Abstract
IKKα and IKKβ, catalytic subunits of IκB kinase (IKK) complex are involved in activation of NF-κB and in mediating a variety of other biological functions. Though these proteins have a high sequence homology, IKKα exhibits different functional characteristics as compared to IKKβ. Earlier, we have demonstrated that cyclin D1 is over-expressed and predominantly localized in the nucleus of IKKα−/− cells, indicating that IKKα regulates turnover and sub-cellular distribution of cyclin D1, which is mediated by IKKα-induced phosphorylation of cyclin D1. Since cyclin D nuclear localization is implicated in tumor development, we examined whether the absence of IKKα leads to tumor development as well. In the current study, we demonstrate that IKKα plays a critical role in tumorigenesis. Though IKKα−/− MEF cells demonstrate a slower anchorage-dependent growth, they are clonogenic in soft agar. These cells are tumorigenic in nude mice. Microarray analysis of IKKα−/− cells indicates a differential expression of genes involved in proliferation and apoptosis. Further, analysis of microarray data of human lung cancer cell lines revealed decreased IKKα RNA expression level as compared to cell lines derived from normal bronchial epithelium. These results suggest that IKKα may function as a tumor suppressor gene. Absence of IKKα may induce tumorigenicity by nuclear localization of cyclin D1 and modulating the expression of genes involved in neoplastic transformation.
INTRODUCTION
Several studies suggest the role of the NF-κB pathway in neoplasia and constitutive activation of the pathway has been demonstrated in a variety of tumor types (1,2). The IκB kinase (IKK) complex is critical for activation of the NF-κB pathway (3–8) and consists of catalytic subunits IKKα and IKKβ, and a regulatory subunit IKKγ/NEMO. Despite of their structural and biochemical similarities, IKKα and IKKβ appear to be functionally distinct (9,10). IKKβ is the major kinase for NF-κB activation by canonical pathway, while the role of IKKα appears redundant in phosphorylation of IκB. However, several recent observation suggest different biological functions of IKKα including phosphorylation of p100 (11), transcriptional regulation of gene expression (12) and a role in post-translational modification of cyclin D1 (13). IKKα−/− mice demonstrate a phenotype characterized by severe defects of skin and limb development, which is distinct from that of IKKβ or IKKγ knockout mice. The epidermal cells in IKKα knockout mice demonstrate increased proliferation with dysregulated epidermal differentiation, a role possibly independent NF-κB (14–18).
Several studies demonstrated that altered cyclin D1 expression or sub-cellular distribution is associated with a variety of neoplasms including breast, colon, esophagus, lung, and mantle cell lymphoma (19–21). Increased expression or nuclear localization of cyclin D1 likely plays a major role in development of these tumors (22–24). Earlier it was shown that GSK-3β phosphorylates cyclin D1 at T286, which is required for nuclear export during the S phase of the cell cycle and subsequent proteolysis (25,26). Recently, we demonstrated that IKKα−/− cells exhibit nuclear localization of cyclin D1 and IKKα is required for phosphorylation of cyclin D1 at T286 (13). Mutation of cyclin D1 at T286 results in constitutive nuclear distribution. Cyclin D1 T286A mutant in murine cells induces cellular transformation in vitro and leads to tumor development in nude mice. These findings suggest that constitutive nuclear localization of cyclin D1 is tumorigenic. Further studies with a splice variant of cyclin D1 (cyclin D1b), which lacks C-terminus (including T286) required for its nuclear export, remains distributed in the nucleus similar to cyclin D1 T286A mutant (27,28). Cells expressing cyclin D1b variant acquire a neoplastic phenotype and clinically this variant has been described in esophageal cancer (28).
Hyperplasia of epidermal cells in IKKα−/− mice also suggested a role of IKKα in cellular proliferation (14,15). While investigating the role of IKKα in cellular proliferation, we demonstrated that cyclin D1 is localized constitutively in the nucleus of IKKα−/− cells (13). As nuclear localization of cyclin D1 is associated with neoplasia, we investigated whether IKKα regulates cellular transformation and tumor development. In this study, we demonstrate that IKKα−/− cells exhibit anchorage-independent growth in soft agar and are tumorigenic in athymic nude mice. Further, experiments suggest that there is decreased expression of IKKα in a panel of lung cancer cell lines by microarray analysis. Comparison of microarray data on IKKα−/− with IKKα reconstituted cells suggest that IKKα regulates tumorigenicity by modulating expression of genes known to play a critical role in neoplastic transformation.
MATERIAL AND METHODS
Cell lines
Wild-type mouse embryonic fibroblast (MEF) were a kind gift from Xiaodong Wong. IKKα−/− and IKKβ−/− cells were kindly provided by Inder M. Verma (15). A Moloney-based retrovirus expressing Myc-tagged IKKα or β-galactosidase was utilized with hygromycin selection to generate stable cell lines from parental IKK knockout cells, expressing IKKα (IKKα+/+), β-galactosidase (IKKα−/−), or IKKγ−/−, and has been described previously (12). The IKKα−/− cells generated were similar to parental IKKα knockout cells and were used to perform different experiments described previously (13). HeLa and 293 cells were obtained from ATCC. All cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% (v/v) fetal bovine serum (FBS).
Western blot analysis
Immunoblotting was performed with IKKα (Oncogene, OP-133), IKKβ (Santa Cruz, sc-7607), cyclin D1 (Santa Cruz, sc-718, sc-72-13G), Actin (Santa Cruz, sc-1615) as described earlier (13,29).
Soft agar growth assay
Wild-type MEF, IKKα−/−, and IKKα+/+ cells were tested for their growth in soft agar plates. IKKβ−/−, IKKγ−/− cells were used as control. One ml of 0.6% Bacto-Agar (Becton Dickinson) in DMEM was poured into 6-well plate. After bottom layer was solidified, 1 ml of cell suspension in 0.4 % agar (2 ml of 3×104/ml cells mixed with 4 ml of 0.6% Bacto-Agar) was poured on the top of solidified bottom layer. Final concentration of FBS in the top layer was 10% in 1X DMEM. Colonies were counted and photographed after 10 to 14 days of incubation at 37 °C.
Tumorigenicity assays
Wild-type MEF, IKKα−/−, and IKKα+/+ cells were harvested and washed twice in 10% FBS-containing DMEM media, and the number of viable cells was counted by trypan blue exclusion. Six week-old immunocompromised athymic female nude mice (Swiss nu/nu nude) obtained from Charles River (Wilmington, MA) were injected subcutaneously with 5×106 cells in a volume of 0.5 ml PBS. The size of the tumor was measured at every two days. Mice were sacrificed when tumor size was more than 2 centimeters in largest dimension. Institutional guidelines and an Animal Research Committee-approved protocol were followed for mouse studies.
