Abstract
Sensory experience and learning alter sensory representations in cerebral cortex. The synaptic mechanisms underlying sensory cortical plasticity have long been sought. Recent work indicates that long-term cortical plasticity is a complex, multicomponent process involving multiple synaptic and cellular mechanisms. Sensory use, disuse, and training drive long-term potentiation and depression (LTP and LTD), homeostatic synaptic plasticity and plasticity of intrinsic excitability, and structural changes including formation, removal, and morphological remodeling of cortical synapses and dendritic spines. Both excitatory and inhibitory circuits are strongly regulated by experience. This review summarizes these findings and proposes that these mechanisms map onto specific functional components of plasticity, which occur in common across the primary somatosensory, visual, and auditory cortices.
Keywords: sensory experience, sensory map, cerebral cortex, long-term potentiation, long-term depression, structural plasticity
INTRODUCTION
A fundamental property of the brain is plasticity, the ability to change in response to experience and use. Plasticity allows the brain to learn and remember patterns in the sensory world, to refine movements, to predict and obtain reward, and to recover function after injury. A major goal of neuroscience has been to understand the cellular and synaptic plasticity mechanisms that underlie information storage, learning, and adaptive behavior in the brain (James 1890, Konorski 1948, Hebb 1949). With the advent of modern electrophysiological, molecular, and imaging techniques, substantial progress has been made in identifying mechanisms for learning and plasticity (Maren 2005, Weinberger 2007, Sossin et al. 2008).
The neocortex is a particularly relevant region for plasticity because it performs sensory, motor, and cognitive tasks with strong learning components. Even basic sensory perception is influenced by prior sensory experience and learning (Gilbert 1998, Dan & Poo 2006, Han et al. 2007). In sensory areas of neocortex, two basic paradigms have been used to study plasticity. First, in experience-dependent map plasticity, the statistical pattern of sensory experience over several days alters topographic sensory maps in primary sensory cortex, in both animals and humans (Hubel & Wiesel 1998, Blake et al. 2002, Rauschecker 2002). Second, in sensory perceptual learning, training on sensory perception or discrimination tasks causes gradual improvement in sensory ability associated with changes in neuronal receptive fields and/or maps in cortical sensory areas (Gilbert 1998, Weinberger 2007). Sensory map plasticity and sensory perceptual learning are not unitary processes, but involve multiple discrete functional components. Many of these components occur with strong similarity across cortical areas, suggesting common underlying mechanisms.
Cellular mechanisms for cortical plasticity have been proposed to include both physiological mechanisms (functional modification of existing synapses and neurons) and structural mechanisms (physical rewiring of cortical circuits by synapse formation, elimination, and morphological change). Early models posited rapid physiological plasticity via NMDA receptor–dependent LTP and LTD, generally followed by slower structural remodeling of cortical microcircuits to consolidate plasticity (Bear et al. 1987, Katz & Shatz 1996, Buonomano & Merzenich 1998). LTP and LTD implement Hebbian synaptic plasticity, which can explain prominent aspects of cortical plasticity (Hebb 1949, Stent 1973). Other models have discounted physiological plasticity mechanisms, and propose that rapid structural rearrangements underlie plasticity (Berardi et al. 2003, Chklovskii et al. 2004, Hensch 2005). Recently, involvement of LTP and LTD in cortical plasticity has begun to receive direct experimental support (Feldman & Brecht 2007). However, new, sensitive methods have also revealed many novel forms of cellular plasticity, both in vivo and in vitro. These include multiple forms of LTP and LTD (Sjostrom et al. 2008), plasticity of intrinsic excitability (Kim & Linden 2007), plasticity of GABAergic circuits (Foeller & Feldman 2004, Hensch 2005), and non-Hebbian plasticity, including homeostatic synaptic scaling and metaplasticity (Abraham & Bear 1996, Turrigiano & Nelson 2004). In vivo time-lapse imaging has revealed that rapid structural plasticity of synapses and dendritic spines is widespread (Alvarez & Sabatini 2007).
This abundance of plasticity mechanisms presents a major challenge for determining how each contributes to cortical plasticity in vivo and whether common mechanisms and principles for plasticity exist across cortical areas. This review summarizes the evidence for these synaptic and cellular plasticity mechanisms in long-lasting sensory cortical plasticity. Rapid, transient forms of plasticity (e.g., sensory adaptation) and lesion-induced plasticity involve distinct mechanisms and are not discussed. Building on the concept that cortical plasticity is composed of multiple functional components, many of which occur in common across cortical areas, I propose a model in which these components of plasticity are each mediated by specific cellular plasticity mechanisms.
COMMON COMPONENTS OF CORTICAL PLASTICITY
Experience- and training-induced cortical plasticity occur with common functional components in S1, V1, and A1, which may reflect common cellular mechanisms. This section summarizes basic forms of plasticity in each area and identifies these components.
Whisker Map Plasticity in S1
Tactile experience drives robust plasticity of the somatotopic map in S1 (Blake et al. 2002), with cellular mechanisms best analyzed in the whisker map in rodent S1. Whiskers are active tactile detectors, represented anatomically by cell clusters, called barrels, in cortical layer (L) 4. An orderly map of whisker receptive fields exists in S1, with neurons in each barrel-related column responding best to deflection of the corresponding whisker. Similar to other primary sensory areas, whisker input from thalamus primarily arrives in L4, which in turn projects to L2/3, and then to L5, which provide corticocortical and subcortical output, respectively (Lubke & Feldmeyer 2007).
Trimming or plucking a subset of whiskers causes S1 neurons to rapidly lose spiking responses to deprived whiskers and to more slowly increase responses to spared whiskers, thus weakening and shrinking the representation of deprived whiskers and strengthening and expanding the representation of spared whiskers within the map (Fox 1992, Diamond et al. 1993, Glazewski & Fox 1996). Plasticity is most robust in young animals but persists substantially into adulthood. Plasticity occurs at multiple sites and layers in S1, with L4 being a primary site of plasticity in neonates (<4–6 days of age), whereas in juveniles and adults, plasticity occurs most rapidly and extensively, and sometimes exclusively, in L2/3 (Diamond et al. 1994, Glazewski & Fox 1996, Stern et al. 2001, Drew & Feldman 2009). Depression of responses to deprived whiskers (termed response depression) and potentiation of responses to spared whiskers (response potentiation) have distinct dynamics, are separable developmentally and genetically, and can be differentially induced by different patterns of whisker deprivation. They therefore represent distinct functional components and mechanisms of plasticity (Glazewski et al. 2000, Fox 2002).
In young adults (<2 months), plasticity involves both response depression and response potentiation, whereas in older animals only response potentiation is present (Fox & Wong 2005). The net effect of this map plasticity is to dynamically reallocate cortical processing space from deprived inputs toward spared inputs, which may optimize sensory processing. For detailed review of S1 map plasticity, see Feldman & Brecht (2005) and Fox (2002).
Ocular Dominance Plasticity in V1
Ocular dominance plasticity occurs in many species (Hubel & Wiesel 1998), but cellular mechanisms have been best analyzed in rodents. Rodent V1 lacks discrete ocular dominance columns but has a small binocular region in which individual neurons exhibit visual responses to both eyes. Monocular deprivation during a critical period (19–32 days of age) causes a rapid loss of responses to the deprived eye, followed by a slower gain of responses to the open eye, leading to a physiological shift in ocular dominance. As in S1, ocular dominance plasticity occurs both in L4 and in L2/3, with separate sites and mechanisms for plasticity in these layers (Daw et al. 1992, Maffei et al. 2004, Liu et al. 2008, Maffei & Turrigiano 2008). Like S1, plasticity can occur in L2/3 before in L4, suggesting that L2/3 is a primary early locus for plasticity (Trachtenberg et al. 2000). Response potentiation and response depression are genetically separable, with similar pharmacological and genetic requirements to S1 (Sawtell et al. 2003, Kaneko et al. 2008b, Liu et al. 2008) and similar layer-specific dynamics and critical periods (Daw et al. 1992, Trachtenberg et al. 2000, Mrsic-Flogel et al. 2007). Ocular dominance plasticity persists in adult rodents but is slower and mediated mostly by response potentiation, like map plasticity in S1 (Sawtell et al. 2003, Hofer et al. 2006, Sato & Stryker 2008). In contrast, adult cats and primates show substantially less adult plasticity.
Physiological changes in ocular dominance in L4 and L2/3 are followed in L4 after several days (in cats) to weeks (in mice) by shrinking and expanding geniculocortical axon arbors representing the closed and open eye, respectively (Antonini & Stryker 1993, Antonini et al. 1999). Whether whisker experience similarly remodels single thalamocortical axons in S1 is not known. For detailed review of ocular dominace plasticity, see Fox & Wong (2005) and Hofer et al. (2006).
Use- and Correlation-Dependent Plasticity Without Deprivation
In another major form of plasticity, repeated activation of a specific sensory input (without deprivation) potentiates neural responses to that input. This is usually most robust in young animals. For example, exposing young rats to auditory stimuli enhances the representation of the presented frequencies and intensities in A1, thus altering auditory tuning curves and the tonotopic map (Keuroghlian & Knudsen 2007). Presentation of high-contrast oriented gratings to young mice similarly drives orientation-specific enhancement of visual responses in V1 (Frenkel et al. 2006). Similar potentiation occurs in adult S1 in response to temporal correlations between inputs, leading to neurons and map regions with strong joint representation of temporally correlated inputs (e.g., Diamond et al. 1993, Wang et al. 1995). In adult V1, temporally correlated, near-simultaneous stimuli drive systematic shifts in visual tuning related to stimulus order and timing (Dan & Poo 2006).
Learning-Related Plasticity in Adults
Whereas map plasticity in juveniles occurs rapidly in response to passive sensory experience, such plasticity is slower and more limited in adults, except when stimuli are actively attended and behaviorally relevant (e.g., during a perceptual learning task) or explicitly paired with positive or negative reinforcement or neuromodulation. Thus, classical conditioning using tone stimuli increases A1 responses to trained frequencies (Weinberger 2007), classical conditioning using whisker stimuli expands the representation of trained whiskers (Siucinska & Kossut 1996, 2004), and perceptual training on visual stimulus location or orientation discrimination tasks alters V1 tuning for the trained feature (Gilbert 1998, Dan & Poo 2006). Training can increase neural responses to reinforced stimuli, shift tuning curves toward (or away from) trained stimuli, or sharpen tuning curves to improve discrimination between stimuli. These changes in neural tuning are generally modest and do not cause large-scale changes in map topography, except with very extensive training (Blake et al. 2002, Karmarkar & Dan 2006).
Homeostatic Plasticity
In a distinct form of plasticity, substantial sensory overuse or deprivation drives compensatory, homeostatic changes that restore, at least partially, cortical activity to a set point level. For example, 24 hours of continuous whisker deflection weakens the S1 representation of the stimulated whisker, whereas visual deprivation increases visual responses in the deprived monocular zone of rodent V1 (Knott et al. 2002, Mrsic-Flogel et al. 2007). Turrigiano & Nelson (2004) propose that such homeostatic plasticity stabilizes mean cortical activity in the face of slowly changing input levels and in response to synaptogenesis and synapse elimination during development (Turrigiano & Nelson 2004). Homeostatic plasticity also occurs during more modest sensory manipulations, such as monocular closure in binocular visual cortex, and thus is likely to contribute to multiple types of cortical plasticity (Mrsic-Flogel et al. 2007, Maffei & Turrigiano 2008).
