Abstract
The brain is a metabolically active organ exhibiting high oxygen consumption and robust production of reactive oxygen species (ROS). The large amounts of ROS are kept in check by an elaborate network of antioxidants, which sometimes fail and lead to neuronal oxidative stress. Thus, ROS are typically categorized as neurotoxic molecules and typically exert their detrimental effects via oxidation of essential macromolecules such as enzymes and cytoskeletal proteins. Most importantly, excessive ROS are associated with decreased performance in cognitive function. However, at physiological concentrations, ROS are involved in functional changes necessary for synaptic plasticity and hence, for normal cognitive function. The fine line of role reversal of ROS from good molecules to bad molecules is far from being fully understood. This review focuses on identifying the multiple sources of ROS in the mammalian nervous system and on presenting evidence for the critical and essential role of ROS in synaptic plasticity and memory. The review also shows that the inability to restrain either age- or pathology-related increases in ROS levels leads to opposite, detrimental effects that are involved in impairments in synaptic plasticity and memory function. Antioxid. Redox Signal. 14, 2013–2054.
I. Introduction
Functionally active neurons exhibit increased oxygen consumption and production of reactive oxygen species (ROS) (164). The powerful oxidative metabolism of the brain generates large amounts of ROS that are kept in check by an elaborate antioxidant system composed of a multitude of enzymes, including superoxide dismutase (SOD), catalase, and peroxidases (64, 163). ROS are typically categorized as neurotoxic molecules and exert their detrimental effects via oxidation of essential molecules such as enzymes and cytoskeletal proteins (69, 142, 285). Excessive ROS also are associated with decreased performance in cognitive tasks in mammals (78, 134, 138, 171, 190, 220, 277, 417), as well as invertebrates (125). During normal physiological aging, ROS production increases and antioxidant defenses decline; hence, ROS levels increase dramatically, resulting in neuronal oxidative stress (12, 22, 204, 282, 302, 337). This is also true of hundreds of pathological conditions that promote oxidative stress, including neurodegenerative diseases such Alzheimer's disease (AD) and Parkinson's disease (PD), as well as posttraumatic and ischemic insults (159). Consistent with this idea, manipulations increasing the presence of superoxide in the brain are associated with worsening of cognitive performance (114, 123), whereas interventions designed to quench superoxide tend to normalize behavioral deficits (115, 190, 255, 277).
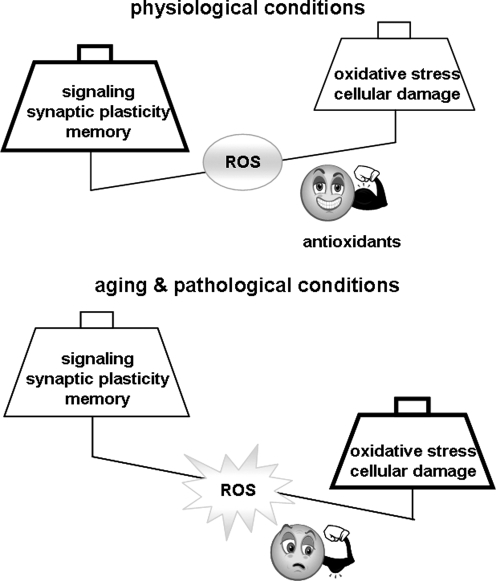
Although changes in redox status are often linked to age-dependent declines in synaptic plasticity and cognitive function, a growing body of evidence from both neuronal and nonneuronal cells suggests that ROS also can function as small physiological molecules involved in functional and structural changes necessary for synaptic plasticity. ROS have been implicated as modulators of hippocampus-dependent and hippocampus-independent memory formation (78, 134, 138). ROS also have been shown to regulate synaptic plasticity-related signaling molecules, receptors, and channels, including N-methyl-d-aspartate (NMDA) receptors (46, 186), calcium (Ca2+) channels (194), potassium channels (17, 149, 299), Ca2+/calmodulin kinase II (CaMKII) (385), the extracellular signal-regulated kinase (ERK) (194, 209, 213, 225), and cyclic adenosine monophosphate (cAMP) response element binding protein (CREB) in the hippocampus (181). In particular, hydrogen peroxide (H2O2) has been shown to promote ryanodine receptor redox modifications, with the subsequent Ca2+ release signal modulating synaptic plasticity via ERK-mediated CREB phosphorylation (213). ROS also have been demonstrated to modulate long-term potentiation (LTP) (228, 229, 231), a form of synaptic plasticity widely studied as a cellular substrate for learning and memory (54, 265) (Figs. 1 and 2). Although ROS have been shown to be necessary for LTP, they also have been implicated with deficient LTP during aging (30, 189) and in mouse models of AD (71). ROS-mediated LTP modulation has been shown to involve other key signaling enzymes, including the serine/threonine family of phosphatases protein phosphatase 2A (259) and 2B (also termed calcineurin) (244).
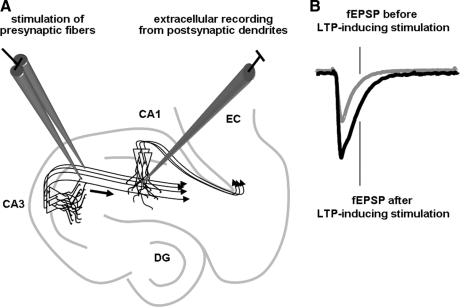
FIG. 1.
LTP in a rodent hippocampal slice. (A) Experimental setup for a typical LTP experiment in a hippocampal slice. The Schaffer collateral pathway is stimulated and fEPSPs are recorded in stratum radiatum (the dendrites of pyramidal neurons) in area CA1. (B) Sample fEPSPs before and after LTP has been induced by high-frequency stimulation of the Schaffer collaterals. fEPSP, field excitatory postsynaptic potential; CA, cornu ammonis; LTP, long-term potentiation.
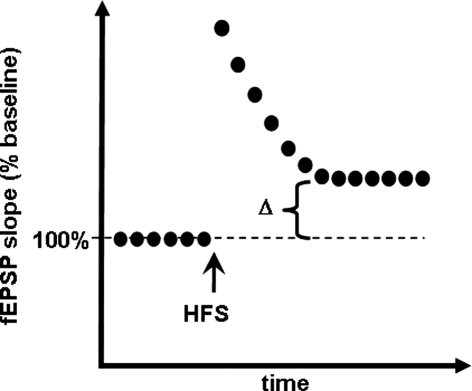
FIG. 2.
Graph of a typical LTP experiment. A schematic graph representing the slope of the fEPSP in hippocampal slices before and after delivery of high-frequency stimulation (indicated by the arrow) of the Schaffer collateral to induce LTP (indicated by Δ) recorded from the dendrites in stratum radiatum of area CA1. HFS, high-frequency stimulation.
Investigating the role of ROS in synaptic structure and function reveals a very interesting yet complex role for these molecules. ROS undoubtedly are very important physiological mediators of plasticity and signaling, but they can become detrimental to neuronal function when they accumulate excessively in the brain. The fine line of role reversal from good molecules to bad molecules of these very highly reactive players is far from being fully understood. This review briefly outlines the important components of learning and memory, and then focuses on identifying the multiple sources of ROS in a mammalian nervous system and on presenting evidence for the critical and essential role of ROS in synaptic plasticity and memory. The review also shows that the inability to restrain age- or pathology-related increases in ROS levels leads to opposite, detrimental effects that are involved in impairing synaptic plasticity and memory function.
II. Basic Components of Learning and Memory
Learning and memory can be grossly defined as the process through which information is obtained, stored, and then retrieved for use by the brain. Much progress has been made in the past two decades toward understanding the molecular mechanisms governing the formation of memories. Multiple publications review these mechanisms, and the reader is referred to these reviews for further details (3, 18, 55, 176, 187, 217, 224, 230, 295, 304, 353, 428, 444). Here we briefly summarize key components of learning and memory processes, as a reference to the subsequent examination of the effect of ROS on such processes (Fig. 3).
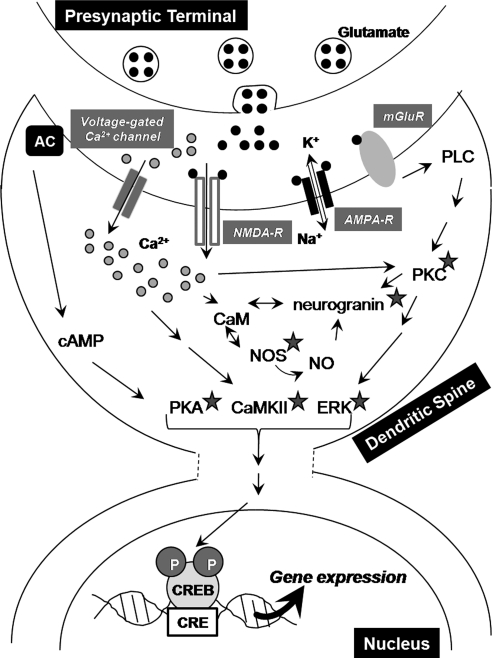
FIG. 3.
Essential cellular components of LTP and memory. Stimulation of presynaptic neurons causes the release of glutamate, which activates AMPA, NMDA, and mGluRs, resulting in massive Ca2+ influx and subsequent production of small messenger molecules such as cAMP and cyclic guanosine monophosphate/NO. These messengers activate multiple protein kinase signaling pathways, including CaMKII, PKA, PKC, and ERK, which ultimately result in the phosphorylation of CREB, which in turn recruits multiple transcription coactivators to initiate a wave of transcription/translation. The newly formed proteins modulate synaptic strength and efficacy via altering the electrical properties of membranes, increasing glutamate receptor expression, changing synaptic morphology, increasing the number of synapses etc., all of which are necessary for long-lasting LTP and the formation of long-term memories. In addition to CREB-mediated gene transcription, some components of these signaling cascades, such as NO and neurogranin, directly contribute to the induction and maintenance of LTP. ROS produced at various sites within the cell (as described in the text) are also essential to the induction of LTP and contribute to these signaling cascades at multiple levels by modifying the molecules marked by grey stars. Note that this is a simplified version of the complex signaling involved in LTP and memory formation. Ca2+, calcium; cAMP, cyclic adenosine monophosphate; CREB, cAMP response element binding protein; ERK, extracellular signal-regulated kinase; mGluR, metabotrobic glutamate receptor; NMDA, N-methyl-d-aspartate; NO, nitric oxide; PKA, protein kinase A; ROS, reactive oxygen species.
Memory formation begins at the neuronal plasma membrane at synapses. At excitatory synapses, stimulation of presynaptic neurons causes the release of glutamate, which activates a variety of glutamate receptors, ionotropic and metabotropic, located on the postsynaptic spines. During periods of robust synaptic activity, enough glutamate is released to cause a large postsynaptic depolarization after activation of the alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionate (AMPA) subtype of glutamate receptors. The postsynaptic depolarization activates the NMDA subtype of glutamate receptor, which allows massive Ca2+ influx into the postsynaptic neuron. These are hallmarks of the induction of LTP, the most intensely studied cellular substrate for memory. Ca2+ influx leads to the production of small messenger molecules such as cAMP, cyclic guanosine monophosphate/nitric oxide (NO), and arachidonic acid. Ca2+ also binds to calmodulin, inducing a conformational change that permits interaction with and activation of other molecules. The small messengers, along with the activated Ca2+/calmodulin complex, activate multiple protein kinase signaling pathways, including CaMKII, cAMP-dependent protein kinase (PKA), protein kinase C (PKC), the Ras pathway, and ERK. One of the downstream targets of PKC is neurogranin/RC3, which is a Ca2+-sensitive calmodulin-binding protein whose calmodulin-binding affinity is attenuated by phosphorylation via PKC and oxidation via NO, thus prolonging the availability of calmodulin long after Ca2+ influx has subsided (33, 192, 250, 261, 386). Neurogranin has been shown to play a critical role in synaptic plasticity and memory. It was shown to be phosphorylated by PKC after induction of LTP (84, 193), and antibodies against the neurogranin phosphorylation domain prevented the induction of LTP (127). Additionally, mouse transgenic knockouts lacking neurogranin have been shown to have deficient spatial learning in the Morris water maze (322), as well as changes in the induction of hippocampal LTP and short-term plasticity (445). The combined activation of these signal transduction cascades ultimately results in the phosphorylation of CREB, which in turn recruits multiple transcription coactivators to initiate a wave of transcription, followed by translation. The newly formed proteins modulate synaptic strength and efficacy via altering the electrical properties of membranes, increasing glutamate receptor expression, changing synaptic morphology, increasing the number of synapses, etc., all of which are necessary for long-lasting LTP and the formation and consolidation of long-term memories.
The formation of memory is therefore dependent on the coordination of complex intracellular signaling pathways. Of particular interest for this review, ROS have been shown to act as small signaling molecules, necessary to modulate the aforementioned cascades. However, as a consequence of the complexity of memory formation, minor disruptions in one or more of the involved signaling cascades, including ROS, can be deleterious to memory formation. In the sections that follow, we will explore the involvement of ROS in memory formation, both under physiological and pathological conditions.
III. Sources of Reactive Oxygen Species
ROS can be produced at multiple sites in a mammalian cell. Mitochondria are the energy machinery of the cell and produce the largest amount of ROS. Nonetheless, other sources, such as monoamine oxidase (MAO) and NO synthase (NOS), produce ROS amounts large enough to participate in a myriad of pathophysiological cellular processes. ROS have been shown to be critical signaling molecules that are essential for the proper induction of synaptic plasticity and memory formation. However, the specific sources of ROS with regard to learning and memory have not been distinctly identified. In this section, we will discuss the potential sources of ROS involved in the physiological signaling role of these molecules. Later in this review, we will address the involvement of ROS and their sources in pathological conditions.
A. The mitochondrial respiratory chain
Similar to every other cell in mammalian systems, neurons use adenosine triphosphate (ATP) as a major energy source to drive cellular processes necessary for its function (426). ATP is produced mainly in mitochondria by the process of oxidative phosphorylation, of which the major byproduct is superoxide (426). Superoxide often plays an important role in modulating signaling pathways, but is relatively short-lived and its usual fate is rapid dismutation to H2O2 by the mitochondrial SOD (SOD-2 or manganese [Mn]-SOD). Given the extremely high metabolic rate of the brain, neurons produce very large amounts of ROS compared to other organs (164). Although the production of ROS ensures the induction of physiological events such as synaptic plasticity and memory, it also puts the brain at a higher risk for oxidative stress, leading to the impairments of the very functions that ROS are necessary for. Thus, the balance between ROS production and clearance is the critical factor in determining whether the beneficial effects of ROS are outweighed by their ability to cause oxidative stress.
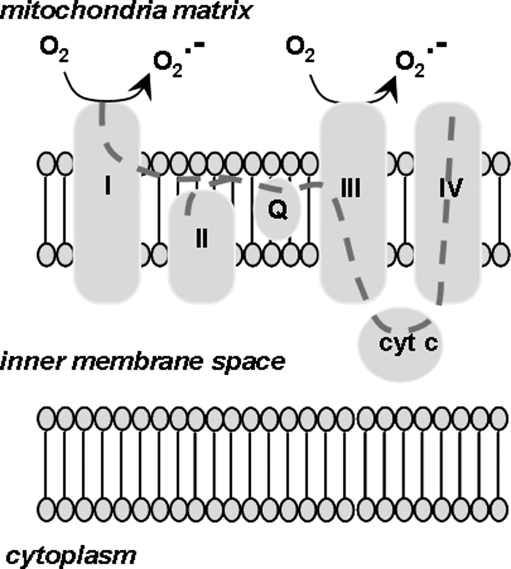
Mitochondrial ROS generation occurs mainly through the leaking of superoxide at complexes I (NADH ubiquinone oxidoreductase) and III (coenzyme Q, bc1 complex, ubiquinone/cytochrome c reductase) of the mitochondrial respiratory chain (398) (Fig. 4).
FIG. 4.
Production of superoxide by mitochondria. A schematic diagram illustrating the various elements of the electron transport chain located in the inner mitochondrial membrane. Note the production of superoxide at complexes I and III. The gray dotted line represents the path of electron transfer through the respiratory chain.
1. Complexes I and III
Complex I, also known as NADH ubiquinone oxidoreductase, is a trans-mitochondrial membrane complex that oxidizes a previously reduced NADH using coenzyme Q10 as the electron acceptor (398). Complex I is the major site of entry of reducing equivalents, followed closely by complex II (398). It is coupled to proton pumping, specifically pumping four protons across the inner mitochondrial membrane, from the matrix to the intermembrane space, thus contributing to the proton gradient that will later fuel the ATP synthase (239). Complex I is the major site of the premature leak of electrons to oxygen, thereby creating the free radical superoxide (469).
Complex III contains two pools of ubiquinone: Qi faces the mitochondrial matrix and Q0 faces the intermembrane space (93, 394). Complex III catalyzes the reduction of ubiquinone in a stepwise fashion. First, two electrons are removed from ubiquinone (Q0) and are passed on to two molecules of cytochrome c to eventually reach complex IV. In a second step, two other electrons are passed on to the Qi site to reduce quinone to quinol. In total, this sequential reaction releases four protons (398). Complex III participates in the generation of the proton gradient across the mitochondrial membrane by an asymmetric absorption/release of protons rather than pumping protons through the membrane. In terms of ROS production, complex III can leak electrons and participate in superoxide formation (394). It is believed that if the electron transfer from ubiquinone to cytochrome c is delayed for any reason, ubiquinone tends to auto-oxidize and release an electron that is free to attack oxygen for superoxide formation (146).
Animal models carrying mutations in the various components of the electron transport chain, as well as pharmacologic inhibitors, have proven very useful in coupling effects with sources of ROS. We focus the remainder of this section on discussing complexes I and III as potential sources of superoxide involved in modulating learning and memory.
2. Mitochondrial superoxide in learning and memory
Several studies have suggested that ROS of mitochondrial origin might impact signaling during synaptic plasticity and memory, although the studies are at best suggestive. The exact source of ROS during synaptic plasticity has yet to be determined. For example, treatment of rat isolated mitochondria with elevated levels of sodium and Ca2+ (thus mimicking the events happening during depolarization of neurons and synaptic transmission of neuronal signals) leads to increased production of superoxide (116). In addition, application of glutamate to cultured forebrain neurons activates NMDA receptors and induces an increase in ROS production that can be blocked by the NMDA receptor channel blocker MK801 (362). NMDA receptor-dependent superoxide production also occluded further superoxide production after uncoupling of mitochondrial proton transport with the inhibitor p-trifluoromethoxy carbonyl cyanide phenyl hydrazone (51). Although the authors of the latter study concluded that their data provided evidence for mitochondrial superoxide production after NMDA-receptor activation, one should not rule out the possibility that p-trifluoromethoxy carbonyl cyanide phenyl hydrazone could be uncoupling proton transport through NADPH oxidase as well (4). Other investigators (113) have used specific inhibitors of complexes I (rotenone) and III (antimycin A) to demonstrate that NMDA receptor-dependent ROS production in cortical neurons originates in the mitochondrial electron transport chain. Although these studies suggest a link between mitochondrial superoxide and synaptic transmission that is essential for plasticity, they have not directly investigated the involvement of mitochondrial ROS in synaptic plasticity.
Perhaps the most direct evidence linking mitochondrial superoxide to synaptic plasticity and memory comes from studies demonstrating that large stimulus-induced increases in cytosolic Ca2+ result in production of superoxide by mitochondria (181, 183). This superoxide acts as a modulator of two kinases, CaMKII and PKA, both known to be involved in the induction of synaptic plasticity. On another hand, in the course of investigating the function of mitochondria in CD8+ T cell functioning, it was demonstrated that incubation with rotenone, a widely used inhibitor of complex I, leads to decreased production of H2O2, Ca2+ influx, and ERK phosphorylation (457), another kinase known to be involved in synaptic plasticity (194, 210).
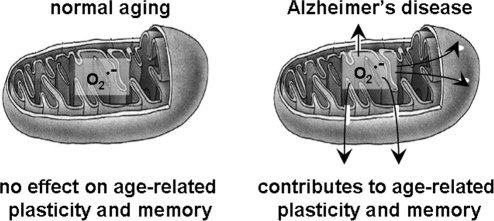
A counter argument to the suggestion that mitochondrial ROS may be involved in synaptic plasticity comes from behavioral and electrophysiological studies in transgenic mice overexpressing SOD-2. These mice have lower levels of mitochondrial superoxide; however, the mice do not exhibit deficits in either LTP or in learning and memory (188), suggesting that mitochondrial superoxide does not contribute in a major way to synaptic plasticity and memory formation under normal physiological conditions. In contrast, mitochondrial superoxide plays a major role in memory dysfunction associated with neuropathological conditions such as aging and AD (115, 277), both discussed later in the review.
Thus, the evidence at this time suggests that there is minimal involvement of mitochondrial ROS in synaptic plasticity and memory, and that other sources of ROS are likely to be involved in these processes.
B. Monoamine oxidase
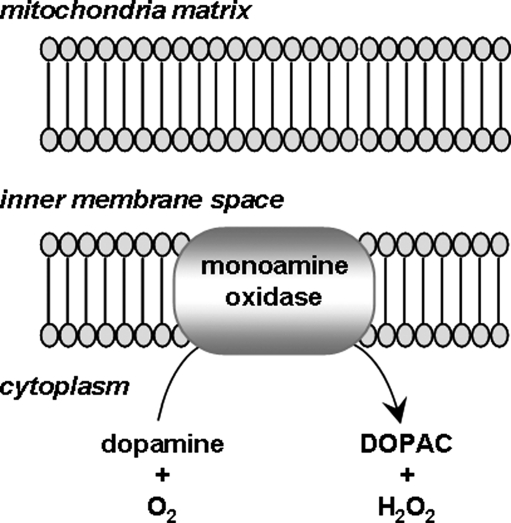
MAOs are flavoproteins located in the outer mitochondrial membrane (426) (Fig. 5). They catalyze the oxidative deamination of monoamine substrates producing H2O2 as a byproduct of the reaction (426), which is widely thought to contribute to oxidative stress resulting in neuronal degeneration. Two subtypes of MAO have been identified, MAO-A and MAO-B (426), and implicated with redox state modulation of both neurons and glia. The role of MAOs as redox modulators of learning and memory has been studied with the use of specific MAO inhibitors. Total inhibition of MAO-A by clorgyline (an MAO-A specific inhibitor) and pargyline (nonselective MAO inhibitor) induced increased locomotion, but no facilitation of spatial memory in the adult rat. In addition, inhibition of MAO-A combined with inhibition of MAO-B with deprenyl did not enhance spatial memory, suggesting that ROS produced by MAO do not alter normal memory function (28). In contrast, deprenyl was shown to improve spatial memory deficits in aged rats (221), where the effect of deprenyl was improved by the addition of estradiol (222). In a model of ischemia/reperfusion, deprenyl significantly reduced oxidative stress and prevented spatial memory deficits when administered before ischemia (220). Deprenyl also has been shown to protect aged, but not young, dogs from spatial memory deficits (173), supporting the notion that MAO-B-induced ROS production does not affect memory under normal conditions, but does contribute to memory-impairments during aging. Similar results were found where deprenyl protected rats against age-related short- and long-term memory impairments (101), iron-induced cognitive impairments (102), ischemia-induced memory deficits (262), and HIV-associated reduced verbal memory (366).
FIG. 5.
Production of H2O2 by monoamine oxidase. A schematic diagram illustrating the production of H2O2 from the monoamine oxidase located in the outer mitochondrial membrane. H2O2, hydrogen peroxide.
Two studies have assessed the effects of coadministration of deprenyl with donepezil, an acetylcholinesterase inhibitor used in the treatment of AD, on cognitive function. Administration of either drug alone improved spatial and associative memory. Both drugs used together at doses that are not individually efficacious improved memory, indicating that deprenyl potentiated the effect of donepezil on cognition (407, 418). Additional studies have shown that deprenyl has long-term beneficial effects in AD on memory modalities that require prefrontal areas of the brain, which are rich in dopamine receptors. In these studies, the effects of deprenyl were delayed, suggesting that the mechanism for memory improvement is through either neuronal rescue or neuroprotection (131). Studies in PD patients failed to demonstrate that deprenyl improved PD-related cognitive dysfunction, consistent with the idea that different mechanisms underlie cognitive impairments in PD and AD (97, 215).
C. NOS: NO (and related gases)
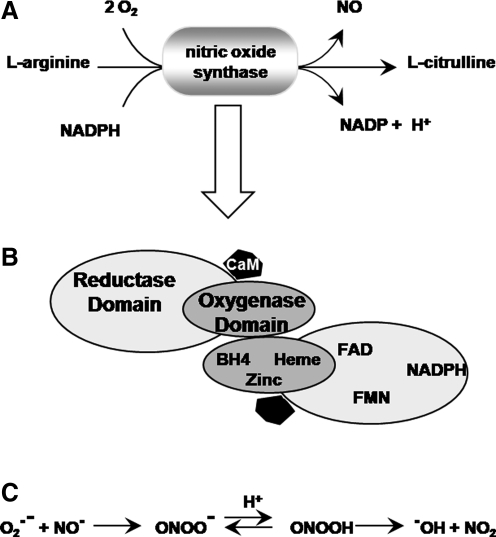
NOS is a flavin-containing Ca2+/calmodulin-dependent enzyme requiring NADPH, tetrahydrobiopterin, and molecular oxygen as cofactors (155) (Fig. 6A, B). The primary function of NOS is to generate NO gas from l-arginine. NO is considered to be a reactive nitrogen species, and upon interaction with superoxide, it can generate the more highly reactive peroxynitrite (350) (Fig. 6C). NO has been studied extensively and shown to be a critical signaling molecule, both for synaptic transmission as well as for synaptic plasticity (369, 470). For example, an inhibitor of NOS that competes for the l-arginine substrate prevents induction of LTP, which can be reversed by adding excessive l-arginine to the bath (369). Using different inhibitors of NOS, several other studies demonstrated the direct involvement of NO in the formation of both spatial and associative memories in multiple species (mice, rats, chicks, and rabbits) (63, 73, 80, 180). It appears that the role of NO depends on the type of memory being studied. For example, using the NOS inhibitor N omega-nitro-l-arginine, it was shown that NO is involved in spatial learning as measured by the radial arm maze and olfactory memory in the social recognition test (56). No effect was observed on the shock avoidance test, suggesting that NO may be involved in some, but not all, types of memory (56). Similarly, inhibition of NOS by N-ω-nitro-l-arginine methyl ester (L-NAME) leads to severe deficits in the acquisition of place-navigation learning, with no effect on either the sensorimotor or motivational processes involved in a related task (124). NO also has been shown to oxidize neurogranin/RC3. Oxidation of neurogranin by NO has been shown to attenuate its calmodulin-binding capacity, thereby leading modulation of both LTP and memory formation (386).
FIG. 6.
Production of nitric oxide by NOS. (A) NOS uses L-arginine and molecular oxygen as precursors to produce NO and L-citrulline. (B) NOS is active as a homodimer and has two domains: an oxygenase domain and a reductase domain. The oxygenase domain contains three bound cofactors (BH4, heme, and zinc) and functions as the active site of the enzyme. The reductase domain contains three cofactors as well (NADPH, FAD, and FMN) and functions primarily in the electron transfer within the enzyme and the regeneration of other cofactors. The oxygenase and reductase domains are linked together via a region that binds calmodulin (CaM), which is required for the activity of the enzyme (and therefore for NO production). (C) Production of peroxynitrite. Peroxinitrite is a short-lived oxidant species formed by the combination of superoxide and nitric oxide radicals. Peroxynitrite anion (ONOO−) is in equilibrium with peroxynitrous acid (ONOOH; pKa = 6.8); both are highly reactive species. Sites of peroxynitrite formation are assumed to be associated with the sources of superoxide (such as the mitochondrial respiratory chain complexes I and III or the NADPH oxidases) because NO is a relatively stable and highly diffusible free radical while superoxide is short-lived and has restricted diffusion across membranes. BH4, tetrahydrobiopterin; NOS, NO synthase.
An interesting enzymatic feature of NOS is that if its substrate l-arginine is either at low levels or absent, it has the capacity to produce ROS instead of NO (174). Specifically, purified NOS has been shown to produce superoxide at the heme domain of the enzyme, in a Ca2+- and calmodulin-dependent manner (174). The frequently used NOS-specific inhibitors L-NAME and N-ω-monomethyl l-arginine (L-NMMA) had different effects on superoxide production. L-NAME inhibited superoxide production in a concentration-dependent manner, whereas L-NMMA had no impact of the generation of superoxide (342). In the presence of l-arginine, L-NMMA resulted in increased generation of superoxide, because NO generation was attenuated.
NOS also has been shown to act as an NADPH:oxygen oxidoreductase, catalyzing the formation of H2O2 at suboptimal concentrations of either the substrate l-arginine or the cofactor tetrahydrobiopterin (343, 447, 448). Similar to superoxide production, different inhibitors of NOS had different effects of H2O2 production. Specifically, N-ω-nitro-l-arginine (L-NNA), its methyl ester L-NAME, and L-NMMA all inhibited NO production from NOS (174), whereas only L-NNA and L-NAME could block the substrate-independent generation of H2O2 (174, 342). According to these results, activation of NOS at suboptimal levels of either substrate or cofactor may result in the formation of ROS instead of NO.
These NOS-induced ROS (either superoxide or H2O2) may contribute to the events underlying LTP and memory formation. Their role in synaptic plasticity, however, as opposed to NO, has not been investigated. The main reason for the poor understanding of the role of NOS-induced ROS is that most studies using pharmacological inhibitors of NOS fail to discriminate between the two enzymatic activities of NOS (NO-producing versus superoxide/H2O2-producing). Thus, in such experiments, disruptions in learning and memory occurring after inhibition of NOS are always attributed to the decrease in NO production. No specific role of NOS-induced superoxide is addressed.
The role of NOS-generated superoxide has been studied by cotransfecting NOS with SOD. NOS expression leads to increased ERK activation, which suggests a role for NOS in synaptic plasticity and learning (434). This role could be attributed to either NO or NOS-generated superoxide. Cotransfection of SOD resulted in the inhibition of the NOS-mediated ERK activation, suggesting that either superoxide or H2O2 (generated by the dismutation of superoxide by SOD) contributes to synaptic plasticity via ERK activation. Mutation studies in the NOS enzyme helped to address this ambiguity. Mutations in NOS that silence the NO-producing domain did not bear any consequence on the NOS-induced ERK activation (434), suggesting that it is a superoxide-mediated event. This is supported by experiments using NOS mutants with an incompetent NADH domain that abolish NOS-mediated ERK activation (434). Collectively, these results suggest that NOS might contribute to synaptic plasticity and memory formation not only via the production of NO, but also via NADPH oxidation and production of superoxide.
Whereas the role of NO in learning and memory processes has been investigated extensively, research investigating the role of endogenous carbon monoxide (CO), a diatomic radical structurally similar to NO, has lagged behind. Although CO is traditionally regarded as an environmental toxic pollutant, it has been shown to be produced endogenously, usually via a reaction catalyzed by the enzyme heme oxygenase (HO) (241, 416). Endogenous CO functions as a signaling molecule in the nervous system and is involved in the regulation of neurotransmitter release [reviewed in ref. (446)]. Similar to NO, CO has been documented to have vasorelaxant properties, and it has been shown to activate guanylate cyclase (397), which is relevant for synaptic plasticity and memory. For example, several studies suggest a role for CO in LTP and memory. Various protoporphyrin inhibitors of HO were shown to block the induction of LTP in a dose-dependent manner and attenuate preexisting LTP in rodent hippocampal slices (396, 467, 468). These effects were also shown to be distinct from the effect of NO on LTP (467). Using similar inhibitors, CO has also been shown to play a role in Ca2+-dependent release of glutamate (388), which is an important component of LTP. On another hand, two studies using HO knockouts (341) or HO inhibitors (286) challenged these results and showed no clear effect of CO on LTP. Instead, the authors suggested that the observed effects of one HO inhibitor (chromium mesoporphyrin IX) may be due to a secondary action on NOS (286). These contradictory results may be in part due to the different concentrations of inhibitors used in the different studies. CO also has been implicated in memory formation, most notably tasks of spatial or avoidance learning (43, 52, 96, 132). On the other hand, no effect of CO has been observed on long-term depression (396), a form of synaptic plasticity manifested as a long-lasting decrease in synaptic strength. For further details, the reader is referred to two reviews covering the physiological role of CO andits role in learning and memory (95, 446). Although these findings suggest an important role for CO produced by HO in synaptic plasticity and memory, further studies are needed to determine whether CO is as important as NO for memory function.
In recent years, interest in other naturally occurring gases as signaling molecules has been revived, most notably hydrogen sulfide (H2S) [reviewed in ref. (219)]. Although H2S is not a radical itself, it is involved in redox signaling because of its connection to cysteine biology. H2S is produced by cystathionine β synthase, cystathionine γ lyase, or 3-mercaptopyruvate sulfurtransferase with cysteine aminotransferase in the brain, cardiac muscle, and vascular endothelium. It acts as a neuromodulator as well as vasodilator. Although its effect on learning and memory is poorly understood, it has been shown to enhance the activity of NMDA receptors and facilitate the induction of LTP. It has also been shown to protect neurons from oxidative stress. Recent progress in the studies of the physiological functions of H2S in neurons can be found in a recent review (219).
D. NADPH oxidase
NADPH oxidase is a large enzymatic complex whose primary function is the oxidation of NADPH with concomitant production of superoxide (Fig. 7). Although NADPH oxidase is better characterized as an enzyme of the immune system, it recently emerged as a neuronal enzyme with a potential role in synaptic plasticity and memory, which will be discussed in the following section.
FIG. 7.
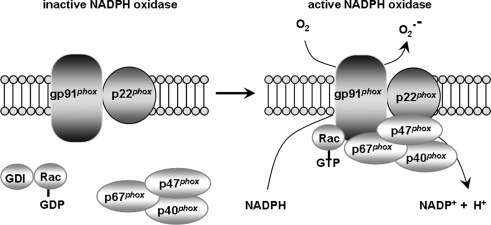
Production of superoxide by NADPH oxidase. When NADPH oxidase is inactive, gp91phox and p22phox are membrane-bound while the four remaining subunits, p67phox, p47phox, p40phox, and Rac are cytosolic. Upon stimulation, the cytosolic subunits translocate to the membrane to form a complex with the membrane-bound components. The assembly of all subunits yields an active enzyme that catalyzes the oxidation of NADPH into NADP+, releasing an electron in the process. The electron is coupled to oxygen to generate superoxide. Although gp91phox is the catalytic core of the enzyme, responsible for the transmission of an electron to oxygen, the presence and correct positioning of all subunits is required for full function of the enzyme. GDI, guanine nucleotide dissociation inhibitor; GDP, guanosine diphosphate; Rac, ras-associated C3 botulinum toxin substrate.
