An overview of the CCP4 software suite for macromolecular crystallography is given.
Keywords: CCP4, macromolecular crystallography, software, collaboration, automation, macromolecular structure determination
Abstract
The CCP4 (Collaborative Computational Project, Number 4) software suite is a collection of programs and associated data and software libraries which can be used for macromolecular structure determination by X-ray crystallography. The suite is designed to be flexible, allowing users a number of methods of achieving their aims. The programs are from a wide variety of sources but are connected by a common infrastructure provided by standard file formats, data objects and graphical interfaces. Structure solution by macromolecular crystallography is becoming increasingly automated and the CCP4 suite includes several automation pipelines. After giving a brief description of the evolution of CCP4 over the last 30 years, an overview of the current suite is given. While detailed descriptions are given in the accompanying articles, here it is shown how the individual programs contribute to a complete software package.
1. Introduction
CCP4 (Collaborative Computational Project, Number 4, 1994 ▶) exists to produce and support a world-leading integrated suite of programs that allows researchers to determine macromolecular structures by X-ray crystallography and other biophysical techniques. CCP4 aims to develop and support the development of cutting-edge approaches to the experimental determination and analysis of protein structure and to integrate these approaches into the CCP4 software suite. CCP4 is a community-based resource that supports the widest possible researcher community, embracing academic, not-for-profit and for-profit research. CCP4 aims to play a key role in the education and training of scientists in experimental structural biology. It encourages the wide dissemination of new ideas, techniques and practice.
In this article, we give an overview of the CCP4 project, past, present and future. We begin with a historical perspective on the growth of the software suite, followed by a summary of the current functionality in the suite. We then discuss ongoing plans for the next generation of the suite which is in development. In this account we focus on the suite as a whole, while other articles in this issue delve deeper into individual programs. We intend that this article could serve as a general literature citation for the use of the CCP4 software suite in structure determination, although we also encourage the citation of individual programs, many of the relevant references for which are included here. While we focus here on the CCP4 software suite, we would emphasize that comparable functionality is available in other software packages such as SHARP/autoSHARP (Vonrhein et al., 2007 ▶), SHELX (Sheldrick, 2008 ▶), ARP/wARP (Langer et al., 2008 ▶), PHENIX (Adams et al., 2010 ▶) and many others.
2. Evolution of the CCP4 software suite
The CCP4 software suite is a collection of programs implementing specific algorithms concerned with macromolecular structure solution from X-ray diffraction data. Significantly, it is a collection of autonomous and independently developed programs. While some have been commissioned by the academic committees overseeing the CCP4 project, the majority originate from the community to address a perceived gap in current functionality or to implement newly developed algorithms. The result is a collection of around 200 programs, ranging from large programs which are effectively packages in themselves to small ‘jiffy’ programs. Over the years the suite has grown continuously, with each major release featuring significant new software (see Table 1 ▶). Unsurprisingly, there is overlap of functionality, with several programs performing a particular task, albeit often using different approaches. The question then is how to combine these programs into a software suite, both in terms of ensuring communication between the different programs and in helping both naïve and experienced users to navigate through the suite.
Table 1. A summary of major CCP4 releases within the last ten years.
| Major release | Date | No. of binaries | Highlights |
|---|---|---|---|
| 6.1 | December 2008 | 238 | Automation pipelines |
| 6.0 | February 2006 | 212 | BP3, Phaser, Pirate |
| 5.0 | May 2004 | 180 | C-based libraries |
| 4.2 | April 2002 | 177 | ACORN, BEAST, PROFESSS |
| 4.1 | January 2001 | 172 | REFMAC5 |
Early on in the history of CCP4, there was an agreement for all programs to use the same file formats for data files. Formats were specified for diffraction data (the LCF format, later replaced by the MTZ format) and for electron-density maps (the CCP4 map format), while for atomic coordinates the PDB format was adopted. A software library was developed to facilitate reading and writing of these data formats and thereby ensure standardization of the formats. Originally supporting only Fortran programs, the library was re-written to support both Fortran and C/C++ as well as scripting languages (Winn et al., 2002 ▶). The CCP4 set of libraries has since expanded to cover a wider range of crystallographic tasks, in particular with the addition of the Clipper library (Cowtan, 2003 ▶), the MMDB library (Krissinel et al., 2004 ▶) and the CCTBX library (Grosse-Kunstleve et al., 2002 ▶) from the PHENIX project (Adams et al., 2010 ▶).
