Abstract
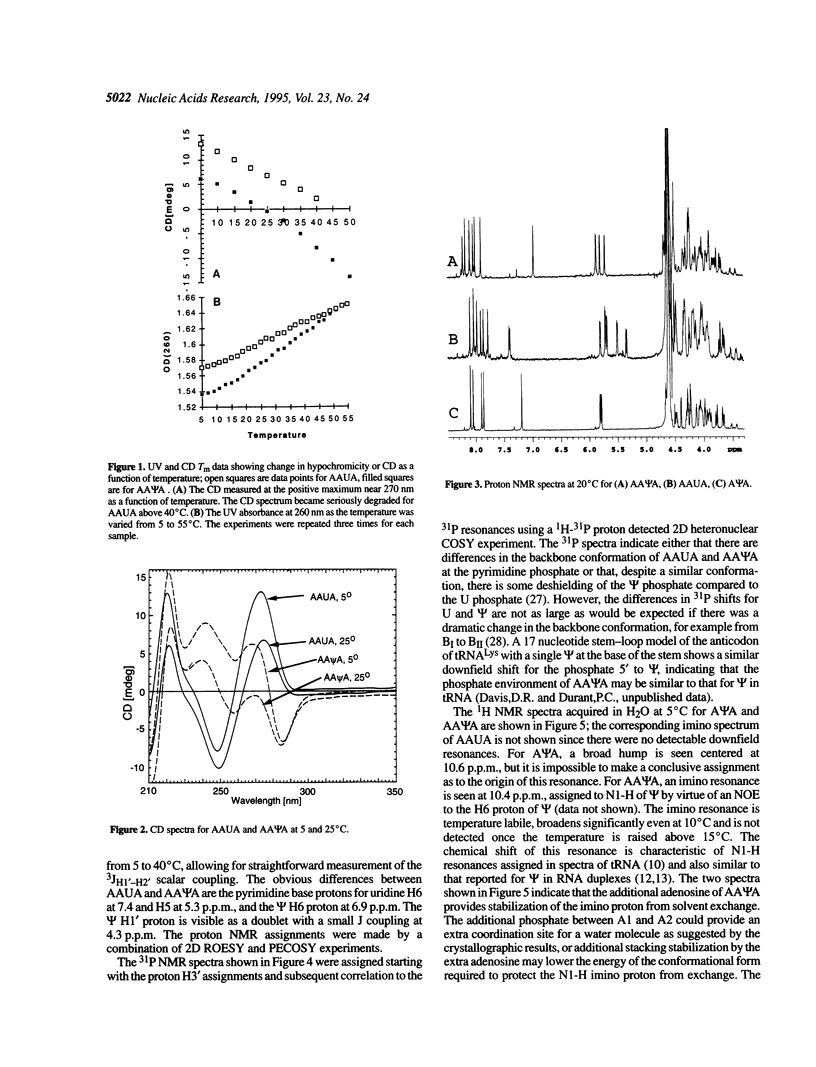
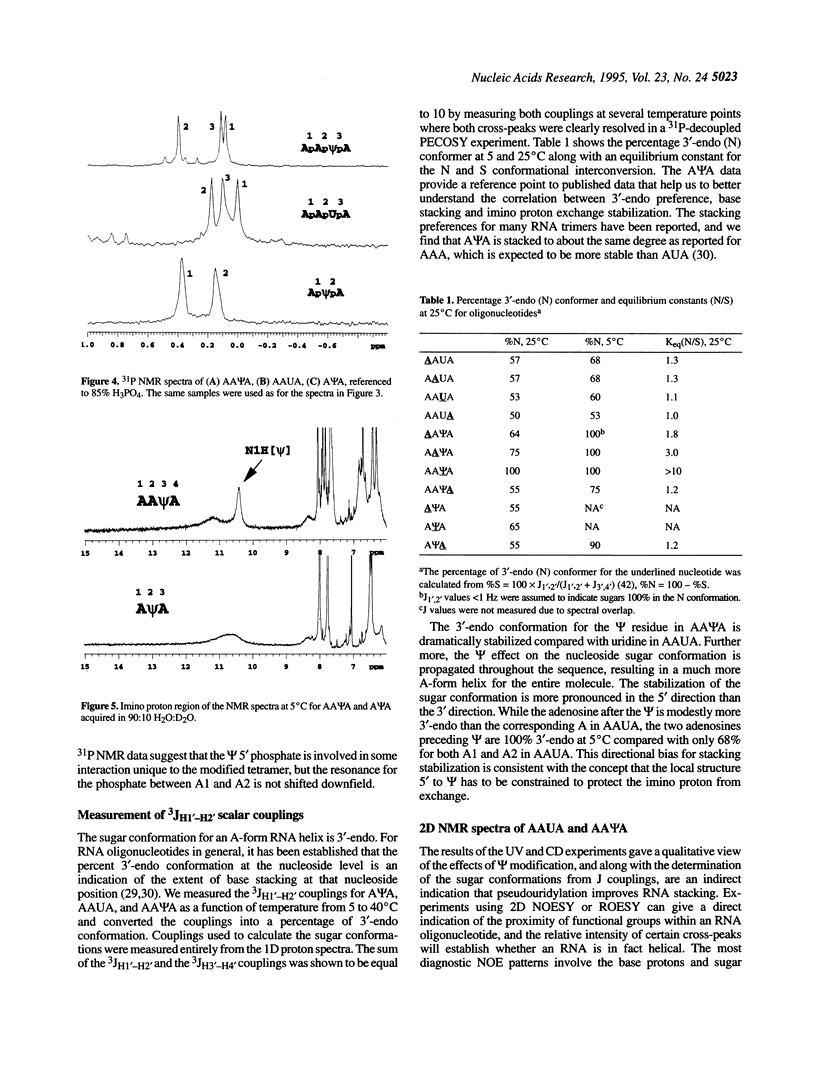
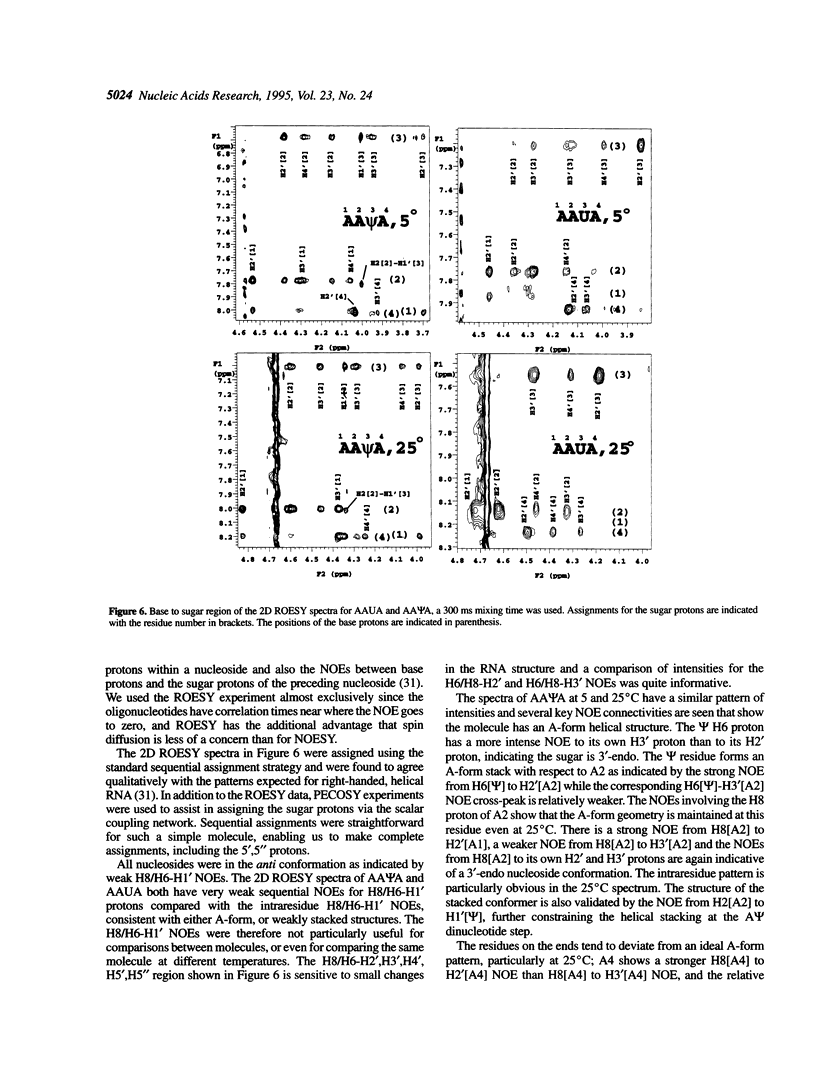
The effect of the modified nucleoside pseudouridine (psi) on RNA structure was compared with uridine. The extent of base stacking in model RNA oligonucleotides was measured by 1H NMR, UV, and CD spectroscopy. The UV and CD results indicate that the model single-stranded oligoribonucleotides AAUA and AA psi A form stacked structures in solution and the CD results for AA psi A are consistent with a general A-form helical conformation. The AA psi A oligomer exhibits a greater degree of UV hypochromicity over the temperature range 5-55 degrees C, consistent with a better stacked, more A-form structure compared with AAUA. The extent of stacking for each nucleotide residue was inferred from the percent 3'-endo sugar conformation as indicated by the H1'-H2' NMR scalar coupling. This indirect indication of stacking was confirmed by sequential NOE experiments. NMR measurements as a function of temperature indicate that pseudouridine forms a more stable base stacking arrangement than uridine, an effect that is propagated throughout the helix to stabilize stacking of neighboring purine nucleosides. The N1-H imino proton in AA psi A exchanges slowly with solvent, suggesting a role for the extra imino proton in stabilizing the conformation of pseudouridine. These results show that the conformational stabilization is an intrinsic property of pseudouridine occurring at the nucleotide level. The characteristics of pseudouridine in these models are consistent with earlier studies on intact rRNA, indicating that pseudouridine probably performs the same stabilizing function in most structural contexts.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altona C., Sundaralingam M. Conformational analysis of the sugar ring in nucleosides and nucleotides. Improved method for the interpretation of proton magnetic resonance coupling constants. J Am Chem Soc. 1973 Apr 4;95(7):2333–2344. doi: 10.1021/ja00788a038. [DOI] [PubMed] [Google Scholar]
- Arnez J. G., Steitz T. A. Crystal structure of unmodified tRNA(Gln) complexed with glutaminyl-tRNA synthetase and ATP suggests a possible role for pseudo-uridines in stabilization of RNA structure. Biochemistry. 1994 Jun 21;33(24):7560–7567. doi: 10.1021/bi00190a008. [DOI] [PubMed] [Google Scholar]
