Abstract
Deep brain stimulation (DBS) has recently been proven to be an effective therapy for medication-refractory symptoms of Parkinson’s disease. As the evidence base continues to evolve, many important issues have surfaced, including: what operation should be performed (brain target[s], unilateral vs bilateral, simultaneous vs staged); when to operate (how early is too early to intervene?), who should be operated on (disease duration, age, symptom profiles and the use of the interdisciplinary screening team); and finally, why to operate (the rationale of surgery vs medication/apomorphine pumps/duodopa pumps/stem cell trials/gene therapy trials). We will address each of these critical issues, as well make the argument that a tailored approach to DBS and DBS targeting will best serve each potential candidate. We will review the multiple peer-reviewed studies and we will emphasize the recently available data from randomized DBS studies. We will argue that moving away from a single DBS target (e.g., subthalamic nucleus DBS) and a single approach to DBS methodology (e.g., bilateral simultaneous operations) is a reasonable next step for the Parkinson’s disease community. Following careful interdisciplinary DBS screening, a physician–patient discussion has the potential to establish a patient-centered and symptom-specific outcome for each potential DBS candidate. The interdisciplinary DBS team can function together to formulate and to consider an optimal and tailored approach. A tailored approach will allow for the consideration of the complex and numerous variables that may contribute to a positive or negative overall DBS outcome. We will review and provide expert commentary on a potential interdisciplinary approach to selecting unilateral or alternatively bilateral subthalamic nucleus or globus pallidus internus DBS. Our approach is aimed to maximize benefit(s) and minimize risk(s) in order to best tailor therapy for an individual patient.
Keywords: candidates, DBS, deep brain stimulation, GPi, Parkinson’s disease, STN
Deep brain stimulation (DBS) has recently proven to be an effective therapy for medication-refractory symptoms of Parkinson’s disease (PD) [1,2]. As the evidence base continues to evolve, many important issues have surfaced, including: what operation should be performed (brain target[s], unilateral vs bilateral, simultaneous vs staged); when to operate (how early is too early to intervene?), who should be operated on (disease duration, age, symptom profiles and the use of the interdisciplinary screening team); and finally, why to operate (the rationale of surgery vs medications/apomorphine pumps/duodopa pumps/stem cell trials/gene therapy trials). We will address each of these critical issues (what, when, who and why), as well as make the argument that a tailored approach to DBS, and to DBS targeting will best serve each potential candidate. Since multiple peer-reviewed studies and experiments have provided a strong rationale for tailoring therapy, we will argue that moving away from a single DBS target (e.g., subthalamic nucleus [STN] DBS) and single approach to DBS methodology (e.g., bilateral simultaneous operations) would be a reasonable next step for the PD community, especially in light of recently available data from multiple randomized DBS trials [3–7]. Following careful interdisciplinary DBS screening, a physician–patient discussion has the potential to establish a patient-centered and symptom-specific outcome for each potential candidate [8]. The interdisciplinary team can then function together to consider an optimal and tailored approach that will maximize the risk–benefit ratio for each patient, and will help to avoid DBS failure(s) [9]. A tailored approach will allow for the consideration of the complex and numerous variables that may contribute to a positive or negative overall DBS outcome [10].
Methods
A complete review of the PD DBS literature using PubMed was performed to establish current medical and surgical practices. Five medium-to-large sized randomized DBS trials were identified and then reviewed in detail to derive pertinent information for tailoring DBS therapy (maximizing benefit and minimizing risk) based on the available data. Those trials have been included and will be referred to for the purposes of this article by the following names: the NEJM Quality of Life Study with bilateral STN DBS versus best medical therapy trial (utilizing the PDQ-39 quality of life as the primary outcome variable) [3]; the VA Cooperative Study comparing best medical therapy to bilateral STN and bilateral globus pallidus internus (GPi) DBS (utilizing the Unified Parkinson Disease Rating Scale [UPDRS] III motor score as the primary outcome variable) [5]; the VA Cooperative Study comparing bilateral STN vs GPi DBS (utilizing the UPDRS III motor score as the primary outcome variable) [4]; the NIH COMPARE study, which examined unilateral STN versus GPi DBS (utilizing the visual analogue mood scale and verbal fluency as co-primary outcome measures) [7]; and the UK PD SURGE trial, which compared medical therapy (including apomorphine pumps) to bilateral STN DBS (PDQ-39 was the primary outcome variable) [6]. When available, data from secondary analyses derived from these randomized cohorts were included [11,12].
What operation should be performed?
Brain targets (ventral intermediate thalamus, STM, GPi, PPN, centromedian thalamic nucleus, zona incerta & others)
A plethora of new information has been emerging that will assist clinicians in selecting the most appropriate brain target for a given PD patient. Early in surgical therapy, many DBS groups implanted the ventral intermediate (VIM) thalamus. However this approach gradually fell out of favor with the realization that VIM was most effective for upper extremity tremor, with much less measured efficacy for the other cardinal motor features of PD (e.g., bradykinesia and rigidity). However, very select patients with upper extremity tremor and without other prominent disabling features of PD are still considered to be appropriate for VIM DBS [13].
Following an era of encouraging results in ablative/lesional brain therapy, first in the VIM thalamus (thalamotomy) and then in the GPi (pallidotomy), the GPi emerged in the early 1990s as a potential target for DBS therapy. The DBS approach was preferred over lesion therapy by most centers, especially those interested in bilateral therapies. The reason for the preference was that bilateral lesions had been commonly associated with unacceptable speech, swallowing and cognitive deficits. However, the GPi target was quickly overtaken by STN DBS [9,14], following the revelation that STN lesions were found to be very effective for the parkinsonian primate [15] and that drastic medication reduction could be realized in some human cases [16]. However, recent trials have demonstrated that GPi DBS is also an effective target for treatment of the levodopa-responsive symptoms and the motor fluctuations associated with PD [4,7]. Small individual differences in motor, mood, cognitive, adverse event and quality of life outcomes have been observed across available comparative studies (e.g., bradykinesia, depressive symptoms, apathy, verbal fluency, falling and speech issues), and more secondary analyses of the respective databases are needed.
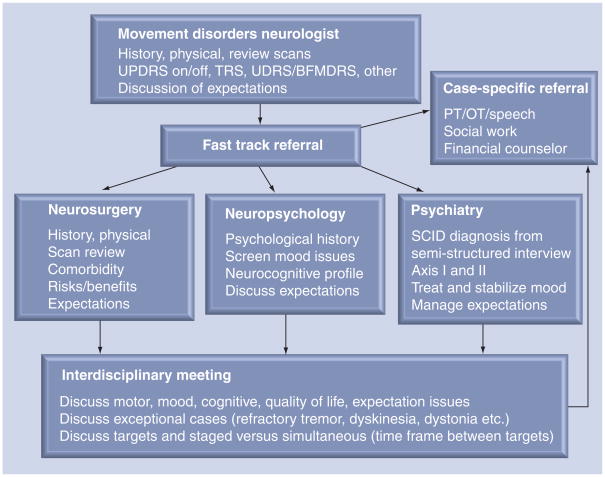
There are potential ways to interpret emerging DBS outcome data to maximize benefits and to minimize risks. An interdisciplinary DBS team (neurosurgery, neurology, neuropsychology and psychiatry) can weigh the benefits and risks of choosing an individual target for a potential DBS patient. Figure 1 shows the pathway at the University of Florida (FL, USA) for the interdisciplinary ‘fast track’ work-up, as well as the details of the follow-up interdisciplinary discussion for any potential DBS patient. We refer to ‘fast track’ at the University of Florida as an interdisciplinary process where patients can be seen and worked-up by multiple specialties on consecutive days. The work-up is then followed by an interdisciplinary discussion. We understand that not all centers have psychiatrists on their DBS teams, and in cases where a psychiatrist is not available, screening is usually performed by a neuropsychologist. In our experience, we have found the psychiatrist and the psychiatry evaluation to be essential to a complete work-up.
