Abstract
Play behavior in juvenile primates, rats, and other species is sexually dimorphic, with males demonstrating more play than females. In mice, sex differences in juvenile play have only been examined in out-bred CD-1 mice. In this strain, contrary to other animals, male mice display less play soliciting than females. Using an established same-sex dyadic interaction test, we examined play in inbred C57BL/6J (B6) 21 day-old mice. When paired with non-siblings, males tended to be more social than females, spending more time exploring the test cage. Females displayed significantly more anogenital sniffing and solicited play more frequently than did males. To determine if the origin of the sex difference was sex chromosome genes or gonadal sex, next we used the four core genotype (FCG) mouse. We found significant interactions between gonadal sex and genotype for several behaviors. Finally, we asked if sibling pairs (as compared to non-siblings) would display qualitative or quantitatively different behavior. In fact, XX females paired with a sibling were more social and less exploratory or investigative, while XY males exhibited less investigative and play soliciting behaviors in tests with siblings. Many neurobehavioral disorders, like autism spectrum disorder (ASD), are sexually dimorphic in incidence and patients interact less than normal with other children. Our results suggest that sex chromosome genes interact with gonadal hormones to shape the development of juvenile social behavior, and that social context can drastically alter sex differences. These data may have relevance for understanding the etiology of sexually dimorphic disorders such as ASD.
Keywords: Juvenile, adolescent, Social Behavior, Play, Sex Chromosomes, Autism
INTRODUCTION
Juvenile social behaviors have been examined in several ways: preference for social novelty (Mcnaughton et al., 2008; Moy et al., 2004; Moy et al., 2009), conditioned place preference (Panksepp & Lahvis, 2007), and direct social encounters with peers (Mcfarlane et al., 2008). While young mice, like rats, exhibit play fighting, social encounters are primarily non-contact interactions; mice do not display the same variety of play fighting behaviors as juvenile rats (Pellis & Pasztor, 1999). In addition, male rats engage in more play fighting (also called rough and tumble play) than females (Auger & Olesen, 2009; Meaney, 1988), and female rat play is also qualitatively different from male play (Pellis et al., 1997; Pellis & Pellis, 1990). In contrast, out-bred CD-1 female mice display more play behavior than males (Laviola & Alleva, 1995; Terranova et al., 1993). In inbred strains play studies have only included males (Mcfarlane et al., 2008; Moy et al., 2007), thus it is unknown if commonly used inbred mice display sex differences in play.
In rats, sexual dimorphisms in play fighting are organized by the exposure of the male brain to androgens during neonatal development (Beatty et al., 1981; Meaney & McEwen, 1986; Meaney & Stewart, 1981). In testicular feminization mutant rats, which lack functional androgen receptors, play behavior is comparable to females (Meaney et al., 1983). Male rats treated as neonates with anti-androgens (Casto et al., 2003; Meaney et al., 1983) show reduced play fighting. Conversely, female rats exposed perinatally to either estradiol or dopamine receptor agonists (Olesen et al., 2005) demonstrate increased play. These results suggest that both androgen and estrogen receptors contribute to sex differences in play fighting behavior in rats (Auger & Olesen, 2009; Pellis, 2002).
Nothing is known about the mechanisms that regulate social play in mice. Moreover, we cannot assume that mice and rats are organized in the same manner (Bonthuis et al., 2010). We use the four core genotype (FCG) mouse model to separate the actions of gonadal hormones and sex chromosome genes on sexually dimorphic behaviors (Arnold & Chen, 2009). In this mouse several adult social behaviors (Gatewood et al., 2006; McPhie-Lalmansingh et al., 2008), differ based upon sex chromosome complement. We hypothesized that sex chromosome genes might also play a role in shaping juvenile social interactions. To test this we assessed sex differences in social interactions in non-sibling C57BL/6J (B6) mice on the day of weaning, postnatal day 21 (PN21). We used a dyadic social interaction test which was developed and previously used to investigate strain differences in juvenile male social behaviors in the context of modeling autism, a sexually dimorphic disease (Mcfarlane et al., 2008). Because autism is a sexually dimorphic disease we are interested in whether sex chromosome genetic differences may be one of its causes. In Experiment 1 sex differences were observed in B6 mice. Next we tested FCG mice to assess the independent roles of gonads and sex chromosome genes. Finally, we asked how tests with siblings, instead of strangers, would affect social interactions.
METHODS
Animals
Mice were maintained on a 12:12 light/dark cycle (lights off at 1200 EST) and food (Harlan Teklad #7912) and water were provided ad libitum. Both the B6 mouse colony and the FCG colony were bred and maintained in the University of Virginia School of Medicine, Jordan Hall Animal Facility. All procedures were conducted in compliance with the University of Virginia Animal Use and Care Committee.
Development and breeding of the Four Core Genotypes (FCG) mouse model has been described (De Vries et al., 2002). Briefly, males carrying a spontaneous mutation of Sry (testes determining gene) on the Y chromosome (Y) were supplemented with an Sry transgene inserted on an autosome. When these males (XY-Sry) are mated with normal C57BL/6J females (XX), they produce offspring with four genotypes: XX females, XY females, XXmales, and XYmales (Table 1). In our colony, the 129 Y- chromosome and Sry transgene have been fully crossed (over 10 generations) into the C57BL/6J (B6) strain. The FCG mice were backcrossed in our laboratory and we confirmed their generational status by examination of B6 microsatellite markers. For both the FCG and B6 colony, twice a year we purchase animals from Jackson Labs and breed them to our mice to reduce the possibility of genetic drift.