1. Structure and regulation of the NADPH oxidase
NADPH oxidase first was identified as a membrane-bound enzymatic complex of the phagosome (24, 240) (Fig. 7). It is made up of six subunits: One Rho-GTPase (usually Rac 1 or Rac 2) and five “phox” subunits (phox stands for phagocytic oxidase): gp91phox (aka NADPH oxidase 2 [Nox2]), p22phox, p40phox, p47phox, and p67phox. In its latent status, gp91phox and p22phox are membrane-bound, whereas the four remaining subunits are cytosolic (24, 240). Upon stimulation, the cytosolic subunits translocate to the membrane to form a complex with the membrane-bound components (240, 404). The assembly of all the subunits yields an active enzyme that catalyzes the oxidation of NADPH into NADP+, releasing an electron in the process (240, 404). The electron is coupled to oxygen to generate superoxide. gp91phox is the catalytic core of the enzyme, responsible for the transmission of an electron to oxygen (240, 404). However, the presence and correct positioning of all subunits is needed for this transfer to occur. p67phox and Rac act as the activators of gp91phox, whereas p47phox acts as the organizer, ensuring that all subunits are properly aligned for optimal function (240, 404). Thus, the regulation of NADPH oxidase occurs via complex interactions between the various subunits, and proper activation and alignment of all subunits is required for full function of the enzyme.
NADPH oxidase also is regulated by multiple signaling pathways. Many of these regulatory pathways also are important for synaptic plasticity and memory (83, 98). Most notably, Ca2+ influx, particularly store-operated Ca2+ entry, has been shown to play a critical role in the activation of NADPH oxidase and the subsequent production of superoxide in neutrophils (62). Given the important role of Ca2+ in synaptic plasticity and memory, Ca2+-dependent NADPH activation and superoxide production could very likely be occuring at synaptic terminals, especially since NADPH oxidase recently was found to be localized to neuronal structures in multiple brain areas, including the hippocampus (216, 373, 415). Such regulation and localization suggest a direct role of NADPH oxidase in synaptic plasticity and memory and will be discussed in further depth in the following two sections.
2. NADPH oxidase in the brain
NADPH oxidase is classically linked to respiratory bursts of superoxide serving as host defense against bacteria in polymorphonuclear neutrophils (24, 240). This function and localization of NADPH oxidase have been studied extensively since it was first described over 40 years ago. However, recent findings indicate that this classical view of NADPH oxidase being exclusively an immunological enzyme as being outdated. Instead, in the past decade substantial evidence has accumulated indicating that NADPH oxidase has nonphagocytic functions. These extra-phagocytic functions of NADPH oxidase include regulating cellular growth and death, regulating endothelial function, and mediating intracellular signaling (154, 356).
The first nonphagocytic NADPH oxidase was discovered in 1999 in vascular smooth muscle cells and was named Nox1. This discovery was followed during the next 10 years by a large body of research investigating the existence and function of nonphagocytic Nox (402). We now know of the existence of at least seven different Nox isoforms (Nox 1–5 and Duox 1 and 2). Although structurally very similar and related, each of these isoforms is expressed in different locations and serves a different cellular function. Of particular interest to this review is the discovery that NADPH oxidase can be localized to multiple brain structures, most notably the hippocampus and amygdala (216, 373), which have primary roles in the formation and maintenance of memory.
NADPH oxidase in neurons was first demonstrated by Tammariello and colleagues, who showed that Nox2 subunits were expressed in rat isolated sympathetic neurons in culture. Expression of Nox2 was shown to be necessary for a proper apoptotic response after insult by nerve growth factor withdrawal (409). Shortly after this study, two immunohistochemical studies in mouse and rat tissue extensively characterized the distribution of various NADPH oxidase subunits in the central nervous system (216, 373). Using a variety of antibodies to stain mouse brain sections, widespread distribution of p40phox, p47phox, p67phox, p22phox, and gp91phox was observed in neurons in all regions of the neuraxis. Particularly prominent regions included the cortex, striatum, thalamus, hippocampus, and amygdala, suggesting that NADPH oxidase may play a role in both normal neuronal function as well as neurodegeneration in the brain. Similar experiments in rat brain tissue yielded results showing strong p47phox and Nox2 immunoreactivities in the cortex and hippocampus (373). In contrast, no particular localization of NADPH oxidase subunits was observed in either the thalamus or the amygdala, but some immunoreactivity was observed in cerebellar neurons. Nox4 and Nox5 were also found localized to the brain, particularly to the cortex, cerebellum, and hippocampal pyramidal cells (424). Nox3 was found highly expressed in the inner ear (27), and finally, a fully functional NADPH oxidase was observed in the lens epithelium (354). Besides their localization to the brain, little is known about the regulation of these different Nox isoforms and whether they are involved in ROS signaling in the brain.
Tejada-Simon and colleagues further characterized the mouse hippocampal NADPH oxidase and found that all of its subunits (including Nox2) are present in cell bodies and dendrites of hippocampal neurons. They also found that these subunits were enriched in synaptoneurosome preparations and that p67phox colocalized with synaptophysin, suggesting that NADPH oxidase is localized at synaptic sites (415). Finally, the authors also showed that hippocampal NADPH oxidase is fully functional, producing superoxide in response to stimulation with phorbol esters (415).
Taken together, these studies clearly demonstrate localization of a functional NADPH oxidase in hippocampal neurons, suggesting that this enzyme might be the source of superoxide required for LTP and memory formation, a subject addressed in the following section.
3. NADPH oxidase in synaptic plasticity
We have argued that given its localization to key brain structures and to its functionality, NADPH oxidase is a prominent candidate as a source for ROS production during synaptic plasticity and memory formation. Another important feature is that NADPH can produce large amounts of superoxide in a very well-controlled manner (349). Thus, ROS production by NADPH oxidase is a punctuate event that can be turned on or off rapidly and specifically in response to particular extracellular stimuli and subsequent signaling events. Signaling events of particular interest to this review include the NMDA receptor-dependent activation of ERK. This type of signaling has been extensively shown to be involved in various forms of synaptic plasticity and memory (340, 405). Inhibiting NADPH oxidase with diphenylene iodonium (DPI) resulted in the inhibition of NMDA receptor-dependent ERK activation, suggesting a direct role for NADPH oxidase in this type of signaling that is associated with synaptic plasticity and memory (225). This observation was supported by studies in transgenic mice lacking the p47phox subunit. These mice do not have a functional NADPH oxidase and do not exhibit NMDA receptor-dependent ERK activation (225). The p47phox knockout mice, along with mice lacking the gp91phox subunit of NADPH oxidase are typically used as models of chronic granulomatous disease, a genetic condition characterized by phagocytic dysfunction (199, 336). In addition, they can be used to study the effect of selective inhibition of NADPH oxidase activity on LTP induction and memory formation. Using these NADPH oxidase mutant mice, it was demonstrated that ablation of NADPH oxidase activity leads to obstruction of the early-phase LTP (223). Nox2 knockout mice also exhibited deficits in posttetanic potentiation, which is a form of short-term synaptic plasticity that is independent of NMDA receptors (223). These studies are unique in that they demonstrate a direct role for NADPH oxidase in LTP induction. This suggests that ROS involved in synaptic plasticity and memory (as will be described later in section IV b) could be generated by the large enzymatic NADPH oxidase complex.
IV. Physiological Roles of ROS
A. Synaptic signaling and LTP
Synaptic plasticity can be defined as the altering of the strength of preexisting synapses or the formation of new synapses. It is thought to be the physiological process that underlies learning and memory at the cellular level (117). One common form of synaptic plasticity is hippocampal LTP, which is defined as a long-lasting increase in synaptic strength and is widely used as a substrate of learning and memory. A wealth of research has been performed to clarify the role played by ROS in LTP induction. Evidence implicating ROS in the proper induction of LTP came from experiments involving the exogenous application of ROS to hippocampal slices, and the use of transgenic mice and pharmacological approaches to block the activity of ROS. Two main types of ROS have been examined, namely, superoxide and H2O2.
LTP studies in the rodent hippocampus have revealed that superoxide accumulates in hippocampal slices after NMDA receptor activation, which is a critical event in the induction of LTP (51). In addition, superoxide was shown to regulate the activities of ERK (209) and PKC (232), both of which are essential for normal LTP (Fig. 8). In hippocampal slices, scavenging superoxide with manganese porphyrin compounds that mimic SOD function blocked high-frequency stimulation–induced LTP (228). On the other hand, as mentioned above, reduction of superoxide production by genetic deletion of various NADPH oxidase subunits results in deficient LTP (223). In vitro production of superoxide via exogenous application of xanthine/xanthine oxidase (X/XO) to hippocampal slices resulted in a transient depression of synaptic transmission that ultimately recovered and resulted in the induction of LTP (231). This late-forming LTP was inhibited by the application of SOD, indicating that it is superoxide dependent (231). In line with these studies, mice overexpressing SOD, the main superoxide scavenger in mammalian cells, exhibit differential effects on LTP formation and maintenance depending on the SOD isoform expressed. Three SOD isoforms are present in cells and there are transgenic mice that overexpress each of them (Fig. 9A). SOD-1 (also known as Cu/Zn-SOD) and the extracellular SOD (EC-SOD) are responsible for scavenging the cytosolic and extracellular pools of superoxide, respectively, whereas SOD-2 (or Mn-SOD) is responsible for scavenging the mitochondrial superoxide pool. Using these mice, it was shown that both EC-SOD and SOD-1 transgenic mice exhibit deficits in LTP (205, 417), but the mechanisms responsible for these deficits are different. EC-SOD transgenic mice have deficient LTP because there is too little superoxide present in the hippocampus (417), whereas the SOD-1 mice have a deficient LTP because of increased H2O2 produced as a result of superoxide dismutation (205). Surprisingly, transgenic mice that overexpress SOD-2 exhibit normal LTP (188), indicating that the mitochondrial pool of superoxide probably does not play a role in LTP induction. This could perhaps be due to the nature of the mitochondrial membrane, which is composed of a phospholipid bilayer that hinders the free diffusion of mitochondrial superoxide to the cytosol (460). Because overexpression of SOD-2 should result in increased H2O2 production, it appears that mitochondrial H2O2 does not contribute to LTP, contrary to its cytoplasmic counterpart, which as discussed later likely plays a complex role in learning and memory. In contrast to superoxide, H2O2 has been recently shown to cross membranes. Specifically, recent evidence indicates that select aquaporin homologs from plants and mammals (aquaporin 8) have the capacity to channel H2O2 across membranes (48). The mammalian nervous system contains predominantly aquaporins 1, 4, and 9 (14, 25, 306) and brain inner-mitochondrial membranes have been shown to specifically contain aquaporin 9 (13). Considering that the permeability of different aquaporin subtypes to H2O2 has not been investigated, to the best of our knowledge, no direct in vivo or in vitro evidence is available to suggest that the brain mitochondrial membrane is permeable to H2O2. Therefore, the idea that excessive superoxide dismutation inside the mitochondria could increase cytoplasmic H2O2 is possible, but highly speculative at this point.
FIG. 8.
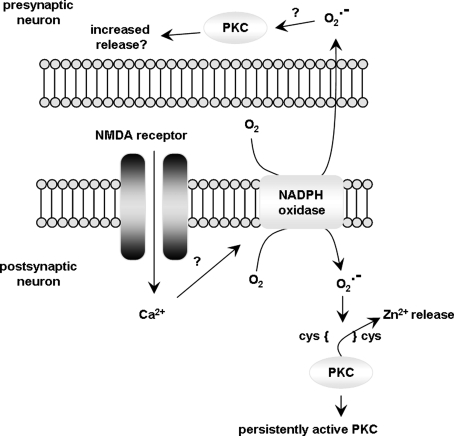
A model for oxidative activation of PKC in LTP. LTP-inducing stimulation results in activation of the NMDA receptor and subsequent Ca2+ influx into the postsynaptic neuron. Ca2+ triggers production of superoxide via NADPH oxidase, which acts directly on cysteine residues in PKC. Oxidation of PKC results in zinc release and autonomously active PKC. It is unknown whether this activation occurs presynaptically or postsynaptically.
FIG. 9.
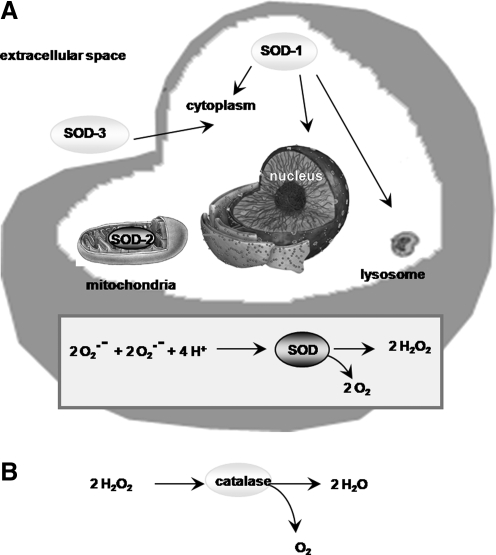
(A) Cellular localization of the three SOD isozymes and the dismutation of superoxide. SODs catalyze the dismutation of the superoxide radical into H2O2. There are three isoforms of SOD. SOD-1 (or Cu/Zn-SOD) is found in all intracellular compartments, including the cytoplasm, the nucleus, lysosomes, and the inner membrane space of mitochondria. SOD-2 (or manganese SOD) is localized primarily in the mitochondrial matrix. SOD-3 (or extracellular SOD) is present mainly in the extracellular space. In the brain, it can also be found on the endothelial cell surface and cytoplasm. (B) Removal of H2O2 by catalase. H2O2 produced in the cell by various reactions, including the dismutation of superoxide by SODs, is converted into water (H2O) by the action of catalase. SOD, superoxide dismutase.
In studies where superoxide was shown to modulate LTP, the addition of catalase to quench the superoxide dismutation product H2O2 (Fig. 9B) results in an attenuation of LTP, indicating that H2O2 also plays a role in LTP (229). However, investigations of the role of H2O2 in the induction of LTP have produced divergent results. On one hand, studies in rat hippocampal slices demonstrate that H2O2 inhibits LTP and causes a long-lasting depression of population spikes and excitatory postsynaptic potentials (EPSPs) (211). On the other hand, H2O2 causes a significant increase in EPSPs in sympathetic preganglionic neurons (252). The apparent discrepancy in these reports can be explained by the large differences in the doses of H2O2 used in the different studies (mM vs. μM). Indeed, Kamsler and Segal have shown that H2O2 can either potentiate or depress high-frequency stimulation–induced LTP in a concentration-dependent manner (204). They also observed a dose-dependent effect of H2O2 on long-term depression. In a subsequent study (205), these authors proposed a model by which H2O2 modulates synaptic plasticity based on the background concentration of H2O2 present in the system at the time of stimulation: in young wild-type mice, the background levels of H2O2 are very low, and therefore stimulation-induced transient increases in H2O2 facilitate the induction of LTP. Exogenous addition of H2O2 pushes the system to a threshold that is detrimental to LTP induction. SOD-1 transgenic mice have a higher ambient H2O2 concentration and maintain an intracellular redox milieu of proteins adapted to functioning in higher H2O2 levels, and is therefore desensitized to the small rise in H2O2 that follows stimulation, resulting in deficient LTP. In such an instance, addition of exogenous H2O2 boosts the small stimulation-induced H2O2 increase to a level sufficient for LTP induction. During aging, the opposite effects occur, as will be discussed later.
In summary, how ROS contribute to LTP depends on the identity of the specific ROS, the location of ROS production, and the concentration of the ROS. Along the same lines, induction of oxidative stress in honeybees by injections of ferrous ammonium citrate causes learning and memory impairments in a dose- and time-dependent manner (125).
LTP is the leading candidate for the cellular basis of learning and memory (117). Hence, in addition to its role in modulating synaptic plasticity at the cellular level, ROS play a prominent role in the formation and maintenance of memory, a subject discussed in the next section.
B. Learning and memory
In the previous section, we discussed the role of ROS in the induction of LTP, a commonly studied cellular substrate for learning and memory (117). The role of ROS also has been studied from a behavioral point of view, especially with respect to the limbic system and cognitive function. These studies show that, similar to their effect on the neuronal circuitry and synaptic plasticity, ROS act as a double-edged sword with respect to memory function. On one hand, ROS have been shown to be an essential signaling component for memory formation; on the other hand, they also have been shown to impair the same neuronal networks necessary for memory function. The dichotomy of ROS function is a recurrent theme in redox regulation of learning and memory and will be further discussed in this section.
ROS have long been linked to impaired learning and memory processes, mainly via their toxic effect on the neuronal circuitry necessary for memory formation (see section V of this review). The beneficial role of ROS on the formation of memory has been implied from LTP studies described in the above section. However, their involvement in memory function was not directly demonstrated until the late 1990s with the generation of transgenic mouse models of the various SOD isoforms, and with mice with genetic deletions for NADPH oxidase subunits.
The earliest evidence for the involvement of ROS, specifically superoxide, in learning and memory came from the study of transgenic mice overexpressing SOD-1 (143). Utilizing a water maze paradigm designed to assess spatial learning and memory, it was shown that SOD-1 transgenic mice display impaired hippocampus-dependent spatial memory function (143). As discussed earlier, hippocampal slices prepared from SOD-1 transgenic mice exhibit deficient LTP (143), indicating that superoxide is necessary both for memory formation and its cellular substrate at the synaptic level. SODs are responsible for dismutating superoxide into H2O2, which is then transformed into water by catalase. Therefore, mice overexpressing SODs have decreased levels of superoxide, but also exhibit increased H2O2 as a byproduct of the dismutation reaction. It is therefore conceivable that at least part of the observed effect on LTP and cognition could be due to increased H2O2 as opposed to the exclusive removal of superoxide. In the same study described above, hippocampal slices from SOD-1 transgenic mice were treated with either an antioxidant spin-trapping agent or catalase and the LTP deficits were reversed, indicating at least a partial role for H2O2 in the LTP impairment (143). The same effect on behavior has not yet been investigated, so it is not clear whether the behavioral deficits observed in mice overexpressing SOD-1 are solely due to superoxide or involve other ROS. EC-SOD mice also have been investigated in the context of learning and memory. Two groups have shown that young EC-SOD mice exhibit impaired memory function (247, 417). Mice overexpressing EC-SOD had impairments in the consolidation of contextual fear memory as assessed by a hippocampus-dependent fear conditioning paradigm (417). In addition, the EC-SOD transgenic mice exhibit significant impairments in the acquisition of spatial memory using the radial arm maze, which also is a hippocampus-dependent task (247). In the latter study, the authors showed that EC-SOD overexpression also impacted other brain regions. They found that learning in the young EC-SOD mice is highly dependent on the motivational state of the mouse as induced by food restriction. Mice with low motivation have impaired learning, whereas mice with high motivation are able to learn, though at a slower rate, and express normal long-term memory. These studies indicate that ROS also affect brain regions distinct from the hippocampus (247). EC-SOD overexpression also was investigated in pharmacological studies where hippocampal slices from the EC-SOD transgenic mice were treated with the copper chelator diethylcarbamate with the intention of lowering EC-SOD activity by neutralizing its cofactor. Such treatment resulted in a recovery of the LTP impairments observed in young EC-SOD transgenic mice (417), but has not been tested in behavioral experiments.
Mice overexpressing SOD-2 have been reported to have no significant behavioral phenotypes as far as learning and memory is concerned (188), which is consistent with the lack of LTP phenotypes displayed by these mice (188). This observation reinforces the idea introduced above that mitochondrial superoxide might be restricted due to sequestration in the mitochondrial membrane. However, we will discuss later that quenching mitochondrial superoxide with SOD-2 is beneficial to AD-related cognitive dysfunction (115, 277).
The source of ROS required for normal learning and memory function has been investigated with NADPH oxidase mutant mice that were described earlier. In addition to its involvement in LTP, NADPH oxidase is required for hippocampus-dependent memory. Specifically, gp91phox mutant mice have mild deficits in spatial memory formation as measured by the Morris water maze task and that p47phox mutant mice have deficits in associative memory as measured with a contextual fear conditioning paradigm (223). These mice also exhibit deficient performance in the rotating rod and open field tests, indicating that other brain areas also may be affected by impairing NADPH oxidase function (223).
In summary, in this section we have described numerous experiments directly linking superoxide and H2O2 in memory acquisition and consolidation in young, healthy mice. In addition to the modulatory role of ROS in the signaling events underlying LTP and memory formation in the young adult mice, ROS also play an important role in the impairment of memory in the aged and diseased brain. In the next section, we will discuss the importance of redox regulation of memory function in the context of physiological aging, neurodegenerative diseases, and ischemic insult.
V. Pathological Release and Effects of ROS
Although ROS contribute to the regulation of long-term functional changes in neurons and appear to be required for normal learning and memory in healthy, young adult animals, the opposite appears to be true of the aged and diseased brain. The high energy demand of the brain, together with its high level of ROS production, places it at risk during conditions of increased stress, such as the ones occurring during aging, in neurodegenerative disorders, and after either traumatic or ischemic insults. Altered ROS levels in the brain are compounded by aging-related reductions in antioxidant defense, leading to a disruption of the fragile ROS balance. In other words, when ROS levels become too high, their ability to trigger oxidative stress outweighs their role as signaling molecules, and the outcome shifts from positive modulation of synaptic plasticity and memory to impairments in these processes. In the following sections, we will discuss the toxic effect of ROS on cognition in the context of physiological aging and oxidative stress conditions such as ischemia and neurodegenerative diseases.
A. ROS in physiological aging
Oxidative stress has long been linked to aging by the free radical theory of aging proposed by Harman in 1956 (169). This theory postulates that the age-dependent accumulation of oxidative damage to cellular macromolecules causes a progressive functional deterioration of cells, tissues and organ systems, which then exhibit functional senescence and ultimately death (169). A variety of functions are altered in an aging individual, including locomotor, reproductive, sensory, immune, and memory. In most cases, there is evidence that oxidative damage contributes to the aging-related decline in these functions. This section will be dedicated to reviewing the evidence implicating ROS and oxidative damage in age-related cognitive decline.
Age-related cognitive decline includes functions such as short-term memory, problem-solving abilities, information processing speed (87), and the cellular mechanisms underlying these mechanisms such as LTP.
LTP impairments have been observed in aged animals (31) and specific deficits in LTP in area cornu ammonis (CA)1 and the dentate gyrus of the hippocampus have been attributed, at least in part, to the rising levels of ROS. Consistent with the oxidative damage theory of aging, overexpression of either EC-SOD or SOD-1 throughout the lifetime of mice protected against age-related LTP deficits (190), and improved performance in spatial memory (203, 247). As mentioned earlier, overexpression of SOD-2, the mitochondrial isoform of SOD, had no effect on either age-related LTP deficits or memory impairments associated with aging (188), suggesting that the mitochondrial superoxide pool does not contribute to these events, perhaps due to limited diffusion across the mitochondrial membrane. A pharmacological approach using antioxidants also has been used to assess the role of oxidative damage in senescence of brain function. Continuous systemic administration of two SOD/catalase mimetics (EUK compounds) in aged mice reduced the age-related oxidative damage to protein, lipids, and DNA, and resulted in better memory performance in a fear conditioning paradigm (255). Administration of a carboxyfullerene SOD mimetic to wild type mice, starting middle age, resulted in improved age-related performance in the Morris water maze and increased the life span of these mice (347). Other dietary antioxidants also have been used to examine synaptic plasticity and memory in aged animals, including vitamin E, coenzyme Q, vitamin C, lipoic acid, berries, the nitrone spin trap α-phenyl-N-tert-butyl nitrone (PBN), and various combinations of these compounds (see section V for details and references). Studies with these compounds largely show decreased markers of oxidation as well as improved synaptic plasticity and memory function in aged animals, generally in spatial and working memory tests. Further, combinations of various antioxidants improve cognition better than larger doses of a single antioxidant, suggesting that synergism may be taking place (281). Also, large doses of single antioxidants such as vitamin E have been associated with toxicity in human patients with chronic diseases (292). Therefore, a combination of multiple antioxidants and supplements might be a better approach for the prevention of age-associated memory dysfunction.
Because oxidative damage accumulates with age, an important prediction of the oxidative stress theory of aging would be that the severity of cognitive decline should correlate with the amount of accumulated oxidative damage (392). This prediction was confirmed in a study where the investigators subjected young (4-month old) and aged (22-month old) mice to a battery of behavioral tests and then measured the extent of oxidative damage in multiple brain regions. In support of ROS involvement in aging-related cognitive decline, they found that age-related spatial memory deficits were directly correlated with the amounts of oxidized proteins in the cortex (134).
Data from clinical trials in humans support the animal research, and show variable antioxidant benefits on memory function depending on the compound and dose used. For example, serum levels of the antioxidants vitamin C, vitamin A, carotenoids, and selenium do not correlate with poor memory performance assessed by a delayed recall test in an elderly multiethnic sample of individuals. Only vitamin E showed benefit in this particular setting (328). There is other research showing similar findings (162, 329). Additional data have attributed beneficial effects on cognitive function to a combination of vitamin C, vitamin E, and carotene (438). These results are supported by studies showing that vitamin E and vitamin C supplementation, and/or a multivitamin regimen results in an improvement in aging-related cognitive decline and vascular cognitive impairment (278). Clinical trials in elderly men receiving β-carotene supplementation suggest that short-term antioxidant supplementation had no effect on cognitive dysfunction, whereas long-term supplementation positively impacted it (156). In a randomized, controlled trial of multivitamin supplementation to an aging population, it was reported that there was no significant effect on age-related cognitive dysfunction. However, the authors did notice a trend toward improved cognition in a particular subgroup of their study population: those older than 75 years were more susceptible to vitamin deficiency (284). Of all the trials assessing the link between antioxidants and cognition, very few measure cognitive decline as opposed to cognitive dysfunction. Of particular interest, a study of antioxidant supplementation in aged, free-living adults showed a 36% reduction in the rate of cognitive decline over 3 years for individuals that fall in the highest to lowest quintiles of vitamin E intake (296). This study suggests that perhaps antioxidants can slow the rate of cognitive decline, as opposed to totally eradicating cognitive dysfunction.
Interpretation of all of the clinical trials collectively becomes quite complicated, especially when some show cognitive benefits of a certain antioxidant versus others not showing a cognitive benefit. The discrepancies could arise from several reasons, such as the presence of confounding factors that were unaccounted for. For example, people who eat diets rich in antioxidants or take vitamin supplements generally tend to lead healthier lifestyles, and therefore the effects of antioxidants in such individuals may be potentiated by other factors such as lower caloric intake and/or physical exercise. Another possibility for the differential results of these studies could be the different duration of antioxidant treatment. This argument is supported by a study showing that long-term β-carotene is beneficial for age-related cognitive dysfunction, whereas short-term treatment is not (156). Other possibilities may include suboptimal dosage and reduced bioavailability of synthetic compounds. Despite the range of variability in these clinical studies, it remains clear that ROS participate in the events leading to cognitive dysfunction through damaging oxidizing effects on protein, lipids, and DNA. Whether dietary antioxidants constitute appropriate therapy remains open for debate.
B. ROS in AD
AD is a progressive neurodegenerative disorder characterized by cognitive dysfunction, memory decline, speech loss, and drastic personality changes (414). The disease progression phenotype varies greatly amongst individuals based on general health and social status. This heterogeneity makes it difficult to pinpoint specific clinical determinants for the onset and progression of AD. Increasing evidence, however, both from animal models as well as human patients, implicates oxidative damage in AD-related cognitive dysfunction (175, 273, 391). For example, subjects with established AD-related cognitive dysfunction have been shown to have an imbalance in oxidant/antioxidant levels (390). Also, Aβ, the major pathological determinant of AD, has been shown extensively to act as an oxidizing molecule (40, 68). It has been linked to increased H2O2 production and decreased cytochrome C oxidase activity in the Tg2576 mouse model of AD (266). Both Aβ and its precursor amyloid precursor protein (APP) have been shown to enter the mitochondria and compromise its function through energy processing dysfunction, causing the release of large amounts of ROS (15, 267). Superoxide has been specifically implicated with Aβ vascular deposition and cerebral hypoperfusion (166), which also are thought to be an integral part of the disease and its ensuing cognitive dysfunction. In addition, superoxide has been shown to mediate cell death by enhancing the impact of presenilin-1 mutations, specifically mitochondrial Ca2+ accumulation and membrane depolarization after exposure to Aβ42 (157). Aβ also can initiate a signaling amplification cascade that culminates in the inactivation of SOD-2, leading to further accumulation of mitochondrial superoxide (16). One particular study demonstrated a global increase in SOD-2 in AD patients, presumably as a compensatory mechanism to the increased oxidative stress originating from mitochondrial superoxide production (272). This increase was less pronounced in hippocampal area CA1 (272), which suffers the most damage during AD (439) and incidentally happens to be one of the major areas involved in the learning and memory processes (439). In addition, the same SOD/catalase mimetics used in the aging studies described above proved effective against Aβ toxicity in cell culture (65). Finally, we have shown that Aβ-induced ERK phosphorylation in organotypic hippocampal cultures was mediated by redox signaling through NADPH oxidase, suggesting similar mechanisms occur during AD (372). Because ERK plays a critical role in LTP (194, 210), the most probable cellular substrate for learning and memory (117), this study indirectly implicated ROS produced by NADPH oxidase in the learning and memory dysfunction associated with AD.
Despite the overwhelming evidence for the pro-oxidant role of Aβ, several other studies demonstrated free radical involvement in AD before Aβ pathology (236, 399), and also have implicated ROS in increases in Aβ levels. In addition, transgenic AD model mice with reduced SOD-2 activity exhibit increased amyloid plaque burden (249) and increased tau phosphorylation (287), both of which are implicated with AD-related cognitive dysfunction. SOD-2 deficiency also has been shown to accelerate the onset of a number of behavioral deficits in the hAPP mouse model of AD (123). Recent studies have further explored the involvement of mitochondrial superoxide in AD-related cognitive dysfunction. We and others concurrently found that overexpression of SOD-2 in the Tg2576 AD mouse model reduced the levels of hippocampal superoxide, presumably from mitochondrial origin, and led to the prevention of both associative and spatial memory deficits as measured by a fear conditioning paradigm and the Morris water maze, respectively (115, 277). Our data are in agreement with several studies that demonstrate the involvement of mitochondrial superoxide, through SOD-2 deficiency, in increasing AD-related cognitive dysfunction and correlating reduced mitochondrial ROS with improved cognition (112, 123, 249, 287). Of particular interest are two studies by Dumont and associates showing that AD-related increases in mitochondrial ROS and impairments in memory function were exacerbated by deficiency in the α-ketoglutarate dehydrogenase mitochondrial enzyme complex (114) and improved by SOD-2 overexpression (115).
In recent years, an increasing number of studies have been focused on characterizing antioxidant therapies to help prevent and/or treat the declining cognitive functions of affected individuals. Intrahippocampal injections of a lentiviral vector carrying the nuclear factor E2-related factor 2 (Nrf2) transcription factor improves spatial learning in the APP/PS1 mouse model of AD concomitant with a change in Aβ solubility consistent with reduced Aβ toxicity (207). Nrf2 binds to and activates the antioxidant response element (ARE) enhancer sequence, which upregulates a cassette of antioxidant enzymes, thereby constituting an important oxidative stress response (305). Nrf2/ARE pathway activity is reduced during AD; therefore, boosting its activity via agents such as tBHQ (118) or tert-butylhydroquinone (208) may prove beneficial for AD-related memory dysfunction. In addition, animal models have demonstrated that dietary supplementation with antioxidant vitamins can decrease oxidative stress and either prevent or reverse the age-related changes in cognitive functions (198, 314, 352, 451). Clinical trials, however, have produced conflicting results concerning the therapeutic efficacy of antioxidant supplementation during AD (121, 122, 258). Similar to the conflicting results of antioxidant therapy during physiological aging, this discrepancy may be due to multiple factors such as reduced bioavailability and/or poor specificity of these treatment regimens. As described above, we and others have shown specific involvement of mitochondrial superoxide in AD-related cognitive dysfunction in mouse models, and therefore targeted mitochondrial antioxidant therapy may prove more useful in the treatment of AD-related cognitive dysfunction.
C. ROS during hypoxia/ischemia and traumatic brain injury
It has long been established that ROS are produced in association with traumatic brain injury (TBI), during ischemia and hypoxia, as well as during the reperfusion and reoxygenation after these events (23, 75, 420, 425). Ischemia is characterized by a transient block in blood flow that leads to decreased glucose and oxygen perfusion to the brain, resulting in energy failure, neuronal dysfunction and death, and impairments in cognitive functions, especially if the focal point of the ischemia was in learning and memory regions of the brain. After an ischemic insult, a core of tissue is usually severely injured and is surrounded by an area called the penumbra, where neurons are impaired, but sufficiently active to maintain membrane potentials (185). Although the precise timing and mechanisms by which ischemia causes neuronal dysfunction and death are not fully understood, there seems to be a consensus that three main events are involved: (a) oxidative stress, (b) glutamate-induced excitotoxicity, and (c) apoptotic-like cell death (111, 257). These three events together lead to the activation of the same cellular machinery involved in the induction of synaptic plasticity. After oxygen and glucose deprivation, neurons fail to generate sufficient ATP leading to loss of ionic gradients and release of glutamate, which activates NMDA receptors that results in excessive Ca2+ entry into neurons. Although NMDA receptor activation (54, 72, 264) and Ca2+ entry (57, 265) are necessary for the induction of LTP, the excessive activation of NMDA receptors leads to toxicity and impairment of LTP. At the same time, the failing of mitochondrial energy metabolism coupled to the rush of oxygen during reperfusion results in increased production of ROS, which adversely affects synaptic plasticity, therefore compounding the effect of ischemia on these events. Given the established critical role of oxidative stress in the events shortly after ischemia, a large body of research effort has been focused on assessing the efficacy of a variety of antioxidants at quenching ROS after ischemic events and at improving ischemia-induced learning and memory impairments. Edaravone (3-methyl-1-phenyl-2-pyrazolin-5-one), a free radical scavenger, has been shown to protect the rat hippocampus from ischemia-induced LTP impairments as well as increases in hydroxyl radical, with a specific very early therapeutic time window (day 0–1 postischemia) (309). In a separate study, edaravone was found to afford significant protection against learning and memory impairments and altered neuronal morphological integrity when administered for 2 days in the acute phase after ischemia (317). Both of these studies suggest that free radical formation after ischemia/reperfusion is a pivotal trigger of brain dysfunction after global ischemic stroke. MAO-B has been shown to participate in the generation of hydroxyl radicals during ischemia (370). Hence, several groups investigated the effects of deprenyl, the MAO-B irreversible inhibitor, and found that it reduced ischemia-induced oxidative stress and prevented spatial memory deficits and apoptotic neuronal cell death (220, 262). One of these studies administered deprenyl before ischemia (220) and the other administered it right after the ischemic insult (262), in the early stages of dysfunction. These results indicate that ROS play a role in ischemia-related cognitive deficits and that therapeutics should be targeted to the time window immediately surrounding the ischemic injury to quench oxidative stress before the occurrence of irreversible damage. In addition, idebenone, a coenzyme Q analog, also ameliorated memory impairment induced by cerebral vascular disturbance in rats (453). Several other antioxidants of either chemical or plant origin (including, but not limited to, L-NAME, flavonoids, and carnosine) also have been used and produced very similar results in terms of improvement of memory function as well as reduction of oxidative stress after ischemia (294, 326, 344, 351, 376), strengthening the postulate that ROS play a critical role in the cognitive impairments associated with ischemia and reperfusion. In addition to ischemia, ROS have been shown to contribute to the secondary injury process after TBI. ROS scavenging compounds have been shown to have neuroprotective properties in various models of experimental brain injury, including TBI. For example, nitrone spin traps such as NXY-059 (91), PBN, and its sulfo-derivative, 2-sulfo-PBN (274), administered after moderate to severe lateral fluid percussion injury in rats have been shown to be neuroprotective and to improve cognition. Vitamin E also has been shown to protect against oxidative damage and learning disability after mild TBI in rats by affecting molecular systems involved in the maintenance of synaptic plasticity, such as brain-derived neurotrophic factor and the silent information regulator 2 (443). Sulforaphane, a naturally occurring compound found in cruciferous vegetables, has been used after TBI, and was demonstrated to reduce cerebral edema (463) and attenuate blood–brain barrier permeability (464) via induction of the Nrf2/ARE pathway, which is primarily a free radical scavenging system. Sulforaphane also has been shown to improve performance in the Morris water maze after TBI (99). These studies suggest a major role for ROS in the learning and memory pathology associated with TBI.
