Abstract
Purpose
Patients with inflammatory breast cancer (IBC) or locally advanced breast cancer (LABC) were randomly assigned to 21-day doxorubicin and cyclophosphamide administered for five cycles (standard arm) versus weekly doxorubicin and daily oral cyclophosphamide administered with granulocyte colony-stimulating factor support for 15 weeks (continuous arm). All patients had subsequent weekly paclitaxel for 12 weeks before surgery.
Patients and Methods
Patients (n = 372) were randomly assigned to the standard arm (n = 186) or the continuous arm (n = 186) stratified by disease type (LABC, n = 256; IBC, n = 116). The primary outcome was microscopic pathologic complete response (pCR) at surgery. Secondary outcomes included disease-free survival, overall survival, and toxicity.
Results
More patients in the standard arm had grade 3 to 4 leukopenia and neutropenia, but there were more instances of stomatitis/pharyngitis and hand-foot skin reaction in the continuous arm. Assessed among 356 eligible patients, pCR was not different between the treatment groups stratified by disease type (P = .42). In subset analysis, higher pCR rates were observed in the continuous arm versus the standard arm only for stage IIIB disease (P = .0057) and in IBC (P = .06). Comparison of overall survival and disease-free survival showed no difference between treatment groups (P = .37 and P = .87, respectively).
Conclusion
No significant clinical benefit was seen for the investigational arm in this trial overall.
INTRODUCTION
A continuous approach with doxorubicin (A) and cyclophosphamide (C) was based on the concept of dose intensity.1,2 Weekly intravenous chemotherapy with methotrexate (M) and fluorouracil (F) combined with daily oral cyclophosphamide was the most dose-intense way to give the older CMF regimen and yielded the best long-term outcomes.3 Beginning in the 1990s, Ellis et al4 began to use a regimen modeled on continuous CMF, substituting weekly doxorubicin for M, deleting F, and adding filgrastim (granulocyte colony-stimulating factor [G-CSF]) to maintain dose intensity for doxorubicin (AC+G). Initial studies included patients in the high-risk adjuvant setting and those receiving neoadjuvant treatment for locally advanced breast cancer (LABC) or inflammatory breast cancer (IBC). Phase II data on neoadjuvant AC+G for 96 evaluable patients are available from Southwest Oncology Group (SWOG) S9625 study.5 Results appear especially encouraging for patients with IBC: in 12 (24%) of 49 patients, a pathologic complete response (pCR) was achieved. Results in estrogen receptor (ER) –negative disease were similar, with pCR in 16 (36%) of 45 patients.
More recently, it has been proposed that dose density, rather than dose intensity, may be critical to outcome with AC chemotherapy.6 Although our continuous regimen does not meet Norton's definition (since individual doses are reduced), it is a dense regimen, providing nearly continuous exposure to potentially effective drug concentrations.
A third supporting concept is that of metronomic dosing proposed by Man et al.7 Preclinical data suggest that continuous or metronomic exposure to cyclophosphamide results in selective cytotoxicity for endothelial cells, leading to death by apoptosis, followed by antiangiogenic effects on tumor cells.7 We hereafter refer to our experimental regimen as continuous rather than metronomic, because the definition of metronomic used by Man et al implies administration of relatively low doses, while we gave doses requiring growth factor support.
This study was initially designed to compare standard (every 3 weeks) AC to the continuous regimen. Following study initiation, both regimens were modified to include administration of weekly paclitaxel after completion of AC, on the basis of a randomized trial showing a benefit in patients with operable disease.8
PATIENTS AND METHODS
Study Design
This randomized phase III study compared patients who had IBC or LABC and were treated with a standard 21-day AC regimen given for five cycles (standard arm) with patients who were treated with weekly AC given with G-CSF support for 15 weeks (continuous arm). All patients subsequently received weekly paclitaxel for 12 weeks before surgery for a planned duration of therapy of 27 weeks. For patients with responding or stable disease, surgery was to take place within 6 weeks of completing chemotherapy. Patients whose tumors progressed during chemotherapy were removed from the study before surgery but are included in the analyses as described in Patients under Results.
In the standard arm, doxorubicin (60 mg/m2) and cyclophosphamide (600 mg/m2) were given intravenously on day 1 every 3 weeks for five cycles (chosen for comparable duration—see below), without prophylactic use of G-CSF (G-CSF was allowed by usual clinical indications). In the continuous arm, doxorubicin (24 mg/m2/wk) was given weekly for 15 doses and cyclophosphamide (60 mg/m2/d) was given orally each day for 15 weeks, with G-CSF (5 μg/kg/d) given on days 2 through 7 each week. Fifteen weeks was the average administered duration of treatment in our previous phase II trial, SWOG 9625. In both treatment arms, paclitaxel (80 mg/m2/wk) was then given intravenously once a week for 12 weeks, followed by surgical resection. Dose reductions were allowed under specific circumstances per protocol.