Figure 1. University of Florida (FL, USA) interdisciplinary work-up and discussion for a patient referred for deep brain stimulation surgery.
OT: Occupational therapy; PT: Physical therapy; SCID: Severe combined immunodeficiency; TRS: Tremor rating scale; UDRS/BFMDRS: Unified Dystonia Rating Scale and Burke-Fahn Marsden Dystonia Rating Scale; UPDRS: Unified Parkinson’s Disease Rating Scale.
There has been a recent surge in interest in pedunculopontine nucleus (PPN) DBS to address levodopa-resistant PD-related gait and balance dysfunction [17,18]. However, there is a paucity of data available to recommend PPN DBS, and thus it is currently limited to the research setting. Important issues that remain to be resolved for PPN DBS are the inclusion/exclusion criteria (on vs off freezing, gait problems, balance problems and combinations of clinical issues), locating the best target region (imaging and physiology), whether preoperative white matter lesions on MRI impact outcome (comorbidities), and to better define and clarify which motor and non-motor features of PD may respond to this approach. Furthermore, it is important to note that Stefani et al. may have inadvertently targeted the peripeduncular nucleus and not the PPN [18,19]. Other recent experimental brain targets for PD DBS have included the centro-median thalamic nucleus [20] and the zona incerta [21], but similar to PPN DBS, these targets remain investigational with little data available to guide clinician–patient discussions.
Unilateral versus bilateral operations
There are no large-scale studies available that directly compare a unilateral versus bilateral approach to PD DBS. The randomized COMPARE DBS cohort data revealed that unilateral STN and unilateral GPi DBS targets were both efficacious in addressing motor dysfunction, however, they had similar but slightly different mood and cognitive side-effect profiles [7]. There remains an open question as to whether a unilateral approach would be a safer and more desirable option for a subset of patients.
An interesting follow-up study from the COMPARE cohort suggested that unilateral GPi DBS had better quality of life outcomes when compared with unilateral STN DBS [12], raising the question that if patients are to be implanted unilaterally, GPi may prove to be a more appropriate target. A follow-up study currently in press revealed that 21 out of the 44 patients (47%) from the COMPARE cohort remained unilateral, and were followed for an average of 3.5 years after surgery [22]. Of these cases, 14 out of 21 (67%) had a GPi target. The most common reason for adding a second-sided DBS was inadequacy in addressing motor symptoms. Satisfaction with motor outcomes was the most common reason for remaining unilateral. Those who chose a second DBS lead had significantly higher baseline UPDRS III motor scores and a significantly lower asymmetric index (meaning that their symptoms were rated as closer to equal on both sides of the body). The logistic regression analysis revealed the odds of proceeding to bilateral DBS was 5.2-times higher for STN compared with GPi. For every 1% increase in asymmetry, the odds of bilateral DBS increased by 0.96 [22]. It is unknown why unilateral GPi DBS may be better in select cases than unilateral STN DBS, but part of the explanation may lie in the mechanisms of dyskinesia suppression (medication reduction in STN vs a more direct suppression of dyskinesia in GPi). The differences between targets will need more scrutiny, as it is likely that STN also has some dyskinesia-suppressing properties.
Simultaneous implantation into two brain sides versus a staged approach
When considering a simultaneous versus staged approach to DBS, some explanation of the different approaches is required. Two DBS leads may be placed in the same intraoperative sitting (simultaneous implantation), or alternatively they may be separated by days, weeks or months. Similarly, the batteries (impulse generators) may be placed on the same day, or alternatively placed days, weeks or even a month following lead insertion. Although there is no data to support the utility for timelines of these various approaches, some DBS interdisciplinary teams feel that extended intraoperative time may be an important factor in increasing complications, particularly in the elderly patient (>70 years old), the frail patient or in the patient with multiple comorbidities. Some teams have also begun to exercise caution in operating on patients over 70 years of age, although this point is highly debatable among the experts.
The ‘pros’ supporting a single intraoperative bilateral lead insertion session include patient convenience, eliminating a second and same-day operation and/or an economic advantage for the healthcare system. The ‘cons’ include longer intraoperative times, the potential for greater complications (including intra- and post-operative mental status changes), and in some countries, decreased compensation for performing DBS in a single intraoperative sitting. It has been hypothesized that a unilateral electrode implantation may be less likely to result in mental adverse effects compared with bilateral electrode implantation due to microlesioning, but further studies are needed to confirm such suspicions. When it comes to battery placement, many surgeons prefer to delay placement for a few weeks and even up to a month in order to limit postoperative confusion, which may occur by prolonging the lead insertion surgery. A delay has the added benefit of allowing brain edema to resolve prior to attempting device programing. It should also be noted that in centers not allowing MRI following a DBS lead insertion, this may impact the ability to place a second contralateral DBS. There is an ongoing debate as to the safety of MRI post-DBS, with many groups, including the National Parkinson Foundation, citing an excellent safety record with MRI and DBS, especially when performed under optimal conditions [23].
Recently, a study was published that explored postoperative stays following unilateral DBS surgery. This study examined factors potentially related to extended stays. Most patients in the 115 patient cohort (79%) had a hospital stay of 1 day following surgery. The most frequent reasons for delayed discharge (>1 day) included mental status change (n = 6) and hemorrhage (n = 5). Those with delayed discharge had significantly lower pre-surgical cognitive screening scores (Mini-Mental State Evaluation [MMSE]), higher presurgical ‘on’ medication motor score and more microelectrode passes compared with the immediate discharge group. In correlation analyses, increased length of hospital stay was significantly associated with more microelectrode passes, higher presurgical ‘on’ medication motor scores and decreasing MMSE scores. When the significant variables from the preliminary analyses were entered into a Poisson regression model, a greater number of microelectrode passes, as well as lower MMSE scores, remained significant predictors of increased length of stay. These issues should be more closely examined in larger studies, studies including a bilateral simultaneous approach and studies also including long-term outcomes [24].
Closed-loop technologies & Parkinson’s disease (improving management)
There are currently no US FDA-approved DBS devices that allow for either scheduled stimulation or responsive (closed-loop) stimulation. There are limited available settings on devices that allow for cycling of current, and these settings have been largely unexplored. Recently, a device has been introduced for epilepsy patients, and there is hope that similar devices may play a role in the management of PD [25]. One reason for the enthusiasm for such devices is that in the PD brain, regions have been identified that predictably oscillate at frequencies such as the β-band. New devices may have the potential to detect this β-band frequency (and other frequencies during rest or during action). These frequency oscillations may prove useful in choosing programing parameters for chronic DBS management [26]. Though closed-loop technologies are interesting, it should be noted that there may be cumulative motor effects from long-term DBS [27].
