Abstract
BMI-1 and EZH2 are polycomb group (PcG) proteins which maintain self-renewal of stem cells, and are overexpressed in leukemia. To investigate the potential of PcG proteins as leukemia-associated antigens, and targets for graft-versus-leukemia (GVL) effects, we studied cells from 86 chronic myeloid leukemia (CML) patients and 25 HLA-A*0201+ sibling donors collected prior to allogeneic stem cell transplantation (SCT). Although BMI-1 overexpression in CD34+ cells of CML patients treated with pharmacotherapy is associated with poor prognosis, we found, conversely, that in CML patients treated with SCT, a higher expression of BMI-1, and correspondingly lower expression of its target for repression, CDKN2A, is associated with improved leukemia-free survival. Cytotoxic T lymphocyte (CTL) responses to BMI-1 peptide were detected in 5 of 25 (20%) donors, and 8 of 19 (42%) HLA-A*0201+ CML patients. BMI-1 generated more total and high avidity immune responses, and was more immunogenic than EZH2. PcG-specific CTLs had memory phenotype, were readily expanded in short-term cultures, and were detected post-SCT in recipients of PcG-specific CTL-positive donors. A higher BMI-1 expression in CML CD34+ progenitors was associated with native BMI-1 immune responses. These immune responses to PcG proteins may target leukemia stem cells and have relevance for disease control by GVL.
Keywords: Chronic myeloid leukemia, BMI-1, graft-versus-leukemia effect, leukemia-associated antigens
Introduction
The polycomb group (PcG) proteins BMI-1 and EZH2 are key regulators of transcription as members of polycomb repressive complexes (PRC) which primarily exert repression of downstream genes. Their activity controls numerous aspects of mammalian cell function, in particular those associated with cell fate, self-renewal and ageing(1, 2). Of these two PcG proteins, BMI-1 has been more widely studied in normal and cancerous hematopoietic cells(3). In normal hematopoiesis, BMI-1 is responsible for the maintenance of long-term self-renewing capacity in hematopoietic stem cells in mice(4–6) as well as in humans(7). In leukemic stem cells, knock-down of BMI-1 causes apoptosis and reduced self-renewal(8). BMI-1 has recently been reported to collaborate with BCR-ABL in leukemic transformation(9). The expression of BMI-1 is higher in CD34+ cells from chronic myeloid leukemia (CML) patients than those from healthy individuals, and BMI-1 expression also increases with the progression of disease from chronic phase (CP) to advanced phase(10, 11). A high expression of BMI-1 in solid tumors(12) and leukemias(10, 13, 14) is associated with more rapid disease progression and poor outcome, implying that an increased level of stem-ness conferred by BMI-1 contributes to leukemogenicity and refractoriness to conventional cytotoxic treatment(3). However, in the setting of allogeneic stem cell transplantation (SCT), a higher expression of BMI-1 in pre-transplant CML cells was found to be associated with superior survival due to a lower incidence and severity of acute graft-versus-host disease (GVHD) in a cohort of patients transplanted in CP-CML (15).
In view of the importance of PcG proteins in leukemia stem cell function, and maintenance(4, 16), and their higher expression in leukemic stem cells compared with normal hematopoietic stem cells, they would be ideal leukemia-associated antigens. Immune responses to BMI-1 and EZH2 have been described in solid tumors(17) and leukemias(18), but their general relevance to disease outcome has not been clearly defined. Here, we describe cytotoxic T lymphocyte (CTL) responses to peptides derived from BMI-1 and EZH2 in patients with CML and their healthy HLA-identical sibling donors. A higher expression of BMI-1 and correspondingly lower expression of its target for repression CDKN2A encoding the tumor suppressor protein p16INK4A, in CML cells pre-SCT was associated with reduced disease-associated death, and improved leukemia-free survival and overall survival post-SCT. We found that immune responses to BMI-1 and EZH2 also occur post-SCT, suggesting that they may be relevant for disease control by graft-versus-leukemia (GVL) effects.
Materials and Methods
Patients and healthy controls
All consecutive CML patients who received T-cell depleted SCT from their HLA-identical sibling between September 1993 and May 2006 in the Hematology Branch, National Heart, Lung and Blood Institute, with available pre-SCT biological material were studied. The pre-SCT disease status of either CP or advanced phase (AdP, accelerated and blast phase) was determined using the International Bone Marrow Transplant Registry criteria(19). All patients and donors gave written informed consent before enrolling in myeloablative (n=84) or non-myeloablative (n=2) Institutional Review Board (IRB)-approved transplantation protocols, details of which have been reported previously(20–22). Current leukemia free survival (LFS) was defined as the survival without evidence of leukemia at the time of the most recent assessment(23). Diagnosis and staging of acute and chronic GVHD was graded according to standard criteria(24, 25). Immune responses were studied in cells from HLA-A*0201+ patients pre-SCT (n=19) and healthy sibling donors (n=25). Furthermore, 8 patients with sufficient biological material were studied post-SCT, and 8 HLA-A*0201+ healthy blood donors were assessed for short-term expansion of leukemia-associated antigen (LAA)-specific CTL.
Sample preparation
Patient cells from leukapheresis products, except in nine patients where bone marrow cells were used, were collected within two months prior to SCT. Donor leukaphereses were collected prior to stem cell mobilization. Post-SCT patient cells were obtained from venous sampling at designated timepoints during clinic follow-up. Mononuclear cells (MNCs) from either leukapheresis product, bone marrow harvest or venous whole blood were isolated using Ficoll-Hypaque density gradient centrifugation (Organon Teknika, Durham, NC) and cryopreserved in RPMI1640 supplemented with 20% fetal calf serum and 10% dimethyl sulfoxide. For gene expression studies, CD34+ cells from thawed MNCs were selected by binding to immunomagnetic beads (MiniMACS, Miltenyi Biotec, Auburn, CA) according to the manufacturer’s instructions as previously reported(20). Total RNA was extracted from CD34+ and CD34-negative cells using the RNeasy kit (Qiagen, Valencia, CA) and reverse transcription performed with the Advantage RT-for-PCR kit (Clontech, Mountain View, CA). In HLA-A*0201+ patients and donors, MNCs were thawed, washed and resuspended in complete media (CM) (RPMI1640 supplemented with 10% pooled human AB serum (Sigma-Aldrich, St Louis, MO)). High resolution HLA Class I typing was performed by sequence-specific polymerase chain reaction (PCR) using genomic DNA (HLA Laboratory, Department of Transfusion Medicine, NIH, Bethesda, MD). The presence of immunoglobulin-G (IgG) and IgM cytomegalovirus (CMV) antibodies in the samples was analyzed by passive latex agglutination (CMVSCAN kit; BD Becton Dickinson Microbiology System, Cockeysville, MD) according to the manufacturer’s instructions.
Peptide synthesis
Peptides were manufactured by Biosynthesis (Lewisville, TX) to a minimum purity of 95%. The identity of each peptide was confirmed by mass spectrometry. Two peptides each derived from BMI-1 (BMI-1 74–82; TLQDIVYKL [BMI-T] and BMI-1 271–279; CLPSPSTPV [BMI-C]) and EZH2 (EZH2 666–674; YMCSFLFNL [EZH-Y] and EZH2 734–742; SQADALKYV [EZH-S])(17, 18) were tested. Comparative studies were performed with peptides from two well-characterized LAAs - Wilms’ tumor 1 (WT1 126–134; RMFPNAPYL) and proteinase 3 (PR1 169–177; VLQELNVTV)(26). In patients and donors who had CMV antibodies, the CMVpp65 -derived peptide NLVPMVATV was used as positive control.