Table 1.
Explanation of the Four Core Genotypes offspring.
| Four Core Genotypes Offspring | ||||
|---|---|---|---|---|
| Genotype | XX female | XY female | XX male | XY male |
| Sry | no | no | yes | yes |
| Gonads | ovaries | ovaries | testes | testes |
| Number of X | 2 | 1 | 2 | 1 |
| Number of Y | 0 | 1 | 0 | 1 |
Sry= testes determining gene on the Y chromosome
Behavioral Testing
All mice were reared by both parents and left largely unhandled (excluding routine cage changes) until PN20. A total of 10 B6 and 28 FCG litters were used. Litter sex ratios were random and were evenly divided among groups, with 8–12 mice per group. Mice were tested for social interactions on PN21 following a protocol similar to one used by McFarlane and colleagues (Mcfarlane et al., 2008). Animals were organized into same sex (all experiments) and same-genotype (Experiments 2 and 3) pairs, and the observer scoring the behavior (KHC) was blind to these variables. On PN20, approximately 30 minutes before the room lights turn off, one of the subjects in each pair was marked by striping its tail with a black marker (Sharpie™). Mice were individually placed into empty cages, similar to their home cages and containing clean bedding, and allowed to habituate in the testing room for one hour without access to food or water. Afterwards, the subjects were returned to their home cages with their siblings and parents. Twenty-four hours later, the tail marking was refreshed as needed, and each mouse was habituated again for one hour in an empty cage. After habituation, mice were paired in a clean empty cage and their behaviors were recorded for 30 minutes under red light illumination.
Behavior Scoring
Behaviors were scored separately for both mice in each pair. All behaviors were scored using Noldus Observer (5.0) software (Noldus, Leesburg, VA, USA). Based on the paradigm of McFarlane et al. (2008) behaviors were grouped into four general categories: Social Interactions, Nonsocial Behaviors, Investigation, and Play Solicitation; we describe these in detail below. Social and nonsocial behaviors were scored for duration of time spent engaged in each behavior. When mice investigate and solicit play, the interactions are fast and brief, thus, these behaviors were scored as frequencies. Typically the animals were more active and more attentive to the new cage than the test partners in the first 10 minutes of the interaction, so, we analyzed data from the first 10 minutes and last 20 minutes of the 30-minute encounter separately.
Social Interactions (Durations)
Side by Side Sitting: sitting and/or sleeping in close contact with the other mouse
Social Grooming: allogrooming the other mouse
Social Other: spending time in close contact with the other mouse while self-grooming
Nonsocial Behaviors (Durations)
Exploring: investigating the walls or floor of the test cage
Self Grooming: grooming any part of its own body while sitting alone
Sitting: sitting alone while the other mouse was engaged in other behaviors
Investigation (Frequencies)
Anogenital Sniff: sniffing the other mouse’s anogenital region
Nose Sniff: sniffing the other mouse’s nose
Follow: walking behind and following the other mouse around the cage
Play Solicitation (Frequencies)
Crawl: crawling over or under the other mouse
Push: pushing between the other mouse, typically with the cage wall behind the mouse exhibiting the pushing
Approach: approaching the other mouse head-on
Reproductive Organ Weights and Plasma Testosterone Levels
To assess reproductive development in the FCG mice, a separate cohort of mice (n=7 for each genotype and sex) were reared under identical conditions. On P21 they were anesthetized using isofluorane and rapidly decapitated. Ovaries and uteri were removed and weighed. Male trunk blood was collected along with gonads. The androgen-target tissues, the seminal vesicles, were too small to collect accurate weights, so instead we assayed plasma testosterone (assayed by the University of Virginia Core Ligand and Assay Laboratory) using a total testosterone radioimmunoassay kit (Siemens Healthcare Diagnostics, Deerfield, IL) optimized for sensitivity (detectible range: 0.07– 9 ng/ml). Samples were run in singlet, and some were pooled from the same litter, same genotypes (n=4 XX M samples and n=5 XY M samples).
Statistical Analysis
All data were analyzed using NCSS Software (2000). Prior to analysis, data points that were greater than two standard deviations from the mean were tested using Grubb’s Outlier test, and any outliers for each of the behavioral measures were removed from the data set (less than 4% of the observations). To compare male and female B6 mice, we conducted a one-way ANOVA with sex as the factor. For FCG behavioral data, we used two-way ANOVAs to assess the contributions of gonadal sex and sex chromosome complement. Paired comparisons were conducted using Fisher’s LSD multiple comparison tests. Gonad weights and testosterone data were compared using Student t-tests.