When should DBS be performed?
What is the current rationale for when to operate?
Deep brain stimulation for PD is currently offered to patients with medication refractory on–off fluctuations, dyskinesia or tremor. These patients may be selected as candidates following an extensive interdisciplinary screening. The screening should aim to elucidate which features of PD are most responsive to dopaminergic therapy (responsive features tend to have the best response to DBS, with the exceptions of tremor, dyskinesia and motor fluctuations), it should identify cognitive/psychiatric issues, and it should assess comorbidities that may increase the risk or limit the effectiveness of therapy [8]. The major unanswered question for DBS therapy has been when to say enough is enough, and to proceed to DBS therapy [28]. Most practitioners agree that when medication intervals become very close in time (within 2–3 h), and on–off fluctuations, dyskinesia or tremor emerge and are difficult to control, then it is time to at least consider the use of DBS therapy. Several recent studies have revealed an advantage of DBS over medical therapy, including the UK PD surge trial [6], (which allowed apomorphine therapy in medication and in DBS groups), the VA Cooperative Study [4] and the NEJM Quality of Life Study [3].
Should the timeline for operating be moved up?
Traditionally many expert PD centers have delayed invasive surgical therapy for as long as possible. This purposeful delay has raised the question, ‘how long is too long?’ DBS has not been shown to have a disease-modifying effect. As DBS is not disease modifying, to proceed to surgical therapy symptoms should, at a minimum, be difficult to manage with medications, and they should have a profound impact on the sufferer’s quality of life. If patients are still working, and DBS has the potential to keep them working, this should be considered in the decision-making process. To date, studies have not shown any advantage for DBS to keep PD patients at work. These types of studies are, however, very complex to perform, and very complex to interpret [29,30], as there are many factors as to why a patient may not return to work.
The many ways to define early DBS & how early is too early?
A recent study suggested that DBS in less advanced cases (lower UPDRS scores) may be feasible and efficacious [29,30]. Trials published in the literature to date have not included patients with short disease durations (1–4 years) or patients without motor fluctuations. The neuroprotective (or disease-modifying) effects of surgery have not been substantiated, and thus cannot be ethically used to argue for earlier intervention in individual patients. There is a solid argument evolving for cost savings with early DBS. However, arguments based on motor, non-motor and quality of life features have less of an evidence base at this time. Better methodologically constructed and adequately powered clinical trials with carefully conceived end points will need to be performed in order to settle the questions surrounding early DBS intervention.
‘Early DBS’ covers several scenarios. Consider the example of a 60-year-old patient diagnosed with PD 2–4 years prior, experiencing life-altering disability from tremor, despite maximal combinations of levodopa, dopamine agonists and anticholinergics. Most clinicians and multidisciplinary surgical teams would agree that this could be a reasonable case for early intervention.
A more controversial case for ‘early’ intervention would be that of a 60-year-old patient with 2-h levodopa dose intervals, experiencing mild dyskinesia, as well as on–off fluctuations (regardless of disease duration), with minimal off time. The patient might desire DBS, believing the surgery to be inevitable, with the goal of minimizing the surgical risks of hemorrhage and cognitive deterioration. Although not all experts would agree that surgery should be performed in such a case, the scenario does represent a rational argument for earlier intervention.
In our opinion, the most controversial scenarios for ‘early’ intervention are:
Patients who have not yet received levodopa or standard of care medication therapy;
Patients who have been diagnosed for less than 5 years;
Patients without motor fluctuations, or patients just beginning to manifest motor fluctuations;
Patients seeking to reduce or discontinue medication with short disease durations (<5 years);
Patients with short disease durations (<5 years) and nondisabling and nonbothersome dyskinesia;
Patients who desire to continue working and desire DBS in the hope of continuing to do so for a longer time period of time (some centers view this as noncontroversial despite a lack of evidence-based studies);
Patients with short disease durations (<5 years) seeking improvement in non-motor manifestations [31].
We believe that patients who fall into these above categories should, in most cases, have DBS performed only in the context of an Institutional Review Board-approved clinical research trial.
There are several reasons for not performing DBS routinely in the above situations. There is a higher chance of confusing a Parkinsonian disorder (such as multiple system atrophy) within the first 5 years of diagnosis, and DBS has not conclusively been shown to benefit patients with ‘Parkinson plus’ syndromes (it should be noted that 5 years is an arbitrary time interval and has not been shown to be an adequate cut-off by formal studies). Furthermore, PD is not one disease, but rather a syndrome. Patients with fewer than 5 years of PD may have an insufficient symptom history for proper surgical stratification. For example, some patients with tremor-predominant PD will continue to function well for decades on dopaminergic therapy alone. Currently, we have no way to easily identify such patients who may never need surgery, and therefore we cannot remove them from the surgical candidate pool.
There are ongoing studies of early intervention underway in the USA and in Europe [32]. Although we expect that this data will shed light on the more controversial issues regarding this debate, it is unlikely to settle it. The data from each study will be specific and based on individual study design. Levodopa and the motor UPDRS are currently used as the best preoperative indicator of symptomatic benefit for DBS in PD. They may be less useful in indicators in ‘early’ scenarios.
Designing early intervention trials to create or revise outcome measurements will be challenging. Early intervention trials will be costly, and the follow-up required longer than what we have come to think of as standard for the field. The data from these trials will also be necessary for the formulation of rational criteria under which early intervention can be considered [31].
Many centers have been shifting toward earlier implantation of DBS devices. Physicians should keep in mind that earlier DBS translates to increased potential for adverse events over the lifetime of a patient and therefore to more programing visits and also to more battery changes. Therefore, patient commitment and patient-perceived benefits must also be carefully considered when deciding whether or not to perform surgery at an earlier stage of PD [31]. There are many flaws in existing studies of early DBS, especially in methodology and outcomes, and future studies will need to carefully examine these issues.
Who should be operated on?
Symptom profiles that may or may not benefit from DBS
Data derived from the available randomized DBS studies suggest that PD patients with medication-refractory and difficult to control on–off fluctuations may be the best candidates for DBS therapy [3–7,28]. In the UK PD Surge trial, the authors collected information on the main reasons why patients considered DBS surgery. Severe ‘offs’, dyskinesia, and tremor were the most commonly cited indications [6]. Although it is now well-established that levodopa-responsive symptoms of PD have the greatest response to surgical interventions, the effect of disease progression may not be routinely included in preoperative patient discussions, where risks and benefits can be thoroughly vetted. Discussions with patients should routinely involve the realization that walking, talking and thinking could worsen not only from stimulation- or lesion-induced effects, but also as a result of disease progression [8,33] (these can occur in both unilateral and bilateral lead implantations and it is unknown whether there is an advantage of one approach over the other). Patients with highly asymmetric symptoms may need only a single-sided DBS operation, and in these cases taking a conservative approach to therapy may improve the risk–benefit ratio (i.e., waiting until after a single lead has been placed and medically/surgically optimizing before proceeding to a second-sided DBS) [34–36].
