Abstract
Acoustic information is brought to the brain by auditory nerve fibers, all of which terminate in the cochlear nuclei, and is passed up the auditory pathway through the principal cells of the cochlear nuclei. A population of neurons variously known as T stellate, type I multipolar, planar multipolar, or chopper cells forms one of the major ascending auditory pathways through the brain stem. T Stellate cells are sharply tuned; as a population they encode the spectrum of sounds. In these neurons, phasic excitation from the auditory nerve is made more tonic by feed forward excitation, coactivation of inhibitory with excitatory inputs, relatively large excitatory currents through NMDA receptors, and relatively little synaptic depression. The mechanisms that make firing tonic also obscure the fine structure of sounds that is represented in the excitatory inputs from the auditory nerve and account for the characteristic chopping response patterns with which T stellate cells respond to tones. In contrast with other principal cells of the ventral cochlear nucleus (VCN), T stellate cells lack a low-voltage-activated potassium conductance and are therefore sensitive to small, steady, neuromodulating currents. The presence of cholinergic, serotonergic and noradrenergic receptors allows the excitability of these cells to be modulated by medial olivocochlear efferent neurons and by neuronal circuits associated with arousal. T Stellate cells deliver acoustic information to the ipsilateral dorsal cochlear nucleus (DCN), ventral nucleus of the trapezoid body (VNTB), periolivary regions around the lateral superior olivary nucleus (LSO), and to the contralateral ventral lemniscal nuclei (VNLL) and inferior colliculus (IC). It is likely that T stellate cells participate in feedback loops through both medial and lateral olivocochlear efferent neurons and they may be a source of ipsilateral excitation of the LSO.
Keywords: ventral cochlear nucleus, brainstem auditory pathways, ion channels, patch-clamp recording
1. Introduction
Acoustic information flows into the brain through the cochlear nuclei where the auditory pathway is subdivided into multiple, parallel ascending pathways. An important and interesting one is through stellate (or multipolar) cells of the VCN1. Recent findings indicate that these cells and the synapses that feed acoustic information to them are specialized, allowing them to carry different acoustic information than the bushy and octopus cells, the two other major groups of principal cells of the VCN. Individual T stellate cells encode the envelope of sounds in the band of frequencies to which they are tuned, cues that are known to be critical for the understanding of speech (Shannon et al., 1995). As a population, T stellate cells encode spectrum, acoustic information that is used not only for understanding but also for localizing sounds (Blackburn and Sachs, 1990; May et al., 1998).
Most T stellate cells occupy the multipolar cell region of the VCN between the nerve root and the octopus cell area, with a few sitting anterior to the nerve root (Osen, 1969; Lorente de Nó, 1981; Brawer et al., 1974; Oertel et al., 1990; Doucet and Ryugo, 1997; Doucet and Ryugo, 2006). T Stellate cells contact numerous targets in the brainstem, including the olivocochlear efferent neurons, ventral and intermediate nuclei of the lateral lemniscus and inferior colliculus. Here we summarize what functions T stellate cells perform and how cellular features support those functions.
2. Definitions of stellate/multipolar/chopper cells
T Stellate cells were identified in a variety of ways and were eventually found to correspond to a single cell type. Early studies named them “multipolar” (on the basis of Nissl staining) and “stellate” (on the basis of Golgi impregnation) cells (Osen, 1969; Brawer et al., 1974; Lorente de Nó, 1981). It then became clear that there were two distinct types of multipolar/stellate cells. Cant showed that “type I” and “type II” multipolar cells in cats differed in their somatic innervation (Cant, 1981). Smith and Rhode made the correlation between anatomical features and responses to tones. They showed that in cats type I multipolar/stellate cells responded to tones with sustained “chopping” whereas type II responded with “onset-chopping,” firing a few times at regular intervals at the onset of a tone but then stopping firing in the continued presence of the tone (Smith and Rhode, 1989). Choppers have been subdivided into “sustained” and “transient” choppers, defined below. There is no evidence to suggest that sustained and transient choppers represent different anatomical cell types. Studies in slices revealed that the axons of some multipolar/stellate cells project through the Trapezoid body (hence T stellate) whereas others project Dorsalward (D stellate) (Oertel et al., 1990). Those multipolar cells whose main axon projects through the trapezoid body, T stellate cells, have axon collaterals that innervate the same isofrequency lamina in the VCN and in the deep layer of the DCN (Fig. 1). The projection patterns of T and D stellate cells in slices corresponded to projection patterns of choppers and onset choppers, respectively (Rhode et al., 1983; Smith and Rhode, 1989). We conclude, therefore that T stellate cells correspond to type I multipolar/choppers and D stellate cells with type II multipolar/onset-choppers. Doucet and Ryugo confirmed the differences in dendritic morphology and projection patterns with extracellular dye injections in vivo but gave them yet other, albeit more elegant, names: “planar” (type I/T stellate/chopper) and “radial” (type II/D stellate/onset chopper) multipolar cells (Doucet et al., 1997; Doucet et al., 2006).
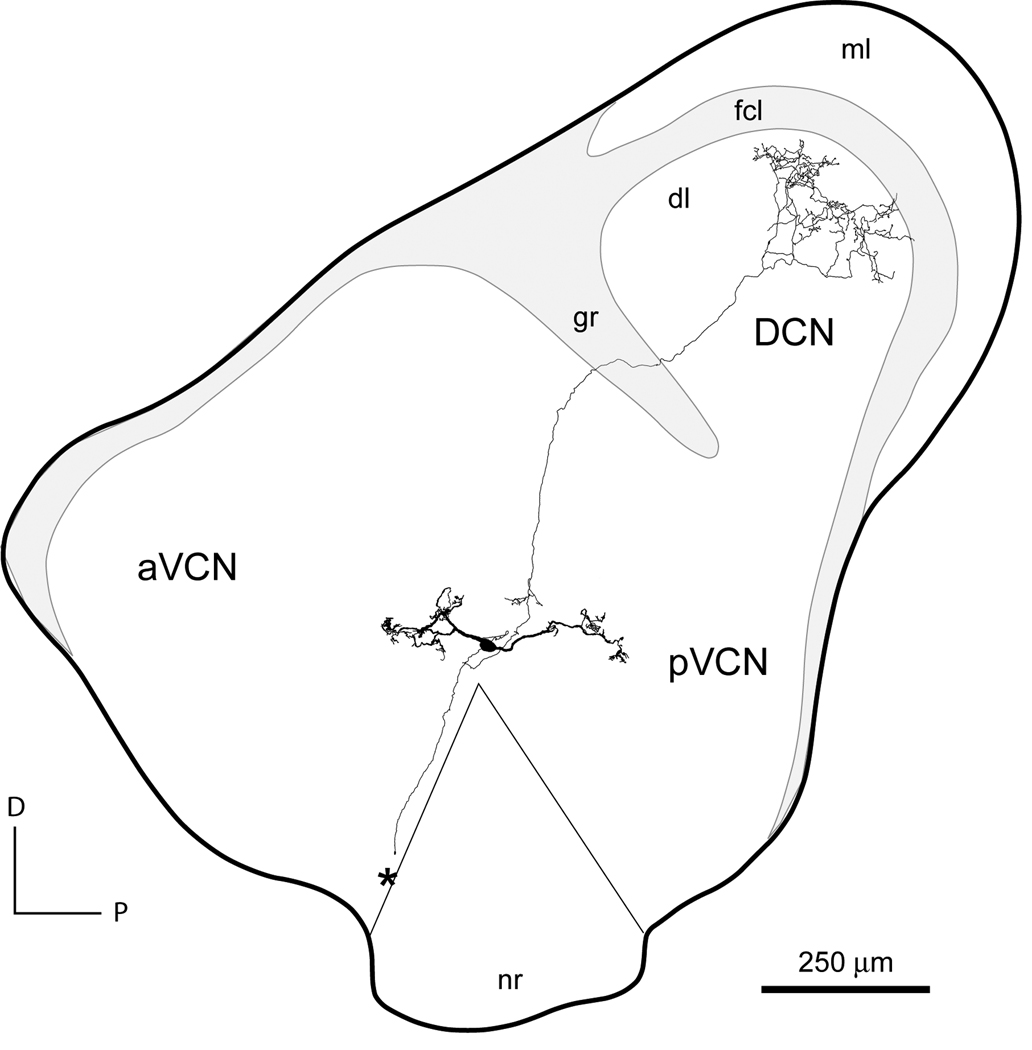
Figure 1.
Reconstruction of a T stellate cell in a slice of the cochlear nuclear complex. The cell was labeled with biocytin in a slice of living tissue. The cell was reconstructed with a camera lucida from sections of the slice that had been processed to visualize cells with methods that have been described (Golding et al., 1995). The soma of this cell lies at the indistinct border between the anteroventral (aVCN) and posteroventral (pVCN) cochlear nuclei, just dorsal to the nerve root (nr). As is characteristic of T stellate cells, its dendrites lie parallel to the path of the ascending and descending branches of auditory nerve fibers with the highly branched tips in the vicinity of overlying granule cells. The axon emanates from the cell body. One branch projects dorsally through the granule cell lamina (gr) into the deep layer (dl) of the dorsal cochlear nucleus (DCN). The terminal arbor occupies an isofrequency lamina that crosses the deep layer but does not extend into the fusiform cell layer (fcl) or the molecular layer (ml). The main axon was cut at the medial surface of the slice where it projected into the trapezoid body (*).