RESULTS
Experiment 1: Males are more interactive than females
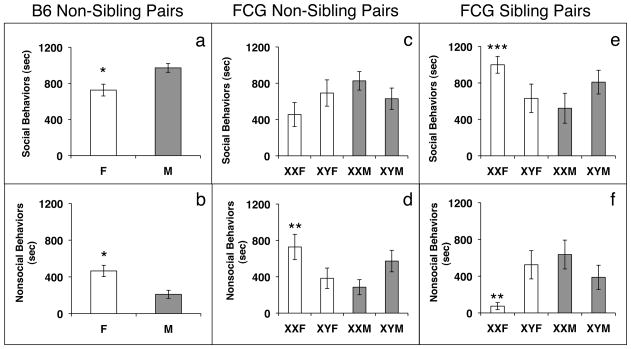
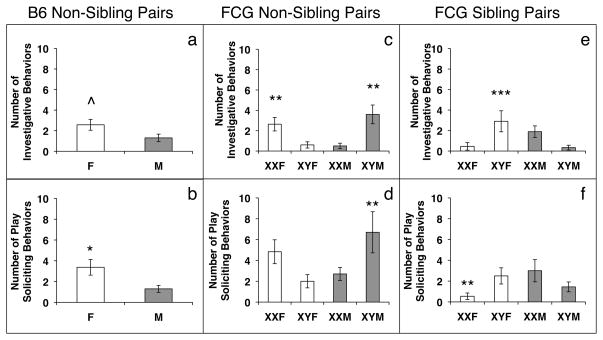
Males engaged in more social interactions than females (F(1,18)=9.48; p<0.01; Figure 1a). When analyzed separately, none of the individual social behaviors revealed any sex differences, thus the combination of social interactions, not any one behavior produced the sex difference (Table 2). In contrast, females spent significantly more time exploring than males (F(1,18)=10.97; p<0.01; Table 2), and spent more time engaged in nonsocial behaviors (F(1,18)=11.96; p<0.01; Figure 1b). In addition, females engaged in more anogenital sniffing than males (F(1,17)=7.94; p<0.03; Table 3). This produced a trend for an overall sex difference in number of investigations, with females investigating more than males (F(1,15)=4.19; p=0.059; Figure 2a). Finally, females approached their partners more than males (F(1,18)=6.94; p<0.02; Table 3), and solicited more play overall (F(1,16)=7.29; p<0.02; Figure 2b).
Figure 1.
Mean +/− SEM time (in seconds) spent engaged in social and nonsocial behaviors during the last 20 minutes of a 30-minute test. Panels a) and b) Data from Experiment 1: social tests with non-sibling partners B6 pairs (n=8 females, 10 males). Panels c) and d) Data from Experiment 2: social tests with non-sibling partners, FCG pairs (n=10–12 per group). Panels e) and f) Data from Experiment 3: social tests with sibling partners, FCG pairs (n=10–12 per group). * Significantly different from males, p<0.01. ** Significantly different from XY females and XX males, p<0.02 or less. *** Significantly different from all other groups, p< 0.03. F=female, M=male, B6=C57BL/6J, FCG= four core genotypes.
Table 2.
Mean +/− SEM time spent engaged in social and nonsocial behaviors by B6 (Exp. 1) or FCG mice paired with nonsiblings (Exp. 2), or siblings (Exp. 3), in a 30-minute social interaction.
| Social Interactions (seconds) | Nonsocial Behaviors (seconds) | ||||||
|---|---|---|---|---|---|---|---|
| Side by Side Sitting | Social Grooming | Social Other | Exploring | Self-Grooming | Sitting | ||
| Exp. 1 (non-sibling partners) | B6 F (n=8) | 498.50 +/− 102.74 | 7.02 +/− 6.70 | 221.14 +/− 62.61 | 351.05 +/− 43.53# | 24.50 +/− 8.48 | 89.31 +/− 28.20 |
| B6 M (n=10) | 712.25 +/− 91.89 | 10.13 +/− 5.61 | 249.40 +/− 56.00 | 157.66 +/− 38.93 | 9.86 +/− 7.99 | 43.72 +/− 25.22 | |
| Exp. 2 (non-sibling partners) | XXF (n=12) | 273.68 +/− 109.03 | 12.65 +/− 6.13 | 168.82 +/− 29.78 | 661.35 +/− 128.92^ | 58.60 +/− 15.51 | 8.65 +/− 4.78 |
| XYF (n=10) | 518.25 +/− 163.56 | 4.75 +/− 2.76 | 169.71 +/− 54.27 | 301.60 +/− 94.88 | 37.22 +/− 16.06 | 49.66 +/− 42.45 | |
| XXM (n=10) | 725.65 +/− 104.63^ | 0.00 +/− 0.00 | 100.78 +/− 26.83 | 250.09 +/− 83.65 | 25.77 +/− 16.26 | 13.65 +/− 8.11 | |
| XYM (n=10) | 394.34 +/− 127.61 | 2.16 +/− 1.46 | 233.19 +/− 76.71 | 489.17 +/− 109.82 | 62.75 +/− 22.31 | 23.28 +/− 10.33 | |
| Exp. 3 (sibling partners) | XXF (n=12) | 776.60 +/− 135.14 | 0.00 +/− 0.00 | 222.72 +/− 64.83 | 64.79 +/− 33.46 | 9.20 +/− 9.20 | 6.63 +/− 6.63 |
| XYF (n=10) | 483.32 +/− 172.45 | 0.58 +/− 0.58 | 146.73 +/− 46.05 | 483.87 +/− 140.84^ | 41.15 +/− 20.74 | 8.28 +/− 5.59 | |
| XXM (n=10) | 398.45 +/− 145.99 | 0.00 +/− 0.00 | 123.33 +/− 36.73 | 596.35 +/− 153.08^ | 24.34 +/− 10.10 | 26.73 +/− 21.22 | |
| XYM (n=10) | 630.58 +/− 156.34 | 0.00 +/− 0.00 | 178.25 +/− 52.64 | 368.93 +/− 128.35 | 17.41 +/− 8.21 | 1.72 +/− 1.30 | |
Data shown are for the last 20 minutes of the test. F= Females, M= Males,
sex effect,
interaction of sex and genotype (p <0.05)
Table 3.