Five Common Components of Sensory Cortical Plasticity
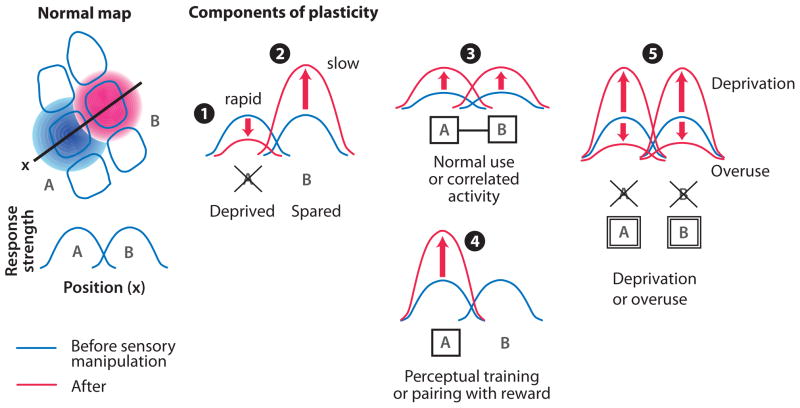
The analysis above suggests five basic components of experience-dependent plasticity that can be identified across S1, V1, and A1. These are illustrated in Figure 1. The first component is rapid response depression to deprived inputs, and the second is slower response potentiation to spared inputs, when a subset of inputs are deprived. These components of plasticity are classically hypothesized to involve Hebbian weakening and strengthening of deprived and spared pathways, and to be driven by competition between active and inactive inputs, because less or no plasticity occurs when all inputs are deprived (Wiesel & Hubel 1965). Response potentiation must involve a competitive process because it is driven heterosynaptically by depriving neighboring inputs. (On the cellular level, this could be accomplished by classical heterosynaptic plasticity or by homeostatic plasticity or metaplasticity affecting all synapses on a neuron.) Whether response depression is a competitive process is less clear. In some cases, response depression requires neighboring, active inputs (Glazewski et al. 1998), which may heterosynaptically depress deprived inputs. However, response depression can also occur when all inputs are deprived (Wallace & Fox 1999, Kaneko et al. 2008b), which is more consistent with noncompetitive, homosynaptic plasticity driven by residual activity on deprived pathways (Rittenhouse et al. 1999, Frenkel & Bear 2004). The third component is potentiation of responses to active inputs during normal sensory use, and in response to temporal correlation between inputs. The fourth is potentiation of responses paired with reinforcement in adults. The third and fourth components are both consistent with Hebbian strengthening of active inputs but differ in dependence on attention or reward. These are driven homosynaptically or cooperatively by activity on active pathways and therefore appear functionally distinct from potentiation of spared inputs during deprivation-induced plasticity. The fifth component is homeostatic regulation of cortical activity in response to substantial increase or decrease in sensory input. The next sections review many of the known cellular plasticity mechanisms in cortex and suggest how they mediate these components of plasticity.
Figure 1.
Common functional components of plasticity in S1, V1, and A1. Left: normal sensory map schematized from the whisker map in S1. Black outlines indicate a map view of barrels. Red and blue shading indicate strength of whisker responses evoked by deflecting two whiskers (“A” and “B”). Below: cortical responses to whiskers A and B along the black transect. Right: Illustration of the five basic components of plasticity defined in the text. 1, 2: Response depression to deprived inputs and response potentiation to spared inputs, in response to deprivation of a subset of inputs. 3: Potentiation of responses during normal sensory use or in response to temporal correlation between inputs. 4: Potentiation of responses after pairing with reward. 5: Homeostatic regulation of responses during substantial decrease or increase in sensory activity.
SYNAPTIC PHYSIOLOGICAL MECHANISMS FOR CORTICAL PLASTICITY
A large number of synaptic plasticity mechanisms are known, many recently discovered (Kim & Linden 2007, Sjostrom et al. 2008). Here we focus on several of the best-studied forms and their roles in cortical plasticity.
Long-Term Depression
LTD implements use-dependent, homosynaptic and heterosynaptic weakening and therefore may mediate response depression to deprived inputs. Multiple forms of LTD exist and may have different roles in plasticity (for review, see Malenka & Bear 2004, Massey & Bashir 2007). In NMDA receptor–dependent LTD (NMDA-LTD), calcium from postsynaptic NMDA receptors activates protein phosphatases including calcineurin, leading to dephosphorylation of specific sites on the AMPA receptor GluR1 subunit and internalization of synaptic AMPA receptors. In cortex, NMDA-LTD (defined by NMDA receptor involvement and AMPA receptor internalization) has been clearly observed at thalamocortical synapses in V1, and likely S1 (Feldman et al. 1998, Crozier et al. 2007), and at other synapses in sensory, anterior cingulate, entorhinal, and perirhinal cortex (Dodt et al. 1999, Toyoda et al. 2006, Deng & Lei 2007, Griffiths et al. 2008). A second major form is metabotropic glutamate receptor–dependent LTD (mGluR-LTD), of which several subforms exist (Egger et al. 1999, Renger et al. 2002, Barbara et al. 2003, Czarnecki et al. 2007). A third form of LTD involves cannabinoid type 1 (CB1) receptors (Chevaleyre et al. 2006). In CB1-LTD, postsynaptic calcium elevation and activation of group I mGluRs drive postsynaptic endocannabinoid synthesis, which signals retrogradely to presynaptic CB1 receptors, driving a long-lasting decrease in release probability (Chevaleyre et al. 2006). CB1-LTD occurs at many neocortical excitatory synapses (Sjostrom et al. 2003, Bender et al. 2006b, Nevian & Sakmann 2006, Crozier et al. 2007, Lafourcade et al. 2007). CB1-LTD is independent of post-synaptic NMDA receptors but may require presynaptic NMDA receptors, which exist at specific neocortical synapses (Sjostrom et al. 2003, Rodriguez-Moreno & Paulsen 2008).
LTD in response depression to deprived inputs
Recent evidence indicates that LTD is a major mechanism for depression of responses to deprived sensory inputs in S1 and V1. Whisker deprivation after P7 drives response depression to deprived whiskers primarily in L2/3, not L4, suggesting LTD at L4–L2/3 excitatory synapses (Glazewski & Fox 1996, Drew & Feldman 2009). This LTD has been directly observed in ex vivo S1 slices from whisker-deprived rats. Whisker deprivation weakens input-output curves of presumptive L4–L2/3 synapses in deprived columns (Allen et al. 2003, Bender et al. 2006a), and glutamate uncaging experiments show that L4–L2/3 synapses are specifically weakened (Shepherd et al. 2003). Deprivation converts normal paired pulse depression into facilitation and slows use-dependent blockade of NMDA-EPSCs by MK-801, indicating a decrease in release probability at these synapses (Bender et al. 2006a). In contrast, neither postsynaptic excitability (Allen et al. 2003) nor measures of postsynaptic responsiveness (mEPSC amplitude, quantal L4–L2/3 synaptic currents, AMPA:NMDA ratio) are altered (Bender et al. 2006a). Deprivation-induced synapse weakening occludes CB1-LTD, which is prominent at L4–L2/3 synapses and which is also expressed as a decrease in presynaptic release probability (Allen et al. 2003, Bender et al. 2006b). These findings suggest that deprivation weakens L4–L2/3 synapses in vivo by CB1-LTD. Consistent with this model, systemic injection of a CB1 antagonist prevents rapid deprivation-induced weakening of L4–L2/3 synapses and prevents depression of responses to deprived whiskers (Li et al. 2007).
In V1, monocular lid suture, which decorrelates retinal activity, drives greater weakening of deprived-eye responses than monocular injection of TTX, which greatly reduces retinal activity levels. This finding is consistent with homosynaptic LTD driven by uncorrelated firing on deprived pathways (Rittenhouse et al. 1999). Monocular deprivation decreases AMPAR surface expression and alters GluR1 phosphorylation similar to NMDA-LTD (Heynen et al. 2003). Knockout of the PKA RIIβ subunit or AKAP150 selectively blocks LTD and reduces or prevents ocular dominance plasticity (Daw et al. 2004, Fischer et al. 2004). Presumptive L4–L2/3 synapses exhibit CB1-LTD in vitro, whereas L4 synapses exhibit NMDA-LTD (Crozier et al. 2007). Systemic pharmacological blockade of CB1 receptors in vivo prevents depression of closed-eye responses in L2/3, but not in L4, suggesting that CB1-LTD is a critical mechanism for response depression in L2/3, whereas other mechanisms, potentially including NMDA-LTD, are active in L4 (Liu et al. 2008). mGluR2-dependent LTD is not likely to be involved because mGluR2 knockout does not disrupt ocular dominance plasticity (Renger et al. 2002).
Despite this strong evidence for LTD in ocular dominance plasticity, several manipulations that block LTD in vitro, including knockout of PKA RIβ and transgenic overexpression of BDNF, do not block ocular dominance plasticity (Hanover et al. 1999, Hensch 2005). Plasticity may proceed in these cases by unblocked forms of LTD, by response potentiation instead of depression, or by compensation by other mechanisms. BDNF involvement in ocular dominance plasticity is complex and is discussed separately below.
Age may be an important factor for LTD involvement in sensory map plasticity. Induction requirements for LTD in vitro change with age (Kemp & Bashir 2001). In young animals (<2 months) whisker and visual deprivation drive both response depression and response potentiation in V1 and S1, whereas in adults, response potentiation occurs solely or primarily (Sawtell et al. 2003, Fox & Wong 2005, Sato & Stryker 2008). Thus, deprivation may drive LTD primarily in young animals, contributing to the more rapid and extensive plasticity at young ages. In adult cortex, NMDA-LTD mediates some aspects of cortical learning not associated with deprivation: In perirhinal cortex, visual experience weakens responses to familiar visual stimuli, a phenomenon that may contribute to visual recognition memory. NMDA-LTD is prominent in adult perirhinal cortex, and peptides that block AMPA receptor internalization block both LTD and visual recognition memory (Griffiths et al. 2008).
Other mechanisms for response depression
Whisker deprivation decreases the probability of synaptic connections between L2/3 pyramidal cells (Cheetham et al. 2007), reduces L2/3 horizontal axonal projections extending toward deprived columns (Broser et al. 2007), and reduces L2/3 input from L4 barrels versus inter-barrel septa (Shepherd et al. 2003). These functional and structural changes in microcircuits and macroscopic projections are appropriate to weaken L2/3 responses to deprived whiskers, and thus could contribute with LTD to rapid response depression in that layer. In V1, rapid ocular dominance plasticity is blocked by several genetic manipulations unrelated to LTD, implicating additional mechanisms in depression of deprived-eye responses (Taha et al. 2002, Yang et al. 2005). These may include structural synaptic plasticity (Mataga et al. 2004) and potentiation of inhibitory circuit function (Maffei et al. 2006), which are discussed below.
Long-Term Potentiation
LTP has been proposed to underlie use-dependent and temporal correlation-dependent strengthening of sensory responses in juveniles, reinforcement-dependent strengthening of responses in adults, and strengthening of spared inputs during deprivation-induced plasticity. Many neocortical excitatory synapses exhibit LTP (Kirkwood & Bear 1994, Castro-Alamancos et al. 1995, Crair & Malenka 1995, Isaac et al. 1997, Markram et al. 1997, Buonomano 1999, Feldman 2000, Sjostrom et al. 2001, Froemke & Dan 2002, Hardingham & Fox 2006). Where characterized, cortical LTP is most often classical NMDA-LTP. In NMDA-LTP, calcium from postsynaptic NMDA receptors and other sources activates kinases including αCaMKII, which drive specific AMPA receptor phosphorylation, and insertion of GluR1-containing AMPA receptors into synapses (Malinow & Malenka 2002). Long-lasting LTP further involves CaMKII autophosphorylation, activation of CREB, and altered gene expression. NMDA-LTP in neocortex can be blocked by viral expression of a GluR1 C-terminal tail construct (GluR1-ct) that prevents activity-dependent GluR1 insertion (Takahashi et al. 2003, Hardingham & Fox 2006, Toyoda et al. 2007). NMDA-LTP causes appearance of AMPA receptor currents at immature synapses that express NMDA but not AMPA receptors (“silent synapses”), thus functionalizing these synapses (Isaac et al. 1997, Rumpel et al. 1998). A second form of neocortical LTP is expressed presynaptically by an increase in release probability, which alters short-term synaptic dynamics (Markram & Tsodyks 1996, Buonomano 1999, Eder et al. 2002). LTP at adult L4-L2/3 synapses in S1 appears to exhibit both a presynaptic component mediated by retrograde nitric oxide (NO) signaling and a postsynaptic component involving GluR1 insertion (Hardingham et al. 2003, Hardingham & Fox 2006). Additional forms of LTP may also occur (Daw et al. 2004).