3. T Stellate cells respond to sound by firing tonically
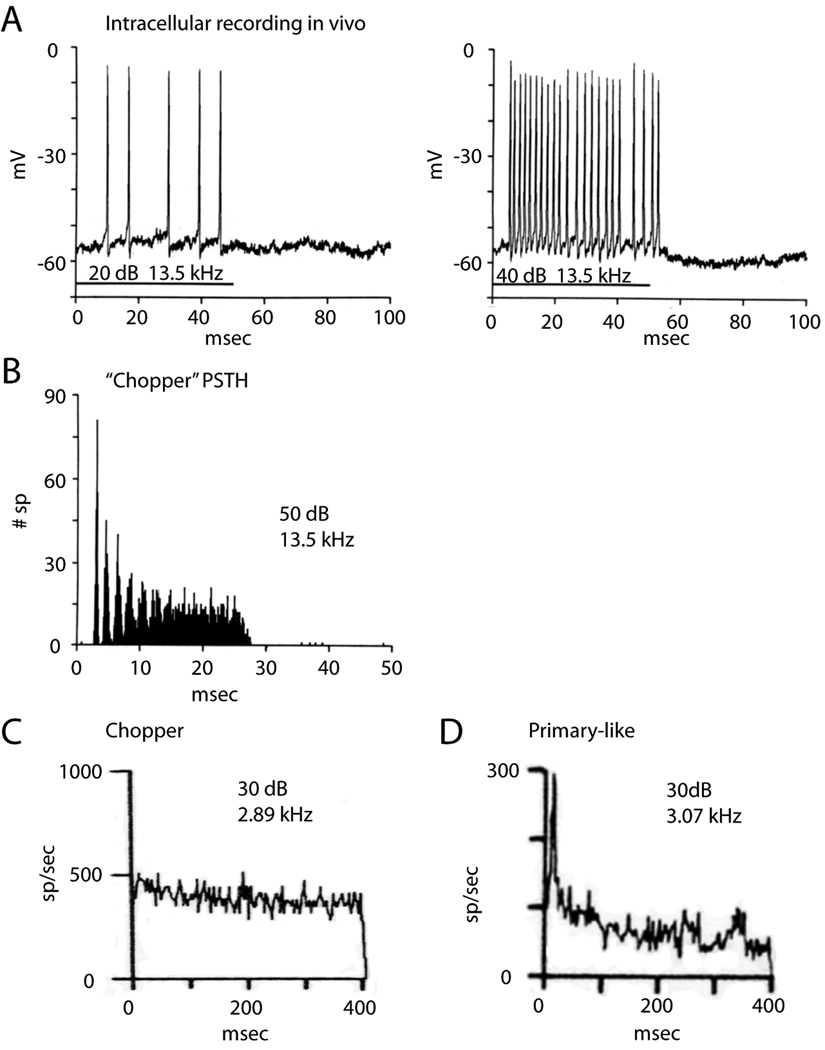
Tones evoke regular, tonic firing in T stellate cells whose rate increases monotonically with intensity (Rhode and Smith, 1986; Young et al., 1988; Blackburn and Sachs, 1989) (Fig. 2A). The timing of action potentials is so reproducible that peristimulus time histograms have modes that are strong and sharp at the onset of the response and weaken as temporal jitter accumulates over the duration of the response to tones (Rhode et al., 1983; Smith et al., 1989; Blackburn et al., 1989) (Fig. 2B). This pattern was termed chopping (Pfeiffer, 1966). Most (70%) choppers respond to tones with regular firing at a constant rate for the duration of the tone as “sustained choppers” (Fig. 2C) (Young et al., 1988; Blackburn et al., 1989). For these neurons, the interspike interval histograms are sharp. In a small proportion of choppers (30%), the “transient choppers,” firing is less regular and the firing rate decreases in the continued presence of a tone. The two populations were distinguished by the coefficient of variation (CV), the ratio of the mean firing rate/standard deviation of the interspike interval. Those with a CV < 0.3 were defined as “sustained choppers”; those with CV >0.3 as “transient choppers” (Young et al., 1988; Blackburn et al., 1989). The chopper whose responses are illustrated in Figure 2C fired with great temporal regularity; the CV remained essentially constant at 0.15. Auditory nerve fibers and the other types of principal cells fire relatively rapidly at the onset of a tone and the rate of their irregular firing decreases to a lower rate over time in the continued presence of a tone, reflecting adaptation (Fig. 2D) (Kiang, 1965; Young et al., 1988).
Figure 2.
T Stellate cells fire tonically in response to tones. A. An intracellular recording from a T stellate cell in a cat shows that the cell fired steadily for the duration of the 50-msec tone at the cell’s characteristic frequency and that the firing became more rapid with increasing intensity. B. Peristimulus time histogram from the same cell as in A generated from responses to 250 repetitions of tones at the characteristic frequency. C. Chopper fires at an almost constant rate for the duration of a 500 msec tone at the characteristic frequency. Bin width for computing histogram was 3.9 ms. D. In contrast with choppers, primary-like neurons fire rapidly at the onset of a tone and then more slowly as they adapt. Figures A and B are by Rhode and Smith, reproduced from the Journal of Neurophysiology, 1986, Am Physiol Soc, used with permission, and C and D are by Blackburn and Sachs, reproduced from the Journal of Neurophysiology, 1989, Am Physiol Soc, used with permission.
Inhibition shapes the temporal response patterns and the tuning of choppers. Glycinergic and GABAergic inhibition that is tuned similarly as excitation reduces peak excitation (Caspary et al., 1994). Choppers also have inhibitory sidebands; sound energy that falls near but outside the frequency range to which choppers increase firing, can cause the firing rate to decrease (Rhode et al., 1986; Palombi and Caspary, 1992; Nelken and Young, 1994; Paolini et al., 2005). Inhibitory sidebands, like the center-surround organization of visual receptive fields, enhance the encoding of spectral peaks and spectral edges. Inhibitory sidebands are produced, at least in part, by input from the more broadly tuned, glycinergic D stellate cells (Ferragamo et al., 1998b) with possible contributions from the periolivary regions (Adams and Warr, 1976; Shore et al., 1991; Ostapoff et al., 1997).
The rate of tonic firing increases monotonically with level both at the onset of tones as well as during the steady state. The firing rate of choppers thus signals level. In contrast, other cells fire more phasically, that is, they fire rapidly at the onset of a tone and then more slowly (primary-like) or stop firing (onset) in the continued presence of a tone. Phasically firing neurons (Fig. 2D) signal changes in level. The tonic firing of choppers is well suited for neurons that encode the spectrum of sounds as a population because the encoding of spectral peaks and valleys is relatively independent of the time after the onset of a sound (Blackburn et al., 1990; May et al., 1998). In neurons whose firing rate slows after the onset, level is encoded not only in the firing rate but also depends on the time after the onset of a sound.
The tonic firing of T stellate cells also makes them well suited for encoding the envelope of sounds. Understanding speech depends on the detection of the envelopes of sounds, especially over the range of <50 Hz (Shannon et al., 1995; Smith et al., 2002). Choppers have been demonstrated to encode amplitude modulation over a wide range of intensities (Frisina et al., 1990; Rhode and Greenberg, 1994).
The phasic firing of auditory nerve fibers is preserved or enhanced in other principal cells of the VCN, the bushy and octopus cells. That phasic firing is functionally important because it enhances formant transitions and provides accurate information about the location of sound sources even in reverberant environments, critical features of hearing (Delgutte and Kiang, 1984; Devore et al., 2009). If the excitatory input to choppers is phasic, however, the question arises how choppers convert phasic to tonic firing.
4. Multiple mechanisms enable T stellate cells to make phasic excitation more tonic
The observation that chopping can be generated in T stellate cells simply by applying steady depolarizing current (Oertel et al., 1988) was initially surprising because excitation by auditory nerve fibers would be expected to be large excitatory synaptic currents at the onset of a tone when auditory nerve fibers fire most rapidly and then to decrease as the firing rate of auditory nerve fibers adapts. It is now clear that five mechanisms conspire to enable tonic firing.
4.1 Feedforward excitation of T stellate cells through other T stellate cells
T Stellate cells innervate their own isofrequency lamina within the multipolar cell area of the VCN (Smith et al., 1989; Oertel et al., 1990; Doucet et al., 1997; Friedland et al., 2003). As most of the cells in the vicinity of T stellate cells in the multipolar cell area are T stellate cells, their targets are probably other T stellate cells. Electrophysiological results confirm that conclusion. Shocks to the root of the auditory nerve evoke not only monosynaptic but also disynaptic excitatory input in slices of the VCN (Ferragamo et al., 1998b).
4.2 Coactivation of phasic excitation with phasic inhibition
It is likely that the coactivation of phasic inhibition reduces phasic excitation (Paolini et al., 2005). T Stellate cells receive inhibition from D stellate cells ipsilaterally and contralaterally (Ferragamo et al., 1998b; Needham and Paolini, 2003). D Stellate cells respond transiently to tones with “onset chopping” patterns (Smith et al., 1989). The rapid firing of D stellate cells at the onset would be expected to produce relatively strong inhibition in T stellate cells at the onset of a tone when excitation from auditory nerve fibers is greatest. The onset of inhibition would be expected to follow the onset of excitation by about 1or 2 ms if auditory nerve fibers are activated synchronously and would therefore be expected to affect T stellate cell responses after one action potential (Oertel et al., 1990). Because D stellate/onset choppers are more broadly tuned than T stellate/chopper cells and often receive input from fibers tuned to higher frequencies, the temporal disparities could be smaller because the cochlear traveling wave delays are shortest for the highest frequencies (Wickesberg, 1996). Inhibition reduces the firing rate within the response area of choppers consistent with choppers receiving input from the more broadly tuned, more phasically responding, glycinergic D stellate cells and from sharply tuned glycinergic neurons in the deep layer of the DCN (Ferragamo et al., 1998b; Wickesberg and Oertel, 1990; Caspary et al., 1994; Palmer et al., 1996). Tuberculoventral neurons whose cell bodies lie in the deep layer of the DCN provide sharply tuned inhibition to T stellate cells (Wickesberg and Oertel, 1988; Wickesberg et al., 1990). The firing patterns of tuberculoventral cells seem to be variable. Cells likely to be tuberculoventral cells have been reported to respond to tones phasically in anesthetized cats (Rhode, 1999). On the other hand, neurons in unanesthetized cats and gerbils cells thought to be tuberculoventral cells have been shown to respond with either phasic and tonic firing patterns (Ding and Voigt, 1997; Shofner and Young, 1985). Glycinergic inhibition may also arise from the ipsilateral periolivary regions and GABAergic inhibition from periolivary regions bilaterally (Adams et al., 1976; Shore et al., 1991; Ostapoff et al., 1997).