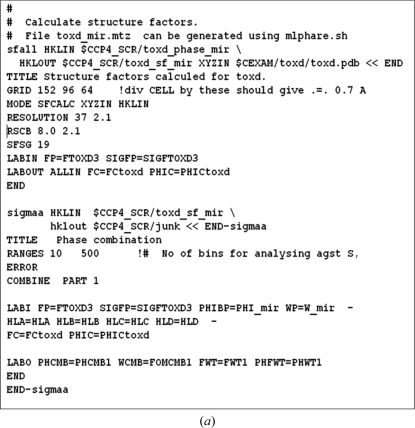
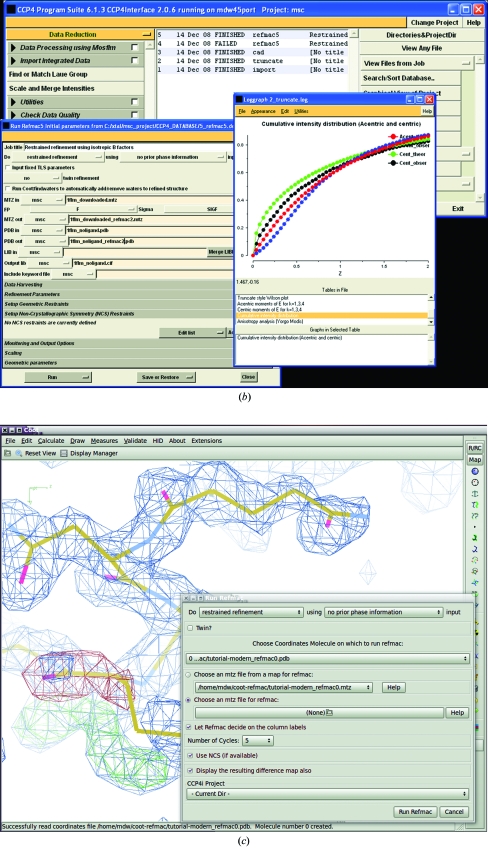
Crystallographic tasks were performed by writing or adapting scripts (e.g. Unix shell or VMS scripts) to link together a number of programs (Fig. 1a ▶) and the suite can still be run in this way. The programs communicate solely via the data files which are passed between them. The user sets program options based on the program documentation and the expected results from earlier steps. A major change was introduced in 2000 with the release of the graphical user interface ccp4i (Fig. 1b ▶; Potterton et al., 2003 ▶). Task interfaces help the user to prepare run scripts. Details of how to run specific programs are largely hidden, as are the jiffy programs used to perform minor functions such as format conversion. Some limited intelligence in the interface code allows program options to be customized according to properties of the data and/or the desired objective. ccp4i interfaces are now available for all of the commonly used CCP4 programs as well as for several non-CCP4 programs (e.g. ARP/wARP; Langer et al., 2008 ▶).
Figure 1.
The changing face of CCP4: (a) a typical script chaining programs together, (b) the ccp4i graphical user interface and (c) the molecular-graphics viewer Coot (Emsley et al., 2010 ▶) from which refinement and validation programs can be launched.
The ccp4i interface also introduced for the first time tools for helping the user to organize data. Jobs that have been run were recorded in a ‘database’ (in reality a directory of files) with tools to access and interpret the files saved there. Jobs are further organized into projects, representing different structure solutions. There are now plans to update the CCP4 GUI (see §4), but the impact of the original ccp4i on the suite should not be underestimated.
In the last few years, two other modes of accessing the CCP4 suite have emerged. On the one hand, the latest version of the suite contains four complementary automation pipelines, namely xia2 (Winter, 2010 ▶), CRANK (Ness et al., 2004 ▶), MrBUMP (Keegan & Winn, 2007 ▶) and BALBES (Long et al., 2008 ▶). These pipelines attempt to perform large sections of the full structure solution (e.g. phasing) without user intervention. This is achieved partly through the use of a large number of trials, trying different protocols and performing parameter scanning. Such an approach can be very powerful, using cheap computer power to make many more attempts than a user would manually. Automation pipelines have been realised in the last few years because of the maturity of the underlying programs and the availability of sufficient computer power to support multiple trials.
