Abstract
The function of regulatory T cells (Treg cells) has been attributed to a growing number of diverse pathways, molecules and processes. Seemingly contradictory conclusions regarding the mechanisms underlying Treg cell suppressive activity have revitalized skeptics in the field who challenge the core validity of the idea of Treg cells as central immune regulators. However, we note that a consensus may be emerging from the data: that multiple Treg cell functions act either directly or indirectly at the site of antigen presentation to create a regulatory milieu that promotes bystander suppression and infectious tolerance. Thus, the versatility and adaptability of the Foxp3+ Treg cells may in fact be the best argument that these cells are ‘multitalented masters of immune regulation’.
Armed with the potential to destroy invading microorganisms and stop aberrant outgrowth of tumor cells, the immune system has extensive built-in mechanisms for preventing attack of healthy self tissues. The first line of such ‘self-tolerance’ is the elimination of self-reactive T lymphocytes and B lymphocytes during negative selection in the thymus and bone marrow, respectively. However, there has long been a belief that the immune system must have peripheral mechanisms in place to deal with immune cells that ‘escape’ central tolerance. For almost 40 years, immunologists have postulated the existence of suppressor T cells that police the immune system to avert unwanted immune responses1-3. However, that phenomenology was cast into doubt as various labs presented unique, hard-to-reproduce systems, each with complexities and idiosyncrasies that raised credibility issues. This situation was not unlike the early years of cytokine biology, during which dozens of activities were found in sera and cell supernatants without consistent molecular or biochemical ‘signatures’. Fortunately, as biochemistry and the molecular biology revolution rescued the field of cytokine biology by identifying genes and biochemical structures tied to the varied biologic activities, the identification of a constellation of cell surface, transcriptional and biochemical markers that uniquely mark ‘regulatory’ T cells (Treg cells) has made possible a rebirth of the suppressor T cell field over the past decade.
The realization that Treg cells have a unique surface expression profile incorporating CD25, CD62L and specific CD45 isoforms4-6, together with the identification of the Treg cell–specific transcription factor Foxp3, catapulted Treg cells from a rare CD4+ T cell subset to what many regard as ‘master regulators’ of immune homeostasis7-9. However, the waters are becoming murky once again. First, there are myriad subpopulations of Treg cells, including the CD4+CD25+ Foxp3+ cells, interleukin 10 (IL-10)–producing ‘Tr1’ cells10, transforming growth factor-β (TGF-β)–producing T helper type 3 cells11, CD8+ T suppressor cells12, natural killer T cells13, CD4−CD8−T cells14 and γθ T cells15. Some of these Treg cells, such as the CD4+Foxp3+ cells, originate in the thymus during ontogeny and are referred to as ‘natural’ Treg cells. Treg cells can also be induced from naive T cells in the periphery16. Some but not all of these peripherally induced ‘adaptive’ Treg cells also express Foxp3. Now it is rare to find a high-profile journal that does not include a new functional activity ascribed to Treg cells. This explosion of molecules, processes, mechanisms of action and phenomenology has led to an undercurrent of cynicism in the field. In this review, we will attempt to make some sense of many (although not all) of the reported functions of Treg cells. We will focus our discussion on Foxp3+ CD4+ natural Treg cells (which develop in the thymus) because these cells are central for immune homeostasis, as illustrated by the fatal consequence of their absence from mice deficient in IL-2, CD25 or Foxp3 or of their depletion from normal adult mice7-9,17,18. Notably, like mice, humans with mutations in FOXP3 develop multiorgan autoimmune diseases with fatal consequences19.
Many investigations have firmly established the involvement of natural Treg cells in controlling autoimmunity, inflammatory disorders such as asthma and colitis, and immune responses to tissue transplants, tumors and various infectious agents20. Treg cells are purported to use many cellular processes to control immune responses. However, to fully consider the varied mechanisms of action of Treg cells, it is critical to consider the two core Treg cell–mediated phenomena: bystander suppression and infectious tolerance.
Although the suppressive activity of Treg cells requires their prior activation through their T cell receptor, once activated, Treg cells suppress in an antigen-nonspecific way called ‘bystander suppression’. Thus, Treg cells with one antigen specificity can suppress effector T cells (Teff cells) with many other distinct antigen specificities.
The phenomenon of infectious tolerance is proposed on the basis of in vivo transfer studies in which one population of suppressor T cells creates a regulatory milieu that promotes the outgrowth of a new population of Treg cells with antigen specificities distinct from those of the original Treg population. For example, in the setting of transplantation and type 1 diabetes, the tolerant state induced by Treg cells is maintained even after loss or removal of the original Treg cell population21,22. Furthermore, new antigen specificities can be acquired by adaptive Treg cell populations as long as the new antigen is present on the same tissue that the antigen recognized by the original Treg cells was21. Thus, through the processes of bystander suppression and infectious tolerance, Treg cells effectively establish a state of dominant and stable tolerance.
Investigations of the mechanisms of Treg cell function have identified not one or two but an ever-growing list of molecules and processes that contribute to Treg cell–suppressive activities (Table 1). Most of these studies have relied on the widely used in vitro suppression assay and a limited number of in vivo disease models. Three complementary approaches have been used to define Treg cell functional biology by examining the influence of Treg cells on other T cells or on antigen-presenting cells (APCs) or by molecular characterization of Treg cell–derived suppressive molecules.
Table 1.