Mean +/− SEM number of investigative and play soliciting behaviors displayed by B6 (Exp. 1) or FCG mice paired with nonsiblings (Exp. 2), or siblings (Exp. 3), in a 30-minute social interaction.
| Investigation | Play Solicitation | ||||||
|---|---|---|---|---|---|---|---|
| Anogenital Sniffs | Nose Sniffs | Follows | Crawls | Pushes | Approaches | ||
| Exp. 1 (non-sibling partners) | B6 F (n=8) | 0.50 +/− 0.13# | 2.25 +/− 0.46 | 6.63 +/− 6.63 | 0.28 +/− 0.19 | 1.63 +/− 0.45 | 1.50 +/− 0.31# |
| B6 M (n=10) | 0.00 +/− 0.12 | 1.00 +/− 0.41 | 1.72 +/− 1.30 | 0.56 +/− 0.17 | 0.44 +/− 0.42 | 0.40 +/− 0.28 | |
| Exp. 2 (non-sibling partners) | XXF (n=12) | 0.67 +/− 0.26 | 1.55 +/− 0.34^ | 0.73 +/− 0.30 | 1.17 +/− 0.37 | 3.00 +/− 0.87^ | 0.67 +/− 0.28 |
| XYF (n=10) | 0.00 +/− 0.00 | 0.50 +/− 0.27 | 0.11 +/− 0.11 | 0.56 +/− 0.24 | 1.10 +/− 0.45 | 0.40 +/− 0.16 | |
| XXM (n=10) | 0.00 +/− 0.00 | 0.50 +/− 0.27 | 0.00 +/− 0.00 | 1.40 +/− 0.43 | 0.89 +/− 0.31 | 0.05 +/− 0.22 | |
| XYM (n=10) | 0.83 +/− 0.38^ | 1.33 +/− 0.29 | 1.56 +/− 0.60^ | 1.70 +/− 0.60 | 2.90 +/− 0.31 | 2.10 +/− 0.80^ | |
| Exp. 3 (sibling partners) | XXF (n=12) | 0.08 +/− 0.08 | 0.09 +/− 0.09 | 0.27 +/− 0.19 | 0.27 +/− 0.27 | 0.83 +/− 0.41 | 0.08+/− 0.19 |
| XYF (n=10) | 0.20 +/− 0.13 | 2.30 +/− 0.93^ | 0.40 +/− 0.22 | 0.90 +/− 0.24 | 1.30 +/− 0.45 | 0.67 +/− 0.37 | |
| XXM (n=10) | 0.00 +/− 0.00 | 1.50 +/− 0.43 | 0.33 +/− 0.17 | 0.90 +/− 0.46 | 2.50 +/− 0.90^ | 0.44 +/− 0.24 | |
| XYM (n=10) | 0.00 +/− 0.00 | 0.22 +/− 0.15 | 0.11 +/− 0.11 | 0.80 +/− 0.47 | 1.00 +/− 0.37 | 0.30 +/− 0.15 | |
Data shown are for the last 20 minutes of the test. F= Females, M= Males,
sex effect,
interaction of sex and genotype (p <0.05), indicates significantly different group
Figure 2.
Mean +/− SEM number of investigative or play soliciting behaviors during the last 20 minutes of a 30-minute test. Panels a) and b) Data from Experiment 1: social tests with non-sibling partners, B6 pairs (n=8 females, 10 males). Panels c) and d) Data from Experiment 2: social tests with non-sibling partners, FCG pairs (n=10–12 per group). Panels e) and f) Data from Experiment 3: social tests with sibling partners, FCG pairs (n=10–12 per group). ^ Females tend to display more investigations than males, p=0.059. * Significantly different from males, p<0.02. ** Significantly different from XY females and XX males, p<0.02 or less. *** Significantly different from XX females and XY males, p<0.004. F=female, M=male, B6=C57BL/6J, FCG= four core genotypes.
Experiment 2: Sex chromosome complement affects interactions with non-siblings
We noted an interaction between sex chromosomes and gonads in time spent engaged in social interactions: XX males spent more time side-by-side sitting (F(1,38)=5.11; p<0.03; Table 2) than XX females. The reverse was true for nonsocial behaviors. An interaction was also present for time spent displaying nonsocial behaviors. XX females spent more time engaged in nonsocial behaviors than XX males or XY females (F(1,42)=6.41; p<0.02; Figure 1d). This finding was reflected in time spent exploring (F(1,38)=7.53; p<0.01; Table 2).
The total number of investigative behaviors also showed an interaction (F(1,36)=15.97; p<0.0002; Figure 2c), XY males and XX females investigated more than XX males and XY females. The XX females displayed more nose-to-nose sniffing than XX males and XY females (F(1,36)=9.96; p<0.004), while the XY males performed more anogenital sniffs (F(1,36)=7.02; p<0.02) and follows than XX males and XY females (F(1,35)=10.71; p<0.003; Table 3). In addition, we found interactions between the two factors in the total number of play soliciting behaviors (F(1,35)=7.63; p<0.009), the number of approaches (F(1,35)=4.50; p<0.04) and the number of pushes (F(1,35)=8.31; p<0.02). For total play soliciting behaviors (Figure 2d), XY males displayed more of these behaviors than XX males and XY females. For the number of approaches (Table 3), XY males displayed more than all other groups. XX females also displayed more pushes than XX males (Table 3).
