Abstract
The transgenic animals with mutant copper/zinc superoxide dismutase (SOD1) DNA develop paralytic motor neuron disease resembling human amyotrophic lateral sclerosis (ALS) patients and are commonly used as models for ALS. In the transgenic (Tg) mice with the G93A mutation of the human SOD1 gene SOD1G93A mice), the loss of ventral root axons and the synapses between the muscles and the motor neurons suggested that the motor neuron degeneration might proceed in a dying-back degeneration pattern. To reveal the relationship between axonal degeneration and the progression of the muscle atrophy in the SOD1G93A mice, we investigated the status of the neuromuscular junction along the disease progression. As a presynaptic or postsynaptic marker of neuromuscular junction (NMJ), anti-synaptic vesicle protein 2 (anti-SV2) antibody and α-bungarotoxin (α-BuTX) were chosen in this study and, as a marker of synaptic cleft, anti-agrin antibody was chosen in this study. In the immunohistochemistry of α-BuTX and anti-SV2 antibody, the percentages of double positive NMJs among α-BuTX single positive were decreased in Tg mice through time from ten weeks. The number of postsynaptic acethylcholine receptor (AChR) clusters did not decrease in Tg mice even at the end stage. Immunohistochemistry of α-BuTX and anti-agrin antibody revealed that the increase of immunopositive area of anti-agrin antibody around the muscle fiber in Tg mice from ten weeks of age. In this study, we revealed that the detachment of nerve terminals started at ten weeks in Tg mice. The levels of AChR did not change throughout 5–20 weeks of age in both groups of mice, and AChR remains clustering at NMJs, suggesting that the muscle abnormality is the result of detachment of nerve terminals.
Key words: amyotrophic lateral sclerosis, neuromuscular junction, α-bungarotoxin, SV-2, agrin.
Introduction
Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disease, typically leading to paralysis, respiratory failure and death within 3–5 years after onset. Most patients are sporadic, but about 20% of familial ALS patients have the mutation in the copper/zinc superoxide dismutase (SOD1) gene, and more than 100 mutations have been reported.1,2 The transgenic animals with mutant SOD1 DNA develop paralytic motor neuron disease resembling human ALS patients and are commonly used as models for ALS.3–6
Transgenic (Tg) mice with the G93A mutation of the human SOD1 gene (SOD1G93A mice were the first established model4 and the most widely studied,7 so many trials for treatment have used this animal model. The mice showed first clinical signs as a tremor at 80–90 days after birth, gradually developed paralysis, and died at 140–150 days. In the anterior horn of the spinal cord, the first histological abnormality was mitochondria swelling in motor neuron at 60 days as a consequence of the abnormal stability.8,9 Then astrocytosis was revealed around 80 days. Lastly motor neuron loss became prominent from 100 days, when the clinical symptoms were already observed. The axonal transport defects were found as the early events of motor neuron disease,10–12 and the inhibition of retrograde axonal transport by overexpression of dynamitin demonstrated motor neuron degeneration.13 Moreover, the loss of ventral root axons and the synapses between the muscles and the motor neurons suggested that the motor neuron degeneration might proceed in a dying-back degeneration pattern.14,15 The first event in ALS is the destruction of the neuromuscular junction (NMJ), more specifically of the postsynaptic apparatus, followed by axonal degeneration and late-onset degeneration of motor neuron cell body.14 However, recent studies have shown that even a complete rescue of motor neuron cell bodies does not cure mice over-expressing mutant forms of the SOD1 enzyme (mSOD1 mice).16–18 Hence, preserving motor neuron cell bodies is therapeutically not sufficient since the rescued motor neurons are unable to recreate destroyed NMJs. To reveal the relationship between axonal degeneration and the progression of the muscle atrophy in the SOD1G93A mice, we investigated the status of the NMJ along the disease progression.
Materials and Methods
All experimental procedures were approved by the Animal Care and Use Committee of Okayama University. The SOD1G93A mice were obtained from Jackson Laboratories (B6SJL-TgN (SOD1-G93A) 1Gur mice) (Bar Harbor, ME) (Gurney et al., 1994), and the line was maintained as hemizygotes by mating SOD1G93A males with C57BL/6J females. The offspring were genotyped by a polymerase chain reaction assay of DNA obtained from tail tissue. They are the high-expressing G93A SOD1 mutant mice that display disease onset at 15 weeks and die approximately four weeks later. Since there is a difference in disease onset between male and female SOD1G93A mice, only males were used in this study.