Peptide-HLA Class I dextramer complexes and immunophenotyping
Freshly thawed MNCs from HLA-A*0201+ patients and donors were stained with peptide/HLA Class I dextramer complexes for 30 min according to the manufacturer (Immunodex, Dako, Glostrup, Denmark)’s instructions using allophycocyanin (APC)- conjugated BMI-C/HLA-A*0201 or EZH-Y/HLA-A*0201, phycoerythrin (PE)-conjugated BMI-T/HLA-A*0201 or EZH-S/HLA-A*0201, with positive control dextramer complexes of CMVpp65-derived peptides NLVPMVATV/HLA-A*0201-APC and VLEETSVML/HLA-A*0201-PE and negative controls human immunodeficiency virus (HIV) envelope-derived peptides SLYNTVATL/HLA-A*0201-APC and ILKEPVHGV/HLA-A*0201-PE. Between 1 – 3 × 106 MNCs were stained in 100μL 5% FCS/phosphate buffered saline (PBS) at room temperature. Subsequently, cells were stained with a titrated panel of directly conjugated monoclonal antibodies to CD3, CD4, CD8, CD27, CD45RO (all from BD Biosciences, San Jose, CA) using fluorescein isothiocyanate (FITC), peridinin chlorophyll protein (PerCP), PE-Cy7, APC-H7 and Pacific Blue as fluorochromes. An amine-reactive dead cell stain detected in the ultra-violet channel (BIVID, Molecular Probes, Invitrogen, Carlsbad, CA) was used to gate out non-viable cells. A minimum of 0.5 × 106 cells were acquired using the LSRII flowcytometer on the FACSDiva software (BD Biosciences, San Jose, CA). All individuals studied were serologically negative for HIV antibodies, and a 2-fold or greater response in comparison to the negative control (HIV dextramers) was defined as a positive immune response.
Detection of functional antigen-specific CD8+ T cells
MNCs from HLA-A*0201+ patients and donors were rested overnight at 37°C in CM after thawing. Costimulatory antibodies anti-CD28 and 49d, (1μg/mL, Fastimmune, BD Biosciences), monensin (Golgistop, BD Biosciences), brefeldin (10μg/mL, Sigma-Aldrich, St Louis, MO) and CD107a-FITC (BD Biosciences) were added. The cells were loaded with 0.1 and 10.0μM of test peptides BMI-T, BMI-C, EZH-Y and EZH-S. Negative control consisted of unstimulated cells, and two positive controls, Staphylococcus enterotoxin B (SEB) (1μg/mL, Toxin Technology, Sarasota, FL) and the CMVpp65 -derived peptide NLVPMVATV, were included for each assay. Cells were incubated for 5 hours at 37°C, and then washed and stained with CD3-FITC, CD8-PerCP and BIVID, fixed and permeabilised and subsequently stained for intra-cellular cytokines with monoclonal antibodies against interferon (IFN)-γ, tumor- necrosis factor (TNF)-α, interleukin (IL)-2, IL-4, IL-10 conjugated with APC or PE (all from BD Biosciences).
Real-time quantitative reverse-transcription polymerase chain reaction (RQ-PCR)
TaqMan™ Assays-on-demand™ probe-and-primer reagents (Applied Biosystems, Foster City, CA) for BMI-1, Hs00180411_m1, EZH2, Hs01016789_m1, and CDKN2A, Hs00233365_m1 were used according to the manufacturer’s instructions. WT1 and BCR-ABL expression were measured as previously described for assessment of minimal residual disease post-SCT(26). ABL expression was used as the endogenous cDNA quantity control as previously described(27). All reactions were performed in triplicate on 10 μL volume using standard conditions with 40 cycles of amplification using the ABI PRISM 7900 sequence detection system.
Short-term expansion of BMI-1 and EZH2-specific CD8+ T cells in vitro and IFN-γELISPOT assay
The frequency of antigen-specific CTLs in freshly thawed MNCs and in MNCs expanded for seven days in-vitro was assessed using a modification of the ELISPOT assay as comprehensively described previously (28, 29). Cells were plated in 96-well round bottom microtiter plates (100,000 MNCs or 25,000 CD8+ T-cells/well) and loaded with test peptides (0.1, 1.0 and 10μM), or medium alone as negative control. Test peptides BMI-T, BMI-C, EZH-Y, EZH-S, PR1, WT1 and control peptides gp100209–217(210M) and CAP1-6D (irrelevant HLA-A*0201-binding peptide- negative controls), and CMVpp65 -derived peptide NLVPMVATV (positive control for CMV-responsive patients/donors) were added directly to each well. Cells were harvested on day 7 for detection of IFN-γ by ELISPOT analysis, and weekly subsequently to assess longer-term expansion following weekly restimulation with the relevant peptide. A positive ELISPOT response was defined as fulfilling 3 criteria: (a) at least 20 spots per 100,000 MNCs or 25,000 CD8+ T-cells, (b) at least 2-fold the number of spots in background negative control wells, and (c) significant by t-test (p<0.05), in the presence of viable negative and positive controls.
Statistical methods
Patient groups were compared using the χ2 test for categorical data or Mann-Whitney U test for continuous data. Survival curves were calculated using the Kaplan-Meier method and patient groups were compared using the log-rank test. Patients were divided into groups delineated by the median cutoffs of RQ-PCR expression values for EZH2, BMI-1 and CDKN2A. RQ-PCR results from CD34+ and CD34-negative populations in patient samples were compared using Spearman’s ρ. P-values were from two-sided tests with values <0.05 considered significant. Data analysis was performed using SPSS 14 for Windows software (SPSS, Chicago, IL) and GraphPad Prism 4.0 (GraphPad Software, San Diego, CA).
Results
Transplant outcome
The characteristics of the patients included in this study, conditioning regimens, GVHD prophylaxis and parameters of transplant outcome have been reported in detail recently (20). In brief, this cohort of CML patients received T-cell depleted grafts (containing 0.2 – 2 × 105/kg T cells) from their HLA-identical sibling donors. The median follow-up time for this cohort after SCT was 62.6 months (range 0.5 – 159.2 months). Patients were transplanted at a median of 12.8 months from diagnosis (range, 1.6–147 months). According to IRB-approved protocols(30), in the absence of GVHD or unless molecular remission was documented, 1 – 5 × 107/kg T-cells were added back on day 30–60 (n=67) or day 100 (n=14). Only 12 patients had T-cells added at both timepoints. On multivariate analysis, the factors associated with improved leukemia free survival (LFS) and overall survival (OS) in this cohort of CML patients were disease phase, CD34+ cell dose of the graft and lymphocyte count at Day 30 post-SCT(21). In particular, there was no difference in LFS, OS or relapse rate post-SCT between older and newer transplant protocols, despite modest improvements in transplant-related mortality with the era of transplantation. In this cohort, we had previously reported that a higher expression of PR3 or ELA2 in pre-SCT leukemic CD34+ cells of AdP-CML patients was associated with a better outcome post-transplantation. However, PR3 and ELA2 expression were not prognostic in CP-CML patients(20).