Molecular mechanisms underlying Treg cell–mediated suppression
| Mechanism | In vitro |
In vivo |
||||||
|---|---|---|---|---|---|---|---|---|
| IBD | Type 1 diabetes | Autoimmune gastritis |
Asthma | Infection | Tumor | Transplantation | ||
| IL-10 | No (refs. 41,48) | Yes (ref. 64) | No (ref. 71) | No (ref. 72) | No (refs. 37,54) | Yes (ref. 64) | Yes (ref. 65)a,b | Yes (ref. 73)c |
| TGF-β | No (refs. 24,41,48) | Yes (ref. 53); no (ref. 55) |
Yes (refs. 74,75)d | No (ref. 76) | Yes (ref. 65)a,b | No | ||
| CTLA-4 | Yes (refs. 77); no (refs. 41,42) |
Yes (ref. 43) | No (ref. 78) | Yes (ref. 77)c | Yes (ref. 79)c | |||
| Granzyme B | Yes (refs. 27-29) | Yes (ref. 80) | ||||||
| Perforin | No (ref. 27); yes (refs. 26,29)b |
Yes (ref. 80) | ||||||
| IFN-γ | No (ref. 41) | Yes (ref. 81)d | ||||||
| IL-9 | Yes (ref. 82) | |||||||
| HO-1 | Yes (refs. 56,57)c; no (ref. 83) |
Yes (ref. 57)c | Yes (ref. 84) | |||||
| cAMP | Yes (ref. 25) | |||||||
| Galectins | Yes (refs. 59,60) | |||||||
| CD39-CD73; adenosine |
Yes (ref. 58) | Yes (ref. 58) | ||||||
| ‘IL-2 sink’ | Yes (ref. 32) | Yes (ref. 32) | ||||||
| IL-35 | Yes (ref. 62) | Yes (ref. 62) | ||||||
Dependence on IL-10 and TGF-β determined in vitro.
Data derived from human studies.
Source of suppressive molecule may or may not be natural Treg cells.
Treg cells in these settings may be natural or adaptive Treg cells.
Treg cells influence other T cells
Both in vitro and in vivo analyses suggest that Treg cells can suppress the proliferation and/or cytokine production of Teff cells. Additionally, Treg cells prevent CD8+ cells from differentiating into cytolytic effector cells in vivo without affecting their proliferation or interferon-γ (IFN-γ) production23. Because the suppression of Teff cell proliferation by Treg cells was observed in vitro in an APC-free system, it was suggested that Treg cells suppress through direct contact with Teff cells24. A mechanism to explain the contact dependence of in vitro suppression has been proposed in a publication showing that Treg cells have large amounts of cytoplamic cAMP and could deliver this potent immunosuppressive compound to Teff cells by contact through gap junctions25. Other studies have shown that Treg cells can kill Teff cells directly in culture through the release of granzyme B and perforin26-29. However, imaging studies have shown that Teff cells and Treg cells do not interact stably during suppression in vivo and in vitro30,31.
Because natural Treg cells constitutively express CD25, the high-affinity receptor for IL-2, it has long been suspected that Treg cells suppress by ‘sopping up’ IL-2 produced by Teff cells thereby preventing their proliferation and differentiation. This hypothesis has been revisited in studies examining the physiological changes in Teff cells after their encounter with Treg cells32. Teff cells undergo apoptosis after being exposed to Treg cells. This form of Teff cell death is distinct from those mediated by granzyme B or perforin mentioned earlier and is dependent on the proapoptotic factor Bim, expressed by Teff cells. Preventing Bim-mediated cell death by either Bim deficiency or forced expression of Bcl-2, a protein shown to counteract the proapoptotic activity of Bim, renders the Teff cells resistant to Treg cell–mediated suppression in vitro and in vivo. In this context, it is notable that the galectins also mediate cell death by a perforin- and granzyme-independent mechanism, which suggests a potential link between these two pathways33,34. Unfortunately, it is difficult to explain bystander suppression or infectious tolerance in vivo on the basis of these Teff cell–killing activities.
In contrast, some studies suggest that Treg cells can alter the differentiation of other T cells. For example, CD4+ Teff cells differentiate into IL-10- or TGF-β-producing adaptive Treg cells in the presence of Treg cells in vitro and in vivo, an observation more consistent with the idea of bystander suppression and infectious tolerance35-37.
Treg cells alter APCs
A second approach to defining Treg cell function has been to evaluate their effect on APCs. It has been shown that Treg cells directly interact with antigen-presenting dendritic cells (DCs) in vivo within hours of transfer. The interaction of Treg cells with DCs profoundly affects the ability of Teff cells to subsequently engage and become activated by the same DCs. Treg cells either abrogate the antigen-presenting activity of the DC or promote the secretion of suppressive factors by the target DC population. It has been shown that Treg cells can stimulate APCs to upregulate the activity of indoleamine 2,3-dioxygenase (IDO), a potent immunosuppressive enzyme associated with pregnancy and tumor immune evasion38,39. In those studies, IDO induction was found to depend on high expression of the inhibitory receptor CTLA-4 on Treg cells40. However, there is no clear evidence of the involvement of IDO in Treg cell function in vivo or in vitro. The effect of CTLA-4 on Treg cell function is also complex. Blocking CTLA-4 has no effect on in vitro suppression by Treg cells, and in most circumstances, Treg cells from CTLA-4-deficient mice show suppressive activity similar to that of cells from their wild-type littermates41,42. However, in vivo CTLA-4 blockade abrogates Treg cell–mediated suppression in mouse models of inflammatory bowl disease (IBD), autoimmune gastritis and transplantation (Table 1). In the setting of IBD, CTLA-4-deficient Treg cells are able to prevent disease. However, unlike the protection afforded by wild-type Treg cells, disease protection mediated by CTLA-4-deficient Treg cells is dependent on IL-10 (ref. 43). That in vivo finding is consistent with in vitro analyses showing that CTLA-4-deficient Treg cells are distinct from wild-type Treg cells in the dependency of their suppressive function on TGF-β42. Finally, mice deficient in the recombination-activating gene product, when reconstituted with a mixture of CTLA-4-deficient and Foxp3-deficient bone marrow cells, live longer than mice reconstituted with either CTLA-4-deficient or Foxp3-deficient bone marrow cells alone, which shows that CTLA-4+ and Foxp3+ cells complement each other's function44. These findings suggest that Treg cells rely not on one mechanism for their function but instead use many alternative mechanisms to control unwanted autoimmune and uncontrolled adaptive immune responses.
