] [PubMed] [Google Scholar]
- 75.Cheng X, Shen D, Samie M, Xu H. Mucolipins: Intracellular TRPML1–3 channels. FEBS Lett. 2010;584:2013–2021. doi: 10.1016/j.febslet.2009.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhu MX, Ma J, Parrington J, Galione A, Evans AM. TPCs: Endolysosomal channels for Ca2+ mobilization from acidic organelles triggered by NAADP. FEBS Lett. 2010;584:1966–1974. doi: 10.1016/j.febslet.2010.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen CS, Bach G, Pagano RE. Abnormal transport along the lysosomal pathway in mucolipidosis, type IV disease. Proc Natl Acad Sci U S A. 1998;95:6373–6378. doi: 10.1073/pnas.95.11.6373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Treusch S, Knuth S, Slaugenhaupt SA, Goldin E, Grant BD, et al. Caenorhabditis elegans functional orthologue of human protein h-mucolipin-1 is required for lysosome biogenesis. Proc Natl Acad Sci U S A. 2004;101:4483–4488. doi: 10.1073/pnas.0400709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vergarajauregui S, Puertollano R. Two di-leucine motifs regulate trafficking of mucolipin-1 to lysosomes. Traffic. 2006;7:337–353. doi: 10.1111/j.1600-0854.2006.00387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Venkatachalam K, Long AA, Elsaesser R, Nikolaeva D, Broadie K, et al. Motor deficit in a Drosophila model of mucolipidosis type IV due to defective clearance of apoptotic cells. Cell. 2008;135:838–851. doi: 10.1016/j.cell.2008.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vergarajauregui S, Connelly PS, Daniels MP, Puertollano R. Autophagic dysfunction in mucolipidosis type IV patients. Hum Mol Genet. 2008;17:2723–2737. doi: 10.1093/hmg/ddn174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Galione A, Morgan AJ, Arredouani A, Davis LC, Rietdorf K, et al. NAADP as an intracellular messenger regulating lysosomal calcium-release channels. Biochem Soc Trans. 2010;38:1424–1431. doi: 10.1042/BST0381424. [DOI] [PubMed] [Google Scholar]
- 83.Saito M, Hanson PI, Schlesinger P. Luminal Chloride-dependent Activation of Endosome Calcium Channels: PATCH CLAMP STUDY OF ENLARGED ENDOSOMES. J Biol Chem. 2007;282:27327–27333. doi: 10.1074/jbc.M702557200. [DOI] [PubMed] [Google Scholar]
- 84.Lange I, Yamamoto S, Partida-Sanchez S, Mori Y, Fleig A, et al. TRPM2 functions as a lysosomal Ca2+-release channel in beta cells. Sci Signal. 2009;2:ra23. doi: 10.1126/scisignal.2000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Saxena SK, Kaur S. Regulation of epithelial ion channels by Rab GTPases. Biochem Biophys Res Commun. 2006;351:582–587. doi: 10.1016/j.bbrc.2006.10.087. [DOI] [PubMed] [Google Scholar]
- 86.Suh BC, Hille B. PIP2 is a necessary cofactor for ion channel function: how and why? Annu Rev Biophys. 2008;37:175–195. doi: 10.1146/annurev.biophys.37.032807.125859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dong XP, Cheng X, Mills E, Delling M, Wang F, et al. The type IV mucolipidosis-associated protein TRPML1 is an endolysosomal iron release channel. Nature. 2008;455:992–996. doi: 10.1038/nature07311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pryor PR, Reimann F, Gribble FM, Luzio JP. Mucolipin-1 is a lysosomal membrane protein required for intracellular lactosylceramide traffic. Traffic. 2006;7:1388–1398. doi: 10.1111/j.1600-0854.2006.00475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dove SK, Cooke FT, Douglas MR, Sayers LG, Parker PJ, et al. Osmotic stress activates phosphatidylinositol-3,5-bisphosphate synthesis. Nature. 1997;390:187–192. doi: 10.1038/36613. [DOI] [PubMed] [Google Scholar]