BMI-1, EZH2 and CDKN2A expression as predictors of SCT outcome in CML patients
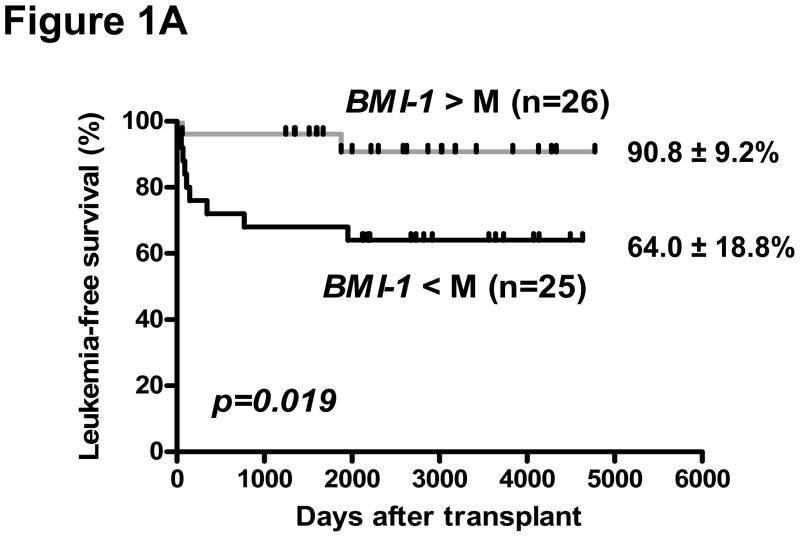
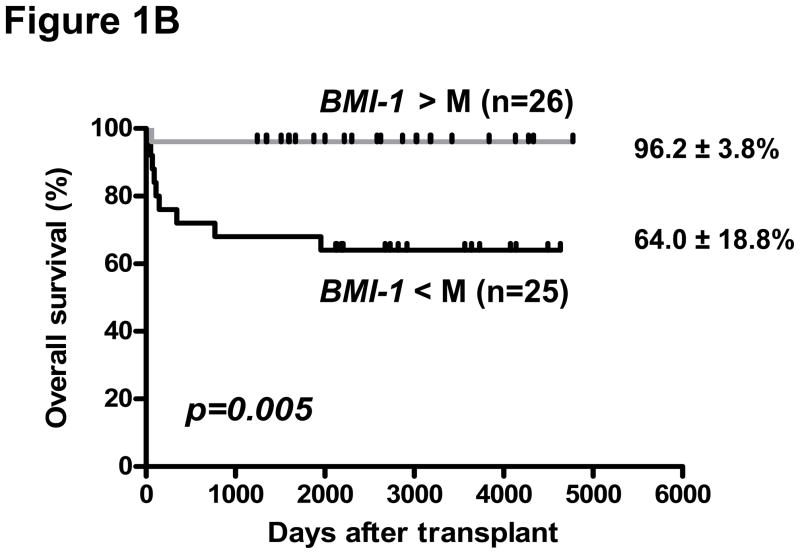
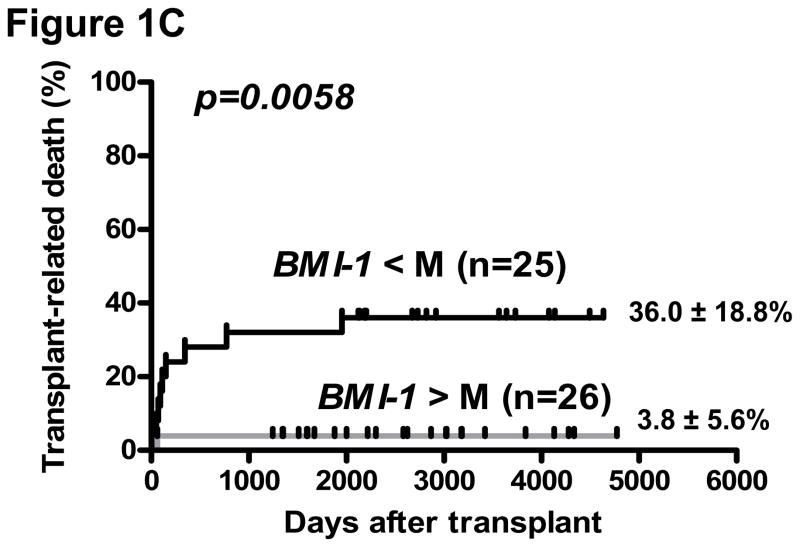
Biological material for isolation of CD34+ cells was available in 82 of 86 CML patients pre-SCT, of which 51 were in CP and 31 AdP-CML (6 blast phase, 25 accelerated phase) at the time of SCT. The expression of EZH2, BMI-1, and its target for repression, CDKN2A (which encodes for p16INK4A) was highly correlated in CD34+ and CD34-negative cells (p<0.005, Supplementary Figure 1). In a univariate analysis of all CML patients in this cohort, a lower CDKN2A expression in CD34-negative cells was associated with greater incidence of chronic GVHD (cGVHD) (25/32 patients below median value with cGVHD [limited = 20, extensive = 5] vs. 16/34 above median with cGVHD [limited = 13; extensive = 3], p= 0.009). However, there was no corresponding association of BMI-1 or EZH2 expression with either acute GVHD or cGVHD in the entire cohort. In a logistic regression model, death-related-to-disease (DRD) was lower in patients with low CDKN2A in either CD34+ or CD34-negative populations (p=0.0001 for both). Correspondingly, a lower CDKN2A in both the CD34+ population (p= 0.013 and p=0.005, respectively) and CD34-negative population (p=0.03 and p=0.048, respectively) was associated with superior LFS and OS in the entire cohort of CML patients. In subgroup analysis of CP-CML patients, a higher expression of BMI-1, and accordingly, lower CDKN2A was associated with improved LFS (p=0.019 and p=0.011, respectively) and OS (p=0.005 and p=0.006, respectively). Transplant-related mortality (TRM) was also lower in CP-CML patients with high BMI-1 (p=0.0058) (Figure 1). There were no significant associations of EZH2 expression with disease outcome.
Figure 1. BMI-1 expression and post-transplant outcome.
Probability of leukemia-free survival (A), overall survival (B) and transplant-related death (C), in CP-CML. Groups are segregated according to the median (M) values of BMI-1 expression.
Ex vivo detection of CD8+ T-cell responses directed against HLA-A*0201-restricted BMI-1 and EZH2 epitopes
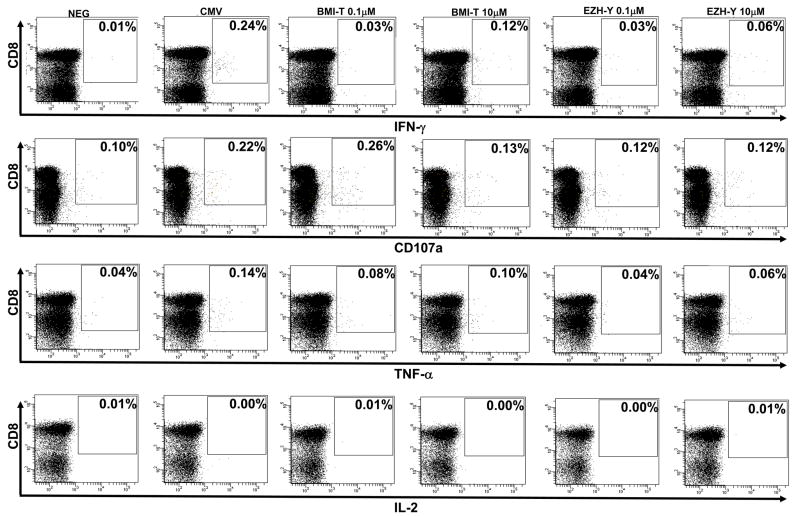
All HLA-A*0201+ patients and donors with available biological material were studied for immune responses to BMI-1- and EZH2-derived peptides. BMI-1-specific CTL responses were detected in 8 of 19 (42%) HLA-A*0201+ CML patients before SCT, and 5 of 25 (20%) healthy sibling donors. Of the two BMI-1 derived peptides, nine CTL responses were to BMI-T and eight to BMI-C. Four individuals had CTL responses to both BMI-1 peptides. On the other hand, native EZH2-specific CTL responses were detected in 6 of 19 (32%) CML patients and 3 of 25 (12%) healthy donors. There were eight EZH-Y-CTL responses and four EZH-S-CTL responses, with three individuals having CTL responses to both EZH2 peptides (Table 1). Polyfunctionality of CTLs was studied in 14 patients and 4 donors where sufficient biological material was available. The majority of detected PcG-specific CTLs were interferon-γ producing only upon exposure to the relevant peptide. However, in the minority, TNF-α and CD107a responses were also detected (Figure 2). IL-2, IL-4 or IL-10 responses were not detected in samples with concordant interferon-γ, TNF-α or CD107a responses to specific peptides.