- Bakin A., Lane B. G., Ofengand J. Clustering of pseudouridine residues around the peptidyltransferase center of yeast cytoplasmic and mitochondrial ribosomes. Biochemistry. 1994 Nov 15;33(45):13475–13483. doi: 10.1021/bi00249a036. [DOI] [PubMed] [Google Scholar]
- Bakin A., Ofengand J. Four newly located pseudouridylate residues in Escherichia coli 23S ribosomal RNA are all at the peptidyltransferase center: analysis by the application of a new sequencing technique. Biochemistry. 1993 Sep 21;32(37):9754–9762. doi: 10.1021/bi00088a030. [DOI] [PubMed] [Google Scholar]
- Cantor C. R., Tinoco I., Jr Absorption and optical rotatory dispersion of seven trinucleoside diphosphates. J Mol Biol. 1965 Aug;13(1):65–77. doi: 10.1016/s0022-2836(65)80080-8. [DOI] [PubMed] [Google Scholar]
- Davis D. R., Poulter C. D. 1H-15N NMR studies of Escherichia coli tRNA(Phe) from hisT mutants: a structural role for pseudouridine. Biochemistry. 1991 Apr 30;30(17):4223–4231. doi: 10.1021/bi00231a017. [DOI] [PubMed] [Google Scholar]
- Deslauriers R., Smith I. C. A proton magnetic resonance study of the influence of base ionization on the conformation of pseudouridine. Can J Biochem. 1972 Jul;50(7):766–774. doi: 10.1139/o72-107. [DOI] [PubMed] [Google Scholar]
- Gasparutto D., Livache T., Bazin H., Duplaa A. M., Guy A., Khorlin A., Molko D., Roget A., Téoule R. Chemical synthesis of a biologically active natural tRNA with its minor bases. Nucleic Acids Res. 1992 Oct 11;20(19):5159–5166. doi: 10.1093/nar/20.19.5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffey R. H., Davis D., Yamaizumi Z., Nishimura S., Bax A., Hawkins B., Poulter C. D. 15N-labeled Escherichia coli tRNAfMet, tRNAGlu, tRNATyr, and tRNAPhe. Double resonance and two-dimensional NMR of N1-labeled pseudouridine. J Biol Chem. 1985 Aug 15;260(17):9734–9741. [PubMed] [Google Scholar]
- Hall K. B., McLaughlin L. W. Properties of a U1/mRNA 5' splice site duplex containing pseudouridine as measured by thermodynamic and NMR methods. Biochemistry. 1991 Feb 19;30(7):1795–1801. doi: 10.1021/bi00221a010. [DOI] [PubMed] [Google Scholar]