LTP driven by normal sensory use
NMDA-LTP is strongly implicated in activity-and use-dependent strengthening of cortical synapses during early development. In S1, whisker experience strengthens developing L4-L2/3 excitatory synapses via NMDA-LTP, as shown by molecular interventions that alter AMPA receptor trafficking. When GluR1 is overexpressed, LTP causes synaptic insertion of GluR1-homomeric (GluR2-lacking) AMPA receptors, which show unusually high current rectification, allowing LTP to be detected electrophysiologically (Shi et al. 2001). Viral expression of GluR1 in developing L2/3 neurons in vivo causes increased rectification at L4-L2/3 synapses. This increased rectification does not occur in whisker-trimmed rats, indicating that whisker experience drives GluR1 insertion into L4-L2/3 synapses. Conversely, viral transfection of GluR1-ct, which prevents delivery of native GluR1 to synapses, prevents experience-dependent enhancement of AMPA currents at L4-L2/3 synapses. Thus, normal whisker experience strengthens developing L4-L2/3 synapses by GluR1 insertion, which likely represents NMDA-LTP (Takahashi et al. 2003). Experience-dependent strengthening is regulated by PSD-95 similarly to NMDA-LTP (Ehrlich & Malinow 2004). Developmental strengthening of thalamocortical synapses also appears to involve insertion of AMPA receptors, including into silent synapses (Isaac et al. 1997).
In mouse V1, daily visual stimulation with high-contrast grating stimuli gradually increases visual responses to trained stimuli. This increased responsiveness is prevented by systemic injection of NMDA antagonist and by viral expression of GluR1-ct, suggesting that responses are strengthened by NMDA-LTP at cortical synapses (Frenkel et al. 2006).
LTP in response potentiation to spared inputs
Involvement of NMDA-LTP in response potentiation during deprivation-induced plasticity has long been hypothesized (Bear et al. 1987, Fox 2002) but remains controversial. Early indirect evidence showed that NMDA receptors were required for ocular dominance and whisker map plasticity (Bear et al. 1990, Rema et al. 1998, Roberts et al. 1998) and that LTP and map plasticity showed similar layer-specific critical periods (Crair & Malenka 1995, Feldman et al. 1999, Jiang et al. 2007). In addition, whisker experience was shown to alter short-term synapse dynamics at several S1 synapses, reminiscent of presynaptically expressed LTP (Finnerty et al. 1999).
More recent, direct evidence links NMDA-LTP with deprivation-induced response potentiation in S1. Studies in knockout mice show that α/δ CREB, α-CaMKII, and α-CaMKII autophosphorylation are all required for response potentiation in L2/3 in vivo, consistent with a requirement for NMDA-LTP (Glazewski et al. 1996, 1999, 2000). Moreover, deprivation of all but one whisker (single whisker experience), which drives response potentiation to the spared whisker in L2/3 of the spared column, increases quantal size, AMPA:NMDA ratio, and AMPA current rectification at L4-L2/3 synapses, relative to deprived columns and to animals with normal whisker experience. It also increases susceptibility to a selective antagonist of GluR2-lacking AMPA receptors, consistent with single whisker experience driving LTP at L4-L2/3 synapses by inserting GluR2-lacking AMPA receptors into synapses (Clem & Barth 2006). Very recently, response potentiation during single whisker experience has been partially blocked by GluR1 knockout and completely blocked by combined GluR1 and neuronal nitric oxide synthase (nNOS) knockout, suggesting that both NMDA-LTP and presynaptic, NO-dependent LTP are involved in response potentiation (Fox et al. 2007).
In V1, LTP involvement in response potentiation during monocular deprivation is less compelling. Several signaling molecules involved in long-lasting LTP are required for ocular dominance plasticity, including ERK, CREB, αCaMKII autophosphorylation, and PKA RIIα (Di Cristo et al. 2001, Mower et al. 2002, Taha et al. 2002, Rao et al. 2004). However, these molecules also participate in other signaling pathways, including neurotrophin signaling, which influence visual system development and critical period timing. Cortex-specific deletion of NR1 prevents response potentiation in adult V1, but whether this represents LTP is not clear (Sawtell et al. 2003). In addition, several manipulations that block LTP, including NO-dependent LTP, do not impair ocular dominance plasticity (Reid et al. 1996, Ruthazer et al. 1996, Daw et al. 2004, Hensch 2005, Hofer et al. 2006). Thus, unlike the case of visual stimulation-driven response potentiation (Frenkel et al. 2006), whether LTP is required for response potentiation during monocular deprivation is not clear. Several compelling alternatives exist, including homeostatic synaptic scaling and experience-dependent structural remodeling (discussed below).
LTP and learning-related plasticity in adults
In hippocampus and amygdala, specific molecular and physiological tools have provided direct evidence that LTP occurs during, and is required for, adult learning (Maren 2005, Sossin et al. 2008). In contrast, evidence for LTP in adult cortical learning remains incomplete. In rat primary motor cortex (M1), synapses on L2/3 horizontal pathways are strengthened by training on a forelimb reaching task, and this strengthening occludes and functionally resembles LTP (Rioult-Pedotti et al. 2000). However, the molecular basis for this strengthening and its role in learning are unknown. Strong evidence for LTP in adult cortical learning comes from V1, where presentation of temporally precise, flashed visual stimuli alters functional synaptic connectivity, visual receptive fields, and visual perception in a manner consistent with induction of spike timing–dependent LTP and LTD (discussed below) (Dan & Poo 2006). Whether LTP contributes to the many other forms of experience-dependent plasticity and perceptual learning in adult V1 is not known.
Evidence for LTP in learning in other neocortical areas remains weak. In piriform, prefrontal, and anterior cingulate cortex, sensory experience or training on specific learning tasks increases the functional strength of specific synapses, but whether this synaptic enhancement represents LTP is not known. In S1, pairing whisker stimulation with tail shock causes NMDA receptor–dependent expansion of trained whisker representations and an increase in the level and autophosphorylation of αCaMKII, suggestive of NMDA-LTP (Skibinska-Kijek et al. 2008).
Spike Timing–Dependent Plasticity and Learning Rules for Plasticity
In the past decade, a dizzying variety of LTP/LTD learning rules have been discovered that vary with cell type, synapse location on dendrites, background network activity, and neuromodulation (Sjostrom et al. 2008). Which learning rules are most relevant in vivo, and which spike train patterns or other aspects of neural activity trigger experience-dependent plasticity in vivo, remain largely unknown.
One learning rule that appears to mediate some types of experience-dependent plasticity in vivo is spike timing–dependent plasticity (STDP), in which the temporal sequence and interval between pre- and postsynaptic spikes drive plasticity. In classical STDP, pre-leading-post firing (0–20-ms interval) drives LTP, and post-leading-pre firing (0 to 20–50-ms interval) drives LTD, although STDP rules vary considerably across synapses and physiological states. STDP occurs at many neocortical synapses in vitro and can be induced experimentally in vivo by pairing sensory stimulation with precisely timed spikes (Meliza & Dan 2006, Jacob et al. 2007). STDP mechanisms are surprisingly diverse, involving NMDA-LTP and NMDA-LTD at some synapses (Froemke et al. 2005), NMDA-LTP and CB1-LTD at others (Sjostrom et al. 2003, Bender et al. 2006b, Nevian & Sakmann 2006) and mGluR-LTD at others (Egger et al. 1999). STDP has powerful, Hebbian-like computational properties that predict development and plasticity of sensory maps (Song & Abbott 2001). For detailed review of STDP, see Caporale & Dan (2008).
STDP is strongly implicated in one form of perceptual learning-related plasticity in V1. Sequentially flashing brief visual stimuli at two nearby retinotopic locations imposes specific spike timing on V1 neurons representing these locations. Repeated presentation of such stimuli to anesthetized adult cats at short, STDP-like intervals (~10 ms) alters the functional strength of synaptic connections between activated neurons and spatially shifts neuronal receptive fields in a manner consistent with STDP. In humans, the same conditioning procedure causes a shift in the perceived location of visual stimuli, again consistent with STDP (Fu et al. 2002). Similar conditioning with sequentially flashed oriented stimuli shifts V1 orientation tuning and alters perception of orientation (Yao & Dan 2001). Thus, STDP drives perceptual learning in V1 in response to appropriate timed visual stimuli. In A1, a similar conditioning procedure shifts frequency tuning of A1 neurons consistent with STDP (Dahmen et al. 2008). Other forms of perceptual learning that do not involve precise stimulus timing are less likely to involve STDP and may involve other mechanisms.
STDP may also drive LTD at L4-L2/3 synapses in response to sensory deprivation. L4-L2/3 synapses in S1 exhibit robust STDP in vitro (Feldman 2000, Nevian & Sakmann 2006) and undergo LTD in vivo in response to whisker deprivation (see above). During normal sensory responses in vivo, L4 neurons fire a few ms before cocolumnar L2/3 neurons, consistent with a serial relay of sensory information from L4 to L2/3. Simulated whisker deprivation acutely causes firing order to reverse, and firing to decorrelate, in deprived columns. These spike timing changes quantitatively predict STDP-LTD induction at L4-L2/3 synapses, suggesting that LTD is induced by STDP in vivo (Celikel et al. 2004). In V1, retinotopic plasticity following focal retinal lesions has also been reported to be more consistent with STDP than with standard correlation-dependent plasticity (Young et al. 2007).
Homeostatic Plasticity
Slower, non-Hebbian forms of plasticity exist that globally adjust synapse strength and neuronal excitability to maintain mean cellular activity at a set point level (Turrigiano & Nelson 2004). This homeostatic plasticity was discovered in cortical cultures in vitro, where experimentally increasing (or decreasing) network activity over hours to days causes a uniform, multiplicative decrease (or increase) in excitatory synapse strength, termed homeostatic synaptic scaling. Opposite plasticity occurs at some inhibitory synapses; here decreased activity causes a reduction in inhibitory synapse strength (Turrigiano & Nelson 2004). Intrinsic excitability, NMDAR content at synapses, and excitatory-inhibitory balance are also homeostatically regulated. Synaptic scaling is mediated by multiple cellular mechanisms that vary by cell type, time course, brain region, and developmental stage. In neocortex, scaling of excitatory synapses onto principal neurons is expressed primarily by regulating AMPA receptor insertion, similar to NMDA-LTP and LTD (Turrigiano & Nelson 2004). How cellular or network activity is read out to drive homeostatic plasticity is not clear. Recent work suggests that glial cells, which detect mean local network activity, trigger one form of synaptic scaling in hippocampus and visual cortex by secreting the cytokine tumor-necrosis factor-α (TNF-α) (Stellwagen & Malenka 2006, Kaneko et al. 2008b).
