Abstract
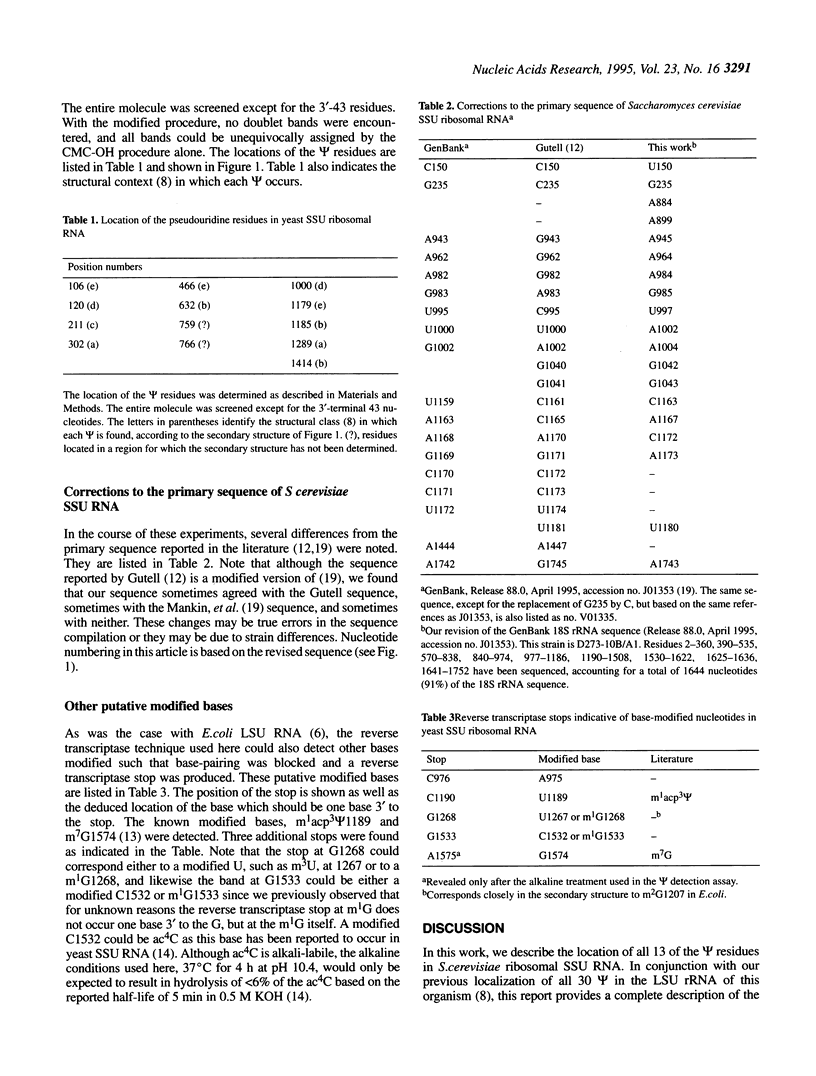
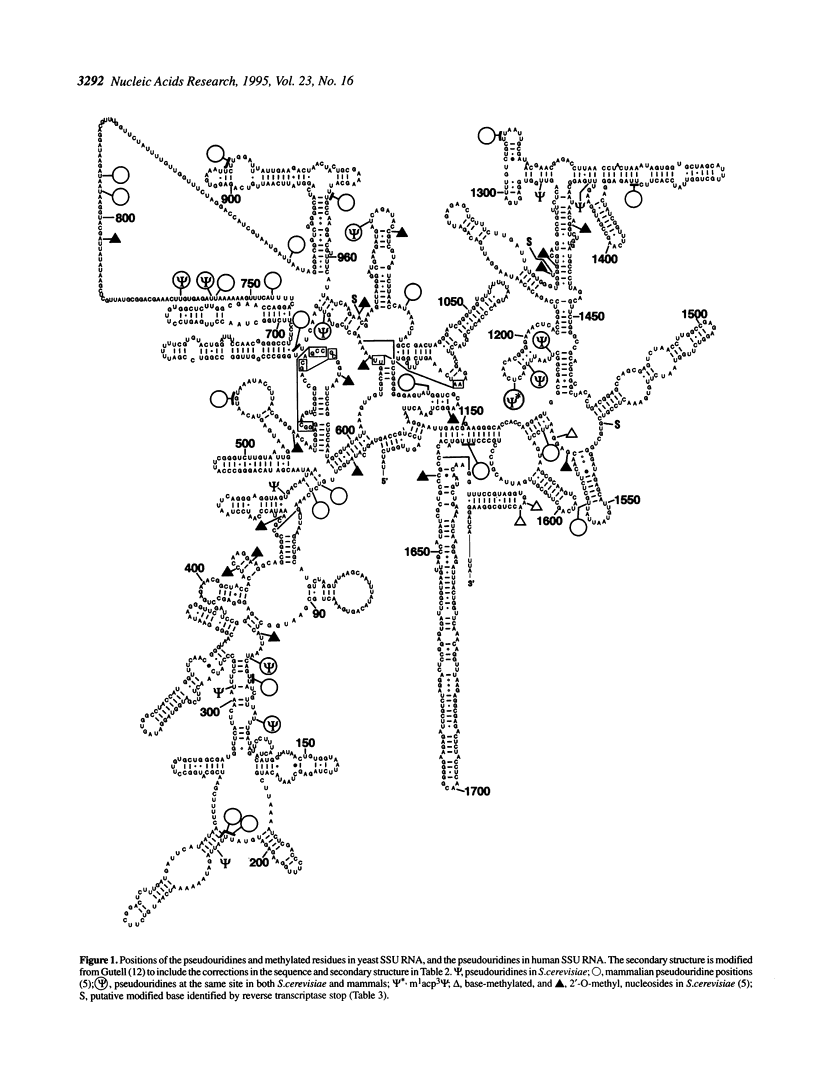
The number and location of all of the pseudouridine (phi) residues in Saccharomyces cerevisiae small subunit (SSU) ribosomal RNA have been determined by a reverse transcriptase sequencing method [Bakin, A. and Ofengand, J., 1993, Biochemistry, 32, 9754-9762]. Thirteen residues were found in addition to the previously described m1acp3 phi 1189. The residues were scattered throughout the molecule with three being in expansion segments. No phi was found in the three highly conserved single-stranded sequence elements common to all SSU RNAs. Specifically, phi 563, the analog of phi 516 (Escherichia coli) and phi 517 (Bacillus subtilis) were not found. Eight of the phi were located identically to those in mammalian SSU RNA and three were near to mammalian phi residues in the secondary structure. There was no discernible correlation between the sites for phi and the known locations of the methylated nucleosides as exists in large subunit (LSU) RNAs. Comparison of the structural context in which phi was found in SSU RNA with that in LSU RNA showed a differential bias suggestive of possible different roles for phi in the two rRNAs. This work also identified the locations of three putative new modified bases in SSU rRNA, and revealed 15 sequence differences between the yeast strain used here and the reported sequence.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bakin A., Kowalak J. A., McCloskey J. A., Ofengand J. The single pseudouridine residue in Escherichia coli 16S RNA is located at position 516. Nucleic Acids Res. 1994 Sep 11;22(18):3681–3684. doi: 10.1093/nar/22.18.3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakin A., Lane B. G., Ofengand J. Clustering of pseudouridine residues around the peptidyltransferase center of yeast cytoplasmic and mitochondrial ribosomes. Biochemistry. 1994 Nov 15;33(45):13475–13483. doi: 10.1021/bi00249a036. [DOI] [PubMed] [Google Scholar]