Table 1.
Immune responses to PcG-specific peptides in HLA-A*0201+ patients and donors
| UPN | Status | Cell source | Immune responses in CD8+ T cells (%)† | |||
|---|---|---|---|---|---|---|
| BMI-T | BMI-C | EZH-Y | EZH-S | |||
| 402 | CP | PB | Neg | Neg | Neg | Neg |
| 199 | CP | PB | Neg | Neg | Neg | 0.23 |
| 41 | CP | BM | 0.05 | 0.08 | Neg | Neg |
| 11 | CP | BM | 0.07 | Neg | 0.05 | 0.05 |
| 319 | CP | PB | Neg | Neg | 0.09 | Neg |
| 1 | CP | PB | Neg | 0.16 | Neg | Neg |
| 177 | CP | PB | 0.15 | 0.10 | 0.09 | Neg |
| 273 | CP | PB | Neg | 0.11 | Neg | Neg |
| 28 | CP | PB | Neg | Neg | Neg | Neg |
| 16 | CP | BM | Neg | Neg | Neg | Neg |
| 223 | CP | PB | 0.11 | 0.13 | Neg | Neg |
| 365 | CP | PB | Neg | Neg | 0.09 | Neg |
| 241 | CP | PB | Neg | Neg | Neg | Neg |
| 17 | AdP | PB | Neg | Neg | Neg | Neg |
| 25 | AdP | PB | 0.12 | Neg | 0.06 | Neg |
| 68 | AdP | PB | Neg | Neg | Neg | Neg |
| 469 | AdP | PB | Neg | Neg | Neg | Neg |
| 483 | AdP | PB | 0.05 | Neg | Neg | Neg |
| 19 | AdP | PB | Neg | Neg | Neg | Neg |
| 94 | Donor | PB | Neg | Neg | Neg | Neg |
| 12 | Donor | PB | Neg | Neg | Neg | Neg |
| 49 | Donor | PB | Neg | Neg | Neg | Neg |
| 327 | Donor | PB | Neg | Neg | Neg | Neg |
| 402 | Donor | PB | Neg | Neg | Neg | Neg |
| 38 | Donor | PB | Neg | Neg | Neg | Neg |
| 283 | Donor | PB | Neg | Neg | Neg | Neg |
| 25 | Donor | PB | Neg | 0.32 | 0.25 | Neg |
| 41 | Donor | PB | Neg | Neg | Neg | Neg |
| 170 | Donor | PB | Neg | Neg | Neg | Neg |
| 11 | Donor | PB | Neg | Neg | Neg | Neg |
| 319 | Donor | PB | Neg | Neg | Neg | Neg |
| 469 | Donor | PB | Neg | Neg | Neg | Neg |
| 262 | Donor | PB | 0.12 | Neg | Neg | Neg |
| 68 | Donor | PB | Neg | Neg | Neg | Neg |
| 1 | Donor | PB | Neg | Neg | Neg | Neg |
| 17 | Donor | PB | Neg | Neg | Neg | Neg |
| 273 | Donor | PB | 0.06 | Neg | Neg | Neg |
| 483 | Donor | PB | Neg | Neg | Neg | Neg |
| 28 | Donor | PB | Neg | Neg | Neg | Neg |
| 19 | Donor | PB | Neg | 0.14 | 0.11 | 0.16 |
| 16 | Donor | PB | Neg | Neg | Neg | Neg |
| 365 | Donor | PB | 0.17 | 0.16 | 0.17 | 0.18 |
| 48 | Donor | PB | Neg | Neg | Neg | Neg |
| 241 | Donor | PB | Neg | Neg | Neg | Neg |
UPN=Unique patient number, CP = chronic phase CML, AdP = advanced phase CML, PB= peripheral blood, BM=bone marrow
Immune responses (percentage of total CD8+ cells, with positive results in bold) to PcG-specific peptide refer to ex-vivo interferon-γ (IFN-γ) production in all cases except UPN 177 (TNF-α production) and UPN 365 and donor for UPN 25 (dextramer positivity)
Figure 2. BMI-1-specific cytotoxic T lymphocytes are polyfunctional.
Representative dot plots from CML patient UPN 25 showing ex-vivo immune responses to BMI-T and EZH-Y peptides using detection of interferon- (IFN)-γ, CD107a degranulation, tumor necrosis factor (TNF)-α and interleukin (IL)-2. A positive response is defined as at least (a) two-fold background (negative control) and (b) 0.05% CD8+ population. Low avidity response to 10μM BMI-T peptide is detected with IFN-γ and TNF-α production. High avidity TNF-α response to 0.1μM BMI-T peptide is accompanied by CD107a degranulation. In comparison, low avidity response to 10μM EZH-Y peptide is manifest with IFN-γ production alone.
Immune responses to PcG-specific peptides were studied in eight patients with available biological material from the first 120 days post-SCT. Four patients received SCT from donors with PcG-CTL responses (UPN 25, UPN 262, UPN 273 and UPN 365), three had donors without PcG-CTL responses (UPN 469, UPN 483 and UPN 283) and one patient (UPN 199) did not have available donor material for study. PcG-specific CTLs were detected post-SCT in 4/4 patients whose donors had detectable PcG-CTLs, and in none of the patients whose donors did not have PcG-CTLs. Donor myeloid and T cell chimerism was achieved in the time period studied, suggesting that post- SCT PcG-CTL responses were donor-derived. These PcG-CTLs were of memory phenotype and could be cultured short-term in ELISPOT assays (Figure 3). However, in three patients (UPN19, UPN 262, UPN273) with available biological material, PcG-CTL responses were no longer detectable in the later period post-SCT (at 13, 12 and 10 months post-SCT respectively) in the absence, or reduction of residual leukemia by PCR in these patients. Concordance of the ELISPOT assay with the detection of PcG-specific CTLs using intracytoplasmic cytokine or dextramer staining was found in 75% of individual responses (9 patients, 7 healthy donors) (Supplementary Table 1). In 50% (5/10) of individuals where direct ex-vivo immune responses to PcG-peptides were detected, PcG-specific CTLs could also be expanded in short-term 7-day cultures. In two of three patients (UPN 273, UPN 365 and UPN 319) with adequate biological material, expansion of PcG-specific CTLs were detected beyond two weeks culture. CML patients whose donors had immune responses to BMI-1 peptides had improved leukemia-free survival post-SCT compared with those whose donors had no BMI-1 immune response (80% vs 60%); however, as this was a small cohort, this difference was not statistically significant.