- 90.Denis V, Cyert MS. Internal Ca(2+) release in yeast is triggered by hypertonic shock and mediated by a TRP channel homologue. J Cell Biol. 2002;156:29–34. doi: 10.1083/jcb.200111004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Grimm C, Jors S, Saldanha SA, Obukhov AG, Pan B, et al. Small molecule activators of TRPML3. Chem Biol. 2010;17:135–148. doi: 10.1016/j.chembiol.2009.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wu L, Bauer CS, Zhen XG, Xie C, Yang J. Dual regulation of voltage-gated calcium channels by PtdIns(4,5)P2. Nature. 2002;419:947–952. doi: 10.1038/nature01118. [DOI] [PubMed] [Google Scholar]
- 93.Su Z, Zhou X, Loukin SH, Saimi Y, Kung C. Mechanical force and cytoplasmic Ca(2+) activate yeast TRPY1 in parallel. J Membr Biol. 2009;227:141–150. doi: 10.1007/s00232-009-9153-9. [DOI] [PubMed] [Google Scholar]
- 94.Shin HW, Hayashi M, Christoforidis S, Lacas-Gervais S, Hoepfner S, et al. An enzymatic cascade of Rab5 effectors regulates phosphoinositide turnover in the endocytic pathway. Journal of Cell Biology. 2005;170:607–618. doi: 10.1083/jcb.200505128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sbrissa D, Ikonomov OC, Fu Z, Ijuin T, Gruenberg J, et al. Core protein machinery for mammalian phosphatidylinositol 3,5-bisphosphate synthesis and turnover that regulates the progression of endosomal transport. Novel Sac phosphatase joins the ArPIKfyve-PIKfyve complex. J Biol Chem. 2007;282:23878–23891. doi: 10.1074/jbc.M611678200. [DOI] [PubMed] [Google Scholar]
- 96.Vicinanza M, D'Angelo G, Di Campli A, De Matteis MA. Function and dysfunction of the PI system in membrane trafficking. EMBO J. 2008;27:2457–2470. doi: 10.1038/emboj.2008.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.James DJ, Khodthong C, Kowalchyk JA, Martin TF. Phosphatidylinositol 4,5-bisphosphate regulates SNARE-dependent membrane fusion. J Cell Biol. 2008;182:355–366. doi: 10.1083/jcb.200801056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Vergarajauregui S, Martina JA, Puertollano R. Identification of the penta-EF-hand protein ALG-2 as a Ca2+-dependent interactor of mucolipin-1. J Biol Chem. 2009;284:36357–36366. doi: 10.1074/jbc.M109.047241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Groffen AJ, Martens S, Arazola Diez R, Cornelisse LN, Lozovaya N, et al. Doc2b is a high-affinity Ca2+ sensor for spontaneous neurotransmitter release. Science. 327:1614–1618. doi: 10.1126/science.1183765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Arac D, Chen X, Khant HA, Ubach J, Ludtke SJ, et al. Close membrane-membrane proximity induced by Ca(2+)-dependent multivalent binding of synaptotagmin-1 to phospholipids. Nat Struct Mol Biol. 2006;13:209–217. doi: 10.1038/nsmb1056. [DOI] [PubMed] [Google Scholar]
- 101.Karacsonyi C, Miguel AS, Puertollano R. Mucolipin-2 localizes to the Arf6-associated pathway and regulates recycling of GPI-APs. Traffic. 2007;8:1404–1414. doi: 10.1111/j.1600-0854.2007.00619.x. [DOI] [PubMed] [Google Scholar]
- 102.Liu JP, Tang Y, Zhou S, Toh BH, McLean C, et al. Cholesterol involvement in the pathogenesis of neurodegenerative diseases. Mol Cell Neurosci. 43:33–42. doi: 10.1016/j.mcn.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 103.Parkinson-Lawrence EJ, Shandala T, Prodoehl M, Plew R, Borlace GN, et al. Lysosomal storage disease: revealing lysosomal function and physiology. Physiology (Bethesda) 25:102–115. doi: 10.1152/physiol.00041.2009. [DOI] [PubMed] [Google Scholar]