Homeostatic plasticity occurs in vivo and may explain homeostatic changes in sensory responses with substantial overuse or deprivation. In the monocular region of V1, contralateral eye closure dramatically reduces sensory activity and leads to increased network excitability and spontaneous firing (Desai et al. 2002, Maffei et al. 2004) and to increased visual responses to the closed eye (Mrsic-Flogel et al. 2007). Multiple homeostatic mechanisms are involved, including scaling up of excitatory synapse strength, cell-type specific changes in inhibitory circuits, and changes in intrinsic excitability, depending on the precise visual deprivation paradigm (Maffei & Turrigiano 2008). Synaptic scaling obeys layer-specific critical periods but persists in L2/3 through adulthood. Homeostatic synaptic and intrinsic plasticity occur in A1 with peripheral hearing loss (Kotak et al. 2005). In S1, synaptic scaling has not yet been observed, but sensory activation drives homeostatic changes in L4 inhibitory circuits (see below) and in glutamate transport by astrocytes (Genoud et al. 2006).
Homeostatic plasticity may also contribute to response potentiation to spared inputs during deprivation-induced map plasticity. In a homeostatic model, deprivation of a subset of inputs drives rapid Hebbian weakening on deprived pathways and more slowly drives a homeostatic increase in global synapse strength and/or intrinsic excitability, which increases responses to spared inputs (and partially offsets weakening of deprived inputs). Consistent with homeostasis, monocular deprivation slowly potentiates deprived-eye responses in neurons in binocular V1 that lack open-eye input, and binocular deprivation slowly potentiates responses to both eyes (Mrsic-Flogel et al. 2007). Moreover, knockout of TNF-α, which prevents homeostatic synaptic scaling in hippocampus, blocks potentiation of open-eye responses during ocular dominance plasticity (Kaneko et al. 2008b). Together, these findings strongly suggest that synaptic scaling is one mechanism driving potentiation of open-eye responses during ocular dominance plasticity.
Metaplasticity
A distinct class of plasticity is metaplasticity, that is, experience-dependent changes in synaptic plasticity rules themselves (Abraham & Bear 1996). In metaplasticity, experience-dependent alterations in inhibitory tone, dendritic excitability, NMDA receptor function, or neuromodulation alter the ability of future stimuli to drive LTP and LTD. In V1, visual experience regulates the capacity for LTP and LTD at L4-L2/3 synapses by regulating NMDA receptor subunit composition (Philpot et al. 2003, 2007). This form of metaplasticity is homeostatic: Visual deprivation biases LTP/LTD learning rules toward LTP so that subsequent activity tends to strengthen synapses and restore mean cortical activity. Such metaplasticity was hypothesized to counteract the inherently unstable, positive-feedback nature of Hebbian synaptic plasticity and may act during monocular deprivation to promote LTP by open-eye inputs, thereby driving response potentiation (Bienenstock et al. 1982, Bear et al. 1987). In S1, single whisker experience both drives NMDA-LTP at L4-L2/3 synapses and induces a form of metaplasticity in which a novel mGluR-LTP appears. This mGluR-LTP is required after initial potentiation to maintain synapse strength in vivo (Clem et al. 2008).
Plasticity of GABAergic Cells and Circuits
Although most research has focused on excitatory synapses and circuits as loci for cortical plasticity, recent findings demonstrate that GABAergic inhibitory neurons and circuits are highly plastic and play several important roles in sensory map plasticity.
Regulation of critical period timing
A major discovery in the past decade was that maturation of specific GABAergic neurons (large, parvalbumin-positive basket cells that make α1GABA-A receptor-containing synapses onto pyramidal neurons) regulates the onset of the critical period in V1 (for review, see Hensch 2005). How these cells control plasticity is not known but may involve setting a permissive excitatory-inhibitory balance or editing pyramidal cell firing patterns to promote excitatory synaptic plasticity.
GABAergic circuits and expression of receptive field plasticity
Both inhibitory synapses and excitatory synapses on inhibitory interneurons are capable of activity-dependent, long-term plasticity (Gaiarsa et al. 2002, Kullmann & Lamsa 2007), which may directly contribute to expression of receptive field plasticity in target neurons. One example of this process is in L4 of V1, where deprivation increases strength of inhibitory synapses from fast-spiking (FS) interneurons onto excitatory cells. This potentiation resembles and occludes LTP at FS to pyramidal cell synapses (LTPi). LTPi is induced by pairing FS cell spikes with subthreshold postsynaptic depolarization but is suppressed by postsynaptic spiking. Thus, visual deprivation may drive LTPi in vivo by reducing postsynaptic spiking, and this potentiation of inhibition may underlie depression of closed-eye responses in L4 during visual deprivation (Maffei et al. 2006). A different role for inhibitory plasticity is proposed in adult A1, where pairing of auditory stimuli with stimulation of the cholinergic nucleus basalis powerfully increases responses to paired tone frequencies. Pairing rapidly decreases inhibition evoked by the paired tone, prior to a gradual increase in tone-evoked excitation, which suggests that transient disinhibition to paired stimuli may provide a physiological “tag” that guides and drives subsequent modification of excitatory networks (Froemke et al. 2007).
Homeostatic plasticity of GABAergic circuits
GABAergic circuits show strong homeostatic plasticity. In S1, whisker deprivation reduces, and classical conditioning increases, levels of GABA, GAD, GABA-A receptors, and GABAergic puncta (reviewed in Foeller & Feldman 2004). Twenty-four hours of continuous whisker stimulation increases inhibitory synapse density and the ratio of inhibitory to excitatory synapses on spines in stimulated L4 barrels (Knott et al. 2002). This change is associated with a reduction in whisker-evoked spiking responses and therefore represents a homeostatic mechanism to decrease cortical activity in response to overstimulation. Conversely, whisker deprivation decreases the magnitude of inhibitory postsynaptic currents onto principal cells and preferentially reduces whisker-evoked activation of fast spiking interneurons (Jiao et al. 2006, Lee et al. 2007), which would enhance whisker responses. In V1, visual deprivation decreases sensory-evoked recruitment of inhibitory networks in L2/3 and homeostatically alters L4 and L2/3 inhibitory circuits to increase excitability (Gandhi et al. 2008, Maffei et al. 2004). These changes are appropriate to preserve both overall cortical activity and excitatory-inhibitory balance, which is tightly regulated to enable proper cortical function.
STRUCTURAL MECHANISMS FOR CORTICAL PLASTICITY
Ultimately, map plasticity is expressed by structural changes in macroscopic axonal projections including thalamocortical and horizontal, cross-columnar axons and, to a lesser extent, dendrites (reviewed in Fox & Wong 2005, Broser et al. 2007). These large-scale structural changes typically lag physiologically measured plasticity by several days or weeks (but can be rapid, see Trachtenberg & Stryker 2001). In contrast, very rapid structural changes (hours to days) occur continuously at the level of spines and synapses. For example, dendritic spines of L5 and L2/3 cortical pyramidal cells appear, disappear, and change shape on this time scale in vivo, and these dynamics are increased by sensory manipulations, including whisker and visual deprivation (Trachtenberg et al. 2002, Oray et al. 2004, Holtmaat et al. 2006, Knott et al. 2006). Spine formation and retraction are associated with synapse formation and elimination (Trachtenberg et al. 2002, Holtmaat et al. 2006). Spines are more dynamic in young adult mice (1–2 months) than in mature mice (4–5 months) and are more dynamic in mature S1 than in V1, paralleling developmental and area-specific capacities for experience-dependent plasticity (Alvarez & Sabatini 2007). Thus, rapid synapse formation and elimination may contribute to rapid components of experience-dependent plasticity. For detailed review, see Zito & Svoboda (2002), Feldman & Brecht (2005), and Alvarez & Sabatini (2007).
Researchers have debated whether structural or physiological synaptic plasticity is the primary mediator of map plasticity. One model of ocular dominance plasticity in V1 proposes that rapid components of plasticity are mediated entirely by structural rearrangement of synapses and spines (Hensch 2005). In support of this model, brief monocular deprivation increases spine dynamics (Oray et al. 2004) and alters spine number in binocular V1 (Mataga et al. 2004). Moreover, structural plasticity is limited by several factors, including chondroitin sulfate proteoglycans (CSPGs) on the extracellular matrix (ECM). Degradation of the ECM by the protease tissue-type plasminogen activator (tPA) occurs during, and is permissive for, ocular dominance plasticity. Enzymatic degradation of CSPGs and blockade of Nogo receptor signaling, which enable spine plasticity and neurite outgrowth, reactivate ocular dominance plasticity in adults (Berardi et al. 2003, Hensch 2005, McGee et al. 2005). How these structural changes implement ocular dominance plasticity is not known. However, a recent study found that monocular deprivation in adults increases spine dynamics and spine number in layer 5 neurons in binocular V1, which would be consistent with formation of excitatory synapses to mediate potentiation of open-eye responses (Hofer et al. 2008).
One prominent hypothesis for how experience drives structural changes and competitive features of cortical plasticity is the neurotrophic hypothesis for ocular dominance plasticity (Berardi et al. 2003). Neurotrophins, including NGF, BDNF, and NT-4, promote axon growth and dendritic proliferation. In the neurotrophic model, right- and left-eye axonal pathways compete in an activity-dependent manner for a limited supply of target-derived neurotrophins, enabling more active axons to extend and form more synapses. Consistent with this model, infusion of BDNF or NT-4 desegregates ocular dominance columns (Cabelli et al. 1995) and prevents ocular dominance plasticity (Gillespie et al. 2000, Lodovichi et al. 2000), and sequestration of endogenous ligands of trkB (the high-affinity receptor for BDNF and NT-4) prevents developmental segregation of columns (Cabelli et al. 1997). However, selective antagonism of trkB during monocular deprivation (by mutating trkB to confer susceptibility to a specific antagonist) does not prevent ocular dominance plasticity measured physiologically (Kaneko et al. 2008a). Thus, the role of neurotrophins in mediating competition between inputs remains unclear. In contrast, it is clear that BDNF does have a major role prior to the critical period in the development of cortical inhibitory circuits, thereby controlling critical period timing (Huang et al. 1999, Gianfranceschi et al. 2003).
A major unanswered question is how these synapse-scale structural changes relate to physiological plasticity of synapses and to macroscopic structural changes in axonal projections. Because spine plasticity can accompany experimentally induced LTP and LTD (Alvarez & Sabatini 2007), one model proposes that activity rapidly regulates existing synapse strength via LTP and LTD, leading to formation and removal of spines and synapses that effectively rewire cortical microcircuits. In turn, this rewiring may lead to slower, macroscopic changes in axons and dendrites (Cline & Haas 2008). However, whether structural modification is linked to LTP and LTD during experience-dependent cortical plasticity or is independent remains unknown. An alternative view is that experience first induces formation of new synapses, which then become substrates for functional selection by LTP and LTD in response to subsequent experience (Hofer et al. 2008).
TOWARD A MECHANISTIC MODEL OF CORTICAL PLASTICITY
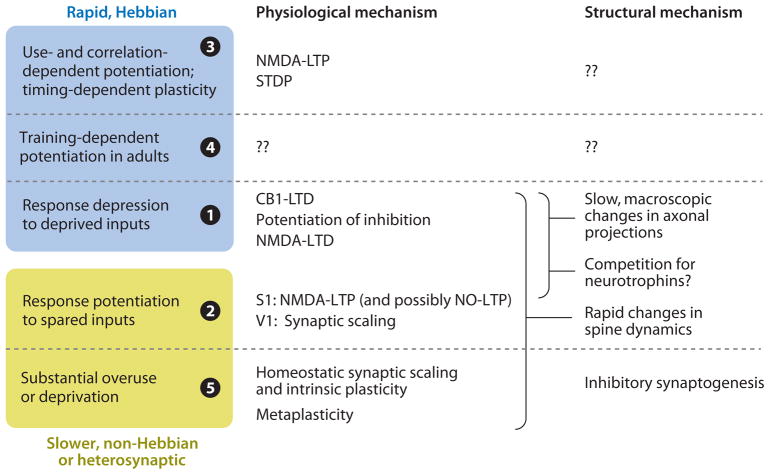
The studies summarized here support a model in which cortical plasticity consists of distinct functional components, each driven by a particular aspect of sensory experience and each involving a specific set of cellular mechanisms and sites of plasticity. Available data have begun to identify mechanisms that mediate each functional component. Figure 2 lists those mechanisms that currently have the most compelling experimental support, as summarized above. Laminar sites of plasticity are ignored here.