D. ROS in multiple disease states
ROS have been implicated with the etiology of multiple disease states, such as PD, diabetes, and homocysteinuria. In the following section, we will address the involvement of oxidative stress specifically in the learning and memory deficits accompanying each of these diseases.
1. Parkinson's disease
PD is a neurodegenerative disease of the basal ganglia, characterized by dopaminergic neuronal loss, mainly in the substantia nigra, and results in bradykinesia, tremor, and postural instability. Although the mechanisms of cell death in PD are not yet fully understood, oxidative stress is known to play an important role. In fact, the oxidation of dopamine during PD generates toxic semiquinones and the accelerated metabolism of dopamine by MAO-B induced the production of excessive H2O2 and hydroxyl radicals (313). Further evidence for the role of oxidative stress in PD comes from studies showing that the two main misfolded proteins of PD, parkin and α-synuclein, are a source of oxidative and nitrative stress (59). PD was found to be associated with increased oxidative damage to DNA (38), lipid peroxidation (107, 108, 458), and protein carbonylation (10, 150). Other studies showed increased brain levels of iron (105, 151) and reduced levels of ferritin (106), which creates a source of free radicals that potentiate the oxidative damage, especially in the substantia nigra. Although there is a large body of literature discussing the beneficial effect of antioxidant therapy for PD, no specific reports directly discuss the involvement of oxidative stress in PD-related learning and memory deficits. In a recent study investigating the effect of deprenyl and tocopherol on the PD-related cognitive dysfunction, the authors reported an improvement in the cognitive functions of the group treated with antioxidants. However, the authors do mention that this could be due to recruitment bias and/or limitations of the mini mental state examination (MMSE) test used to assess mental function (423).
2. Diabetes
Growing data report learning and memory problems (135, 161, 441) as well as LTP (21, 202) deficits associated with diabetes mellitus. Expression of neuronal NOS mRNA and protein in the hippocampus of diabetic rats is decreased (357), suggesting a critical role for NO in synaptic plasticity associated with diabetes. Supporting studies, using the active avoidance learning test in rats, demonstrated the involvement of NO imbalance in the occurrence of diabetes-induced learning deficits (234). Multiple studies using a variety of antioxidants to treat and/or prevent the cognitive dysfunction associated with diabetes also demonstrated an involvement of ROS in these events. For example, melatonin and vitamin E have been shown to improve diabetes-induced spatial memory impairments (421). Lycopene also has been shown to decrease markers of oxidative stress associated with diabetes and to attenuate spatial memory deficits (235). Thus, ROS likely play a role in cognitive dysfunction associated with diabetes.
3. Homocysteinuria
Homocysteinuria is a metabolic disorder characterized by deficiency of the enzyme cystathione β-synthase activity leading to accumulation of homocysteine. Homocysteine causes free radical formation and severe neurological dysfunction, which is thought to be due to the increased oxidative stress (263). Administration of homocysteine to adult rats caused severe memory impairments in the step-down inhibitory avoidance test that was prevented by pretreatment with vitamins C and E, suggesting that ROS play a critical role in the onset of memory dysfunction during homocysteinuria (358). Using the same model system it was shown that homocysteinuria caused severe learning and memory deficits in the passive avoidance and Morris water maze tests, and that these deficits were reversed by chronic melatonin treatment, which also significantly reduced the levels of lipid peroxidation (35). Taken together, these findings suggest that homocysteinuria causes cognitive deficits via increased oxidative stress.
VI. Antioxidant Defenses Against Pathological ROS
In this review we have discussed extensively the important role played by ROS both as cellular messengers in physiological events such as learning and memory and as toxic molecules in pathological events such as ischemia, aging, and neurodegeneration. Because of this dichotomy in the function of ROS in mammals, the balance between ROS formation and scavenging is of utmost importance for proper neuronal function. Multiple sources of ROS were discussed earlier in the review. The following section will be dedicated to presenting the variety of antioxidant mechanisms, which constitute first line of defense when ROS start exceeding their physiological levels. Antioxidant mechanisms include a variety of enzymes, such as SOD, catalase, glutathione peroxidase (GPx), and glutathione reductase (GR). Organisms can also synthesize nonenzymatic antioxidants such as glutathione (GSH), coenzyme Q, and uric acid. In addition, antioxidants can be obtained through the diet, including vitamins and minerals (such as ascorbate, carotenoids, tocopherol, flavonoids, selenium, manganese, copper, and zinc), and a variety of herbs (such as curcumin, gingko, and grape-seed extract).
A. Antioxidant enzymes
1. Superoxide dismutases
SODs are a class of metalloenzymes involved in catalyzing the dismutation of the superoxide radical into oxygen and H2O2 and, as such, constitute a very important element of the antioxidant defenses of organisms (136) (Fig. 9A). In mammals, including humans, there are three isoforms of the enzyme that are encoded by three different genes. The SOD isoforms differ with regard to their localization as well as the metal bound to them. SOD-1 is a cytoplasmic enzyme with copper and zinc acting as cofactors, and therefore also is known as Cu/Zn-SOD. SOD-2 is a mitochondrial enzyme with manganese at its core and hence, is also referred to as Mn-SOD. SOD-3 also is a Cu/Zn enzyme, but is localized extracellularly and is often called EC-SOD. The three SOD isoforms have some structural differences, with SOD-1 being functional as a dimer, whereas SOD-2 and EC-SOD are tetramers. The physiological importance of all three SODs is illustrated by the multiple pathologies exhibited in mutant mice lacking each isoform. SOD-1 knockout mice develop hepatocellular carcinoma, age-related mass loss, increased formation of cataracts, and a reduced life span (119, 298). SOD-2 knockout mice survive only a few days postbirth and then die of severe oxidative stress (251). EC-SOD knockout mice do not exhibit an obvious major phenotype, but do develop hypersensitivity to free radical-induced injury (371). The physiological importance of the SODs is also illustrated by studies demonstrating that upregulation of SODs serve to attenuate neuropathological conditions such as ischemic injuries (212) and AD (115, 277).
The use of SOD transgenic mice and/or pharmacological mimetics has been instrumental in determining the functions of these enzymes and the role of superoxide in synaptic plasticity and memory. In line with the dual effect of superoxide and H2O2 as crucial signaling molecules in healthy organisms, but involved in oxidative stress in the aging and/or diseased brain, the effects of overexpressing SODs were found to be dependent on the age of the animals.
a. Synaptic plasticity and memory in young mice
LTP studies in hippocampal slices from young EC-SOD transgenic mice revealed impairments in LTP, without affecting normal synaptic transmission (417). The LTP impairments persisted even after treatment with catalase to quench excessive H2O2, indicating that superoxide is crucial for LTP under physiological conditions (417). In line with its effect on LTP induction, significant deficits in the acquisition of spatial memory were observed in mice overexpressing EC-SOD (247). These mice also are deficient in the consolidation of contextual fear memory, which requires the hippocampus (190). LTP impairments in slices from EC-SOD transgenic mice can be reversed by treating the slices with the copper chelator diethylcarbamate, raising the possibility that targeting the cofactor of EC-SOD modulates its action on memory formation (417). Similar to the studies of EC-SOD transgenic mice, SOD-1-overexpressing mice, which have a higher level of H2O2, have normal baseline synaptic physiology, but are deficient in LTP and spatial memory formation (143). The effects of SOD-1 overexpression on LTP were reversed by H2O2 quenching antioxidants such as catalase and spin trap reagents (143). Whether the same H2O2 antioxidant treatments improve the impaired memory in the SOD-1 mice has not been investigated.
The differential impact of catalase on the actions of SOD-1 and EC-SOD indicates that these enzymes are involved in synaptic plasticity and memory formation using different mechanisms; SOD-1-induced LTP impairments are due to high ambient H2O2 levels, whereas EC-SOD-induced LTP impairments are due to decreased levels of superoxide. Despite these mechanistic differences, the conclusion remains the same in that free radical balance, under physiological conditions, is crucial for proper synaptic plasticity and memory formation.
Whether mitochondrial superoxide contributes to LTP and memory formation is debatable, especially since it has been suggested that the mitochondrial membrane may not allow the free diffusion of mitochondrial superoxide (460). SOD-2 transgenic mice have been characterized extensively and, contrary to its cytoplasmic and extracellular counterparts, SOD-2 overepxression does not affect either LTP or memory formation in healthy young mice (188).
Collectively, these studies indicate that the different SOD isoforms, and consequently the different pools of superoxide they act on, affect synaptic plasticity and memory via different mechanisms, but lead to the same conclusion that free radicals are important for synaptic plasticity and memory formation under physiological conditions.
b. Synaptic plasticity and memory in the aged and diseased brain
It appears that high levels of SOD enzymes are detrimental to normal synaptic function in young animals, but that they act in an opposite manner in the aged or diseased brain. During aging there are significant impairments in LTP that are accompanied by deficits in cognitive abilities and memory formation (31). Overexpression of EC-SOD improved LTP in aged mice and catalase treatment did not alter this improvement, indicating that EC-SOD improves LTP in aged mice via quenching superoxide independently of H2O2 (190). EC-SOD overexpression also improved age-related spatial memory deficits. Associative memory measured by a fear conditioning paradigm was not improved in the aged EC-SOD transgenic mice (190). Other studies have shown that age-related deficits in associative memory may not present themselves unless the interval between training and testing is greater than 24 h (152), which may explain why associative memory was not improved in the aged EC-SOD transgenic mice. In addition to improving aging-related spatial memory impairments, EC-SOD also has been shown to be beneficial to TBI-related cognitive dysfunction (332).
The level of key synaptosomal proteins in the hippocampus is significantly reduced in mice overexpressing SOD-1 (140, 387), suggesting that the beneficial quenching of superoxide may be outweighed by the predominant presence of H2O2, which has been shown to possess neurotoxic effects. This idea is supported by studies of SOD-1 overexpression during AD or TBI. These studies specifically showed that AD-related cognitive dysfunction and loss of memory after TBI not only were exacerbated by SOD-1 overexpression (41, 170), but also could be reduced by SOD-1 deficiency (41, 140). These results are surprising in light of the findings that SOD-1 overexpression can reverse the age-related decline in LTP (205). Using a theta-burst protocol, which should represent more closely the physiological processes during learning and memory, improvements in LTP have been reported in slices from aged mice overexpressing SOD-1, possibly due to increases in H2O2 in these mice (205). The authors of this study proposed a model by which the redox milieu present in SOD-1 transgenic mice has been adapted to working at high H2O2 levels and, as such, the combined increase in H2O2 from superoxide dismutation and from age-related mitochondrial leakage, while detrimental to a normal system, becomes beneficial to this system (205). Consistent with this notion, aged EC-SOD transgenic mice exhibit better spatial memory than wild-type littermates (203). Thus, excessive production of H2O2 might improve memory in aged mice.
Surprisingly, there was no effect of overexpression of SOD-2 on synaptic plasticity and memory in aged mice (188). SOD-2 overexpression appears to be beneficial under conditions of either acute toxicity or increased oxidative stress. For example, SOD-2 overexpression was shown to protect against 6-hydroxydopamine- and methamphetamine-induced brain injury (74, 271). In addition, SOD-2 overexpression reverses the AD-related cognitive deficits (115, 277). This will be discussed in more detail later in the review.
Taken together, these studies show that the functional involvement of SODs in synaptic plasticity and memory depends on the age of the animal as well as the isoform of SOD. Another important factor may be the nature of oxidative insult initiating the events, as well as the area of the brain affected.
2. Catalase
Catalase is an antioxidant enzyme found in nearly all living organisms. Its main function is to catalyze the decomposition of H2O2 to water and oxygen (82) (Fig. 9B). Catalase is an iron enzyme; it contains four porphyrin heme groups that allow it to interact with H2O2. Studies investigating the role of catalase in the mechanisms underlying memory function have done so in relation to H2O2 metabolism. For example, LTP in hippocampal slices from adult or aged wild-type mice was examined in the presence or absence of an enzymatic treatment of either catalase or SOD-1. In line with Kamsler and Segal's model of H2O2 modulation of synaptic plasticity (205), increased SOD-1 had no effect on LTP from young adult mice, but made LTP impairments worse in old mice (436). It was also found that catalase inhibited LTP in slices from the young adult mice, but reversed age-related LTP deficits in older mice, indicating that in wild-type mice, H2O2 plays a critical role in synaptic plasticity at young ages, but becomes detrimental during aging (436). This study supported earlier findings that demonstrated a dual role for H2O2 in the regulation of LTP that is mediated by the protein phosphatase calcineurin. H2O2 was found to be necessary in young animals, but became detrimental in old animals (204). Several studies investigated the contribution of brain catalase to ethanol-induced learning reinforcement. It was found that catalase is involved in the acquisition of ethanol-induced conditioned place preference (133), as well as learning in the social recognition test (270). Additional supporting evidence of the involvement of catalase in ethanol-induced behavioral reinforcements came from studies of conditioned taste aversion combined with a catalase inhibitor (aminotriazole). These effects are thought to be mediated by acetaldehyde, which is an ethanol metabolite directly produced in the brain by the catalase system (20, 308, 346). In studies directly relevant to aging and neurodegeneration, it was shown that aging-related cognitive dysfunction can be reversed by treatment with synthetic SOD/catalase mimetics, further supporting the idea that free radicals are involved in learning and memory (90, 255).
3. GPx, glutathione reductase, and related enzymes
GPx is a general nomenclature attributed to a group of antioxidant enzymes dedicated mainly to reducing H2O2 to water and lipid hydroperoxides to their corresponding alcohol. GPx reduces H2O2 to water by oxidizing GSH. Then, the oxidized glutathione disulfide (GSSG) is regenerated back to its reduced form by the action of GR (Fig. 10). GR is constitutively active and inducible upon oxidative stress; therefore, GSH is found almost always in its reduced form, ready to counteract the deleterious oxidant effects (26).
FIG. 10.
Schematic representation of the glutathione synthesis and redox system. GSH is a tripeptide synthesized from glutamate, cysteine, and glycine by a two-step reaction requiring ATP. GSH is a major scavenger of H2O2. H2O2 is transformed into two molecules of water (H2O), whereas two GSH molecules are oxidized (GSSG) by the action of the seleno-enzyme glutathione peroxidase. GSSG can be converted back to GSH by the NADPH-dependent glutathione reductase. ATP, adenosine triphosphate; GSH, glutathione; GSSG, oxidized glutathione.
Up to eight isoforms of GPx have been identified in humans thus far, with specific antioxidant mechanisms attributed to each. For example, the cytoplasmic homotetrameric GPx1, the most abundant of these peroxidases, has been shown to preferentially quench H2O2, whereas the ubiquitous but less abundant monomeric GPx4 plays an important role in detoxification of membrane lipids. With the exception of increased cataract occurrence, GPx1 knockout mice are phenotypically normal, indicating that this enzyme is not crucial for survival. However, genetic deletion of GPx4 is embryonic lethal, illustrating the different function of the different GPx isoforms (297). GPx levels and/or activity are used widely as an index of oxidative stress and assessment of therapeutic efficacy. In studies of oxidative stress-induced synaptic plasticity and memory impairments, impaired cognition is typically found to be correlated with reduced levels of GPx that are brought back to normal upon certain antioxidant treatments [examples in refs. (86, 172, 301)]. This is in agreement with the evidence discussed in this review, implicating ROS dysfunction with cognitive diseases.
Additional studies using GPx transgenic mice have addressed more directly the role of this antioxidant enzyme in oxidative stress-induced cognitive deficits. For example, moderate increases in GPx1 were shown to reverse hypoxia-induced functional damage to hippocampal slices. Control slices subjected to hypoxia suffered an irreversible loss of EPSPs with an inability to generate LTP. GPx overexpression resulted in a significant recovery of synaptic transmission and LTP (141). In addition, GPx overexpression has been shown to be beneficial to TBI-induced cognitive deficits in the absence of any effect on the lesion anatomy and brain volume. Further, GPx protected hippocampal neurons via its antioxidant effect on early oxidative stress (419). The effect of GPx and GR as antioxidants cannot be dissociated from the main precursor for the system, namely GSH (discussed in details later in this review). In fact, boosting GSH levels can result in increasing the activity of both GPx and GR, as well as other antioxidants (300). In addition, the effectiveness of these enzymes in neuroprotection and improvement of cognitive function depends on the availability of both GSH and GSSG as reducing equivalents (110, 283). In certain instances, increased activity of GPx has been shown to correlate positively with memory impairments, which appears counterintuitive (66). However, the levels of GPx are a better indicator of oxidative stress-induced impaired memory function, which also resulted in the activation of antioxidant defenses, including the increased activity of GPx.
A group of enzymes functionally related to the GSH system are thiol-disulfide oxidoreductases that are primarily involved in the catalysis of thiol-disulfide interchange reactions. This group includes thioltransferase (glutaredoxin [GRX]), thioredoxin (TRX), and protein disulfide isomerase (437). Whereas TRX and protein disulfide isomerase have broad substrate specificities (289), GRX specifically reduces GSH-containing mixed disulfides with greater efficiency than TRX (153, 289). The TRX system contains, aside from TRX, an NADPH-dependent TRX reductase, which reduces the oxidized form of TRX. The GRX system uses GSH as a reducing agent, which then enters the GSH system and is regenerated with GR [reviewed in ref. (201)]. Two gene isoforms of each GRX and TRX have been identified in mammals and a summary of their function can be found in the following review (179). Both GRX and TRX are endogenous antioxidants that contribute to protection against oxidative stress, specifically the formation of intra- and intermolecular disulfide bonds in proteins. As such, both of these systems may modulate memory formation. However, studies specifically investigating the direct involvement of GRX and TRX in synaptic plasticity and memory are limited. TRX upregulation, along with increased ERK-mediated Nrf2 phosphorylation and a reduction in free radical levels, contributes to the beneficial effects of acetyl-L-carnitine on hypoxia-induced spatial memory impairment (29). Additionally, GRX1 and TRX1 are deregulated in AD and contribute to Aβ-induced neurotoxicity (9). However, these studies did not specifically address the involvement of GRX1 and TRX1 in memory dysfunction associated with AD. GRX also was shown to be essential for maintaining the integrity of complex I of the mitochondrial respiratory chain after toxic insult by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine, a neurotoxin that produces PD-like symptoms through inhibition of complex I (214). This finding suggests that GRX also may be involved in PD pathology; however, its involvement in learning and memory can only be inferred.
Another group of related enzymes, peroxiredoxins (PRXs), is involved in the decomposition of H2O2, organic hydroperoxides, and peroxinitrite (79). PRXs are related to TRX because distinct isoforms of TRX are cofactors for PRX. PRX has been shown to contribute its antioxidative capability in the realm of aging- and AD-related cognitive dysfunction. Specifically, PRX II-deficient mice were shown to have increased mitochondrial ROS generation and pronounced age-related LTP impairments compared to their wild-type littermates (218). These mice also exhibited an impairment in the activation of synaptic plasticity-related signaling pathways involving CREB, CaMKII, and ERK, indicating that PRX II is necessary for maintaining redox homeostasis (218). Dietary vitamin E alleviated a number of PRX II deficiency-related impairments, including a restoration of the cognitive decline in aged PRX II-deficient mice (218), suggesting that PRX II helps maintain hippocampal synaptic plasticity against age-related oxidative damage. In a differential proteomic analysis, PRX 1 from aged rat temporal cortex tissue is carbonylated, suggesting that this enzyme contributes to the decline in cognitive function that occurs during aging (431). PRX II also has been shown to be upregulated in response to increase Aβ levels during AD, with the purpose of protecting neurons against Aβ-induced toxicity (456). Conversely, reducing the levels of Aβ in the brains of senescence accelerated mice resulted in reduced PRX II levels (338). Although these findings do not directly link PRX II to AD-related cognitive dysfunction, they do support the idea that PRX II plays a crucial antioxidant role during aging and AD.
This section reviewed evidence for the involvement of the glutathione system, including GSH, GPx, GR, and related enzymes, in reducing oxidative stress and improving cognitive dysfunction, providing more evidence that oxidative stress is associated with neurotoxicity and memory impairment.
4. Metallothionein
Metallothionein (MT) is a family of cycteine-rich low-molecular-weight proteins that are ubiquitous in eukaryotes. Their primary function is to bind essential physiological (copper, zinc, and selenium) and nonessential xenobiotic (cadmium, mercury, silver, and arsenic) heavy metals via their cysteine residues. A significant literature is available (167, 177, 200, 243, 310, 325, 368) describing the physical structure, biochemistry, and molecular biology of MT, as well as their role in protecting cells and organisms from environmental toxicant damage (metal toxicity), but also from oxidative stress. Specific evidence documenting the involvement of MT in synaptic plasticity and memory is also available (248, 459); however, it is not clear whether this involvement is related to their antioxidative capabilities and therefore will not be discussed here.
B. Antioxidant molecules (nonenzymatic)
Although behavioral outcomes of increased brain oxidative stress are usually discussed with reference to enzymatic dysfunctions in the antioxidant system, several molecules with redox-dependent activity are involved in the synaptic plasticity that plays a role in learning and memory. These nonenzymatic molecules are discussed in this section.
1. Ascorbate (vitamin C)
Ascorbate (vitamin C) is a potent antioxidant that can neutralize free radicals by donating a hydrogen atom. Vitamin C itself becomes a free radical in the process. However, this does not pose a major concern, as the vitamin C radical is very stable (nonreactive) due to its resonance structure (Fig. 11). Moreover, the reduced form of vitamin C can be readily regenerated by the action of NADH and NADPH-reductases. Excess vitamin C radical becomes a problem in the presence of metal ions such as iron (Fe) or copper (Cu), where it can turn into a powerful pro-oxidant. Normally ferrous iron (II) (Fe2+) is oxidized by H2O2 to produce ferric iron (III) (Fe3+), along with the highly reactive hydroxyl radical and a hydroxyl anion. This is known as the Fenton reaction (Fig. 12). After the Fenton reaction, Fe3+ can be reduced back to Fe2+ by the action of H2O2 to produce a peroxyl radical and a proton in the process. The presence of ascorbate can accelerate the latter free radical producing reaction by acting as an electron acceptor for Fe3+, instead of H2O2 (Fig. 11). Organisms have developed defenses against this process, such as having iron bound to the transport proteins ferritin and transferritin. However, the exogenous introduction of metal ions from environmental pollutants can cause failure in this defense mechanism.
FIG. 11.
Vitamin C. Vitamin C (or ascorbate) is an antioxidant molecule that neutralizes free radicals (radical•) by donating an electron and becoming a free radical itself. However, the ascorbate radical is stable due to its resonance structure (shown by the dashed lines in the chemical structure). Vitamin C also is returned readily to its nonradical form via the action of either NADH- or NADPH-dependent reductases. In the presence of metal ions, however, the production of ascorbate radical can become overwhelming. For example, ascorbate acts as an electron acceptor for Fe3+, instead of H2O2, generating an ascorbate radical in the process. Copper ion (Cu2+) can also react with ascorbate, with 80 times more efficiency than Fe3+. Thus, Vitamin C can be a powerful antioxidant as long as metal ions are not present, but small amounts of vitamin C in the presence of metal ions can make vitamin C a dangerous prooxidant. Organisms have developed defenses against this process, such as having iron and copper bound to the transport proteins such as ferritin, transferritin, and caeruloplasmin. However, the exogenous introduction of metal ions from environmental pollutants can cause the failure of this defense mechanism.
FIG. 12.
Fenton chemistry. Ferrous iron (II) (Fe2+) is oxidized by H2O2 to produce ferric iron (III)(Fe3+), along with the highly reactive hydroxyl radical (OH•) and a hydroxyl anion (OH−). After the Fenton reaction, Fe3+ can be reduced back to Fe2+ by the action of H2O2 to produce a peroxyl radical (HOO•) and a proton in the process.
Vitamin C is an essential dietary supplement because it is required for collagen synthesis and primates have lost the ability to synthesize it. However, its antioxidant properties make it an increasingly desired dietary addition. These antioxidant properties have been explored both in vitro and in vivo and their effect on protein oxidation, lipid peroxidation, and DNA damage have been well characterized. In the interest of this review, we will focus on the role vitamin C plays in the learning and memory processes.
The vast majority of studies investigating the role of vitamin C in learning and memory have done so in the setting of memory dysfunction. In other words, these studies investigate the usefulness and effectiveness of vitamin C in reversing memory deficits induced by various oxidative stress-related conditions, ranging from chemical induction to disease states. Very few studies have addressed the role of vitamin C in normal learning and memory. One study from the early 1980s compared short-term and immediate memory performance of 20 individuals at times when their plasma level of vitamin C was high (owing to supplementation) versus times with low plasma levels of vitamin C (5). This study found no differences in the memory performance, nor did it find any differences in alertness, concentration, or mood. The purpose of this study primarily was to determine the effect of vitamin C on alertness, and therefore the memory test used was a simple procedure consisting of repeating the last five digits of a series of numbers played through headphones. This task was designed to test immediate short-term memory, stemming from concentration as opposed to actual learning (5). Two more recent studies addressed the antioxidant role of vitamin C in learning and memory (374, 422). It was demonstrated that although acute injections of ascorbate had no effect on the learning ability of rats measured with passive avoidance test, short- and long-term supplementation with vitamin C facilitated acquisition and retrieval of learning and memory in rats (374). Tveden-Nyborg et al. went a step further and investigated the effect of chronic vitamin C deficiency on spatial memory as measured by the Morris water maze and also counted hippocampal neurons to assess whether ascorbate had an effect on neurodegeneration (422). In this study, two groups of newborn guinea pigs were randomly assigned to either a vitamin C-sufficient diet or a deficient diet (minimally adequate to prevent scurvy) for 2 months. The objective of the study was to assess the effect of vitamin C deficiency on the neonatal brain, because it is more susceptible to oxidative damage than the adult mature brain. The results showed a significant reduction in spatial memory, compounded by a lower number of neurons in all regions of the hippocampus (422). Collectively, these animal studies indicate that ascorbate, at least partially, contributes to protection of neurons from free-radical-induced memory impairments. Clinical trials investigating the benefits of vitamin C on cognition have yielded more complicated results and are discussed in section V under physiological aging and AD.
2. Tocopherol (vitamin E)
Vitamin E is a generic term referring to a group of tocopherols and tocotrienols, of which α-tocopherol is the best studied. Vitamin E is a fat-soluble vitamin known for its role as an antioxidant, involved in stopping ROS from forming in membranes undergoing lipid peroxidation chain reactions. The role of vitamin E as a systemic antioxidant has been studied extensively, with conflicting results as to its beneficial effects. Similar to ascorbate, the involvement of vitamin E in learning and memory has been for the most part investigated under therapeutics for either ROS-induced disease states or physiological aging (discussed above in section V). Very few studies have addressed the role of vitamin E in normal memory function. In the early 1970s, it was reported that there was no significant effect of vitamin E deficiency on the learning ability of rats, although they needed more trials to acquire a conditioned response (238). The most striking effect of vitamin E deficiency in these experiments was the impaired retention of a one-trial experience, a task dependent on reference memory (238). In similar experiments, the effect of vitamin E deficiency on reference and working memory were subtle at best (367). Studies examining the effect of long-term vitamin E deficiency and vitamin E supplementation in rats found that it had no effect on the acquisition and maintenance of memory tasks, but did impact their learning (195). Collectively, the results of these studies have not conclusively demonstrated whether vitamin E acts as an antioxidant in learning and memory, which may seem surprising given that memory processes depend on the redox status of neurons. In fact, two more recent related studies (449, 450) show a direct role of vitamin E in the modulation of LTP, which is the substrate of learning and memory formation (117). In these studies (449, 450), vitamin E deficiency resulted in LTP impairments and that vitamin E induced a slowly developing long-lasting increase in EPSPs that was independent of NMDA receptor activation. The same effect was not observed with ascorbate. Although these studies clearly argue for a role for vitamin E in LTP (and thus memory formation), the ascorbate study suggests that this effect may not be mediated by ROS (449, 450). Collectively these studies demonstrate that the involvement of vitamin E as an antioxidant in the learning and memory process is not clear and is at best inferred.
3. Glutathione
Glutathione (GSH, l-γ-glutamyl-l-cysteinylglycine) is the predominant cytoplasmic antioxidant molecule in cells. GSH serves to provide reducing equivalents for the maintenance of proper oxidant/antioxidant homeostasis and is required for viability and proper functioning of cells. GSH is a tripeptide synthesized from the amino acids glutamate, glycine, and cysteine (19) (Fig. 11). Given that glycine and glutamate are plentiful in cells, it is cysteine availability that controls the production rate of GSH. This is supported by studies in transgenic flies showing that neuronal overexpression of the rate-limiting enzyme for GSH synthesis increased lifespan by up to 50% (316). The main roles of GSH are to scavenge peroxynitrite and hydroxyl radicals, as well as to convert H2O2 to water. A GSH radical is formed in the process; however, it is readily neutralized by combination with another GSH radical to produce GSSG. GSSG can be converted back to GSH by NADPH-dependent GSH reductase (26). Each antioxidant is converted into a radical upon scavenging another radical. This is a concept that was described above for both for vitamin C and E, and it is also true of GSH. All of these antioxidants can recharge each other as such: GSH regenerates vitamin C, which regenerates vitamin E, creating a chain of antioxidant molecule dependency.
With regard to learning and memory, the role of GSH has mainly been investigated after chemical depletion and its effect discussed in the context of schizophrenia, where a deficiency in GSH is common and is associated with cognitive decline. It was demonstrated that low levels of GSH negatively impact LTP and paired-pulse facilitation, indicating that low GSH content can impair short- and long-term forms of synaptic plasticity (11). The effect of GSH depletion in the presence of dopamine, which is a potent pro-oxidant, was studied by inducing GSH depletion in rats by injecting buthionine sulfoximide (BSO) either before or after dopamine administration, and then assessing the spatial memory of rats using the Morris water maze. These investigators found that GSH depletion before the oxidant insult introduced by dopamine led to significant impairments in memory, indicating that GSH is a necessary component of the antioxidant machinery dedicated to maintain a healthy balance of free radicals necessary for proper memory function (389). A specific role for GSH in the acquisition, but not retention, of spatial memory in maze tasks was demonstrated by inducing GSH depletion in rats with systemic diethylmaleate injections and then examining metabolism and cognitive performance in the passive avoidance and Morris water maze tests. These studies showed that GSH depletion resulted in reduced levels of GSH and GPx activity in the hippocampus. It also resulted in reduced acquisition of spatial memory when the drug was administered before training, but did not affect retention of already acquired memory when the drug was administered after memory acquisition (94). More complex assessments of spatial and working memory were performed after transitory GSH depletion by BSO injection in either young normal or mutant rats (osteogenic disorder Shionogi [ODS]—incapable of synthesizing ascorbate). Ascorbate is known to compensate for GSH depletion, and therefore the ODS group served to dissect the direct effect of GSH on cognitive abilities. BSO-ODS rats exhibited impaired spatial memory whether they were tested at a young age, immediately after GSH restoration to physiological levels, or at an older age, long after treatment cessation. In contrast, BSO-normal rats did not exhibit memory impairments at an early age, possibly due to ascorbate compensation, but they did exhibit selective spatial memory impairments at an advanced age when the task required integration of multimodal cues, such as visual and olfactory cues. Although the results of this study were exclusively discussed in the context of schizophrenia, they clearly show that GSH depletion causes complex behavioral impairments associated with increased brain oxidative stress (70). In a more recent study, GSH depletion was induced with 2-cyclohexene-1-one both in rats and mice and then their memory was assessed in the Y-maze test. In both species, GSH depletion caused a significant impairment in short-term spatial recognition memory, suggesting that GSH is necessary for proper spatial memory function, possibly explaining its involvement in psychiatric diseases such as schizophrenia (104). Collectively, these studies outline an important role for GSH in spatial memory formation. On a related note, administration of reduced GSH into the antennal lobes of honeybees prevented oxidative stress-induced impairments in olfactory learning and memory (125), indicating that GSH plays a critical role in learning not only in mammals, but also in invertebrates.
4. Coenzyme Q
Coenzyme Q, also known as coenzyme Q10, ubiquinone, ubidecarenone, or simply Q, is a benzoquinone found in most eukaryotic cells, primarily in the mitochondria. It participates in the electron transport chain in its capacity of electron acceptor/donor and essentially functions as an electron transferring molecule (426) (Fig. 4). Electron transferring ability means that coenzyme Q can act as an antioxidant. Because of this antioxidant ability, coenzyme Q is widely used as a dietary supplement taken to enhance bioenergetics and ameliorate all side effects of increased oxidative stress during aging and a multitude of pathological conditions. Coenzyme Q has been explored as a therapeutic avenue for heart failure (327), mitochondrial dysfunction (160), migraine headaches (47), and neurodegenerative disorders associated with cognitive dysfunction (39). Idebenone, a benzoquinone commercially marketed as a synthetic analog of ubiquinone, also has been extensively studied for its antioxidant properties. We review here the role of both in learning and memory mechanisms.