Experiment 3: Behavioral patterns with siblings are reversed compared to non-siblings
When mice were tested with siblings, we noted many of the same significant interactions as in Experiment 2, however the direction of the differences between the four genotypes was often reversed. For example, in Experiment 2 we found an interaction for social interactions, with XX males spending more time doing these behaviors than XX females. In Experiment 3 we found the opposite effect: XX females spent more time displaying social behaviors than mice in any other group (F(1,38)=5.84; p<0.03; Figure 1e). This difference was not driven by any individual social behavior, as there were no gonadal or sex chromosome differences found for any of the social behaviors when examined separately (Table 2). We also observed an interaction between gonad type and sex chromosome complement for nonsocial behaviors. In this case XX males and XY females (the hetero-sex chromosome mice) displayed more nonsocial behaviors (F(1,38)=7.92; p<0.01; Figure 1f) and, spent more time exploring than XX females (F(1,37)=7.19; p<0.02; Table 2). Again this pattern was nearly reciprocal to that noted in the FCG non-siblings pairs (Experiment 2).
An interaction between gonads and sex chromosomes was detected in the number of investigations, with XY females displaying more investigative behaviors than XX females and XY males (F(1,35)=9.93; p<0.004; Figure 2e). This was caused by XY females who performed more nose-to-nose sniffing than the XX females or XY males (F(1,36)=11.22; p<0.002; Table 3). There was also an interaction in the number of play behaviors. XX males and XY females solicited more play than XX females (F(1,35)=6.39; p<0.02; Figure 2f). XX males also showed a significantly more pushes as compared to XX females (F(1,37)=4.25; p<0.05; Table 3).
Because only one significant sex difference was noted in the first 10 minutes of the 30-minute behavioral tests (in Experiment 1, number of anogenital sniffs; p<0.02), we limited the preceding data presentation to the last 20 minutes of the interaction. Summary data for the first 10 minutes and the complete 30 minutes are provided in Supplementary Table 1.
Juvenile mice of all genotypes are reproductively immature
No differences were found in the weights of the uteri and ovaries between XX versus XY females. These estrogen target tissues were uniformly tiny, indicating that estradiol levels were pre-pubertal in females of both genotypes. XY males had significantly higher testes weights than XX males (Table 4; p<0.0001). However, seminal vesicles were not visible in males of either genotype, and there were no differences in testosterone levels between XX and XY males. Moreover, measured levels were about one fifth the levels normally detected in adult males. These findings are consistent with data reported by others using adult FCG males and similar models (Arnold & Chen, 2009; Koopman et al., 1991). In adults, larger testes in XY males of the FCG do no reflect differences in androgen secretion (Gatewood et al., 2006), but adult XX males do have decreased sperm production (Koopman et al., 1991).
Table 4.
Mean +/− SEM gonad weights from PN21 FCG mice and testosterone levels from P21 FCG males. XY males had significantly higher testes weights than XX males (* p<0.0001), no differences were found in male testosterone levels, ovarian or uterine weights of the females. (numbers per group)
| Genotype | Testosterone (ng/ml) | Testes Weight (mg) | Ovarian Weight (mg) | Uterine Weight (mg) |
|---|---|---|---|---|
| XX F | - | - | 5 +/− 0.40 (7) | 6 +/− 1.20 (7) |
| XY F | - | - | 4 +/− 0.70 (7) | 5 +/− 0.40 (7) |
| XXM | 0.31 +/− 0.09 (4) | 16 +/− 0.70 (7) | - | - |
| XY M | 0.36 +/− 0.03 (5) | 31 +/− 2.10 (7) * | - | - |
DISCUSSION
Here we report sex differences in juvenile social play behaviors as measured by same-sex dyadic interactions in B6 mice. When paired with a non-sibling, females tended to be less social than males, spending more time exploring the test cage. Females also displayed more anogenital sniffing and solicited play more frequently. Males, on the other hand, spent the majority of their time in close physical contact with their partner. Our results are markedly different than those in rats. Male rats show distinct play behaviors that differ both quantitatively and qualitatively from the play of females (reviewed in (Auger & Olesen, 2009). In both play tests with pairs (Panksepp, 1981), as well as observations made in group-housed males (Meaney & Mcewen, 1986; Meaney & Stewart, 1981), male rats show more social play and play-fighting behaviors than do females. However, it is possible that group observation tests, using whole litters, detect more sex differences than dyadic tests (Auger & Olesen, 2009). Although we found sex differences in a paired test, it may be important in the future to compare male and female mice using group (litter) observations.
Because sex differences were apparent in normal inbred mice, we asked if these differences could be attributed to sex chromosome genes and/or gonads by testing FCG mice in the same test. Again, XX females were less social than other genotypes, but they did not differ statistically from XY males on any social or nonsocial behavioral measure. Additionally, in contrast to our findings in normal B6 mice, our FCG “normal” females and males (XX females and XY males) spent more time investigating their partner than hetero-chromosomal mice (XY females and XX males). In addition, XY males solicited play more than mice in any other group. Overall, the interactions between gonadal sex and sex chromosomes were responsible for differences, suggesting that sex chromosome genes are not acting alone to influence these behaviors. Since we found no differences in circulating hormones on PN21, developmental hormones are likely interacting with sex chromosome genes to differentiate social interactions. From these experiments, we cannot concluded which hormone(s) during development are influencing mouse juvenile interactions, but the data on play in rats suggest that both androgens and estrogens are involved (Auger & Olesen, 2009; Pellis, 2002). Future studies in which hormones are manipulated during the neonatal period will help clarify how sex differences in these behaviors arise.