4.3 Absence of low-voltage-activated potassium conductance
T Stellate cells lack a low-voltage-activated potassium conductance that helps to make firing transient in bushy and octopus cells (Manis and Marx, 1991; Bal and Oertel, 2001; Ferragamo and Oertel, 2002; McGinley and Oertel, 2006; Cao et al., 2007). In contrast with other principal cells of the VCN, bushy and octopus cells, the biophysical properties of T stellate cells allow them to fire steadily at rates that reflect the magnitude of depolarization (Oertel et al., 1990; Ferragamo et al., 2002).
4.4 Activation of NMDA receptors
Excitation of T stellate cells activates relatively large currents through NMDA receptors (Cao and Oertel, 2010). NMDA receptors require depolarization through AMPA receptors for the relief of the block by Mg2+200 ; they therefore amplify excitation through AMPA receptors (Nowak et al., 1984). In being slower and longer lasting than currents through AMPA receptors, NMDA receptors amplify excitation through AMPA receptors not only in amplitude but also over time. The long-lasting excitation through NMDA receptors thus reduces the phasic nature of excitation and obscures the time course of excitation through AMPA receptors.
4.5 Relatively little synaptic depression
The excitatory inputs of T stellate cells show less synaptic depression than those on phasically firing bushy and octopus cells. Trains of shocks to excitatory inputs, largely from auditory nerve fibers but possibly including some from other T stellate cells, show less synaptic depression than those to bushy or octopus cells (Wu and Oertel, 1987; Chanda and Xu-Friedman, 2010; Cao and Oertel, 2010). The excitation of T stellate cells therefore adapts less than in other neurons of the VCN.
5. The mechanisms that enhance tonic firing obscure the encoding of temporal fine structure of sounds
The onset of the chopper response is dominated by excitation from auditory nerve fibers and would thus be expected to reflect the timing of the arrival of signals through auditory nerve fibers, similarly as in other principal cells of the VCN. Surprisingly, the latency between the onset of a tone and the first spike of chopper responses to tones has a small standard deviation but is about 1 msec longer than that of the other principal cells (van Gisbergen et al., 1975; Young et al., 1988). These authors suggest that the longer first spike latency reflects a longer integration time in choppers than in other principal cells. The integration window, defined by the biophysical properties of the membrane, is indeed longer for T stellate than for bushy or octopus cells (McGinley et al., 2006). It is also possible that the timing of the onset of the response to tones in choppers is affected by inhibition that is activated by higher frequencies and shorter traveling wave delays (Wickesberg, 1996). After an initial volley of excitation, feedforward excitation, the activation of NMDA receptors, and inhibition would be expected to sum with incoming excitation and to obscure the fine structure of sounds that is encoded in the ongoing firing of auditory nerve fibers and in the activation of AMPA receptors. After the onset, the timing of firing of choppers is determined by the biophysical properties of T stellate cells rather than by the fine structure of incoming excitation.
6. T Stellate cells are affected by neuromodulatory as well as driving inputs
T Stellate cells differ from other principal cells of the VCN in their sensitivity to neuromodulatory currents. Their relatively high input resistances allow small currents to cause relatively large voltage changes to produce firing (Fujino and Oertel, 2001). T Stellate cells lack the low-voltage-activated potassium conductance that reduces repetitive firing in bushy and octopus cells (Ferragamo et al., 2002; McGinley et al., 2006). The low-voltage-activated potassium conductance also makes bushy and octopus cells relatively insensitive to steady currents in comparison with T stellate cells (Oertel and Fujino, 2001; McGinley et al., 2006). Furthermore, in T stellate cells the hyperpolarization-activated conductance is activated at more hyperpolarized potentials than in bushy or octopus cells so that a smaller fraction is activated at rest. In having relatively high input resistances, T stellate cells respond with relatively large voltage changes to small modulatory currents. As the voltage-sensitivity of the hyperpolarization-activated inward current can be altered through G-protein coupled receptors, the modulation of the hyperpolarization241 activated inward current can itself make T stellate cells more excitable (Rodrigues and Oertel, 2006). T Stellate cells have been shown to be sensitive to neuromodulatory currents (Fujino et al., 2001).
6.1 Sources of driving inputs
Auditory nerve fibers provide the driving glutamateric excitation for T stellate cells on proximal dendrites and at the soma (Cant, 1981; Ferragamo et al., 1998b; Alibardi, 1998). Consistent with their narrow tuning, only a few (5 or 6 in mice) auditory nerve fibers contact a T stellate cell (Ferragamo et al., 1998b; Cao and Oertel, 2010). In addition they receive glutamatergic excitation from other T stellate cells and glycinergic inhibition from D stellate cells (Ferragamo et al., 1998b). T Stellate cells also receive glycinergic inhibition from tuberculoventral cells in the deep layer of the DCN (Wickesberg et al., 1990; Zhang and Oertel, 1993). As the synaptic responses are well defined in intracellular recordings, these probably arise near the cell body in proximal dendrites as illustrated in Figure 3A (Ferragamo et al., 1998).
Figure 3.
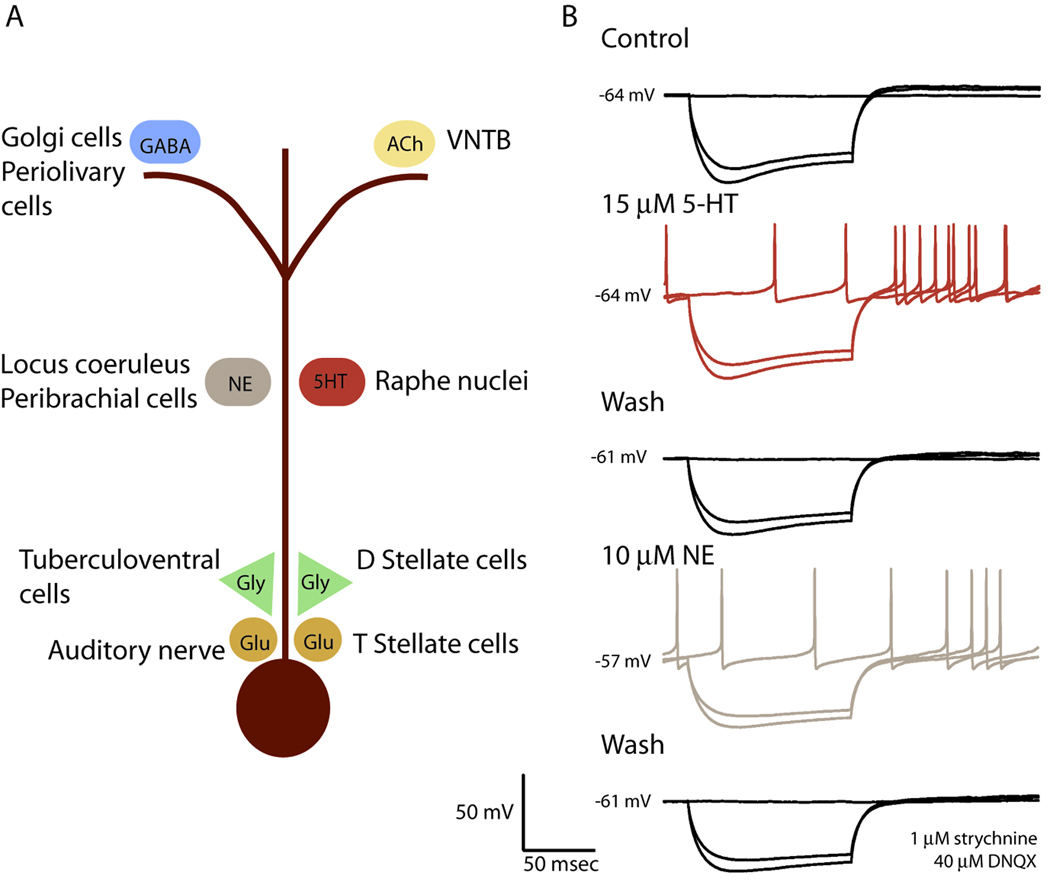
T Stellate cells integrate neuromodulatory with driving inputs. A. Indirect evidence indicates that inputs to T stellate cells are to some extent spatially segregated. Intracellular recordings with electrodes at the soma reveal glutamatergic (Glu) and glycinergic (Gly) synaptic responses. The rapid rise of those synaptic responses is consistent with their being generated near the soma. There are several known sources for these excitatory and inhibitory inputs. Even GABAergic synaptic responses mediated through GABAA receptors are so slow and small that individual PSPs are difficult to resolve suggesting that they are generated distally in dendrites, which is also consistent with the location of GABAergic terminals from Golgi and Periolivary cells in granule cell regions. T Stellate cells have nicotinic and muscarinic acetylcholine receptors. Cholinergic fibers terminate near the tips of T stellate cell dendrites near the granule cell domains . Noradrenergic and serotonergic fibers course throughout the VCN suggesting that they can contact dendrites of T stellate cells either or both proximally or distally. B. A T stellate cell was excited by the bath-application of neuromodulators. A whole-cell patch-clamp recording made with a pipette that contained a gluconate-based, GTP-containing solution in the presence of 1 µM strychnine and 40 µM 6,7-dinitroquinoxaline-2,3-dione (DNQX) in the extracellular saline solution to block glutamatergic and glycinergic synaptic responses, shows that both serotonin and norepinephrine reversibly evoked spontaneous firing. The application of serotonin did not change the resting potential, shown by the numbers at the left of the traces in mV, and responses to hyperpolarizing current pulses (− 0.4 and − 0.5 nA) indicate that the change in input resistance was too small to be detected. Serotonin increased firing after the end of the hyperpolarizing pulse. After the serotonin was washed out, norepinephrine increased spontaneous and anode break firing in the same cell. All effects were reversible. Details of the methods have been described previously (Fujino and Oertel, 2001).