A general statement that seems to be true of the involvement of quinone in learning and memory is that these compounds do not really affect cognitive abilities under physiological conditions, but rather improve cognitive dysfunction induced by a variety of pathological conditions. One study of the late 1980s directly linked quinones to synaptic plasticity by determining a role for idebenone in enhancing LTP in hippocampal slices (196). Two different concentrations of ubiquinone were tested on several physiological functions that are known to be impaired during aging, including cognitive dysfunction. These investigators supplemented their mice with coenzyme Q at a young age and then assessed their spatial learning and memory at several age points. Although the amount of ubiquinones was increased in the cerebral cortex of treated mice, the low dose treatment did not alter ubiquinone levels. Surprisingly, at higher doses, age-related decreases in learning and memory were made worse, indicating that low coenzyme Q levels had no discernable impact on cognitive dysfunction, whereas higher levels impaired cognitive function (403). These results suggest that under physiological conditions ubiquinone supplementation is not beneficial, but rather can be detrimental to memory. In a similar study investigating the role of coenzyme Q, vitamin E, or both on brain function of aged mice, it was observed that only mice treated with both coenzyme Q and vitamin E exhibited memory improvements (281). Similar experiments with higher doses of coenzyme Q were conducted, and unlike the previous study, there was no adverse effect on memory. On the other hand, the investigators also did not observe any memory improvements. They concluded that coenzyme Q is only beneficial for memory when paired with vitamin E, perhaps because they work in concert, possibly via mutual regeneration of antioxidant function (281). Similar to previous studies, rats fed a ubiquinone-rich diet did not exhibit improvement in the early stages of the Morris water maze test, but did exhibit improvements in the later stages of the test (312). After hyperoxia to induce oxidative stress, rats fed coenzyme Q showed improved memory function over control rats. Repeating the same studies in vitamin E-deficient rats or rats supplemented with vitamin E showed the same pattern of improvement and indicated no synergistic effect of coenzyme Q with vitamin E (312), in contrast to previous studies that demonstrated that coenzyme Q can regenerate the antioxidant powers of vitamin E (281).
Two other conditions in which ubiquinones proved to be beneficial for learning and memory were after ischemia and scopolamine-induced amnesia, which was studied in the context of cholinergic dysfunction during AD. Idebenone ameliorated memory impairment induced by cerebral vascular disturbance in rats. More specifically, the authors demonstrated that idebenone treatment after the acquisition trial of passive avoidance learning caused shortening in the latencies of the retention test trial, that is, idebenone reversed the retrograde amnesia caused by cerebral ischemia (453). In a different study, in embolized rats, idebenone improved learning and memory abilities in the radial arm test (227), as well as passive avoidance test (226), indicating that quinones have a positive effect on learning impairments caused by brain hypofunction.
Perhaps the largest body of literature investigating the effect of quinones in the mechanisms governing memory function is in studies related to AD (AD). Idebenone has long been investigated as a possible therapy for AD with mild, but generally positive, results, suggesting that quinones are involved in preserving the oxidant balance necessary for proper cognitive function. Several groups have studied scopolamine-induced amnesia, which induces deficits in the cholinergic system as seen in AD patients. Idebenone treatment improved scopolamine-induced spatial memory deficits (256) and working memory deficits (452). Using a delayed alternation task in rats, it was shown that idebenone treatment improved short-term memory dysfunction induced by scopolamine, but it did not alter memory in control rats (454). These observations were extended to a serotonin-deficient rat model, where idebenone improved the retardation of discrimination learning induced by central serotonergic dysfunction (455). Because nerve growth factor (NGF) is necessary for the maintenance of cholinergic neurons, its depletion mimics cholinergic deficiency as observed in AD. Unfortunately, NGF cannot be used directly as a therapy because of its inability to cross the blood brain barrier. However, idebenone administration stimulated NGF synthesis in vivo, resulting in an amelioration of the behavioral deficits induced by lesions in the basal forebrain cholinergic system. In these studies, idebenone was beneficial as an NGF stimulator rather than as an antioxidant (307).
An interesting question in the AD field is whether oxidative stress contributes to learning and memory deficits caused by Aβ42. Rats given continuous intracerebroventricular infusion of Aβ42 were treated with either idebenone or vitamin E and then their memory was assessed in the Y-maze, the Morris water maze, and the passive avoidance test. It was observed that both idebenone and vitamin E improved the performance of the Aβ42-treated rats in the Y-maze and Morris water maze, but not in the passive avoidance test (451). These results suggest that quinones might be effective antioxidants to improve learning and memory deficits associated with AD. Finally, streptozotocin (STZ)-treated rats display markers of oxidative damage (increases in thiobarbituric reactive substance, GSH, protein carbonyls, and the activities of the GSH peroxidase and reductase enzymes) and a decline in the level of ATP in the hippocampus and cerebral cortex. Supplementing STZ-infused rats with coenzyme Q reversed all the aforementioned markers, indicating that ubiquinone has neuroprotective effects on cognitive impairments and oxidative damage associated with STZ toxicity (197). In a multicenter, randomized, double-blind, placebo-controlled study in AD patients, treatment with idebenone was effective in delaying memory impairments associated with AD, with no significant side effects (42). In a more recent study, treatment of AD patients with idebenone in the predementia stage resulted in short- and long-term memory improvements in 37% of the patients (427). On a concluding note, although the precise mechanism of action of ubiquinone remains unknown and it does not appear to alter learning and memory under normal physiological conditions (147), it appears to play a central role as an antioxidant molecule involved in restoring the redox balance necessary for improvement of cognitive dysfunction associated with a multitude of pathological conditions, especially AD.
5. Carotenoids
Carotenoids are phytochemicals produced in plants, with β-carotene and lycopene being the most investigated (321). Many epidemiologic studies have made the association of high dietary carotenoid intake with a lower incidence of chronic disease, but the biological mechanisms underlying such protection are not understood. There are several theories that have been put forward to explain the protective effect of carotenoids (321): (a) they can be converted to retinoids and thus acquire pro-vitamin A activity, (b) they can act as potent antioxidants, (c) they can modulate the function of lipooxygenases, and (d) they can modulate expression of genes involved in cell–cell interactions. In the interest of this review, we will focus on the role of carotenoids as antioxidants with an emphasis on their involvement in the regulation of learning and memory. The antioxidant properties of carotenoids lie in their ability to physically quench singlet oxygen and to trap peroxyl radicals. Singlet oxygen is not a free radical but it has electrons in an excited state that may adversely affect molecules containing double bonds. Carotenoids are the most effective singlet oxygen quenchers (109), which results in the formation of excited carotenoids. The excited carotenoids have the capacity to rapidly dissipate newly acquired energy through a series of rotational and vibrational interactions with the solvent, resulting in regeneration of the unexcited carotenoid that can be reused for further cycles of singlet oxygen quenching (67). Several carotenoids, with β-carotene in particular, have the capability of scavenging peroxyl radicals by the formation of a transitory carotenoid adduct radical (67). This radical is highly stable and undergoes decay to a nonradical species, thereby terminating the actions of harmful peroxyl radicals. This latter process destroys carotenoids (442). The antioxidant properties of carotenoids vary depending on the system being used to study them as well as the concentration used. For example, carotenoids can protect cells against oxidant-induced lipid peroxidation in a pro-vitamin A-independent fashion (275). In transformed thymocytes, carotenoids act as potent antioxidants when oxygen tension is low, but turn into pro-oxidants when oxygen tension is high (324). In addition, supraphysiological concentrations of carotenoids may result in pro-oxidative effects (145). These pro-oxidative effects can be prevented by the coadministration of other antioxidants such as vitamin E (323). Many clinical studies have been undertaken to assess the antioxidant ability of carotenoids and produced conflicting results. It was demonstrated that a diet deficient in carotenoids, but otherwise adequate for all other nutrients, resulted in diminished markers of antioxidant capacity in the blood (315). Conversely, supplementing the diet with β-carotene has been shown to increase plasma levels of antioxidant molecules (288). In one study using the oxygen radical absorbance capacity test, no positive role for carotenoids on plasma levels of antioxidants was demonstrated (76). Another report supported this finding by showing that there was no effect of carotenoids on the total antioxidant capacity of the plasma (58). The differences in results between these studies could be due to either different types of supplementation or different age groups and genders of the study individuals. Humans have the capacity to absorb intact carotenoids from the intestines; however, such absorption may differ depending on the type of supplementation offered (within the food as opposed to capsules). Certain forms may be more readily bioavailable than others. For example, lycopene has been shown to be better extracted from heated tomatoes as opposed to raw ones (395). Thus, special care should be put into selecting the right concentrations and form of carotenoids for each particular study setting.
Carotenoids have been investigated as an antioxidant in various pathological conditions associated with learning and memory dysfunction. One carotenoid, retinoic acid (an essential growth factor derived from vitamin A), has been extensively studied for its modulation of the signaling events involved in learning and memory. In fact, retinoic acid activates gene transcription through nuclear receptors, thereby influencing LTP and neurogenesis, particularly in the hippocampus (279, 293). This suggests a role for retinoic acid in learning and memory processes, but it appears to be distinct from the ability of carotenoids to act as antioxidants. Because this review is dedicated to the redox regulation of memory mechanisms, we will limit the discussion in this section to studies specifically addressing the antioxidant function of carotenoids.
A number of epidemiological studies have been undertaken to assess the association between carotenoid levels and either age- or disease-related declines in cognitive function. With the exception of one study, which delineated a pro-oxidant effect of vitamin A in the substantia nigra and striatum at clinical doses (103), all of the other studies showed either neutral or positive correlations with cognitive dysfunction. Engelhart et al. examined whether high plasma levels of vitamins A and E together were associated with lower prevalence of cognitive decline. They performed a cross-sectional study within another AD study and found that if a univariate model was used, high plasma vitamin A and E could be correlated to lower cognitive decline. However, when adjustments were made with respect to age, gender, and total cholesterol, the correlation weakened, and it was concluded that there was no association between plasma levels of vitamin A and E and cognitive decline (122). The same question was examined in a cohort of elderly woman and no correlation between plasma carotenoids and tocopherols and cognitive deficits was observed (206). The same group explored the effect of β-carotene supplementation on cognitive function in men and found that short-term supplementation (mean duration of 1 year) did not affect cognition, whereas long-term supplementation (mean duration of 18 years) provided cognitive benefits (156). The latter study illustrates that careful consideration should be given to every study parameter (such as treatment duration) before conclusions can be drawn.
Five additional studies have described beneficial effects of carotenoids on cognitive deficits. The interaction between carotenoids and the AD susceptibility gene apolipoprotein E was investigated and it was found that among high-functioning older persons, β-carotene supplementation offered protection from cognitive decline, even in persons with greater susceptibility evidenced by the presence of the APO-E4 allele (191). The relationship between plasma carotenoid levels and the MMSE was examined and it was found that lower levels of carotenoids correlated with cognitive impairments (7). As part of the cache county study, high antioxidant intake from food combined with supplementation with vitamin E, vitamin C, and carotene may delay cognitive decline in the elderly (438). Although this study did not dissect a specific role for carotenoids in this protection, it did suggest that carotene is potentially a beneficial antioxidant involved in protection of cognitive abilities. In addition, a significant association between higher carotenoid levels and docosahexanoic acid with higher MMSE scores has been reported, supporting a protective role for both nutrients in aging and AD-related cognitive impairments (433). Finally, the involvement of lycopene in diabetes-associated cognitive decline in rats was examined, and this particular carotenoid has a significant therapeutic potential in diabetes-induced learning and memory impairments (235).
A large body of research has centered on the interesting and widely beneficial effects of a naturally occurring carotenoid chemical called crocin. Crocin is found in the flower Crocus sativus L., more commonly known as saffron. Crocin is the ingredient primarily responsible for the color of saffron. It is a diester formed from a disaccharide (gentiobiose) and the dicarboxylic acid crocetin. Aside from being a potent antioxidant (311, 465), it also has been reported to have anticarcinogenic (1, 88), antidepressant (8), and aphrodisiac properties (184). Given its potent antioxidant capacity, a role for crocin in modulating learning and memory has been proposed. Recent behavioral and electrophysiological studies have shown a beneficial effect of crocin on ethanol-induced LTP deficits and learning and memory impairments. In several separate studies, Saito and associates have demonstrated that crocin can prevent ethanol-induced impairment of hippocampal synaptic plasticity, both in vitro in rat slices (401) and in vivo in anesthetized rats (400). They further investigated the underlying mechanism of these effects and found that crocin specifically antagonized the inhibitory effect of ethanol on NMDA receptor-mediated responses in hippocampal neurons (2). Using the same ethanol-induced impairment model, they also demonstrated that although crocin had no effects on memory registration, consolidation, or retrieval in normal mice, it did improve the ethanol-induced impairments of memory function in all of its constituents (461). In a more recent study (333), crocin counteracted delay-dependent recognition memory deficits in the normal rat, suggesting that this carotenoid can modulate storage and/or retrieval of information. It also attenuated scopolamine-induced spatial memory deficits in the radial arm maze. This study not only demonstrated the beneficial effect of crocin on learning and memory impairments induced by ethanol, but it also suggested that crocin is a compound that can modulate the mechanisms underlying normal recognition and spatial memory (333).
In conclusion, although carotenoids have well-documented antioxidant effects, there continues to be a great deal of uncertainty as to their beneficial role in the mechanisms underlying learning and memory deficits. Depending on the conditions of their use (administration, absorption, duration of administration, etc.), carotenoids appear to offer protection against learning and memory deficits associated with physiological aging as well as a multitude of pathological conditions. The mechanism responsible for the beneficial effects of carotenoids on cognitive function remains to be elucidated.
6. Melatonin
Melatonin is primarily a pineal hormone derived from its precursor serotonin. Its main function is in sleep cycle regulation. Melatonin also is a very powerful scavenger of hydroxyl radicals, making it a potent antioxidant molecule (168). Unlike vitamin C and GSH, which are only effective in aqueous phase, and vitamin E, which is only effective in lipid phase, melatonin can be functional in both phases (412). Melatonin also easily crosses cell membranes and the blood–brain barrier (360), making it a powerful candidate for protecting nuclear and mitochondrial DNA and the central nervous system from damage induced by oxidative stress (359). Many biological effects of melatonin are produced through activation of melatonin receptors (61), so it is important to distinguish its action as an antioxidant on learning and memory.
The discovery of melatonin as an antioxidant was made in 1993 (412). Melatonin is a direct scavenger of hydroxyl and peroxyl radicals. It can also neutralize superoxide, singlet oxygen, H2O2, and hypochlorous acid (335). Melatonin also inhibits peroxynitrite formation by inhibiting NOS in brain tissue (245). Melatonin differs from other antioxidants in two important aspects. First, melatonin does not undergo redox cycling; that is, once oxidized, it cannot be regenerated to its reduced form. This happens because melatonin forms stable intermediates upon reacting with free radicals, which makes it a terminal antioxidant (410). Second, the antioxidant action of melatonin involves the donation of two electrons, not one electron, thereby ensuring that melatonin does not become a free radical in the process (410). Melatonin is a much more potent antioxidant than many traditionally used antioxidants. For example, it is twice as effective as vitamin E in protecting cell membranes from lipid peroxidation (330), five times more potent than GSH in scavenging hydroxyl radicals (331), and 60 times more effective than vitamins C and E in providing DNA protection (345). Besides its own ability to scavenge a variety of free radicals, several of its metabolites are themselves potent antioxidants (361, 411). Melatonin can also boost expression and/or activity of other antioxidants, most notably GSH peroxidase (up to eightfold increase), SOD, and catalase (233, 276).
Because the brain has lower GSH concentrations than other organs (164), melatonin plays a particularly important antioxidant role in the central nervous system. In fact, melatonin receptors have been shown to play an important role in the mechanisms of learning and memory in mice (242), and melatonin has been shown to alter the cellular processes associated with memory, such as LTP.
a. Melatonin and LTP
Using rat hippocampal slices, it was shown that melatonin (100 μM) has the ability to block the induction of LTP in an NMDA receptor-independent manner (92). In addition, in studies in the suprachiasmatic nucleus, melatonin prevented LTP induction via an inhibitory effect on CaMKII autophosphorylation (139). Moreover, a specific role for the circadian clock, and therefore melatonin levels, in modulating synaptic plasticity in the hippocampus has been demonstrated (81). Another group showed that melatonin inhibits hippocampal LTP via a mechanism involving MT2-receptor-mediated regulation of the adenylyl cyclase-cAMP-PKA pathway (430). Finally, it has been reported that melatonin significantly alters synaptic transmission and LTP in area CA1, but has only modest actions in area CA3 in mouse hippocampal slices (318). Taken together, these findings suggest that melatonin inhibits hippocampal LTP.
In addition to its impact on hippocampal synaptic plasticity, melatonin was shown to prevent the induction of neocortical LTP and impair spatial performance in the radial maze (393). Neonatal rats given low doses of melatonin exhibited impaired hippocampal LTP and displayed learning and memory deficits. Melatonin also exacerbated LTP impairments as well as learning and memory deficits induced by lead (77). Finally, in a study designed to assess if dark rearing (i.e., high levels of melatonin) affects hippocampal synaptic plasticity, melatonin administered early postnatally prevents the induction of LTP (408).
There seems to be a consensus that melatonin inhibits LTP induction. This may seem surprising as it might be expected that an antioxidant should improve cognition rather than making it worse. However, these studies (77, 92, 139, 318, 393, 408, 430) were performed in the absence of excessive oxidative stress. Going back to the first part of this review, we described numerous studies indicating that ROS are important signaling molecules necessary for proper LTP induction, and therefore scavenging radicals with melatonin likely explains the detrimental effects of melatonin on synaptic plasticity.
b. Melatonin and learning and memory
Similar to what has been observed in LTP studies, behavioral studies indicate that melatonin has a detrimental effect on learning and memory performance (242, 355, 375, 466). On the other hand, in studies performed under situations of oxidative stress, the effect of melatonin on learning and memory deficits is for the most part positive. This is consistent with the idea that melatonin quenches bad ROS under stress situations to produce a positive effect on learning and memory. The studies describing the beneficial effects of melatonin and memory function can be largely classified in four major subcategories, depending on the pathological condition causing the oxidative stress-induced learning and memory deficits: Alzheimer's disease, alcohol poisoning, excitotoxicity/trauma/ischemia, and other oxidative stress conditions.
1. Alzheimer's disease
The effect of melatonin on AD-related decreases in learning and memory function has been studied using multiple animal models of the disease and various types of behavioral tests, as well as measurements of oxidative stress markers. These animal models include intracerebroventricular injections of either STZ (377, 379) or amyloid β peptides (381–383), transgenic APP695 mice (128, 144), transgenic APP/PS1 mice (314), and scopolamine-induced cognitive dysfunction (6). Behavioral tests included passive avoidance (6, 128, 379, 382), shuttle box (382), water maze (144, 253, 314, 381–383), open field activity (144, 314), radial arm water maze (314), Y maze (314), elevated plus maze (314), and circular platform (314). Oxidative stress markers included malondialdehyde (379), GSH (379, 383), lipid peroxidation (383), SOD (253, 314, 383), choline acetyltransferase activity (128), acetylcholinesterase (6), GPx (314), catalase (314), MAO (253), and ER stress-related proteins including BiP/GRP78 and CHOP/GADD153 (253). With the exception of one study (413), all studies uniformly showed that melatonin has a positive effect on memory performance and affords protection against oxidative stress-induced damage associated with AD.
2. Alcohol poisoning
It has been shown that either aged or chronic ethanol-treated mice exhibit poor retention of memory in step-down passive avoidance and in elevated plus-maze task (352). Chronic melatonin treatment reversed cognitive deficits in aged and ethanol-intoxicated mice, but failed to modulate the retention performance of young mice, both effects being consistent with the antioxidant properties of melatonin (352). In another study of the effect of melatonin supplementation on chronic ethanol-induced learning and memory dysfunctions and markers of oxidative stress (37), it was found that melatonin significantly reduced lipid peroxidation and elevated GSH levels with a concomitant improvement in learning (Morris water maze and passive avoidance) that was more substantial in older as opposed to younger rats (37). The effects of melatonin on acute ethanol-induced increases in oxidative stress (lipid peroxidation, GPx, SOD) and impairments in learning and memory (Morris water maze) have been studied. These investigators found that melatonin reversed increases in the markers of oxidative stress without a significant effect on spatial memory (148). Surprisingly, in this study melatonin alone had a positive effect on water maze performance (148).
3. Excitotoxicity/trauma/ischemia
Trauma and excitotoxi-city induce cognitive dysfunction resulting from hippocampal damage. Melatonin exhibited a protective effect against head trauma-induced hippocampal damage and spatial memory deficits in immature rats (319). It also prevented learning disabilities associated with ibotenate (a glutamate analog)-induced excitotoxicity (60). During ischemia and cerebral hypoperfusion, melatonin plays a similar protective role (100, 246), highlighting its important antioxidant protective role against learning and memory deficits induced by oxidative stress.
4. Other
Melatonin reversed oxidative stress and improved the learning and memory deficits induced by either ovariectomy (129, 130) or hyperhomocysteinemia (34, 35) in adult rats. Melatonin also reduced memory impairments and oxidative damage in mouse models of senescence (85, 384) and diabetes (421). Lastly, melatonin improved stress-related declines in learning and memory (363), as well as deficits associated with exposure to the neurotoxic industrial solvent thinner (36).
In conclusion, melatonin is a potent antioxidant that shows significant positive effects on learning and memory performance under oxidative stress conditions. Its negative effects on learning and memory under normal physiological conditions recapitulate a recurring theme in this review: ROS are important signaling molecules for synaptic plasticity, and therefore quenching balanced physiological levels of ROS may lead to detrimental rather than beneficial effects with respect to memory function. Moreover, care should be taken in selecting the proper concentration for treatment as it has been shown that blood concentrations which are 10 times normal levels can cleave heme molecules to liberate iron and induce oxidative stress (89). Even higher levels of melatonin concentrations can deplete reduced glutathione levels and promote fas-induced cell death (440).
7. Lipoic acid
Lipoic acid is an organosulfur compound that was first postulated to be an effective antioxidant because it prevented symptoms of vitamin E deficiency (334). The mechanism of action for lipoic acid to act as an antioxidant is not well understood. It has been shown that dihydrolipoic acid (the reduced form of lipoic acid) can regenerate oxidized antioxidants, such as GSH, vitamin C, and vitamin E (50, 320), thereby permitting them to enter the circulation to quench more free radicals. Lipoic acid also has been shown to possess the ability to scavenge ROS in vitro; however, there is no clear evidence that this actually occurs in vivo. Recent findings suggest that lipoic acid can boost mitochondrial membrane potential and oxygen consumption, while decreasing mitochondrial production of oxidants (280). This occurs by lipoic acid amplifying key antioxidant protective mechanisms via PPARγ coactivator-1α (PGC-1α) mediation (280).
Lipoic acid has been studied extensively as a therapeutic antioxidant to protect against the damaging effects of oxidative stress on synaptic plasticity and memory. Studies have shown that lipoic acid significantly reversed oxidative stress-induced deficits in LTP (282, 429) and learning and memory in a variety of pathological conditions, such as aging (126, 254, 291, 339), radiation poisoning (268, 269), AD (348, 377, 380), and diabetes (49). In an attempt to identify the underlying mechanism of lipoic acid protection of cognitive functions, α-lipoic acid enhances Ca2+ entry through presynaptic N- and P/Q-type channels to cause an increase in evoked glutamate release from rat cerebrocortical synaptosomes (432). This facilitation of glutamate release by α-lipoic acid was found to be due, at least in part, to activation of PKA and PKC (432). Collectively, these studies support the hypothesis that ROS are involved in the modulation of learning and memory processes.
C. Minerals
Given that they are essential cofactors for antioxidant enzymes, minerals such as selenium, zinc, and manganese play an important antioxidant role, especially in the mechanisms underlying learning and memory processes.
1. Selenium
Although it is toxic in large doses (462), selenium is an essential micronutrient for animals. In humans, selenium is a trace element nutrient that functions as cofactor for multiple enzymes, most notably GSH peroxidases and certain forms of TRX reductase found in animals and some plants (178, 365). This confers to selenium an indirect role as an antioxidant and neuroprotectant mineral. In fact, selenium has been shown to protect the brain from oxidative damage in various models of neurodegeneration. For example, rats treated with intracerebroventricular STZ are a model for oxidative damage and sporadic AD, and display severe deficits in the passive avoidance and Morris water maze tests that is accompanied by high levels of lipid peroxidation, protein carbonylation, reduced GSH, GPx, GR, and ATP in the hippocampus and cortex. Selenium, in line with its powerful antioxidant function, prevented all of the alterations induced by STZ, including the cognitive deficits and the oxidative damage (198). Selenium also has been shown to be neuroprotective and beneficial for memory deficits induced by lead poisoning (237) and psychosis, without affecting baseline behavior (260). The beneficial effects of selenium also were explored in fluoride toxicity (462) and ischemia models (158), where it was shown to enhance ROS scavenging and improve short-term memory impairments. In addition, organic selenium compounds enhanced long-term memory in a novel object recognition test (364). However, memory in the active avoidance shuttle box test was normal in rats that were selenium and vitamin E deficient (32). In a more recent study, using the same model it was shown that the absence of selenium can cause changes in synaptic function in area CA3 of the hippocampus, and inferred that it must play a role in learning and memory (435). These studies suggest that the effects of selenium as an antioxidant beneficial to learning and memory is dependent on the task, as well as the concentration used. Consistent with this idea, it was demonstrated that at higher concentration, selenium could cause toxicity (462).
2. Zinc
After iron, zinc is the second most abundant metal in humans. Zinc is an essential micronutrient for a wide variety of biological functions and has been shown to be a component of more than 1200 enzymes, either acting as a cofactor or as a structural element (137). It is actually the only metal found in all enzyme classes. In the brain, zinc is packaged into vesicles of glutamatergic neurons (53) and plays a critical role in brain excitability (165) as well as synaptic plasticity and learning (303). Similar to selenium, zinc has antioxidant functions, but not without controversy (290). Selenium is an integral component of GSH peroxidases and therefore acts as a general antioxidant, whereas zinc is an antioxidant only at specific sites (45). Although zinc is an integral component of the antioxidant enzyme SOD-1, it plays a structural role as opposed to being a cofactor. In support of this notion, zinc deficiency does not affect SOD-1 activity. On the contrary, SOD-1 activity is depressed with high zinc intake (45). The antioxidant properties of zinc stem mainly from competition with iron and copper for binding to certain proteins, thereby displacing redox-active metals. Zinc also can bind the sulfhydryl groups in proteins, thereby protecting them from oxidation (45). There is a large body of literature discussing the involvement of zinc in synaptic plasticity and memory (44, 303), but this is beyond the scope of this review. To our knowledge, none of these reports have discussed the role of zinc in learning and memory in the context of its antioxidant abilities.
3. Manganese
Manganese is an essential trace element (120) acting as a cofactor for multiple classes of enzymes, including, but not limited to, oxidoreductases, transferases, and hydrolases, most of which are involved in redox homeostasis. The best known manganese-containing enzymes are SOD-2 and arginase (406). As discussed earlier, SOD-2 is the main superoxide scavenger of mitochondria and as such is one of the most characterized antioxidant enzymes. The involvement of manganese in oxidative stress-induced synaptic plasticity and memory stems mainly from its association with SOD-2, which was described earlier in the review.
VII. Conclusions
In this review we have provided extensive evidence demonstrating a dual role for ROS in the brain (Fig. 13). Under physiological conditions, ROS act as essential signaling molecules, necessary for the proper formation of learning and memory processes. However, during aging, ischemia, trauma, or neurodegenerative diseases, the levels of ROS increase to levels higher than the antioxidant machinery of the cells can handle, and therefore their beneficial signaling role becomes outweighed by the ambiance of oxidative damage that they create. We also have reviewed evidence showing that the specific species of ROS act differently in various systems. For example, both cytosolic and extracellular superoxide have been shown to be involved in synaptic plasticity and memory. Mitochondrial superoxide, however, failed to show any involvement in age-dependent synaptic plasticity and memory dysfunction, perhaps due to limited diffusion of mitochondrial superoxide across the mitochondrial membrane. Mitochondrial superoxide proved to be a key player in conditions of extreme oxidative stress such as occurring during AD. This is not surprising, in light of the wealth of literature documenting mitochondrial dysfunction (and hence increased probability for leakage of mitochondrial free radicals through damaged membranes) as an integral part of AD (Fig. 14).
FIG. 13.
Dual roles for ROS in the brain. Under physiological conditions, ROS act as essential signaling molecules, necessary for the proper synaptic plasticity and normal memory. However, during aging, ischemia, trauma, and neurodegenerative diseases, the levels of ROS increase to levels higher than the antioxidant machinery of the cells can handle. Thus, the beneficial roles of ROS are overwhelmed by the ambiance of oxidative damage that ROS create.
FIG. 14.
Why does mitochondrial superoxide impair learning and memory during AD but not during normal aging? In contrast to cytosolic and extracellular superoxide, which contribute to normal synaptic plasticity and memory, mitochondrial superoxide does not appear to impact either normal synaptic plasticity and memory or age-dependent impairments in synaptic plasticity and memory. Perhaps this is due to limited diffusion of mitochondrial superoxide across the mitochondrial membrane. During AD, mitochondrial dysfunction is prevalent, hence increasing the probability for leakage of mitochondrial-produced free radicals through damaged membranes. Thus, mitochondrial superoxide plays a critical role in extreme oxidative stress that occurs during neurodegenerative diseases such AD. AD, Alzheimer's disease.
Abbreviations Used
- AD
Alzheimer's disease
- ATP
adenosine triphosphate
- BH4
tetrahydrobiopterin
- BSO
buthionine sulfoximide
- CA
cornu ammonis
- Ca2+
calcium
- cAMP
cyclic adenosine monophosphate
- CREB
cAMP response element binding protein
- EC-SOD
extracellular superoxide dismutase
- ERK
extracellular signal regulated kinase
- fEPSP
field excitatory postsynaptic potential
- GDI
guanine nucleotide dissociation inhibitor
- GDP
guanosine diphosphate
- GPx
glutathione peroxidase
- GR
glutathione reductase
- GRX
glutaredoxin
- GSH
glutathione
- GSSG
oxidized glutathione
- H2O2
hydrogen peroxide
- HFS
high-frequency stimulation
- L-NAME
N-ω-nitro-l-arginine methyl ester
- L-NMMA
N-ω-monomethyl l-arginine
- L-NNA
N-ω-nitro-l-arginine
- LTP
long-term potentiation
- MAO
monoamine oxidase
- mGluR
metabotrobic glutamate receptor
- MMSE
mini mental state examination
- Mn
manganese
- MT
metallothionein
- NMDA
N-methyl-d-aspartate
- NO
nitric oxide
- NOS
NO synthase
- Nox
NADPH oxidase
- Nrf2
nuclear factor E2-related factor 2
- ODS
osteogenic disorder Shionogi
- PBN
α-phenyl-N-tert-butyl nitrone
- PD
Parkinson's disease
- PKA
protein kinase A
- PRX
peroxiredoxin
- Rac
ras-associated C3 botulinum toxin substrate
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- STZ
streptozotocin
- TBI
traumatic brain injury
- TRX
thioredoxin
Acknowledgments
C.A.M. is supported by a fellowship of the American Health Assistance Foundation, Alzheimer's Disease Research. This work also was supported by NIH grant NS034007 and Alzheimer's Association Investigator Initiated Research Grant 09-131756 (to E.K.). The authors apologize to their fellow scientists whose work could not be cited due to space limitations.