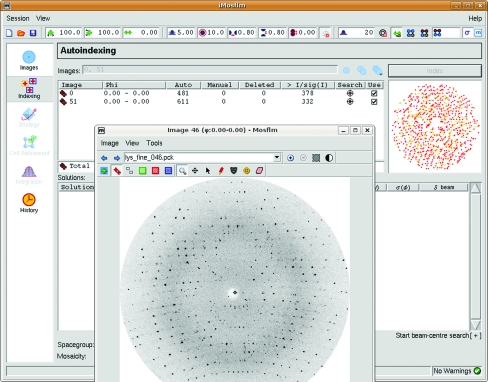
On the other hand, graphical programs for interactive use have become more powerful. Rather than simply reviewing the results of previously run programs and performing interactive model editing, Coot (Emsley et al., 2010 ▶) can launch separate refinement and validation programs (Fig. 1c ▶). Similarly, iMOSFLM can be used to interface the data-processing programs POINTLESS and SCALA. In some ways this is a completely different scenario to the automation pipelines. User interaction is paramount, with crystallography programs acting as tools to be invoked. The user can become familiar with the data and structure and use this to make intelligent decisions. Such an approach has also become possible because of the maturity of the invoked programs and the availability of sufficient computer power to run the programs interactively.
3. Overview of current functionality
In this section, we give an overview of the current functionality of the CCP4 software suite (corresponding to release series 6.1 at the time of writing). We summarize the automation pipelines and individual programs included in the suite; many more details can be found in the accompanying articles in this issue. We present the functionality in the traditional manner, starting at data processing and ending at validation. However, it is becoming increasingly apparent that these neat categories are breaking down.
3.1. Data processing
The earliest starting point for entry into the CCP4 suite is a set of X-ray diffraction images. The data-reduction program MOSFLM (Leslie, 2006 ▶) will take a set of diffraction images, identify spots on each image, index the diffraction pattern and thus identify the Bragg peaks, and integrate the spots. The output is a list of integrated intensities and their standard uncertainties labelled by the h, k, l indices. Associated information includes the batch number of the image from which the intensity was obtained, whether the peak was full or partial and the symmetry operation that relates the particular observation to the chosen asymmetric unit. MOSFLM continues to be improved, with support added recently for Pilatus detectors, addition of automatic backstop masking etc. The most visible change is the replacement of the old X-windows-based interface with the Tcl-based iMOSFLM interface (Fig. 2 ▶), which guides the user in a stepwise manner through the stages of data processing.
Figure 2.
The iMOSFLM interface, showing the main window and a display of one selected image.
POINTLESS is a relatively new program whose primary purpose is to identify the Laue group of a crystal from an unmerged data set (Evans, 2006 ▶). The program will also attempt to identify the space group from an analysis of systematic absences. A secondary purpose is to test the choice of indexing and re-index a data set if necessary.
Given a choice of space group, the program SCALA (Evans, 2006 ▶) will refine the parameters of a scaling function for an unmerged data set, apply scales to each observation of a reflection and merge all observations of a reflection to give an average intensity. It will also provide an improved estimate of the standard uncertainty of each intensity. The new program CTRUNCATE (which replaces the older TRUNCATE; Stein, unpublished program) can then convert the intensities to structure-factor amplitudes, although downstream programs increasingly use the mean intensities directly. Perhaps more importantly, CTRUNCATE will analyse a data set for signs of twinning, translational noncrystallographic symmetry (NCS), anisotropy and other notable features, since it is best to identify problems before attempting phasing. The program SFCHECK (Vaguine et al., 1999 ▶) will also provide an analysis of a data set, including testing for twinning and translational NCS, estimating the optical resolution and the anisotropy, and plotting the radial and angular completeness.
The previous steps of data processing are automated by the xia2 pipeline (Winter, 2010 ▶). From a directory of images, xia2 will identify the type of experiment (multi-wedge, multi-pass, multi-wavelength) and process accordingly. The pipeline will determine the point group, space group and correct indexing. Multiple processing pipelines using alternative underlying programs are supported. At the end, the user should have a set of merged structure-factor amplitudes suitable for input to phasing.
3.2. Experimental phasing
CCP4 includes the CRANK pipeline (Ness et al., 2004 ▶), which covers experimental phasing and beyond, and interfaces with several CCP4 and non-CCP4 programs. Heavy-atom substructure detection is performed by AFRO/CRUNCH2 (de Graaff et al., 2001 ▶) or by SHELXC/D (Sheldrick, 2008 ▶) and initial phasing is carried out by BP3 (Pannu et al., 2003 ▶; Pannu & Read, 2004 ▶) or SHELXE (Sheldrick, 2008 ▶). Phase improvement is carried out by SOLOMON (Abrahams & Leslie, 1996 ▶), DM (Cowtan et al., 2001 ▶) or Pirate (Cowtan, 2000 ▶) and automated model building by Buccaneer (Cowtan, 2006 ▶; Cowtan, 2008 ▶) or ARP/wARP (Langer et al., 2008 ▶). CRANK thus supports a range of underlying software handling the communication of data and allowing the user to trial different combinations.