Figure 2.
Summary of physiological and structural plasticity mechanisms that have been experimentally linked with different components of experience-dependent plasticity. Additional undiscovered mechanisms are also likely. Blue box: rapid components of plasticity that are functionally consistent with homosynaptic, Hebbian plasticity. Yellow box: slower components that either are non-Hebbian in direction or require heterosynaptic competition between inputs. Numbers refer to plasticity components described in Figure 1. See text for citations and details.
As reviewed above, potentiation of responses during normal sensory use involves NMDA-LTP. Because of LTP’s cooperative nature, it seems likely that LTP also contributes to cooperative strengthening of temporally correlated inputs, but this remains unconfirmed. Response potentiation driven by precisely timed sensory stimuli occurs via STDP rules, suggesting that spike timing–dependent LTP and LTD are involved. Thus, use- and correlation-dependent response potentiation may involve LTP. How reward drives cortical plasticity during reinforcement-based conditioning remains mechanistically unknown. Response depression to deprived sensory inputs involves CB1-LTD in L2/3, potentiation of inhibition in L4, and perhaps NMDA-LTD. Response potentiation to spared inputs involves NMDA-LTP (and perhaps presynaptic, NO-dependent LTP) in S1 but in V1 is more strongly linked to homeostatic plasticity including synaptic scaling. Homeostatic plasticity inherently implements competition between inputs by strengthening spared pathways in response to deprivation of any one major input. In contrast, how deprivation of one pathway drives LTP of spared inputs is not known and may be secondary to metaplasticity or disinhibition (Bienenstock et al. 1982, Froemke et al. 2007). Whether S1 and V1 mechanisms for this component are really distinct, and why, is not known. Homeostatic responses to substantial overuse or underuse involve multiple cellular homeostatic mechanisms that regulate excitatory and inhibitory synapse strength and intrinsic excitability. GABAergic circuits show especially strong homeostatic regulation in vivo, both structurally and functionally, which may serve to maintain the precise balance between excitation and inhibition that is characteristic of sensory cortex. Deprivation also drives metaplasticity of LTP/LTD learning rules. Thus, a common theme is that rapid, homosynaptic components of plasticity involve Hebbian LTP and LTD at cortical synapses, whereas slower homeostatic or competitive components are likely to involve homeostatic cellular plasticity mechanisms and metaplasticity.
In addition to these physiological mechanisms for plasticity, rapid experience-dependent structural plasticity of spines and synapses is widespread and is therefore likely to play a major role in plasticity. A causal role for structural plasticity is clearest in V1, where ocular dominance plasticity is limited by endogenous factors that restrict structural plasticity of spines and synapses. However, it is not clear which types or sites of rapid structural plasticity mediate which specific functional components of plasticity, or whether neurotrophin signaling is a proximal driver of structural plasticity. The relationship of rapid spine-level structural plasticity to macroscopic changes in axonal projections and to synaptic physiological changes is also unknown.
In the past 10 years, sensitive physiological and anatomical techniques have revealed many novel sites and mechanisms for experience-driven plasticity, as well as many similarities and differences across cortical areas. These discoveries lead inescapably to the view that cortical plasticity involves multiple cellular mechanisms, each working at distinct synaptic loci, time scales, and developmental stages. However, this complexity raises a tremendous scientific challenge (Kim & Linden 2007): Will cellular mechanisms for experience-dependent plasticity be hopelessly numerous and idiosyncratic? Or will broad principles emerge? This review attempts to provide a first draft of the correspondence between cellular plasticity mechanisms and systems-level features of plasticity to determine if such principles exist.
Glossary
- N-methyl-D-aspartate (NMDA) receptor
a subtype of glutamate receptor that triggers induction of LTP and LTD at many synapses
- Long-term potentiation (LTP)
rapid, long-lasting increase in synaptic strength induced by a specific neural activity pattern; usually brief, strongly correlated pre- and postsynaptic activity
- Long-term depression (LTD)
rapid, long-lasting decrease in synaptic strength induced by a specific neural activity pattern; usually sustained, weakly correlated pre- and postsynaptic activity
- Hebbian synaptic plasticity
rapid, long-lasting changes in synapse strength, in which consistently correlated pre- and postsynaptic activity drives synapse strengthening, and weakly correlated activity drives synapse weakening
- S1
primary somatosensory cortex
- V1
primary visual cortex
- A1
primary auditory cortex
- Critical period
defined period within early postnatal development with a heightened or exclusive capacity for plasticity
- Homeostatic plasticity
a form of plasticity that acts to restore or maintain cellular or network activity at a set point level
- Heterosynaptic plasticity
synaptic plasticity in which activity at one set of synapses drives plasticity on a different set of synapses on the same postsynaptic cell
- Metaplasticity
a persistent change in cellular plasticity rules induced by prior activity or neuromodulation
- Homosynaptic plasticity
synaptic plasticity in which activity at one set of synapses drives plasticity at those same synapses
- α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor
subtype of glutamate receptor that mediates the bulk of fast excitatory transmission in the brain
- mGluR
metabotropic glutamate receptor
- CB1 receptor
type 1 cannabinoid receptor
- Endocannabinoid
neurotransmitter derived from membrane lipid precursors that activates cannabinoid and related receptors and regulates synaptic transmission
- STDP
spike timing–dependent plasticity
Footnotes
DISCLOSURE STATEMENT
The author is not aware of any biases that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- Abraham WC, Bear MF. Metaplasticity: the plasticity of synaptic plasticity. Trends Neurosci. 1996;19:126–30. doi: 10.1016/s0166-2236(96)80018-x. [DOI] [PubMed] [Google Scholar]
- Allen CB, Celikel T, Feldman DE. Long-term depression induced by sensory deprivation during cortical map plasticity in vivo. Nat Neurosci. 2003;6:291–99. doi: 10.1038/nn1012. [DOI] [PubMed] [Google Scholar]
- Alvarez VA, Sabatini BL. Anatomical and physiological plasticity of dendritic spines. Annu Rev Neurosci. 2007;30:79–97. doi: 10.1146/annurev.neuro.30.051606.094222. [DOI] [PubMed] [Google Scholar]
- Antonini A, Fagiolini M, Stryker MP. Anatomical correlates of functional plasticity in mouse visual cortex. J Neurosci. 1999;19:4388–406. doi: 10.1523/JNEUROSCI.19-11-04388.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonini A, Stryker MP. Rapid remodeling of axonal arbors in the visual cortex. Science. 1993;260:1819–21. doi: 10.1126/science.8511592. [DOI] [PubMed] [Google Scholar]
- Barbara JG, Auclair N, Roisin MP, Otani S, Valjent E, et al. Direct and indirect interactions between cannabinoid CB1 receptor and group II metabotropic glutamate receptor signaling in layer V pyramidal neurons from the rat prefrontal cortex. Eur J Neurosci. 2003;17:981–90. doi: 10.1046/j.1460-9568.2003.02533.x. [DOI] [PubMed] [Google Scholar]
- Bear MF, Cooper LN, Ebner FF. A physiological basis for a theory of synapse modification. Science. 1987;237:42–48. doi: 10.1126/science.3037696. [DOI] [PubMed] [Google Scholar]
- Bear MF, Kleinschmidt A, Gu QA, Singer W. Disruption of experience-dependent synaptic modifications in striate cortex by infusion of an NMDA receptor antagonist. J Neurosci. 1990;10:909–25. doi: 10.1523/JNEUROSCI.10-03-00909.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender KJ, Allen CB, Bender VA, Feldman DE. Synaptic basis for whisker deprivation-induced synaptic depression in rat somatosensory cortex. J Neurosci. 2006a;26:4155–65. doi: 10.1523/JNEUROSCI.0175-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender VA, Bender KJ, Brasier DJ, Feldman DE. Two coincidence detectors for spike timing-dependent plasticity in somatosensory cortex. J Neurosci. 2006b;26:4166–77. doi: 10.1523/JNEUROSCI.0176-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berardi N, Pizzorusso T, Ratto GM, Maffei L. Molecular basis of plasticity in the visual cortex. Trends Neurosci. 2003;26:369–78. doi: 10.1016/S0166-2236(03)00168-1. [DOI] [PubMed] [Google Scholar]
- Bienenstock EL, Cooper LN, Munro PW. Theory for the development of neuron selectivity: orientation specificity and binocular interaction in visual cortex. J Neurosci. 1982;2:32–48. doi: 10.1523/JNEUROSCI.02-01-00032.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake DT, Byl NN, Merzenich MM. Representation of the hand in the cerebral cortex. Behav Brain Res. 2002;135:179–84. doi: 10.1016/s0166-4328(02)00163-8. [DOI] [PubMed] [Google Scholar]
- Broser P, Grinevich V, Osten P, Sakmann B, Wallace DJ. Critical period plasticity of axonal arbors of layer 2/3 pyramidal neurons in rat somatosensory cortex: layer-specific reduction of projections into deprived cortical columns. Cereb Cortex. 2007;18:1588–603. doi: 10.1093/cercor/bhm189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonomano DV. Distinct functional types of associative long-term potentiation in neocortical and hippocampal pyramidal neurons. J Neurosci. 1999;19:6748–54. doi: 10.1523/JNEUROSCI.19-16-06748.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonomano DV, Merzenich MM. Cortical plasticity: from synapses to maps. Annu Rev Neurosci. 1998;21:149–86. doi: 10.1146/annurev.neuro.21.1.149. [DOI] [PubMed] [Google Scholar]
- Cabelli RJ, Hohn A, Shatz CJ. Inhibition of ocular dominance column formation by infusion of NT-4/5 or BDNF. Science. 1995;267:1662–66. doi: 10.1126/science.7886458. [DOI] [PubMed] [Google Scholar]
- Cabelli RJ, Shelton DL, Segal RA, Shatz CJ. Blockade of endogenous ligands of trkB inhibits formation of ocular dominance columns. Neuron. 1997;19:63–76. doi: 10.1016/s0896-6273(00)80348-7. [DOI] [PubMed] [Google Scholar]
- Caporale N, Dan Y. Spike timing-dependent plasticity: a Hebbian learning rule. Annu Rev Neurosci. 2008;31:25–46. doi: 10.1146/annurev.neuro.31.060407.125639. [DOI] [PubMed] [Google Scholar]