References
- 1.Abdullaev JF. Caballero-Ortega H. Riveron-Negrete L. Pereda-Miranda R. Rivera-Luna R. Manuel HJ. Perez-Lopez I. Espinosa-Aguirre JJ. In vitro evaluation of the chemopreventive potential of saffron. Rev Invest Clin. 2002;54:430–436. [PubMed] [Google Scholar]
- 2.Abe K. Sugiura M. Shoyama Y. Saito H. Crocin antagonizes ethanol inhibition of NMDA receptor-mediated responses in rat hippocampal neurons. Brain Res. 1998;787:132–138. doi: 10.1016/s0006-8993(97)01505-9. [DOI] [PubMed] [Google Scholar]
- 3.Abraham WC. Williams JM. LTP maintenance and its protein synthesis-dependence. Neurobiol Learn Mem. 2008;89:260–268. doi: 10.1016/j.nlm.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Abramov AY. Jacobson J. Wientjes F. Hothersall J. Canevari L. Duchen MR. Expression and modulation of an NADPH oxidase in mammalian astrocytes. J Neurosci. 2005;25:9176–9184. doi: 10.1523/JNEUROSCI.1632-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adam K. Lack of effect on mental efficiency of extra vitamin C. Am J Clin Nutr. 1981;34:1712–1716. doi: 10.1093/ajcn/34.9.1712. [DOI] [PubMed] [Google Scholar]
- 6.Agrawal R. Tyagi E. Shukla R. Nath C. Effect of insulin and melatonin on acetylcholinesterase activity in the brain of amnesic mice. Behav Brain Res. 2008;189:381–386. doi: 10.1016/j.bbr.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 7.Akbaraly NT. Faure H. Gourlet V. Favier A. Berr C. Plasma carotenoid levels and cognitive performance in an elderly population: results of the EVA Study. J Gerontol A Biol Sci Med Sci. 2007;62:308–316. doi: 10.1093/gerona/62.3.308. [DOI] [PubMed] [Google Scholar]
- 8.Akhondzadeh S. Fallah-Pour H. Afkham K. Jamshidi AH. Khalighi-Cigaroudi F. Comparison of Crocus sativus L. and imipramine in the treatment of mild to moderate depression: a pilot double-blind randomized trial [ISRCTN45683816] BMC Complement Altern Med. 2004;4:12. doi: 10.1186/1472-6882-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akterin S. Cowburn RF. Miranda-Vizuete A. Jimenez A. Bogdanovic N. Winblad B. Cedazo-Minguez A. Involvement of glutaredoxin-1 and thioredoxin-1 in beta-amyloid toxicity and Alzheimer's disease. Cell Death Differ. 2006;13:1454–1465. doi: 10.1038/sj.cdd.4401818. [DOI] [PubMed] [Google Scholar]
- 10.Alam ZI. Daniel SE. Lees AJ. Marsden DC. Jenner P. Halliwell B. A generalised increase in protein carbonyls in the brain in Parkinson's but not incidental Lewy body disease. J Neurochem. 1997;69:1326–1329. doi: 10.1046/j.1471-4159.1997.69031326.x. [DOI] [PubMed] [Google Scholar]
- 11.Almaguer-Melian W. Cruz-Aguado R. Bergado JA. Synaptic plasticity is impaired in rats with a low glutathione content. Synapse. 2000;38:369–374. doi: 10.1002/1098-2396(20001215)38:4<369::AID-SYN1>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 12.Ames BN. Shigenaga MK. Hagen TM. Oxidants, antioxidants, and the degenerative diseases of aging. Proc Natl Acad Sci USA. 1993;90:7915–7922. doi: 10.1073/pnas.90.17.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amiry-Moghaddam M. Lindland H. Zelenin S. Roberg BA. Gundersen BB. Petersen P. Rinvik E. Torgner IA. Ottersen OP. Brain mitochondria contain aquaporin water channels: evidence for the expression of a short AQP9 isoform in the inner mitochondrial membrane. FASEB J. 2005;19:1459–1467. doi: 10.1096/fj.04-3515com. [DOI] [PubMed] [Google Scholar]
- 14.Amiry-Moghaddam M. Ottersen OP. The molecular basis of water transport in the brain. Nat Rev Neurosci. 2003;4:991–1001. doi: 10.1038/nrn1252. [DOI] [PubMed] [Google Scholar]
- 15.Anandatheerthavarada HK. Biswas G. Robin MA. Avadhani NG. Mitochondrial targeting and a novel transmembrane arrest of Alzheimer's amyloid precursor protein impairs mitochondrial function in neuronal cells. J Cell Biol. 2003;161:41–54. doi: 10.1083/jcb.200207030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anantharaman M. Tangpong J. Keller JN. Murphy MP. Markesbery WR. Kiningham KK. St. Clair DK. Beta-amyloid mediated nitration of manganese superoxide dismutase: implication for oxidative stress in a APPNLH/NLH X PS-1P264L/P264L double knock-in mouse model of Alzheimer's disease. Am J Pathol. 2006;168:1608–1618. doi: 10.2353/ajpath.2006.051223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Angelova P. Muller W. Oxidative modulation of the transient potassium current IA by intracellular arachidonic acid in rat CA1 pyramidal neurons. Eur J Neurosci. 2006;23:2375–2384. doi: 10.1111/j.1460-9568.2006.04767.x. [DOI] [PubMed] [Google Scholar]
- 18.Anwyl R. Metabotropic glutamate receptor-dependent long-term potentiation. Neuropharmacology. 2009;56:735–740. doi: 10.1016/j.neuropharm.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 19.Aoyama K. Watabe M. Nakaki T. Regulation of neuronal glutathione synthesis. J Pharmacol Sci. 2008;108:227–238. doi: 10.1254/jphs.08r01cr. [DOI] [PubMed] [Google Scholar]
- 20.Aragon CM. Spivak K. Amit Z. Blockade of ethanol induced conditioned taste aversion by 3-amino-1,2,4-triazole: evidence for catalase mediated synthesis of acetaldehyde in rat brain. Life Sci. 1985;37:2077–2084. doi: 10.1016/0024-3205(85)90579-x. [DOI] [PubMed] [Google Scholar]
- 21.Artola A. Kamal A. Ramakers GM. Biessels GJ. Gispen WH. Diabetes mellitus concomitantly facilitates the induction of long-term depression and inhibits that of long-term potentiation in hippocampus. Eur J Neurosci. 2005;22:169–178. doi: 10.1111/j.1460-9568.2005.04205.x. [DOI] [PubMed] [Google Scholar]
- 22.Auerbach JM. Segal M. Peroxide modulation of slow onset potentiation in rat hippocampus. J Neurosci. 1997;17:8695–8701. doi: 10.1523/JNEUROSCI.17-22-08695.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Awasthi D. Church DF. Torbati D. Carey ME. Pryor WA. Oxidative stress following traumatic brain injury in rats. Surg Neurol. 1997;47:575–581. doi: 10.1016/s0090-3019(96)00461-2. [DOI] [PubMed] [Google Scholar]
- 24.Babior BM. NADPH oxidase. Curr Opin Immunol. 2004;16:42–47. doi: 10.1016/j.coi.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 25.Badaut J. Petit JM. Brunet JF. Magistretti PJ. Charriaut-Marlangue C. Regli L. Distribution of Aquaporin 9 in the adult rat brain: preferential expression in catecholaminergic neurons and in glial cells. Neuroscience. 2004;128:27–38. doi: 10.1016/j.neuroscience.2004.05.042. [DOI] [PubMed] [Google Scholar]
- 26.Ballatori N. Krance SM. Notenboom S. Shi S. Tieu K. Hammond CL. Glutathione dysregulation and the etiology and progression of human diseases. Biol Chem. 2009;390:191–214. doi: 10.1515/BC.2009.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Banfi B. Malgrange B. Knisz J. Steger K. Dubois-Dauphin M. Krause KH. NOX3, a superoxide-generating NADPH oxidase of the inner ear. J Biol Chem. 2004;279:46065–46072. doi: 10.1074/jbc.M403046200. [DOI] [PubMed] [Google Scholar]
- 28.Barbelivien A. Nyman L. Haapalinna A. Sirvio J. Inhibition of MAO-A activity enhances behavioural activity of rats assessed using water maze and open arena tasks. Pharmacol Toxicol. 2001;88:304–312. [PubMed] [Google Scholar]
- 29.Barhwal K. Hota SK. Jain V. Prasad D. Singh SB. Ilavazhagan G. Acetyl-l-carnitine (ALCAR) prevents hypobaric hypoxia-induced spatial memory impairment through extracellular related kinase-mediated nuclear factor erythroid 2-related factor 2 phosphorylation. Neuroscience. 2009;161:501–514. doi: 10.1016/j.neuroscience.2009.02.086. [DOI] [PubMed] [Google Scholar]
- 30.Barnes CA. Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. J Comp Physiol Psychol. 1979;93:74–104. doi: 10.1037/h0077579. [DOI] [PubMed] [Google Scholar]
- 31.Barnes CA. Long-term potentiation and the ageing brain. Philos Trans R Soc Lond B Biol Sci. 2003;358:765–772. doi: 10.1098/rstb.2002.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bastug M. Ayhan S. Turan B. The effect of altered selenium and vitamin E nutritional status on learning and memory of third-generation rats. Biol Trace Elem Res. 1998;64:151–160. doi: 10.1007/BF02783331. [DOI] [PubMed] [Google Scholar]
- 33.Baudier J. Deloulme JC. Van Dorsselaer A. Black D. Matthes HW. Purification and characterization of a brain-specific protein kinase C substrate, neurogranin (p17). Identification of a consensus amino acid sequence between neurogranin and neuromodulin (GAP43) that corresponds to the protein kinase C phosphorylation site and the calmodulin-binding domain. J Biol Chem. 1991;266:229–237. [PubMed] [Google Scholar]
- 34.Baydas G. Koz ST. Tuzcu M. Nedzvetsky VS. Melatonin prevents gestational hyperhomocysteinemia-associated alterations in neurobehavioral developments in rats. J Pineal Res. 2008;44:181–188. doi: 10.1111/j.1600-079X.2007.00506.x. [DOI] [PubMed] [Google Scholar]
- 35.Baydas G. Ozer M. Yasar A. Tuzcu M. Koz ST. Melatonin improves learning and memory performances impaired by hyperhomocysteinemia in rats. Brain Res. 2005;1046:187–194. doi: 10.1016/j.brainres.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 36.Baydas G. Ozveren F. Akdemir I. Tuzcu M. Yasar A. Learning and memory deficits in rats induced by chronic thinner exposure are reversed by melatonin. J Pineal Res. 2005;39:50–56. doi: 10.1111/j.1600-079X.2005.00212.x. [DOI] [PubMed] [Google Scholar]
- 37.Baydas G. Yasar A. Tuzcu M. Comparison of the impact of melatonin on chronic ethanol-induced learning and memory impairment between young and aged rats. J Pineal Res. 2005;39:346–352. doi: 10.1111/j.1600-079X.2005.00257.x. [DOI] [PubMed] [Google Scholar]
- 38.Beal MF. Aging, energy, and oxidative stress in neurodegenerative diseases. Ann Neurol. 1995;38:357–366. doi: 10.1002/ana.410380304. [DOI] [PubMed] [Google Scholar]
- 39.Beal MF. Coenzyme Q10 as a possible treatment for neurodegenerative diseases. Free Radic Res. 2002;36:455–460. doi: 10.1080/10715760290021315. [DOI] [PubMed] [Google Scholar]
- 40.Behl C. Davis JB. Lesley R. Schubert D. Hydrogen peroxide mediates amyloid beta protein toxicity. Cell. 1994;77:817–827. doi: 10.1016/0092-8674(94)90131-7. [DOI] [PubMed] [Google Scholar]
- 41.Beni SM. Tsenter J. Alexandrovich AG. Galron-Krool N. Barzilai A. Kohen R. Grigoriadis N. Simeonidou C. Shohami E. CuZn-SOD deficiency, rather than overexpression, is associated with enhanced recovery and attenuated activation of NF-kappaB after brain trauma in mice. J Cereb Blood Flow Metab. 2006;26:478–490. doi: 10.1038/sj.jcbfm.9600209. [DOI] [PubMed] [Google Scholar]
- 42.Bergamasco B. Scarzella L. La Commare P. Idebenone, a new drug in the treatment of cognitive impairment in patients with dementia of the Alzheimer type. Funct Neurol. 1994;9:161–168. [PubMed] [Google Scholar]
- 43.Bernabeu R. Princ F. de Stein ML. Fin C. Juknat AA. Batile A. Izquierdo I. Medina JH. Evidence for the involvement of hippocampal CO production in the acquisition and consolidation of inhibitory avoidance learning. Neuroreport. 1995;6:516–518. doi: 10.1097/00001756-199502000-00027. [DOI] [PubMed] [Google Scholar]
- 44.Bertoni-Freddari C. Mocchegiani E. Malavolta M. Casoli T. Di Stefano G. Fattoretti P. Synaptic and mitochondrial physiopathologic changes in the aging nervous system and the role of zinc ion homeostasis. Mech Ageing Dev. 2006;127:590–596. doi: 10.1016/j.mad.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 45.Bettger WJ. Zinc and selenium, site-specific versus general antioxidation. Can J Physiol Pharmacol. 1993;71:721–724. doi: 10.1139/y93-108. [DOI] [PubMed] [Google Scholar]
- 46.Betzen C. White R. Zehendner CM. Pietrowski E. Bender B. Luhmann HJ. Kuhlmann CR. Oxidative stress upregulates the NMDA receptor on cerebrovascular endothelium. Free Radic Biol Med. 2009;47:1212–1220. doi: 10.1016/j.freeradbiomed.2009.07.034. [DOI] [PubMed] [Google Scholar]
- 47.Bianchi A. Salomone S. Caraci F. Pizza V. Bernardini R. D'Amato CC. Role of magnesium, coenzyme Q10, riboflavin, and vitamin B12 in migraine prophylaxis. Vitam Horm. 2004;69:297–312. doi: 10.1016/S0083-6729(04)69011-X. [DOI] [PubMed] [Google Scholar]
- 48.Bienert GP. Moller AL. Kristiansen KA. Schulz A. Moller IM. Schjoerring JK. Jahn TP. Specific aquaporins facilitate the diffusion of hydrogen peroxide across membranes. J Biol Chem. 2007;282:1183–1192. doi: 10.1074/jbc.M603761200. [DOI] [PubMed] [Google Scholar]
- 49.Biessels GJ. Smale S. Duis SE. Kamal A. Gispen WH. The effect of gamma-linolenic acid-alpha-lipoic acid on functional deficits in the peripheral and central nervous system of streptozotocin-diabetic rats. J Neurol Sci. 2001;182:99–106. doi: 10.1016/s0022-510x(00)00456-1. [DOI] [PubMed] [Google Scholar]
- 50.Biewenga GP. Haenen GR. Bast A. The pharmacology of the antioxidant lipoic acid. Gen Pharmacol. 1997;29:315–331. doi: 10.1016/s0306-3623(96)00474-0. [DOI] [PubMed] [Google Scholar]
- 51.Bindokas VP. Jordan J. Lee CC. Miller RJ. Superoxide production in rat hippocampal neurons: selective imaging with hydroethidine. J Neurosci. 1996;16:1324–1336. doi: 10.1523/JNEUROSCI.16-04-01324.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bing O. Grundemar L. Ny L. Moller C. Heilig M. Modulation of carbon monoxide production and enhanced spatial learning by tin protoporphyrin. Neuroreport. 1995;6:1369–1372. doi: 10.1097/00001756-199507100-00002. [DOI] [PubMed] [Google Scholar]
- 53.Bitanihirwe BK. Cunningham MG. Zinc: the brain's dark horse. Synapse. 2009;63:1029–1049. doi: 10.1002/syn.20683. [DOI] [PubMed] [Google Scholar]
- 54.Bliss TV. Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 55.Blundon JA. Zakharenko SS. Dissecting the components of long-term potentiation. Neuroscientist. 2008;14:598–608. doi: 10.1177/1073858408320643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bohme GA. Bon C. Lemaire M. Reibaud M. Piot O. Stutzmann JM. Doble A. Blanchard JC. Altered synaptic plasticity and memory formation in nitric oxide synthase inhibitor-treated rats. Proc Natl Acad Sci USA. 1993;90:9191–9194. doi: 10.1073/pnas.90.19.9191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bolshakov VY. Siegelbaum SA. Postsynaptic induction and presynaptic expression of hippocampal long-term depression. Science. 1994;264:1148–1152. doi: 10.1126/science.7909958. [DOI] [PubMed] [Google Scholar]
- 58.Borel P. Grolier P. Boirie Y. Simonet L. Verdier E. Rochette Y. Alexandre-Gouabau MC. Beaufrere B. Lairon D. Azais-Braesco V. Oxidative stress status and antioxidant status are apparently not related to carotenoid status in healthy subjects. J Lab Clin Med. 1998;132:61–66. doi: 10.1016/s0022-2143(98)90026-9. [DOI] [PubMed] [Google Scholar]
- 59.Bossy-Wetzel E. Schwarzenbacher R. Lipton SA. Molecular pathways to neurodegeneration. Nat Med 10. 2004;Suppl:S2–S9. doi: 10.1038/nm1067. [DOI] [PubMed] [Google Scholar]
- 60.Bouslama M. Renaud J. Olivier P. Fontaine RH. Matrot B. Gressens P. Gallego J. Melatonin prevents learning disorders in brain-lesioned newborn mice. Neuroscience. 2007;150:712–719. doi: 10.1016/j.neuroscience.2007.09.030. [DOI] [PubMed] [Google Scholar]
- 61.Boutin JA. Audinot V. Ferry G. Delagrange P. Molecular tools to study melatonin pathways and actions. Trends Pharmacol Sci. 2005;26:412–419. doi: 10.1016/j.tips.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 62.Brechard S. Tschirhart EJ. Regulation of superoxide production in neutrophils: role of calcium influx. J Leukoc Biol. 2008;84:1223–1237. doi: 10.1189/jlb.0807553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brennan PA. Kishimoto J. Local inhibition of nitric oxide synthase activity in the accessory olfactory bulb does not prevent the formation of an olfactory memory in mice. Brain Res. 1993;619:306–312. doi: 10.1016/0006-8993(93)91625-3. [DOI] [PubMed] [Google Scholar]
- 64.Brigelius-Flohe R. Tissue-specific functions of individual glutathione peroxidases. Free Radic Biol Med. 1999;27:951–965. doi: 10.1016/s0891-5849(99)00173-2. [DOI] [PubMed] [Google Scholar]
- 65.Bruce AJ. Malfroy B. Baudry M. beta-Amyloid toxicity in organotypic hippocampal cultures: protection by EUK-8, a synthetic catalytic free radical scavenger. Proc Natl Acad Sci USA. 1996;93:2312–2316. doi: 10.1073/pnas.93.6.2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brugge K. Nichols S. Saitoh T. Trauner D. Correlations of glutathione peroxidase activity with memory impairment in adults with Down syndrome. Biol Psychiatry. 1999;46:1682–1689. doi: 10.1016/s0006-3223(99)00026-8. [DOI] [PubMed] [Google Scholar]
- 67.Burton GW. Ingold KU. Beta-carotene: an unusual type of lipid antioxidant. Science. 1984;224:569–573. doi: 10.1126/science.6710156. [DOI] [PubMed] [Google Scholar]
- 68.Butterfield DA. Griffin S. Munch G. Pasinetti GM. Amyloid beta-peptide and amyloid pathology are central to the oxidative stress and inflammatory cascades under which Alzheimer's disease brain exists. J Alzheimers Dis. 2002;4:193–201. doi: 10.3233/jad-2002-4309. [DOI] [PubMed] [Google Scholar]
- 69.Butterfield DA. Kanski J. Brain protein oxidation in age-related neurodegenerative disorders that are associated with aggregated proteins. Mech Ageing Dev. 2001;122:945–962. doi: 10.1016/s0047-6374(01)00249-4. [DOI] [PubMed] [Google Scholar]
- 70.Cabungcal JH. Preissmann D. Delseth C. Cuenod M. Do KQ. Schenk F. Transitory glutathione deficit during brain development induces cognitive impairment in juvenile and adult rats: relevance to schizophrenia. Neurobiol Dis. 2007;26:634–645. doi: 10.1016/j.nbd.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 71.Cai F. Wang F. Lin FK. Liu C. Ma LQ. Liu J. Wu WN. Wang W. Wang JH. Chen JG. Redox modulation of long-term potentiation in the hippocampus via regulation of the glycogen synthase kinase-3beta pathway. Free Radic Biol Med. 2008;45:964–970. doi: 10.1016/j.freeradbiomed.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 72.Calabresi P. Pisani A. Mercuri NB. Bernardi G. Long-term potentiation in the striatum is unmasked by removing the voltage-dependent magnesium block of NMDA receptor channels. Eur J Neurosci. 1992;4:929–935. doi: 10.1111/j.1460-9568.1992.tb00119.x. [DOI] [PubMed] [Google Scholar]
- 73.Calixto AV. Duarte FS. Duzzioni M. Nascimento Hackl LP. Faria MS. De Lima TC. Role of ventral hippocampal nitric oxide/cGMP pathway in anxiety-related behaviors in rats submitted to the elevated T-maze. Behav Brain Res. 2010;207:112–117. doi: 10.1016/j.bbr.2009.09.037. [DOI] [PubMed] [Google Scholar]
- 74.Callio J. Oury TD. Chu CT. Manganese superoxide dismutase protects against 6-hydroxydopamine injury in mouse brains. J Biol Chem. 2005;280:18536–18542. doi: 10.1074/jbc.M413224200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Camara AK. Aldakkak M. Heisner JS. Rhodes SS. Riess ML. An J. Heinen A. Stowe DF. ROS scavenging before 27 degrees C ischemia protects hearts and reduces mitochondrial ROS, Ca2+ overload, and changes in redox state. Am J Physiol Cell Physiol. 2007;292:C2021–C2031. doi: 10.1152/ajpcell.00231.2006. [DOI] [PubMed] [Google Scholar]
- 76.Cao G. Russell RM. Lischner N. Prior RL. Serum antioxidant capacity is increased by consumption of strawberries, spinach, red wine or vitamin C in elderly women. J Nutr. 1998;128:2383–2390. doi: 10.1093/jn/128.12.2383. [DOI] [PubMed] [Google Scholar]
- 77.Cao XJ. Wang M. Chen WH. Zhu DM. She JQ. Ruan DY. Effects of chronic administration of melatonin on spatial learning ability and long-term potentiation in lead-exposed and control rats. Biomed Environ Sci. 2009;22:70–75. doi: 10.1016/S0895-3988(09)60025-8. [DOI] [PubMed] [Google Scholar]
- 78.Carney JM. Starke-Reed PE. Oliver CN. Landum RW. Cheng MS. Wu JF. Floyd RA. Reversal of age-related increase in brain protein oxidation, decrease in enzyme activity, and loss in temporal and spatial memory by chronic administration of the spin-trapping compound N-tert-butyl-alpha-phenylnitrone. Proc Natl Acad Sci USA. 1991;88:3633–3636. doi: 10.1073/pnas.88.9.3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chae HZ. Kim IH. Kim K. Rhee SG. Cloning, sequencing, and mutation of thiol-specific antioxidant gene of Saccharomyces cerevisiae. J Biol Chem. 1993;268:16815–16821. [PubMed] [Google Scholar]
- 80.Chapman PF. Atkins CM. Allen MT. Haley JE. Steinmetz JE. Inhibition of nitric oxide synthesis impairs two different forms of learning. Neuroreport. 1992;3:567–570. doi: 10.1097/00001756-199207000-00005. [DOI] [PubMed] [Google Scholar]
- 81.Chaudhury D. Wang LM. Colwell CS. Circadian regulation of hippocampal long-term potentiation. J Biol Rhythms. 2005;20:225–236. doi: 10.1177/0748730405276352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chelikani P. Fita I. Loewen PC. Diversity of structures and properties among catalases. Cell Mol Life Sci. 2004;61:192–208. doi: 10.1007/s00018-003-3206-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chen Q. Powell DW. Rane MJ. Singh S. Butt W. Klein JB. McLeish KR. Akt phosphorylates p47phox and mediates respiratory burst activity in human neutrophils. J Immunol. 2003;170:5302–5308. doi: 10.4049/jimmunol.170.10.5302. [DOI] [PubMed] [Google Scholar]
- 84.Chen SJ. Sweatt JD. Klann E. Enhanced phosphorylation of the postsynaptic protein kinase C substrate RC3/neurogranin during long-term potentiation. Brain Res. 1997;749:181–187. doi: 10.1016/s0006-8993(96)01159-6. [DOI] [PubMed] [Google Scholar]
- 85.Cheng S. Ma C. Qu H. Fan W. Pang J. He H. Differential effects of melatonin on hippocampal neurodegeneration in different aged accelerated senescence prone mouse-8. Neuro Endocrinol Lett. 2008;29:91–99. [PubMed] [Google Scholar]
- 86.Chiu CS. Deng JS. Hsieh MT. Fan MJ. Lee MM. Chueh FS. Han CK. Lin YC. Peng WH. Yam (Dioscorea pseudojaponica Yamamoto) ameliorates cognition deficit and attenuates oxidative damage in senescent mice induced by D-galactose. Am J Chin Med. 2009;37:889–902. doi: 10.1142/S0192415X09007296. [DOI] [PubMed] [Google Scholar]
- 87.Christensen H. What cognitive changes can be expected with normal ageing? Aust NZ J Psychiatry. 2001;35:768–775. doi: 10.1046/j.1440-1614.2001.00966.x. [DOI] [PubMed] [Google Scholar]
- 88.Chryssanthi DG. Lamari FN. Iatrou G. Pylara A. Karamanos NK. Cordopatis P. Inhibition of breast cancer cell proliferation by style constituents of different Crocus species. Anticancer Res. 2007;27:357–362. [PubMed] [Google Scholar]
- 89.Clapp-Lilly KL. Smith MA. Perry G. Harris PL. Zhu X. Duffy LK. Melatonin acts as antioxidant and pro-oxidant in an organotypic slice culture model of Alzheimer's disease. Neuroreport. 2001;12:1277–1280. doi: 10.1097/00001756-200105080-00044. [DOI] [PubMed] [Google Scholar]
- 90.Clausen A. Doctrow S. Baudry M. Prevention of cognitive deficits and brain oxidative stress with superoxide dismutase/catalase mimetics in aged mice. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Clausen F. Marklund N. Lewen A. Hillered L. The nitrone free radical scavenger NXY-059 is neuroprotective when administered after traumatic brain injury in the rat. J Neurotrauma. 2008;25:1449–1457. doi: 10.1089/neu.2008.0585. [DOI] [PubMed] [Google Scholar]
- 92.Collins DR. Davies SN. Melatonin blocks the induction of long-term potentiation in an N-methyl-d-aspartate independent manner. Brain Res. 1997;767:162–165. doi: 10.1016/s0006-8993(97)00733-6. [DOI] [PubMed] [Google Scholar]
- 93.Crofts AR. Barquera B. Gennis RB. Kuras R. Guergova-Kuras M. Berry EA. Mechanism of ubiquinol oxidation by the bc(1) complex: different domains of the quinol binding pocket and their role in the mechanism and binding of inhibitors. Biochemistry. 1999;38:15807–15826. doi: 10.1021/bi990962m. [DOI] [PubMed] [Google Scholar]
- 94.Cruz-Aguado R. Almaguer-Melian W. Diaz CM. Lorigados L. Bergado J. Behavioral and biochemical effects of glutathione depletion in the rat brain. Brain Res Bull. 2001;55:327–333. doi: 10.1016/s0361-9230(01)00484-1. [DOI] [PubMed] [Google Scholar]
- 95.Cutajar MC. Edwards TM. Evidence for the role of endogenous carbon monoxide in memory processing. J Cogn Neurosci. 2007;19:557–562. doi: 10.1162/jocn.2007.19.4.557. [DOI] [PubMed] [Google Scholar]
- 96.Cutajar MC. Edwards TM. Ng KT. Inhibition of endogenous carbon monoxide production induces transient retention losses in the day-old chick when trained using a single trial passive avoidance learning task. Neurobiol Learn Mem. 2005;83:243–250. doi: 10.1016/j.nlm.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 97.Dalrymple-Alford JC. Jamieson CF. Donaldson IM. Effects of selegiline (deprenyl) on cognition in early Parkinson's disease. Clin Neuropharmacol. 1995;18:348–359. doi: 10.1097/00002826-199508000-00007. [DOI] [PubMed] [Google Scholar]
- 98.Dang PM. Fontayne A. Hakim J. El Benna J. Perianin A. Protein kinase C zeta phosphorylates a subset of selective sites of the NADPH oxidase component p47phox and participates in formyl peptide-mediated neutrophil respiratory burst. J Immunol. 2001;166:1206–1213. doi: 10.4049/jimmunol.166.2.1206. [DOI] [PubMed] [Google Scholar]
- 99.Dash PK. Zhao J. Orsi SA. Zhang M. Moore AN. Sulforaphane improves cognitive function administered following traumatic brain injury. Neurosci Lett. 2009;460:103–107. doi: 10.1016/j.neulet.2009.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.De Butte M. Fortin T. Pappas BA. Pinealectomy: behavioral and neuropathological consequences in a chronic cerebral hypoperfusion model. Neurobiol Aging. 2002;23:309–317. doi: 10.1016/s0197-4580(01)00277-9. [DOI] [PubMed] [Google Scholar]
- 101.de Lima MN. Laranja DC. Caldana F. Bromberg E. Roesler R. Schroder N. Reversal of age-related deficits in object recognition memory in rats with l-deprenyl. Exp Gerontol. 2005;40:506–511. doi: 10.1016/j.exger.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 102.de Lima MN. Laranja DC. Caldana F. Grazziotin MM. Garcia VA. Dal Pizzol F. Bromberg E. Schroder N. Selegiline protects against recognition memory impairment induced by neonatal iron treatment. Exp Neurol. 2005;196:177–183. doi: 10.1016/j.expneurol.2005.07.017. [DOI] [PubMed] [Google Scholar]
- 103.de Oliveira MR. Oliveira MW. Behr GA. Hoff ML. da Rocha RF. Moreira JC. Evaluation of the effects of vitamin A supplementation on adult rat substantia nigra and striatum redox and bioenergetic states: mitochondrial impairment, increased 3-nitrotyrosine and alpha-synuclein, but decreased D2 receptor contents. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:353–362. doi: 10.1016/j.pnpbp.2008.12.020. [DOI] [PubMed] [Google Scholar]
- 104.Dean O. Bush AI. Berk M. Copolov DL. van den BM. Glutathione depletion in the brain disrupts short-term spatial memory in the Y-maze in rats and mice. Behav Brain Res. 2009;198:258–262. doi: 10.1016/j.bbr.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 105.Dexter DT. Carayon A. Javoy-Agid F. Agid Y. Wells FR. Daniel SE. Lees AJ. Jenner P. Marsden CD. Alterations in the levels of iron, ferritin and other trace metals in Parkinson's disease and other neurodegenerative diseases affecting the basal ganglia. Brain. 1991;114(Pt 4):1953–1975. doi: 10.1093/brain/114.4.1953. [DOI] [PubMed] [Google Scholar]
- 106.Dexter DT. Carayon A. Vidailhet M. Ruberg M. Agid F. Agid Y. Lees AJ. Wells FR. Jenner P. Marsden CD. Decreased ferritin levels in brain in Parkinson's disease. J Neurochem. 1990;55:16–20. doi: 10.1111/j.1471-4159.1990.tb08814.x. [DOI] [PubMed] [Google Scholar]
- 107.Dexter DT. Carter CJ. Wells FR. Javoy-Agid F. Agid Y. Lees A. Jenner P. Marsden CD. Basal lipid peroxidation in substantia nigra is increased in Parkinson's disease. J Neurochem. 1989;52:381–389. doi: 10.1111/j.1471-4159.1989.tb09133.x. [DOI] [PubMed] [Google Scholar]
- 108.Dexter DT. Holley AE. Flitter WD. Slater TF. Wells FR. Daniel SE. Lees AJ. Jenner P. Marsden CD. Increased levels of lipid hydroperoxides in the parkinsonian substantia nigra: an HPLC and ESR study. Mov Disord. 1994;9:92–97. doi: 10.1002/mds.870090115. [DOI] [PubMed] [Google Scholar]
- 109.Di Mascio P. Kaiser S. Sies H. Lycopene as the most efficient biological carotenoid singlet oxygen quencher. Arch Biochem Biophys. 1989;274:532–538. doi: 10.1016/0003-9861(89)90467-0. [DOI] [PubMed] [Google Scholar]
- 110.Donahue AN. Aschner M. Lash LH. Syversen T. Sonntag WE. Growth hormone administration to aged animals reduces disulfide glutathione levels in hippocampus. Mech Ageing Dev. 2006;127:57–63. doi: 10.1016/j.mad.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 111.Doyle KP. Simon RP. Stenzel-Poore MP. Mechanisms of ischemic brain damage. Neuropharmacology. 2008;55:310–318. doi: 10.1016/j.neuropharm.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Du H. Guo L. Fang F. Chen D. Sosunov AA. McKhann GM. Yan Y. Wang C. Zhang H. Molkentin JD. Gunn-Moore FJ. Vonsattel JP. Arancio O. Chen JX. Yan SD. Cyclophilin D deficiency attenuates mitochondrial and neuronal perturbation and ameliorates learning and memory in Alzheimer's disease. Nat Med. 2008;14:1097–1105. doi: 10.1038/nm.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dugan LL. Sensi SL. Canzoniero LM. Handran SD. Rothman SM. Lin TS. Goldberg MP. Choi DW. Mitochondrial production of reactive oxygen species in cortical neurons following exposure to N-methyl-d-aspartate. J Neurosci. 1995;15:6377–6388. doi: 10.1523/JNEUROSCI.15-10-06377.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dumont M. Ho DJ. Calingasan NY. Xu H. Gibson G. Beal MF. Mitochondrial dihydrolipoyl succinyltransferase deficiency accelerates amyloid pathology and memory deficit in a transgenic mouse model of amyloid deposition. Free Radic Biol Med. 2009;47:1019–1027. doi: 10.1016/j.freeradbiomed.2009.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dumont M. Wille E. Stack C. Calingasan NY. Beal MF. Lin MT. Reduction of oxidative stress, amyloid deposition, and memory deficit by manganese superoxide dismutase overexpression in a transgenic mouse model of Alzheimer's disease. FASEB J. 2009;23:2459–2466. doi: 10.1096/fj.09-132928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dykens JA. Isolated cerebral and cerebellar mitochondria produce free radicals when exposed to elevated CA2+ and Na+: implications for neurodegeneration. J Neurochem. 1994;63:584–591. doi: 10.1046/j.1471-4159.1994.63020584.x. [DOI] [PubMed] [Google Scholar]
- 117.Eccles JC. Mechanisms of long-term memory. J Physiol (Paris) 1986;81:312–317. [PubMed] [Google Scholar]
- 118.Eftekharzadeh B. Maghsoudi N. Khodagholi F. Stabilization of transcription factor Nrf2 by tBHQ prevents oxidative stress-induced amyloid beta formation in NT2N neurons. Biochimie. 2010;92:245–253. doi: 10.1016/j.biochi.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 119.Elchuri S. Oberley TD. Qi W. Eisenstein RS. Jackson RL. Van Remmen H. Epstein CJ. Huang TT. CuZnSOD deficiency leads to persistent and widespread oxidative damage and hepatocarcinogenesis later in life. Oncogene. 2005;24:367–380. doi: 10.1038/sj.onc.1208207. [DOI] [PubMed] [Google Scholar]
- 120.Emsley J. Nature's Building Blocks. An A-Z Guide to the Elements. Oxford: Oxford University Press; 2002. Manganese; pp. 249–253. [Google Scholar]
- 121.Engelhart MJ. Geerlings MI. Ruitenberg A. van Swieten JC. Hofman A. Witteman JC. Breteler MM. Dietary intake of antioxidants and risk of Alzheimer disease. JAMA. 2002;287:3223–3229. doi: 10.1001/jama.287.24.3223. [DOI] [PubMed] [Google Scholar]
- 122.Engelhart MJ. Ruitenberg A. Meijer J. Kiliaan A. van Swieten JC. Hofman A. Witteman JC. Breteler MM. Plasma levels of antioxidants are not associated with Alzheimer's disease or cognitive decline. Dement Geriatr Cogn Disord. 2005;19:134–139. doi: 10.1159/000082884. [DOI] [PubMed] [Google Scholar]
- 123.Esposito L. Raber J. Kekonius L. Yan F. Yu GQ. Bien-Ly N. Puolivali J. Scearce-Levie K. Masliah E. Mucke L. Reduction in mitochondrial superoxide dismutase modulates Alzheimer's disease-like pathology and accelerates the onset of behavioral changes in human amyloid precursor protein transgenic mice. J Neurosci. 2006;26:5167–5179. doi: 10.1523/JNEUROSCI.0482-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Estall LB. Grant SJ. Cicala GA. Inhibition of nitric oxide (NO) production selectively impairs learning and memory in the rat. Pharmacol Biochem Behav. 1993;46:959–962. doi: 10.1016/0091-3057(93)90228-l. [DOI] [PubMed] [Google Scholar]
- 125.Farooqui T. Iron-induced oxidative stress modulates olfactory learning and memory in honeybees. Behav Neurosci. 2008;122:433–447. doi: 10.1037/0735-7044.122.2.433. [DOI] [PubMed] [Google Scholar]