Another relevant observation is the potential of Treg cells to activate TGF-β on the surfaces of DCs. Latent TGF-β binds to DCs through interaction with αVβ8 integrins on the basis of an Arg-Gly-Asp motif. This binding is essential for the provision of a substrate for TGF-β activation and prevention of colitis45. Evidence has been presented that furin, an enzyme purported to cleave latent TGF-β, is made by T cells after stimulation with IL-12 and thus might influence the creation of inflammatory and regulatory milieus46. Additionally, TGF-β, together with a subpopulation of DCs, promotes the differentiation of Teff cells into Foxp3+ Treg cells47. Thus, elaborate cross-talk between Treg cells and DCs lead to DC silencing, local TGF-β activation and expansion of the Treg cell repertoire, all of which help to actively establish and reinforce immune quiescence and self-tolerance.
Treg cell–derived suppressor molecules
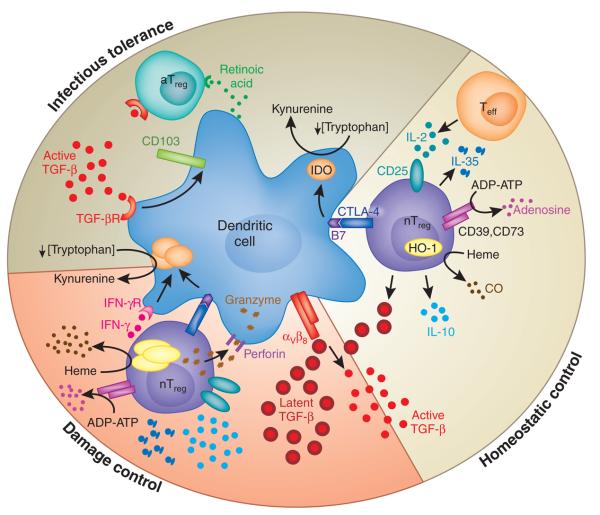
A third and perhaps the most straightforward approach to understanding Treg cell biology has been to identify Treg cell–specific molecules that are directly responsible for their potent tolerogenic effect. Like the other two approaches, this approach has led to complex and contradictory results. Many studies seem to definitively describe a mechanism of action that is essential for Treg cell function, whether IL-10, TGF-β, CTLA-4, granzyme B, perforin, IFN-γ, IL-9, heme oxygenase-1 (HO-1), cAMP, CD39, galectins or IL-35 (Table 1). Yet with the possible exception of CTLA-4 and TGF-β (the latter of which is also essential for Treg cell development), disruption of the genes encoding these molecules does not result in the catastrophic effects found in Treg cell–deficient mice. In an effort to categorize the various pathways and perhaps identify common characteristics, several are summarized below (Fig. 1).
Figure 1.
A three-tiered model of the functions of Treg cells in maintaining normal immune homeostasis. This model shows mechanisms potentially used by Treg cells for homeostatic control in the steady state, during ‘damage control’ at the site of inflammation and for infectious tolerance after the resolution of an immune response. aTreg, adaptive Treg cell; TGF- βR, TGF-β receptor; nTreg, natural Treg cell; CO, carbon monoxide; IFN-γR, IFN-γ receptor.
Many studies have shown that Treg cells can make suppressive cytokines such as IL-10 and TGF-β. Early in vitro studies demonstrated that neutralizing antibodies to IL-10 or TGF-β do not block Treg cell activity and that Treg cells from mice lacking IL-10 or TGF-β show similar suppressive activity24,41,48. It should be pointed out, however, that although often dismissed, there were some early suggestions that TGF-β is involved in Treg cell suppressive function in vitro49,50. In contrast to those in vitro studies, it has been found that neutralizing IL-10 or TGF-β abolishes Treg cell suppression in vivo in mouse models of IBD, type 1 diabetes, leishmania skin infection and transplantation (Table 1). In a few in vivo models, expression of IL-10 or TGF-β specifically in Treg cells is required for Treg cell function51-53. However, in some other situations, the source of these suppressive cytokines is not limited to Treg cells37,54,55.
HO-1, which catalyzes the formation of carbon monoxide through heme degradation, has been linked to Treg cell function56,57. Similarly, CD39 and CD73, which are ‘preferentially’ expressed on the surfaces of Treg cells, catalyze the generation of adenosine from the extracellular nucleotide ATP or ADP58. Adenosine can bind to its receptor A2A expressed on Teff cells to suppress their responses. Additionally, it has been suggested that several members of the galectin family of carbohydrate-binding proteins are involved in Treg cell function59-61. It has also been proposed that another cytokine, IL-35, is responsible for close-range suppression by Treg cells. IL-35, a heterodimeric protein composed of the IL-12 p35 chain and Ebi3, is regulated in Treg cells by Foxp3 (ref. 62). Treg cells lacking either p35 or Ebi3 are less suppressive in vitro and are unable to control IBD induced by Teff cells. Thus, it would seem that many molecules can mediate Treg cell functions both in vitro and in certain in vivo settings.
The ‘big picture’
Although the results presented above seem overwhelming and confusing at first glance, the following theme emerges from the vast array of data obtained in various disease settings: Treg cells probably use many mechanisms to control unwanted immune responses in vivo. Depending on the nature of the immune response, the eliciting agent, the immunological makeup of the host and the site of suppression, certain mechanism(s) may seem to dominate.