We analyzed disease progressions by a rotarod task every seven days. We measured the time until mice fell from the rod or after an arbitrary limit of 600 seconds. We performed three trials, and recorded the average time for every mouse. For histological studies and Western blot analysis, SOD1G93A mice and non-transgenic mice were deeply anesthetized at five, ten, 15 and 20 weeks of age (each group n=5), and transcardially perfused with heparinized phosphate-buffered saline (PBS) (pH 7.4). The right soleus muscles were frozen for Western blot examination, and the left side muscles were quickly frozen by liquid nitrogen for histological studies. The right side muscles were cut on cryostat at a thickness of 10 µm at −20°C. Frozen transverse sections were fixed in 4% paraformaldehyde for 15 min, and stained with hematoxylin and eosin (HE) for morphological assessment. For immunohistochemistry, the sections were incubated with 5% bovine serum albumin for one hour at room temperature to block the non-specific binding of antibodies. Sections were reacted with primary antibodies overnight at 4°C. The following primary antibodies were used: anti-SV2 (sc-11936, Santa Cruz Biotechnology, CA, USA), anti-agrin (MAB5204, Chemicon, Temecula, CA, USA), and α-bungarotoxin (BuTX) biotin-XX-conjugate (B-1196, Molecular Probes, Eugene, OR, USA). After washing with PBS, the sections were exposed to appropriate secondary antibodies conjugated fluorescent dye for one hour at room temperature, then washed with PBS again, and mounted. A set of sections was treated without the primary antibody to ascertain the specific detection by the antibodies used. Sections were examined and photographed through a microscope (Olympus BX 51) under epifluorescent illumination. We evaluated the remaining neuromuscular junctions (NMJs) as counting α-BuTX and SV2-double positive synapses with a diameter above 20 µm. Counting was performed by investigators blinded to the genotype. Multiple group comparisons of the differences in quantitative measurements were made by Student’s t-test. Statistical significance was accepted at the level of p<0.05. The protein level for acethylcholine receptor (AChR) was analyzed by SDS-PAGE and transferred to a PVDF membrane (Millipore Corporation, Bedford, MA, USA). The blots were blocked in 5% dry milk in Tris-buffered saline for one hour at room temperature. The proteins were detected by incubating the blots overnight at 4°C with the first primary antibodies, followed by HRP-conjugated secondary antibody (GE Healthcare Bio-sciences Corp., Piscataway, NJ, USA) in blocking solution for one hour at room temperature. After washing, the blot was visualized with enhanced chemiluminescent detection kit (Vector) and exposed to X-ray films (Agfa-Gevaert N.V., Belgium). Densitometric evaluation of reactive protein bands was performed using Scion imaging software. The membrane was washed by stripping buffer, then β-tubulin (T-4026, Sigma Aldrich co., St. Louis, MO, USA) used as an internal control.
Results
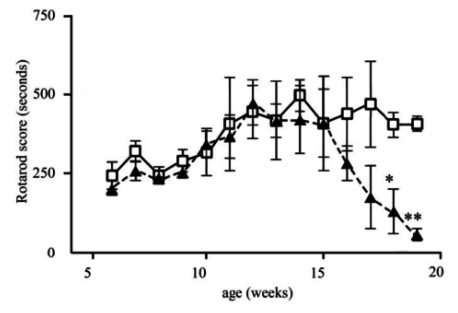
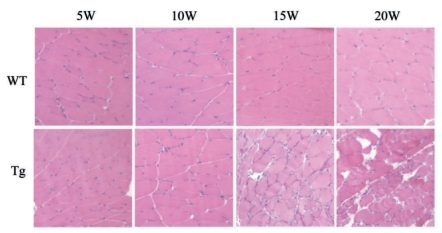
Clinical study showed that SOD1G93A mice (Tg mice) revealed foot tremor from about 15 weeks of age and the rotarod score significantly decreased at 18 (*p<0.05) and 19 (**p<0.01) weeks of age, and died around 20 weeks (Figure 1). Histological findings of soleus muscles with HE staining showed that neurogenic muscular fiber atrophy was already present at ten weeks of age and became severe with time from 15 and 20 weeks in Tg mice (Figure 2, lower panels).