In the last experiment, we asked whether the familiarity of partner had an impact on social behavior. Interestingly, sibling pairs of XX females were more social than any other group, and they spent less time exploring and investigating than XY females and XX males. XX females spent the majority of time sitting side-by-side with their sisters. As was the case in Experiment 2, there were no differences between XX females and XY males. XY males behaved the same whether they were tested with a sibling, as compared to a non-sibling, and did not investigate or play often with their sibling partner. While it is not necessarily surprising that the juveniles would interact differently with siblings than non-siblings, it is interesting that the XX females were the only group to show significant differences in social behaviors when paired with siblings. Adult XX females, tested in a resident-intruder paradigm with a bulbectomized male, display longer latencies to follow the intruder, sniffed the intruder less, and exhibited more digging and avoidance behavior than XY females (McPhie-Lalmansingh et al., 2008). Therefore, XX females may be more responsive to environmental and social surroundings than mice of other genotypes. Social isolation (Terranova et al., 1993), litter sex ratios (Laviola & Alleva, 1995), and spacing of litters (Branchi et al., 2009) have all been examined as factors that influence juvenile mouse social behavior, but, to our knowledge, ours is the first study to demonstrate that juvenile social interactions with siblings differ from those with non-siblings.
This is the first examination of the role of sex chromosome genes in juvenile behaviors. Our lab has reported that other social behaviors are influenced by interactions of sex chromosome genes with gonadal sex in adult mice. Gonadectomized XX females are significantly slower than XY females to follow an intruder in a social interaction test (McPhie-Lalmansingh et al., 2008). In addition, gonadectomized XX females implanted with testosterone are less aggressive than XY females (Gatewood et al., 2006), who behave similarly to testosterone treated XX and XY males. Taken together these data support the idea that changes to sex chromosome complement, along with gonadal hormones during development, affect mouse social behavior.
It is not clear why the patterns of the sex differences we noted in B6 mice were different in the FCG mice, but there are several obvious differences between these lines. One interesting distinction is the origin of the Y chromosome in the FCG. The FCG autosomes and X chromosomes are completely crossed in the B6 background; their Y chromosome, with the spontaneous Sry mutation, is from the 129/Sv strain. Thus, the XY B6 male is not genetically identical to the XY FCG male. This could produce differences in male offspring behavior (reviewed in Curley & Mashoodh, 2010), as strain differences in social behaviors have been previously shown (Moy et al., 2009). Recently, paternal Y chromosomes from different strains have been shown to influence the behavior of female offspring (Nelson et al., 2010), which in the FCG could also affect XX versus XY female behavior. In addition, it is possible that the FCG breeders provide qualitatively and/or quantitatively different parental care as compared with B6 breeders. The difference between the pairs is restricted to the post-natal period since in both cases XX B6 females are the dams. Variations both in parent-offspring contact (Parent & Meaney, 2008) as well as sibling interactions could have significant influences on juvenile social behavior (Curley et al., 2010; Laviola & Alleva, 1995).
Our findings show that B6 mice differ from CD-1 strain in which females were more social and explored less than males (Terranova et al., 1993). However, we used different testing procedures; for example the CD-1 study included repeated tests throughout the juvenile period and some mice were housed alone after weaning. Importantly, our findings that B6 females solicited play more frequently than males are consistent with this study and other subsequent studies by the same group (Laviola & Alleva, 1995). Whereas we are the first to directly compare play behavior in non-siblings vs. siblings, other experiments using males only and a testing procedure similar to ours noted differences in amount of investigation by B6 mice depending on whether they tested with siblings (Yang et al., 2007) or non-siblings (Mcfarlane et al., 2008).
In rodents, as in humans, juvenile play behaviors may be important for the development of social skills and other adaptive behaviors in adults (Panksepp et al., 1984). There are already several genes implicated in the development of social play, including the X-linked MeCP2 gene (Guy et al., 2001; Kurian et al., 2008), which is involved in Rett Syndrome in humans, Kdm5c an X-inactivation escapee which has been linked to X-linked mental retardation, (Xu & Andreassi, 2010), and the genes for vasopressin receptors 1a (Bielsky et al., 2005) and 1b (Scattoni et al., 2008), which are implicated in autism spectrum disorders (ASD). It is important to note that there are sex differences in ASD: this disorder is about 4 times more common in males than females (Auyeung et al., 2010; Giarelli et al., 2010), and it is not known what the cause of this dimorphism is. While future studies are needed to understand how sex differences in social behavior develop, our results show that sex chromosome genes, along with strain differences in Y chromosome genes, can influence juvenile social behavior. Thus, sex chromosome genes may be important contributors to the development of ASD and other neurobehavioral diseases.
Supplementary Material
Acknowledgments
The authors thank Aileen Wills and Savera Shetty for their assistance in genotyping the mice. This work was supported by NIH RO1 MH086711. KHC was supported by NIH T32 HD007323. The University of Virginia Center for Reproduction Ligand Assay and Analysis Core is supported by the Eunice Kennedy Shriver NICHD/NIH (SCCPIR) Grant U54-HD28934.