This phenomenon is most vividly exemplified by the IBD model63. Reliance on IL-10 for Treg cell–mediated control of IBD varies depending on how the disease is elicited and the timing of Treg cell treatment. Control of more aggressive forms of disease induced by memory T cells or pathogenic bacteria and reversion of established disease requires IL-10 in addition to CTLA-4 and TGF-β. In contrast, in the absence of one of the suppressive mechanisms (such as CTLA-4), Treg cells rely more on alternative suppressive functions such as IL-10 and TGF-β in vitro and in vivo42,43. Thus, it is possible that Treg cell–mediated control of IBD, a disease at the highly inflammation-prone mucosal surface, may require a wider array of suppressive mechanisms operating in synergy and thus that the presence of IL-10, TGF-β, CTLA-4, and IL-35 as well as IL-2 deprivations are necessary for complete protection. This scenario is mirrored in the transplant setting. Immune responses to organ transplants are one of the most vigorous forms of immune responses because of the exceptionally high frequency of alloreactive T cells. Thus, Treg cells may depend on many suppressive mechanisms working in concert to keep alloimmune responses under control. In contrast, one or two mechanisms may suffice to control slowly progressing organ-specific autoimmune diseases such as type 1 diabetes and autoimmune gastritis. In these disease settings, when one pathway is blocked, alternative mechanisms may fully compensate; therefore, Treg cell function might not be dependent on any one particular mechanism.
The site of Treg cell action in vivo is certainly not limited to lymphoid organs. Treg cells have been detected at sites of inflammation, and in many situations, their ability to migrate to and remain in inflamed tissues is important for their function in vivo50. Moreover, the molecular mechanisms of suppression used by Treg cells seem to differ depending on their localization in nonlymphoid versus lymphoid tissues. Treg cell–mediated prevention of IBD induced by the transfer of naive T cells is confined to lymphoid organs and is dependent on TGF-β and CTLA-4. In contrast, Treg cell–mediated control of colitogenic bacteria–induced IBD requires the presence of Treg cells in lymph nodes and in the inflamed colon, as well as IL-10 (ref. 64). In addition, control of an acute immune response to leishmania is located mainly in lymphoid organs and is independent of IL-10, whereas control of a chronic immune response in the infected skin requires local retention of Treg cells and their production of IL-10 (ref. 64). Finally, tumor-infiltrating Treg cells suppress tumor immunity by a mechanism dependent on TGF-β and IL-10, unlike Treg cells isolated from the peripheral blood of the same person65. These data from various disease models collectively demonstrate differential requirements for distinct suppressive functions of Treg cells at different tissue sites. In general, Treg cells rely on more suppressive mechanisms to control responses at sites of inflammation than in lymphoid organs.
On the basis of the experimental evidence collected so far, we propose a three-tiered ‘sequential’ model of Treg cell function in vivo (Fig. 1). In the steady state, Treg cells exert ‘homeostatic control’ over the immune system in lymphoid organs to prevent the potential outgrowth of auto-reactive T cells. By virtue of their constitutive expression of CD25, Treg cells effectively ‘sop up’ IL-2 produced by Teff cells, thereby terminating local incidental Teff cell activation while boosting Treg cell fitness and function. This phenomenon helps to explain some of the antigen-non-specific functions of Treg cells noted in several in vivo systems. Treg cells also function in an antigen-specific way. The Treg cell antigen receptor repertoire is skewed toward recognition of self antigens66. In addition, Treg cells show greater sensitivity to antigens than do their Teff cell counterparts47,67. These properties allow Treg cells to effectively patrol the lymphoid organs to prevent potential priming of autoreactive T cells. Most likely the type of Treg cell target in this setting is self antigen–presenting DCs. This contention is supported by the observation that Treg cells are stably conjugated with tissue antigen–bearing DCs during in vivo suppression in lymph nodes. In addition, complete Treg cell ablation in adult mice leads to the expansion of populations of activated DCs and to subsequent fatal multiorgan autoimmune disease18. The molecular mechanisms used by Treg cells during this stage probably involve TGF-β and CTLA-4, as suggested by the IBD studies mentioned above63. In addition, the massive lymphoproliferation noted in CTLA-4-deficient and TGF-β-deficient mice also supports the idea of an essential function for Treg cells in normal immune homeostasis68,69. Other mechanisms involving IL-10, IDO, HO-1 and CD39-CD73 may also contribute to Treg cell function at this stage, but they are probably not essential, as indicated by the lack of overt changes in immune homeostasis in their absence. It is notable that one attribute of the galectins is their ability to bind to multiple ligands and soluble molecules because of the ‘sticky’ nature of the lectin moieties. Thus, it is possible that the galectins, by forming functional scaffolds that bind several of the small molecules purported to be mediators of Treg cell suppression, help to establish a local immunosuppressive milieu that influences many cell types, such as Teff cells, DCs, macrophages and B cells, to actively maintain immunological quiescence.
When steady-state self-tolerance is breached, as after Toll-like receptor engagement during infection70 and in the presence of heightened frequencies of self-reactive Teff cells in autoimmune and transplantation settings, Treg cells become further activated to engage the second tier of ‘damage control’. After being activated, Treg cells shed CD62L and upregulate the chemokine receptors and adhesion molecules needed to leave lymphoid organs and gain entry into inflamed tissues. Once in inflamed tissue, Treg cells reactivated by tissue DCs upregulate CD25 and therefore become more effective at competing with Teff cells for IL-2. Activated Treg cells express more IL-10, CTLA-4, IL-35, TGF-β, HO-1 and CD39-CD73 and so are more potent suppressors. In addition, chronically activated Treg cells have high expression of cytotoxic granules such as granzyme B, which enable Treg cells to actively kill APCs and Teff cells in extreme conditions that lead to resolution of the immune response.