- 126.Farr SA. Poon HF. Dogrukol-Ak D. Drake J. Banks WA. Eyerman E. Butterfield DA. Morley JE. The antioxidants alpha-lipoic acid and N-acetylcysteine reverse memory impairment and brain oxidative stress in aged SAMP8 mice. J Neurochem. 2003;84:1173–1183. doi: 10.1046/j.1471-4159.2003.01580.x. [DOI] [PubMed] [Google Scholar]
- 127.Fedorov NB. Pasinelli P. Oestreicher AB. DeGraan PN. Reymann KG. Antibodies to postsynaptic PKC substrate neurogranin prevent long-term potentiation in hippocampal CA1 neurons. Eur J Neurosci. 1995;7:819–822. doi: 10.1111/j.1460-9568.1995.tb00685.x. [DOI] [PubMed] [Google Scholar]
- 128.Feng Z. Chang Y. Cheng Y. Zhang BL. Qu ZW. Qin C. Zhang JT. Melatonin alleviates behavioral deficits associated with apoptosis and cholinergic system dysfunction in the APP 695 transgenic mouse model of Alzheimer's disease. J Pineal Res. 2004;37:129–136. doi: 10.1111/j.1600-079X.2004.00144.x. [DOI] [PubMed] [Google Scholar]
- 129.Feng Z. Cheng Y. Zhang JT. Long-term effects of melatonin or 17 beta-estradiol on improving spatial memory performance in cognitively impaired, ovariectomized adult rats. J Pineal Res. 2004;37:198–206. doi: 10.1111/j.1600-079X.2004.00158.x. [DOI] [PubMed] [Google Scholar]
- 130.Feng Z. Zhang JT. Long-term melatonin or 17beta-estradiol supplementation alleviates oxidative stress in ovariectomized adult rats. Free Radic Biol Med. 2005;39:195–204. doi: 10.1016/j.freeradbiomed.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 131.Filip V. Kolibas E. Selegiline in the treatment of Alzheimer's disease: a long-term randomized placebo-controlled trial. Czech and Slovak Senile Dementia of Alzheimer Type Study Group. J Psychiatry Neurosci. 1999;24:234–243. [PMC free article] [PubMed] [Google Scholar]
- 132.Fin C. Schmitz PK. Da Silva RC. Bernabeu R. Medina JH. Izquierdo I. Intrahippocampal, but not intra-amygdala, infusion of an inhibitor of heme oxygenase causes retrograde amnesia in the rat. Eur J Pharmacol. 1994;271:227–229. doi: 10.1016/0014-2999(94)90284-4. [DOI] [PubMed] [Google Scholar]
- 133.Font L. Miquel M. Aragon CM. Involvement of brain catalase activity in the acquisition of ethanol-induced conditioned place preference. Physiol Behav. 2008;93:733–741. doi: 10.1016/j.physbeh.2007.11.026. [DOI] [PubMed] [Google Scholar]
- 134.Forster MJ. Dubey A. Dawson KM. Stutts WA. Lal H. Sohal RS. Age-related losses of cognitive function and motor skills in mice are associated with oxidative protein damage in the brain. Proc Natl Acad Sci USA. 1996;93:4765–4769. doi: 10.1073/pnas.93.10.4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Fox MA. Chen RS. Holmes CS. Gender differences in memory and learning in children with insulin-dependent diabetes mellitus (IDDM) over a 4-year follow-up interval. J Pediatr Psychol. 2003;28:569–578. doi: 10.1093/jpepsy/jsg047. [DOI] [PubMed] [Google Scholar]
- 136.Fridovich I. Superoxide radical and superoxide dismutases. Annu Rev Biochem. 1995;64:97–112. doi: 10.1146/annurev.bi.64.070195.000525. [DOI] [PubMed] [Google Scholar]
- 137.Fuchs O. Babusiak M. Vyoral D. Petrak J. Role of zinc in eukaryotic cells, zinc transporters and zinc-containing proteins. Review article. Sb Lek. 2003;104:157–170. [PubMed] [Google Scholar]
- 138.Fukui K. Onodera K. Shinkai T. Suzuki S. Urano S. Impairment of learning and memory in rats caused by oxidative stress and aging, and changes in antioxidative defense systems. Ann NY Acad Sci. 2001;928:168–175. doi: 10.1111/j.1749-6632.2001.tb05646.x. [DOI] [PubMed] [Google Scholar]
- 139.Fukunaga K. Horikawa K. Shibata S. Takeuchi Y. Miyamoto E. Ca2+/calmodulin-dependent protein kinase II-dependent long-term potentiation in the rat suprachiasmatic nucleus and its inhibition by melatonin. J Neurosci Res. 2002;70:799–807. doi: 10.1002/jnr.10400. [DOI] [PubMed] [Google Scholar]
- 140.Fullerton HJ. Ditelberg JS. Chen SF. Sarco DP. Chan PH. Epstein CJ. Ferriero DM. Copper/zinc superoxide dismutase transgenic brain accumulates hydrogen peroxide after perinatal hypoxia ischemia. Ann Neurol. 1998;44:357–364. doi: 10.1002/ana.410440311. [DOI] [PubMed] [Google Scholar]
- 141.Furling D. Ghribi O. Lahsaini A. Mirault ME. Massicotte G. Impairment of synaptic transmission by transient hypoxia in hippocampal slices: improved recovery in glutathione peroxidase transgenic mice. Proc Natl Acad Sci USA. 2000;97:4351–4356. doi: 10.1073/pnas.060574597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gabbita SP. Lovell MA. Markesbery WR. Increased nuclear DNA oxidation in the brain in Alzheimer's disease. J Neurochem. 1998;71:2034–2040. doi: 10.1046/j.1471-4159.1998.71052034.x. [DOI] [PubMed] [Google Scholar]
- 143.Gahtan E. Auerbach JM. Groner Y. Segal M. Reversible impairment of long-term potentiation in transgenic Cu/Zn-SOD mice. Eur J Neurosci. 1998;10:538–544. doi: 10.1046/j.1460-9568.1998.00058.x. [DOI] [PubMed] [Google Scholar]
- 144.Garcia T. Ribes D. Colomina MT. Cabre M. Domingo JL. Gomez M. Evaluation of the protective role of melatonin on the behavioral effects of aluminum in a mouse model of Alzheimer's disease. Toxicology. 2009;265:49–55. doi: 10.1016/j.tox.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 145.Gaziano JM. Hatta A. Flynn M. Johnson EJ. Krinsky NI. Ridker PM. Hennekens CH. Frei B. Supplementation with beta-carotene in vivo and in vitro does not inhibit low density lipoprotein oxidation. Atherosclerosis. 1995;112:187–195. doi: 10.1016/0021-9150(94)05414-e. [DOI] [PubMed] [Google Scholar]
- 146.Gille L. Nohl H. The ubiquinol/bc1 redox couple regulates mitochondrial oxygen radical formation. Arch Biochem Biophys. 2001;388:34–38. doi: 10.1006/abbi.2000.2257. [DOI] [PubMed] [Google Scholar]
- 147.Gillis JC. Benefield P. McTavish D. Idebenone. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use in age-related cognitive disorders. Drugs Aging. 1994;5:133–152. doi: 10.2165/00002512-199405020-00007. [DOI] [PubMed] [Google Scholar]
- 148.Gonenc S. Uysal N. Acikgoz O. Kayatekin BM. Sonmez A. Kiray M. Aksu I. Gulecer B. Topcu A. Semin I. Effects of melatonin on oxidative stress and spatial memory impairment induced by acute ethanol treatment in rats. Physiol Res. 2005;54:341–348. [PubMed] [Google Scholar]
- 149.Gong L. Gao TM. Huang H. Tong Z. Redox modulation of large conductance calcium-activated potassium channels in CA1 pyramidal neurons from adult rat hippocampus. Neurosci Lett. 2000;286:191–194. doi: 10.1016/s0304-3940(00)01121-6. [DOI] [PubMed] [Google Scholar]
- 150.Good PF. Hsu A. Werner P. Perl DP. Olanow CW. Protein nitration in Parkinson's disease. J Neuropathol Exp Neurol. 1998;57:338–342. doi: 10.1097/00005072-199804000-00006. [DOI] [PubMed] [Google Scholar]
- 151.Gorell JM. Ordidge RJ. Brown GG. Deniau JC. Buderer NM. Helpern JA. Increased iron-related MRI contrast in the substantia nigra in Parkinson's disease. Neurology. 1995;45:1138–1143. doi: 10.1212/wnl.45.6.1138. [DOI] [PubMed] [Google Scholar]
- 152.Gould TJ. Feiro OR. Age-related deficits in the retention of memories for cued fear conditioning are reversed by galantamine treatment. Behav Brain Res. 2005;165:160–171. doi: 10.1016/j.bbr.2005.06.040. [DOI] [PubMed] [Google Scholar]
- 153.Gravina SA. Mieyal JJ. Thioltransferase is a specific glutathionyl mixed disulfide oxidoreductase. Biochemistry. 1993;32:3368–3376. doi: 10.1021/bi00064a021. [DOI] [PubMed] [Google Scholar]
- 154.Griendling KK. Novel NAD(P)H oxidases in the cardiovascular system. Heart. 2004;90:491–493. doi: 10.1136/hrt.2003.029397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Griffith OW. Stuehr DJ. Nitric oxide synthases: properties and catalytic mechanism. Annu Rev Physiol. 1995;57:707–736. doi: 10.1146/annurev.ph.57.030195.003423. [DOI] [PubMed] [Google Scholar]
- 156.Grodstein F. Kang JH. Glynn RJ. Cook NR. Gaziano JM. A randomized trial of beta carotene supplementation and cognitive function in men: the Physicians' Health Study II. Arch Intern Med. 2007;167:2184–2190. doi: 10.1001/archinte.167.20.2184. [DOI] [PubMed] [Google Scholar]
- 157.Guo Q. Fu W. Holtsberg FW. Steiner SM. Mattson MP. Superoxide mediates the cell-death-enhancing action of presenilin-1 mutations. J Neurosci Res. 1999;56:457–470. doi: 10.1002/(SICI)1097-4547(19990601)56:5<457::AID-JNR2>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 158.Gupta R. Singh M. Sharma A. Neuroprotective effect of antioxidants on ischaemia and reperfusion-induced cerebral injury. Pharmacol Res. 2003;48:209–215. doi: 10.1016/s1043-6618(03)00102-6. [DOI] [PubMed] [Google Scholar]
- 159.Gutteridge JM. Free radicals in disease processes: a compilation of cause and consequence. Free Radic Res Commun. 1993;19:141–158. doi: 10.3109/10715769309111598. [DOI] [PubMed] [Google Scholar]
- 160.Haas RH. The evidence basis for coenzyme Q therapy in oxidative phosphorylation disease. Mitochondrion. 2007;7(Suppl):S136–S145. doi: 10.1016/j.mito.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 161.Hagen JW. Barclay CR. Anderson BJ. Feeman DJ. Segal SS. Bacon G. Goldstein GW. Intellective functioning and strategy use in children with insulin-dependent diabetes mellitus. Child Dev. 1990;61:1714–1727. [PubMed] [Google Scholar]
- 162.Haller J. Weggemans RM. Ferry M. Guigoz Y. Mental health: minimental state examination and geriatric depression score of elderly Europeans in the SENECA study of 1993. Eur J Clin Nutr. 1996;50(Suppl 2):S112–S116. [PubMed] [Google Scholar]
- 163.Halliwell B. Drug antioxidant effects. A basis for drug selection? Drugs. 1991;42:569–605. doi: 10.2165/00003495-199142040-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Halliwell B. Reactive oxygen species and the central nervous system. J Neurochem. 1992;59:1609–1623. doi: 10.1111/j.1471-4159.1992.tb10990.x. [DOI] [PubMed] [Google Scholar]
- 165.Hambidge KM. Krebs NF. Zinc deficiency: a special challenge. J Nutr. 2007;137:1101–1105. doi: 10.1093/jn/137.4.1101. [DOI] [PubMed] [Google Scholar]
- 166.Hamel E. Nicolakakis N. Aboulkassim T. Ongali B. Tong XK. Oxidative stress and cerebrovascular dysfunction in mouse models of Alzheimer's disease. Exp Physiol. 2008;93:116–120. doi: 10.1113/expphysiol.2007.038729. [DOI] [PubMed] [Google Scholar]
- 167.Hamer DH. Metallothionein. Annu Rev Biochem. 1986;55:913–951. doi: 10.1146/annurev.bi.55.070186.004405. [DOI] [PubMed] [Google Scholar]
- 168.Hardeland R. Antioxidative protection by melatonin: multiplicity of mechanisms from radical detoxification to radical avoidance. Endocrine. 2005;27:119–130. doi: 10.1385/endo:27:2:119. [DOI] [PubMed] [Google Scholar]
- 169.Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 170.Harris-Cerruti C. Kamsler A. Kaplan B. Lamb B. Segal M. Groner Y. Functional and morphological alterations in compound transgenic mice overexpressing Cu/Zn superoxide dismutase and amyloid precursor protein [correction] Eur J Neurosci. 2004;19:1174–1190. doi: 10.1111/j.1460-9568.2004.03188.x. [DOI] [PubMed] [Google Scholar]
- 171.Harrison FE. Hosseini AH. Dawes SM. Weaver S. May JM. Ascorbic acid attenuates scopolamine-induced spatial learning deficits in the water maze. Behav Brain Res. 2009;205:550–558. doi: 10.1016/j.bbr.2009.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.He XL. Zhou WQ. Bi MG. Du GH. Neuroprotective effects of icariin on memory impairment and neurochemical deficits in senescence-accelerated mouse prone 8 (SAMP8) mice. Brain Res. 2010;1334:73–83. doi: 10.1016/j.brainres.2010.03.084. [DOI] [PubMed] [Google Scholar]
- 173.Head E. Hartley J. Kameka AM. Mehta R. Ivy GO. Ruehl WW. Milgram NW. The effects of L-deprenyl on spatial short term memory in young and aged dogs. Prog Neuropsychopharmacol Biol Psychiatry. 1996;20:515–530. doi: 10.1016/0278-5846(96)00014-0. [DOI] [PubMed] [Google Scholar]
- 174.Heinzel B. John M. Klatt P. Bohme E. Mayer B. Ca2+/calmodulin-dependent formation of hydrogen peroxide by brain nitric oxide synthase. Biochem J. 1992;281(Pt 3):627–630. doi: 10.1042/bj2810627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hensley K. Hall N. Subramaniam R. Cole P. Harris M. Aksenov M. Aksenova M. Gabbita SP. Wu JF. Carney JM. Brain regional correspondence between Alzheimer's disease histopathology and biomarkers of protein oxidation. J Neurochem. 1995;65:2146–2156. doi: 10.1046/j.1471-4159.1995.65052146.x. [DOI] [PubMed] [Google Scholar]
- 176.Hidalgo C. Carrasco MA. Munoz P. Nunez MT. A role for reactive oxygen/nitrogen species and iron on neuronal synaptic plasticity. Antioxid Redox Signal. 2007;9:245–255. doi: 10.1089/ars.2007.9.245. [DOI] [PubMed] [Google Scholar]
- 177.Hidalgo J. Aschner M. Zatta P. Vasak M. Roles of the metallothionein family of proteins in the central nervous system. Brain Res Bull. 2001;55:133–145. doi: 10.1016/s0361-9230(01)00452-x. [DOI] [PubMed] [Google Scholar]
- 178.Hill KE. McCollum GW. Boeglin ME. Burk RF. Thioredoxin reductase activity is decreased by selenium deficiency. Biochem Biophys Res Commun. 1997;234:293–295. doi: 10.1006/bbrc.1997.6618. [DOI] [PubMed] [Google Scholar]
- 179.Holmgren A. Johansson C. Berndt C. Lonn ME. Hudemann C. Lillig CH. Thiol redox control via thioredoxin and glutaredoxin systems. Biochem Soc Trans. 2005;33:1375–1377. doi: 10.1042/BST0331375. [DOI] [PubMed] [Google Scholar]
- 180.Holscher C. Rose SP. An inhibitor of nitric oxide synthesis prevents memory formation in the chick. Neurosci Lett. 1992;145:165–167. doi: 10.1016/0304-3940(92)90012-v. [DOI] [PubMed] [Google Scholar]
- 181.Hongpaisan J. Winters CA. Andrews SB. Calcium-dependent mitochondrial superoxide modulates nuclear CREB phosphorylation in hippocampal neurons. Mol Cell Neurosci. 2003;24:1103–1115. doi: 10.1016/j.mcn.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 182. This reference has been deleted.
- 183.Hongpaisan J. Winters CA. Andrews SB. Strong calcium entry activates mitochondrial superoxide generation, upregulating kinase signaling in hippocampal neurons. J Neurosci. 2004;24:10878–10887. doi: 10.1523/JNEUROSCI.3278-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Hosseinzadeh H. Ziaee T. Sadeghi A. The effect of saffron, Crocus sativus stigma, extract and its constituents, safranal and crocin on sexual behaviors in normal male rats. Phytomedicine. 2008;15:491–495. doi: 10.1016/j.phymed.2007.09.020. [DOI] [PubMed] [Google Scholar]
- 185.Hossmann KA. Viability thresholds and the penumbra of focal ischemia. Ann Neurol. 1994;36:557–565. doi: 10.1002/ana.410360404. [DOI] [PubMed] [Google Scholar]
- 186.Hota SK. Hota KB. Prasad D. Ilavazhagan G. Singh SB. Oxidative-stress-induced alterations in Sp factors mediate transcriptional regulation of the NR1 subunit in hippocampus during hypoxia. Free Radic Biol Med. 2010;49:178–191. doi: 10.1016/j.freeradbiomed.2010.03.027. [DOI] [PubMed] [Google Scholar]
- 187.Howland JG. Wang YT. Synaptic plasticity in learning and memory: stress effects in the hippocampus. Prog Brain Res. 2008;169:145–158. doi: 10.1016/S0079-6123(07)00008-8. [DOI] [PubMed] [Google Scholar]
- 188.Hu D. Cao P. Thiels E. Chu CT. Wu GY. Oury TD. Klann E. Hippocampal long-term potentiation, memory, and longevity in mice that overexpress mitochondial superoxide dismutase. Neurobiol Learn Mem. 2007;87:372–384. doi: 10.1016/j.nlm.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Hu D. Klann E. Thiels E. Superoxide dismutase and hippocampal function: age and isozyme matter. Antioxid Redox Signal. 2007;9:201–210. doi: 10.1089/ars.2007.9.201. [DOI] [PubMed] [Google Scholar]
- 190.Hu D. Serrano F. Oury TD. Klann E. Aging-dependent alterations in synaptic plasticity and memory in mice that overexpress extracellular superoxide dismutase. J Neurosci. 2006;26:3933–3941. doi: 10.1523/JNEUROSCI.5566-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Hu P. Bretsky P. Crimmins EM. Guralnik JM. Reuben DB. Seeman TE. Association between serum beta-carotene levels and decline of cognitive function in high-functioning older persons with or without apolipoprotein E 4 alleles: MacArthur studies of successful aging. J Gerontol A Biol Sci Med Sci. 2006;61:616–620. doi: 10.1093/gerona/61.6.616. [DOI] [PubMed] [Google Scholar]
- 192.Huang KP. Huang FL. Chen HC. Characterization of a 7.5-kDa protein kinase C substrate (RC3 protein, neurogranin) from rat brain. Arch Biochem Biophys. 1993;305:570–580. doi: 10.1006/abbi.1993.1463. [DOI] [PubMed] [Google Scholar]
- 193.Huang KP. Huang FL. Jager T. Li J. Reymann KG. Balschun D. Neurogranin/RC3 enhances long-term potentiation and learning by promoting calcium-mediated signaling. J Neurosci. 2004;24:10660–10669. doi: 10.1523/JNEUROSCI.2213-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Huddleston AT. Tang W. Takeshima H. Hamilton SL. Klann E. Superoxide-induced potentiation in the hippocampus requires activation of ryanodine receptor type 3 and ERK. J Neurophysiol. 2008;99:1565–1571. doi: 10.1152/jn.00659.2007. [DOI] [PubMed] [Google Scholar]
- 195.Ichitani Y. Okaichi H. Yoshikawa T. Ibata Y. Learning behaviour in chronic vitamin E-deficient and -supplemented rats: radial arm maze learning and passive avoidance response. Behav Brain Res. 1992;51:157–164. doi: 10.1016/s0166-4328(05)80209-8. [DOI] [PubMed] [Google Scholar]
- 196.Ishihara K. Katsuki H. Sugimura M. Satoh M. Idebenone and vinpocetine augment long-term potentiation in hippocampal slices in the guinea pig. Neuropharmacology. 1989;28:569–573. doi: 10.1016/0028-3908(89)90135-4. [DOI] [PubMed] [Google Scholar]
- 197.Ishrat T. Khan MB. Hoda MN. Yousuf S. Ahmad M. Ansari MA. Ahmad AS. Islam F. Coenzyme Q10 modulates cognitive impairment against intracerebroventricular injection of streptozotocin in rats. Behav Brain Res. 2006;171:9–16. doi: 10.1016/j.bbr.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 198.Ishrat T. Parveen K. Khan MM. Khuwaja G. Khan MB. Yousuf S. Ahmad A. Shrivastav P. Islam F. Selenium prevents cognitive decline and oxidative damage in rat model of streptozotocin-induced experimental dementia of Alzheimer's type. Brain Res. 2009;1281:117–127. doi: 10.1016/j.brainres.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 199.Jackson SH. Gallin JI. Holland SM. The p47phox mouse knock-out model of chronic granulomatous disease. J Exp Med. 1995;182:751–758. doi: 10.1084/jem.182.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Kagi JH. Schaffer A. Biochemistry of metallothionein. Biochemistry. 1988;27:8509–8515. doi: 10.1021/bi00423a001. [DOI] [PubMed] [Google Scholar]
- 201.Kalinina EV. Chernov NN. Saprin AN. Involvement of thio-, peroxi-, and glutaredoxins in cellular redox-dependent processes. Biochemistry (Mosc) 2008;73:1493–1510. doi: 10.1134/s0006297908130099. [DOI] [PubMed] [Google Scholar]
- 202.Kamal A. Biessels GJ. Gispen WH. Ramakers GM. Synaptic transmission changes in the pyramidal cells of the hippocampus in streptozotocin-induced diabetes mellitus in rats. Brain Res. 2006;1073–1074:276–280. doi: 10.1016/j.brainres.2005.12.070. [DOI] [PubMed] [Google Scholar]
- 203.Kamsler A. Avital A. Greenberger V. Segal M. Aged SOD overexpressing mice exhibit enhanced spatial memory while lacking hippocampal neurogenesis. Antioxid Redox Signal. 2007;9:181–189. doi: 10.1089/ars.2007.9.181. [DOI] [PubMed] [Google Scholar]
- 204.Kamsler A. Segal M. Hydrogen peroxide modulation of synaptic plasticity. J Neurosci. 2003;23:269–276. doi: 10.1523/JNEUROSCI.23-01-00269.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Kamsler A. Segal M. Paradoxical actions of hydrogen peroxide on long-term potentiation in transgenic superoxide dismutase-1 mice. J Neurosci. 2003;23:10359–10367. doi: 10.1523/JNEUROSCI.23-32-10359.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Kang JH. Grodstein F. Plasma carotenoids and tocopherols and cognitive function: a prospective study. Neurobiol Aging. 2008;29:1394–1403. doi: 10.1016/j.neurobiolaging.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Kanninen K. Heikkinen R. Malm T. Rolova T. Kuhmonen S. Leinonen H. Yla-Herttuala S. Tanila H. Levonen AL. Koistinaho M. Koistinaho J. Intrahippocampal injection of a lentiviral vector expressing Nrf2 improves spatial learning in a mouse model of Alzheimer's disease. Proc Natl Acad Sci USA. 2009;106:16505–16510. doi: 10.1073/pnas.0908397106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Kanninen K. Malm TM. Jyrkkanen HK. Goldsteins G. Keksa-Goldsteine V. Tanila H. Yamamoto M. Yla-Herttuala S. Levonen AL. Koistinaho J. Nuclear factor erythroid 2-related factor 2 protects against beta amyloid. Mol Cell Neurosci. 2008;39:302–313. doi: 10.1016/j.mcn.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 209.Kanterewicz BI. Knapp LT. Klann E. Stimulation of p42 and p44 mitogen-activated protein kinases by reactive oxygen species and nitric oxide in hippocampus. J Neurochem. 1998;70:1009–1016. doi: 10.1046/j.1471-4159.1998.70031009.x. [DOI] [PubMed] [Google Scholar]
- 210.Kanterewicz BI. Urban NN. McMahon DB. Norman ED. Giffen LJ. Favata MF. Scherle PA. Trzskos JM. Barrionuevo G. Klann E. The extracellular signal-regulated kinase cascade is required for NMDA receptor-independent LTP in area CA1 but not area CA3 of the hippocampus. J Neurosci. 2000;20:3057–3066. doi: 10.1523/JNEUROSCI.20-09-03057.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Katsuki H. Nakanishi C. Saito H. Matsuki N. Biphasic effect of hydrogen peroxide on field potentials in rat hippocampal slices. Eur J Pharmacol. 1997;337:213–218. doi: 10.1016/s0014-2999(97)01323-x. [DOI] [PubMed] [Google Scholar]
- 212.Keller JN. Kindy MS. Holtsberg FW. St. Clair DK. Yen HC. Germeyer A. Steiner SM. Bruce-Keller AJ. Hutchins JB. Mattson MP. Mitochondrial manganese superoxide dismutase prevents neural apoptosis and reduces ischemic brain injury: suppression of peroxynitrite production, lipid peroxidation, and mitochondrial dysfunction. J Neurosci. 1998;18:687–697. doi: 10.1523/JNEUROSCI.18-02-00687.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Kemmerling U. Munoz P. Muller M. Sanchez G. Aylwin ML. Klann E. Carrasco MA. Hidalgo C. Calcium release by ryanodine receptors mediates hydrogen peroxide-induced activation of ERK and CREB phosphorylation in N2a cells and hippocampal neurons. Cell Calcium. 2007;41:491–502. doi: 10.1016/j.ceca.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 214.Kenchappa RS. Ravindranath V. Glutaredoxin is essential for maintenance of brain mitochondrial complex I: studies with MPTP. FASEB J. 2003;17:717–719. doi: 10.1096/fj.02-0771fje. [DOI] [PubMed] [Google Scholar]
- 215.Kieburtz K. McDermott M. Como P. Growdon J. Brady J. Carter J. Huber S. Kanigan B. Landow E. Rudolph A, et al. The effect of deprenyl and tocopherol on cognitive performance in early untreated Parkinson's disease. Parkinson Study Group. Neurology. 1994;44:1756–1759. doi: 10.1212/wnl.44.9.1756. [DOI] [PubMed] [Google Scholar]
- 216.Kim MJ. Shin KS. Chung YB. Jung KW. Cha CI. Shin DH. Immunohistochemical study of p47Phox and gp91Phox distributions in rat brain. Brain Res. 2005;1040:178–186. doi: 10.1016/j.brainres.2005.01.066. [DOI] [PubMed] [Google Scholar]
- 217.Kim SJ. Linden DJ. Ubiquitous plasticity and memory storage. Neuron. 2007;56:582–592. doi: 10.1016/j.neuron.2007.10.030. [DOI] [PubMed] [Google Scholar]
- 218.Kim SU. Jin MH. Kim YS. Lee SH. Cho YS. Cho KJ. Lee KS. Kim YI. Kim GW. Kim JM. Lee TH. Lee YH. Shong M. Kim HC. Chang KT. Yu DY. Lee DS. Peroxiredoxin II preserves cognitive function against age-linked hippocampal oxidative damage. Neurobiol Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.05.017. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 219.Kimura H. Hydrogen sulfide: from brain to gut. Antioxid Redox Signal. 2010;12:1111–1123. doi: 10.1089/ars.2009.2919. [DOI] [PubMed] [Google Scholar]
- 220.Kiray M. Bagriyanik HA. Pekcetin C. Ergur BU. Uysal N. Protective effects of deprenyl in transient cerebral ischemia in rats. Chin J Physiol. 2008;51:275–281. [PubMed] [Google Scholar]
- 221.Kiray M. Bagriyanik HA. Pekcetin C. Ergur BU. Uysal N. Ozyurt D. Buldan Z. Deprenyl and the relationship between its effects on spatial memory, oxidant stress and hippocampal neurons in aged male rats. Physiol Res. 2006;55:205–212. doi: 10.33549/physiolres.930742. [DOI] [PubMed] [Google Scholar]
- 222.Kiray M. Uysal N. Sonmez A. Acikgoz O. Gonenc S. Positive effects of deprenyl and estradiol on spatial memory and oxidant stress in aged female rat brains. Neurosci Lett. 2004;354:225–228. doi: 10.1016/j.neulet.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 223.Kishida KT. Hoeffer CA. Hu D. Pao M. Holland SM. Klann E. Synaptic plasticity deficits and mild memory impairments in mouse models of chronic granulomatous disease. Mol Cell Biol. 2006;26:5908–5920. doi: 10.1128/MCB.00269-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Kishida KT. Klann E. Sources and targets of reactive oxygen species in synaptic plasticity and memory. Antioxid Redox Signal. 2007;9:233–244. doi: 10.1089/ars.2007.9.ft-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Kishida KT. Pao M. Holland SM. Klann E. NADPH oxidase is required for NMDA receptor-dependent activation of ERK in hippocampal area CA1. J Neurochem. 2005;94:299–306. doi: 10.1111/j.1471-4159.2005.03189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Kiyota Y. Hamajo K. Miyamoto M. Nagaoka A. Effect of idebenone (CV-2619) on memory impairment observed in passive avoidance task in rats with cerebral embolization. Jpn J Pharmacol. 1985;37:300–302. doi: 10.1254/jjp.37.300. [DOI] [PubMed] [Google Scholar]
- 227.Kiyota Y. Miyamoto M. Nagaoka A. [Ameliorating effects of idebenone and indeloxazine hydrochloride on impairment of radial maze learning in cerebral embolized rats] Nippon Yakurigaku Zasshi. 1989;93:197–202. doi: 10.1254/fpj.93.197. [DOI] [PubMed] [Google Scholar]
- 228.Klann E. Cell-permeable scavengers of superoxide prevent long-term potentiation in hippocampal area CA1. J Neurophysiol. 1998;80:452–457. doi: 10.1152/jn.1998.80.1.452. [DOI] [PubMed] [Google Scholar]
- 229.Klann E. Roberson ED. Knapp LT. Sweatt JD. A role for superoxide in protein kinase C activation and induction of long-term potentiation. J Biol Chem. 1998;273:4516–4522. doi: 10.1074/jbc.273.8.4516. [DOI] [PubMed] [Google Scholar]
- 230.Klann E. Sweatt JD. Altered protein synthesis is a trigger for long-term memory formation. Neurobiol Learn Mem. 2008;89:247–259. doi: 10.1016/j.nlm.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Knapp LT. Klann E. Potentiation of hippocampal synaptic transmission by superoxide requires the oxidative activation of protein kinase C. J Neurosci. 2002;22:674–683. doi: 10.1523/JNEUROSCI.22-03-00674.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Knapp LT. Klann E. Role of reactive oxygen species in hippocampal long-term potentiation: contributory or inhibitory? J Neurosci Res. 2002;70:1–7. doi: 10.1002/jnr.10371. [DOI] [PubMed] [Google Scholar]
- 233.Kotler M. Rodriguez C. Sainz RM. Antolin I. Menendez-Pelaez A. Melatonin increases gene expression for antioxidant enzymes in rat brain cortex. J Pineal Res. 1998;24:83–89. doi: 10.1111/j.1600-079x.1998.tb00371.x. [DOI] [PubMed] [Google Scholar]
- 234.Kucukatay V. Hacioglu G. Ozkaya G. Agar A. Yargicoglu P. The effect of diabetes mellitus on active avoidance learning in rats: the role of nitric oxide. Med Sci Monit. 2009;15:BR88–BR93. [PubMed] [Google Scholar]
- 235.Kuhad A. Sethi R. Chopra K. Lycopene attenuates diabetes-associated cognitive decline in rats. Life Sci. 2008;83:128–134. doi: 10.1016/j.lfs.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 236.Lahiri DK. Xu Y. Klaunig J. Baiyewu O. Ogunniyi A. Hall K. Hendrie H. Sahota A. Effect of oxidative stress on DNA damage and beta-amyloid precursor proteins in lymphoblastoid cell lines from a Nigerian population. Ann NY Acad Sci. 1999;893:331–336. doi: 10.1111/j.1749-6632.1999.tb07848.x. [DOI] [PubMed] [Google Scholar]
- 237.Lai J. Yin S. Xu Q. Hu S. [Protection of zinc and selenium content of brain and blood on learning and memory exposed to lead in growing rats] Wei Sheng Yan Jiu. 2004;33:218–220. [PubMed] [Google Scholar]
- 238.Lal H. Pogacar S. Daly PR. Puri SK. Behavioral and neuropathological manifestations of nutritionally induced central nervous system “aging” in the rat. Prog Brain Res. 1973;40:128–140. [PubMed] [Google Scholar]
- 239.Lambert AJ. Brand MD. Superoxide production by NADH:ubiquinone oxidoreductase (complex I) depends on the pH gradient across the mitochondrial inner membrane. Biochem J. 2004;382:511–517. doi: 10.1042/BJ20040485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- 241.Landaw SA. Callahan EW., Jr. Schmid R. Catabolism of heme in vivo: comparison of the simultaneous production of bilirubin and carbon monoxide. J Clin Invest. 1970;49:914–925. doi: 10.1172/JCI106311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Larson J. Jessen RE. Uz T. Arslan AD. Kurtuncu M. Imbesi M. Manev H. Impaired hippocampal long-term potentiation in melatonin MT2 receptor-deficient mice. Neurosci Lett. 2006;393:23–26. doi: 10.1016/j.neulet.2005.09.040. [DOI] [PubMed] [Google Scholar]
- 243.Lazo JS. Kondo Y. Dellapiazza D. Michalska AE. Choo KH. Pitt BR. Enhanced sensitivity to oxidative stress in cultured embryonic cells from transgenic mice deficient in metallothionein I and II genes. J Biol Chem. 1995;270:5506–5510. doi: 10.1074/jbc.270.10.5506. [DOI] [PubMed] [Google Scholar]
- 244.Lee JE. Kim H. Jang H. Cho EJ. Youn HD. Hydrogen peroxide triggers the proteolytic cleavage and the inactivation of calcineurin. J Neurochem. 2007;100:1703–1712. doi: 10.1111/j.1471-4159.2006.04340.x. [DOI] [PubMed] [Google Scholar]
- 245.Leon J. Acuna-Castroviejo D. Sainz RM. Mayo JC. Tan DX. Reiter RJ. Melatonin and mitochondrial function. Life Sci. 2004;75:765–790. doi: 10.1016/j.lfs.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 246.Letechipia-Vallejo G. Lopez-Loeza E. Espinoza-Gonzalez V. Gonzalez-Burgos I. Olvera-Cortes ME. Morali G. Cervantes M. Long-term morphological and functional evaluation of the neuroprotective effects of post-ischemic treatment with melatonin in rats. J Pineal Res. 2007;42:138–146. doi: 10.1111/j.1600-079X.2006.00395.x. [DOI] [PubMed] [Google Scholar]
- 247.Levin ED. Christopher NC. Crapo JD. Memory decline of aging reduced by extracellular superoxide dismutase overexpression. Behav Genet. 2005;35:447–453. doi: 10.1007/s10519-004-1510-y. [DOI] [PubMed] [Google Scholar]
- 248.Levin ED. Perraut C. Pollard N. Freedman JH. Metallothionein expression and neurocognitive function in mice. Physiol Behav. 2006;87:513–518. doi: 10.1016/j.physbeh.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 249.Li F. Calingasan NY. Yu F. Mauck WM. Toidze M. Almeida CG. Takahashi RH. Carlson GA. Flint BM. Lin MT. Gouras GK. Increased plaque burden in brains of APP mutant MnSOD heterozygous knockout mice. J Neurochem. 2004;89:1308–1312. doi: 10.1111/j.1471-4159.2004.02455.x. [DOI] [PubMed] [Google Scholar]
- 250.Li J. Pak JH. Huang FL. Huang KP. N-methyl-d-aspartate induces neurogranin/RC3 oxidation in rat brain slices. J Biol Chem. 1999;274:1294–1300. doi: 10.1074/jbc.274.3.1294. [DOI] [PubMed] [Google Scholar]
- 251.Li Y. Huang TT. Carlson EJ. Melov S. Ursell PC. Olson JL. Noble LJ. Yoshimura MP. Berger C. Chan PH. Wallace DC. Epstein CJ. Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nat Genet. 1995;11:376–381. doi: 10.1038/ng1295-376. [DOI] [PubMed] [Google Scholar]
- 252.Lin HH. Chen CH. Hsieh WK. Chiu TH. Lai CC. Hydrogen peroxide increases the activity of rat sympathetic preganglionic neurons in vivo and in vitro. Neuroscience. 2003;121:641–647. doi: 10.1016/s0306-4522(03)00517-7. [DOI] [PubMed] [Google Scholar]
- 253.Ling ZQ. Tian Q. Wang L. Fu ZQ. Wang XC. Wang Q. Wang JZ. Constant illumination induces Alzheimer-like damages with endoplasmic reticulum involvement and the protection of melatonin. J Alzheimers Dis. 2009;16:287–300. doi: 10.3233/JAD-2009-0949. [DOI] [PubMed] [Google Scholar]
- 254.Liu J. Head E. Gharib AM. Yuan W. Ingersoll RT. Hagen TM. Cotman CW. Ames BN. Memory loss in old rats is associated with brain mitochondrial decay and RNA/DNA oxidation: partial reversal by feeding acetyl-l-carnitine and/or R-alpha-lipoic acid. Proc Natl Acad Sci USA. 2002;99:2356–2361. doi: 10.1073/pnas.261709299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Liu R. Liu IY. Bi X. Thompson RF. Doctrow SR. Malfroy B. Baudry M. Reversal of age-related learning deficits and brain oxidative stress in mice with superoxide dismutase/catalase mimetics. Proc Natl Acad Sci USA. 2003;100:8526–8531. doi: 10.1073/pnas.1332809100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Liu XJ. Wu WT. Effects of ligustrazine, tanshinone II A, ubiquinone, and idebenone on mouse water maze performance. Zhongguo Yao Li Xue Bao. 1999;20:987–990. [PubMed] [Google Scholar]