In vivo BrdU labeling
Athymic mice bearing tumor were injected with 350 μl of BrdU (10 mg/ml) or PBS as control twice, 24 and 4 hours prior to sacrificing. Frozen tumor sections were prepared, and BrdU staining was performed using BrdU In-Situ Detection Kit (BD Biosciences) following a method as suggested by the manufacturer. Diaminobenzidine (DAB) was used as chromogen.
Immunofluorescence microscopy
Paraffin embedded tumor sections were prepared from tumors induced by injection of IKKα−/− cells in athymic mice. These sections were deparaffinised and boiled with antigen retrieval solution for 1 hour. Antigen staining was performed with overnight incubation of these sections with antibody directed against either cyclin D1 (Santa Cruz, sc-72-13G) or IKKα (Oncogene, OP-133). Then secondary antibody conjugated with Texas Red was added to the section. After extensive washing, sections were subjected to immunofluorescence microscopy.
Microarray assays and analysis
Wild-type MEF, IKKα−/−, and IKKα reconstituted cells grown under normal condition were harvested and total RNA was extracted using Rneasy Mini Kit (Qiagen). Two independent experiments were performed. Sentrix® Beadchip (Illumina, Inc.) arrays for gene expression were used on each sample. For this, each RNA sample was amplified using the Ambion Illumina RNA amplification kit with biotin UTP (Enzo) labeling. The Ambion Illumina RNA amplification kit uses T7 oligo(dT) primer to generate single stranded cDNA followed by a second strand synthesis to generate double stranded cDNA. The cDNA was then column purified. In vitro transcription was done to synthesize biotin labeled cRNA using T7 RNA polymerase. The cRNA was then purified on the column and checked for the size and yield using the Bio-Rad Experion system. 1.5 μg of cRNA was hybridized for each array using standard Illumina protocols with streptavidin-Cy3 (Amersham) being used for detection. Slides were scanned on an Illumina Beadstation. The summarized mean bead intensities for each probe were generated by BeadStudio software. These intensity values represent expression level for each probe and were used for further normalization. Quantile normalization was done for within-array normalizations by using “affy” package in Bioconductor. Another package from Bioconductor, “limma”, was used for statistical analysis (30). Briefly, the normalized data for each replicates from Wild-type MEF, IKKα−/−, and IKKα +/+ cells were fitted into a linear model. An empirical Bayes method was applied to moderate standard error of estimated log fold changes. To assess differentially expressed genes, hypothesis test was performed and p-value was adjusted using Benjamini and Hochberg’s method. The cutoff for adjusted p-value is p <0.01.
To assess the functional importance of the genes differentially expressed between IKKα−/−, IKKα +/+, and MEF cells, we examined the expression data on all altered genes found in IKKα−/− cells in comparison to IKKα+/+ and MEF cells using Ingenuity Pathway Analysis software. Microarray assays of lung cancer cell lines and normal lung epithelial cells were performed using Affymetrix HG-U133A and HG-U133B GeneChips together, or HG-U133 plus 2 GeneChip according to the manufacturer’s instructions (Affymetrix, Santa Clara, California). The A and B arrays consist of 22,283 (HG-U133A) and 22,645 (HG-U133B) probe sets which together amount to 18,281 unique genes based on Unigene build 199. The Plus 2 array consists of 54,676 probe sets corresponding to 21,270 unique genes. Only the genes common to both sets were used. Analysis was performed using Affymetrix Microarray Suite 5.0 and in-house Visual Basic software MATRIX 1.26. Array data were median-normalized and sample classes were compared by calculating log ratios for each gene with a t-test providing univariate statistical significance.
Complete microarray data are available in Gene Expression Omnibus: http://www.ncbi.nlm.nih.gov/geo, accession GSE4824 (for the cell line microarray data) and in ArrayExpress: http://www.ebi.ac.uk/arrayexpress/, accession E-MEXP-231(for primary lung cancer microarray data).
Clustering Analysis
Differentially expressed genes between IKKα−/− and wild-type cells were combined with genes that were differentially expressed between IKKα−/− and IKKα+/+ cells. The resultant 3077 genes were used for clustering analysis. We calculated expression ratios by using normalized signals from IKKα−/− and IKKα+/+ cells divided by normalized signals of wild-type cells. We used Cluster 3.0 to perform the analysis, applied complete linkage, and used Java TreeView to visualize the results.
Reverse transcription-polymerase chain reaction (RT-PCR)
RT-PCR was performed to verify expression of genes identified by microarray analysis of wild-type MEF, IKKα−/−, and IKKα+/+ cells using RT-PCR kit (Invitrogen). Total RNA was extracted from these cells, and mRNA levels were determined in triplicate in a 50 μl volume with specific primer sets for 24, 30, and 35 cycles using GeneAmp® PCR System 9700 (Applied Biosystem). The oligonucleotide primers used to analyze transcripts were as the following: Kangai1 (13 to 254 nt: 5′-TGT GTC AAA GTC ACC AAG T-3′ and 5′-AGC AAG CAG CGG ACC TCA T-3′), Cxcl12 (1 to 223 nt: 5′-TCG TCG CCG TGC TGG CCC T-3′ and 5′-ACC GGG GTG TCC CCG AGA G-3′), TEAD2 (142 to 406 nt: 5′-GAC ATT GAG CAG AGT TTT C-3′ and 5′-ACA TGG TGG CCA TCG TCT G-3′), Birc2 (118 to 364 nt: 5′-AAG TTT GAC TTT TCG TGT GA-3′ and 5′-GCA GAC TGG CTG AAA GCA GA-3′), Pem (58 to 255 nt: 5′-GAA GAC TCG GAA GAA CAG C-3′ and 5′-TCA TTT TGT TCC TGC TCC A-3′), Glipr1 (5 to 255 nt: 5′-AGG TCA TCC TTG CTG TGA TA-3′ and 5′-TGA ATG CAG CTG TGG GTT GT-3′), Mrpplf3 (4 to 263 nt: 5′-CTC CCT TCT TCG ATT CAA C-3′ and 5′-TTG CAT CTC ATG GGG CTT T-3′), Itgb7 (87 to 437 nt: 5′-GGA GGC CGC AGA ATG GGA G-3′ and 5′-GAG GAA GCG GAC CCG GAA-3′), and GAPDH (825 to 900 nt: 5′-TGA AGC AGG CAT CTG AGG G-3′ and 5′-TTG AAG TCG CAG GAG ACA ACC-3′), and 18S (5′-GTA ACC CGT TGA ACC CCA TT-3′ and 5′-CCA TCC AAT CGG TAG TAG CG-3′).