Infectious tolerance is established during the final stage to stabilize the tolerant state. Tissue destruction associated with the immune response leads to the presentation of newly exposed tissue antigens on the Treg cell–silenced DCs. Together with TGF-β, also a byproduct of immune activation, these tolerogenic DCs induce the differentiation of Teff cells into adaptive Treg cells, thus expanding the Treg cell antigen receptor repertoire. In the intestine, it has been shown that CD103+ DCs have the unique ability to induce adaptive Treg cells because of their ability to produce retinoic acid, which is needed to induce naive T cells to differentiate into Foxp3-expressing Treg cells47. TGF-β induces CD103 expression45; thus, TGF-β may contribute to the induction and/or maintenance of these specialized DCs. This model is consistent with the ‘hygiene hypothesis’, proposed to explain the reciprocal rates of infection and atopic and autoimmune disease. Improved hygienic standards and a decrease in both infections and the resulting active immune responses may also deprive people of the opportunity to expand Treg cell antigen receptor repertoires through infectious tolerance and thereby lead to higher rates of allergy and autoimmune disease.
Overall, Treg cells probably use many mechanisms in vivo to prevent and terminate immune responses and to establish a regulatory milieu that will promote a long-lasting, durable state of tolerance. Short-range mechanisms such as cytokine deprivation and the secretion of molecules such as IL-10, adenosine, IL-35, galectins and carbon monoxide ‘shut down’ local Teff cells during adaptive immune responses. These pathways are designed to act quickly and locally and in many cases are sufficient to dampen immunity. However, in the long term, Treg cells create a regulatory environment by producing TGF-β and/or by substantially changing DC functions to promote new Treg cell production. These processes promote bystander suppression and are critical to the creation of infectious tolerance that can spread beyond the local tissue and exert long-lasting influence over the immune system. It is notable that the some of the mechanisms that Treg cells use to suppress immunity also enhance Treg cell function. For example, by ‘sopping up’ IL-2, Treg cells simultaneously starve Teff cells and promote their own population expansion and survival. Similarly, the direct or indirect production of TGF-β by Treg cells ‘feeds back’ to stabilize and enhance Foxp3 expression in Treg cells, thereby boosting their suppressive function.
Concluding remarks
In conclusion, in vitro and in vivo Treg cell function cannot be attributed to a single dominant pathway or molecule. Instead, Treg cells use many suppressive mechanisms that can target various cell types depending on the circumstances and their surroundings. The versatility and adaptability of Treg cells makes them true ‘masters of immune regulation’. The chief function of Treg cells in a normal person is to maintain immune homeostasis in the lymphoid organs. After the onset of an immune response, Treg cells use additional suppressive strategies to resolve inflammation and limit tissue damage. The dynamic interaction between Treg cells and DCs is vital for Treg cell function. This model provides a conceptual framework for the ongoing quest to discover more suppressive mechanisms used by Treg cells, which are intricately involved in the pathogenesis of autoimmune disease, infectious disease, cancer and the rejection of transplanted organs. Better understanding of their biology will create opportunities for the development of new therapeutic intervention in these disease settings.
Footnotes
Reprints and permissions information is available online at http://npg.nature.com/reprintsandpermissions
References
- 1.Gershon RK, Kondo K. Cell interactions in the induction of tolerance: the role of thymic lymphocytes. Immunology. 1970;18:723–737. [PMC free article] [PubMed] [Google Scholar]
- 2.Benacerraf B, Kapp JA, Debre P, Pierce CW, de la Croix F. The stimulation of specific suppressor T cells in genetic non-responder mice by linear random copolymers of l-amino acids. Transplant. Rev. 1975;26:21–38. doi: 10.1111/j.1600-065x.1975.tb00173.x. [DOI] [PubMed] [Google Scholar]
- 3.Bach JF, Boitard C, Yasunami R, Dardenne M. Control of diabetes in NOD mice by suppressor cells. J. Autoimmun. 1990;3(Suppl 1):97–100. doi: 10.1016/s0896-8411(09)90017-8. [DOI] [PubMed] [Google Scholar]
- 4.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor α-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 5.Herbelin A, Gombert JM, Lepault F, Bach JF, Chatenoud L. Mature mainstream TCR αβ+CD4+ thymocytes expressing L-selectin mediate “active tolerance” in the nonobese diabetic mouse. J. Immunol. 1998;161:2620–2628. [PubMed] [Google Scholar]