- 257.Lo EH. Moskowitz MA. Jacobs TP. Exciting, radical, suicidal: how brain cells die after stroke. Stroke. 2005;36:189–192. doi: 10.1161/01.STR.0000153069.96296.fd. [DOI] [PubMed] [Google Scholar]
- 258.Luchsinger JA. Tang MX. Shea S. Mayeux R. Antioxidant vitamin intake and risk of Alzheimer disease. Arch Neurol. 2003;60:203–208. doi: 10.1001/archneur.60.2.203. [DOI] [PubMed] [Google Scholar]
- 259.Maalouf M. Rho JM. Oxidative impairment of hippocampal long-term potentiation involves activation of protein phosphatase 2A and is prevented by ketone bodies. J Neurosci Res. 2008;86:3322–3330. doi: 10.1002/jnr.21782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Machado MS. Rosa RM. Dantas AS. Reolon GK. Appelt HR. Braga AL. Henriques JA. Roesler R. An organic selenium compound attenuates apomorphine-induced stereotypy in mice. Neurosci Lett. 2006;410:198–202. doi: 10.1016/j.neulet.2006.09.079. [DOI] [PubMed] [Google Scholar]
- 261.Mahoney CW. Pak JH. Huang KP. Nitric oxide modification of rat brain neurogranin. Identification of the cysteine residues involved in intramolecular disulfide bridge formation using site-directed mutagenesis. J Biol Chem. 1996;271:28798–28804. doi: 10.1074/jbc.271.46.28798. [DOI] [PubMed] [Google Scholar]
- 262.Maia FD. Pitombeira BS. Araujo DT. Cunha GM. Viana GS. l-Deprenyl prevents lipid peroxidation and memory deficits produced by cerebral ischemia in rats. Cell Mol Neurobiol. 2004;24:87–100. doi: 10.1023/B:CEMN.0000012727.59502.c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Makhro AV. Mashkina AP. Solenaya OA. Trunova OA. Kozina LS. Arutyunian AV. Bulygina ER. Prenatal hyperhomocysteinemia as a model of oxidative stress of the brain. Bull Exp Biol Med. 2008;146:33–35. doi: 10.1007/s10517-008-0233-0. [DOI] [PubMed] [Google Scholar]
- 264.Malenka RC. Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 265.Malenka RC. Nicoll RA. Long-term potentiation—a decade of progress? Science. 1999;285:1870–1874. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- 266.Manczak M. Anekonda TS. Henson E. Park BS. Quinn J. Reddy PH. Mitochondria are a direct site of A beta accumulation in Alzheimer's disease neurons: implications for free radical generation and oxidative damage in disease progression. Hum Mol Genet. 2006;15:1437–1449. doi: 10.1093/hmg/ddl066. [DOI] [PubMed] [Google Scholar]
- 267.Manczak M. Park BS. Jung Y. Reddy PH. Differential expression of oxidative phosphorylation genes in patients with Alzheimer's disease: implications for early mitochondrial dysfunction and oxidative damage. Neuromolecular Med. 2004;5:147–162. doi: 10.1385/NMM:5:2:147. [DOI] [PubMed] [Google Scholar]
- 268.Manda K. Ueno M. Anzai K. Memory impairment, oxidative damage and apoptosis induced by space radiation: ameliorative potential of alpha-lipoic acid. Behav Brain Res. 2008;187:387–395. doi: 10.1016/j.bbr.2007.09.033. [DOI] [PubMed] [Google Scholar]
- 269.Manda K. Ueno M. Moritake T. Anzai K. Radiation-induced cognitive dysfunction and cerebellar oxidative stress in mice: protective effect of alpha-lipoic acid. Behav Brain Res. 2007;177:7–14. doi: 10.1016/j.bbr.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 270.Manrique HM. Miquel M. Aragon CM. Brain catalase mediates potentiation of social recognition memory produced by ethanol in mice. Drug Alcohol Depend. 2005;79:343–350. doi: 10.1016/j.drugalcdep.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 271.Maragos WF. Jakel R. Chesnut D. Pocernich CB. Butterfield DA. St Clair D. Cass WA. Methamphetamine toxicity is attenuated in mice that overexpress human manganese superoxide dismutase. Brain Res. 2000;878:218–222. doi: 10.1016/s0006-8993(00)02707-4. [DOI] [PubMed] [Google Scholar]
- 272.Marcus DL. Strafaci JA. Freedman ML. Differential neuronal expression of manganese superoxide dismutase in Alzheimer's disease. Med Sci Monit. 2006;12:BR8–BR14. [PubMed] [Google Scholar]
- 273.Markesbery WR. Lovell MA. Four-hydroxynonenal, a product of lipid peroxidation, is increased in the brain in Alzheimer's disease. Neurobiol Aging. 1998;19:33–36. doi: 10.1016/s0197-4580(98)00009-8. [DOI] [PubMed] [Google Scholar]
- 274.Marklund N. Clausen F. McIntosh TK. Hillered L. Free radical scavenger posttreatment improves functional and morphological outcome after fluid percussion injury in the rat. J Neurotrauma. 2001;18:821–832. doi: 10.1089/089771501316919184. [DOI] [PubMed] [Google Scholar]
- 275.Martin KR. Failla ML. Smith JC., Jr Beta-carotene and lutein protect HepG2 human liver cells against oxidant-induced damage. J Nutr. 1996;126:2098–2106. doi: 10.1093/jn/126.9.2098. [DOI] [PubMed] [Google Scholar]
- 276.Martin M. Macias M. Escames G. Leon J. Acuna-Castroviejo D. Melatonin but not vitamins C and E maintains glutathione homeostasis in t-butyl hydroperoxide-induced mitochondrial oxidative stress. FASEB J. 2000;14:1677–1679. doi: 10.1096/fj.99-0865fje. [DOI] [PubMed] [Google Scholar]
- 277.Massaad CA. Washington TM. Pautler RG. Klann E. Overexpression of SOD-2 reduces hippocampal superoxide and prevents memory deficits in a mouse model of Alzheimer's disease. Proc Natl Acad Sci USA. 2009;106:13576–13581. doi: 10.1073/pnas.0902714106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Maxwell CJ. Hicks MS. Hogan DB. Basran J. Ebly EM. Supplemental use of antioxidant vitamins and subsequent risk of cognitive decline and dementia. Dement Geriatr Cogn Disord. 2005;20:45–51. doi: 10.1159/000085074. [DOI] [PubMed] [Google Scholar]
- 279.McCaffery P. Zhang J. Crandall JE. Retinoic acid signaling and function in the adult hippocampus. J Neurobiol. 2006;66:780–791. doi: 10.1002/neu.20237. [DOI] [PubMed] [Google Scholar]
- 280.McCarty MF. Barroso-Aranda J. Contreras F. The “rejuvenatory” impact of lipoic acid on mitochondrial function in aging rats may reflect induction and activation of PPAR-gamma coactivator-1alpha. Med Hypotheses. 2009;72:29–33. doi: 10.1016/j.mehy.2008.07.043. [DOI] [PubMed] [Google Scholar]
- 281.McDonald SR. Sohal RS. Forster MJ. Concurrent administration of coenzyme Q10 and alpha-tocopherol improves learning in aged mice. Free Radic Biol Med. 2005;38:729–736. doi: 10.1016/j.freeradbiomed.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 282.McGahon BM. Martin DS. Horrobin DF. Lynch MA. Age-related changes in LTP and antioxidant defenses are reversed by an alpha-lipoic acid-enriched diet. Neurobiol Aging. 1999;20:655–664. doi: 10.1016/s0197-4580(99)00050-0. [DOI] [PubMed] [Google Scholar]
- 283.McLean CW. Mirochnitchenko O. Claus CP. Noble-Haeusslein LJ. Ferriero DM. Overexpression of glutathione peroxidase protects immature murine neurons from oxidative stress. Dev Neurosci. 2005;27:169–175. doi: 10.1159/000085989. [DOI] [PubMed] [Google Scholar]
- 284.McNeill G. Avenell A. Campbell MK. Cook JA. Hannaford PC. Kilonzo MM. Milne AC. Ramsay CR. Seymour DG. Stephen AI. Vale LD. Effect of multivitamin and multimineral supplementation on cognitive function in men and women aged 65 years and over: a randomised controlled trial. Nutr J. 2007;6:10. doi: 10.1186/1475-2891-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Mecocci P. MacGarvey U. Kaufman AE. Koontz D. Shoffner JM. Wallace DC. Beal MF. Oxidative damage to mitochondrial DNA shows marked age-dependent increases in human brain. Ann Neurol. 1993;34:609–616. doi: 10.1002/ana.410340416. [DOI] [PubMed] [Google Scholar]
- 286.Meffert MK. Haley JE. Schuman EM. Schulman H. Madison DV. Inhibition of hippocampal heme oxygenase, nitric oxide synthase, and long-term potentiation by metalloporphyrins. Neuron. 1994;13:1225–1233. doi: 10.1016/0896-6273(94)90060-4. [DOI] [PubMed] [Google Scholar]
- 287.Melov S. Adlard PA. Morten K. Johnson F. Golden TR. Hinerfeld D. Schilling B. Mavros C. Masters CL. Volitakis I. Li QX. Laughton K. Hubbard A. Cherny RA. Gibson B. Bush AI. Mitochondrial oxidative stress causes hyperphosphorylation of tau. PLoS ONE. 2007;2:e536. doi: 10.1371/journal.pone.0000536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Meydani M. Martin A. Ribaya-Mercado JD. Gong J. Blumberg JB. Russell RM. Beta-carotene supplementation increases antioxidant capacity of plasma in older women. J Nutr. 1994;124:2397–2403. doi: 10.1093/jn/124.12.397. [DOI] [PubMed] [Google Scholar]
- 289.Mieyal JJ. Srinivasan V. Starke DW. Gravina SA. Mieyal P. Glutathionyl specificity of thioltransferase: mechanistic and physiological applications. In: Packer L, editor; Cadenas E, editor. Biothiols in health and disease. New York: Marcel Decker; 1995. pp. 305–372. [Google Scholar]
- 290.Milbury PE, editor; Richer AC, editor. Understanding the Antioxidant Controversy: Scrutinizing the Fountain of Youth. Westport, CT: Greenwood Publishing Group; 2007. [Google Scholar]
- 291.Milgram NW. Araujo JA. Hagen TM. Treadwell BV. Ames BN. Acetyl-L-carnitine and alpha-lipoic acid supplementation of aged beagle dogs improves learning in two landmark discrimination tests. FASEB J. 2007;21:3756–3762. doi: 10.1096/fj.07-8531com. [DOI] [PubMed] [Google Scholar]
- 292.Miller ER., III Pastor-Barriuso R. Dalal D. Riemersma RA. Appel LJ. Guallar E. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005;142:37–46. doi: 10.7326/0003-4819-142-1-200501040-00110. [DOI] [PubMed] [Google Scholar]
- 293.Mingaud F. Mormede C. Etchamendy N. Mons N. Niedergang B. Wietrzych M. Pallet V. Jaffard R. Krezel W. Higueret P. Marighetto A. Retinoid hyposignaling contributes to aging-related decline in hippocampal function in short-term/working memory organization and long-term declarative memory encoding in mice. J Neurosci. 2008;28:279–291. doi: 10.1523/JNEUROSCI.4065-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Mishima K. Pu F. Kaneko T. Egashira N. Iwasaki K. Fujiwara M. Post-ischemic administration [correction of administeration] but not pre-ischemic administration [correction of administeration] of NG-nitro-L-arginine prevents spatial memory impairments and apoptosis by an inhibition of a delayed increase in NOx- in the hippocampus following repeated cerebral ischemia. Neuropharmacology. 2003;44:533–540. doi: 10.1016/s0028-3908(02)00404-5. [DOI] [PubMed] [Google Scholar]
- 295.Miyamoto E. Molecular mechanism of neuronal plasticity: induction and maintenance of long-term potentiation in the hippocampus. J Pharmacol Sci. 2006;100:433–442. doi: 10.1254/jphs.cpj06007x. [DOI] [PubMed] [Google Scholar]
- 296.Morris MC. Evans DA. Bienias JL. Tangney CC. Wilson RS. Vitamin E and cognitive decline in older persons. Arch Neurol. 2002;59:1125–1132. doi: 10.1001/archneur.59.7.1125. [DOI] [PubMed] [Google Scholar]
- 297.Muller FL. Lustgarten MS. Jang Y. Richardson A. Van Remmen H. Trends in oxidative aging theories. Free Radic Biol Med. 2007;43:477–503. doi: 10.1016/j.freeradbiomed.2007.03.034. [DOI] [PubMed] [Google Scholar]
- 298.Muller FL. Song W. Liu Y. Chaudhuri A. Pieke-Dahl S. Strong R. Huang TT. Epstein CJ. Roberts LJ. Csete M. Faulkner JA. Van Remmen H. Absence of CuZn superoxide dismutase leads to elevated oxidative stress and acceleration of age-dependent skeletal muscle atrophy. Free Radic Biol Med. 2006;40:1993–2004. doi: 10.1016/j.freeradbiomed.2006.01.036. [DOI] [PubMed] [Google Scholar]
- 299.Muller W. Bittner K. Differential oxidative modulation of voltage-dependent K+ currents in rat hippocampal neurons. J Neurophysiol. 2002;87:2990–2995. doi: 10.1152/jn.2002.87.6.2990. [DOI] [PubMed] [Google Scholar]
- 300.Murali G. Panneerselvam C. Modulatory role of glutathione monoester in augmenting age-associated neuronal antioxidant system. Exp Aging Res. 2008;34:419–436. doi: 10.1080/03610730802271906. [DOI] [PubMed] [Google Scholar]
- 301.Muriach M. Lopez-Pedrajas R. Barcia JM. Sanchez-Villarejo MV. Almansa I. Romero FJ. Cocaine causes memory and learning impairments in rats. Involvement of nuclear factor kappa B and oxidative stress, and prevention by topiramate. J Neurochem. 2010;114:675–684. doi: 10.1111/j.1471-4159.2010.06794.x. [DOI] [PubMed] [Google Scholar]
- 302.Murray CA. Lynch MA. Evidence that increased hippocampal expression of the cytokine interleukin-1 beta is a common trigger for age- and stress-induced impairments in long-term potentiation. J Neurosci. 1998;18:2974–2981. doi: 10.1523/JNEUROSCI.18-08-02974.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Nakashima AS. Dyck RH. Zinc and cortical plasticity. Brain Res Rev. 2009;59:347–373. doi: 10.1016/j.brainresrev.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 304.Nguyen PV. Woo NH. Regulation of hippocampal synaptic plasticity by cyclic AMP-dependent protein kinases. Prog Neurobiol. 2003;71:401–437. doi: 10.1016/j.pneurobio.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 305.Nguyen T. Huang HC. Pickett CB. Transcriptional regulation of the antioxidant response element. Activation by Nrf2 and repression by MafK. J Biol Chem. 2000;275:15466–15473. doi: 10.1074/jbc.M000361200. [DOI] [PubMed] [Google Scholar]
- 306.Nielsen S. Smith BL. Christensen EI. Agre P. Distribution of the aquaporin CHIP in secretory and resorptive epithelia and capillary endothelia. Proc Natl Acad Sci USA. 1993;90:7275–7279. doi: 10.1073/pnas.90.15.7275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Nitta A. Murakami Y. Furukawa Y. Kawatsura W. Hayashi K. Yamada K. Hasegawa T. Nabeshima T. Oral administration of idebenone induces nerve growth factor in the brain and improves learning and memory in basal forebrain-lesioned rats. Naunyn Schmiedebergs Arch Pharmacol. 1994;349:401–407. doi: 10.1007/BF00170887. [DOI] [PubMed] [Google Scholar]
- 308.Nizhnikov ME. Molina JC. Spear NE. Central reinforcing effects of ethanol are blocked by catalase inhibition. Alcohol. 2007;41:525–534. doi: 10.1016/j.alcohol.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Noor JI. Ikeda T. Mishima K. Aoo N. Ohta S. Egashira N. Iwasaki K. Fujiwara M. Ikenoue T. Short-term administration of a new free radical scavenger, edaravone, is more effective than its long-term administration for the treatment of neonatal hypoxic-ischemic encephalopathy. Stroke. 2005;36:2468–2474. doi: 10.1161/01.STR.0000185653.49740.c6. [DOI] [PubMed] [Google Scholar]
- 310.Nordberg M. Nordberg GF. Toxicological aspects of metallothionein. Cell Mol Biol. 2000;46:451–463. [PubMed] [Google Scholar]
- 311.Ochiai T. Shimeno H. Mishima K. Iwasaki K. Fujiwara M. Tanaka H. Shoyama Y. Toda A. Eyanagi R. Soeda S. Protective effects of carotenoids from saffron on neuronal injury in vitro and in vivo. Biochim Biophys Acta. 2007;1770:578–584. doi: 10.1016/j.bbagen.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 312.Ohwada K. Takeda H. Yamazaki M. Isogai H. Nakano M. Shimomura M. Fukui K. Urano S. Pyrroloquinoline quinone (PQQ) prevents cognitive deficit caused by oxidative stress in rats. J Clin Biochem Nutr. 2008;42:29–34. doi: 10.3164/jcbn.2008005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Olanow CW. Oxidation reactions in Parkinson's disease. Neurology. 1990;40(10 Suppl 3) suppl 32–37; discussion 37–39. [PubMed] [Google Scholar]
- 314.Olcese JM. Cao C. Mori T. Mamcarz MB. Maxwell A. Runfeldt MJ. Wang L. Zhang C. Lin X. Zhang G. Arendash GW. Protection against cognitive deficits and markers of neurodegeneration by long-term oral administration of melatonin in a transgenic model of Alzheimer disease. J Pineal Res. 2009;47:82–96. doi: 10.1111/j.1600-079X.2009.00692.x. [DOI] [PubMed] [Google Scholar]
- 315.Omaye ST. Burri BJ. Swendseid ME. Henning SM. Briggs LA. Bowen HT. Ota RB. Blood antioxidants changes in young women following beta-carotene depletion and repletion. J Am Coll Nutr. 1996;15:469–474. doi: 10.1080/07315724.1996.10718626. [DOI] [PubMed] [Google Scholar]
- 316.Orr WC. Radyuk SN. Prabhudesai L. Toroser D. Benes JJ. Luchak JM. Mockett RJ. Rebrin I. Hubbard JG. Sohal RS. Overexpression of glutamate-cysteine ligase extends life span in Drosophila melanogaster. J Biol Chem. 2005;280:37331–37338. doi: 10.1074/jbc.M508272200. [DOI] [PubMed] [Google Scholar]
- 317.Otani H. Togashi H. Jesmin S. Sakuma I. Yamaguchi T. Matsumoto M. Kakehata H. Yoshioka M. Temporal effects of edaravone, a free radical scavenger, on transient ischemia-induced neuronal dysfunction in the rat hippocampus. Eur J Pharmacol. 2005;512:129–137. doi: 10.1016/j.ejphar.2005.01.050. [DOI] [PubMed] [Google Scholar]
- 318.Ozcan M. Yilmaz B. Carpenter DO. Effects of melatonin on synaptic transmission and long-term potentiation in two areas of mouse hippocampus. Brain Res. 2006;1111:90–94. doi: 10.1016/j.brainres.2006.06.117. [DOI] [PubMed] [Google Scholar]
- 319.Ozdemir D. Tugyan K. Uysal N. Sonmez U. Sonmez A. Acikgoz O. Ozdemir N. Duman M. Ozkan H. Protective effect of melatonin against head trauma-induced hippocampal damage and spatial memory deficits in immature rats. Neurosci Lett. 2005;385:234–239. doi: 10.1016/j.neulet.2005.05.055. [DOI] [PubMed] [Google Scholar]
- 320.Packer L. Witt EH. Tritschler HJ. Alpha-lipoic acid as a biological antioxidant. Free Radic Biol Med. 1995;19:227–250. doi: 10.1016/0891-5849(95)00017-r. [DOI] [PubMed] [Google Scholar]
- 321.Paiva SA. Russell RM. Beta-carotene and other carotenoids as antioxidants. J Am Coll Nutr. 1999;18:426–433. doi: 10.1080/07315724.1999.10718880. [DOI] [PubMed] [Google Scholar]
- 322.Pak JH. Huang FL. Li J. Balschun D. Reymann KG. Chiang C. Westphal H. Huang KP. Involvement of neurogranin in the modulation of calcium/calmodulin-dependent protein kinase II, synaptic plasticity, and spatial learning: a study with knockout mice. Proc Natl Acad Sci USA. 2000;97:11232–11237. doi: 10.1073/pnas.210184697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Palozza P. Calviello G. Bartoli GM. Prooxidant activity of beta-carotene under 100% oxygen pressure in rat liver microsomes. Free Radic Biol Med. 1995;19:887–892. doi: 10.1016/0891-5849(95)00094-e. [DOI] [PubMed] [Google Scholar]
- 324.Palozza P. Luberto C. Calviello G. Ricci P. Bartoli GM. Antioxidant and prooxidant role of beta-carotene in murine normal and tumor thymocytes: effects of oxygen partial pressure. Free Radic Biol Med. 1997;22:1065–1073. doi: 10.1016/s0891-5849(96)00498-4. [DOI] [PubMed] [Google Scholar]
- 325.Park JD. Liu Y. Klaassen CD. Protective effect of metallothionein against the toxicity of cadmium and other metals(1) Toxicology. 2001;163:93–100. doi: 10.1016/s0300-483x(01)00375-4. [DOI] [PubMed] [Google Scholar]
- 326.Pekcetin C. Kiray M. Ergur BU. Tugyan K. Bagriyanik HA. Erbil G. Baykara B. Camsari UM. Carnosine attenuates oxidative stress and apoptosis in transient cerebral ischemia in rats. Acta Biol Hung. 2009;60:137–148. doi: 10.1556/ABiol.60.2009.2.1. [DOI] [PubMed] [Google Scholar]
- 327.Pepe S. Marasco SF. Haas SJ. Sheeran FL. Krum H. Rosenfeldt FL. Coenzyme Q10 in cardiovascular disease. Mitochondrion. 2007;7(Suppl):S154–S167. doi: 10.1016/j.mito.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 328.Perkins AJ. Hendrie HC. Callahan CM. Gao S. Unverzagt FW. Xu Y. Hall KS. Hui SL. Association of antioxidants with memory in a multiethnic elderly sample using the Third National Health and Nutrition Examination Survey. Am J Epidemiol. 1999;150:37–44. doi: 10.1093/oxfordjournals.aje.a009915. [DOI] [PubMed] [Google Scholar]
- 329.Perrig WJ. Perrig P. Stahelin HB. The relation between antioxidants and memory performance in the old and very old. J Am Geriatr Soc. 1997;45:718–724. doi: 10.1111/j.1532-5415.1997.tb01476.x. [DOI] [PubMed] [Google Scholar]
- 330.Pieri C. Marra M. Moroni F. Recchioni R. Marcheselli F. Melatonin: a peroxyl radical scavenger more effective than vitamin E. Life Sci. 1994;55:L271–L276. doi: 10.1016/0024-3205(94)00666-0. [DOI] [PubMed] [Google Scholar]
- 331.Pieri C. Moroni F. Marra M. Marcheselli F. Recchioni R. Melatonin is an efficient antioxidant. Arch Gerontol Geriatr. 1995;20:159–165. doi: 10.1016/0167-4943(94)00593-v. [DOI] [PubMed] [Google Scholar]
- 332.Pineda JA. Aono M. Sheng H. Lynch J. Wellons JC. Laskowitz DT. Pearlstein RD. Bowler R. Crapo J. Warner DS. Extracellular superoxide dismutase overexpression improves behavioral outcome from closed head injury in the mouse. J Neurotrauma. 2001;18:625–634. doi: 10.1089/089771501750291864. [DOI] [PubMed] [Google Scholar]
- 333.Pitsikas N. Zisopoulou S. Tarantilis PA. Kanakis CD. Polissiou MG. Sakellaridis N. Effects of the active constituents of Crocus sativus L., crocins on recognition and spatial rats' memory. Behav Brain Res. 2007;183:141–146. doi: 10.1016/j.bbr.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 334.Podda M. Tritschler HJ. Ulrich H. Packer L. Alpha-lipoic acid supplementation prevents symptoms of vitamin E deficiency. Biochem Biophys Res Commun. 1994;204:98–104. doi: 10.1006/bbrc.1994.2431. [DOI] [PubMed] [Google Scholar]
- 335.Poeggeler B. Saarela S. Reiter RJ. Tan DX. Chen LD. Manchester LC. Barlow-Walden LR. Melatonin—a highly potent endogenous radical scavenger and electron donor: new aspects of the oxidation chemistry of this indole accessed in vitro. Ann NY Acad Sci. 1994;738:419–420. doi: 10.1111/j.1749-6632.1994.tb21831.x. [DOI] [PubMed] [Google Scholar]
- 336.Pollock JD. Williams DA. Gifford MA. Li LL. Du X. Fisherman J. Orkin SH. Doerschuk CM. Dinauer MC. Mouse model of X-linked chronic granulomatous disease, an inherited defect in phagocyte superoxide production. Nat Genet. 1995;9:202–209. doi: 10.1038/ng0295-202. [DOI] [PubMed] [Google Scholar]
- 337.Poon HF. Calabrese V. Scapagnini G. Butterfield DA. Free radicals and brain aging. Clin Geriatr Med. 2004;20:329–359. doi: 10.1016/j.cger.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 338.Poon HF. Farr SA. Banks WA. Pierce WM. Klein JB. Morley JE. Butterfield DA. Proteomic identification of less oxidized brain proteins in aged senescence-accelerated mice following administration of antisense oligonucleotide directed at the Abeta region of amyloid precursor protein. Brain Res Mol Brain Res. 2005;138:8–16. doi: 10.1016/j.molbrainres.2005.02.020. [DOI] [PubMed] [Google Scholar]
- 339.Poon HF. Farr SA. Thongboonkerd V. Lynn BC. Banks WA. Morley JE. Klein JB. Butterfield DA. Proteomic analysis of specific brain proteins in aged SAMP8 mice treated with alpha-lipoic acid: implications for aging and age-related neurodegenerative disorders. Neurochem Int. 2005;46:159–168. doi: 10.1016/j.neuint.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 340.Poser S. Storm DR. Role of Ca2+-stimulated adenylyl cyclases in LTP and memory formation. Int J Dev Neurosci. 2001;19:387–394. doi: 10.1016/s0736-5748(00)00094-0. [DOI] [PubMed] [Google Scholar]
- 341.Poss KD. Thomas MJ. Ebralidze AK. O'Dell TJ. Tonegawa S. Hippocampal long-term potentiation is normal in heme oxygenase-2 mutant mice. Neuron. 1995;15:867–873. doi: 10.1016/0896-6273(95)90177-9. [DOI] [PubMed] [Google Scholar]
- 342.Pou S. Keaton L. Surichamorn W. Rosen GM. Mechanism of superoxide generation by neuronal nitric-oxide synthase. J Biol Chem. 1999;274:9573–9580. doi: 10.1074/jbc.274.14.9573. [DOI] [PubMed] [Google Scholar]
- 343.Pou S. Pou WS. Bredt DS. Snyder SH. Rosen GM. Generation of superoxide by purified brain nitric oxide synthase. J Biol Chem. 1992;267:24173–24176. [PubMed] [Google Scholar]
- 344.Pu F. Mishima K. Irie K. Motohashi K. Tanaka Y. Orito K. Egawa T. Kitamura Y. Egashira N. Iwasaki K. Fujiwara M. Neuroprotective effects of quercetin and rutin on spatial memory impairment in an 8-arm radial maze task and neuronal death induced by repeated cerebral ischemia in rats. J Pharmacol Sci. 2007;104:329–334. doi: 10.1254/jphs.fp0070247. [DOI] [PubMed] [Google Scholar]
- 345.Qi W. Reiter RJ. Tan DX. Garcia JJ. Manchester LC. Karbownik M. Calvo JR. Chromium(III)-induced 8-hydroxydeoxyguanosine in DNA and its reduction by antioxidants: comparative effects of melatonin, ascorbate, and vitamin E. Environ Health Perspect. 2000;108:399–402. doi: 10.1289/ehp.00108399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Quertemont E. Escarabajal MD. De Witte P. Role of catalase in ethanol-induced conditioned taste aversion: a study with 3-amino-1,2,4-triazole. Drug Alcohol Depend. 2003;70:77–83. doi: 10.1016/s0376-8716(02)00341-1. [DOI] [PubMed] [Google Scholar]
- 347.Quick KL. Ali SS. Arch R. Xiong C. Wozniak D. Dugan LL. A carboxyfullerene SOD mimetic improves cognition and extends the lifespan of mice. Neurobiol Aging. 2008;29:117–128. doi: 10.1016/j.neurobiolaging.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 348.Quinn JF. Bussiere JR. Hammond RS. Montine TJ. Henson E. Jones RE. Stackman RW., Jr Chronic dietary alpha-lipoic acid reduces deficits in hippocampal memory of aged Tg2576 mice. Neurobiol Aging. 2007;28:213–225. doi: 10.1016/j.neurobiolaging.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 349.Quinn MT. Gauss KA. Structure and regulation of the neutrophil respiratory burst oxidase: comparison with nonphagocyte oxidases. J Leukoc Biol. 2004;76:760–781. doi: 10.1189/jlb.0404216. [DOI] [PubMed] [Google Scholar]
- 350.Radi R. Beckman JS. Bush KM. Freeman BA. Peroxynitrite oxidation of sulfhydryls. The cytotoxic potential of superoxide and nitric oxide. J Biol Chem. 1991;266:4244–4250. [PubMed] [Google Scholar]
- 351.Raghavendra M. Trigunayat A. Singh RK. Mitra S. Goel RK. Acharya SB. Effect of ethanolic extract of root of Pongamia pinnata (L) pierre on oxidative stress, behavioral and histopathological alterations induced by cerebral ischemia—reperfusion and long-term hypoperfusion in rats. Indian J Exp Biol. 2007;45:868–876. [PubMed] [Google Scholar]
- 352.Raghavendra V. Kulkarni SK. Possible antioxidant mechanism in melatonin reversal of aging and chronic ethanol-induced amnesia in plus-maze and passive avoidance memory tasks. Free Radic Biol Med. 2001;30:595–602. doi: 10.1016/s0891-5849(00)00447-0. [DOI] [PubMed] [Google Scholar]
- 353.Ramakers GM. Pasinelli P. Hens JJ. Gispen WH. De Graan PN. Protein kinase C in synaptic plasticity: changes in the in situ phosphorylation state of identified pre- and postsynaptic substrates. Prog Neuropsychopharmacol Biol Psychiatry. 1997;21:455–486. doi: 10.1016/s0278-5846(97)00013-4. [DOI] [PubMed] [Google Scholar]
- 354.Rao PV. Maddala R. John F. Zigler JS., Jr Expression of nonphagocytic NADPH oxidase system in the ocular lens. Mol Vis. 2004;10:112–121. [PubMed] [Google Scholar]
- 355.Rawashdeh O. de Borsetti NH. Roman G. Cahill GM. Melatonin suppresses nighttime memory formation in zebrafish. Science. 2007;318:1144–1146. doi: 10.1126/science.1148564. [DOI] [PubMed] [Google Scholar]
- 356.Ray R. Shah AM. NADPH oxidase and endothelial cell function. Clin Sci (Lond) 2005;109:217–226. doi: 10.1042/CS20050067. [DOI] [PubMed] [Google Scholar]
- 357.Reagan LP. McEwen BS. Diabetes, but not stress, reduces neuronal nitric oxide synthase expression in rat hippocampus: implications for hippocampal synaptic plasticity. Neuroreport. 2002;13:1801–1804. doi: 10.1097/00001756-200210070-00022. [DOI] [PubMed] [Google Scholar]
- 358.Reis EA. Zugno AI. Franzon R. Tagliari B. Matte C. Lammers ML. Netto CA. Wyse AT. Pretreatment with vitamins E and C prevent the impairment of memory caused by homocysteine administration in rats. Metab Brain Dis. 2002;17:211–217. doi: 10.1023/a:1019982223034. [DOI] [PubMed] [Google Scholar]
- 359.Reiter RJ. Acuna-Castroviejo D. Tan DX. Burkhardt S. Free radical-mediated molecular damage. Mechanisms for the protective actions of melatonin in the central nervous system. Ann NY Acad Sci. 2001;939:200–215. [PubMed] [Google Scholar]
- 360.Reiter RJ. Tan DX. Leon J. Kilic U. Kilic E. When melatonin gets on your nerves: its beneficial actions in experimental models of stroke. Exp Biol Med (Maywood) 2005;230:104–117. doi: 10.1177/153537020523000205. [DOI] [PubMed] [Google Scholar]
- 361.Reiter RJ. Tan DX. Terron MP. Flores LJ. Czarnocki Z. Melatonin and its metabolites: new findings regarding their production and their radical scavenging actions. Acta Biochim Pol. 2007;54:1–9. [PubMed] [Google Scholar]
- 362.Reynolds IJ. Hastings TG. Glutamate induces the production of reactive oxygen species in cultured forebrain neurons following NMDA receptor activation. J Neurosci. 1995;15:3318–3327. doi: 10.1523/JNEUROSCI.15-05-03318.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Rimmele U. Spillmann M. Bartschi C. Wolf OT. Weber CS. Ehlert U. Wirtz PH. Melatonin improves memory acquisition under stress independent of stress hormone release. Psychopharmacology (Berl) 2009;202:663–672. doi: 10.1007/s00213-008-1344-z. [DOI] [PubMed] [Google Scholar]
- 364.Rosa RM. Flores DG. Appelt HR. Braga AL. Henriques JA. Roesler R. Facilitation of long-term object recognition memory by pretraining administration of diphenyl diselenide in mice. Neurosci Lett. 2003;341:217–220. doi: 10.1016/s0304-3940(03)00187-3. [DOI] [PubMed] [Google Scholar]
- 365.Rotruck JT. Pope AL. Ganther HE. Swanson AB. Hafeman DG. Hoekstra WG. Selenium: biochemical role as a component of glutathione peroxidase. Science. 1973;179:588–590. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- 366.Sacktor N. Schifitto G. McDermott MP. Marder K. McArthur JC. Kieburtz K. Transdermal selegiline in HIV-associated cognitive impairment: pilot, placebo-controlled study. Neurology. 2000;54:233–235. doi: 10.1212/wnl.54.1.233. [DOI] [PubMed] [Google Scholar]
- 367.Sarter M. van der LA. Vitamin E deprivation in rats: some behavioral and histochemical observations. Neurobiol Aging. 1987;8:297–307. doi: 10.1016/0197-4580(87)90068-6. [DOI] [PubMed] [Google Scholar]
- 368.Sato M. Bremner I. Oxygen free radicals and metallothionein. Free Radic Biol Med. 1993;14:325–337. doi: 10.1016/0891-5849(93)90029-t. [DOI] [PubMed] [Google Scholar]
- 369.Schuman EM. Madison DV. A requirement for the intercellular messenger nitric oxide in long-term potentiation. Science. 1991;254:1503–1506. doi: 10.1126/science.1720572. [DOI] [PubMed] [Google Scholar]
- 370.Seif-El-Nasr M. Atia AS. Abdelsalam RM. Effect of MAO-B inhibition against ischemia-induced oxidative stress in the rat brain. Comparison with a rational antioxidant. Arzneimittelforschung. 2008;58:160–167. doi: 10.1055/s-0031-1296487. [DOI] [PubMed] [Google Scholar]
- 371.Sentman ML. Granstrom M. Jakobson H. Reaume A. Basu S. Marklund SL. Phenotypes of mice lacking extracellular superoxide dismutase and copper- and zinc-containing superoxide dismutase. J Biol Chem. 2006;281:6904–6909. doi: 10.1074/jbc.M510764200. [DOI] [PubMed] [Google Scholar]
- 372.Serrano F. Chang A. Hernandez C. Pautler RG. Sweatt JD. Klann E. NADPH oxidase mediates beta-amyloid peptide-induced activation of ERK in hippocampal organotypic cultures. Mol Brain. 2:31–2009. doi: 10.1186/1756-6606-2-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Serrano F. Kolluri NS. Wientjes FB. Card JP. Klann E. NADPH oxidase immunoreactivity in the mouse brain. Brain Res. 2003;988:193–198. doi: 10.1016/s0006-8993(03)03364-x. [DOI] [PubMed] [Google Scholar]
- 374.Shahidi S. Komaki A. Mahmoodi M. Atrvash N. Ghodrati M. Ascorbic acid supplementation could affect passive avoidance learning and memory in rat. Brain Res Bull. 2008;76:109–113. doi: 10.1016/j.brainresbull.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 375.Shaji AV. Kulkarni SK. Evidence of GABAergic modulation in melatonin-induced short-term memory deficits and food consumption. Methods Find Exp Clin Pharmacol. 1998;20:311–319. doi: 10.1358/mf.1998.20.4.485685. [DOI] [PubMed] [Google Scholar]
- 376.Shang YZ. Miao H. Cheng JJ. Qi JM. Effects of amelioration of total flavonoids from stems and leaves of Scutellaria baicalensis Georgi on cognitive deficits, neuronal damage and free radicals disorder induced by cerebral ischemia in rats. Biol Pharm Bull. 2006;29:805–810. doi: 10.1248/bpb.29.805. [DOI] [PubMed] [Google Scholar]
- 377.Sharma M. Briyal S. Gupta YK. Effect of alpha lipoic acid, melatonin and trans resveratrol on intracerebroventricular streptozotocin induced spatial memory deficit in rats. Indian J Physiol Pharmacol. 2005;49:395–402. [PubMed] [Google Scholar]
- 378. This reference has been deleted.