6.2 Neuromodulatory inputs
T Stellate cells also receive modulatory inputs. In contrast with the driving inputs, the neuromodulatory inputs raise or lower the excitability of T stellate cells subtly on a slower time scale, without fast postsynaptic potentials (PSPs) or postsynaptic currents (PSCs). This is interesting because it implies that the conductances associated with the neuromodulatory inputs affect the strength and time course of the driving inputs only minimally. Neuromodulatory inputs that are mediated through G-protein coupled receptors are generally slow even if they arise through terminals near the soma. Anatomical and electrophysiological evidence reviewed below suggests that some neuromodulatory inputs are mediated by ionotropic receptors and arise on distal dendrites as illustrated schematically in Figure 3A.
GABA may play a neuromodulatory role. GABAergic inhibitory PSPs or inhibitory PSCs have never been observed although T stellate cells have GABAA receptors and bicuculline, a blocker of GABAA receptors, increases firing in response to trains of shocks to the auditory nerve in slices (Wu and Oertel, 1986; Oertel and Wickesberg, 1993; Ferragamo et al., 1998b). The specificity of bicuculline indicates that T stellate cells receive inhibition through GABAA receptors even when GABAergic IPSPs and IPSCs have not been observed (Ferragamo et al., 1998b). One explanation for these findings is that dendritic filtering obscures GABAergic IPSPs and IPSCs. Golgi cells are GABAergic and lie within the granule cell domains around the VCN and terminate near the fine distal dendrites of T stellate cells (Ferragamo et al., 1998a). Terminals that contain glutamic acid decarboxylase (GAD) have been observed near cell bodies in the multipolar cell area (Adams and Mugnaini, 1987). These could be glycinergic terminals at which GAD is colocalized with glycine and that are functionally glycinergic as in the DCN (Kolston et al., 1992; Golding and Oertel, 1997). They could also arise from GABAergic neurons in the ipsilateral lateral nucleus of the trapezoid body (LNTB) and dorsomedial periolivary nucleus, neurons whose cell bodies are absent in slices of the cochlear nuclei (Adams et al., 1976; Shore et al., 1991).
Acetylcholine modulates firing of T stellate cells. Cholinergic inputs from collateral branches of olivocochlear neurons in the VNTB terminate in the vicinity of the granule cells, where the tips of dendrites of T stellate cells lie, but distant from most cell bodies in the multipolar cell area (Brown et al., 1991; Sherriff and Henderson, 1994; Osen and Roth, 1969; Motts et al., 2008) (Fig. 3). T Stellate cells, in contrast with D stellate cells, have both nicotinic and muscarinic acetylcholine receptors (Fujino et al., 2001). Cholinergic inputs to T stellate cells, together with their olivocochlear efferent actions, can enhance the encoding of spectral peaks in noise (Fujino et al., 2001).
Norepinephrine (NE) and serotonin (5-HT) are associated with arousal. Both noradrenergic and serotonergic fibers terminate in the vicinity of T stellate cells (Klepper and Herbert, 1991; Thompson et al., 1994; Thompson et al., 1995; Thompson and Thompson, 2001; Thompson, 2003a; Thompson, 2003b). As these fibers have swellings even in the body of the VCN where the principal cells are located, serotonergic and noradrenergic inputs could potentially contact T stellate cells anywhere on the soma or dendrites. T Stellate cells have receptors that sense NE and 5-HT. Figure 3B shows that bath application of 15 µM 5-HT (replicated in 4 cells) or 10 µM NE (replicated in 5 cells) in the presence of blockers of glutamatergic and glycinergic inputs result in increased firing in a T stellate cell. Excitatory synaptic responses would be expected to be slow since adrenergic receptors and most serotonergic receptors are G-protein coupled and act through second messengers. NE and 5-HT can affect either or both presynaptic terminals or postsynaptic cells; in the cell illustrated in Figure 3B these neuromodulators were acting postsynaptically because glutamateric and glycinergic inputs were blocked pharmacologically. Norepinephrine has been shown to affect the release probability at the young calyx of Held (Leao and von Gersdorff, 2001). Iontophoretic application of noradrenaline increased the firing rate of choppers (Kössl and Vater, 1989; Ebert, 1996). 5-HT has been shown to excite or inhibit choppers in vivo (Ebert and Ostwald, 1992).
7. T Stellate cells form a major ascending auditory pathway through the brain stem
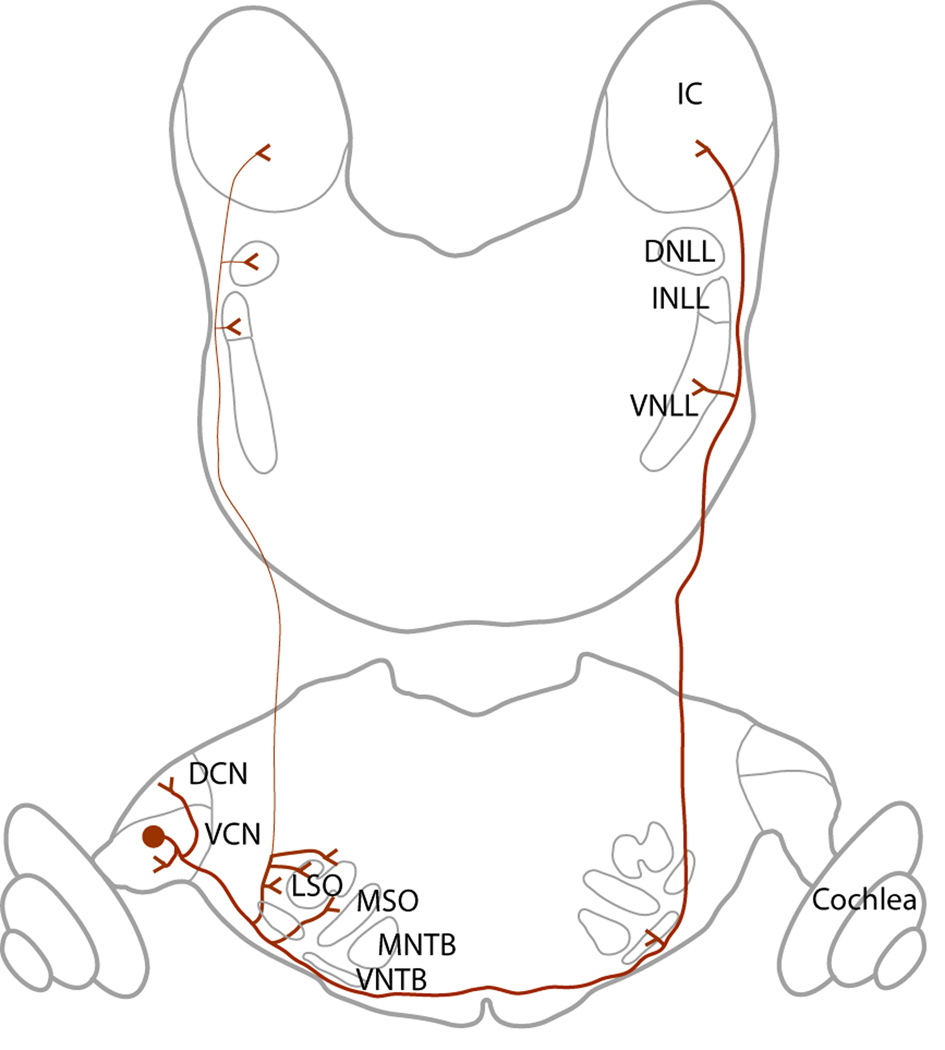
The prominence of T stellate cells in the brainstem auditory pathways can be appreciated by their projections. T Stellate cells have targets within the cochlear nuclei and in addition form one of the major ascending pathways through the brainstem (reviewed by Doucet and Ryugo, 2006). Their projections are summarized in Figure 4.
Figure 4.
T Stellate cells project widely. Local collaterals innervate the ventral (VCN) and dorsal (DCN) cochlear nuclei. The main axon projects out through the trapezoid body, innervates the region around the ipsilateral lateral superior olivary nucleus (LSO), crosses the midline, innervates the contralateral ventral nucleus of the trapezoid body (VNTB), ventral nucleus of the lateral lemniscus (VNLL) and ultimately terminates in the contralateral inferior colliculus. Occasionally T stellate cells innervate the ipsilateral intermediate nucleus of the lateral lemniscus (INLL), dorsal nucleus of the lateral lemniscus (DNLL) and the ipsilateral inferior colliculus.
Axons of T stellate cells exit the VCN through the trapezoid body, cross the midline and ultimately terminate in the contralateral inferior colliculus (Adams, 1979). Through collateral branches they innervate several brainstem auditory nuclei, including the DCN, the lateral superior olive, the contralateral VNTB, and the contralateral VNLL (Warr, 1969; Smith et al., 1993; Thompson, 1998; Doucet and Ryugo, 2003). A small proportion of T stellate cells also projects to the ipsilateral intermediate and dorsal nuclei of the lateral lemniscus and inferior colliculus (IC) (Adams, 1979; Thompson, 1998).