Patient Population
Women with histologically confirmed breast carcinoma (by core-needle biopsy) were eligible if they had stage IIB, IIIA, or IIIB disease and were candidates for neoadjuvant chemotherapy. Patients with metastatic disease were excluded. Human epidermal growth factor receptor 2 (HER2), ER, and progesterone receptor (PgR) were reported by using local institutional standards. Patients could not have received prior radiation therapy, chemotherapy, hormonal therapy, or prior definitive surgery for breast cancer. Patients had to have adequate cardiac, hematologic, renal, and hepatic function, and a Zubrod performance status of 0 to 2. Known HIV-positive patients and pregnant or nursing women were ineligible. Patients with hypertension or those older than age 60 years had to have a normal baseline multiple-gated acquisition scan or echocardiogram and no history of angina pectoris. Concurrent anticancer therapy was not allowed while participating in this study. Patients were followed for a minimum of 5 years or until death. The protocol was approved by the governing institutional review boards at all participating institutions, and written informed consent was obtained before study registration.
Statistical Methodology and Analysis
pCR was defined as the absence of microscopic invasive tumor at the primary site and in axillary lymph nodes at the time of surgery. Patients who had disease progression, death, or initiation of nonprotocol treatment before surgery were classified as not having a pCR for the purpose of the intent-to-treat analysis. The primary objective was to compare pCR rates by using Fisher's exact test. Additional analyses included logistic regression with adjustment for hormone receptor status (ER- or PgR-positive v ER- or PgR-negative). Adverse events were compared by χ2 tests of the contingency tables formed by treatment group and each adverse event.
The target sample size was 350 patients, assuming a two-sided 0.05 significance level with 90% power to detect a difference in pCR rate and assuming 15% in the standard arm versus 30% in the continuous arm. Patients were randomly assigned after stratification by disease status (IBC v LABC).
We also assessed disease-free survival (DFS), the time from registration to disease progression, disease recurrence, or death due to any cause, as well as overall survival (OS), time from registration to death due to any cause. The Kaplan-Meier method was used for displaying estimated survival by groups. Log-rank tests were used to assess the difference between the two survival curves. Cox regression analyses were stratified by disease type and used to estimate the hazard ratios (HRs) and 95% CIs. HRs were defined for the continuous versus the standard arm with a value > 1.0 indicating worse survival for the continuous arm.
A single interim analysis was performed for the independent Data Monitoring Committee, which did not meet requirements for early stopping. The final analysis was set at α = .045 (two-sided) to accommodate the interim analysis. Preliminary analysis was performed before the evaluation of end points for all patients for presentation at the 42nd Annual Meeting of the American Society of Clinical Oncology in 2006.9
RESULTS
Patients
The trial was conducted by the SWOG and included participation by other cooperative groups, specifically the North Central Cancer Treatment Group (NCCTG), Cancer and Leukemia Group B (CALGB), Eastern Cooperative Oncology Group (ECOG), National Surgical Adjuvant Breast and Bowel Project (NSABP), Radiation Therapy Oncology Group (RTOG), and the American College of Surgeons Oncology Group (ACOSOG). A total of 399 patients were randomly assigned between May 1, 2001, and December 31, 2005, at 55 clinical study centers. The first 27 patients enrolled in the study did not receive paclitaxel, which the protocol was amended to include on October 1, 2002; a separate report on this group is available in the Appendix (online only). This article includes only the 372 patients on the amended protocol with paclitaxel added.
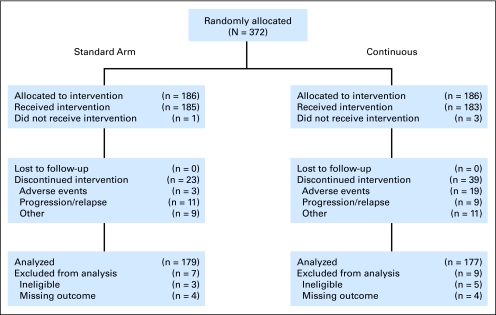
Treatment groups were well balanced with 186 patients randomly assigned to each of the two arms. Figure 1 is a schematic of patient disposition. Three patients in the continuous arm and one patient in the standard arm never started treatment but are included in the intent-to-treat analyses. A total of 62 patients (17%) discontinued treatment early, 22 due to adverse events, 20 due to progression or relapse, and 20 for other reasons.
Fig 1.
CONSORT flow diagram. All 372 patients randomly allocated after the protocol amendment to include taxane treatment are shown.