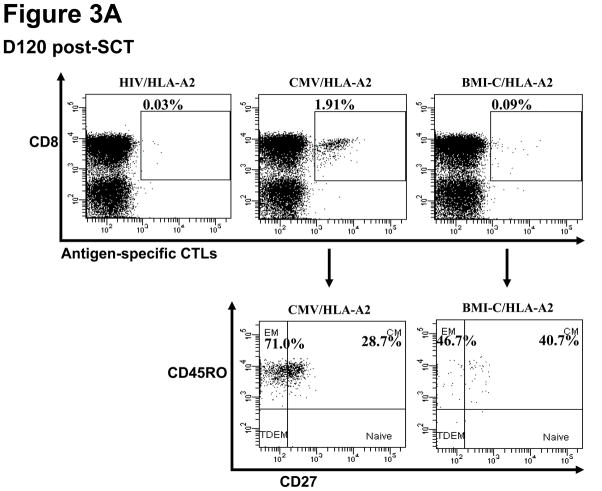
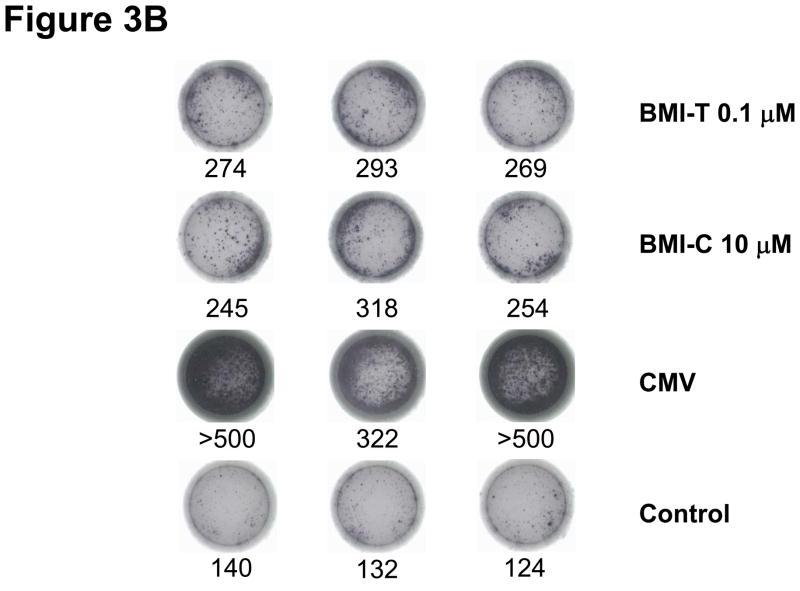
Figure 3. Immune responses to BMI-1-specific peptides detected post-transplant.
Representative immune responses to BMI-T and BMI-C peptides in CML patient UPN 365 at D120 post-transplant. (A) Phenotypic characterization of BMI-1- specific and CMV-specific cytotoxic T lymphocytes (CTLs) using dextramers, with HIV dextramers as negative controls (EM= effector memory; CM= central memory; TDEM = terminally differentiated effector memory CTLs) (B) ELISPOT short-term expansion of BMI-1-specific CTLs, with interferon (IFN)-γ production to BMI-T and BMI-C peptides with similar avidity as in the donor pre-transplant. Numbers below wells refer to IFN-γ spots.
CTL responses to two concentrations of peptide were studied to assess the avidity of responses. A high avidity CTL response was defined as that provoked by a low concentration of peptide (0.1μM), whereas a low avidity response required the higher concentration of 10 μM peptide as previously reported(28, 31). More high avidity responses were found upon stimulation with BMI-1 peptides compared to EZH2-peptides. BMI-T peptides generated the greatest frequency of high avidity CTL responses (Figure 4). There was no appreciable association of PcG expression on CML cells, or CML disease phase with the frequency of higher or lower avidity CTL responses. As PcG protein expression is important in the maintenance of self-renewal in leukemia stem cells, a relationship between the expression of BMI-1 and EZH2 in CD34+ CML cells and PcG-specific CTL responses was sought. There was a significantly higher expression of BMI-1 in CD34+ cells of patients with detectable BMI-1-CTL responses (p=0.02). On the other hand, there was no significant association between EZH2 expression and EZH2-CTL responses (Supplementary Figure 2).
Figure 4. Avidity of CD8+ T-cell responses to peptides derived from polycomb group proteins (PcG) BMI-1 and EZH2.
High- and low-avidity CD8+ T-cell responses are determined by stimulation with 0.1μM or 10μM peptide respectively(28, 31). Results are shown as the ratios of high- to low-avidity T-cell responses from healthy donors (black circles), patients pre-(open circles), and post-allogeneic stem cell transplant (post-SCT) (red circles). Ratios are calculated as IFN-γ+ CD8+ T-cells (%) with 0.1μM peptide/IFN-γ+ CD8+ T-cells (%) with 10μM peptide. Bars represent the median high/low avidity ratio for each peptide. Symbols above the dashed line are samples with majority high avidity responses.
Comparison of immune responses to BMI-1 and EZH2 with established leukemia-associated antigens
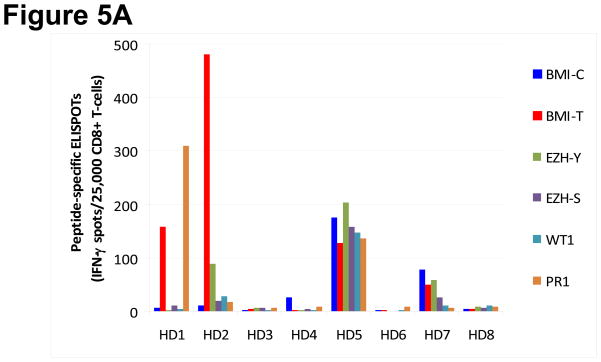
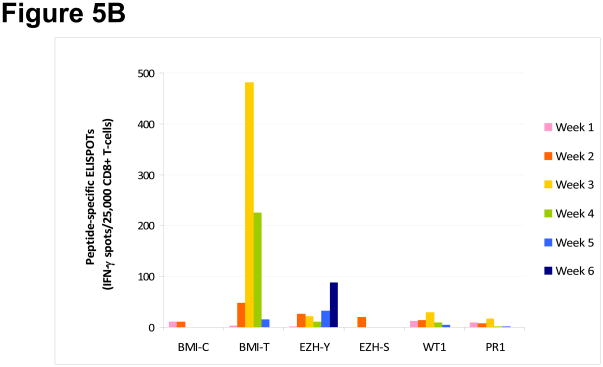
In an additional 8 healthy HLA-A*0201+ blood donors, the frequency of immune responses to BMI-1 and EZH2 was compared to that obtained with well-characterized LAAs WT1 and PR1 peptides using ELISPOT assay for short-term peptide-specific CTL expansion weekly for up to six weeks. Under the same conditions, the short-term expansion of PcG-specific CTLs was not inferior to that obtained by cells stimulated with WT1 or PR1. Five of 8 (63%) healthy blood donors mounted immune responses to BMI-1 peptides, and 3 to EZH2 peptides. WT1- and PR1-specific CTL expansions were found in 2 and 3 donors respectively (Figure 5).
Figure 5. Peptide specific CD8+ T-cell expansion in response to leukemia-associated antigens (LAAs) in healthy donors.
(A) Specific cytotoxic T lymphocyte (CTL) expansion following incubation with BMI-T, BMI-C, EZH-Y, EZH-S, WT1 and PR1 peptides was assessed simultaneously in eight healthy blood donors (HD) at weekly intervals using ELISPOT assay. Individual samples are plotted along the x-axis and the maximal response observed during this time period for each LAA is plotted on the y-axis. (B) Pattern of longitudinal expansion of individual LAA-specific CTLs in representative sample HD2 over 6 weeks. The y-axis denotes the maximum number of interferon (IFN)-γ producing spots positive per 25,000 CD8+ cells plated.