- Castro-Alamancos MA, Donoghue JP, Connors BW. Different forms of synaptic plasticity in somatosensory and motor areas of the neocortex. J Neurosci. 1995;15:5324–33. doi: 10.1523/JNEUROSCI.15-07-05324.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celikel T, Szostak VA, Feldman DE. Modulation of spike timing by sensory deprivation during induction of cortical map plasticity. Nat Neurosci. 2004;7:534–41. doi: 10.1038/nn1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheetham CE, Hammond MS, Edwards CE, Finnerty GT. Sensory experience alters cortical connectivity and synaptic function site specifically. J Neurosci. 2007;27:3456–65. doi: 10.1523/JNEUROSCI.5143-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevaleyre V, Takahashi KA, Castillo PE. Endocannabinoid-mediated synaptic plasticity in the CNS. Annu Rev Neurosci. 2006;29:37–76. doi: 10.1146/annurev.neuro.29.051605.112834. [DOI] [PubMed] [Google Scholar]
- Chklovskii DB, Mel BW, Svoboda K. Cortical rewiring and information storage. Nature. 2004;431:782–88. doi: 10.1038/nature03012. [DOI] [PubMed] [Google Scholar]
- Clem RL, Barth A. Pathway-specific trafficking of native AMPARs by in vivo experience. Neuron. 2006;49:663–70. doi: 10.1016/j.neuron.2006.01.019. [DOI] [PubMed] [Google Scholar]
- Clem RL, Celikel T, Barth AL. Ongoing in vivo experience triggers synaptic metaplasticity in the neocortex. Science. 2008;319:101–4. doi: 10.1126/science.1143808. [DOI] [PubMed] [Google Scholar]
- Cline H, Haas K. The regulation of dendritic arbor development and plasticity by glutamatergic synaptic input: a review of the synaptotrophic hypothesis. J Physiol. 2008;586:1509–17. doi: 10.1113/jphysiol.2007.150029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crair MC, Malenka RC. A critical period for long-term potentiation at thalamocortical synapses. Nature. 1995;375:325–28. doi: 10.1038/375325a0. [DOI] [PubMed] [Google Scholar]
- Crozier RA, Wang Y, Liu CH, Bear MF. Deprivation-induced synaptic depression by distinct mechanisms in different layers of mouse visual cortex. Proc Natl Acad Sci USA. 2007;104:1383–88. doi: 10.1073/pnas.0609596104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czarnecki A, Birtoli B, Ulrich D. Cellular mechanisms of burst firing-mediated long-term depression in rat neocortical pyramidal cells. J Physiol. 2007;578:471–79. doi: 10.1113/jphysiol.2006.123588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahmen JC, Hartley DE, King AJ. Stimulus-timing-dependent plasticity of cortical frequency representation. J Neurosci. 2008;28:13629–39. doi: 10.1523/JNEUROSCI.4429-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dan Y, Poo MM. Spike timing-dependent plasticity: from synapse to perception. Physiol Rev. 2006;86:1033–48. doi: 10.1152/physrev.00030.2005. [DOI] [PubMed] [Google Scholar]
- Daw N, Rao Y, Wang XF, Fischer Q, Yang Y. LTP and LTD vary with layer in rodent visual cortex. Vision Res. 2004;44:3377–80. doi: 10.1016/j.visres.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Daw NW, Fox K, Sato H, Czepita D. Critical period for monocular deprivation in the cat visual cortex. J Neurophysiol. 1992;67:197–202. doi: 10.1152/jn.1992.67.1.197. [DOI] [PubMed] [Google Scholar]
- Deng PY, Lei S. Long-term depression in identified stellate neurons of juvenile rat entorhinal cortex. J Neurophysiol. 2007;97:727–37. doi: 10.1152/jn.01089.2006. [DOI] [PubMed] [Google Scholar]
- Desai NS, Cudmore RH, Nelson SB, Turrigiano GG. Critical periods for experience-dependent synaptic scaling in visual cortex. Nat Neurosci. 2002;5:783–89. doi: 10.1038/nn878. [DOI] [PubMed] [Google Scholar]
- Diamond ME, Armstrong-James M, Ebner FF. Experience-dependent plasticity in adult rat barrel cortex. Proc Natl Acad Sci USA. 1993;90:2082–86. doi: 10.1073/pnas.90.5.2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond ME, Huang W, Ebner FF. Laminar comparison of somatosensory cortical plasticity. Science. 1994;265:1885–88. doi: 10.1126/science.8091215. [DOI] [PubMed] [Google Scholar]
- Di Cristo G, Berardi N, Cancedda L, Pizzorusso T, Putignano E, et al. Requirement of ERK activation for visual cortical plasticity. Science. 2001;292:2337–40. doi: 10.1126/science.1059075. [DOI] [PubMed] [Google Scholar]
- Dodt H, Eder M, Frick A, Zieglgansberger W. Precisely localized LTD in the neocortex revealed by infrared-guided laser stimulation. Science. 1999;286:110–13. doi: 10.1126/science.286.5437.110. [DOI] [PubMed] [Google Scholar]
- Drew PJ, Feldman DE. Intrinsic signal imaging of deprivation-induced contraction of whisker representations in rat somatosensory cortex. Cereb Cortex. 2009;19:331–48. doi: 10.1093/cercor/bhn085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eder M, Zieglgansberger W, Dodt HU. Neocortical long-term potentiation and long-term depression: site of expression investigated by infrared-guided laser stimulation. J Neurosci. 2002;22:7558–68. doi: 10.1523/JNEUROSCI.22-17-07558.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger V, Feldmeyer D, Sakmann B. Coincidence detection and changes of synaptic efficacy in spiny stellate neurons in rat barrel cortex. Nat Neurosci. 1999;2:1098–105. doi: 10.1038/16026. [DOI] [PubMed] [Google Scholar]
- Ehrlich I, Malinow R. Postsynaptic density 95 controls AMPA receptor incorporation during long-term potentiation and experience-driven synaptic plasticity. J Neurosci. 2004;24:916–27. doi: 10.1523/JNEUROSCI.4733-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman DE. Timing-based LTP and LTD at vertical inputs to layer II/III pyramidal cells in rat barrel cortex. Neuron. 2000;27:45–56. doi: 10.1016/s0896-6273(00)00008-8. [DOI] [PubMed] [Google Scholar]
- Feldman DE, Brecht M. Map plasticity in somatosensory cortex. Science. 2005;310:810–15. doi: 10.1126/science.1115807. [DOI] [PubMed] [Google Scholar]
- Feldman DE, Nicoll RA, Malenka RC. Synaptic plasticity at thalamocortical synapses in developing rat somatosensory cortex: LTP, LTD, and silent synapses. J Neurobiol. 1999;41:92–101. [PubMed] [Google Scholar]
- Feldman DE, Nicoll RA, Malenka RC, Isaac JT. Long-term depression at thalamocortical synapses in developing rat somatosensory cortex. Neuron. 1998;21:347–57. doi: 10.1016/s0896-6273(00)80544-9. [DOI] [PubMed] [Google Scholar]
- Finnerty GT, Roberts LS, Connors BW. Sensory experience modifies the short-term dynamics of neocortical synapses. Nature. 1999;400:367–71. doi: 10.1038/22553. [DOI] [PubMed] [Google Scholar]
- Fischer QS, Beaver CJ, Yang Y, Rao Y, Jakobsdottir KB, et al. Requirement for the RIIbeta isoform of PKA, but not calcium-stimulated adenylyl cyclase, in visual cortical plasticity. J Neurosci. 2004;24:9049–58. doi: 10.1523/JNEUROSCI.2409-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foeller E, Feldman DE. Synaptic basis for developmental plasticity in somatosensory cortex. Curr Opin Neurobiol. 2004;14:89–95. doi: 10.1016/j.conb.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Fox K. A critical period for experience-dependent synaptic plasticity in rat barrel cortex. J Neurosci. 1992;12:1826–38. doi: 10.1523/JNEUROSCI.12-05-01826.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox K. Anatomical pathways and molecular mechanisms for plasticity in the barrel cortex. Neuroscience. 2002;111:799–814. doi: 10.1016/s0306-4522(02)00027-1. [DOI] [PubMed] [Google Scholar]
- Fox K, Dachtler J, Wright N. The role of nitric oxide and GluR1 in experience-dependent plasticity in barrel cortex. Soc Neurosci Abstr. 2007:48.11. [Google Scholar]
- Fox K, Wong RO. A comparison of experience-dependent plasticity in the visual and somatosensory systems. Neuron. 2005;48:465–77. doi: 10.1016/j.neuron.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Frenkel MY, Bear MF. How monocular deprivation shifts ocular dominance in visual cortex of young mice. Neuron. 2004;44:917–23. doi: 10.1016/j.neuron.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Frenkel MY, Sawtell NB, Diogo AC, Yoon B, Neve RL, Bear MF. Instructive effect of visual experience in mouse visual cortex. Neuron. 2006;51:339–49. doi: 10.1016/j.neuron.2006.06.026. [DOI] [PubMed] [Google Scholar]
- Froemke RC, Dan Y. Spike-timing-dependent synaptic modification induced by natural spike trains. Nature. 2002;416:433–38. doi: 10.1038/416433a. [DOI] [PubMed] [Google Scholar]
- Froemke RC, Merzenich MM, Schreiner CE. A synaptic memory trace for cortical receptive field plasticity. Nature. 2007;450:425–29. doi: 10.1038/nature06289. [DOI] [PubMed] [Google Scholar]
- Froemke RC, Poo MM, Dan Y. Spike-timing-dependent synaptic plasticity depends on dendritic location. Nature. 2005;434:221–25. doi: 10.1038/nature03366. [DOI] [PubMed] [Google Scholar]
- Fu YX, Djupsund K, Gao H, Hayden B, Shen K, Dan Y. Temporal specificity in the cortical plasticity of visual space representation. Science. 2002;296:1999–2003. doi: 10.1126/science.1070521. [DOI] [PubMed] [Google Scholar]
- Gaiarsa JL, Caillard O, Ben-Ari Y. Long-term plasticity at GABAergic and glycinergic synapses: mechanisms and functional significance. Trends Neurosci. 2002;25:564–70. doi: 10.1016/s0166-2236(02)02269-5. [DOI] [PubMed] [Google Scholar]
- Gandhi SP, Yanagawa Y, Stryker MP. Delayed plasticity of inhibitory neurons in developing visual cortex. Proc Natl Acad Sci USA. 2008;105:16797–802. doi: 10.1073/pnas.0806159105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genoud C, Quairiaux C, Steiner P, Hirling H, Welker E, Knott GW. Plasticity of astrocytic coverage and glutamate transporter expression in adult mouse cortex. PLoS Biol. 2006;4:e343. doi: 10.1371/journal.pbio.0040343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianfranceschi L, Siciliano R, Walls J, Morales B, Kirkwood A, et al. Visual cortex is rescued from the effects of dark rearing by overexpression of BDNF. Proc Natl Acad Sci USA. 2003;100:12486–91. doi: 10.1073/pnas.1934836100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert CD. Adult cortical dynamics. Physiol Rev. 1998;78:467–85. doi: 10.1152/physrev.1998.78.2.467. [DOI] [PubMed] [Google Scholar]
- Gillespie DC, Crair MC, Stryker MP. Neurotrophin-4/5 alters responses and blocks the effect of monocular deprivation in cat visual cortex during the critical period. J Neurosci. 2000;20:9174–86. doi: 10.1523/JNEUROSCI.20-24-09174.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazewski S, Barth AL, Wallace H, McKenna M, Silva A, Fox K. Impaired experience-dependent plasticity in barrel cortex of mice lacking the alpha and delta isoforms of CREB. Cereb Cortex. 1999;9:249–56. doi: 10.1093/cercor/9.3.249. [DOI] [PubMed] [Google Scholar]