References
- Arnold AP, Chen X. What does the “four core genotypes” mouse model tell us about sex differences in the brain and other tissues? Front Neuroendocrinol. 2009;30:1–9. doi: 10.1016/j.yfrne.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auger AP, Olesen KM. Brain sex differences and the organisation of juvenile social play behaviour. J Neuroendocrinol. 2009;21:519–525. doi: 10.1111/j.1365-2826.2009.01871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auyeung B, Taylor K, Hackett G, Baron-Cohen S. Foetal testosterone and autistic traits in 18 to 24-month-old children. Mol Autism. 2010;1:11. doi: 10.1186/2040-2392-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty WW, Dodge AM, Traylor KL, Meaney MJ. Temporal boundary of the sensitive period for hormonal organization of social play in juvenile rats. Physiol Behav. 1981;26:241–243. doi: 10.1016/0031-9384(81)90017-2. [DOI] [PubMed] [Google Scholar]
- Bielsky IF, Hu SB, Ren X, Terwilliger EF, Young LJ. The V1a vasopressin receptor is necessary and sufficient for normal social recognition: a gene replacement study. Neuron. 2005;47:503–513. doi: 10.1016/j.neuron.2005.06.031. [DOI] [PubMed] [Google Scholar]
- Bonthuis PJ, Cox KH, Searcy BT, Kumar P, Tobet S, Rissman EF. Of mice and rats: key species variations in the sexual differentiation of brain and behavior. Front Neuroendocrinol. 2010;31:341–358. doi: 10.1016/j.yfrne.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branchi I, D’Andrea I, Gracci F, Santucci D, Alleva E. Birth spacing in the mouse communal nest shapes adult emotional and social behavior. Physiol Behav. 2009;96:532–539. doi: 10.1016/j.physbeh.2008.12.003. [DOI] [PubMed] [Google Scholar]
- Casto JM, Ward OB, Bartke A. Play, copulation, anatomy, and testosterone in gonadally intact male rats prenatally exposed to flutamide. Physiol Behav. 2003;79:633–641. doi: 10.1016/s0031-9384(03)00120-3. [DOI] [PubMed] [Google Scholar]
- Curley JP, Jensen CL, Mashoodh R, Champagne FA. Social influences on neurobiology and behavior: Epigenetic effects during development. Psychoneuroendocrinology. 2010 doi: 10.1016/j.psyneuen.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curley JP, Mashoodh R. Parent-of-origin and trans-generational germline influences on behavioral development: the interacting roles of mothers, fathers, and grandparents. Dev Psychobiol. 2010;52:312–330. doi: 10.1002/dev.20430. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Rissman EF, Simerly RB, Yang LY, Scordalakes EM, Auger CJ, Swain A, Lovell-Badge R, Burgoyne PS, Arnold AP. A model system for study of sex chromosome effects on sexually dimorphic neural and behavioral traits. J Neurosci. 2002;22:9005–9014. doi: 10.1523/JNEUROSCI.22-20-09005.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatewood JD, Wills A, Shetty S, Xu J, Arnold AP, Burgoyne PS, Rissman EF. Sex chromosome complement and gonadal sex influence aggressive and parental behaviors in mice. J Neurosci. 2006;26:2335–2342. doi: 10.1523/JNEUROSCI.3743-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giarelli E, Wiggins LD, Rice CE, Levy SE, Kirby RS, Pinto-Martin J, Mandell D. Sex differences in the evaluation and diagnosis of autism spectrum disorders among children. Disabil Health J. 2010;3:107–116. doi: 10.1016/j.dhjo.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy J, Hendrich B, Holmes M, Martin JE, Bird A. A mouse Mecp2-null mutation causes neurological symptoms that mimic Rett syndrome. Nat Genet. 2001;27:322–326. doi: 10.1038/85899. [DOI] [PubMed] [Google Scholar]
- Koopman P, Gubbay J, Vivian N, Goodfellow P, Lovell-Badge R. Male development of chromosomally female mice transgenic for Sry. Nature. 1991;351:117–121. doi: 10.1038/351117a0. [DOI] [PubMed] [Google Scholar]
- Kurian JR, Bychowski ME, Forbes-Lorman RM, Auger CJ, Auger AP. Mecp2 organizes juvenile social behavior in a sex-specific manner. J Neurosci. 2008;28:7137–7142. doi: 10.1523/JNEUROSCI.1345-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laviola G, Alleva E. Sibling effects on the behavior of infant mouse litters (Mus domesticus) J Comp Psychol. 1995;109:68–75. doi: 10.1037/0735-7036.109.1.68. [DOI] [PubMed] [Google Scholar]
- McFarlane HG, Kusek GK, Yang M, Phoenix JL, Bolivar VJ, Crawley JN. Autism-like behavioral phenotypes in BTBR T+tf/J mice. Genes Brain Behav. 2008;7:152–163. doi: 10.1111/j.1601-183X.2007.00330.x. [DOI] [PubMed] [Google Scholar]
- McNaughton CH, Moon J, Strawderman MS, Maclean KN, Evans J, Strupp BJ. Evidence for social anxiety and impaired social cognition in a mouse model of fragile X syndrome. Behav Neurosci. 2008;122:293–300. doi: 10.1037/0735-7044.122.2.293. [DOI] [PubMed] [Google Scholar]