Discussion
The treatment and cure of CML by SCT over the last few decades is based on the unique susceptibility of this leukemia to immune attack(32). Although tyrosine kinase inhibitors (TKIs) are now the frontline treatment for CML, a significant proportion of CP patients do not achieve optimal responses and require alternative therapy(33). Furthermore, even patients who obtain a good response to TKIs remain at risk of drug resistance, and disease progression to AdP-CML as leukemic stem cells are not adequately targeted by TKIs (34, 35). An important aspect of CML immunotherapy research is to identify epitopes on leukemia stem cells, which are of relevance to disease biology. As PcG proteins, especially BMI-1, are increasingly overexpressed with disease progression in CML(10), and essential for CML stem cell maintenance(8), we selected to study immune responses to defined peptides derived from PcG proteins BMI-1 and EZH2(17) in both CML patients and healthy donors. The frequency of immune responses to PcG peptides was comparable to those found for other LAAs such as proteinase 3, WT1(36, 37), and preferentially expressed antigen of melanoma (PRAME)(28). Of the PcG protein-derived peptides studied, BMI-1 peptides appeared more immunogenic, generating greater frequencies of total as well as higher avidity responses compared to EZH2 peptides. Notably, a higher expression of BMI-1, and correspondingly lower expression of its target for repression, CDKN2A, in CML cells were both significantly associated with better leukemia-free survival post-SCT. These findings suggest that higher expression of BMI-1 on CML cells may render them more susceptible to GVL effects contributed by immune responses to BMI-1, and may improve CML elimination in a post-SCT setting. BMI-1-specific immune responses may be particularly relevant as BMI-1 is both important in CML stem cell self-renewal and maintenance(8), and is progressively overexpressed in AdP(10). As our cohort is relatively small, these findings were not significant in multivariate analysis, and the impact of BMI-1 may have been obscured by the powerful predictors of disease phase, CD34+ cell dose of the graft and lymphocyte count at Day 30 post-SCT(21). Our patients, who received T-cell depleted SCT, did not have an association of BMI-1 expression with acute GVHD, contrary to T-replete SCT patients in CML(15). Nevertheless, the role of immune responses, as opposed to the indirect predictive power of the presence or absence of GVHD, as prognostic factors for relapse in SCT requires prospective study in a larger patient cohort.
It is notable that unlike BMI-1 and its direct target for repression, CDKN2A, the expression of EZH2 was not associated with outcome. We found that the pattern of EZH2 expression post-SCT did not consistently correlate with BCR-ABL, WT1 or BMI-1 expression in the limited number of patients who could be studied (Supplementary Figure 3). Recent transgenic mouse studies dissecting the function of individual members of polycomb repressive complexes (PRC) found that the relationship between BMI-1 and EZH2 (components of PRC1 and PRC2 respectively) is not necessarily hierarchical in hematopoietic stem cells (38). However, EZH2 overexpression was found in cells from CML patients with primary resistance to imatinib,(39) thus validating the targeting of EZH2-overexpressing cells, which may help eliminate clones which have the propensity to primary TKI resistance.
Although we demonstrated low frequency of PcG-specific CTLs concordantly by both ex-vivo detection and short term culture in the majority of healthy donors and patients, and expanded them for several weeks, we were unable to establish T cell lines from individuals with PcG-specific CTLs. Other investigators demonstrated leukemia-specific killing using BMI-1-specific T cell lines derived from fresh MNCs of healthy donors(18). Thus, although we could not perform direct cytotoxicity assays using cells from individuals in our cohort, we can assume that the PcG-specific CTLs we found have relevance for GVL effects based on existing reports from other groups(17, 18). In many of the individuals studied, both PcG-specific and CMV-specific (positive control) CTL responses comprised both CD8 high and CD8 low cells. CD8 is a critical coreceptor in T-cell receptor (TCR) complex formation during T cell activation, and prolonged encounter with specific antigen has been shown in previous studies of acute and chronic infection to induce CD8 downregulation as a mechanism to control the activation signal through the TCR-CD8 complex (40, 41). CTLs specific for other LAAs such as WT1 and PRAME also comprise CD8 low populations, which have been shown to have high avidity for the cognate antigen (28, 37). We found that the frequency of PcG-specific CTLs post-SCT tended to correlate with the expression of PcG proteins. Thus, in the post-SCT period when the disease burden was low, with the decrease of antigenic stimulus, CTLs fall below the level of detection(42). As previously reported for other LAA-specific CTLs, we detected immune responses to BMI-1- or EZH2-specific CTLs in the post-SCT patients receiving steroid therapy for GVHD in doses less than 1mg/kg prednisolone(42).
LAA immune responses directed towards autologous proteins such as PcG proteins, proteinase 3 or WT1, expressed by a proportion of normal cells, but overexpressed by leukemia cells, may theoretically give rise to auto-immune damage to normal tissues, or become tolerized. Unlike immune responses to proteinase 3, where there is an inverse correlation between leukemic expression of proteinase 3 and native PR1-specific response, suggesting that tolerance may be shaped against the protein (20, 31), we found that a higher expression of BMI-1 in CML CD34+ progenitors is associated with a higher incidence of native BMI-1-specific immune responses. Thus, epitopes derived from BMI-1 may be less tolerogenic in vivo in patients with established disease. In comparison to CTLs directed against other LAAs(28), we found polyfunctional PcG-specific CTLs in a proportion of individuals with immune responses, secreting not only IFN-γ but also TNF-α and showing evidence of degranulation in response to PcG-derived peptides. These findings support the notion that PcG-specific CTLs exert significant immune responses. Despite their low frequencies, the finding that an appreciable proportion of PcG-specific CTLs have a central memory phenotype indicate that they retain the potential to further expand and proliferate. We found PcG-specific CTLs in healthy donors, and in a proportion similar to that described for other LAAs(28). These donors had normal peripheral blood counts and were able to donate sufficient numbers of stem cells for their sibling’s SCT, leading to good engraftment, suggesting that the presence of PcG-specific CTLs did not affect the functionality of normal hematopoietic stem cells.
Our findings have several therapeutic implications. Firstly, although SCT is used less frequently to treat CML nowadays, transplantation still has an important place in the management of patients resistant to TKI and in AdP disease. The increased leukemic expression of PcG proteins in high risk SCT recipients makes them relevant antigens for GVL. The close association of PcG proteins with stem-ness and the maintenance of a leukemic functional phenotype render PcG-derived antigens significant biological targets. Results from a recent clinical trial on adoptive immunotherapy demonstrated the ability of resistant leukemia to escape potent allogeneic CTLs through the generation of functional leukemic clones downregulating the targeted CTL epitope, which subsequently caused patient death from disease relapse(43). Targeting of proteins essential to leukemia stem cell survival would strengthen immunotherapeutic strategies. Secondly, for CML patients with persisting molecular disease after TKI treatment, enhancing immune responses against PcG-proteins with vaccine therapy may help eliminate residual leukemia. Thus PcG protein-derived antigens, in particular BMI-1 epitopes, deserve further study as targets for immunotherapy in CML, as immune responses against these, described by our group and others as occuring in healthy individuals, have the potential to augment current treatments by targeting leukemia stem cells.
Supplementary Material
Acknowledgments
This research was supported by the Intramural Research Program of the National Institutes of Health, at the NHLBI
Footnotes
Conflict of interest
The authors have no competing financial interests in relation to the work described.