RESULTS
IKKα−/− cells form colonies in soft agar
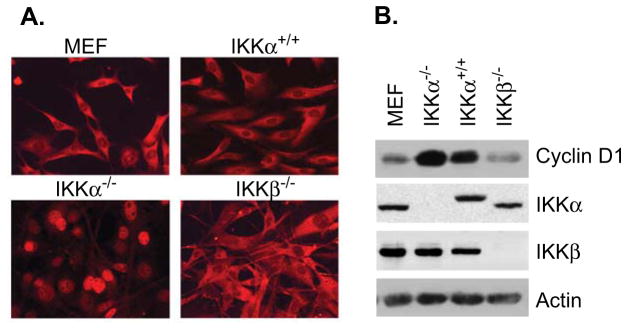
We previously demonstrated that IKKα regulates subcellular distribution and turnover of cyclin D1 by phosphorylation at T286 (13). Immunofluorescent staining indicated that cyclin D1 was distributed in the cytoplasm of wild-type MEF and IKKβ−/− cells (Figure 1A). However, IKKα knockout cells showed that cyclin D1 is predominantly localized in the nucleus and remained nuclear throughout different phases of the cell cycle. IKKα−/− cells reconstituted with IKKα (IKKα+/+) demonstrated predominantly cytoplasmic distribution of cyclin D1, similar to MEF cells. These results indicate that IKKα is involved in regulating cyclin D1 localization. Expression of IKKα and IKKβ in these cells was confirmed by Western blot analysis (Figure 1B). Cyclin D1 level in IKKα knockout cells was higher than that observed in wild-type MEF and IKKα reconstituted cells, indicating that cyclin D1 nuclear localization suggests reduced cytoplasmic proteosome-mediated degradation due to lack of nuclear export (Figure 1B).
Figure 1. Nuclear localization of cyclin D1 in IKKα−/− cells.
(A) Wild-type MEF, IKKα−/−, IKKβ−/− and IKKα+/+ cells were stained with anti-cyclin D1 antibody followed by a secondary antibody conjugated with Texas-Red. (B) Expression of cyclin D1 and IKK proteins was detected by Western blot analysis in indicated cells.
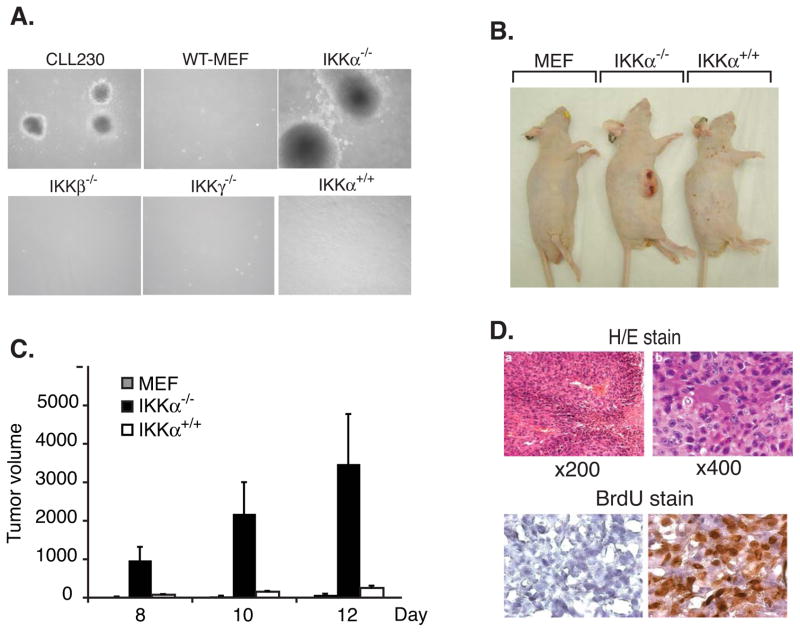
Cyclin D1, an important regulator for cell cycle initiation and G1/S transition, is implicated in the pathogenesis of a variety of tumors. Constitutive nuclear expression of cyclin D1 T286A and a splice variant cyclin D1b is associated with a neoplastic phenotype as suggested by growth in soft agar and tumor formation in nude mice (27,28,31,32). As our work indicated that IKKα is required for nuclear export of cyclin D1, we addressed whether nuclear localization of cyclin D1 in IKKα−/− cells leads to the acquisition of a neoplastic phenotype. To determine the ability of IKKα knockout cells to grow in anchorage independent manner, soft agar colony assays were performed. For these experiments, wild-type MEF, IKKα−/−, IKKβ−/−, IKKγ−/− and IKKα+/+ cells were used. The assays revealed that wild-type MEF, IKKβ−/−, and IKKγ−/− cells do not grow in soft agar. In contrast, IKKα−/− cells were able to grow in anchorage independent manner to form colonies in soft agar. IKKα reconstituted cells grew in soft agar but the number of colonies observed was far lower than IKKα−/− cells (Table 1 and Figure 2A). These results indicate that in the absence of IKKα, there is nuclear localization of cyclin D1 and these cells acquire the ability to form colonies in soft agar.
Table 1.
Induction of colonies in soft agar
| Cell lines | Number of colonies | ||
|---|---|---|---|
| Experiment # 1 | Experiment # 2 | Experiment # 3 | |
| WT MEF | 0 | 0 | 0 |
| IKKα−/− | 205 | 190 | 200 |
| IKKβ−/− | 0 | 0 | 0 |
| IKKγ−/− | 0 | 0 | 0 |
| IKKα+/+ | 13 | 7 | 10 |
0.5 × 104 cells were plated in soft agar as described in methods and number of colonies was counted at two weeks
Figure 2. IKKα−/− cells show tumorigenic potential.
(A) IKKα−/− cells grow anchorage-independently in soft agar. Soft agar assays were performed with wild-type MEF, IKKα−/−, IKKβ−/−, IKKγ−/−, and IKKα reconstituted cells. After 14 days, colonies were counted and photographed. CLL230 cells were used as a positive control. (B) IKKα−/− cells induce tumors in athymic nude mice. Six week-old athymic nude mice were injected with 5×106 indicated cells subcutaneously and observed for development of tumor. The figure shows a representative appearance of tumor at twelve days following inoculation of cells (C) Tumor size was measured at 8, 10, and 12 days following injection of the cells and mice not developing tumor were observed up to three weeks. The figure shows average volume (mm3) on indicated days (SD±SEM). (D) Top panel: Paraffin sections from tumor induced by IKKα−/− cells were stained with hematoxylin and eosin. Sections are shown at 100X (a) and 400X (b). Bottom panel: Athymic mice with tumors induced by injection of IKKα−/− cells were injected with BrdU or PBS as described in methods. The frozen sections from these tumors were stained with avidin-conjugated anti-BrdU antibody followed by streptavidin-conjugated hydrogen peroxide. DAB was used as final chromogen.