- Hall K. B., McLaughlin L. W. Properties of pseudouridine N1 imino protons located in the major groove of an A-form RNA duplex. Nucleic Acids Res. 1992 Apr 25;20(8):1883–1889. doi: 10.1093/nar/20.8.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue H., Hayase Y., Imura A., Iwai S., Miura K., Ohtsuka E. Synthesis and hybridization studies on two complementary nona(2'-O-methyl)ribonucleotides. Nucleic Acids Res. 1987 Aug 11;15(15):6131–6148. doi: 10.1093/nar/15.15.6131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov V. I., Minchenkova L. E., Minyat E. E., Frank-Kamenetskii M. D., Schyolkina A. K. The B to A transition of DNA in solution. J Mol Biol. 1974 Aug 25;87(4):817–833. doi: 10.1016/0022-2836(74)90086-2. [DOI] [PubMed] [Google Scholar]
- Kawai G., Yamamoto Y., Kamimura T., Masegi T., Sekine M., Hata T., Iimori T., Watanabe T., Miyazawa T., Yokoyama S. Conformational rigidity of specific pyrimidine residues in tRNA arises from posttranscriptional modifications that enhance steric interaction between the base and the 2'-hydroxyl group. Biochemistry. 1992 Feb 4;31(4):1040–1046. doi: 10.1021/bi00119a012. [DOI] [PubMed] [Google Scholar]
- Khare D., Orban J. Synthesis of backbone deuterium labelled [r(CGCGAAUUCGCG)]2 and HPLC purification of synthetic RNA. Nucleic Acids Res. 1992 Oct 11;20(19):5131–5136. doi: 10.1093/nar/20.19.5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalak J. A., Dalluge J. J., McCloskey J. A., Stetter K. O. The role of posttranscriptional modification in stabilization of transfer RNA from hyperthermophiles. Biochemistry. 1994 Jun 28;33(25):7869–7876. doi: 10.1021/bi00191a014. [DOI] [PubMed] [Google Scholar]
- Lane B. G., Ofengand J., Gray M. W. Pseudouridine in the large-subunit (23 S-like) ribosomal RNA. The site of peptidyl transfer in the ribosome? FEBS Lett. 1992 May 4;302(1):1–4. doi: 10.1016/0014-5793(92)80269-m. [DOI] [PubMed] [Google Scholar]
- Lee C. H., Tinoco I., Jr Studies of the conformation of modified dinucleoside phosphates containing 1,N6-ethenoadenosine and 2'-O-methylcytidine by 360-MHz 1H nuclear magnetic resonance spectroscopy. Investigation of the solution conformations of dinucleoside phosphates. Biochemistry. 1977 Dec 13;16(25):5403–5414. doi: 10.1021/bi00644a001. [DOI] [PubMed] [Google Scholar]