Figure 1.
Clinical scores of rotarod tests. Note the decrease of the score after 15 weeks in Tg mice (filled triangles) as compared to wild-type (open squares) (*p<0.05, **p<0.01).
Figure 2.
HE staining of soleus muscles in wild-type (WT) and Tg mice. Note a subtle change in 10 weeks of Tg mice, with deteriorating 15–20 weeks of age.
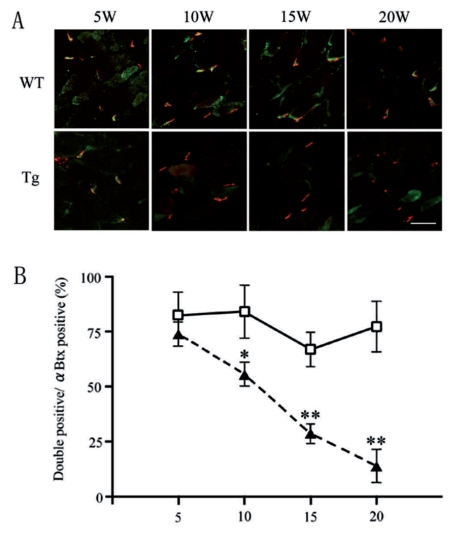
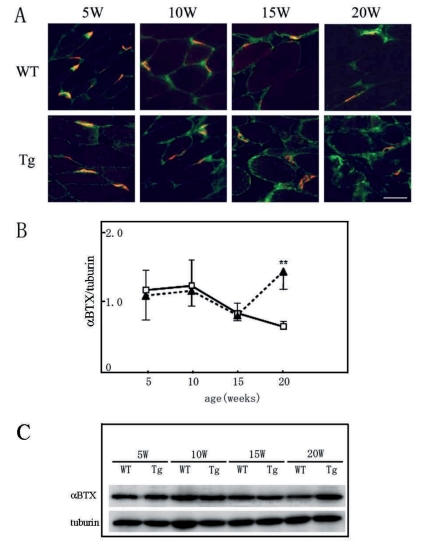
As a presynaptic or postsynaptic marker of NMJ, anti-SV2 antibody and α-BuTX were chosen in this study, which detects synaptic vesicles of axon terminals and AChR of muscles, respectively. As a marker of synaptic cleft, anti-agrin antibody was chosen in this study, which detects anchoring proteins of NMJs. We estimated functional NMJs with double positive for both pre- and postsynaptic markers (Figure 3A). The percentages of double positive NMJs among α-BuTX single positive were not significantly different between WT and Tg mice at five weeks (82.2±9.3% and 67.2±16.0% respectively), which then decreased in Tg mice through the time from ten weeks (62.5±13.8% at ten weeks, 28.7±8.8% at 15 weeks, 13.9±10.4% at 20 weeks) (Figure 3B, filled triangles). The number of postsynaptic AChR clusters did not decrease in Tg mice even at the end stage (20 weeks). Immunohistochemistry of α-BuTX and anti-agrin antibody revealed that there was no increase of the expression levels of agrin throughout the time course in the control muscle, and the immunopositive area of anti-agrin antibody almost co-localized with the immunopositive area of α-BuTX. However, we found the increase of immunopositive area of anti-agrin antibody around the muscle fiber in Tg mice from ten weeks of age. (Figure 4A). In Western blot analysis, the intensity level of α-BuTX did not significantly differ between wild-type and Tg mice throughout 5–20 weeks of age (Figure 4B and C).
Figure 3.
Double immunohistochemistry of anti-SV2 antibody (green) and α-BuTX (red) in soleus muscles in wild-type (WT) and Tg mice. (A) Note the decrease of double positive NMJs from ten weeks in Tg (A, lower panels), and (B) the progressive decrease of the percentages of double positive NMJs in Tg mice (filled triangles) from ten weeks compared with WT mice (open squares). (*p<0.05, **p<0.01).
Figure 4.
Double immunohistochemistry of agrin (green) and α-BuTX (red) (A and B), and Western blotting of AChR of soleus muscles (C). Note the increase of agrin in the muscle spaces at 20 weeks in Tg mice (A, B; filled triangles), while no change in α-BuTX between WT and Tg mice in Western blot (C).