IKKα−/− cells develop tumor in nude mice
We next determined whether IKKα−/− cells induced tumors in immune-deficient mice. Wild type MEF, IKKα−/− and IKKα+/+ cells, were subcutaneously injected into athymic nude mice and observed for tumor formation. Consistent with the soft agar assays, the wild-type MEF cells did not form any tumors in nude mice. In contrast, tumors were observed in all nude mice injected with IKKα−/− cells, and tumor growth was visible as early as 7day after inoculation. Reconstitution of IKKα−/− cells with IKKα abrogated the ability of these cells to induce tumor in these mice (Table 2 and Figure 2B). The result indicates that IKKα is important for suppression of tumorigenic potential of mouse embryo fibroblasts. The tumors induced by IKKα−/− cells grew at a rapid rate as shown in figure 2C. Histological examination of IKKα−/−-induced tumor revealed increased cellularity, mitotic figures and areas of necrosis; hallmarks of high-grade tumors (Figure 2D, upper panels). Vascular proliferation was also observed in tumors indicating neo-angiogenesis. Further, we performed BrdU incorporation in tumor cells to determine the number of cells undergoing active cycling. Athymic nude mice with IKKα−/− cells induced tumors were injected with either BrdU or PBS as a control, and tumor sections were stained to assess the number of cells incorporating BrdU. The results showed that the majority of tumor cell population incorporated BrdU, indicating a high mitotic activity in these cells (Figure 2D, lower panels). Taken together, in vivo tumorigenicity assay of IKKα−/− cells is consistent with soft agar colony assay and suggest that IKKα-mediated cyclin D1 nuclear localization leads to tumorigenicity of these cells
Table 2.
Induction of tumors in nude mice
| Cell line | Number of mice developing tumor | |
|---|---|---|
| Experiment # 1 | Experiment # 2 | |
| WT MEF | 0 of 5 | 0 of 4 |
| IKKα−/− | 5 of 5 | 5 of 5 |
| IKKα+/+ | 0 of 5 | 0 of 5 |
Athymic nude mice were injected with 5×106 cells, subcutaneously in flank area and development of tumor was observed three times a week for three weeks. All mice injected with IKKα cells had visible tumor growth by day 8.
Cyclin D1 remains nuclear in tumor tissue
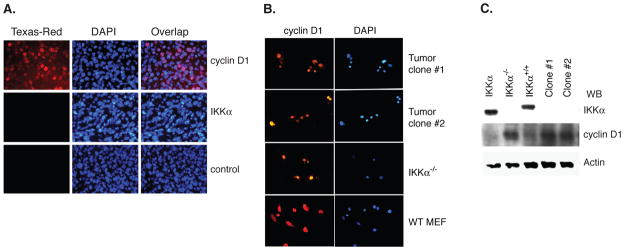
It is possible that in vivo growth of IKKα−/− cells result in altered subcellular distribution of cyclin D1. We evaluated whether tumor cells growing in nude mice preserve nuclear only distribution of cyclin D1. Immunohistochemical analysis of tumor section showed that cyclin D1 was localized in the nucleus, while there was no expression of IKKα, a phenotype similar to the injected IKKα−/− cells. These findings suggest the tumor is derived from the injected IKKα−/− cells and these cells do not acquire the capability to alter subcellular distribution of cyclin D1 while growing in in vivo environment (Figure 3A). To further confirm these findings, clonal cells were isolated from these tumors to determine whether cultured tumor cells retain phenotypic characteristic of injected IKKα−/− cells. Immunofluorescence staining of two separate clones isolated from tumors of different mice, revealed that both exhibited cyclin D1 in the nucleus (Figure. 3B). Again, in this experiment, wild-type MEF and IKKα−/− cells were used as controls. Western blot analysis showed that clonal tumor cells do not express IKKα, and cyclin D1 levels were increased, as compared to those of wild type and IKKα reconstituted cells and similar to IKKα−/− cells (Figure 3C). These results suggest that tumor induced by IKKα−/− cells retains nuclear localization of cyclin D1 in in vivo microenvironment, and nuclear distribution of cyclin D1 likely plays a critical role in tumorigenic potential of these cells.
Figure 3. Analysis of IKKα and cyclin D1 in tumors induced by IKKα−/− cells.
(A) IKKα−/− cells induced tumors were immunostained with either cyclin D1 (1:50) or IKKα antibody (1:50), followed by secondary antibody conjugated to Texas-Red. The nucleus was stained with DAPI. Control indicates immunofluorescent staining without primary antibody (B) Tumor cells were isolated from IKKα−/− cell induced tumors and cultured and multiple clones were isolated and propagated. These cells derived from tumors were stained for cyclin D1 localization using immunofluorescent microscopy. DAPI was used to stain the nucleus. Wild-type MEF and IKKα−/− cells were used as control for this experiment. (C) Western blot analysis of IKKα and cyclin D1 in tumor cell population was performed. Actin expression was used as a control.