Less is known about the cognitive and mood sequela of DBS surgery. The most common cognitive issue that has emerged has been reduction in verbal fluency (patients may complain they cannot get words out of their mouth). This problem has been demonstrated in multiple studies, including recently by a reliable change index that was employed to be sure the effect was actually DBS specific [37,38]. Interestingly, very few DBS studies have carefully controlled for ‘lesional’ effects and separated them from stimulation-induced effects. In the COMPARE cohort, turning the stimulators on and off at 7 months postimplantation revealed a persistent negative effect (lesional effect) on fluency, particularly in the STN group [7]. Interestingly, stimulation-induced effects were also observed in both STN and GPi, particularly when adjusting the stimulation to more ventral (deeper) DBS contacts [7,39].
The most commonly observed mood effect following DBS surgery has been a slight improvement in depressive symptoms. Recent data has suggested that this improvement may not be persistent, and the VA Cooperative Study Data at 24 months actually suggested slight worsening of mood in the STN DBS group [4]. Interestingly, anger has emerged as a common side effect post-DBS surgery, and has been recently shown to be more of a ‘lesional’ effect of both STN and GPi DBS in PD [7,40].
Several recent papers have cited problems with impulsivity in STN DBS patients and data has revealed that DBS tends to speed up decisions during high-conflict situations. Impulsive and compulsive behaviors, dopamine dysregulation and emergent issues when dual or multitasking have led to a re-examination of the cognitive and behavioral effects of DBS [41–45]. Teasing out these effects may need a more rigorous approach with more complex and more loaded (more difficult tasks for patients) neuropsychological testing.
Finally, one must also consider whether a medication reduction (more commonly seen in STN) may be useful in cases with medication-related psychosis or behavioral issues, which may impact target choice or unilateral versus bilateral implantation.
Interdisciplinary team screening & discussion
The inclusion and exclusion criteria for DBS have continued to evolve for various disorders and syndromes, and they continue to evolve in PD. The first step to ensure success in PD is to select the appropriate candidate. Patient selection for DBS should, in the best case scenario, utilize a multidisciplinary team, and preferably an interdisciplinary team. The preoperative detailed assessment by multiple disciplines should be a prerequisite for surgical consideration. An adequate team usually includes a movement disorders neurologist, a neuropsychologist and a neurosurgeon. Our group also recommends that a psychiatrist be involved if possible, especially since many of the urgent and emergent issues in DBS therapy involve psychiatry. Physical therapists, occupational therapists, speech therapists, social workers and financial counselors should be involved on a case-by-case basis. Each member of the interdisciplinary screening team should independently evaluate the patient and a meeting should be convened to review and discuss findings. The review should be inclusive of the past history, medical imaging studies, and if available, a video examination. Standardized rating scales should be performed (such as the UPDRS, with an on–off dopaminergic evaluation) and comorbidities should be discussed. Candidacy should also be determined based on an individual patient’s desired expectations and goals [8,33,46,47]. Any level of cognitive impairment should be identified and discussed by the interdisciplinary team, as this may affect outcome. The patient should be contacted and apprised of the interdisciplinary discussion(s). Figure 1 summarizes the interdisciplinary University of Florida ‘fast track’ process.
Development of a patient-centered outcome
The focus of research on outcomes from medical procedures has shifted in recent years from purely objective scales to an emphasis on patients’ perceptions of potential outcome(s). The majority of clinicians, patients and caregivers report improvements following DBS surgery. Reports of improvement derived directly from questions asked to patients, clinicians and caregivers often fail to correlate. In addition, patient perceptions of outcome are often associated with non-motor factors, including anxiety, perceived social support and nonphysical domains of quality of life. Patients’ perceptions may thus be less associated with motor and physical factors. Clinicians’ perceptions seem to be more closely tied to motor symptoms and other physical factors. Finally, caregivers’ perceptions may not be correlated to many of the outcome variables we typically measure. The differences between patients [29,48], clinicians and caregivers all underscore the importance of anxiety, perceived social support and other non-motor factors as important considerations in preoperative DBS discussions [49].
Why should we operate?
When to say enough is enough in fluctuating Parkinson’s disease patients
The increasing use of DBS for PD has drawn the attention of both the medical and lay communities and has resulted in an appropriately increased scrutiny of outcomes. We have celebrated successes but also been challenged by patients who have undergone surgery with results falling short of expectations – a group now referred to as ‘DBS failures’ [10]. We now have a developing evidence base that compares DBS with standard medical therapy. The large VA Cooperative multicenter study [50], along with the NEJM Quality of Life Study [3] and the UK PD Surge Trial [6], have helped us to appreciate the potential benefits of DBS over best medical therapy. The improvements in ‘on time’ of 4–6 h have helped us draw a better picture of when “enough is enough with medicines, and when it is appropriate to proceed to DBS surgery” [28]. The results of all three of these studies collectively suggest that DBS is superior to best medical management for the treatment of on–off fluctuations in well-selected moderate-to-advanced PD patients. We addressed some of these issues in a recent article, concluding, that the “general neurologists and general practitioners should refer moderate to advanced on–off fluctuating PD patients to experienced surgical centers for complete interdisciplinary screening for DBS surgery. Although with the current technology many PD patients may not be appropriate surgical candidates, DBS surgery carried out on the right patient by an experienced team can provide magnificent benefits. These benefits, we now understand, may extend beyond what can be achieved with medicines alone, and this information should give practitioners solace when saying enough is enough” [28].
Exceptional cases: medication-refractory dyskinesia & tremor in otherwise less than optimal candidates
In optimal circumstances, potential PD DBS candidates report to their neurologist’s office 12 h following cessation of their dopaminergic medications. Patients are then evaluated with a UPDRS scale in the ‘off’ state. Afterwards, they are challenged with a suprathreshold dose of dopaminergic medication and reevaluated in their best ‘on’ state. Optimal surgical candidates typically demonstrate at least a 30% improvement in the motor portion of the UPDRS [51]. However, there may be patients who fail to achieve a 30% improvement with levodopa or dopaminergic medications, but have alternative and potentially responsive indications for DBS (dyskinesia, ‘on/off’ motor fluctuation and medication-refractory tremor), some of whom may not fulfill cognitive criteria to receive DBS surgery. Each of these cases should be individually evaluated by the interdisciplinary DBS team to determine the potential for surgical candidacy. Medication-refractory tremor and severe dyskinesia may, in select cases, be appropriate indications for DBS candidates. In cases where cognitive issues may otherwise negate surgical candidacy, many centers have been utilizing unilateral GPi DBS, although more data will be required on this subject in order to assess the efficacy and safety of this approach [8,10].
It should be emphasized that the UPDRS is a clinical research tool that should not be used in isolation. Insurance companies and interdisciplinary teams should not deny DBS based on less than 30% improvement or on UPDRS on or off scores that do not exceed arbitrary limits (such as a common cut-off score of 30). Each patient should be individually evaluated for appropriateness of DBS.
DBS versus apomorphine, duodopa, stem cell trials & gene therapy trials
There is little available data comparing DBS to other surgical interventions, such as apomorphine pumps, duodopa pumps or to stem cell and gene-therapy approaches. The lack of data often leaves the clinician in a difficult position when attempting to compare standard versus investigational therapies for an individual patient. The UK PD Surge trial did provide some limited data suggesting that in patients still having difficulties post-apomorphine pump placement, DBS may represent a reasonable approach [6]. Future comparative trials, especially of apomorphine and duodopa therapy (compared with DBS) may help to elucidate which phenotype of patients may be the most appropriate for each type of therapy. The lack of data and lack of approval beyond research for stem cells and gene therapy make comparison with DBS difficult.