CCP4 includes a number of additional individual programs, each of which has its own particular strength. The long-standing CCP4 program MLPHARE for phasing still works in straightforward cases and is fast to use. ACORN (Jia-xing et al., 2005 ▶; Dodson & Woolfson, 2009 ▶) uses ab initio methods for the determination of phases starting from a small fragment which could be a single heavy atom. The use of ab initio methods usually requires atomic resolution data, since it assumes atomicity of the electron density. However, a variant of the so-called free-lunch algorithm (Jia-xing et al., 2005 ▶) allows the temporary generation of phases to atomic resolution which the ACORN method can utilize. The OASIS program (Wu et al., 2009 ▶) also uses ab initio methods to break the phase ambiguity in SAD/SIR phasing.
Phaser (McCoy et al., 2007 ▶) can obtain phase estimates starting from known heavy-atom positions and SAD data. Log-likelihood gradient (LLG) maps are used to automatically find additional sites for anomalous scatterers and to detect anisotropy in existing anomalous scatterers. Phaser can also use a partial model, for example from a molecular-replacement solution that is hard to refine, as a source of phase information to help locate weak anomalous scatterers and thus improved phases. The latter reflects the view of experimental phasing and molecular replacement as just two sources of phase information rather than two separate techniques.
3.3. Molecular replacement
CCP4 includes two pipelines for molecular replacement (MR): MrBUMP (Keegan & Winn, 2007 ▶) and BALBES (Long et al., 2008 ▶). Both start from processed data and a target sequence and aim to deliver a molecular-replacement solution consisting of positioned and partially refined models. BALBES uses its own database of protein molecules and domains taken from the PDB and customized for MR, while MrBUMP uses public databases and a set of widely available bioinformatics tools to generate possible search models.
BALBES is based around the MR program MOLREP (Vagin & Teplyakov, 1997 ▶, 2010 ▶), while MrBUMP can also use the program Phaser (McCoy et al., 2007 ▶). Both MOLREP and Phaser are also available as stand-alone programs in CCP4. As well as providing rotation and translation functions, whereby a search model is positioned in the unit cell to give an initial estimate of the phases, these programs provide additional functionality, including a significant contribution to automated decision-making. For instance, a single run of Phaser can search for several copies each of several components in the structure of a complex, testing different possible search orders and trying different possible choices of space group.
The search model for MR may be an ensemble of structures, a set of models from an NMR structure or an electron-density map. Phases for the target may be available, so that the search model is to be fitted into electron density, or there may be density available from an electron-microscopy experiment. The MR step can be followed by rigid-body refinement and the packing of the MR solution can be checked. Much of this functionality is common to Phaser and MOLREP, but there are a number of differences in implementation, so that both may prove useful in certain circumstances.
A crucial component of MR is the selection and preparation of search models. The program CHAINSAW (Stein, 2008 ▶) takes as input a sequence alignment which relates residues in the search model to residues in the target protein and uses this information to edit the search model appropriately. The output model is labelled according to the target sequence. MOLREP (Lebedev et al., 2008 ▶) can take as input the target sequence and performs its own alignment to the search model in order to edit the search model.
3.4. Phase improvement and automated model building
Having obtained initial phases from experimental phasing, the next step is phase improvement (density modification) to give a map that can be built into. When phases come from molecular replacement, phase improvement may also be useful to reduce model bias. For a long time, the main CCP4 phase-improvement programs were DM (Cowtan et al., 2001 ▶) and SOLOMON (Abrahams & Leslie, 1996 ▶), which covered the standard techniques of solvent flattening/flipping, histogram matching and NCS averaging. More recently, statistically based methods have been incorporated into the program Pirate (Cowtan, 2000 ▶). Pirate can give better results, but has been found to be inconveniently slow. The latest program Parrot (Cowtan, 2010 ▶) achieves similar improvements but is also fast and automated.
Given an electron-density map, automated model building is provided in CCP4 by Buccaneer (Cowtan, 2006 ▶, 2008 ▶). This finds candidate Cα positions, builds these into chain fragments, joins the fragments together and docks a sequence. NCS can be used to rebuild and complete related chains. Since version 1.4, there is support for model (re)building after molecular replacement and for supplying known structural elements such as heavy atoms. The CCP4 suite includes an interface for alternating cycles of model building with Buccaneer with cycles of model refinement with REFMAC5. The supplementary program Sloop (Cowtan, unpublished program) builds missing loops using fragments taken from the Richardson’s Top500 library of structures (Lovell et al., 2003 ▶) to fill gaps in the chain. The chance of finding a good fit falls with increasing size of the gap, but the method may work for loops of up to eight residues in length.