- Glazewski S, Chen CM, Silva A, Fox K. Requirement for alpha-CaMKII in experience-dependent plasticity of the barrel cortex. Science. 1996;272:421–23. doi: 10.1126/science.272.5260.421. [DOI] [PubMed] [Google Scholar]
- Glazewski S, Fox K. Time course of experience-dependent synaptic potentiation and depression in barrel cortex of adolescent rats. J Neurophysiol. 1996;75:1714–29. doi: 10.1152/jn.1996.75.4.1714. [DOI] [PubMed] [Google Scholar]
- Glazewski S, Giese KP, Silva A, Fox K. The role of alpha-CaMKII autophosphorylation in neocortical experience-dependent plasticity. Nat Neurosci. 2000;3:911–18. doi: 10.1038/78820. [DOI] [PubMed] [Google Scholar]
- Glazewski S, McKenna M, Jacquin M, Fox K. Experience-dependent depression of vibrissae responses in adolescent rat barrel cortex. Eur J Neurosci. 1998;10:2107–16. doi: 10.1046/j.1460-9568.1998.00222.x. [DOI] [PubMed] [Google Scholar]
- Griffiths S, Scott H, Glover C, Bienemann A, Ghorbel MT, et al. Expression of long-term depression underlies visual recognition memory. Neuron. 2008;58:186–94. doi: 10.1016/j.neuron.2008.02.022. [DOI] [PubMed] [Google Scholar]
- Han YK, Kover H, Insanally MN, Semerdjian JH, Bao S. Early experience impairs perceptual discrimination. Nat Neurosci. 2007;10:1191–97. doi: 10.1038/nn1941. [DOI] [PubMed] [Google Scholar]
- Hanover JL, Huang ZJ, Tonegawa S, Stryker MP. Brain-derived neurotrophic factor overexpression induces precocious critical period in mouse visual cortex. J Neurosci. 1999;19:RC40. doi: 10.1523/JNEUROSCI.19-22-j0003.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham N, Fox K. The role of nitric oxide and GluR1 in presynaptic and postsynaptic components of neocortical potentiation. J Neurosci. 2006;26:7395–404. doi: 10.1523/JNEUROSCI.0652-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham N, Glazewski S, Pakhotin P, Mizuno K, Chapman PF, et al. Neocortical long-term potentiation and experience-dependent synaptic plasticity require alpha-calcium/calmodulin-dependent protein kinase II autophosphorylation. J Neurosci. 2003;23:4428–36. doi: 10.1523/JNEUROSCI.23-11-04428.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebb DO. The Organization of Behavior. New York: Wiley; 1949. [Google Scholar]
- Hensch TK. Critical period plasticity in local cortical circuits. Nat Rev Neurosci. 2005;6:877–88. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- Heynen AJ, Yoon BJ, Liu CH, Chung HJ, Huganir RL, Bear MF. Molecular mechanism for loss of visual cortical responsiveness following brief monocular deprivation. Nat Neurosci. 2003;6:854–62. doi: 10.1038/nn1100. [DOI] [PubMed] [Google Scholar]
- Hofer SB, Mrsic-Flogel TD, Bonhoeffer T, Hubener M. Lifelong learning: ocular dominance plasticity in mouse visual cortex. Curr Opin Neurobiol. 2006;16:451–59. doi: 10.1016/j.conb.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Hofer SB, Mrsic-Flogel TD, Bonhoeffer T, Hübener M. Experience leaves a lasting structural trace in cortical circuits. Nature. 2008 doi: 10.1038/nature07487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtmaat A, Wilbrecht L, Knott GW, Welker E, Svoboda K. Experience-dependent and cell-type-specific spine growth in the neocortex. Nature. 2006;441:979–83. doi: 10.1038/nature04783. [DOI] [PubMed] [Google Scholar]
- Huang ZJ, Kirkwood A, Pizzorusso T, Porciatti V, Morales B, et al. BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell. 1999;98:739–55. doi: 10.1016/s0092-8674(00)81509-3. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Early exploration of the visual cortex. Neuron. 1998;20:401–12. doi: 10.1016/s0896-6273(00)80984-8. [DOI] [PubMed] [Google Scholar]
- Isaac JT, Crair MC, Nicoll RA, Malenka RC. Silent synapses during development of thalamocortical inputs. Neuron. 1997;18:269–80. doi: 10.1016/s0896-6273(00)80267-6. [DOI] [PubMed] [Google Scholar]
- Jacob V, Brasier DJ, Erchova I, Feldman D, Shulz DE. Spike timing-dependent synaptic depression in the in vivo barrel cortex of the rat. J Neurosci. 2007;27:1271–84. doi: 10.1523/JNEUROSCI.4264-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James W. The Principles of Psychology. New York: Holt; 1890. [Google Scholar]
- Jiang B, Trevino M, Kirkwood A. Sequential development of long-term potentiation and depression in different layers of the mouse visual cortex. J Neurosci. 2007;27:9648–52. doi: 10.1523/JNEUROSCI.2655-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao Y, Zhang C, Yanagawa Y, Sun QQ. Major effects of sensory experiences on the neocortical inhibitory circuits. J Neurosci. 2006;26:8691–701. doi: 10.1523/JNEUROSCI.2478-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko M, Hanover JL, England PM, Stryker MP. TrkB kinase is required for recovery, but not loss, of cortical responses following monocular deprivation. Nat Neurosci. 2008a;11:497–504. doi: 10.1038/nn2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko M, Stellwagen D, Malenka RC, Stryker MP. Tumor necrosis factor-alpha mediates one component of competitive, experience-dependent plasticity in developing visual cortex. Neuron. 2008b;58:673–80. doi: 10.1016/j.neuron.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmarkar UR, Dan Y. Experience-dependent plasticity in adult visual cortex. Neuron. 2006;52:577–85. doi: 10.1016/j.neuron.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–38. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- Kemp N, Bashir ZI. Long-term depression: a cascade of induction and expression mechanisms. Prog Neurobiol. 2001;65:339–65. doi: 10.1016/s0301-0082(01)00013-2. [DOI] [PubMed] [Google Scholar]
- Keuroghlian AS, Knudsen EI. Adaptive auditory plasticity in developing and adult animals. Prog Neurobiol. 2007;82:109–21. doi: 10.1016/j.pneurobio.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Linden DJ. Ubiquitous plasticity and memory storage. Neuron. 2007;56:582–92. doi: 10.1016/j.neuron.2007.10.030. [DOI] [PubMed] [Google Scholar]
- Kirkwood A, Bear MF. Hebbian synapses in visual cortex. J Neurosci. 1994;14:1634–45. doi: 10.1523/JNEUROSCI.14-03-01634.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knott GW, Holtmaat A, Wilbrecht L, Welker E, Svoboda K. Spine growth precedes synapse formation in the adult neocortex in vivo. Nat Neurosci. 2006;9:1117–24. doi: 10.1038/nn1747. [DOI] [PubMed] [Google Scholar]
- Knott GW, Quairiaux C, Genoud C, Welker E. Formation of dendritic spines with GABAergic synapses induced by whisker stimulation in adult mice. Neuron. 2002;34:265–73. doi: 10.1016/s0896-6273(02)00663-3. [DOI] [PubMed] [Google Scholar]
- Konorski J. Conditioned Reflexes and Neuron Organization. Cambridge, UK: Cambridge Univ. Press; 1948. [Google Scholar]
- Kotak VC, Fujisawa S, Lee FA, Karthikeyan O, Aoki C, Sanes DH. Hearing loss raises excitability in the auditory cortex. J Neurosci. 2005;25:3908–18. doi: 10.1523/JNEUROSCI.5169-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullmann DM, Lamsa KP. Long-term synaptic plasticity in hippocampal interneurons. Nat Rev Neurosci. 2007;8:687–99. doi: 10.1038/nrn2207. [DOI] [PubMed] [Google Scholar]
- Lafourcade M, Elezgarai I, Mato S, Bakiri Y, Grandes P, Manzoni OJ. Molecular components and functions of the endocannabinoid system in mouse prefrontal cortex. PLoS ONE. 2007;2:e709. doi: 10.1371/journal.pone.0000709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Land PW, Simons DJ. Layer- and cell-type-specific effects of neonatal whisker-trimming in adult rat barrel cortex. J Neurophysiol. 2007;97:4380–85. doi: 10.1152/jn.01217.2006. [DOI] [PubMed] [Google Scholar]
- Li L, Sylwestrak E, Drew PJ, Feldman DE. Endocannabinoid signaling is required for normal development and deprivation-induced plasticity of the whisker map in the rat’s somatosensory (barrel) cortex. Soc Neurosci Abstr. 2007:134.18. [Google Scholar]
- Liu CH, Heynen AJ, Shuler MG, Bear MF. Cannabinoid receptor blockade reveals parallel plasticity mechanisms in different layers of mouse visual cortex. Neuron. 2008;58:340–45. doi: 10.1016/j.neuron.2008.02.020. [DOI] [PubMed] [Google Scholar]
- Lodovichi C, Berardi N, Pizzorusso T, Maffei L. Effects of neurotrophins on cortical plasticity: same or different? J Neurosci. 2000;20:2155–65. doi: 10.1523/JNEUROSCI.20-06-02155.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubke J, Feldmeyer D. Excitatory signal flow and connectivity in a cortical column: focus on barrel cortex. Brain Struct Funct. 2007;212:3–17. doi: 10.1007/s00429-007-0144-2. [DOI] [PubMed] [Google Scholar]
- Maffei A, Nataraj K, Nelson SB, Turrigiano GG. Potentiation of cortical inhibition by visual deprivation. Nature. 2006;443:81–84. doi: 10.1038/nature05079. [DOI] [PubMed] [Google Scholar]
- Maffei A, Nelson SB, Turrigiano GG. Selective reconfiguration of layer 4 visual cortical circuitry by visual deprivation. Nat Neurosci. 2004;7:1353–59. doi: 10.1038/nn1351. [DOI] [PubMed] [Google Scholar]
- Maffei A, Turrigiano GG. Multiple modes of network homeostasis in visual cortical layer 2/3. J Neurosci. 2008;28:4377–84. doi: 10.1523/JNEUROSCI.5298-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci. 2002;25:103–26. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- Maren S. Synaptic mechanisms of associative memory in the amygdala. Neuron. 2005;47:783–86. doi: 10.1016/j.neuron.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Markram H, Lubke J, Frotscher M, Sakmann B. Regulation of synaptic efficacy by coincidence of postsynaptic APs and EPSPs. Science. 1997;275:213–15. doi: 10.1126/science.275.5297.213. [DOI] [PubMed] [Google Scholar]
- Markram H, Tsodyks M. Redistribution of synaptic efficacy between neocortical pyramidal neurons. Nature. 1996;382:807–10. doi: 10.1038/382807a0. [DOI] [PubMed] [Google Scholar]
- Massey PV, Bashir ZI. Long-term depression: multiple forms and implications for brain function. Trends Neurosci. 2007;30:176–84. doi: 10.1016/j.tins.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Mataga N, Mizuguchi Y, Hensch TK. Experience-dependent pruning of dendritic spines in visual cortex by tissue plasminogen activator. Neuron. 2004;44:1031–41. doi: 10.1016/j.neuron.2004.11.028. [DOI] [PubMed] [Google Scholar]
