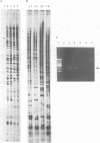
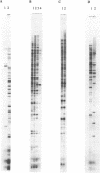
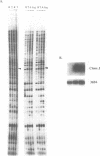
Abstract
Differential display has been developed as a tool to detect and characterize altered gene expression in eukaryotic cells. The basic principle is to systematically amplify messenger RNAs and then distribute their 3' termini on a denaturing polyacrylamide gel. Here we provide methodological details and examine in depth the specificity, sensitivity and reproducibility of the method. We show that the number of anchored oligo-dT primers can be reduced from twelve to four that are degenerate at the penultimate base from the 3' end. We also demonstrate that using optimized conditions described here, multiple RNA samples from related cells can be displayed simultaneously. Therefore process-specific rather than cell-specific genes could be more accurately identified. These results enable further streamlining of the technique and make it readily applicable to a broad spectrum of biological systems.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Band V., Sager R. Distinctive traits of normal and tumor-derived human mammary epithelial cells expressed in a medium that supports long-term growth of both cell types. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1249–1253. doi: 10.1073/pnas.86.4.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basset P., Bellocq J. P., Wolf C., Stoll I., Hutin P., Limacher J. M., Podhajcer O. L., Chenard M. P., Rio M. C., Chambon P. A novel metalloproteinase gene specifically expressed in stromal cells of breast carcinomas. Nature. 1990 Dec 20;348(6303):699–704. doi: 10.1038/348699a0. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Clark J. M. Novel non-templated nucleotide addition reactions catalyzed by procaryotic and eucaryotic DNA polymerases. Nucleic Acids Res. 1988 Oct 25;16(20):9677–9686. doi: 10.1093/nar/16.20.9677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyomarsi K., Sandoval L., Band V., Pardee A. B. Synchronization of tumor and normal cells from G1 to multiple cell cycles by lovastatin. Cancer Res. 1991 Jul 1;51(13):3602–3609. [PubMed] [Google Scholar]
- Lee S. W., Tomasetto C., Sager R. Positive selection of candidate tumor-suppressor genes by subtractive hybridization. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2825–2829. doi: 10.1073/pnas.88.7.2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang P., Averboukh L., Keyomarsi K., Sager R., Pardee A. B. Differential display and cloning of messenger RNAs from human breast cancer versus mammary epithelial cells. Cancer Res. 1992 Dec 15;52(24):6966–6968. [PubMed] [Google Scholar]
- Liang P., Pardee A. B. Differential display of eukaryotic messenger RNA by means of the polymerase chain reaction. Science. 1992 Aug 14;257(5072):967–971. doi: 10.1126/science.1354393. [DOI] [PubMed] [Google Scholar]
- Lin P. F., Lieberman H. B., Yeh D. B., Xu T., Zhao S. Y., Ruddle F. H. Molecular cloning and structural analysis of murine thymidine kinase genomic and cDNA sequences. Mol Cell Biol. 1985 Nov;5(11):3149–3156. doi: 10.1128/mcb.5.11.3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez J., Georgoff I., Martinez J., Levine A. J. Cellular localization and cell cycle regulation by a temperature-sensitive p53 protein. Genes Dev. 1991 Feb;5(2):151–159. doi: 10.1101/gad.5.2.151. [DOI] [PubMed] [Google Scholar]
- Steeg P. S., Bevilacqua G., Kopper L., Thorgeirsson U. P., Talmadge J. E., Liotta L. A., Sobel M. E. Evidence for a novel gene associated with low tumor metastatic potential. J Natl Cancer Inst. 1988 Apr 6;80(3):200–204. doi: 10.1093/jnci/80.3.200. [DOI] [PubMed] [Google Scholar]
- Welsh J., Chada K., Dalal S. S., Cheng R., Ralph D., McClelland M. Arbitrarily primed PCR fingerprinting of RNA. Nucleic Acids Res. 1992 Oct 11;20(19):4965–4970. doi: 10.1093/nar/20.19.4965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. G., Kubelik A. R., Livak K. J., Rafalski J. A., Tingey S. V. DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Res. 1990 Nov 25;18(22):6531–6535. doi: 10.1093/nar/18.22.6531. [DOI] [PMC free article] [PubMed] [Google Scholar]