Discussion
In this study, we revealed that the detachment of nerve terminals started at ten weeks, which is still asymptomatic stage of the disease course in Tg mice. At ten weeks of age, Tg mice looked normal and showed the same scores as wild-type mice in rotarod test, thus histological abnormality in soleus muscles preceded the clinical signs. Muscle fiber changes also started at ten weeks in Tg mice. Muscle atrophy progressed with age (Figure 2) along with the loss of functional NMJs before motor neurons remained in the spinal cord. These data suggest that a part of spinal motor neurons may lose their function with territorial muscle fibers even though the neurons showed normal morphologies. With close analysis of NMJ proteins, the SV2 that is the presynaptic marker of the NMJs decreased with disease progression (Figure 3). Decrease of the SV2 starts at ten weeks of age, indicating that nerve terminals detach from NMJs from the time of ten weeks of age of Tg mice. Agrin is a large extracellular matrix proteoglycan, which potentiates the clustering AChR by interacting with several postsynaptic molecules, such as dystroglycan, integrins, and muscle-specific tyrosine kinase (MuSK) and organizes the postsynaptic differentiation.19 There are alternative splicing isoforms of agrin, derived from motoneurons or muscles.20 The immunoreactivities of agrin in the muscle spaces developed stronger with the disease progression of Tg mice (Figure 4). Muscle agrin plays an important role in preserving the transverse orientation in denervated muscles, though neural agrin more actively aggregates the AChR.21 To our surprise, agrin expression at NMJs did not decrease in atrophic muscles in Tg mice, but increased at 20 weeks of age (Figure 4B). Because the present antibody recognized both nerve- and muscle-derived agrins, the expression levels of muscle agrin may increase during the disease progression in Tg mice. The levels of AChR did not change throughout 5–20 weeks of age in both groups of mice, and AChR remains clustering at NMJs (Figure 4), suggesting that the muscle abnormality is the result of detachment of nerve terminals.
A previous report showed that an axotomy resulted in the nerve terminal degeneration, where AChR remained clustering at the original sites.15 Subsequently, regenerating axons differentiated into nerve terminals when they contacted original synaptic basal lamina.15 The detachment characteristics in the current Tg mice were similar to such a distal axonopathy,14,15 but the regeneration and the recomposition of the functional NMJs never happened in the current Tg mice. This difference may be profoundly related to the pathogenesis of this ALS model mice, which could be explained by the defect of axonal transport in Tg mice.10
References
- 1.Brown RH., Jr Amyotrophic lateral sclerosis: recent insights from genetics and transgenic mice. Cell. 1995;80:687–92. doi: 10.1016/0092-8674(95)90346-1. [DOI] [PubMed] [Google Scholar]
- 2.Pasinelli P, Brown RH. Molecular biology of amyotrophic lateral sclerosis: insights from genetics. Nat Rev Neurosci. 2006;7:710–23. doi: 10.1038/nrn1971. [DOI] [PubMed] [Google Scholar]
- 3.Bruijn LI, Becher MW, Lee MK, et al. ALS-linked SOD1 mutant G85R mediates damage to astrocytes and promotes rapidly progressive disease with SOD1-containing inclusions. Neuron. 1997;18:327–38. doi: 10.1016/s0896-6273(00)80272-x. [DOI] [PubMed] [Google Scholar]
- 4.Gurney ME, Pu H, Chiu AY, et al. Motor neuron degeneration in mice that express a human Cu, Zn superoxide dismutase mutation. Science. 1994;264:1772–5. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- 5.Nagai M, Aoki M, Miyoshi I, et al. Rats expressing human cytosolic copper-zinc superoxide dismutase transgenes with amyotrophic lateral sclerosis: associated mutations develop motor neuron disease. J Neurosci. 2001;21:9246–54. doi: 10.1523/JNEUROSCI.21-23-09246.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wong PC, Pardo CA, Borchelt DR, et al. An adverse property of a familial ALS-linked SOD1 mutation causes motor neuron disease characterized by vacuolar degeneration of mitochondria. Neuron. 1995;14:1105–16. doi: 10.1016/0896-6273(95)90259-7. [DOI] [PubMed] [Google Scholar]