Expert commentary & key issues
The best management of PD DBS will rely on maximizing benefit and minimizing risk. The interdisciplinary process should be designed to identify all of the critical preoperative symptoms, issues and indications that deserve attention, and these areas should be used to guide target choices and optimal approaches. At our own center we have developed a highly flexible and modifiable approach for choosing DBS targets. Our approach is based on the available studies (particularly the randomized studies) [3–7,11,12], as well as on our own biases based on experience gleaned from hundreds of DBS surgeries. Practitioners should be aware that no algorithm has the capability to absolutely address an individual patient or an individual patient’s risk:benefit profile. Our current approach is focused on choosing potential targets and choosing bilateral versus unilateral therapy in an effort to maximize benefit and minimize risk in individual DBS patients. Patients should understand that STN and GPi DBS are both effective for the treatment of PD. However, there may be subtle and important differences that may influence target selection and unilateral versus bilateral stimulation. In addition, it should be appreciated that adequate DBS programing can be critical to outcome (adjustments in pulse width, voltage, frequency and choosing monopolar versus bipolar modes).
Key issues in maximizing DBS benefit
The maximizing of DBS benefits may be influenced by target selection and by laterality.
Utilizing unilateral DBS
Highly asymmetric PD features and especially patients with lower UPDRS motor scores may be addressed with unilateral DBS [22,34]. If a single DBS lead is utilized, there is some evidence that quality of life improvements may be more robust with unilateral GPi DBS [12] and the chances of staying unilateral may also be better with GPi [22].
Tremor, rigidity, bradykinesia & dystonia
Most available randomized data support similar improvements for unilateral and bilateral STN or GPi DBS for tremor, rigidity, bradykinesia and dystonia [4]. One study revealed a slight benefit in rigidity with unilateral STN DBS [7]. However, some centers have leaned toward STN DBS for tremor control, although this point has not been strongly supported by the available randomized studies [4,7]. When implanting DBS devices for tremor, many centers attempt to place the active contacts on the DBS lead more posterior in both the GPi and the STN targets. However, when moving posterior in the GPi, however, one may encounter capsular side effects. Therefore, the capsular side-effect profile, along with the overall larger size of the GPi target (thus requiring larger electrical fields for effective stimulation) have led some centers to lean toward STN DBS in severe tremor cases.
Levodopa-responsive gait & balance issues
Only levodopa-responsive gait and balance issues have the potential to improve following DBS therapy [10]. Following DBS, gait and balance may decline due to surgical effects, or due to disease progression, and patients should understand these possibilities prior to the operation. An interesting finding of the VA Cooperative Study was that the stand–walk–sit test improved in the GPi but not STN groups, when tested in an off-medication off-stimulation state [4]. It is unclear whether this is a clinically relative benefit for GPi DBS.
Medication reduction/increase
In patients who require a dopaminergic medication reduction, STN DBS may be preferable. However, in those who may need to increase medications or keep the dosages stable, GPi seems to be a better option. There was a trend toward medication reduction in unilateral STN but not GPi DBS [7], and STN was superior to GPi in a bilateral randomized comparison (408 mg reduction for the STN group and 243 mg reduction for GPi) [4].
On time, on–off fluctuations & dyskinesia
Some groups favor GPi over STN DBS when selecting patients who may benefit from enhanced on time, improved on–off fluctuations, or for dyskinesia reduction – however, the available motor data has not supported an advantage for GPi. UPDRS III change scores in unilateral and bilateral DBS have been similar between targets [4,7]. In addition, diaries have revealed only one more hour of quality on time for GPi versus STN DBS (non-significant difference). Off-stimulation off-medication scores seem to improve with GPi and worsen with STN, but this finding is hard to interpret, especially because of short washout periods [4]. GPi has been posited to have more of a dyskinesia-suppression mechanism of action, as opposed to STN, which seems to achieve most of its dyskinesia improvement via a medication-reduction mechanism. Several groups have observed that STN may also have dyskinesia-suppression qualities. More information to clarify these differences will be helpful in guiding choices for clinicians and patients.
Quality of life
Quality of life has shown consistent improvement with all DBS targets. If unilateral DBS is sought, more benefits may be evident with GPi DBS [12]. The NEJM Quality of Life Study revealed improvements for bilateral STN DBS in PDQ-39 scores, including mobility, activities of daily living, emotional well being, stigma and body discomfort [3]. The UK PD Surge bilateral STN DBS study demonstrated improved mobility, activities of daily living and body discomfort [6]. Finally, in the VA Cooperative Study, bilateral STN and GPi DBS improved quality of life, except for the communication subscore (worsened in both groups), and the social support subscore (worsened in GPi and improved in STN) [4].
Key issues in minimizing risks
The minimization of risks may also be closely associated with target choice and may be associated with whether unilateral or bilateral DBS surgery is chosen for an individual patient.
Hypophonia, dysarthria & swallowing
Concerns over worsening hyophonia, dysarthria and swallowing issues may sway an interdisciplinary team toward unilateral DBS and perhaps GPi as a target, but more data is required to clarify this issue. Dysarthria and hypophonia issues have been reported to occur in the majority of all bilateral STN DBS cases, and therefore, this issue should be thoroughly discussed with each patient (it is largely unknown if the same effects occur in the GPi target). It is important to keep in mind that despite the majority of patients subjectively reporting issues in speech following DBS, several studies show some objective improvement on UPDRS ratings and this issue requires clarity and better outcome measures [52,53]. It is unknown what the effects of DBS will be on pre-existing swallowing and aspiration problems, but in most centers these patients would be excluded from DBS consideration.
Verbal fluency, cognition & mood
Several pre- versus post-operative DBS studies have documented emerging cognitive and impulse control issues. It is important that all patients undergo detailed preoperative neuropsychiatric evaluations, and if possible, also be evaluated by a psychiatrist. The follow-up to the NEJM Quality of Life Study documented postoperative issues in executive function, verbal fluency and stroop testing. Ten people in their study had severe psychiatric side effects following DBS [11]. In the VA Cooperative Study, there were declines in many areas in both the STN and the GPi targets. However, visuomotor processing speed was worse for STN DBS [4].
Verbal fluency is the most common and most consistent cognitive deficit seen following DBS surgery [4,7,11]. There is some notion that verbal fluency may be less affected by using unilateral DBS, and even less affected when using the GPi target when compared with the STN target. In the COMPARE cohort, the unilateral STN group declined more than the unilateral GPi group in letter fluency (p = 0.03). Interestingly, when adjusting the stimulating contact more ventral (deeper) patients were more confused, less energetic, less happy and more sad. Patients were also less energetic when stimulated dorsally (one contact up from optimal) and when off. The letter fluency in STN was worsened regardless of whether the active contact was ventral, dorsal or off. The issue of target site selection for verbal fluency as well as other cognitive/mood issues will need more supporting data. Furthermore, the notion of using this information to program devices (i.e., move one contact ventral when cognitive or mood problems arise) will need more investigation.