RAPPER (Furnham et al., 2006 ▶) provides a conformational search algorithm for protein modelling, which can produce an ensemble of models satisfying a wide variety of restraint information. In the context of CCP4, restraints on the modelling are provided by the electron density and/or the locations of the Cα atoms. The ccp4i interface includes modes for loop building or for building the entire structure.
3.5. Refinement and model completion
The aim of macromolecular crystallography is to produce a model of the macromolecule of interest which explains the diffraction images as accurately and completely as possible. Both the form of the model and the parameters of the model need to be defined. Refinement is the process of optimizing the values of the model parameters and in CCP4 is performed by the program REFMAC5 (Murshudov et al., 1997 ▶). REFMAC5 will refine atomic coordinates and atomic isotropic or anisotropic displacement parameters (Murshudov et al., 1999 ▶), as well as group parameters for rigid-body refinement and TLS refinement (Winn et al., 2001 ▶, 2003 ▶). It will also refine scaling parameters and a mask-based bulk-solvent correction.
When good-quality experimental phases are available, these can be included as additional data (Pannu et al., 1998 ▶). More recently, it has become possible to refine directly against anomalous data for the cases of SAD (Skubák et al., 2004 ▶) and SIRAS (Skubák et al., 2009 ▶) without the need for estimated phases and phase probabilities. REFMAC5 will also now refine against twinned data (Lebedev et al., 2006 ▶), automatically recognising the twin laws and estimating the corresponding twin fractions.
The nonprotein contents of the crystal are often of most interest, such as bound ligands, cofactors, metal sites etc. Correct refinement at moderate or low resolution requires a knowledge of the ideal geometry together with associated uncertainties. In REFMAC5 this is handled through a dictionary of possible ligands (Vagin et al., 2004 ▶), with details held in mmCIF format. Dictionary files can be created through the tools SKETCHER and JLIGAND.
Refinement goes hand-in-hand with rounds of model building which add/subtract parts of the model and apply large structural changes that are beyond the reach of refinement. In addition to the automated procedures of Buccaneer and RAPPER described above, there are many model-building tools in Coot (Emsley et al., 2010 ▶). A ccp4i interface to the popular ARP/wARP model-building package (Langer et al., 2008 ▶) has also been available for many years.
3.6. Validation, deposition and publication
Validation is the process of ensuring that all aspects of the model are supported by the diffraction data, as well as conforming with known features of protein chemistry. Although validation has traditionally been viewed as something that is performed at the end of structure determination, just before deposition, it is now appreciated that validation is an integral part of the process of structure solution, which should be carried out continually. CCP4 includes a wide variety of validation tools, all of which should be run to gain a complete picture of model quality. Coot (Emsley et al., 2010 ▶) has a dedicated drop-down menu of validation tools which can and should be applied as the model is being built. Coot can also extract warnings about particular links or outliers from a REFMAC5 log file. Warnings associated with specific atoms or residues are linked directly to the model as viewed in Coot.
The ccp4i ‘Validation and Deposition’ module contains further validation tools. As mentioned above, SFCHECK (Vaguine et al., 1999 ▶) provides a number of measures of data quality, but if a model is provided it will also assess the agreement of the model with the data. Sequins (Cowtan, unpublished program) validates the assigned sequence against electron density (generated from experimental phases or from phases calculated from a side-chain omit process) and warns of misplaced side chains or register errors. RAMPAGE (which is part of the RAPPER package; Furnham et al., 2006 ▶) provides Ramachandran plots based on updated ϕ–ψ propensities. PROCHECK is also included, although the Ramachandran plots are no longer generated, having been superseded by RAMPAGE. R500 (Henrick, unpublished program) checks the stereochemistry in a given PDB file against expected values and lists outliers in REMARK 500 records.
The quaternary structure of the protein can be analysed with PISA (Krissinel & Henrick, 2007 ▶). This considers all possible interfaces in the crystal structure, estimates the free energy of dissociation, taking into account solvation and entropy effects, and predicts which interfaces are likely to be of biological significance.