7.1 T Stellate cells bring acoustic input to the DCN
It is likely that T stellate cells deliver the bulk of acoustic input to the deep layer of the ipsilateral DCN (Fig. 1). In mice the density of stellate cell terminals is considerably greater than that of auditory nerve fiber terminals (Cao et al., 2008). T Stellate cells innervate their own isofrequency lamina in the DCN (Smith et al., 1989; Oertel et al., 1990; Doucet et al., 1997; Friedland et al., 2003). It has been suggested that the DCN plays a role in using spectral cues to localize sounds (reviewed by Oertel and Young, 2004). T Stellate cells that encode spectrum effectively are particularly well suited to provide the acoustic input to neurons in the DCN that are involved in making use of spectral cues for localizing sound sources.
7.2 T Stellate cells could bring ipsilateral excitation to the LSO
T Stellate cells project to the lateral superior olive (LSO) raising the possibility that they provide ipsilateral, excitatory, acoustic input to the principal cells of the LSO (Thompson and Thompson, 1991a; Thompson, 1998; Doucet and Ryugo, 2003). LSO neurons detect interaural intensity differences by summing ipsilateral excitation with contralateral inhibition (Tollin and Yin, 2002b; Tollin and Yin, 2002a). What the source is of ipsilateral excitation to the principal cells of the LSO is unclear. It is possible that small spherical bushy cells excite LSO neurons (Cant and Casseday, 1986). However, T stellate cells also project to the LSO (Thompson and Thompson, 1991b; Doucet et al., 2003; Thompson, 1998). T Stellate cells have small diameter axons whose relatively slow propagation could balance the rapid propagation over a longer distance and through an additional synapse from the contralateral globular bushy cells through the medial nucleus of the trapezoid body (MNTB) (Joris and Yin, 1995). It is noteworthy that ipsilateral excitation in the LSO evokes tonic firing in chopping patterns (Tsuchitani and Johnson, 1985). The balance of tonic excitation and more phasic inhibition through globular bushy cells would be expected to result in a balance between excitation and inhibition that changes over the time of a tonal stimulus.
7.3. T Stellate cells take part in olivocochlear efferent feedback loops
T Stellate cell input to the VNTB contributes to medial olivocochlear (MOC) feedback loops. Many cholinergic olivocochlear efferent neurons arise from the contralateral VNTB (Sherriff et al., 1994; Motts et al., 2008). T Stellate cells provide acoustic input to neurons in the contralateral VNTB (Warr, 1982; Robertson and Winter, 1988; Thompson et al., 1991a; Warr, 1992; Smith et al., 1993; de Venecia et al., 2005). They also receive input from at least some of those cholinergic neurons through the crossed MOCs (Spangler et al., 1987; Brown et al., 1988; Winter et al., 1989; Shore et al., 1991; Brown and Benson, 1992; Sherriff et al., 1994; Warr and Beck, 1996). As cholinergic inputs excite T stellate cells, the connections between T stellate cells and the VNTB neurons form a positive feedback loop (Fujino et al., 2001). T Stellate cells are also influenced by medial olivocochlear efferent neurons through the cochlea. This feedback loop is negative because the activation of efferents reduces firing in auditory nerve fibers which in turn reduces excitation of T stellate cells (Wiederhold and Kiang, 1970; Warren, III and Liberman, 1989).
It is also likely that T stellate cells are involved in lateral olivocochlear feedback loops. T Stellate cells terminate in the vicinity of lateral olivocochlear efferent (LOC) neurons (Warr, 1982; Thompson and Thompson, 1988; Thompson et al., 1991a; Doucet et al., 2003). As the excitation of T stellate cells is affected through auditory nerve fibers whose activity is influenced by LOC efferent neurons, T stellate cells are part of a feedback loop in which they regulate the activity of LOC neurons which in turn affect excitability of the auditory nerve fibers that drive responses to sound in T stellate cells. LOC neurons are involved in balancing excitability in the circuits associated with inputs from the two ears (Darrow et al., 2006).
T Stellate cells also feed acoustic input to neurons in the VNLL and to the inferior colliculus. The functional consequences of these direct and indirect connections of T stellate cells with the inferior colliculus are not well understood. They do suggest that the list of integrative functions performed by T stellate cells will continue to grow.
8. Birds have neurons that share many of the features of T stellate cells
The cochlear nuclei have been studied extensively in birds. Nucleus angularis receives innervation from auditory nerve fibers and contains neurons that bear a strong resemblance to T stellate cells. The heterogeneous dendritic morphologies indicate that nucleus angularis holds multiple types and that not all neurons in nucleus angularis bear homology to T stellate cells (Soares and Carr, 2001). Neurons in nucleus angularis project to the lemniscal nuclei and to the inferior colliculus (Takahashi and Konishi, 1988b; Takahashi and Konishi, 1988a). They also project to olivocochlear neurons (Conlee and Parks, 1986; Takahashi et al., 1988a; Yang et al., 1999). Extracellular recordings show that the nucleus angularis of barn owls contains neurons that are sharply tuned and respond to tones as choppers but other response patterns are also present (Sullivan and Konishi, 1984; Sullivan, 1985; Köppl and Carr, 2003).
In slices some neurons in nucleus angularis also respond to depolarizing current pulses with steady, tonic firing (Soares et al., 2002). Like T stellate cells in mammals, cells in nucleus angularis have excitatory synaptic responses with relatively large NMDA components (MacLeod and Carr, 2005). Furthermore, in contrast with neurons in nucleus magnocellularis that resemble mammalian bushy cells, inputs to cells in nucleus angularis not only have less synaptic depression but they sometimes show facilitation (MacLeod et al., 2007).
Owls use interaural timing cues to localize sounds in the azimuth and they use intensity or spectral cues in asymmetrically placed ears to localize sounds in elevation. Neurons in nucleus angularis process the spectral, but not interaural timing, cues that owls use to localize sound sources (Takahashi et al., 1984). The role of neurons in nucleus angularis goes beyond localizing sounds, however. Carr and her colleagues have suggested that neurons in nucleus angularis play a role in recognizing and discriminating sounds (Köppl et al., 2003; MacLeod et al., 2005; MacLeod et al., 2007).
9. Summary
As a population, T stellate cells encode the spectrum of sounds. They receive acoustic input from auditory nerve fibers whose phasic firing emphasizes changes in intensity and convert them to more tonic responses. Several mechanisms contribute to that transformation: Feedforward excitation through other T stellate cells, coactivation of excitation and inhibition, reduction in synaptic depression, and the amplification of excitatory synaptic current over time through NMDA receptors. They deliver that information to nuclei that make use of spectral information. T Stellate cells terminate in the DCN, to olivocochlear efferent neurons, to the lateral superior olive, to the contralateral nuclei of the lateral lemniscus, and to the contralateral inferior colliculus. These targets use spectral information to localize sounds, to adjust the sensitivity of the inner ear, and to recognize and understand sounds. Birds also process sounds through neurons that resemble T stellate cells in their projections and also in their cellular properties, attesting to the fundamental importance that T stellate-like cells have for hearing in vertebrates.
Research Highlights.
T Stellate cells encode the spectrum and envelope of sounds.
These cells convert phasic excitation from the auditory nerve to tonic firing.
Five mechanisms, some cellular and some as circuits, conspire to produce tonic firing.
T Stellate cells sense driving inputs through glutamaterigic and glycinergic receptors.
T stellate cells are modulated through GABA, ACh, 5-HT, and NE receptors.
Acknowledgements
Many colleagues contributed to this work over many years. We are especially grateful to Shu Hui Wu, Robert Wickesberg, Nace Golding, and Aldo Rodrigues. We also thank Ravi Kochhar, Inge Siggelkow, Jo Ann Ekleberry, and members of the office staff whose help and support has been critical and longstanding. We also thank Jennifer Seifert for her professional editorial input. This work was supported by a grant from the NIH DC00176.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Abbreviations: VCN, ventral cochlear nucleus; DCN, dorsal cochlear nucleus; VNTB, ventral nucleus of the trapezoid body; LSO, lateral superior olive; MSO, medial superior olive; IC, inferior colliculus; MOC, medial olivocochlear efferent neurons; LOC, lateral olivocochlear efferent neurons; CV, coefficient of variation; NMDA, N-methyl-D-aspartate; AMPA, α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate; DNQX, 6,7-dinitroquinoxaline-2,3-dione; GABA, γ-amino butyric acid; GAD, glutamic acid decarboxylase; CV, coefficient of variation; PSP, postsynaptic potential; PSC, postsynaptic current; NE, norepinephrine; 5-HT, serotonin.