- McPhie-Lalmansingh AA, Tejada LD, Weaver JL, Rissman EF. Sex chromosome complement affects social interactions in mice. Horm Behav. 2008;54:565–570. doi: 10.1016/j.yhbeh.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney MJ. The sexual differentiation of social play. Trends Neurosci. 1988;11:54–58. doi: 10.1016/0166-2236(88)90164-6. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, McEwen BS. Testosterone implants into the amygdala during the neonatal period masculinize the social play of juvenile female rats. Brain Res. 1986;398:324–328. doi: 10.1016/0006-8993(86)91492-7. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Stewart J. Neonatal-androgens influence the social play of prepubescent rats. Horm Behav. 1981;15:197–213. doi: 10.1016/0018-506x(81)90028-3. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Stewart J, Poulin P, McEwen BS. Sexual differentiation of social play in rat pups is mediated by the neonatal androgen-receptor system. Neuroendocrinology. 1983;37:85–90. doi: 10.1159/000123524. [DOI] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Perez A, Barbaro RP, Johns JM, Magnuson TR, Piven J, Crawley JN. Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes Brain Behav. 2004;3:287–302. doi: 10.1111/j.1601-1848.2004.00076.x. [DOI] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Young NB, Nonneman RJ, Grossman AW, Murphy DL, D’Ercole AJ, Crawley JN, Magnuson TR, Lauder JM. Social approach in genetically engineered mouse lines relevant to autism. Genes Brain Behav. 2009;8:129–142. doi: 10.1111/j.1601-183X.2008.00452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Young NB, Perez A, Holloway LP, Barbaro RP, Barbaro JR, Wilson LM, Threadgill DW, Lauder JM, Magnuson TR, Crawley JN. Mouse behavioral tasks relevant to autism: phenotypes of 10 inbred strains. Behav Brain Res. 2007;176:4–20. doi: 10.1016/j.bbr.2006.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson VRS, Sabrina H, Nadeau, Joseph H. Transgenerational genetic effects of the paternal Y chromosome on daughters’ phenotypes. Epigenomics. 2010;2:513–521. doi: 10.2217/epi.10.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen KM, Jessen HM, Auger CJ, Auger AP. Dopaminergic activation of estrogen receptors in neonatal brain alters progestin receptor expression and juvenile social play behavior. Endocrinology. 2005;146:3705–3712. doi: 10.1210/en.2005-0498. [DOI] [PubMed] [Google Scholar]
- Panksepp J. The ontogeny of play in rats. Dev Psychobiol. 1981;14:327–332. doi: 10.1002/dev.420140405. [DOI] [PubMed] [Google Scholar]
- Panksepp J, Siviy S, Normansell L. The psychobiology of play: theoretical and methodological perspectives. Neurosci Biobehav Rev. 1984;8:465–492. doi: 10.1016/0149-7634(84)90005-8. [DOI] [PubMed] [Google Scholar]
- Panksepp JB, Lahvis GP. Social reward among juvenile mice. Genes Brain Behav. 2007;6:661–671. doi: 10.1111/j.1601-183X.2006.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent CI, Meaney MJ. The influence of natural variations in maternal care on play fighting in the rat. Dev Psychobiol. 2008;50:767–776. doi: 10.1002/dev.20342. [DOI] [PubMed] [Google Scholar]
- Pellis SM. Sex differences in play fighting revisited: traditional and nontraditional mechanisms of sexual differentiation in rats. Arch Sex Behav. 2002;31:17–26. doi: 10.1023/a:1014070916047. [DOI] [PubMed] [Google Scholar]
- Pellis SM, Field EF, Smith LK, Pellis VC. Multiple differences in the play fighting of male and female rats. Implications for the causes and functions of play. Neurosci Biobehav Rev. 1997;21:105–120. doi: 10.1016/0149-7634(95)00060-7. [DOI] [PubMed] [Google Scholar]
- Pellis SM, Pasztor TJ. The developmental onset of a rudimentary form of play fighting in C57 mice. Dev Psychobiol. 1999;34:175–182. [PubMed] [Google Scholar]
- Pellis SM, Pellis VC. Differential rates of attack, defense, and counterattack during the developmental decrease in play fighting by male and female rats. Dev Psychobiol. 1990;23:215–231. doi: 10.1002/dev.420230303. [DOI] [PubMed] [Google Scholar]
- Scattoni ML, McFarlane HG, Zhodzishsky V, Caldwell HK, Young WS, Ricceri L, Crawley JN. Reduced ultrasonic vocalizations in vasopressin 1b knockout mice. Behav Brain Res. 2008;187:371–378. doi: 10.1016/j.bbr.2007.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terranova ML, Laviola G, Alleva E. Ontogeny of amicable social behavior in the mouse: gender differences and ongoing isolation outcomes. Dev Psychobiol. 1993;26:467–481. doi: 10.1002/dev.420260805. [DOI] [PubMed] [Google Scholar]
- Xu J, Andreassi M. Reversible histone methylation regulates brain gene expression and behavior. Horm Behav. 2010 doi: 10.1016/j.yhbeh.2010.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Zhodzishsky V, Crawley JN. Social deficits in BTBR T+tf/J mice are unchanged by cross-fostering with C57BL/6J mothers. Int J Dev Neurosci. 2007;25:515–521. doi: 10.1016/j.ijdevneu.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.