- Limbach P. A., Crain P. F., McCloskey J. A. Summary: the modified nucleosides of RNA. Nucleic Acids Res. 1994 Jun 25;22(12):2183–2196. doi: 10.1093/nar/22.12.2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maden B. E. The numerous modified nucleotides in eukaryotic ribosomal RNA. Prog Nucleic Acid Res Mol Biol. 1990;39:241–303. doi: 10.1016/s0079-6603(08)60629-7. [DOI] [PubMed] [Google Scholar]
- Roy S., Papastavros M. Z., Sanchez V., Redfield A. G. Nitrogen-15-labeled yeast tRNAPhe: double and two-dimensional heteronuclear NMR of guanosine and uracil ring NH groups. Biochemistry. 1984 Sep 11;23(19):4395–4400. doi: 10.1021/bi00314a024. [DOI] [PubMed] [Google Scholar]
- Samuelsson T., Olsson M. Transfer RNA pseudouridine synthases in Saccharomyces cerevisiae. J Biol Chem. 1990 May 25;265(15):8782–8787. [PubMed] [Google Scholar]
- Schweizer M. P., Thedford R., Slama J. Synthesis and conformational properties of diribonucleoside monophosphates containing modified nucleosides as found in transfer RNA. Biochim Biophys Acta. 1971 Mar 11;232(2):217–226. doi: 10.1016/0005-2787(71)90573-9. [DOI] [PubMed] [Google Scholar]
- Scott J. F., Zamecnik P. C. Some optical properties of diadenosine-5'-phosphates. Proc Natl Acad Sci U S A. 1969 Dec;64(4):1308–1314. doi: 10.1073/pnas.64.4.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg S., Misch A., Sprinzl M. Compilation of tRNA sequences and sequences of tRNA genes. Nucleic Acids Res. 1993 Jul 1;21(13):3011–3015. doi: 10.1093/nar/21.13.3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varani G., Tinoco I., Jr RNA structure and NMR spectroscopy. Q Rev Biophys. 1991 Nov;24(4):479–532. doi: 10.1017/s0033583500003875. [DOI] [PubMed] [Google Scholar]
- Warshaw M. M., Tinoco I., Jr Absorption and optical rotatory dispersion of six dinucleoside phosphates. J Mol Biol. 1965 Aug;13(1):54–64. doi: 10.1016/s0022-2836(65)80079-1. [DOI] [PubMed] [Google Scholar]
- Zerfass K., Beier H. Pseudouridine in the anticodon G psi A of plant cytoplasmic tRNA(Tyr) is required for UAG and UAA suppression in the TMV-specific context. Nucleic Acids Res. 1992 Nov 25;20(22):5911–5918. doi: 10.1093/nar/20.22.5911. [DOI] [PMC free article] [PubMed] [Google Scholar]