- 6.Hall BM, Pearce NW, Gurley KE, Dorsch SE. Specific unresponsiveness in rats with prolonged cardiac allograft survival after treatment with cyclosporine. III. Further characterization of the CD4+ suppressor cell and its mechanisms of action. J. Exp. Med. 1990;171:141–157. doi: 10.1084/jem.171.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 8.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 9.Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat. Immunol. 2003;4:337–342. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 10.Roncarolo MG, et al. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunol. Rev. 2006;212:28–50. doi: 10.1111/j.0105-2896.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- 11.Faria AM, Weiner HL. Oral tolerance. Immunol. Rev. 2005;206:232–259. doi: 10.1111/j.0105-2896.2005.00280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang CC, et al. Tolerization of dendritic cells by TS cells: the crucial role of inhibitory receptors ILT3 and ILT4. Nat. Immunol. 2002;3:237–243. doi: 10.1038/ni760. [DOI] [PubMed] [Google Scholar]
- 13.Kronenberg M. Toward an understanding of NKT cell biology: progress and paradoxes. Annu. Rev. Immunol. 2005;23:877–900. doi: 10.1146/annurev.immunol.23.021704.115742. [DOI] [PubMed] [Google Scholar]
- 14.Zhang ZX, Yang L, Young KJ, DuTemple B, Zhang L. Identification of a previously unknown antigen-specific regulatory T cell and its mechanism of suppression. Nat. Med. 2000;6:782–789. doi: 10.1038/77513. [DOI] [PubMed] [Google Scholar]
- 15.Hayday A, Tigelaar R. Immunoregulation in the tissues by γθ T cells. Nat. Rev. Immunol. 2003;3:233–242. doi: 10.1038/nri1030. [DOI] [PubMed] [Google Scholar]
- 16.Bluestone JA, Abbas AK. Natural versus adaptive regulatory T cells. Nat. Rev. Immunol. 2003;3:253–257. doi: 10.1038/nri1032. [DOI] [PubMed] [Google Scholar]
- 17.Malek TR, Yu A, Vincek V, Scibelli P, Kong L. CD4 regulatory T cells prevent lethal autoimmunity in IL-2Rβ-deficient mice. Implications for the nonredundant function of IL-2. Immunity. 2002;17:167–178. doi: 10.1016/s1074-7613(02)00367-9. [DOI] [PubMed] [Google Scholar]
- 18.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat. Immunol. 2007;8:191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 19.Gambineri E, Torgerson TR, Ochs HD. Immune dysregulation, polyendocrinopathy, enteropathy, and X-linked inheritance (IPEX), a syndrome of systemic autoimmunity caused by mutations of FOXP3, a critical regulator of T-cell homeostasis. Curr. Opin. Rheumatol. 2003;15:430–435. doi: 10.1097/00002281-200307000-00010. [DOI] [PubMed] [Google Scholar]
- 20.Sakaguchi S. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu. Rev. Immunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 21.Qin S, et al. “Infectious” transplantation tolerance. Science. 1993;259:974–977. doi: 10.1126/science.8094901. [DOI] [PubMed] [Google Scholar]
- 22.Tarbell KV, et al. Dendritic cell-expanded, islet-specific CD4+ CD25+ CD62L+ regulatory T cells restore normoglycemia in diabetic NOD mice. J. Exp. Med. 2007;204:191–201. doi: 10.1084/jem.20061631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mempel TR, et al. Regulatory T cells reversibly suppress cytotoxic T cell function independent of effector differentiation. Immunity. 2006;25:129–141. doi: 10.1016/j.immuni.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 24.Piccirillo CA, Shevach EM. Cutting edge: control of CD8+ T cell activation by CD4+CD25+ immunoregulatory cells. J. Immunol. 2001;167:1137–1140. doi: 10.4049/jimmunol.167.3.1137. [DOI] [PubMed] [Google Scholar]
- 25.Bopp T, et al. Cyclic adenosine monophosphate is a key component of regulatory T cell-mediated suppression. J. Exp. Med. 2007;204:1303–1310. doi: 10.1084/jem.20062129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grossman WJ, et al. Human T regulatory cells can use the perforin pathway to cause autologous target cell death. Immunity. 2004;21:589–601. doi: 10.1016/j.immuni.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 27.Gondek DC, Lu LF, Quezada SA, Sakaguchi S, Noelle RJ. Cutting edge: contact-mediated suppression by CD4+CD25+ regulatory cells involves a granzyme B-dependent, perforin-independent mechanism. J. Immunol. 2005;174:1783–1786. doi: 10.4049/jimmunol.174.4.1783. [DOI] [PubMed] [Google Scholar]
- 28.Zhao DM, Thornton AM, Dipaolo RJ, Shevach EM. Activated CD4+CD25+ T cells selectively kill B lymphocytes. Blood. 2006;107:3925–3932. doi: 10.1182/blood-2005-11-4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qin HY, et al. A novel mechanism of regulatory T cell-mediated down-regulation of autoimmunity. Int. Immunol. 2006;18:1001–1015. doi: 10.1093/intimm/dxl035. [DOI] [PubMed] [Google Scholar]
- 30.Tang Q, et al. Visualizing regulatory T cell control of autoimmune responses in non-obese diabetic mice. Nat. Immunol. 2006;7:83–92. doi: 10.1038/ni1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tang Q, Krummel MF. Imaging the function of regulatory T cells in vivo. Curr. Opin. Immunol. 2006;18:496–502. doi: 10.1016/j.coi.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 32.Pandiyan P, Zheng L, Ishihara S, Reed J, Lenardo MJ. CD4+CD25+Foxp3+ regulatory T cells induce cytokine deprivation-mediated apoptosis of effector CD4+ T cells. Nat. Immunol. 2007;8:1353–1362. doi: 10.1038/ni1536. [DOI] [PubMed] [Google Scholar]
- 33.Valenzuela HF, et al. O-glycosylation regulates LNCaP prostate cancer cell susceptibility to apoptosis induced by galectin-1. Cancer Res. 2007;67:6155–6162. doi: 10.1158/0008-5472.CAN-05-4431. [DOI] [PubMed] [Google Scholar]
- 34.Toscano MA, et al. Differential glycosylation of TH1, TH2 and TH-17 effector cells selectively regulates susceptibility to cell death. Nat. Immunol. 2007;8:825–834. doi: 10.1038/ni1482. [DOI] [PubMed] [Google Scholar]