Mood, as measured by self-report indexes, such as the Beck Depression Inventory, has been observed to mildly improve following DBS surgery. However, in the recent VA Cooperative study, bilateral GPi mildly improved mood, and bilateral STN mildly worsened it [4] following 24 months of follow-up.
It is unknown whether GPi may be superior in patients with preoperative cognitive, mood, impulse control disorders and dopamine dysregulation syndromes, and this is an area that will require clarification. The original notion of operating patients with impulse control disorders and dopamine dysregulation bilaterally in the STN in the hopes that medication reduction would alleviate symptoms has not proven to be universally true. One interesting recent finding has been the observation of increased anger following both STN and GPi DBS [7,40].
There has been important information recently published regarding suicide in STN DBS [54]. Potential important factors identified in this study included “postoperative depression, being single, a previous history of impulse control disorders, compulsive medication use, and being younger” [54]. Patients in at-risk categories should be followed closely if they proceed to DBS or they develop postoperative depression.
One interesting notion would be to utilize clinical outcomes data, imaging and field modeling to select a DBS contact in the clinic to maximize motor effects and minimize cognitive/mood side effects for a particular patient. It is also important for DBS programers to be aware that lesion effects may occur from damage along the entire trajectory of the path of the DBS lead and that when an effect is lesional it will not respond to parameter or DBS contact adjustment.
Levodopa-unresponsive gait issues
Centers should not offer DBS to patients who are intent on improving levodopa-unresponsive gait or balance issues [10].
Age
Age should be treated on a case-by-case basis but older age, especially when accompanied by severe atrophy may sway some centers to recommend unilateral DBS, and to even consider GPi DBS (which may have a better quality of life outcome when performed unilaterally). The VA Cooperative Study enrolled over 20% of their particpants from a group of individuals older than 70 years of age, and it will be interesting to follow their target specific outcomes [4].
Weight gain
Weight gain seems to occur slightly less frequently following unilateral surgery of either target, and this may be important for a subset of patients concerned about this issue. It should be noted that although weight gain occurs in the majority of patients, weight loss may also occur in 10–20% of DBS patients and it is currently unknown why this weight loss occurs.
Battery changes
Data from the VA Cooperative Study revealed differences in average voltages (3.95–3.16 V), pulse widths (95.7–75.9 μs) and frequencies (168–165 Hz) when comparing GPi to STN DBS [4]. Therefore, the frequency of battery changes will probably be slightly less when using STN (it is not clear whether this is clinically relevant). The reason underpinning the difference is thought to be smaller target size and, therefore, a relatively smaller electrical requirement to achieve symptom control.
Adverse events
The VA Cooperative Study revealed that of the 335 significant adverse events, 77 were in bilateral GPi and 83 in bilateral STN, and there was no difference between targets [4]. The COMPARE study revealed similar findings for unilateral DBS, but when separating out adverse events into mood and cognitive categories, greater numbers were seen in the STN group (particularly anxiety, confusion, irritability, aggressiveness, obsessive–compulsive disorder and mania) [7]. The UK PD Surge Trial only examined STN DBS but had similar adverse event rates [6] as the VA Cooperative Study. The NEJM Quality of Life Study reported only 13 significant adverse events (STN DBS only) [3], but this was probably due simply to differences in how adverse events were defined and recorded.
Five-year view of tailoring DBS for Parkinson’s disease
The horizon for tailoring DBS for PD over the next 5 years will probably include advances in clinical management and advances in research. Clinically, we anticipate that more data will become available to help assist interdisciplinary teams in deciding: who should get DBS; what brain target(s) should be implanted; whether patients should be implanted unilaterally versus bilaterally; and what specific cognitive, mood and motor profiles may sway a change in surgical target or surgical approach. In the research realm, we anticipate greater clarity, especially in explaining the cognitive and neuropsychiatric effects of DBS. We also anticipate advances in imaging, targeting and DBS hardware. Over the next 5 years there is a good chance that we will observe a clinical use for diffusion tensor imaging and for DBS field modeling. These advances will aid in better predicting the benefits and the side effects of the therapy. Smart devices, which provide physiological output, may become available and assist clinicians and researchers in DBS placement, optimization and DBS programing. Finally, there is some hope that PPN DBS, or alternatively, another neuromodulation approach, may emerge to treat levodopa-resistant PD symptoms (gait, balance, speech, swallowing and cognition).
Acknowledgments
We would like to acknowledge Ihtsham Haq for his help in refining our thoughts on the early DBS section of the manuscript.
Footnotes
For reprint orders, please contact reprints@expert-reviews.com
Financial & competing interests disclosure
We would like to acknowledge the support of the University of Florida National Parkinson Foundation Center of Excellence and the Greene and Jacobus family funds.
Michael Okun serves as a consultant for the National Parkinson Foundation, and has received research grants from NIH, NPF, the Michael J Fox Foundation, the Parkinson Alliance, Medtronic peer reviewed fellowship training grants, and the UF Foundation. Michael Okun has in the past received honoraria for DBS educational talks prior to 2010, but currently receives no support (since July 2009). Michael Okun has received royalties for publications with Demos, Manson and Cambridge (movement disorders books). Michael Okun has potential royalty interest in the COMPRESS tool for DBS. Michael Okun has participated in CME activities on movement disorders sponsored by the USF CME office.
Kelly Foote has received research grants from NIH, NPF, Medtronic peer reviewed fellowship training grants and the UF Foundation. Kelly Foote has in the past received honoraria for DBS educational talks, but currently receives no support. Kelly Foote has potential royalty interest in the COMPRESS tool for DBS. Kelly Foote has participated in CME activities on movement disorders sponsored by the USF CME office.