References
- Adams JC. Ascending projections to the inferior colliculus. J. Comp. Neurol. 1979;183:519–538. doi: 10.1002/cne.901830305. [DOI] [PubMed] [Google Scholar]
- Adams JC, Mugnaini E. Patterns of glutamate decarboxylase immunostaining in the feline cochlear nuclear complex studied with silver enhancement and electron microscopy. Journal.of.Comparative.Neurology. 1987;262:375–401. doi: 10.1002/cne.902620305. [DOI] [PubMed] [Google Scholar]
- Adams JC, Warr WB. Origins of axons in the cat's acoustic striae determined by injection of horseradish peroxidase into severed tracts. J. Comp. Neurol. 1976;170:107–121. doi: 10.1002/cne.901700108. [DOI] [PubMed] [Google Scholar]
- Alibardi L. Ultrastructural and immunocytochemical characterization of neurons in the rat ventral cochlear nucleus projecting to the inferior colliculus. Ann. Anat. 1998;180:415–426. doi: 10.1016/S0940-9602(98)80102-7. [DOI] [PubMed] [Google Scholar]
- Bal R, Oertel D. Potassium currents in octopus cells of the mammalian cochlear nuclei. J. Neurophysiol. 2001;86:2299–2311. doi: 10.1152/jn.2001.86.5.2299. [DOI] [PubMed] [Google Scholar]
- Blackburn CC, Sachs MB. Classification of unit types in the anteroventral cochlear nucleus: PST histograms and regularity analysis. J. Neurophysiol. 1989;62:1303–1329. doi: 10.1152/jn.1989.62.6.1303. [DOI] [PubMed] [Google Scholar]
- Blackburn CC, Sachs MB. The representations of the steady-state vowel sound /e/ in the discharge patterns of cat anteroventral cochlear nucleus neurons. J.Neurophysiol. 1990;63:1191–1212. doi: 10.1152/jn.1990.63.5.1191. [DOI] [PubMed] [Google Scholar]
- Brawer JR, Morest DK, Kane EC. The neuronal architecture of the cochlear nucleus of the cat. J.Comp.Neurol. 1974;155:251–300. doi: 10.1002/cne.901550302. [DOI] [PubMed] [Google Scholar]
- Brown MC, Benson TE. Transneuronal labeling of cochlear nucleus neurons by HRP-labeled auditory nerve fibers and olivocochlear branches in mice. J.Comp.Neurol. 1992;321:645–665. doi: 10.1002/cne.903210411. [DOI] [PubMed] [Google Scholar]
- Brown MC, Liberman MC, Benson TE, Ryugo DK. Brainstem branches from olivocochlear axons in cats and rodents. J. Comp. Neurol. 1988;278:591–603. doi: 10.1002/cne.902780410. [DOI] [PubMed] [Google Scholar]
- Brown MC, Pierce S, Berglund AM. Cochlear-nucleus branches of thick (medial) olivocochlear fibers in the mouse: a cochleotopic projection. J.Comp.Neurol. 1991;303:300–315. doi: 10.1002/cne.903030211. [DOI] [PubMed] [Google Scholar]
- Cant NB. The fine structure of two types of stellate cells in the anterior division of the anteroventral cochlear nucleus of the cat. Neurosci. 1981;6:2643–2655. doi: 10.1016/0306-4522(81)90109-3. [DOI] [PubMed] [Google Scholar]
- Cant NB, Casseday JH. Projections from the anteroventral cochlear nucleus to the lateral and medial superior olivary nuclei. J. Comp. Neurol. 1986;247:457–476. doi: 10.1002/cne.902470406. [DOI] [PubMed] [Google Scholar]
- Cao XJ, McGinley MJ, Oertel D. Connections and synaptic function in the posteroventral cochlear nucleus of deaf jerker mice. J. Comp. Neurol. 2008;510:297–308. doi: 10.1002/cne.21788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao XJ, Oertel D. Auditory nerve fibers excite targets through synapses that vary in convergence, strength and short-term plasticity. J. Neurophysiol. 2010 doi: 10.1152/jn.00451.2010. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao XJ, Shatadal S, Oertel D. Voltage-sensitive conductances of bushy cells of the mammalian ventral cochlear nucleus. J. Neurophysiol. 2007;97:3961–3975. doi: 10.1152/jn.00052.2007. [DOI] [PubMed] [Google Scholar]
- Caspary DM, Backoff PM, Finlayson PG, Palombi PS. Inhibitory inputs modulate discharge rate within frequency receptive fields of anteroventral cochlear nucleus neurons. J. Neurophysiol. 1994;72:2124–2133. doi: 10.1152/jn.1994.72.5.2124. [DOI] [PubMed] [Google Scholar]
- Chanda S, Xu-Friedman MA. A Low-affinity antagonist reveals saturation and desensitization in mature synapses in the auditory brainstem. J. Neurophysiol. 2010 doi: 10.1152/jn.00751.2009. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conlee JW, Parks TN. Origin of ascending auditory projections to the nucleus mesencephalicus lateralis pars dorsalis in the chicken. Brain Res. 1986;367:96–113. doi: 10.1016/0006-8993(86)91583-0. [DOI] [PubMed] [Google Scholar]
- Darrow KN, Maison SF, Liberman MC. Cochlear efferent feedback balances interaural sensitivity. Nat.Neurosci. 2006;9:1474–1476. doi: 10.1038/nn1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Venecia RK, Liberman MC, Guinan JJ, Jr, Brown MC. Medial olivocochlear reflex interneurons are located in the posteroventral cochlear nucleus: a kainic acid lesion study in guinea pigs. J.Comp. Neurol. 2005;487:345–360. doi: 10.1002/cne.20550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgutte B, Kiang NY. Speech coding in the auditory nerve: IV. Sounds with consonant-like dynamic characteristics. J.Acoust.Soc.Am. 1984;75:897–907. doi: 10.1121/1.390599. [DOI] [PubMed] [Google Scholar]
- Devore S, Ihlefeld A, Hancock K, Shinn-Cunningham B, Delgutte B. Accurate sound localization in reverberant environments is mediated by robust encoding of spatial cues in the auditory midbrain. Neuron. 2009;62:123–134. doi: 10.1016/j.neuron.2009.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding J, Voigt HF. Intracellular response properties of units in the dorsal cochlear nucleus of unanesthetized decerebrate gerbil. J.Neurophysiol. 1997;77:2549–2572. doi: 10.1152/jn.1997.77.5.2549. [DOI] [PubMed] [Google Scholar]
- Doucet JR, Ryugo DK. Projections from the ventral cochlear nucleus to the dorsal cochlear nucleus in rats. J.Comp.Neurol. 1997;385:245–264. [PubMed] [Google Scholar]
- Doucet JR, Ryugo DK. Axonal pathways to the lateral superior olive labeled with biotinylated dextran amine injections in the dorsal cochlear nucleus of rats. J. Comp. Neurol. 2003;461:452–465. doi: 10.1002/cne.10722. [DOI] [PubMed] [Google Scholar]
- Doucet JR, Ryugo DK. Structural and functional classes of multipolar cells in the ventral cochlear nucleus. Anat Rec.A Discov.Mol. Cell Evol. Biol. 2006;288:331–344. doi: 10.1002/ar.a.20294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert U. Noradrenalin enhances the activity of cochlear nucleus neurons in the rat. Eur.J. Neurosci. 1996;8:1306–1314. doi: 10.1111/j.1460-9568.1996.tb01299.x. [DOI] [PubMed] [Google Scholar]
- Ebert U, Ostwald J. Serotonin modulates auditory information processing in the cochlear nucleus of the rat. Neurosci. Lett. 1992;145:51–54. doi: 10.1016/0304-3940(92)90201-h. [DOI] [PubMed] [Google Scholar]
- Ferragamo M, Golding NL, Gardner SM, Oertel D. Golgi cells in the superficial granule cell domain overlying the ventral cochlear nucleus: Morphology and eletrophysiology in slices. J.Comp.Neurol. 1998a;400:519–528. [PubMed] [Google Scholar]
- Ferragamo MJ, Golding NL, Oertel D. Synaptic inputs to stellate cells in the ventral cochlear nucleus. J.Neurophysiol. 1998b;79:51–63. doi: 10.1152/jn.1998.79.1.51. [DOI] [PubMed] [Google Scholar]
- Ferragamo MJ, Oertel D. Octopus cells of the mammalian ventral cochlear nucleus sense the rate of depolarization. J. Neurophysiol. 2002;87:2262–2270. doi: 10.1152/jn.00587.2001. [DOI] [PubMed] [Google Scholar]
- Friedland DR, Pongstaporn T, Doucet JR, Ryugo DK. Ultrastructural examination of the somatic innervation of ventrotubercular cells in the rat. J. Comp. Neurol. 2003;459:77–89. doi: 10.1002/cne.10603. [DOI] [PubMed] [Google Scholar]
- Frisina RD, Smith RL, Chamberlain SC. Encoding of amplitude modulation in the gerbil cochlear nucleus: I. A hierarchy of enhancement. Hear.Res. 1990;44:99–122. doi: 10.1016/0378-5955(90)90074-y. [DOI] [PubMed] [Google Scholar]
- Fujino K, Oertel D. Cholinergic modulation of stellate cells in the mammalian ventral cochlear nucleus. J.Neurosci. 2001;21:7372–7383. doi: 10.1523/JNEUROSCI.21-18-07372.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golding NL, Oertel D. Physiological identification of the targets of cartwheel cells in the dorsal cochlear nucleus. J Neurophysiol. 1997;78:248–260. doi: 10.1152/jn.1997.78.1.248. [DOI] [PubMed] [Google Scholar]
- Golding NL, Robertson D, Oertel D. Recordings from slices indicate that octopus cells of the cochlear nucleus detect coincident firing of auditory nerve fibers with temporal precision. J.Neurosci. 1995;15:3138–3153. doi: 10.1523/JNEUROSCI.15-04-03138.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joris PX, Yin TC. Envelope coding in the lateral superior olive. I . Sensitivity to interaural time differences. J.Neurophysiol. 1995;73:1043–1062. doi: 10.1152/jn.1995.73.3.1043. [DOI] [PubMed] [Google Scholar]