References
- 1.Sparmann A, van LM. Polycomb silencers control cell fate, development and cancer. Nat Rev Cancer. 2006 Nov;6(11):846–56. doi: 10.1038/nrc1991. [DOI] [PubMed] [Google Scholar]
- 2.Pietersen AM, van LM. Stem cell regulation by polycomb repressors: postponing commitment. Curr Opin Cell Biol. 2008 Apr;20(2):201–7. doi: 10.1016/j.ceb.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 3.Rajasekhar VK, Begemann M. Concise review: roles of polycomb group proteins in development and disease: a stem cell perspective. Stem Cells. 2007 Oct;25(10):2498–510. doi: 10.1634/stemcells.2006-0608. [DOI] [PubMed] [Google Scholar]
- 4.Park IK, Qian D, Kiel M, Becker MW, Pihalja M, Weissman IL, et al. Bmi-1 is required for maintenance of adult self-renewing haematopoietic stem cells. Nature. 2003 May 15;423(6937):302–5. doi: 10.1038/nature01587. [DOI] [PubMed] [Google Scholar]
- 5.Iwama A, Oguro H, Negishi M, Kato Y, Morita Y, Tsukui H, et al. Enhanced self-renewal of hematopoietic stem cells mediated by the polycomb gene product Bmi-1. Immunity. 2004 Dec;21(6):843–51. doi: 10.1016/j.immuni.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 6.Lessard J, Sauvageau G. Bmi-1 determines the proliferative capacity of normal and leukaemic stem cells. Nature. 2003 May 15;423(6937):255–60. doi: 10.1038/nature01572. [DOI] [PubMed] [Google Scholar]
- 7.Rizo A, Dontje B, Vellenga E, de HG, Schuringa JJ. Long-term maintenance of human hematopoietic stem/progenitor cells by expression of BMI1. Blood. 2008 Mar 1;111(5):2621–30. doi: 10.1182/blood-2007-08-106666. [DOI] [PubMed] [Google Scholar]
- 8.Rizo A, Olthof S, Han L, Vellenga E, de HG, Schuringa JJ. Repression of BMI1 in normal and leukemic human CD34+ cells impairs self-renewal and induces apoptosis. Blood. 2009 Aug 20;114(8):1498–505. doi: 10.1182/blood-2009-03-209734. [DOI] [PubMed] [Google Scholar]
- 9.Rizo A, Horton SJ, Olthof S, Dontje B, Ausema A, van OR, et al. BMI1 collaborates with BCR-ABL in leukemic transformation of human CD34+ cells. Blood. 2010 doi: 10.1182/blood-2010-02-270660. e-pub ahead of print 19 Aug 2010. [DOI] [PubMed] [Google Scholar]
- 10.Mohty M, Yong AS, Szydlo RM, Apperley JF, Melo JV. The polycomb group BMI-1 gene is a molecular marker for predicting prognosis of chronic myeloid leukemia. Blood. 2007 Jul 1;110(1):380–3. doi: 10.1182/blood-2006-12-065599. [DOI] [PubMed] [Google Scholar]
- 11.Bhattacharyya J, Mihara K, Yasunaga S, Tanaka H, Hoshi M, Takihara Y, et al. BMI-1 expression is enhanced through transcriptional and posttranscriptional regulation during the progression of chronic myeloid leukemia. Ann Hematol. 2009 Apr 1;88(4):333–40. doi: 10.1007/s00277-008-0603-8. [DOI] [PubMed] [Google Scholar]
- 12.Glinsky GV, Berezovska O, Glinskii AB. Microarray analysis identifies a death-from-cancer signature predicting therapy failure in patients with multiple types of cancer. J Clin Invest. 2005 Jun;115(6):1503–21. doi: 10.1172/JCI23412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chowdhury M, Mihara K, Yasunaga S, Ohtaki M, Takihara Y, Kimura A. Expression of Polycomb-group (PcG) protein BMI-1 predicts prognosis in patients with acute myeloid leukemia. Leukemia. 2007 May;21(5):1116–22. doi: 10.1038/sj.leu.2404623. [DOI] [PubMed] [Google Scholar]
- 14.Mihara K, Chowdhury M, Nakaju N, Hidani S, Ihara A, Hyodo H, et al. Bmi-1 is useful as a novel molecular marker for predicting progression of myelodysplastic syndrome and patient prognosis. Blood. 2006 Jan 1;107(1):305–8. doi: 10.1182/blood-2005-06-2393. [DOI] [PubMed] [Google Scholar]
- 15.Mohty M, Szydlo RM, Yong AS, Apperley JF, Goldman JM, Melo JV. Association between BMI-1 expression, acute graft-versus-host disease, and outcome following allogeneic stem cell transplantation from HLA-identical siblings in chronic myeloid leukemia. Blood. 2008 Sep 1;112(5):2163–6. doi: 10.1182/blood-2008-04-148130. [DOI] [PubMed] [Google Scholar]
- 16.Kamminga LM, Bystrykh LV, de BA, Houwer S, Douma J, Weersing E, et al. The Polycomb group gene Ezh2 prevents hematopoietic stem cell exhaustion. Blood. 2006 Mar 1;107(5):2170–9. doi: 10.1182/blood-2005-09-3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steele JC, Torr EE, Noakes KL, Kalk E, Moss PA, Reynolds GM, et al. The polycomb group proteins, BMI-1 and EZH2, are tumour-associated antigens. Br J Cancer. 2006 Nov 6;95(9):1202–11. doi: 10.1038/sj.bjc.6603369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fujii N, Turtle CJ, Campregher PV, Warren EH. Generation of CD8+ cytotoxic T cell clones recognizing BMI1-derived peptides. Blood. 2008;112 Abstract 2909. [Google Scholar]
- 19.Speck B, Bortin MM, Champlin R, Goldman JM, Herzig RH, McGlave PB, et al. Allogeneic bone-marrow transplantation for chronic myelogenous leukaemia. Lancet. 1984 Mar 24;1(8378):665–8. doi: 10.1016/s0140-6736(84)92179-2. [DOI] [PubMed] [Google Scholar]
- 20.Yong AS, Rezvani K, Savani BN, Eniafe R, Mielke S, Goldman JM, et al. High PR3 or ELA2 expression by CD34+ cells in advanced phase chronic myeloid leukemia is associated with improved outcome following allogeneic stem cell transplantation and may improve PR1 peptide driven graft-versus-leukemia effects. Blood. 2007 Jul 15;110(2):770–5. doi: 10.1182/blood-2007-02-071738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Savani BN, Rezvani K, Mielke S, Montero A, Kurlander R, Carter CS, et al. Factors associated with early molecular remission after T cell-depleted allogeneic stem cell transplantation for chronic myelogenous leukemia. Blood. 2006 Feb 15;107(4):1688–95. doi: 10.1182/blood-2005-05-1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sloand E, Childs RW, Solomon S, Greene A, Young NS, Barrett AJ. The graft-versus-leukemia effect of nonmyeloablative stem cell allografts may not be sufficient to cure chronic myelogenous leukemia. Bone Marrow Transplant. 2003 Nov;32(9):897–901. doi: 10.1038/sj.bmt.1704231. [DOI] [PubMed] [Google Scholar]
- 23.Craddock C, Szydlo RM, Klein JP, Dazzi F, Olavarria E, van RF, et al. Estimating leukemia-free survival after allografting for chronic myeloid leukemia: a new method that takes into account patients who relapse and are restored to complete remission. Blood. 2000 Jul 1;96(1):86–90. [PubMed] [Google Scholar]