Analysis of gene expression regulated by IKKα
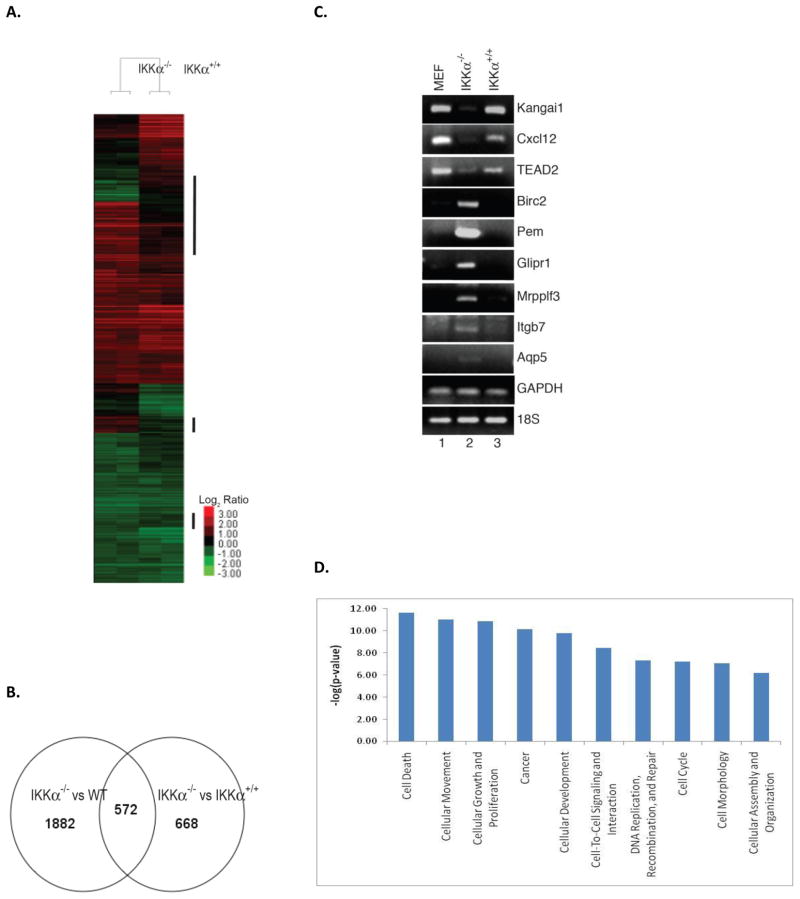
We evaluated genes with altered expression pattern dependent on IKKα and could potentially play a role in tumorigenesis, in a systematic manner by microarray analysis as described above in methods, 40k mouse cDNA microarray (Illumina) was used. Wild-type MEF, IKKα−/−, and IKKα reconstituted cells grown under normal condition were harvested without any stimulation. Total RNA was extracted and reverse transcribed into cDNA using Cy5 (unstimulated)- and Cy3 (stimulated)-labeled dCTP for two-color microarray analysis. Two independent experiments were performed using RNA purified at separate times. Changes in gene expression in IKKα knockout cells as compared to wild-type MEF and IKKα reconstituted cells were analyzed (Figure 4A). The results showed that expression of 527 genes out of 40,000 was altered in IKKα−/− cells as compared to MEF and IKKα reconstituted cells (Figure 4B). Upregulated genes in IKKα knockout cells include proliferin (Plf) (33), mitogen regulated protein, proliferin3 (Mrpplf3) (34), EGF-like-domain, multiple 9 (Egfl9) (35), which are involved in cell growth and proliferation; anti-apoptotic baculoviral IAP repeat-containing 2 and 3 (Birc2/Birc3) (36), and placenta and embryonic expression gene onco-fetal gene (Pem) (37). Down regulated genes included tumor suppressor genes such as suppression of tumorigenicity 6, prostate (Kai1) (38) and multiple endocrine neoplasia 1 (Men1) (39). Other notable genes regulated by IKKα included chemokine (C-X-C motif) ligand 12 (Cxcl12) (40), integrin alpha 3 (Itga3) (41) and integrin β7 (Ingb7) (42).
Figure 4. Microarray analysis of IKKα-regulated genes.
(A) Gene expression analysis of IKKα−/− cells. Clustering analysis of 3077 genes that were differentially expressed in IKKα−/− cells in comparison with either wild-type cells or IKKα+/+ cells. The color code represents log2 ratio against normalized wild-type signals. Samples from IKKα−/− and IKKα+/+ cells were clustered into two different groups. Gene clusters where expressions were altered only in IKKα−/− cells but not in IKKα+/+ cells were highlighted. (B) VennDiagram shows common genes whose expressions were changed in IKKα−/− cells, when comparing with either wild-type or IKKα+/+ cells. (C) Validation of microarray results using RT-PCR. Total RNA from cell lines was subjected to a semi-quantitative RT-PCR analysis using specific DNA oligonucleotides. GAPDH and 18S were used as internal controls. (D) The most differentially expressed genes are plotted against their transformed p-values from Fisher’s Exact Test.
RT-PCR analysis of the expression of genes identified by microarray
To verify expression change in genes of IKKα−/− cells identified by microarray analysis, semi-quantitative RT-PCR assay was performed. Total RNA was prepared from wild-type MEF, IKKα−/−, and IKKα reconstituted cells and subjected to RT-PCR analysis with specific DNA oliogonucleotide primers to selected genes. The RT-PCR analysis showed that expression of Kai1, Cxcl12, and TEAD2 genes was down regulated, while Birc2, Pem, Glipr1, Mrpplf3, and Itgb7 gene expression was upregulated (Figure 4C). Control genes GAPDH and 18S were similarly expressed in wild-type MEF, IKKα−/− and IKKα reconstituted cells. Notably, increased expression level of Pem (78-fold) and Glipr1 (32-fold) identified by microarray assays was confirmed by RT-PCR analysis. These results indicate that RNA expression levels of most genes as evaluated by RT-PCR were consistent with results obtained by microarray analysis. Further, up-regulation of pro-proliferative and anti-apoptotic genes and down-regulation of tumor suppressor genes observed in IKKα−/− cells is consistent with tumorigenic potential of these cells.
The analysis of all 527 altered genes using Ingenuity Pathway Analysis software shows that the most differentially expressed genes are those associated with signature characteristics of neoplastic cells including apoptosis, cell migration, and cell growth (Figure 4D).
Decreased expression of IKKα in human lung cancer cells
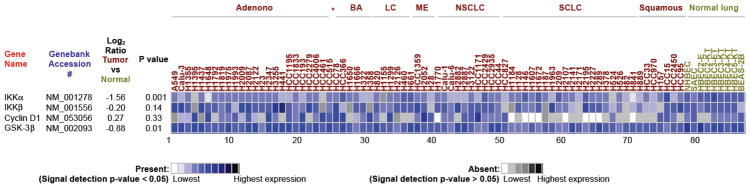
Based on results obtained with mouse cells, we reasoned whether IKKα plays a role in development of human cancers. To evaluate this possibility, we looked at expression pattern of IKKα in 66 human lung cancer cell lines and 8 immortalized lung epithelial cell lines from normal individuals. The analysis was performed using Affymetrix chips as described in methods. Expression data revealed that average IKKα expression level in human lung cancer cells was about three fold lower than that of control cells. The difference was statistically significant (p < 0.0001) (Figure 5). Next, we determined whether IKKα-regulated genes identified by mouse cDNA arrays demonstrate a similar expression pattern in human lung cancer cells. We specifically looked at expression of genes evaluated by RT-PCR in mouse cells. Expression of tumor suppressor gene, Kai1 was similarly decreased in human lung cancer as compared to control cells (3.8 fold).
Figure 5. Lung cancer cells exhibits decreased level of IKKα compared to normal bronchial epithelial cell lines.
Microarray data of 76 human lung cancer cell lines and 9 from those normal individuals were analyzed for expression of IKKα, IKKβ, cyclin D1, and GSK-3β genes. Cell lines derived from patients with different morphology of tumors were analyzed and are shown as: Lane 1–25, adenocarcinoma; Lane 26 (*) adenosquamous carcinoma; Lane 27–31, bronchio-alveolar carcinoma; Lane 32–37, large cell carcinoma; Lane 38–40, mesothelioma; Lane 41–50, non-small cell lung cancer (not otherwise characterized): Lane 51–72, small cell lung cancer; Lane 73–76, squamous cell carcinoma; Lane 78–86, cell lines from normal bronchial epithelium.