- Bakin A., Ofengand J. Four newly located pseudouridylate residues in Escherichia coli 23S ribosomal RNA are all at the peptidyltransferase center: analysis by the application of a new sequencing technique. Biochemistry. 1993 Sep 21;32(37):9754–9762. doi: 10.1021/bi00088a030. [DOI] [PubMed] [Google Scholar]
- Brand R. C., Klootwijk J., Sibum C. P., Planta R. J. Pseudouridylation of yeast ribosomal precursor RNA. Nucleic Acids Res. 1979 Sep 11;7(1):121–134. doi: 10.1093/nar/7.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHN W. E. 5-Ribosyl uracil, a carbon-carbon ribofuranosyl nucleoside in ribonucleic acids. Biochim Biophys Acta. 1959 Apr;32:569–571. doi: 10.1016/0006-3002(59)90644-4. [DOI] [PubMed] [Google Scholar]
- COHN W. E. Pseudouridine, a carbon-carbon linked ribonucleoside in ribonucleic acids: isolation, structure, and chemical characteristics. J Biol Chem. 1960 May;235:1488–1498. [PubMed] [Google Scholar]
- Fitch I., Dahmann C., Surana U., Amon A., Nasmyth K., Goetsch L., Byers B., Futcher B. Characterization of four B-type cyclin genes of the budding yeast Saccharomyces cerevisiae. Mol Biol Cell. 1992 Jul;3(7):805–818. doi: 10.1091/mbc.3.7.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutell R. R. Collection of small subunit (16S- and 16S-like) ribosomal RNA structures. Nucleic Acids Res. 1993 Jul 1;21(13):3051–3054. doi: 10.1093/nar/21.13.3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane B. G., Ofengand J., Gray M. W. Pseudouridine and O2'-methylated nucleosides. Significance of their selective occurrence in rRNA domains that function in ribosome-catalyzed synthesis of the peptide bonds in proteins. Biochimie. 1995;77(1-2):7–15. doi: 10.1016/0300-9084(96)88098-9. [DOI] [PubMed] [Google Scholar]
- Lane B. G., Ofengand J., Gray M. W. Pseudouridine in the large-subunit (23 S-like) ribosomal RNA. The site of peptidyl transfer in the ribosome? FEBS Lett. 1992 May 4;302(1):1–4. doi: 10.1016/0014-5793(92)80269-m. [DOI] [PubMed] [Google Scholar]