- 35.Jonuleit H, et al. Infectious tolerance: human CD25+ regulatory T cells convey suppressor activity to conventional CD4+ T helper cells. J. Exp. Med. 2002;196:255–260. doi: 10.1084/jem.20020394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dieckmann D, Bruett CH, Ploettner H, Lutz MB, Schuler G. Human CD4+CD25+ regulatory, contact-dependent T cells induce interleukin 10-producing, contact-independent type 1-like regulatory T cells. J. Exp. Med. 2002;196:247–253. doi: 10.1084/jem.20020642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kearley J, Barker JE, Robinson DS, Lloyd CM. Resolution of airway inflammation and hyperreactivity after in vivo transfer of CD4+CD25+ regulatory T cells is interleukin 10 dependent. J. Exp. Med. 2005;202:1539–1547. doi: 10.1084/jem.20051166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Munn DH, Mellor AL. IDO and tolerance to tumors. Trends Mol. Med. 2004;10:15–18. doi: 10.1016/j.molmed.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 39.Mellor AL, Munn D. Policing pregnancy: Tregs help keep the peace. Trends Immunol. 2004;25:563–565. doi: 10.1016/j.it.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 40.Puccetti P, Grohmann U. IDO and regulatory T cells: a role for reverse signalling and non-canonical NF-κB activation. Nat. Rev. Immunol. 2007;7:817–823. doi: 10.1038/nri2163. [DOI] [PubMed] [Google Scholar]
- 41.Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J. Exp. Med. 1998;188:287–296. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tang Q, et al. Distinct roles of CTLA-4 and TGF-β in CD4+CD25+ regulatory T cell function. Eur. J. Immunol. 2004;34:2996–3005. doi: 10.1002/eji.200425143. [DOI] [PubMed] [Google Scholar]
- 43.Read S, et al. Blockade of CTLA-4 on CD4+CD25+ regulatory T cells abrogates their function in vivo. J. Immunol. 2006;177:4376–4383. doi: 10.4049/jimmunol.177.7.4376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chikuma S, Bluestone JA. Expression of CTLA-4 and FOXP3 in cis protects from lethal lymphoproliferative disease. Eur. J. Immunol. 2007;37:1285–1289. doi: 10.1002/eji.200737159. [DOI] [PubMed] [Google Scholar]
- 45.Travis MA, et al. Loss of integrin αvβ8 on dendritic cells causes autoimmunity and colitis in mice. Nature. 2007;449:361–365. doi: 10.1038/nature06110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pesu M, Muul L, Kanno Y, O'Shea JJ. Proprotein convertase furin is preferentially expressed in T helper 1 cells and regulates interferon γ. Blood. 2006;108:983–985. doi: 10.1182/blood-2005-09-3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Coombes JL, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-β and retinoic acid-dependent mechanism. J. Exp. Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Takahashi T, et al. Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int. Immunol. 1998;10:1969–1980. doi: 10.1093/intimm/10.12.1969. [DOI] [PubMed] [Google Scholar]
- 49.Nakamura K, Kitani A, Strober W. Cell contact-dependent immunosuppression by CD4+CD25+ regulatory T cells is mediated by cell surface-bound transforming growth factor β. J. Exp. Med. 2001;194:629–644. doi: 10.1084/jem.194.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.You S, Thieblemont N, Alyanakian MA, Bach JF, Chatenoud L. Transforming growth factor-β and T-cell-mediated immunoregulation in the control of autoimmune diabetes. Immunol. Rev. 2006;212:185–202. doi: 10.1111/j.0105-2896.2006.00410.x. [DOI] [PubMed] [Google Scholar]
- 51.Belkaid Y, Piccirillo CA, Mendez S, Shevach EM, Sacks DL. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 2002;420:502–507. doi: 10.1038/nature01152. [DOI] [PubMed] [Google Scholar]
- 52.Maloy KJ, et al. CD4+CD25+ TR cells suppress innate immune pathology through cytokine-dependent mechanisms. J. Exp. Med. 2003;197:111–119. doi: 10.1084/jem.20021345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li MO, Wan YY, Flavell RA. T cell-produced transforming growth factor-β1 controls T cell tolerance and regulates Th1- and Th17-cell differentiation. Immunity. 2007;26:579–591. doi: 10.1016/j.immuni.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 54.Wilson MS, et al. Suppression of allergic airway inflammation by helminth-induced regulatory T cells. J. Exp. Med. 2005;202:1199–1212. doi: 10.1084/jem.20042572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kullberg MC, et al. TGF-β1 production by CD4+ CD25+ regulatory T cells is not essential for suppression of intestinal inflammation. Eur. J. Immunol. 2005;35:2886–2895. doi: 10.1002/eji.200526106. [DOI] [PubMed] [Google Scholar]
- 56.Choi BM, Pae HO, Jeong YR, Kim YM, Chung HT. Critical role of heme oxygenase-1 in Foxp3-mediated immune suppression. Biochem. Biophys. Res. Commun. 2005;327:1066–1071. doi: 10.1016/j.bbrc.2004.12.106. [DOI] [PubMed] [Google Scholar]
- 57.Xia ZW, et al. Heme oxygenase-1-mediated CD4+CD25high regulatory T cells suppress allergic airway inflammation. J. Immunol. 2006;177:5936–5945. doi: 10.4049/jimmunol.177.9.5936. [DOI] [PubMed] [Google Scholar]
- 58.Deaglio S, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J. Exp. Med. 2007;204:1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kubach J, et al. Human CD4+CD25+ regulatory T cells: proteome analysis identifies galectin-10 as a novel marker essential for their anergy and suppressive function. Blood. 2007;110:1550–1558. doi: 10.1182/blood-2007-01-069229. [DOI] [PubMed] [Google Scholar]