However, except possibly for Pem and Plf whose orthologs could not be identified in the Affymetrix array, the other genes evaluated by RT-PCR did not show such differential expression between tumor and control, presumably because of differences of species, cell types, and technical issues related to microarrays. Looking at array data on primary lung cancers, we could not confirm these findings (downregulation of IKK-alpha and KAI1), again presumably due to differences of species, cell types, tumor heterogeneity, etc. Further studies will therefore be needed to fully characterize the molecular pathways involved in IKK-alpha inactivation in these and other cancers.
Our panel of lung cancer cell lines included 14 cell lines derived from squamous cell and 27 from patients with non-squamous morphology. Cell lines derived from squamous cell lung cancer cells had 2.7-fold lower expression of IKKα, while those from non-squamous morphology had 2.6-fold decrease as compared to control lung epithelial cells. These results suggest that expression of IKKα is lower in lung cancer cells irrespective of histological subtypes (Figure 5). This preliminary data is consistent with tumor suppressor role of IKKα and raises a possibility that IKKα functions as a tumor suppressor gene.
DISCUSSION
In this study, we evaluated the role of IKKα in cellular transformation and tumorigenesis. IKKα−/− cells express cyclin D1 in a nuclear pattern unlike cells with preserved expression of IKKα. These IKKα knockout cells demonstrate the ability to form colonies in soft agar, a characteristic of cells with neoplastic phenotype. This was further confirmed by ability of these cells to induce tumors in 100% of athymic nude mice injected with these cells. Reconstitution of IKKα in these cells markedly limited their ability to grow in soft agar and abrogated ability to induce tumor in nude mice. These results strongly implicate role of IKKα as a tumor suppressor. Further experiments looking at global pattern of gene expression by micro-array demonstrated that there is over-expression of known genes with oncogenic potential and reduced expression of tumor suppressor genes. In addition, we tested expression of IKKα in a panel of lung cancer cell lines by cDNA microarray. Results obtained were consistent with those observed with mouse cells. Decreased expression of IKKα in lung cancer cell lines, when taken together with data from murine cells suggest that lack of IKKα in lung cancer cell lines may be involved in development of lung cancer in these patients.
D-type cyclins are critical for recruitment of post embryonic cells into cycling and mediate initial pRb phosphorylation in partner with CDK4/CDK6. Over-expression and altered subcellular distribution are implicated in the pathogenesis of a variety of tumors including breast, lung, colon, and mantle cell lymphoma (43–44). There is strong experimental evidence to support role of cycle D1 in tumorigenesis. Cyclin D1 is exported out of the nucleus during the S phase of cell cycle and degraded, a process dependent on its phosphorylation at T286 residue (25). Earlier, it was shown that GSK-3β mediates this phosphorylation (26). We demonstrated that IKKα is critical for phosphorylation of cyclin D1 at same threonine 286 residue (13) and in the absence of IKKα, cyclin D1 remains in the nucleus. Expression of cyclin D1-T286A mutant, which remains localized to the nucleus of cells leads to a neoplastic phenotype (31). Similar results were observed with a splice variant of cyclin D1 (cyclin D1b), in which carboxy terminus including T286 is deleted (27). Although T286A mutation of cyclin D1 is not described in tumors, transgenic cyclin D1-T286A mice developed B-cell lymphoma (32). On the other hand, expression of cyclin D1b has been observed in esophageal cancer (28). These studies, strongly suggest that nuclear localization of cyclin D1 leads to transformation of cells to a neoplastic phenotype and raises the possibility that a similar mechanism may be involved in development of some cancer types in humans. Earlier studies have not demonstrated the underlying mechanism responsible for altered expression and/or sub-cellular distribution of cyclin D1 in most of the tumors with the exception of t(11:14) for mantle cell lymphoma and isolation of cyclin D1b from esophageal cancer cell line.
We report here that IKKα knockout cells with nuclear localization of cyclin D1 are tumorigenic. These findings are consistent with the above work demonstrating that nuclear distribution of cyclin D1 is associated with neoplastic phenotype. We found the lack of IKKα is associated with nuclear localization of cyclin D1 and ability of these cells to transform to tumors. It is likely this mechanism is involved in a broader array of human cancers, as suggested by lower expression of IKKα in lung cancer cell lines. These findings provide a novel mechanism for development of neoplasia and may explain the observed alteration in cyclin D1 in a variety of cancers, in which there is increased expression of cyclin D1 without a known gain of function mutation in cyclin D1 gene, or other intrinsic abnormalities of cyclin D1 expression such as those induced by gene amplification or translocation. It is likely that an IKKα upstream defect is responsible for this phenotype. This could be due to mutations involving IKKα gene or yet undefined mechanism.
Recently, a study by Liu et al identified mutations involving IKKα gene in exon 15 in eight of nine poorly differentiated squamous cell carcinoma of skin (45). Their findings further support the role of IKKα as tumor suppression gene. Though, we have not performed a mutational analysis on our lung cancer cell lines yet, a similar mechanism is a distinct possibility.
The oncogenic potential of cyclin D1b and the cyclin D1-T286A mutant has been attributed to the constitutive nuclear localization of cyclin D, lack of nuclear export and subsequent proteolysis thus leading to increased levels of cyclin D. Our data is consistent with this observation. Several possible mechanisms have been put forward to explain oncogenicity of cyclin D1 under these conditions (46,47). One likely explanation could be constitutive activation of CDK4/6 kinase activity and pRb phosphorylation leading to deregulation of G1/S checkpoint. Alternatively, it is possible that cyclin D effect is not a direct consequence of its activity as classical cyclin, instead mediated by its role as a transcription factor or as a regulatory protein for other transcription factors such as C/EBPβ, which has been implicated in the development of cancer (48). Several studies indicating cyclin D1 activity as regulator of transcription in addition to its well defined role in cell cycle support this as a possibility.