- 379.Sharma M. Gupta YK. Effect of chronic treatment of melatonin on learning, memory and oxidative deficiencies induced by intracerebroventricular streptozotocin in rats. Pharmacol Biochem Behav. 2001;70:325–331. doi: 10.1016/s0091-3057(01)00611-6. [DOI] [PubMed] [Google Scholar]
- 380.Sharma M. Gupta YK. Effect of alpha lipoic acid on intracerebroventricular streptozotocin model of cognitive impairment in rats. Eur Neuropsychopharmacol. 2003;13:241–247. doi: 10.1016/s0924-977x(03)00008-7. [DOI] [PubMed] [Google Scholar]
- 381.Shen Y. Zhang G. Liu L. Xu S. Suppressive effects of melatonin on amyloid-beta-induced glial activation in rat hippocampus. Arch Med Res. 2007;38:284–290. doi: 10.1016/j.arcmed.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 382.Shen YX. Wei W. Yang J. Liu C. Dong C. Xu SY. Improvement of melatonin to the learning and memory impairment induced by amyloid beta-peptide 25–35 in elder rats. Acta Pharmacol Sin. 2001;22:797–803. [PubMed] [Google Scholar]
- 383.Shen YX. Xu SY. Wei W. Sun XX. Liu LH. Yang J. Dong C. The protective effects of melatonin from oxidative damage induced by amyloid beta-peptide 25–35 in middle-aged rats. J Pineal Res. 2002;32:85–89. doi: 10.1034/j.1600-079x.2002.1819.x. [DOI] [PubMed] [Google Scholar]
- 384.Shen YX. Xu SY. Wei W. Sun XX. Yang J. Liu LH. Dong C. Melatonin reduces memory changes and neural oxidative damage in mice treated with D-galactose. J Pineal Res. 2002;32:173–178. doi: 10.1034/j.1600-079x.2002.1o850.x. [DOI] [PubMed] [Google Scholar]
- 385.Shetty PK. Huang FL. Huang KP. Ischemia-elicited oxidative modulation of Ca2+/calmodulin-dependent protein kinase II. J Biol Chem. 2008;283:5389–5401. doi: 10.1074/jbc.M708479200. [DOI] [PubMed] [Google Scholar]
- 386.Sheu FS. Mahoney CW. Seki K. Huang KP. Nitric oxide modification of rat brain neurogranin affects its phosphorylation by protein kinase C and affinity for calmodulin. J Biol Chem. 1996;271:22407–22413. doi: 10.1074/jbc.271.37.22407. [DOI] [PubMed] [Google Scholar]
- 387.Shin JH. London J. Le Pecheur M. Hoger H. Pollak D. Lubec G. Aberrant neuronal and mitochondrial proteins in hippocampus of transgenic mice overexpressing human Cu/Zn superoxide dismutase 1. Free Radic Biol Med. 2004;37:643–653. doi: 10.1016/j.freeradbiomed.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 388.Shinomura T. Nakao S. Mori K. Reduction of depolarization-induced glutamate release by heme oxygenase inhibitor: possible role of carbon monoxide in synaptic transmission. Neurosci Lett. 1994;166:131–134. doi: 10.1016/0304-3940(94)90468-5. [DOI] [PubMed] [Google Scholar]
- 389.Shukitt-Hale B. Erat SA. Joseph JA. Spatial learning and memory deficits induced by dopamine administration with decreased glutathione. Free Radic Biol Med. 1998;24:1149–1158. doi: 10.1016/s0891-5849(97)00399-7. [DOI] [PubMed] [Google Scholar]
- 390.Smith CD. Carney JM. Starke-Reed PE. Oliver CN. Stadtman ER. Floyd RA. Markesbery WR. Excess brain protein oxidation and enzyme dysfunction in normal aging and in Alzheimer disease. Proc Natl Acad Sci USA. 1991;88:10540–10543. doi: 10.1073/pnas.88.23.10540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Smith MA. Richey Harris PL. Sayre LM. Beckman JS. Perry G. Widespread peroxynitrite-mediated damage in Alzheimer's disease. J Neurosci. 1997;17:2653–2657. doi: 10.1523/JNEUROSCI.17-08-02653.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Sohal RS. Mockett RJ. Orr WC. Mechanisms of aging: an appraisal of the oxidative stress hypothesis. Free Radic Biol Med. 2002;33:575–586. doi: 10.1016/s0891-5849(02)00886-9. [DOI] [PubMed] [Google Scholar]
- 393.Soto-Moyano R. Burgos H. Flores F. Valladares L. Sierralta W. Fernandez V. Perez H. Hernandez P. Hernandez A. Melatonin administration impairs visuo-spatial performance and inhibits neocortical long-term potentiation in rats. Pharmacol Biochem Behav. 2006;85:408–414. doi: 10.1016/j.pbb.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 394.St Pierre J. Buckingham JA. Roebuck SJ. Brand MD. Topology of superoxide production from different sites in the mitochondrial electron transport chain. J Biol Chem. 2002;277:44784–44790. doi: 10.1074/jbc.M207217200. [DOI] [PubMed] [Google Scholar]
- 395.Stahl W. Sies H. Uptake of lycopene and its geometrical isomers is greater from heat-processed than from unprocessed tomato juice in humans. J Nutr. 1992;122:2161–2166. doi: 10.1093/jn/122.11.2161. [DOI] [PubMed] [Google Scholar]
- 396.Stevens CF. Wang Y. Reversal of long-term potentiation by inhibitors of haem oxygenase. Nature. 1993;364:147–149. doi: 10.1038/364147a0. [DOI] [PubMed] [Google Scholar]
- 397.Stone JR. Marletta MA. Soluble guanylate cyclase from bovine lung: activation with nitric oxide and carbon monoxide and spectral characterization of the ferrous and ferric states. Biochemistry. 1994;33:5636–5640. doi: 10.1021/bi00184a036. [DOI] [PubMed] [Google Scholar]
- 398.Stowe DF. Camara AK. Mitochondrial reactive oxygen species production in excitable cells: modulators of mitochondrial and cell function. Antioxid Redox Signal. 2009;11:1373–1414. doi: 10.1089/ars.2008.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Su JH. Deng G. Cotman CW. Neuronal DNA damage precedes tangle formation and is associated with up-regulation of nitrotyrosine in Alzheimer's disease brain. Brain Res. 1997;774:193–199. doi: 10.1016/s0006-8993(97)81703-9. [DOI] [PubMed] [Google Scholar]
- 400.Sugiura M. Shoyama Y. Saito H. Abe K. Crocin (crocetin di-gentiobiose ester) prevents the inhibitory effect of ethanol on long-term potentiation in the dentate gyrus in vivo. J Pharmacol Exp Ther. 1994;271:703–707. [PubMed] [Google Scholar]
- 401.Sugiura M. Shoyama Y. Saito H. Abe K. The effects of ethanol and crocin on the induction of long-term potentiation in the CA1 region of rat hippocampal slices. Jpn J Pharmacol. 1995;67:395–397. doi: 10.1254/jjp.67.395. [DOI] [PubMed] [Google Scholar]
- 402.Suh YA. Arnold RS. Lassegue B. Shi J. Xu X. Sorescu D. Chung AB. Griendling KK. Lambeth JD. Cell transformation by the superoxide-generating oxidase Mox1. Nature. 1999;401:79–82. doi: 10.1038/43459. [DOI] [PubMed] [Google Scholar]
- 403.Sumien N. Heinrich KR. Shetty RA. Sohal RS. Forster MJ. Prolonged intake of coenzyme Q10 impairs cognitive functions in mice. J Nutr. 2009;139:1926–1932. doi: 10.3945/jn.109.110437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Sumimoto H. Miyano K. Takeya R. Molecular composition and regulation of the Nox family NAD(P)H oxidases. Biochem Biophys Res Commun. 2005;338:677–686. doi: 10.1016/j.bbrc.2005.08.210. [DOI] [PubMed] [Google Scholar]
- 405.Sweatt JD. The neuronal MAP kinase cascade: a biochemical signal integration system subserving synaptic plasticity and memory. J Neurochem. 2001;76:1–10. doi: 10.1046/j.1471-4159.2001.00054.x. [DOI] [PubMed] [Google Scholar]
- 406.Sykes AG. Advances in Inorganic Chemistry. London, UK: Academic Press; 1998. [Google Scholar]
- 407.Takahata K. Minami A. Kusumoto H. Shimazu S. Yoneda F. Effects of selegiline alone or with donepezil on memory impairment in rats. Eur J Pharmacol. 2005;518:140–144. doi: 10.1016/j.ejphar.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 408.Talaei SA. Sheibani V. Salami M. Light deprivation improves melatonin related suppression of hippocampal plasticity. Hippocampus. 2010;20:447–455. doi: 10.1002/hipo.20650. [DOI] [PubMed] [Google Scholar]
- 409.Tammariello SP. Quinn MT. Estus S. NADPH oxidase contributes directly to oxidative stress and apoptosis in nerve growth factor-deprived sympathetic neurons. J Neurosci. 2000;20:RC53. doi: 10.1523/JNEUROSCI.20-01-j0006.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Tan DX. Manchester LC. Reiter RJ. Qi WB. Karbownik M. Calvo JR. Significance of melatonin in antioxidative defense system: reactions and products. Biol Signals Recept. 2000;9:137–159. doi: 10.1159/000014635. [DOI] [PubMed] [Google Scholar]
- 411.Tan DX. Manchester LC. Terron MP. Flores LJ. Reiter RJ. One molecule, many derivatives: a never-ending interaction of melatonin with reactive oxygen and nitrogen species? J Pineal Res. 2007;42:28–42. doi: 10.1111/j.1600-079X.2006.00407.x. [DOI] [PubMed] [Google Scholar]
- 412.Tan DX. Reiter RJ. Manchester LC. Yan MT. El Sawi M. Sainz RM. Mayo JC. Kohen R. Allegra M. Hardeland R. Chemical and physical properties and potential mechanisms: melatonin as a broad spectrum antioxidant and free radical scavenger. Curr Top Med Chem. 2002;2:181–197. doi: 10.2174/1568026023394443. [DOI] [PubMed] [Google Scholar]
- 413.Tang F. Nag S. Shiu SY. Pang SF. The effects of melatonin and Ginkgo biloba extract on memory loss and choline acetyltransferase activities in the brain of rats infused intracerebroventricularly with beta-amyloid 1–40. Life Sci. 2002;71:2625–2631. doi: 10.1016/s0024-3205(02)02105-7. [DOI] [PubMed] [Google Scholar]
- 414.Tanzi RE. Bertram L. Twenty years of the Alzheimer's disease amyloid hypothesis: a genetic perspective. Cell. 2005;120:545–555. doi: 10.1016/j.cell.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 415.Tejada-Simon MV. Serrano F. Villasana LE. Kanterewicz BI. Wu GY. Quinn MT. Klann E. Synaptic localization of a functional NADPH oxidase in the mouse hippocampus. Mol Cell Neurosci. 2005;29:97–106. doi: 10.1016/j.mcn.2005.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Tenhunen R. Marver HS. Schmid R. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc Natl Acad Sci USA. 1968;61:748–755. doi: 10.1073/pnas.61.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Thiels E. Urban NN. Gonzalez-Burgos GR. Kanterewicz BI. Barrionuevo G. Chu CT. Oury TD. Klann E. Impairment of long-term potentiation and associative memory in mice that overexpress extracellular superoxide dismutase. J Neurosci. 2000;20:7631–7639. doi: 10.1523/JNEUROSCI.20-20-07631.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Tsunekawa H. Noda Y. Mouri A. Yoneda F. Nabeshima T. Synergistic effects of selegiline and donepezil on cognitive impairment induced by amyloid beta (25–35) Behav Brain Res. 2008;190:224–232. doi: 10.1016/j.bbr.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 419.Tsuru-Aoyagi K. Potts MB. Trivedi A. Pfankuch T. Raber J. Wendland M. Claus CP. Koh SE. Ferriero D. Noble-Haeusslein LJ. Glutathione peroxidase activity modulates recovery in the injured immature brain. Ann Neurol. 2009;65:540–549. doi: 10.1002/ana.21600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Turrens JF. Beconi M. Barilla J. Chavez UB. McCord JM. Mitochondrial generation of oxygen radicals during reoxygenation of ischemic tissues. Free Radic Res Commun. 1991;12–13(Pt 2):681–689. doi: 10.3109/10715769109145847. [DOI] [PubMed] [Google Scholar]
- 421.Tuzcu M. Baydas G. Effect of melatonin and vitamin E on diabetes-induced learning and memory impairment in rats. Eur J Pharmacol. 2006;537:106–110. doi: 10.1016/j.ejphar.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 422.Tveden-Nyborg P. Johansen LK. Raida Z. Villumsen CK. Larsen JO. Lykkesfeldt J. Vitamin C deficiency in early postnatal life impairs spatial memory and reduces the number of hippocampal neurons in guinea pigs. Am J Clin Nutr. 2009;90:540–546. doi: 10.3945/ajcn.2009.27954. [DOI] [PubMed] [Google Scholar]
- 423.Uc EY. McDermott MP. Marder KS. Anderson SW. Litvan I. Como PG. Auinger P. Chou KL. Growdon JC. Incidence of and risk factors for cognitive impairment in an early Parkinson disease clinical trial cohort. Neurology. 2009;73:1469–1477. doi: 10.1212/WNL.0b013e3181bf992f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Vallet P. Charnay Y. Steger K. Ogier-Denis E. Kovari E. Herrmann F. Michel JP. Szanto I. Neuronal expression of the NADPH oxidase NOX4, and its regulation in mouse experimental brain ischemia. Neuroscience. 2005;132:233–238. doi: 10.1016/j.neuroscience.2004.12.038. [DOI] [PubMed] [Google Scholar]
- 425.Vanden Hoek TL. Becker LB. Shao Z. Li C. Schumacker PT. Reactive oxygen species released from mitochondria during brief hypoxia induce preconditioning in cardiomyocytes. J Biol Chem. 1998;273:18092–18098. doi: 10.1074/jbc.273.29.18092. [DOI] [PubMed] [Google Scholar]
- 426.Voet D, editor; Voet JG, editor; Pratt CW, editor. Fundamentals of Biochemistry. Life at the Molecular Level. New York: John Wiley and Sons; 2008. [Google Scholar]
- 427.Voronkova KV. Meleshkov MN. Use of Noben (idebenone) in the treatment of dementia and memory impairments without dementia. Neurosci Behav Physiol. 2009;39:501–506. doi: 10.1007/s11055-009-9148-0. [DOI] [PubMed] [Google Scholar]
- 428.Waltereit R. Weller M. Signaling from cAMP/PKA to MAPK and synaptic plasticity. Mol Neurobiol. 2003;27:99–106. doi: 10.1385/MN:27:1:99. [DOI] [PubMed] [Google Scholar]
- 429.Wang HL. Chen XT. Yin ST. Liu J. Tang ML. Wu CY. Ruan DY. Opposite effects of alpha-lipoic acid on antioxidation and long-term potentiation in control and chronically lead-exposed rats. Naunyn Schmiedebergs Arch Pharmacol. 2008;378:303–310. doi: 10.1007/s00210-008-0307-6. [DOI] [PubMed] [Google Scholar]
- 430.Wang LM. Suthana NA. Chaudhury D. Weaver DR. Colwell CS. Melatonin inhibits hippocampal long-term potentiation. Eur J Neurosci. 2005;22:2231–2237. doi: 10.1111/j.1460-9568.2005.04408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Wang Q. Zhao X. He S. Liu Y. An M. Ji J. Differential proteomics analysis of specific carbonylated proteins in the temporal cortex of aged rats: the deterioration of antioxidant system. Neurochem Res. 2010;35:13–21. doi: 10.1007/s11064-009-0023-8. [DOI] [PubMed] [Google Scholar]
- 432.Wang SJ. Chen HH. Presynaptic mechanisms underlying the alpha-lipoic acid facilitation of glutamate exocytosis in rat cerebral cortex nerve terminals. Neurochem Int. 2007;50:51–60. doi: 10.1016/j.neuint.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 433.Wang W. Shinto L. Connor WE. Quinn JF. Nutritional biomarkers in Alzheimer's disease: the association between carotenoids, n-3 fatty acids, and dementia severity. J Alzheimers Dis. 2008;13:31–38. doi: 10.3233/jad-2008-13103. [DOI] [PubMed] [Google Scholar]
- 434.Wang W. Wang S. Nishanian EV. Del Pilar CA. Wesley RA. Danner RL. Signaling by eNOS through a superoxide-dependent p42/44 mitogen-activated protein kinase pathway. Am J Physiol Cell Physiol. 2001;281:C544–C554. doi: 10.1152/ajpcell.2001.281.2.C544. [DOI] [PubMed] [Google Scholar]
- 435.Wang Y. Su M. Tian DP. Effects of prolonged selenium deficiency on synaptic structures in CA3 area of hippocampus in the third generation rats. Zhonghua Bing Li Xue Za Zhi. 2005;34:302–304. [PubMed] [Google Scholar]
- 436.Watson JB. Arnold MM. Ho YS. O'Dell TJ. Age-dependent modulation of hippocampal long-term potentiation by antioxidant enzymes. J Neurosci Res. 2006;84:1564–1574. doi: 10.1002/jnr.21040. [DOI] [PubMed] [Google Scholar]
- 437.Wells WW. Yang Y. Deits TL. Gan ZR. Thioltransferases. Adv Enzymol Relat Areas Mol Biol. 1993;66:149–201. doi: 10.1002/9780470123126.ch4. [DOI] [PubMed] [Google Scholar]
- 438.Wengreen HJ. Munger RG. Corcoran CD. Zandi P. Hayden KM. Fotuhi M. Skoog I. Norton MC. Tschanz J. Breitner JC. Welsh-Bohmer KA. Antioxidant intake and cognitive function of elderly men and women: the Cache County Study. J Nutr Health Aging. 2007;11:230–237. [PubMed] [Google Scholar]
- 439.West MJ. Kawas CH. Martin LJ. Troncoso JC. The CA1 region of the human hippocampus is a hot spot in Alzheimer's disease. Ann NY Acad Sci. 2000;908:255–259. doi: 10.1111/j.1749-6632.2000.tb06652.x. [DOI] [PubMed] [Google Scholar]
- 440.Wolfler A. Caluba HC. Abuja PM. Dohr G. Schauenstein K. Liebmann PM. Prooxidant activity of melatonin promotes fas-induced cell death in human leukemic Jurkat cells. FEBS Lett. 2001;502:127–131. doi: 10.1016/s0014-5793(01)02680-1. [DOI] [PubMed] [Google Scholar]
- 441.Wolters CA. Yu SL. Hagen JW. Kail R. Short-term memory and strategy use in children with insulin-dependent diabetes mellitus. J Consult Clin Psychol. 1996;64:1397–1405. doi: 10.1037//0022-006x.64.6.1397. [DOI] [PubMed] [Google Scholar]
- 442.Woodall AA. Britton G. Jackson MJ. Carotenoids and protection of phospholipids in solution or in liposomes against oxidation by peroxyl radicals: relationship between carotenoid structure and protective ability. Biochim Biophys Acta. 1997;1336:575–586. doi: 10.1016/s0304-4165(97)00007-x. [DOI] [PubMed] [Google Scholar]
- 443.Wu A. Ying Z. Gomez-Pinilla F. Vitamin E protects against oxidative damage and learning disability after mild traumatic brain injury in rats. Neurorehabil Neural Repair. 2010;24:290–298. doi: 10.1177/1545968309348318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Wu H. Zhou Y. Xiong ZQ. Transducer of regulated CREB and late phase long-term synaptic potentiation. FEBS J. 2007;274:3218–3223. doi: 10.1111/j.1742-4658.2007.05891.x. [DOI] [PubMed] [Google Scholar]
- 445.Wu J. Li J. Huang KP. Huang FL. Attenuation of protein kinase C and cAMP-dependent protein kinase signal transduction in the neurogranin knockout mouse. J Biol Chem. 2002;277:19498–19505. doi: 10.1074/jbc.M109082200. [DOI] [PubMed] [Google Scholar]
- 446.Wu L. Wang R. Carbon monoxide: endogenous production, physiological functions, and pharmacological applications. Pharmacol Rev. 2005;57:585–630. doi: 10.1124/pr.57.4.3. [DOI] [PubMed] [Google Scholar]
- 447.Xia Y. Dawson VL. Dawson TM. Snyder SH. Zweier JL. Nitric oxide synthase generates superoxide and nitric oxide in arginine-depleted cells leading to peroxynitrite-mediated cellular injury. Proc Natl Acad Sci USA. 1996;93:6770–6774. doi: 10.1073/pnas.93.13.6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Xia Y. Tsai AL. Berka V. Zweier JL. Superoxide generation from endothelial nitric-oxide synthase. A Ca2+/calmodulin-dependent and tetrahydrobiopterin regulatory process. J Biol Chem. 1998;273:25804–25808. doi: 10.1074/jbc.273.40.25804. [DOI] [PubMed] [Google Scholar]
- 449.Xie Z. Sastry BR. Induction of hippocampal long-term potentiation by alpha-tocopherol. Brain Res. 1993;604:173–179. doi: 10.1016/0006-8993(93)90365-t. [DOI] [PubMed] [Google Scholar]
- 450.Xie Z. Sastry BR. Impairment of long-term potentiation in rats fed with vitamin E-deficient diet. Brain Res. 1995;681:193–196. doi: 10.1016/0006-8993(95)00271-q. [DOI] [PubMed] [Google Scholar]
- 451.Yamada K. Tanaka T. Han D. Senzaki K. Kameyama T. Nabeshima T. Protective effects of idebenone and alpha-tocopherol on beta-amyloid-(1-42)-induced learning and memory deficits in rats: implication of oxidative stress in beta-amyloid-induced neurotoxicity in vivo. Eur J Neurosci. 1999;11:83–90. doi: 10.1046/j.1460-9568.1999.00408.x. [DOI] [PubMed] [Google Scholar]
- 452.Yamamoto T. Yatsugi S. Ohno M. Furuya Y. Kitajima I. Ueki S. Minaprine improves impairment of working memory induced by scopolamine and cerebral ischemia in rats. Psychopharmacology (Berl) 1990;100:316–322. doi: 10.1007/BF02244599. [DOI] [PubMed] [Google Scholar]
- 453.Yamazaki N. Kiyota Y. Take Y. Miyamoto M. Nagawa Y. Nagaoka A. Effects of idebenone on memory impairment induced in ischemic and embolization models of cerebrovascular disturbance in rats. Arch Gerontol Geriatr. 1989;8:213–224. doi: 10.1016/0167-4943(89)90004-6. [DOI] [PubMed] [Google Scholar]
- 454.Yamazaki N. Nagaoka A. Nagawa Y. [Effect of idebenone on scopolamine-induced impairment of short-term memory in rats] Yakubutsu Seishin Kodo. 1985;5:321–328. [PubMed] [Google Scholar]
- 455.Yamazaki N. Nomura M. Nagaoka A. Nagawa Y. Idebenone improves learning and memory impairment induced by cholinergic or serotonergic dysfunction in rats. Arch Gerontol Geriatr. 1989;8:225–239. doi: 10.1016/0167-4943(89)90005-8. [DOI] [PubMed] [Google Scholar]
- 456.Yao J. Taylor M. Davey F. Ren Y. Aiton J. Coote P. Fang F. Chen JX. Yan SD. Gunn-Moore FJ. Interaction of amyloid binding alcohol dehydrogenase/Abeta mediates up-regulation of peroxiredoxin II in the brains of Alzheimer's disease patients and a transgenic Alzheimer's disease mouse model. Mol Cell Neurosci. 2007;35:377–382. doi: 10.1016/j.mcn.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 457.Yi JS. Holbrook BC. Michalek RD. Laniewski NG. Grayson JM. Electron transport complex I is required for CD8+ T cell function. J Immunol. 2006;177:852–862. doi: 10.4049/jimmunol.177.2.852. [DOI] [PubMed] [Google Scholar]
- 458.Yoritaka A. Hattori N. Uchida K. Tanaka M. Stadtman ER. Mizuno Y. Immunohistochemical detection of 4-hydroxynonenal protein adducts in Parkinson disease. Proc Natl Acad Sci USA. 1996;93:2696–2701. doi: 10.1073/pnas.93.7.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.Yoshida M. Watanabe C. Horie K. Satoh M. Sawada M. Shimada A. Neurobehavioral changes in metallothionein-null mice prenatally exposed to mercury vapor. Toxicol Lett. 2005;155:361–368. doi: 10.1016/j.toxlet.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 460.Zeevalk GD. Bernard LP. Song C. Gluck M. Ehrhart J. Mitochondrial inhibition and oxidative stress: reciprocating players in neurodegeneration. Antioxid Redox Signal. 2005;7:1117–1139. doi: 10.1089/ars.2005.7.1117. [DOI] [PubMed] [Google Scholar]
- 461.Zhang Y. Shoyama Y. Sugiura M. Saito H. Effects of Crocus sativus L. on the ethanol-induced impairment of passive avoidance performances in mice. Biol Pharm Bull. 1994;17:217–221. doi: 10.1248/bpb.17.217. [DOI] [PubMed] [Google Scholar]
- 462.Zhang Z. Shen X. Xu X. Effects of selenium on the damage of learning-memory ability of mice induced by fluoride. Wei Sheng Yan Jiu. 2001;30:144–146. [PubMed] [Google Scholar]
- 463.Zhao J. Moore AN. Clifton GL. Dash PK. Sulforaphane enhances aquaporin-4 expression and decreases cerebral edema following traumatic brain injury. J Neurosci Res. 2005;82:499–506. doi: 10.1002/jnr.20649. [DOI] [PubMed] [Google Scholar]
- 464.Zhao J. Moore AN. Redell JB. Dash PK. Enhancing expression of Nrf2-driven genes protects the blood brain barrier after brain injury. J Neurosci. 2007;27:10240–10248. doi: 10.1523/JNEUROSCI.1683-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.Zheng YQ. Liu JX. Wang JN. Xu L. Effects of crocin on reperfusion-induced oxidative/nitrative injury to cerebral microvessels after global cerebral ischemia. Brain Res. 2007;1138:86–94. doi: 10.1016/j.brainres.2006.12.064. [DOI] [PubMed] [Google Scholar]
- 466.Zhu LQ. Wang SH. Ling ZQ. Wang DL. Wang JZ. Effect of inhibiting melatonin biosynthesis on spatial memory retention and tau phosphorylation in rat. J Pineal Res. 2004;37:71–77. doi: 10.1111/j.1600-079X.2004.00136.x. [DOI] [PubMed] [Google Scholar]
- 467.Zhuo M. Laitinen JT. Li XC. Hawkins RD. On the respective roles of nitric oxide and carbon monoxide in long-term potentiation in the hippocampus. Learn Mem. 1999;6:63–76. [PMC free article] [PubMed] [Google Scholar]
- 468.Zhuo M. Small SA. Kandel ER. Hawkins RD. Nitric oxide and carbon monoxide produce activity-dependent long-term synaptic enhancement in hippocampus. Science. 1993;260:1946–1950. doi: 10.1126/science.8100368. [DOI] [PubMed] [Google Scholar]
- 469.Zoccarato F. Cavallini L. Bortolami S. Alexandre A. Succinate modulation of H2O2 release at NADH:ubiquinone oxidoreductase (complex I) in brain mitochondria. Biochem J. 2007;406:125–129. doi: 10.1042/BJ20070215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 470.Zorumski CF. Izumi Y. Nitric oxide and hippocampal synaptic plasticity. Biochem Pharmacol. 1993;46:777–785. doi: 10.1016/0006-2952(93)90484-e. [DOI] [PubMed] [Google Scholar]