- McGee AW, Yang Y, Fischer QS, Daw NW, Strittmatter SM. Experience-driven plasticity of visual cortex limited by myelin and Nogo receptor. Science. 2005;309:2222–26. doi: 10.1126/science.1114362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meliza CD, Dan Y. Receptive-field modification in rat visual cortex induced by paired visual stimulation and single-cell spiking. Neuron. 2006;49:183–89. doi: 10.1016/j.neuron.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Mower AF, Liao DS, Nestler EJ, Neve RL, Ramoa AS. cAMP/Ca2+ response element-binding protein function is essential for ocular dominance plasticity. J Neurosci. 2002;22:2237–45. doi: 10.1523/JNEUROSCI.22-06-02237.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrsic-Flogel TD, Hofer SB, Ohki K, Reid RC, Bonhoeffer T, Hubener M. Homeostatic regulation of eye-specific responses in visual cortex during ocular dominance plasticity. Neuron. 2007;54:961–72. doi: 10.1016/j.neuron.2007.05.028. [DOI] [PubMed] [Google Scholar]
- Nevian T, Sakmann B. Spine Ca2+ signaling in spike-timing-dependent plasticity. J Neurosci. 2006;26:11001–13. doi: 10.1523/JNEUROSCI.1749-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oray S, Majewska A, Sur M. Dendritic spine dynamics are regulated by monocular deprivation and extracellular matrix degradation. Neuron. 2004;44:1021–30. doi: 10.1016/j.neuron.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Philpot BD, Cho KK, Bear MF. Obligatory role of NR2 A for metaplasticity in visual cortex. Neuron. 2007;53:495–502. doi: 10.1016/j.neuron.2007.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philpot BD, Espinosa JS, Bear MF. Evidence for altered NMDA receptor function as a basis for metaplasticity in visual cortex. J Neurosci. 2003;23:5583–88. doi: 10.1523/JNEUROSCI.23-13-05583.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao Y, Fischer QS, Yang Y, McKnight GS, LaRue A, Daw NW. Reduced ocular dominance plasticity and long-term potentiation in the developing visual cortex of protein kinase A RII alpha mutant mice. Eur J Neurosci. 2004;20:837–42. doi: 10.1111/j.1460-9568.2004.03499.x. [DOI] [PubMed] [Google Scholar]
- Rauschecker JP. Cortical map plasticity in animals and humans. Prog Brain Res. 2002;138:73–88. doi: 10.1016/S0079-6123(02)38072-5. [DOI] [PubMed] [Google Scholar]
- Reid SN, Daw NW, Czepita D, Flavin HJ, Sessa WC. Inhibition of nitric oxide synthase does not alter ocular dominance shifts in kitten visual cortex. J Physiol. 1996;494:511–17. doi: 10.1113/jphysiol.1996.sp021509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rema V, Armstrong-James M, Ebner FF. Experience-dependent plasticity of adult rat S1 cortex requires local NMDA receptor activation. J Neurosci. 1998;18:10,196–206. doi: 10.1523/JNEUROSCI.18-23-10196.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renger JJ, Hartman KN, Tsuchimoto Y, Yokoi M, Nakanishi S, Hensch TK. Experience-dependent plasticity without long-term depression by type 2 metabotropic glutamate receptors in developing visual cortex. Proc Natl Acad Sci USA. 2002;99:1041–46. doi: 10.1073/pnas.022618799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rioult-Pedotti MS, Friedman D, Donoghue JP. Learning-induced LTP in neocortex. Science. 2000;290:533–36. doi: 10.1126/science.290.5491.533. [DOI] [PubMed] [Google Scholar]
- Rittenhouse CD, Shouval HZ, Paradiso MA, Bear MF. Monocular deprivation induces homosynaptic long-term depression in visual cortex. Nature. 1999;397:347–50. doi: 10.1038/16922. [DOI] [PubMed] [Google Scholar]
- Roberts EB, Meredith MA, Ramoa AS. Suppression of NMDA receptor function using antisense DNA block ocular dominance plasticity while preserving visual responses. J Neurophysiol. 1998;80:1021–32. doi: 10.1152/jn.1998.80.3.1021. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Moreno A, Paulsen O. Spike timing-dependent long-term depression requires presynaptic NMDA receptors. Nat Neurosci. 2008;11:744–45. doi: 10.1038/nn.2125. [DOI] [PubMed] [Google Scholar]
- Rumpel S, Hatt H, Gottmann K. Silent synapses in the developing rat visual cortex: evidence for postsynaptic expression of synaptic plasticity. J Neurosci. 1998;18:8863–74. doi: 10.1523/JNEUROSCI.18-21-08863.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruthazer ES, Gillespie DC, Dawson TM, Snyder SH, Stryker MP. Inhibition of nitric oxide synthase does not prevent ocular dominance plasticity in kitten visual cortex. J Physiol. 1996;494(Pt. 2):519–27. doi: 10.1113/jphysiol.1996.sp021510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M, Stryker MP. Distinctive features of adult ocular dominance plasticity. J Neurosci. 2008;28:10278–86. doi: 10.1523/JNEUROSCI.2451-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawtell NB, Frenkel MY, Philpot BD, Nakazawa K, Tonegawa S, Bear MF. NMDA receptor-dependent ocular dominance plasticity in adult visual cortex. Neuron. 2003;38:977–85. doi: 10.1016/s0896-6273(03)00323-4. [DOI] [PubMed] [Google Scholar]
- Shepherd GM, Pologruto TA, Svoboda K. Circuit analysis of experience-dependent plasticity in the developing rat barrel cortex. Neuron. 2003;38:277–89. doi: 10.1016/s0896-6273(03)00152-1. [DOI] [PubMed] [Google Scholar]
- Shi S, Hayashi Y, Esteban JA, Malinow R. Subunit-specific rules governing AMPA receptor trafficking to synapses in hippocampal pyramidal neurons. Cell. 2001;105:331–43. doi: 10.1016/s0092-8674(01)00321-x. [DOI] [PubMed] [Google Scholar]
- Siucinska E, Kossut M. Short-lasting classical conditioning induces reversible changes of representational maps of vibrissae in mouse SI cortex—a 2DG study. Cereb Cortex. 1996;6:506–13. doi: 10.1093/cercor/6.3.506. [DOI] [PubMed] [Google Scholar]
- Siucinska E, Kossut M. Experience-dependent changes in cortical whisker representation in the adult mouse: a 2-deoxyglucose study. Neuroscience. 2004;127:961–71. doi: 10.1016/j.neuroscience.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Sjostrom PJ, Rancz EA, Roth A, Hausser M. Dendritic excitability and synaptic plasticity. Physiol Rev. 2008;88:769–840. doi: 10.1152/physrev.00016.2007. [DOI] [PubMed] [Google Scholar]
- Sjostrom PJ, Turrigiano GG, Nelson SB. Rate, timing, and cooperativity jointly determine cortical synaptic plasticity. Neuron. 2001;32:1149–64. doi: 10.1016/s0896-6273(01)00542-6. [DOI] [PubMed] [Google Scholar]
- Sjostrom PJ, Turrigiano GG, Nelson SB. Neocortical LTD via coincident activation of presynaptic NMDA and cannabinoid receptors. Neuron. 2003;39:641–54. doi: 10.1016/s0896-6273(03)00476-8. [DOI] [PubMed] [Google Scholar]
- Skibinska-Kijek A, Radwanska A, Kossut M. Alpha calcium/calmodulin dependent protein kinase II in learning-dependent plasticity of mouse somatosensory cortex. Neuroscience. 2008;151:750–57. doi: 10.1016/j.neuroscience.2007.11.012. [DOI] [PubMed] [Google Scholar]
- Song S, Abbott LF. Cortical development and remapping through spike timing-dependent plasticity. Neuron. 2001;32:339–50. doi: 10.1016/s0896-6273(01)00451-2. [DOI] [PubMed] [Google Scholar]
- Sossin WS, Lacaille J-C, Castellucci VF, Belleville S, editors. Essence of Memory. Vol. 169. Oxford, UK: Elsevier; 2008. [DOI] [PubMed] [Google Scholar]
- Stellwagen D, Malenka RC. Synaptic scaling mediated by glial TNF-alpha. Nature. 2006;440:1054–59. doi: 10.1038/nature04671. [DOI] [PubMed] [Google Scholar]
- Stent GS. A physiological mechanism for Hebb’s postulate of learning. Proc Natl Acad Sci USA. 1973;70:997–1001. doi: 10.1073/pnas.70.4.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern EA, Maravall M, Svoboda K. Rapid development and plasticity of layer 2/3 maps in rat barrel cortex in vivo. Neuron. 2001;31:305–15. doi: 10.1016/s0896-6273(01)00360-9. [DOI] [PubMed] [Google Scholar]
- Taha S, Hanover JL, Silva AJ, Stryker MP. Autophosphorylation of alphaCaMKII is required for ocular dominance plasticity. Neuron. 2002;36:483–91. doi: 10.1016/s0896-6273(02)00966-2. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Svoboda K, Malinow R. Experience strengthening transmission by driving AMPA receptors into synapses. Science. 2003;299:1585–88. doi: 10.1126/science.1079886. [DOI] [PubMed] [Google Scholar]
- Toyoda H, Wu LJ, Zhao MG, Xu H, Zhuo M. Time-dependent postsynaptic AMPA GluR1 receptor recruitment in the cingulate synaptic potentiation. Dev Neurobiol. 2007;67:498–509. doi: 10.1002/dneu.20380. [DOI] [PubMed] [Google Scholar]
- Toyoda H, Zhao MG, Zhuo M. NMDA receptor-dependent long-term depression in the anterior cingulate cortex. Rev Neurosci. 2006;17:403–13. doi: 10.1515/revneuro.2006.17.4.403. [DOI] [PubMed] [Google Scholar]
- Trachtenberg JT, Chen BE, Knott GW, Feng G, Sanes JR, et al. Long-term in vivo imaging of experience-dependent synaptic plasticity in adult cortex. Nature. 2002;420:788–94. doi: 10.1038/nature01273. [DOI] [PubMed] [Google Scholar]
- Trachtenberg JT, Stryker MP. Rapid anatomical plasticity of horizontal connections in the developing visual cortex. J Neurosci. 2001;21:3476–82. doi: 10.1523/JNEUROSCI.21-10-03476.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachtenberg JT, Trepel C, Stryker MP. Rapid extragranular plasticity in the absence of thalamocortical plasticity in the developing primary visual cortex. Science. 2000;287:2029–32. doi: 10.1126/science.287.5460.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano GG, Nelson SB. Homeostatic plasticity in the developing nervous system. Nat Rev Neurosci. 2004;5:97–107. doi: 10.1038/nrn1327. [DOI] [PubMed] [Google Scholar]
- Wallace H, Fox K. Local cortical interactions determine the form of cortical plasticity. J Neurobiol. 1999;41:58–63. [PubMed] [Google Scholar]
- Wang X, Merzenich MM, Sameshima K, Jenkins WM. Remodelling of hand representation in adult cortex determined by timing of tactile stimulation. Nature. 1995;378:71–75. doi: 10.1038/378071a0. [DOI] [PubMed] [Google Scholar]
- Weinberger NM. Associative representational plasticity in the auditory cortex: a synthesis of two disciplines. Learn Mem. 2007;14:1–16. doi: 10.1101/lm.421807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH. Comparison of the effects of unilateral and bilateral eye closure on cortical unit responses in kittens. J Neurophysiol. 1965;28:1029–40. doi: 10.1152/jn.1965.28.6.1029. [DOI] [PubMed] [Google Scholar]
- Yang Y, Fischer QS, Zhang Y, Baumgartel K, Mansuy IM, Daw NW. Reversible blockade of experience-dependent plasticity by calcineurin in mouse visual cortex. Nat Neurosci. 2005;8:791–96. doi: 10.1038/nn1464. [DOI] [PubMed] [Google Scholar]
- Yao H, Dan Y. Stimulus timing-dependent plasticity in cortical processing of orientation. Neuron. 2001;32:315–23. doi: 10.1016/s0896-6273(01)00460-3. [DOI] [PubMed] [Google Scholar]
- Young JM, Waleszczyk WJ, Wang C, Calford MB, Dreher B, Obermayer K. Cortical reorganization consistent with spike timing-but not correlation-dependent plasticity. Nat Neurosci. 2007;10:887–95. doi: 10.1038/nn1913. [DOI] [PubMed] [Google Scholar]
- Zito K, Svoboda K. Activity-dependent synaptogenesis in the adult Mammalian cortex. Neuron. 2002;35:1015–17. doi: 10.1016/s0896-6273(02)00903-0. [DOI] [PubMed] [Google Scholar]