Oncogenic potential of IKKα−/− cells is likely mediated by lack of IKKα to phosphorylate cyclin D1 and with similar downstream mechanisms to that observed with cyclin D1 T286A or cyclin D1b variant. However, direct effect of IKKα on other pathways remains a possibility. IKKα has been demonstrated to regulate transcription of κB responsive and nuclear hormone receptor genes (12,49). In another study, expression of c-fos has been demonstrated as directly regulated by IKKα at transcriptional level independent of the NF-κB pathway (50). These studies suggest a broader role of IKKα as a transcription regulator. Our microarray and RT-PCR data demonstrate up regulation of pro-mitotic and angiogenesis genes and suppression of anti-apoptotic genes and tumor suppressor genes such as Kai 1 and Men 1. Whether altered expression of these genes is directly mediated by IKKα or through its effect on cyclin D remains to be defined. Repression of raf, ERK and VEGF-A was noted in epidermal cells by IKKα in the study demonstrating mutations involving IKKα in squamous cell cancers. These findings suggest that several pro-mitogenic/angiogenic pathways may be repressed by IKKα. However, authors in this study (45) did not look at cyclin D1 level or subcellular distribution, therefore, possibility remains that activation of mitogenic kinases observed is mediated by nuclear cyclin D in these patients as well. Further studies are needed to define underlying mechanism of tumor suppressor activity of IKKα.
Our finding of decreased IKKα expression in lung cancer cell lines and study by Liu et al quoted above (45) strongly suggest that IKKα is a tumor suppressor gene of broader clinical significance. IKKα gene is located on chromosome 10 near PTEN. Genetic changes involving this area are common in different malignancies. It is likely that these may involve IKKα gene leading to development of different neoplastic disorders. A comprehensive analysis of IKKα gene expression different type and degrees of cancers will provide new insight whether IKKα contributes to induction and progression of tumors. We are currently evaluating this using breast cancer cell lines and tissue samples from patients.
In summary, through current work we provide evidence that lack of IKKα leads to a neoplastic phenotype, which is not observed after reconstitution of IKKα. Acquisition of this neoplastic phenotype is associated with nuclear localization of cyclin D1. Our findings reported here in association with other studies suggest that decreased levels or activity of IKKα may be responsible for increased cyclin D1 observed in various tumors. Our findings warrant further studies to explore the role of IKKα as a tumor suppressor gene of wide clinical significance.
Acknowledgments
We appreciate the help from A. Herrera for preparation of figures and Chandrani Mukhopadhyay for technical help. This work was supported by a NIH grant R01 AI041860 to UNV.
References
- 1.Basseres DS, Baldwin AS. Oncogene. 2006;25:6817–6830. doi: 10.1038/sj.onc.1209942. [DOI] [PubMed] [Google Scholar]
- 2.Karin M, Cao Y, Greten FR, Li ZW. Nat Rev Cancer. 2002;2:301–310. doi: 10.1038/nrc780. [DOI] [PubMed] [Google Scholar]
- 3.DiDonato JA, Hayakawa M, Rothwarf DM, Zandi E, Karin M. Nature. 1997;388:548–554. doi: 10.1038/41493. [DOI] [PubMed] [Google Scholar]
- 4.Mercurio F, Zhu H, Murray BW, Shevchenko A, Bennett BL, Li J, Young DB, Barbosa M, Mann M, Manning, et al. Science. 1997;278:860–866. doi: 10.1126/science.278.5339.860. [DOI] [PubMed] [Google Scholar]
- 5.Woronicz JD, Gao X, Cao Z, Rothe M, Goeddel DV. Science. 1997;278:866–869. doi: 10.1126/science.278.5339.866. [DOI] [PubMed] [Google Scholar]
- 6.Zandi E, Rothwarf DM, Delhase M, Hayakawa M, Karin M. Cell. 1997;91:243–252. doi: 10.1016/s0092-8674(00)80406-7. [DOI] [PubMed] [Google Scholar]
- 7.Rothwarf DM, Zandi E, Natoli G, Karin M. Nature. 1998;395:297–300. doi: 10.1038/26261. [DOI] [PubMed] [Google Scholar]
- 8.Yamaoka S, Courtois G, Bessia C, Whiteside ST, Weil R, Agou F, Kirk HE, Kay RJ, Israel A. Cell. 1998;93:1231–1240. doi: 10.1016/s0092-8674(00)81466-x. [DOI] [PubMed] [Google Scholar]
- 9.Zandi E, Chen Y, Karin M. Science. 1998;281:1360–1363. doi: 10.1126/science.281.5381.1360. [DOI] [PubMed] [Google Scholar]
- 10.Kwak YT, Guo J, Shen J, Gaynor RB. J Biol Chem. 2000;275:14752–14759. doi: 10.1074/jbc.m001039200. [DOI] [PubMed] [Google Scholar]
- 11.Senftleben U, Cao Y, Xiao G, Greten FR, Krahn G, Bonizzi G, Chen Y, Hu Y, Fong A, Sun SC, Karin M. Science. 2001;293:1495–1499. doi: 10.1126/science.1062677. [DOI] [PubMed] [Google Scholar]
- 12.Yamamoto Y, Verma UN, Prajapati S, Kwak YT, Gaynor RB. Nature. 2003;423:655–659. doi: 10.1038/nature01576. [DOI] [PubMed] [Google Scholar]
- 13.Kwak YT, Li R, Becerra CR, Tripathy D, Frenkel EP, Verma UN. J Biol Chem. 2005;280:33945–33952. doi: 10.1074/jbc.M506206200. [DOI] [PubMed] [Google Scholar]
- 14.Hu Y, Baud Ver, Delhase M, Zhang P, Deerinck T, Ellisman M, Johnson R, Karin M. Science. 1999;284:316–320. doi: 10.1126/science.284.5412.316. [DOI] [PubMed] [Google Scholar]
- 15.Li Q, Lu Q, Hwang JY, Buscher D, Lee KF, Izpisua-Belmonte JC, Verma IM. Genes Dev. 1999;13:1322–1328. doi: 10.1101/gad.13.10.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tanaka M, Fuentes ME, Yamaguchi K, Durnin MH, Dalrymple SA, Hardy KL, Goeddel DV. Immunity. 1999;10:421–429. doi: 10.1016/s1074-7613(00)80042-4. [DOI] [PubMed] [Google Scholar]