- Kiang NY. Discharge Patterns of Single Fibers in the Cat's Auditory Nerve. Cambridge, Massachusetts: The M.I.T. Press; 1965. [Google Scholar]
- Klepper A, Herbert H. Distribution and origin of noradrenergic and serotonergic fibers in the cochlear nucleus and inferior colliculus of the rat. Brain Res. 1991;557:190–201. doi: 10.1016/0006-8993(91)90134-h. [DOI] [PubMed] [Google Scholar]
- Kolston J, Osen KK, Hackney CM, Ottersen OP, Storm-Mathisen J. An atlas of glycine- and GABA-like immunoreactivity and colocalization in the cochlear nuclear complex of the guinea pig. Anatomy.&.Embryology. 1992;186:443–465. doi: 10.1007/BF00185459. [DOI] [PubMed] [Google Scholar]
- Köppl C, Carr CE. Computational diversity in the cochlear nucleus angularis of the barn owl. J. Neurophysiol. 2003;89:2313–2329. doi: 10.1152/jn.00635.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kössl M, Vater M. Noradrenaline enhances temporal auditory contrast and neuronal timing precision in the cochlear nucleus of the mustached bat. J.Neurosci. 1989;9:4169–4178. doi: 10.1523/JNEUROSCI.09-12-04169.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leao RM, von Gersdorff H. Noradrenaline increases high-frequency firing at the calyx of held synapse during development by inhibiting glutamate release. J.Neurophysiol. 2001;87:2297–2306. doi: 10.1152/jn.2002.87.5.2297. [DOI] [PubMed] [Google Scholar]
- Lorente de Nó R. The Primary Acoustic Nuclei. New York: Raven Press; 1981. [Google Scholar]
- MacLeod KM, Carr CE. Synaptic physiology in the cochlear nucleus angularis of the chick. J. Neurophysiol. 2005;93:2520–2529. doi: 10.1152/jn.00898.2004. [DOI] [PubMed] [Google Scholar]
- MacLeod KM, Horiuchi TK, Carr CE. A role for short-term synaptic facilitation and depression in the processing of intensity information in the auditory brain stem. J. Neurophysiol. 2007;97:2863–2874. doi: 10.1152/jn.01030.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manis PB, Marx SO. Outward currents in isolated ventral cochlear nucleus neurons. J.Neurosci. 1991;11:2865–2880. doi: 10.1523/JNEUROSCI.11-09-02865.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May BJ, Prell GS, Sachs MB. Vowel representations in the ventral cochlear nucleus of the cat: effects of level, background noise, and behavioral state. J.Neurophysiol. 1998;79:1755–1767. doi: 10.1152/jn.1998.79.4.1755. [DOI] [PubMed] [Google Scholar]
- McGinley MJ, Oertel D. Rate thresholds determine the precision of temporal integration in principal cells of the ventral cochlear nucleus. Hear. Res. 2006;216–217:52–63. doi: 10.1016/j.heares.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Motts SD, Slusarczyk AS, Sowick CS, Schofield BR. Distribution of cholinergic cells in guinea pig brainstem. Neurosci. 2008;154:186–195. doi: 10.1016/j.neuroscience.2007.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needham K, Paolini AG. Fast inhibition underlies the transmission of auditory information between cochlear nuclei. J Neurosci. 2003;23:6357–6361. doi: 10.1523/JNEUROSCI.23-15-06357.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelken I, Young ED. Two separate inhibitory mechanisms shape the responses of dorsal cochlear nucleus type IV units to narrowband and wideband stimuli. J.Neurophysiol. 1994;71:2446–2462. doi: 10.1152/jn.1994.71.6.2446. [DOI] [PubMed] [Google Scholar]
- Nowak L, Bregetovski P, Ascher P, Herbet P, Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984;307:462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Oertel D, Fujino K. Role of biophysical specialization in cholinergic modulation in neurons of the ventral cochlear nuclei. Audiol.Neurootol. 2001;6:161–166. doi: 10.1159/000046825. [DOI] [PubMed] [Google Scholar]
- Oertel D, Wickesberg RE. Glycinergic inhibition in the cochlear nuclei: evidence for tuberculoventral neurons being glycinergic. In: Merchan MA, Juiz JM, Godfrey DA, editors. The Mammalian Cochlear Nuclei: Organization and Function. New York: Plenum Publishing Corp.; 1993. [Google Scholar]
- Oertel D, Wu SH, Garb MW, Dizack C. Morphology and physiology of cells in slice preparations of the posteroventral cochlear nucleus of mice. J.Comp.Neurol. 1990;295:136–154. doi: 10.1002/cne.902950112. [DOI] [PubMed] [Google Scholar]
- Oertel D, Wu SH, Hirsch JA. Electrical characteristics of cells and neuronal circuitry in the cochlear nuclei studied with intracellular recordings from brain slices. In: Edelman GM, Gall WE, Cowan WM, editors. Auditory Function. New York: John Wiley & Sons, Inc.; 1988. pp. 313–336. [Google Scholar]
- Oertel D, Young ED. What's a cerebellar circuit doing in the auditory system? Trends Neurosci. 2004;27:104–110. doi: 10.1016/j.tins.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Osen KK. Cytoarchitecture of the cochlear nuclei in the cat. J.Comp.Neurol. 1969;136:453–484. doi: 10.1002/cne.901360407. [DOI] [PubMed] [Google Scholar]
- Osen KK, Roth K. Histochemical localization of cholinesterases in the cochlear nuclei of the cat, with notes on the origin of acetylcholinesterase-positive afferents and the superior olive. Brain Res. 1969;16:165–185. doi: 10.1016/0006-8993(69)90092-4. [DOI] [PubMed] [Google Scholar]
- Ostapoff EM, Benson CG, Saint Marie RL. GABA- and glycine-immunoreactive projections from the superior olivary complex to the cochlear nucleus in guinea pig. J.Comp.Neurol. 1997;381:500–512. doi: 10.1002/(sici)1096-9861(19970519)381:4<500::aid-cne9>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Palmer AR, Jiang D, Marshall DH. Responses of ventral cochlear nucleus onset and chopper units as a function of signal bandwidth. J.Neurophysiol. 1996;75:780–794. doi: 10.1152/jn.1996.75.2.780. [DOI] [PubMed] [Google Scholar]
- Palombi PS, Caspary DM. GABAA receptor antagonist bicuculline alters response properties of posteroventral cochlear nucleus neurons. J. Neurophysiol. 1992;67:738–746. doi: 10.1152/jn.1992.67.3.738. [DOI] [PubMed] [Google Scholar]
- Paolini AG, Clarey JC, Needham K, Clark GM. Balanced inhibition and excitation underlies spike firing regularity in ventral cochlear nucleus chopper neurons. Eur.J. Neurosci. 2005;21:1236–1248. doi: 10.1111/j.1460-9568.2005.03958.x. [DOI] [PubMed] [Google Scholar]
- Pfeiffer RR. Classification of response patterns of spike discharges for units in the cochlear nucleus: tone-burst stimulation. Exp.Brain Res. 1966;1:220–235. doi: 10.1007/BF00234343. [DOI] [PubMed] [Google Scholar]
- Rhode WS. Vertical cell responses to sound in cat dorsal cochlear nucleus. J.Neurophysiol. 1999;82:1019–1032. doi: 10.1152/jn.1999.82.2.1019. [DOI] [PubMed] [Google Scholar]
- Rhode WS, Greenberg S. Encoding of amplitude modulation in the cochlear nucleus of the cat. J.Neurophysiol. 1994;71:1797–1825. doi: 10.1152/jn.1994.71.5.1797. [DOI] [PubMed] [Google Scholar]
- Rhode WS, Oertel D, Smith PH. Physiological response properties of cells labeled intracellularly with horseradish peroxidase in cat ventral cochlear nucleus. J.Comp.Neurol. 1983;213:448–463. doi: 10.1002/cne.902130408. [DOI] [PubMed] [Google Scholar]
- Rhode WS, Smith PH. Encoding timing and intensity in the ventral cochlear nucleus of the cat. J.Neurophysiol. 1986;56:261–286. doi: 10.1152/jn.1986.56.2.261. [DOI] [PubMed] [Google Scholar]
- Robertson D, Winter IM. Cochlear nucleus inputs to olivocochlear neurones revealed by combined anterograde and retrograde labelling in the guinea pig. Brain Res. 1988;462:47–55. doi: 10.1016/0006-8993(88)90583-5. [DOI] [PubMed] [Google Scholar]
- Rodrigues ARA, Oertel D. Hyperpolarization-activated currents regulate excitability in stellate cells of the mammalian ventral cochlear nucleus. J.Neurophysiol. 2006;95:76–87. doi: 10.1152/jn.00624.2005. [DOI] [PubMed] [Google Scholar]
- Shannon RV, Zeng FG, Kamath V, Wygonski J, Ekelid M. Speech recognition with primarily temporal cues. Science. 1995;270:303–304. doi: 10.1126/science.270.5234.303. [DOI] [PubMed] [Google Scholar]
- Sherriff FE, Henderson Z. Cholinergic neurons in the ventral trapezoid nucleus project to the cochlear nuclei in the rat. Neurosci. 1994;58:627–633. doi: 10.1016/0306-4522(94)90086-8. [DOI] [PubMed] [Google Scholar]
- Shofner WP, Young ED. Excitatory/inhibitory response types in the cochlear nucleus: relationships to discharge patterns and responses to electrical stimulation of the auditory nerve. J.Neurophysiol. 1985;54:917–939. doi: 10.1152/jn.1985.54.4.917. [DOI] [PubMed] [Google Scholar]
- Shore SE, Helfert RH, Bledsoe SC, Jr, Altschuler RA, Godfrey DA. Descending projections to the dorsal and ventral divisions of the cochlear nucleus in guinea pig. Hear. Res. 1991;52:255–268. doi: 10.1016/0378-5955(91)90205-n. [DOI] [PubMed] [Google Scholar]