- 60.Garin MI, et al. Galectin-1: a key effector of regulation mediated by CD4+CD25+ T cells. Blood. 2007;109:2058–2065. doi: 10.1182/blood-2006-04-016451. [DOI] [PubMed] [Google Scholar]
- 61.Terness P, et al. Tolerance signaling molecules and pregnancy: IDO, galectins, and the renaissance of regulatory T cells. Am. J. Reprod. Immunol. 2007;58:238–254. doi: 10.1111/j.1600-0897.2007.00510.x. [DOI] [PubMed] [Google Scholar]
- 62.Collison LW, et al. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007;450:566–569. doi: 10.1038/nature06306. [DOI] [PubMed] [Google Scholar]
- 63.Izcue A, Coombes JL, Powrie F. Regulatory T cells suppress systemic and mucosal immune activation to control intestinal inflammation. Immunol. Rev. 2006;212:256–271. doi: 10.1111/j.0105-2896.2006.00423.x. [DOI] [PubMed] [Google Scholar]
- 64.Belkaid Y, Blank RB, Suffia I. Natural regulatory T cells and parasites: a common quest for host homeostasis. Immunol. Rev. 2006;212:287–300. doi: 10.1111/j.0105-2896.2006.00409.x. [DOI] [PubMed] [Google Scholar]
- 65.Strauss L, et al. A unique subset of CD4+CD25highFoxp3++ T cells secreting interleukin-10 and transforming growth factor-β1 mediates suppression in the tumor micro-environment. Clin. Cancer Res. 2007;13:4345–4354. doi: 10.1158/1078-0432.CCR-07-0472. [DOI] [PubMed] [Google Scholar]
- 66.Hsieh CS, et al. Recognition of the peripheral self by naturally arising CD25+ CD4+ T cell receptors. Immunity. 2004;21:267–277. doi: 10.1016/j.immuni.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 67.Jordan MS, et al. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat. Immunol. 2001;2:301–306. doi: 10.1038/86302. [DOI] [PubMed] [Google Scholar]
- 68.Waterhouse P, et al. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270:985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 69.Shull MM, et al. Targeted disruption of the mouse transforming growth factor-β1 gene results in multifocal inflammatory disease. Nature. 1992;359:693–699. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299:1033–1036. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- 71.Balasa B, Van Gunst K, Jung N, Katz JD, Sarvetnick N. IL-10 deficiency does not inhibit insulitis and accelerates cyclophosphamide-induced diabetes in the nonobese diabetic mouse. Cell. Immunol. 2000;202:97–102. doi: 10.1006/cimm.2000.1658. [DOI] [PubMed] [Google Scholar]
- 72.Suri-Payer E, Cantor H. Differential cytokine requirements for regulation of auto-immune gastritis and colitis by CD4+CD25+ T cells. J. Autoimmun. 2001;16:115–123. doi: 10.1006/jaut.2000.0473. [DOI] [PubMed] [Google Scholar]
- 73.Hara M, et al. IL-10 is required for regulatory T cells to mediate tolerance to alloantigens in vivo. J. Immunol. 2001;166:3789–3796. doi: 10.4049/jimmunol.166.6.3789. [DOI] [PubMed] [Google Scholar]
- 74.Green EA, Gorelik L, McGregor CM, Tran EH, Flavell RA. CD4+CD25+ T regulatory cells control anti-islet CD8+ T cells through TGF-β-TGF-β receptor interactions in type 1 diabetes. Proc. Natl. Acad. Sci. USA. 2003;100:10878–10883. doi: 10.1073/pnas.1834400100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Belghith M, et al. TGF-β-dependent mechanisms mediate restoration of self-tolerance induced by antibodies to CD3 in overt autoimmune diabetes. Nat. Med. 2003;9:1202–1208. doi: 10.1038/nm924. [DOI] [PubMed] [Google Scholar]
- 76.Piccirillo CA, et al. CD4+CD25+ regulatory T cells can mediate suppressor function in the absence of transforming growth factor β1 production and responsiveness. J. Exp. Med. 2002;196:237–246. doi: 10.1084/jem.20020590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Takahashi T, et al. Immunologic self-tolerance maintained by CD25+CD4+ regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J. Exp. Med. 2000;192:303–310. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Salomon B, Bluestone JA. Complexities of CD28/B7: CTLA-4 costimulatory pathways in autoimmunity and transplantation. Annu. Rev. Immunol. 2001;19:225–252. doi: 10.1146/annurev.immunol.19.1.225. [DOI] [PubMed] [Google Scholar]
- 79.Kingsley CI, Karim M, Bushell AR, Wood KJ. CD25+CD4+ regulatory T cells prevent graft rejection: CTLA-4- and IL-10-dependent immunoregulation of alloresponses. J. Immunol. 2002;168:1080–1086. doi: 10.4049/jimmunol.168.3.1080. [DOI] [PubMed] [Google Scholar]
- 80.Cao X, et al. Granzyme B and perforin are important for regulatory T cell-mediated suppression of tumor clearance. Immunity. 2007;27:635–646. doi: 10.1016/j.immuni.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 81.Sawitzki B, et al. IFN-γ production by alloantigen-reactive regulatory T cells is important for their regulatory function in vivo. J. Exp. Med. 2005;201:1925–1935. doi: 10.1084/jem.20050419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lu LF, et al. Mast cells are essential intermediaries in regulatory T-cell tolerance. Nature. 2006;442:997–1002. doi: 10.1038/nature05010. [DOI] [PubMed] [Google Scholar]
- 83.Zelenay S, Chora A, Soares MP, Demengeot J. Heme oxygenase-1 is not required for mouse regulatory T cell development and function. Int. Immunol. 2007;19:11–18. doi: 10.1093/intimm/dxl116. [DOI] [PubMed] [Google Scholar]
- 84.Sato K, et al. Carbon monoxide generated by heme oxygenase-1 suppresses the rejection of mouse-to-rat cardiac transplants. J. Immunol. 2001;166:4185–4194. doi: 10.4049/jimmunol.166.6.4185. [DOI] [PubMed] [Google Scholar]