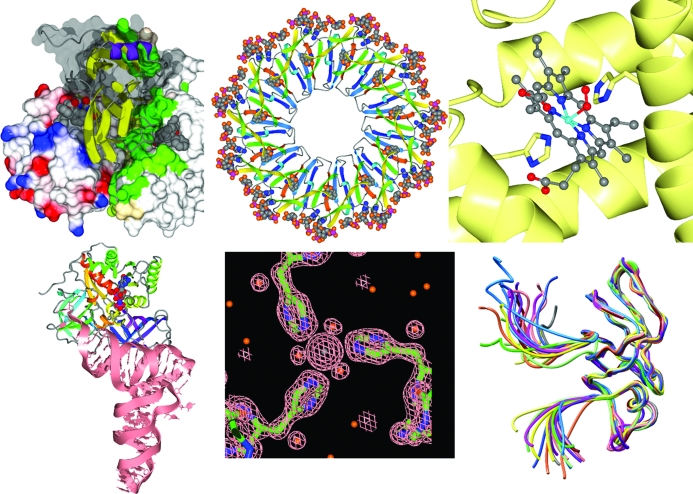
The CCP4 molecular-graphics program CCP4mg (Potterton et al., 2002 ▶, 2004 ▶) provides a simple means of generating publication-quality images and movies. As well as displaying coordinates in a wide variety of styles, CCP4mg can display molecular surfaces, electron density, arbitrary vectors and labels. The latest versions are built on the Qt toolkit, giving an enhanced look and feel (Fig. 3 ▶). Structures and views can be transferred between CCP4mg and Coot.
Figure 3.
A montage of images from the molecular-graphics program CCP4mg (Potterton et al., 2004 ▶).
3.7. Jiffies and utilities
In addition to the main functionality described above, the CCP4 suite contains a large number of utilities for performing format conversions and various analyses. Reflection data processed in other software packages can be imported with the utilities COMBAT, POINTLESS, SCALEPACK2MTZ, DTREK2SCALA and DTREK2MTZ, while data can be exchanged with other structure-solution packages with CONVERT2MTZ, F2MTZ, CIF2MTZ, MTZ2VARIOUS and MTZ2CIF. There are several useful utilities based on the Clipper library (Cowtan, 2003 ▶), such as CPHASEMATCH, which will compare two phase sets and look for changes in origin or hand. There are also many useful utilities for analysing coordinate files. New programs based on the MMDB library (Krissinel et al., 2004 ▶) include NCONT for listing atom contacts and PDB_MERGE for combining two PDB files.
4. Future plans
At the heart of the CCP4 suite are the set of algorithms encoded in individual programs. As always, we include new programs in each major release of the suite and will continue to do so. Since the source of novel software is usually independent developers, the additions to the suite are not centrally planned. Nevertheless, some current themes are clearly recognisable, such as automated model building, in particular for low-resolution data.
CCP4 also aims to enhance its functionality related to the maintenance and use of data on small molecules (ligands). Firstly, a considerably larger library of chemical compounds will be provided with the suite. Extended search functions will be provided to allow the efficient retrieval of known compounds or their close analogues. Secondly, existing functions for generating restraint data for new ligands will be enhanced by the inclusion of relevant software such as PRODRG (Schüttelkopf & van Aalten, 2004 ▶) into the suite, as well as by the development of new methods for structure reconstruction on the basis of partial similarity to structures in the library. Functionality will be available through a graphical front-end application, JLIGAND.
In addition to the core programs, the infrastructure of CCP4 continues to evolve to support the latest working practices. The current CCP4 GUI, ccp4i, was a major innovation and has served us well for over ten years (Potterton et al., 2003 ▶). While it continues to provide a useful interface to the CCP4 suite, there are increasing demands from automation pipelines and users alike. In particular, there is a requirement to provide help on what to try next, advice which can be useful to both scientists and automated software. This depends on a robust assessment of the experimental data and the results of previous processing, which in turn requires good data management. We aim to address these issues through the development of a next-generation CCP4 interface.
There will also be changes in the way that CCP4 is delivered to the end user. We have all become used to automated updates to the software we use (e.g. Windows Update, Synaptic for Debian-based Linux or application-specific updates such as for Firefox). Some CCP4 programs do alert the users to the availability of newer versions and CCP4mg (Potterton et al., 2002 ▶, 2004 ▶) will update the version on request. A CCP4-wide update mechanism is more difficult given the heterogeneous nature of the suite, but efforts in this direction are under way. A specific example of a remotely maintained crystallography platform is given by the US-based SBGrid Consortium.