- Maden B. E. The numerous modified nucleotides in eukaryotic ribosomal RNA. Prog Nucleic Acid Res Mol Biol. 1990;39:241–303. doi: 10.1016/s0079-6603(08)60629-7. [DOI] [PubMed] [Google Scholar]
- Mankin A. S., Skryabin K. G., Rubtsov P. M. Identification of ten additional nucleotides in the primary structure of yeast 18S rRNA. Gene. 1986;44(1):143–145. doi: 10.1016/0378-1119(86)90054-5. [DOI] [PubMed] [Google Scholar]
- Raué H. A., Klootwijk J., Musters W. Evolutionary conservation of structure and function of high molecular weight ribosomal RNA. Prog Biophys Mol Biol. 1988;51(2):77–129. doi: 10.1016/0079-6107(88)90011-9. [DOI] [PubMed] [Google Scholar]
- SCANNELL J. P., CRESTFIELD A. M., ALLEN F. W. Methylation studies on various uracil derivatives and on an isomer of uridine isolated from ribonucleic acids. Biochim Biophys Acta. 1959 Apr;32:406–412. doi: 10.1016/0006-3002(59)90613-4. [DOI] [PubMed] [Google Scholar]
- Santer M., Santer U., Nurse K., Bakin A., Cunningham P., Zain M., O'Connell D., Ofengand J. Functional effects of a G to U base change at position 530 in a highly conserved loop of Escherichia coli 16S RNA. Biochemistry. 1993 Jun 1;32(21):5539–5547. doi: 10.1021/bi00072a007. [DOI] [PubMed] [Google Scholar]
- Thomas G., Gordon J., Rogg H. N4-Acetylcytidine. A previously unidentified labile component of the small subunit of eukaryotic ribosomes. J Biol Chem. 1978 Feb 25;253(4):1101–1105. [PubMed] [Google Scholar]
- Wrzesinski J., Bakin A., Nurse K., Lane B. G., Ofengand J. Purification, cloning, and properties of the 16S RNA pseudouridine 516 synthase from Escherichia coli. Biochemistry. 1995 Jul 11;34(27):8904–8913. doi: 10.1021/bi00027a043. [DOI] [PubMed] [Google Scholar]
- YU C. T., ALLEN F. W. Studies on an isomer of uridine isolated from ribonucleic acids. Biochim Biophys Acta. 1959 Apr;32:393–406. doi: 10.1016/0006-3002(59)90612-2. [DOI] [PubMed] [Google Scholar]