- 24.Gratwohl A, Hermans J, Apperley J, Arcese W, Bacigalupo A, Bandini G, et al. Acute graft-versus-host disease: grade and outcome in patients with chronic myelogenous leukemia. Working Party Chronic Leukemia of the European Group for Blood and Marrow Transplantation. Blood. 1995 Jul 15;86(2):813–8. [PubMed] [Google Scholar]
- 25.Sullivan KM, Agura E, Anasetti C, Appelbaum F, Badger C, Bearman S, et al. Chronic graft-versus-host disease and other late complications of bone marrow transplantation. Semin Hematol. 1991 Jul;28(3):250–9. [PubMed] [Google Scholar]
- 26.Rezvani K, Yong AS, Mielke S, Savani BN, Musse L, Superata J, et al. Leukemia-associated antigen-specific T-cell responses following combined PR1 and WT1 peptide vaccination in patients with myeloid malignancies. Blood. 2008 Jan 1;111(1):236–42. doi: 10.1182/blood-2007-08-108241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yong AS, Szydlo RM, Goldman JM, Apperley JF, Melo JV. Molecular profiling of CD34+ cells identifies low expression of CD7, along with high expression of proteinase 3 or elastase, as predictors of longer survival in patients with CML. Blood. 2006 Jan 1;107(1):205–12. doi: 10.1182/blood-2005-05-2155. [DOI] [PubMed] [Google Scholar]
- 28.Rezvani K, Yong AS, Tawab A, Jafarpour B, Eniafe R, Mielke S, et al. Ex vivo characterization of polyclonal memory CD8+ T-cell responses to PRAME-specific peptides in patients with acute lymphoblastic leukemia and acute and chronic myeloid leukemia. Blood. 2009 Mar 5;113(10):2245–55. doi: 10.1182/blood-2008-03-144071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weber G, Karbach J, Kuci S, Kreyenberg H, Willasch A, Koscielniak E, et al. WT1 peptide-specific T cells generated from peripheral blood of healthy donors: possible implications for adoptive immunotherapy after allogeneic stem cell transplantation. Leukemia. 2009 Sep;23(9):1634–42. doi: 10.1038/leu.2009.70. [DOI] [PubMed] [Google Scholar]
- 30.Montero A, Savani BN, Shenoy A, Read EJ, Carter CS, Leitman SF, et al. T cell depleted peripheral blood stem cell allotransplantation with T cell add back for patients with hematological malignancies: effect of chronic GVHD on outcome. Biol Blood Marrow Transplant. 2006 Dec;12(12):1318–25. doi: 10.1016/j.bbmt.2006.08.034. [DOI] [PubMed] [Google Scholar]
- 31.Molldrem JJ, Lee PP, Kant S, Wieder E, Jiang W, Lu S, et al. Chronic myelogenous leukemia shapes host immunity by selective deletion of high-avidity leukemia-specific T cells. J Clin Invest. 2003 Mar;111(5):639–47. doi: 10.1172/JCI16398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gratwohl A, Heim D. Current role of stem cell transplantation in chronic myeloid leukaemia. Best Pract Res Clin Haematol. 2009 Sep;22(3):431–43. doi: 10.1016/j.beha.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 33.Marin D, Milojkovic D, Olavarria E, Khorashad JS, de LH, Reid AG, et al. European LeukemiaNet criteria for failure or sub-optimal response reliably identify patients with CML in early chronic phase treated with imatinib whose eventual outcome is poor. Blood. 2008 Dec 1;112(12):4437–44. doi: 10.1182/blood-2008-06-162388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiang X, Zhao Y, Smith C, Gasparetto M, Turhan A, Eaves A, et al. Chronic myeloid leukemia stem cells possess multiple unique features of resistance to BCR-ABL targeted therapies. Leukemia. 2007 May;21(5):926–35. doi: 10.1038/sj.leu.2404609. [DOI] [PubMed] [Google Scholar]
- 35.Konig H, Holtz M, Modi H, Manley P, Holyoake TL, Forman SJ, et al. Enhanced BCR-ABL kinase inhibition does not result in increased inhibition of downstream signaling pathways or increased growth suppression in CML progenitors. Leukemia. 2008 Apr;22(4):748–55. doi: 10.1038/sj.leu.2405086. [DOI] [PubMed] [Google Scholar]
- 36.Gannage M, Abel M, Michallet AS, Delluc S, Lambert M, Giraudier S, et al. Ex vivo characterization of multiepitopic tumor-specific CD8 T cells in patients with chronic myeloid leukemia: implications for vaccine development and adoptive cellular immunotherapy. J Immunol. 2005 Jun 15;174(12):8210–8. doi: 10.4049/jimmunol.174.12.8210. [DOI] [PubMed] [Google Scholar]
- 37.Rezvani K, Brenchley JM, Price DA, Kilical Y, Gostick E, Sewell AK, et al. T-cell responses directed against multiple HLA-A*0201-restricted epitopes derived from Wilms’ tumor 1 protein in patients with leukemia and healthy donors: identification, quantification, and characterization. Clin Cancer Res. 2005 Dec 15;11(24 Pt 1):8799–807. doi: 10.1158/1078-0432.CCR-05-1314. [DOI] [PubMed] [Google Scholar]
- 38.Majewski IJ, Ritchie ME, Phipson B, Corbin J, Pakusch M, Ebert A, et al. Opposing roles of polycomb repressive complexes in hematopoietic stem and progenitor cells. Blood. 2010 Aug 5;116(5):731–9. doi: 10.1182/blood-2009-12-260760. [DOI] [PubMed] [Google Scholar]
- 39.de Lavallade H, Finetti P, Carbuccia N, Khorashad JS, Charbonnier A, Foroni L, et al. A gene expression signature of primary resistance to imatinib in chronic myeloid leukemia. Leuk Res. 2010 Feb;34(2):254–7. doi: 10.1016/j.leukres.2009.09.026. [DOI] [PubMed] [Google Scholar]
- 40.Schmitz JE, Forman MA, Lifton MA, Concepcion O, Reimann KA, Jr, Crumpacker CS, et al. Expression of the CD8alpha beta-heterodimer on CD8(+) T lymphocytes in peripheral blood lymphocytes of human immunodeficiency virus- and human immunodeficiency virus+ individuals. Blood. 1998 Jul 1;92(1):198–206. [PubMed] [Google Scholar]
- 41.Park JH, Adoro S, Lucas PJ, Sarafova SD, Alag AS, Doan LL, et al. ‘Coreceptor tuning’: cytokine signals transcriptionally tailor CD8 coreceptor expression to the self-specificity of the TCR. Nat Immunol. 2007 Oct;8(10):1049–59. doi: 10.1038/ni1512. [DOI] [PubMed] [Google Scholar]
- 42.Kapp M, Stevanovic S, Fick K, Tan SM, Loeffler J, Opitz A, et al. CD8+ T-cell responses to tumor-associated antigens correlate with superior relapse-free survival after allo-SCT. Bone Marrow Transplant. 2009 Mar;43(5):399–410. doi: 10.1038/bmt.2008.426. [DOI] [PubMed] [Google Scholar]
- 43.Warren EH, Fujii N, Akatsuka Y, Chaney CN, Mito JK, Loeb KR, et al. Therapy of relapsed leukemia after allogeneic hematopoietic cell transplantation with T cells specific for minor histocompatibility antigens. Blood. 2010 May 13;115(19):3869–78. doi: 10.1182/blood-2009-10-248997. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.