- 7.Bendotti C, Carri MT. Lessons from models of SOD1-linked familial ALS. Trends Mol Med. 2004;10:393–400. doi: 10.1016/j.molmed.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 8.Bendotti C, Calvaresi N, Chiveri L, et al. Early vacuolization and mitochondrial damage in motor neurons of FALS mice are not associated with apoptosis or with changes in cytochrome oxidase histochemical reactivity. J Neurol Sci. 2001;191:25–33. doi: 10.1016/s0022-510x(01)00627-x. [DOI] [PubMed] [Google Scholar]
- 9.Bergemalm D, Jonsson PA, Graffmo KS, et al. Overloading of stable and exclusion of unstable human superoxide dismutase-1 variants in mitochondria of murine amyotrophic lateral sclerosis models. J Neurosci. 2006;26:4147–54. doi: 10.1523/JNEUROSCI.5461-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Warita H, Itoyama Y, Abe K. Selective impairment of fast anterograde axonal transport in the peripheral nerves of asymptomatic transgenic mice with a G93A mutant SOD1 gene. Brain Res. 1999;819:120–31. doi: 10.1016/s0006-8993(98)01351-1. [DOI] [PubMed] [Google Scholar]
- 11.Williamson TL, Cleveland DW. Slowing of axonal transport is a very early event in the toxicity of ALS-linked SOD1 mutants to motor neurons. Nat Neurosci. 1999;2:50–6. doi: 10.1038/4553. [DOI] [PubMed] [Google Scholar]
- 12.Zhang B, Tu P, Abtahian F, et al. Neurofilaments and orthograde transport are reduced in ventral root axons of transgenic mice that express human SOD1 with a G93A mutation. J Cell Biol. 1997;139:1307–15. doi: 10.1083/jcb.139.5.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.LaMonte BH, Wallace KE, Holloway BA, et al. Disruption of dynein/dynactin inhibits axonal transport in motor neurons causing late-onset progressive degeneration. Neuron. 2002;34:715–27. doi: 10.1016/s0896-6273(02)00696-7. [DOI] [PubMed] [Google Scholar]
- 14.Fischer LR, Culver DG, Tennant P, et al. Amyotrophic lateral sclerosis is a distal axonopathy: evidence in mice and man. Exp Neurol. 2004;185:232–40. doi: 10.1016/j.expneurol.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 15.Pun S, Santos AF, Saxena S, et al. Selective vulnerability and pruning of phasic motoneuron axons in motoneuron disease alleviated by CNTF. Nat Neurosci. 2006;9:408–19. doi: 10.1038/nn1653. [DOI] [PubMed] [Google Scholar]
- 16.Gould TW, Buss RR, Vinsant S, et al. Complete dissociation of motor neuron death from motor dysfunction by Bax deletion in a mouse model of ALS. J Neurosci. 2006;26:8774–86. doi: 10.1523/JNEUROSCI.2315-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rouaux C, Panteleeva I, René F, et al. Sodium valproate exerts neuroprotective effects in vivo through CREB-binding protein-dependent mechanisms but does not improve survival in an amyotrophic lateral sclerosis mouse model. J Neurosci. 2007;27:5535–45. doi: 10.1523/JNEUROSCI.1139-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dupuis L, Loeffler J-P. Neuromuscular junction destruction during amyotrophic lateral sclerosis: insights from transgenic models. Curr Opin Pharmacol. 2009;9:341–6. doi: 10.1016/j.coph.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 19.Sanes JR, Lichtman JW. Induction, assembly, maturation and maintenance of a postsynaptic apparatus. Nat Rev Neurosci. 2001;2:791–805. doi: 10.1038/35097557. [DOI] [PubMed] [Google Scholar]
- 20.Burgess RW, Nguyen QT, Son YJ, et al. Alternatively spliced isoforms of nerve- and muscle-derived agrin: their roles at the neuromuscular junction. Neuron. 1999;23:33–44. doi: 10.1016/s0896-6273(00)80751-5. [DOI] [PubMed] [Google Scholar]
- 21.Bezakova G, Lomo T. Muscle activity and muscle agrin regulate the organization of cytoskeletal proteins and attached acetylcholine receptor (AChR) aggregates in skeletal muscle fibers. J Cell Biol. 2001;153:1453–63. doi: 10.1083/jcb.153.7.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]