The CCP4 suite is downloaded to a user’s machine or a local server before being run. This is in contrast to many biology software tools, which are web-based. Reasons for running CCP4 locally include the wallclock time of jobs, the detailed control required and the size of data files. Nevertheless, there is increasing usage of web servers for crystallographic tasks. A server at York (http://www.ysbl.york.ac.uk/YSBLPrograms/index.jsp) runs a number of CCP4 programs, including BALBES and Buccaneer, while CCP4 programs are included in a number of other services, for example the ARP/wARP server at Hamburg (http://cluster.embl-hamburg.de/ARPwARP/remote-http.html). Plans are under way to make more CCP4 functionality available via the web.
Finally, the coming years will see increasing integration of crystallography with other techniques, both experimental and theoretical. CCP4 aims to contribute towards efforts, such as the European infrastructure project INSTRUCT, to ease the transfer of data to and from these other domains.
Acknowledgments
CCP4 aims to be a community effort and as such we are grateful to the many many people from the community that have contributed over the years, whether in terms of code, bug reports or simply feedback. CCP4 is supported by the BBSRC through grant BB/F0202281. We are also grateful to our industrial users for support over many years. GNM is supported by Wellcome Trust Grant No. 064405/Z/01/A. NSP is supported by the Netherlandse Organisatie voor Wetenschappelijk Onderzoek (NWO) grant No. 700.55.425.
References
- Abrahams, J. P. & Leslie, A. G. W. (1996). Acta Cryst. D52, 30–42. [DOI] [PubMed]
- Adams, P. D. et al. (2010). Acta Cryst. D66, 213–221.
- Collaborative Computational Project, Number 4 (1994). Acta Cryst. D50, 760–763.
- Cowtan, K. (2000). Acta Cryst. D56, 1612–1621. [DOI] [PubMed]
- Cowtan, K. D., Zhang, K. Y. J. & Main, P. (2001). International Tables for Crystallography, Vol. F, edited by E. Arnold & M. G. Rossmann, ch. 25.2.5. Dordrecht: Kluwer Academic Publishers.
- Cowtan, K. (2003). IUCr Comput. Comm. Newsl. 2, 4–9.
- Cowtan, K. (2006). Acta Cryst. D62, 1002–1011. [DOI] [PubMed]
- Cowtan, K. (2008). Acta Cryst. D64, 83–89. [DOI] [PMC free article] [PubMed]
- Cowtan, K. (2010). Acta Cryst. D66, 470–478. [DOI] [PMC free article] [PubMed]
- Dodson, E. J. & Woolfson, M. M. (2009). Acta Cryst. D65, 881–891. [DOI] [PubMed]
- Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. (2010). Acta Cryst. D66, 486–501. [DOI] [PMC free article] [PubMed]
- Evans, P. (2006). Acta Cryst. D62, 72–82. [DOI] [PubMed]
- Furnham, N., Doré, A. S., Chirgadze, D. Y., de Bakker, P. I. W., Depristo, M. A. & Blundell, T. L. (2006). Structure, 14, 1313–1320. [DOI] [PubMed]
- Graaff, R. A. G. de, Hilge, M., van der Plas, J. L. & Abrahams, J. P. (2001). Acta Cryst. D57, 1857–1862. [DOI] [PubMed]
- Grosse-Kunstleve, R. W., Sauter, N. K., Moriarty, N. W. & Adams, P. D. (2002). J. Appl. Cryst. 35, 126–136.