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Benabid AL. Deep brain stimulation for Parkinson’s disease. Curr Opin Neurobiol. 2003;13(6):696–706. doi: 10.1016/j.conb.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Benabid AL, Chabardes S, Seigneuret E, et al. Functional neurosurgery: past, present, and future. Clin Neurosurg. 2005;52:265–270. [PubMed] [Google Scholar]
- 3••.Deuschl G, Schade-Brittinger C, Krack P, et al. A randomized trial of deep-brain stimulation for Parkinson’s disease. N Engl J Med. 2006;355(9):896–908. doi: 10.1056/NEJMoa060281. Important randomized deep brain stimulation (DBS) trial that explored quality of life improvements with bilateral subthalamic nucleus (STN) DBS. [DOI] [PubMed] [Google Scholar]
- 4••.Follett KA, Weaver FM, Stern M, et al. Pallidal versus subthalamic deep-brain stimulation for Parkinson’s disease. N Engl J Med. 2010;362(22):2077–2091. doi: 10.1056/NEJMoa0907083. Largest randomized trial of STN and globus pallidus internus DBS performed to date. The results demonstrated that both targets performed well at 2 years for the motor symptoms of Parkinson’s disease. [DOI] [PubMed] [Google Scholar]
- 5•.Weaver FM, Follett K, Stern M, et al. Bilateral deep brain stimulation vs best medical therapy for patients with advanced Parkinson disease: a randomized controlled trial. JAMA. 2009;301(1):63–73. doi: 10.1001/jama.2008.929. Demonstrated the superiority of DBS over medications in a select group of fluctuating Parkinson’s disease patients enrolled in the large Veterans Affairs trial. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams A, Gill S, Varma T, et al. Deep brain stimulation plus best medical therapy versus best medical therapy alone for advanced Parkinson’s disease (PD SURG trial): a randomised, open-label trial. Lancet Neurol. 9(6):581–591. doi: 10.1016/S1474-4422(10)70093-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7••.Okun MS, Fernandez HH, Wu SS, et al. Cognition and mood in Parkinson’s disease in subthalamic nucleus versus globus pallidus interna deep brain stimulation: the COMPARE trial. Ann Neurol. 2009;65(5):586–595. doi: 10.1002/ana.21596. Randomized blinded trial looking at mood and cognition in unilateral STN and globus pallidus internus DBS. Results were similar in both targets. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okun MS, Fernandez HH, Rodriguez RL, Foote KD. Identifying candidates for deep brain stimulation in Parkinson’s disease: the role of the primary care physician. Geriatrics. 2007;62(5):18–24. [PubMed] [Google Scholar]
- 9.Okun MS, Foote KD. Subthalamic nucleus vs globus pallidus interna deep brain stimulation, the rematch: will pallidal deep brain stimulation make a triumphant return? Arch Neurol. 2005;62(4):533–536. doi: 10.1001/archneur.62.4.533. [DOI] [PubMed] [Google Scholar]
- 10•.Okun MS, Tagliati M, Pourfar M, et al. Management of referred deep brain stimulation failures: a retrospective analysis from 2 movement disorders centers. Arch Neurol. 2005;62(8):1250–1255. doi: 10.1001/archneur.62.8.noc40425. Explored the reasons for DBS failure in a group of patients referred to two expert DBS centers. [DOI] [PubMed] [Google Scholar]
- 11•.Witt K, Daniels C, Reiff J, et al. Neuropsychological and psychiatric changes after deep brain stimulation for Parkinson’s disease: a randomised, multicentre study. Lancet Neurol. 2008;7(7):605–614. doi: 10.1016/S1474-4422(08)70114-5. Explored the neuropsychological consequences of STN DBS. [DOI] [PubMed] [Google Scholar]
- 12.Zahodne LB, Okun MS, Foote KD, et al. Greater improvement in quality of life following unilateral deep brain stimulation surgery in the globus pallidus as compared to the subthalamic nucleus. J Neurol. 2009;256(8):1321–1329. doi: 10.1007/s00415-009-5121-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hariz MI, Krack P, Alesch F, et al. Multicentre European study of thalamic stimulation for parkinsonian tremor: a 6 year follow-up. J Neurol Neurosurg Psychiatry. 2008;79(6):694–699. doi: 10.1136/jnnp.2007.118653. [DOI] [PubMed] [Google Scholar]
- 14.Okun MS, Vitek JL. Lesion therapy for Parkinson’s disease and other movement disorders: update and controversies. Mov Disord. 2004;19(4):375–389. doi: 10.1002/mds.20037. [DOI] [PubMed] [Google Scholar]
- 15.Bergman H, Wichmann T, DeLong MR. Reversal of experimental parkinsonism by lesions of the subthalamic nucleus. Science. 1990;249(4975):1436–1438. doi: 10.1126/science.2402638. [DOI] [PubMed] [Google Scholar]
- 16.Benabid AL, Chabardes S, Mitrofanis J, Pollak P. Deep brain stimulation of the subthalamic nucleus for the treatment of Parkinson’s disease. Lancet Neurol. 2009;8(1):67–81. doi: 10.1016/S1474-4422(08)70291-6. [DOI] [PubMed] [Google Scholar]
- 17.Moro E, Hamani C, Poon YY, et al. Unilateral pedunculopontine stimulation improves falls in Parkinson’s disease. Brain. 2010;133(Pt 1):215–224. doi: 10.1093/brain/awp261. [DOI] [PubMed] [Google Scholar]
- 18•.Stefani A, Lozano AM, Peppe A, et al. Bilateral deep brain stimulation of the pedunculopontine and subthalamic nuclei in severe Parkinson’s disease. Brain. 2007;130(Pt 6):1596–1607. doi: 10.1093/brain/awl346. Explored pedunculopontine nucleus DBS as a rescue strategy for patients with bilateral STN DBS. [DOI] [PubMed] [Google Scholar]
- 19.Yelnik J. PPN or PPD, what is the target for deep brain stimulation in Parkinson’s disease? Brain. 2007;130(Pt 9):e79. doi: 10.1093/brain/awm138. [DOI] [PubMed] [Google Scholar]
- 20.Goff LK, Jouve L, Melon C, Salin P. Rationale for targeting the thalamic centre-median parafascicular complex in the surgical treatment of Parkinson’s disease. Parkinsonism Relat Disord. 2009;15(Suppl 3):S167–S170. doi: 10.1016/S1353-8020(09)70807-7. [DOI] [PubMed] [Google Scholar]
- 21.Plaha P, Khan S, Gill SS. Bilateral stimulation of the caudal zona incerta nucleus for tremor control. J Neurol Neurosurg Psychiatry. 2008;79(5):504–513. doi: 10.1136/jnnp.2006.112334. [DOI] [PubMed] [Google Scholar]
- 22•.Taba HA, Wu SS, Foote KD, et al. A closer look at unilateral vs bilateral DBS: results of the National Institutes of Health COMPARE cohort. J Neurosurg. 2010 doi: 10.3171/2010.8.JNS10312. (Epub ahead of print). Explored a group of patients from a randomized DBS trial and the reasons why they did not require a second DBS device. [DOI] [PubMed] [Google Scholar]
- 23.Tagliati M, Jankovic J, Pagan F, Susatia F, Isaias IU, Okun MS. Safety of MRI in patients with implanted deep brain stimulation devices. Neuroimage. 2009;47(Suppl 2):T53–T57. doi: 10.1016/j.neuroimage.2009.04.044. [DOI] [PubMed] [Google Scholar]
- 24.Mikos A, Pavon J, Bowers D, et al. Factors related to extended hospital stays following deep brain stimulation for Parkinson’s disease. Parkinsonism Relat Disord. 2010;16(5):324–328. doi: 10.1016/j.parkreldis.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 25.Morrell M. Brain stimulation for epilepsy: can scheduled or responsive neurostimulation stop seizures? Curr Opin Neurol. 2006;19(2):164–168. doi: 10.1097/01.wco.0000218233.60217.84. [DOI] [PubMed] [Google Scholar]