- 17.Hu Y, Baud V, Oga T, Kim KI, Yoshida K, Karin M. Nature. 2001;410:710–714. doi: 10.1038/35070605. [DOI] [PubMed] [Google Scholar]
- 18.Sil AK, Maeda S, Sano Y, Roop DR, Karin M. Nature. 2004;428:660–664. doi: 10.1038/nature02421. [DOI] [PubMed] [Google Scholar]
- 19.Betticher DC, Thatcher N, Altermatt HJ, Hoban P, Ryder WD, Heighway J. Oncogene. 1995;11:1005–1011. [PubMed] [Google Scholar]
- 20.Hibberts NA, Simpson DJ, Bicknell JE, Broome JC, Hoban PR, Clayton RN, Farrell WE. Clin Cancer Res. 1999;5:2133–2139. [PubMed] [Google Scholar]
- 21.Bani-Hani K, Martin IG, Hardie LJ, Mapstone N, Briggs JA, Forman D, Wild CP. J Natl Cancer Inst. 2000;92:1316–1321. doi: 10.1093/jnci/92.16.1316. [DOI] [PubMed] [Google Scholar]
- 22.Diehl JA. Cancer biology & therapy. 2002;1:226–231. doi: 10.4161/cbt.72. [DOI] [PubMed] [Google Scholar]
- 23.Chen CH, Shen J, Lee WJ, Chow SN. Int J Gynecol Cancer. 2005;15:878–883. doi: 10.1111/j.1525-1438.2005.00150.x. [DOI] [PubMed] [Google Scholar]
- 24.Weroha SJ, Li SA, Tawfik O, Li JJ. Carcinogenesis. 2006;27:491–498. doi: 10.1093/carcin/bgi278. [DOI] [PubMed] [Google Scholar]
- 25.Diehl JA, Zindy F, Sherr CJ. Genes Dev. 1997;11:957–972. doi: 10.1101/gad.11.8.957. [DOI] [PubMed] [Google Scholar]
- 26.Diehl JA, Cheng M, Roussel MF, Sherr CJ. Genes Dev. 1998;12:3499–3511. doi: 10.1101/gad.12.22.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Solomon DA, Wang Y, Fox SR, Lambeck TC, Giesting S, Lan Z, Senderowicz AM, Conti CJ, Knudsen ES. J Biol Chem. 2003;278:30339–30347. doi: 10.1074/jbc.M303969200. [DOI] [PubMed] [Google Scholar]
- 28.Lu F, Gladden AB, Diehl JA. Cancer Res. 2003;63:7056–7061. [PubMed] [Google Scholar]
- 29.Verma UN, Yamamoto Y, Prajapati S, Gaynor RB. J Biol Chem. 2004;279:3509–3515. doi: 10.1074/jbc.M309300200. [DOI] [PubMed] [Google Scholar]
- 30.Smyth GK. Statistical applications in genetics and molecular biology. 2004;3:Article3. doi: 10.2202/1544-6115.1027. [DOI] [PubMed] [Google Scholar]
- 31.Alt JR, Cleveland JL, Hannink M, Diehl JA. Genes Dev. 2000;14:3102–3114. doi: 10.1101/gad.854900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gladden AB, Woolery R, Aggarwal P, Wasik MA, Diehl JA. Oncogene. 2006;25:998–1007. doi: 10.1038/sj.onc.1209147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Linzer DI, Nathans D. Proc Natl Acad Sci U S A. 1984;81:4255–4259. doi: 10.1073/pnas.81.14.4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Connor AM, Waterhouse P, Khokha R, Denhardt DT. Biochim Biophys Acta. 1989;1009:75–82. doi: 10.1016/0167-4781(89)90081-x. [DOI] [PubMed] [Google Scholar]
- 35.Bonaldo MF, Lennon G, Soares MB. Genome Res. 1996;6:791–806. doi: 10.1101/gr.6.9.791. [DOI] [PubMed] [Google Scholar]
- 36.Rothe M, Pan MG, Henzel WJ, Ayres TM, Goeddel DV. Cell. 1995;83:1243–1252. doi: 10.1016/0092-8674(95)90149-3. [DOI] [PubMed] [Google Scholar]
- 37.Wilkinson MF, Kleeman J, Richards J, MacLeod CL. Dev Biol. 1990;141:451–455. doi: 10.1016/0012-1606(90)90400-d. [DOI] [PubMed] [Google Scholar]
- 38.Nagira M, Imai T, Ishikawa I, Uwabe KI, Yoshie O. Cell Immunol. 1994;157:144–157. doi: 10.1006/cimm.1994.1212. [DOI] [PubMed] [Google Scholar]
- 39.Stewart C, Parente F, Piehl F, Farnebo F, Quincey D, Silins G, Bergman L, Carle GF, Lemmens I, Grimmond S, Xian CZ, Khodei S, Teh BT, Lagercrantz J, Siggers P, Calender A, Van de Vem V, Kas K, Weber G, Hayward N, Gaudray P, Larsson C. Oncogene. 1998;17:2485–2493. doi: 10.1038/sj.onc.1202164. [DOI] [PubMed] [Google Scholar]
- 40.Tashiro K, Tada H, Heilker R, Shirozu M, Nakano T, Honjo T. Science. 1993;261:600–603. doi: 10.1126/science.8342023. [DOI] [PubMed] [Google Scholar]
- 41.Tamura RN, Cooper HM, Collo G, Quaranta V. Proc Natl Acad Sci U S A. 1991;88:10183–10187. doi: 10.1073/pnas.88.22.10183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hu MC, Crowe DT, Weissman IL, Holzmann B. Proc Natl Acad Sci U S A. 1992;89:8254–8258. doi: 10.1073/pnas.89.17.8254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yu Q, Geng Y, Sicinski P. Nature. 2001;411:1017–1021. doi: 10.1038/35082500. [DOI] [PubMed] [Google Scholar]
- 44.Pelosio P, Barbareschi M, Bonoldi E, Marchetti A, Verderio P, Caffo O, Bevilacqua P, Boracchi P, Buttitta F, Borbazza R, Palma PD, Gasparini G. Ann Oncol. 1996;7:695–703. doi: 10.1093/oxfordjournals.annonc.a010718. [DOI] [PubMed] [Google Scholar]
- 45.Liu B, Park E, Zhu F, Bustos T, Liu J, Shen J, Fischer SM, Hu Y. Proc Natl Acad Sci U S A. 2006;103:17202–17207. doi: 10.1073/pnas.0604481103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Coqueret O. Gene. 2002;299:35–55. doi: 10.1016/s0378-1119(02)01055-7. [DOI] [PubMed] [Google Scholar]
- 47.Gladden AB, Diehl JA. J Cell Biochem. 2005;96:906–913. doi: 10.1002/jcb.20613. [DOI] [PubMed] [Google Scholar]
- 48.Lamb J, Ramaswamy S, Ford HL, Contreras B, Martinez RV, Kittrell FS, Zahnow CA, Patterson N, Golub TR, Ewen ME. Cell. 2003;114:323–334. doi: 10.1016/s0092-8674(03)00570-1. [DOI] [PubMed] [Google Scholar]
- 49.Park KJ, Krishnan V, O’Malley BW, Yamamoto Y, Gaynor RB. Mol Cell. 2005;18:71–82. doi: 10.1016/j.molcel.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 50.Anest V, Cogswell PC, Baldwin AS., Jr J Biol Chem. 2004;279:31183–31189. doi: 10.1074/jbc.M404380200. [DOI] [PubMed] [Google Scholar]