- Smith PH, Joris PX, Banks MI, Yin TCT. Responses of cochlear nucleus cells and projections of their axons. In: Merchan MA, Juiz JM, Godfrey DA, Mugnaini E, editors. The Mammalian Cochlear Nuclei, Organization and Function. New York and London: Plenum Press; 1993. pp. 349–360. [Google Scholar]
- Smith PH, Rhode WS. Structural and functional properties distinguish two types of multipolar cells in the ventral cochlear nucleus. J.Comp.Neurol. 1989;282:595–616. doi: 10.1002/cne.902820410. [DOI] [PubMed] [Google Scholar]
- Smith ZM, Delgutte B, Oxenham AJ. Chimaeric sounds reveal dichotomies in auditory perception. Nature. 2002;416:87–90. doi: 10.1038/416087a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares D, Carr CE. The cytoarchitecture of the nucleus angularis of the barn owl (Tyto alba) J. Comp. Neurol. 2001;429:192–205. doi: 10.1002/1096-9861(20000108)429:2<192::aid-cne2>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Soares D, Chitwood RA, Hyson RL, Carr CE. Intrinsic neuronal properties of the chick nucleus angularis. J. Neurophysiol. 2002;88:152–162. doi: 10.1152/jn.2002.88.1.152. [DOI] [PubMed] [Google Scholar]
- Spangler KM, Cant NB, Henkel CK, Farley GR, Warr WB. Descending projections from the superior olivary complex to the cochlear nucleus of the cat. J.Comp.Neurol. 1987;259:452–465. doi: 10.1002/cne.902590311. [DOI] [PubMed] [Google Scholar]
- Sullivan WE. Classification of response patterns in cochlear nucleus of barn owl: correlation with functional response properties. J.Neurophysiol. 1985;53:201–216. doi: 10.1152/jn.1985.53.1.201. [DOI] [PubMed] [Google Scholar]
- Sullivan WE, Konishi M. Segregation of stimulus phase and intensity coding in the cochlear nucleus of the barn owl. J.Neurosci. 1984;4:1787–1799. doi: 10.1523/JNEUROSCI.04-07-01787.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Moiseff A, Konishi M. Time and intensity cues are processed independently in the auditory system of the owl. J.Neurosci. 1984;4:1781–1786. doi: 10.1523/JNEUROSCI.04-07-01781.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi TT, Konishi M. Projections of nucleus angularis and nucleus laminaris to the lateral lemniscal nuclear complex of the barn owl. J.Comp.Neurol. 1988a;274:212–238. doi: 10.1002/cne.902740207. [DOI] [PubMed] [Google Scholar]
- Takahashi TT, Konishi M. Projections of the cochlear nuclei and nucleus laminaris to the inferior colliculus of the barn owl. J.Comp.Neurol. 1988b;274:190–211. doi: 10.1002/cne.902740206. [DOI] [PubMed] [Google Scholar]
- Thompson AM. Heterogeneous projections of the cat posteroventral cochlear nucleus. J Comp Neurol. 1998;390:439–453. doi: 10.1002/(sici)1096-9861(19980119)390:3<439::aid-cne10>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Thompson AM. A medullary source of norepinephrine in cat cochlear nuclear complex. Exp.Brain Res. 2003a;153:486–490. doi: 10.1007/s00221-003-1681-4. [DOI] [PubMed] [Google Scholar]
- Thompson AM. Pontine sources of norepinephrine in the cat cochlear nucleus. J. Comp. Neurol. 2003b;457:374–383. doi: 10.1002/cne.10540. [DOI] [PubMed] [Google Scholar]
- Thompson AM, Moore KR, Thompson GC. Distribution and origin of serotoninergic afferents to guinea pig cochlear nucleus. J. Comp. Neurol. 1995;351:104–116. doi: 10.1002/cne.903510110. [DOI] [PubMed] [Google Scholar]
- Thompson AM, Thompson GC. Neural connections identified with PHA-L anterograde and HRP retrograde tract-tracing techniques. J.Neurosci.Meth. 1988;25:13–17. doi: 10.1016/0165-0270(88)90115-x. [DOI] [PubMed] [Google Scholar]
- Thompson AM, Thompson GC. Posteroventral cochlear nucleus projections to olivocochlear neurons. J. Comp. Neurol. 1991a;303:267–285. doi: 10.1002/cne.903030209. [DOI] [PubMed] [Google Scholar]
- Thompson AM, Thompson GC. Projections from the posteroventral cochlear nucleus to the superior olivary complex in guinea pig: Light and EM observations with the PHA-L method. J.Comp.Neurol. 1991b;311:495–508. doi: 10.1002/cne.903110405. [DOI] [PubMed] [Google Scholar]
- Thompson AM, Thompson GC. Serotonin projection patterns to the cochlear nucleus. Brain Res. 2001;907:195–207. doi: 10.1016/s0006-8993(01)02483-0. [DOI] [PubMed] [Google Scholar]
- Thompson GC, Thompson AM, Garrett KM, Britton BH. Serotonin and serotonin receptors in the central auditory system. Otolaryngol.Head Neck Surg. 1994;110:93–102. doi: 10.1177/019459989411000111. [DOI] [PubMed] [Google Scholar]
- Tollin DJ, Yin TC. The coding of spatial location by single units in t he lateral superior olive of the cat. I. Spatial receptive fields in azimuth. J. Neurosci. 2002a;22:1454–1467. doi: 10.1523/JNEUROSCI.22-04-01454.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollin DJ, Yin TC. The coding of spatial location by single units in the lateral superior olive of the cat. II. The determinants of spatial receptive fields in azimuth. J. Neurosci. 2002b;22:1468–1479. doi: 10.1523/JNEUROSCI.22-04-01468.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchitani C, Johnson DH. The effects of ipsilateral tone burst stimulus level on the discharge patterns of cat lateral superior olivary units. J Acoust.Soc Am. 1985;77:1484–1496. doi: 10.1121/1.392043. [DOI] [PubMed] [Google Scholar]
- van Gisbergen JA, Grashuis JL, Johannesma PI, Vendrik AJ. Statistical analysis and interpretation of the initial response of cochlear nucleus neurons to tone bursts. Exp.Brain Res. 1975;23:407–423. doi: 10.1007/BF00238023. [DOI] [PubMed] [Google Scholar]
- Warr WB. Fiber degeneration following lesions in the posteroventral cochlear nucleus of the cat. Exp.Neurol. 1969;23:140–155. doi: 10.1016/0014-4886(69)90040-5. [DOI] [PubMed] [Google Scholar]
- Warr WB. Contributions to Sensory Physiology. New York: Academic Press; 1982. Parallel ascending Pathways from the cochlear nucleus: Neuroanatomical evidence of functional specialization; pp. 1–38. [Google Scholar]
- Warr WB. Organization of olivocochlear efferent systems in mammals. In: Webster DB, Popper AN, Fay RR, editors. The Mammalian Auditory Pathway: Neuroanatomy. New York: Springer; 1992. pp. 410–448. [Google Scholar]
- Warr WB, Beck JE. Multiple projections from the ventral nucleus of the trapezoid body in the rat. Hear.Res. 1996;93:83–101. doi: 10.1016/0378-5955(95)00198-0. [DOI] [PubMed] [Google Scholar]
- Warren EH, III, Liberman MC. Effects of contralateral sound on auditory-nerve responses. I. Contributions of cochlear efferents. Hear.Res. 1989;37:89–104. doi: 10.1016/0378-5955(89)90032-4. [DOI] [PubMed] [Google Scholar]
- Wickesberg RE. Rapid inhibition in the cochlear nuclear complex of the chinchilla. J.Acoust.Soc.Am. 1996;100:1691–1702. doi: 10.1121/1.416067. [DOI] [PubMed] [Google Scholar]
- Wickesberg RE, Oertel D. Tonotopic projection from the dorsal to the anteroventral cochlear nucleus of mice. J.Comp.Neurol. 1988;268:389–399. doi: 10.1002/cne.902680308. [DOI] [PubMed] [Google Scholar]
- Wickesberg RE, Oertel D. Delayed, frequency-specific inhibition in the cochlear nuclei of mice: A mechanism for monaural echo suppression. J.Neurosci. 1990;10:1762–1768. doi: 10.1523/JNEUROSCI.10-06-01762.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiederhold ML, Kiang NY. Effects of electric stimulation of the crossed olivocochlear bundle on single auditory-nerve fibers in the cat. J. Acoust.Soc. Am. 1970;48:950–965. doi: 10.1121/1.1912234. [DOI] [PubMed] [Google Scholar]
- Winter IM, Robertson D, Cole KS. Descending projections from auditory brainstem nuclei to the cochlea and cochlear nucleus of the guinea pig. J.Comp.Neurol. 1989;280:143–157. doi: 10.1002/cne.902800110. [DOI] [PubMed] [Google Scholar]
- Wu SH, Oertel D. Inhibitory circuitry in the ventral cochlear nucleus is probably mediated by glycine. J.Neurosci. 1986;6:2691–2706. doi: 10.1523/JNEUROSCI.06-09-02691.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SH, Oertel D. Maturation of synapses and electrical properties. Hear. Res. 1987;30:99–110. doi: 10.1016/0378-5955(87)90187-0. [DOI] [PubMed] [Google Scholar]
- Yang L, Monsivais P, Rubel EW. The superior olivary nucleus and its influence on nucleus laminaris: a source of inhibitory feedback for coincidence detection in the avian auditory brainstem. J. Neurosci. 1999;19:2313–2325. doi: 10.1523/JNEUROSCI.19-06-02313.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young ED, Robert JM, Shofner WP. Regularity and latency of units in ventral cochlear nucleus: implications for unit classification and generation of response properties. J.Neurophysiol. 1988;60:1–29. doi: 10.1152/jn.1988.60.1.1. [DOI] [PubMed] [Google Scholar]
- Zhang S, Oertel D. Tuberculoventral cells of the dorsal cochlear nucleus of mice: intracellular recordings in slices. J.Neurophysiol. 1993;69:1409–1421. doi: 10.1152/jn.1993.69.5.1409. [DOI] [PubMed] [Google Scholar]