- 26.Bronte-Stewart H, Barberini C, Koop MM, Hill BC, Henderson JM, Wingeier B. The STN β-band profile in Parkinson’s disease is stationary and shows prolonged attenuation after deep brain stimulation. Exp Neurol. 2009;215(1):20–28. doi: 10.1016/j.expneurol.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 27.Temperli P, Ghika J, Villemure JG, Burkhard PR, Bogousslavsky J, Vingerhoets FJ. How do parkinsonian signs return after discontinuation of subthalamic DBS? Neurology. 2003;60(1):78–81. doi: 10.1212/wnl.60.1.78. [DOI] [PubMed] [Google Scholar]
- 28.Okun MS, Foote KD. Enough is enough: moving on to deep brain stimulation in patients with fluctuating Parkinson disease. Arch Neurol. 2009;66(6):778–780. doi: 10.1001/archneurol.2009.82. [DOI] [PubMed] [Google Scholar]
- 29.Schupbach WM, Agid Y. Psychosocial adjustment after deep brain stimulation in Parkinson’s disease. Nat Clin Pract Neurol. 2008;4(2):58–59. doi: 10.1038/ncpneuro0714. [DOI] [PubMed] [Google Scholar]
- 30.Schupbach WM, Maltete D, Houeto JL, et al. Neurosurgery at an earlier stage of Parkinson disease: a randomized, controlled trial. Neurology. 2007;68(4):267–271. doi: 10.1212/01.wnl.0000250253.03919.fb. [DOI] [PubMed] [Google Scholar]
- 31.Haq I, Foote KD, Okun MS. Is earlier better? What do we mean when we advocate for early DBS in Parkinson disease? Appl Neurol. 2008:24–31. [Google Scholar]
- 32.Charles PD, Gill CE, Davis TL, Konrad PE, Benabid AL. Is deep brain stimulation neuroprotective if applied early in the course of PD? Nat Clin Pract Neurol. 2008;4(8):424–426. doi: 10.1038/ncpneuro0848. [DOI] [PubMed] [Google Scholar]
- 33.Okun MS, Foote KD. A mnemonic for Parkinson disease patients considering DBS: a tool to improve perceived outcome of surgery. Neurologist. 2004;10(5):290. doi: 10.1097/01.nrl.0000138737.97544.7c. [DOI] [PubMed] [Google Scholar]
- 34.Alberts JL, Hass CJ, Vitek JL, Okun MS. Are two leads always better than one: an emerging case for unilateral subthalamic deep brain stimulation in Parkinson’s disease. Exp Neurol. 2008;214(1):1–5. doi: 10.1016/j.expneurol.2008.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alberts JL, Okun MS, Vitek JL. The persistent effects of unilateral pallidal and subthalamic deep brain stimulation on force control in advanced Parkinson’s patients. Parkinsonism Relat Disord. 2008;14(6):481–488. doi: 10.1016/j.parkreldis.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tabbal SD, Ushe M, Mink JW, et al. Unilateral subthalamic nucleus stimulation has a measurable ipsilateral effect on rigidity and bradykinesia in Parkinson disease. Exp Neurol. 2008;211(1):234–242. doi: 10.1016/j.expneurol.2008.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zahodne LB, Okun MS, Foote KD, et al. Cognitive declines one year after unilateral deep brain stimulation surgery in Parkinson’s disease: a controlled study using reliable change. Clin Neuropsychol. 2009;23(3):385–405. doi: 10.1080/13854040802360582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.York MK, Dulay M, Macias A, et al. Cognitive declines following bilateral subthalamic nucleus deep brain stimulation for the treatment of Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2008;79(7):789–795. doi: 10.1136/jnnp.2007.118786. [DOI] [PubMed] [Google Scholar]
- 39.Mikos A, Bowers D, Noecker AM, et al. Patient-specific analysis of the relationship between the volume of tissue activated during DBS and verbal fluency. Neuroimage. 2010 doi: 10.1016/j.neuroimage.2010.03.068. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Burdick AP, Fernandez HH, Foote KD, et al. Target specificity for post-DBS anger. Presented at: Movement Disorders, Movement Disorders Society 14th Annual Congress; Buenos Aires, Argentina. 13–17 June (2010). [Google Scholar]
- 41.Frank MJ, Samanta J, Moustafa AA, Sherman SJ. Hold your horses: impulsivity, deep brain stimulation, and medication in parkinsonism. Science. 2007;318(5854):1309–1312. doi: 10.1126/science.1146157. [DOI] [PubMed] [Google Scholar]
- 42.Ballanger B, van Eimeren T, Moro E, et al. Stimulation of the subthalamic nucleus and impulsivity: release your horses. Ann Neurol. 2009;66(6):817–824. doi: 10.1002/ana.21795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alberts JL, Voelcker-Rehage C, Hallahan K, Vitek M, Bamzai R, Vitek JL. Bilateral subthalamic stimulation impairs cognitive-motor performance in Parkinson’s disease patients. Brain. 2008;131(Pt 12):3348–3360. doi: 10.1093/brain/awn238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hershey T, Mink JW. Using functional neuroimaging to study the brain’s response to deep brain stimulation. Neurology. 2006;66(8):1142–1143. doi: 10.1212/01.wnl.0000216425.34178.dd. [DOI] [PubMed] [Google Scholar]
- 45.Hershey T, Wu J, Weaver PM, et al. Unilateral vs. bilateral STN DBS effects on working memory and motor function in Parkinson disease. Exp Neurol. 2008;210(2):402–408. doi: 10.1016/j.expneurol.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Okun MS, Fernandez HH, Pedraza O, et al. Development and initial validation of a screening tool for Parkinson disease surgical candidates. Neurology. 2004;63(1):161–163. doi: 10.1212/01.wnl.0000133122.14824.25. [DOI] [PubMed] [Google Scholar]
- 47.Okun MS, Fernandez HH, Foote KD, Murphy TK, Goodman WK. Avoiding deep brain stimulation failures in Tourette syndrome. J Neurol Neurosurg Psychiatry. 2008;79(2):111–112. doi: 10.1136/jnnp.2007.135715. [DOI] [PubMed] [Google Scholar]
- 48.Schupbach M, Gargiulo M, Welter ML, et al. Neurosurgery in Parkinson disease: a distressed mind in a repaired body? Neurology. 2006;66(12):1811–1816. doi: 10.1212/01.wnl.0000234880.51322.16. [DOI] [PubMed] [Google Scholar]
- 49.Okun MS, Fogel A, Skoblar B, et al. Patient centered outcomes for deep BRAIN stimulation. Presented at: 60th Annual Meeting of the American Academy of Neurology; Chicago, IL, USA. 12–19 April (2008). [Google Scholar]
- 50.Follett K, Weaver F, Stern M, et al. Multisite randomized trial of deep brain stimulation. Arch Neurol. 2005;62(10):1643–1644. doi: 10.1001/archneur.62.10.1643-b. [DOI] [PubMed] [Google Scholar]
- 51.Defer GL, Widner H, Marie RM, Remy P, Levivier M. Core assessment program for surgical interventional therapies in Parkinson’s disease (CAPSIT-PD) Mov Disord. 1999;14(4):572–584. doi: 10.1002/1531-8257(199907)14:4<572::aid-mds1005>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 52.Tabbal SD, Revilla FJ, Mink JW, et al. Safety and efficacy of subthalamic nucleus deep brain stimulation performed with limited intraoperative mapping for treatment of Parkinson’s disease. Neurosurgery. 2007;61(3 Suppl):119–127. doi: 10.1227/01.neu.0000289725.97211.51. [DOI] [PubMed] [Google Scholar]
- 53.Limousin P, Krack P, Pollak P, et al. Electrical stimulation of the subthalamic nucleus in advanced Parkinson’s disease. N Engl J Med. 1998;339(16):1105–1111. doi: 10.1056/NEJM199810153391603. [DOI] [PubMed] [Google Scholar]
- 54.Voon V, Krack P, Lang AE, et al. A multicentre study on suicide outcomes following subthalamic stimulation for Parkinson’s disease. Brain. 2008;131(Pt 10):2720–2728. doi: 10.1093/brain/awn214. [DOI] [PMC free article] [PubMed] [Google Scholar]