- Jia-xing, Y., Woolfson, M. M., Wilson, K. S. & Dodson, E. J. (2005). Acta Cryst. D61, 1465–1475. [DOI] [PubMed]
- Keegan, R. M. & Winn, M. D. (2007). Acta Cryst. D63, 447–457. [DOI] [PubMed]
- Krissinel, E. & Henrick, K. (2007). J. Mol. Biol. 372, 774–797. [DOI] [PubMed]
- Krissinel, E. B., Winn, M. D., Ballard, C. C., Ashton, A. W., Patel, P., Potterton, E. A., McNicholas, S. J., Cowtan, K. D. & Emsley, P. (2004). Acta Cryst. D60, 2250–2255. [DOI] [PubMed]
- Langer, G., Cohen, S. X., Lamzin, V. S. & Perrakis, A. (2008). Nature Protoc. 3, 1171–1179. [DOI] [PMC free article] [PubMed]
- Lebedev, A. A., Vagin, A. A. & Murshudov, G. N. (2006). Acta Cryst. D62, 83–95. [DOI] [PubMed]
- Lebedev, A. A., Vagin, A. A. & Murshudov, G. N. (2008). Acta Cryst. D64, 33–39. [DOI] [PMC free article] [PubMed]
- Leslie, A. G. W. (2006). Acta Cryst. D62, 48–57. [DOI] [PubMed]
- Long, F., Vagin, A. A., Young, P. & Murshudov, G. N. (2008). Acta Cryst. D64, 125–132. [DOI] [PMC free article] [PubMed]
- Lovell, S. C., Davis, I. W., Arendall, W. B. III, de Bakker, P. I. W., Word, J. M., Prisant, M. G., Richardson, J. S. & Richardson, D. C. (2003). Proteins, 50, 437–450. [DOI] [PubMed]
- McCoy, A. J., Grosse-Kunstleve, R. W., Adams, P. D., Winn, M. D., Storoni, L. C. & Read, R. J. (2007). J. Appl. Cryst. 40, 658–674. [DOI] [PMC free article] [PubMed]
- Murshudov, G. N., Vagin, A. A. & Dodson, E. J. (1997). Acta Cryst. D53, 240–255. [DOI] [PubMed]
- Murshudov, G. N., Vagin, A. A., Lebedev, A., Wilson, K. S. & Dodson, E. J. (1999). Acta Cryst. D55, 247–255. [DOI] [PubMed]
- Ness, S. R., de Graaff, R. A. G., Abrahams, J. P. & Pannu, N. S. (2004). Structure, 12, 1753–1761. [DOI] [PubMed]
- Pannu, N. S., McCoy, A. J. & Read, R. J. (2003). Acta Cryst. D59, 1801–1808. [DOI] [PubMed]
- Pannu, N. S., Murshudov, G. N., Dodson, E. J. & Read, R. J. (1998). Acta Cryst. D54, 1285–1294. [DOI] [PubMed]
- Pannu, N. S. & Read, R. J. (2004). Acta Cryst. D60, 22–27. [DOI] [PubMed]
- Potterton, E., Briggs, P., Turkenburg, M. & Dodson, E. (2003). Acta Cryst. D59, 1131–1137. [DOI] [PubMed]
- Potterton, E., McNicholas, S., Krissinel, E., Cowtan, K. & Noble, M. (2002). Acta Cryst. D58, 1955–1957. [DOI] [PubMed]
- Potterton, L., McNicholas, S., Krissinel, E., Gruber, J., Cowtan, K., Emsley, P., Murshudov, G. N., Cohen, S., Perrakis, A. & Noble, M. (2004). Acta Cryst. D60, 2288–2294. [DOI] [PubMed]
- Schüttelkopf, A. W. & van Aalten, D. M. F. (2004). Acta Cryst. D60, 1355–1363. [DOI] [PubMed]
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Skubák, P., Murshudov, G. N. & Pannu, N. S. (2004). Acta Cryst. D60, 2196–2201. [DOI] [PubMed]
- Skubák, P., Murshudov, G. & Pannu, N. S. (2009). Acta Cryst. D65, 1051–1061. [DOI] [PMC free article] [PubMed]
- Stein, N. (2008). J. Appl. Cryst. 41, 641–643.
- Vagin, A. A., Steiner, R. A., Lebedev, A. A., Potterton, L., McNicholas, S., Long, F. & Murshudov, G. N. (2004). Acta Cryst. D60, 2184–2195. [DOI] [PubMed]
- Vagin, A. & Teplyakov, A. (1997). J. Appl. Cryst. 30, 1022–1025.
- Vagin, A. & Teplyakov, A. (2010). Acta Cryst. D66, 22–25. [DOI] [PubMed]
- Vaguine, A. A., Richelle, J. & Wodak, S. J. (1999). Acta Cryst. D55, 191–205. [DOI] [PubMed]
- Vonrhein, C., Blanc, E., Roversi, P. & Bricogne, G. (2007). Methods Mol. Biol. 364, 215–230. [DOI] [PubMed]
- Winn, M. D., Ashton, A. W., Briggs, P. J., Ballard, C. C. & Patel, P. (2002). Acta Cryst. D58, 1929–1936. [DOI] [PubMed]
- Winn, M. D., Isupov, M. N. & Murshudov, G. N. (2001). Acta Cryst. D57, 122–133. [DOI] [PubMed]
- Winn, M. D., Murshudov, G. N. & Papiz, M. Z. (2003). Methods Enzymol. 374, 300–321. [DOI] [PubMed]
- Winter, G. (2010). J. Appl. Cryst. 43, 186–190.
- Wu, L.-J., Zhang, T., Gu, Y.-X., Zheng, C.-D. & Fan, H.-F. (2009). Acta Cryst. D65, 1213–1216. [DOI] [PubMed]