Eight (three in the standard arm; five in the continuous arm) of the 372 randomly assigned patients were deemed ineligible on the basis of entry criteria and were excluded from all analyses, leaving 364 patients for baseline analysis. Four of the eight ineligible patients did not have the required type of biopsy, two had metastatic disease, one had incomplete eligibility information, and one had absolute neutrophil count < 1,500. For the toxicity analyses, we also excluded the four patients who never started treatment and two for whom toxicity was not assessed, leaving a total of 358 patients. Of the 364 eligible patients evaluated for baseline characteristics, eight patients (four per arm) were missing an evaluation of the primary outcome, pCR. These were excluded from the efficacy analyses but were included in the survival analyses. A total of 356 eligible patients with outcomes were included in the analysis of pCR (179, standard arm; 177, continuous arm).
Baseline Characteristics
Table 1 provides patient characteristics, which were well balanced across treatment groups for most characteristics, but imbalance was seen in the distribution of hormone receptor status and tumor stage. Age ranged from 22 to 77 years, with a median of 52 years. Most patients had LABC (68%) versus IBC (32%).
Table 1.
Patient and Tumor Characteristics for Eligible Patients
| Characteristic | Standard Arm (n = 183) |
Continuous Arm(n = 181) |
Overall (n = 364) |
|||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |
| Age, years | ||||||
| Median | 51 | 52 | 52 | |||
| Range | 22-77 | 27-76 | 22-77 | |||
| Race/ethnicity (reported) | ||||||
| White | 127 | 69 | 129 | 71 | 256 | 70 |
| Black | 18 | 10 | 25 | 14 | 43 | 12 |
| Asian | 8 | 4 | 7 | 4 | 15 | 4 |
| Pacific Islander | 3 | 2 | 0 | 0 | 15 | 4 |
| Native American | 2 | 1 | 3 | 2 | 5 | 1 |
| Multiracial | 3 | 2 | 2 | 1 | 5 | 1 |
| Unknown | 22 | 13 | 15 | 8 | 37 | 10 |
| Hispanic (reported) | ||||||
| Yes | 32 | 17 | 20 | 11 | 52 | 14 |
| No | 127 | 69 | 132 | 73 | 259 | 71 |
| Unknown | 24 | 13 | 29 | 16 | 53 | 15 |
| Disease type | ||||||
| Locally advanced | 126 | 69 | 123 | 68 | 249 | 68 |
| Inflammatory | 57 | 31 | 58 | 32 | 115 | 32 |
| Hormone receptors | ||||||
| Positive | 85 | 46 | 100 | 55 | 185 | 51 |
| Negative | 89 | 49 | 752 | 41 | 164 | 45 |
| Unknown | 9 | 5 | 6 | 3 | 15 | 4 |
| HER2 status | ||||||
| Positive | 47 | 26 | 45 | 25 | 92 | 25 |
| Negative | 118 | 64 | 118 | 65 | 236 | 65 |
| Unknown | 18 | 10 | 18 | 10 | 36 | 10 |
| Stage | ||||||
| IIB | 35 | 19 | 22 | 12 | 57 | 16 |
| IIIA | 56 | 31 | 57 | 31 | 113 | 31 |
| IIIB | 88 | 48 | 95 | 52 | 183 | 50 |
| Unknown | 4 | 2 | 7 | 4 | 11 | 3 |
Abbreviation: HER2, human epidermal growth factor receptor 2.
Safety
The adverse events deemed probably, possibly, or definitely related to study treatment are given in Table 2. Only those with more than one grade 3 to 5 adverse event are shown. Overall, there was greater toxicity on the standard arm than on the continuous arm (P < .001), although more patients discontinued treatment early on the continuous arm due to toxicity (Fig 1). The standard arm had greater toxicity from neutropenia and febrile neutropenia. The continuous arm had more stomatitis/pharyngitis and hand-foot syndrome.
Table 2.
Toxicity Profile
| Adverse Event | Grade |
|||||||
|---|---|---|---|---|---|---|---|---|
| Standard Arm (n = 181) |
Continuous Arm (n = 177) |
|||||||
| ≤ 2 | 3 | 4 | 5 | ≤ 2 | 3 | 4 | 5 | |
| Any adverse event | 64 | 53 | 64 | 0 | 85 | 74 | 17 | 1 |
| Anemia | 173 | 7 | 1 | 0 | 170 | 7 | 0 | 0 |
| Anorexia | 178 | 3 | 0 | 0 | 177 | 0 | 0 | 0 |
| Anxiety/agitation | 179 | 2 | 0 | 0 | 176 | 1 | 0 | 0 |
| Bone pain | 181 | 0 | 0 | 0 | 174 | 3 | 0 | 0 |
| Constipation/bowel obstruction | 180 | 1 | 0 | 0 | 176 | 1 | 0 | 0 |
| Depression | 179 | 1 | 1 | 0 | 175 | 2 | 0 | 0 |
| Diarrhea without colostomy | 179 | 2 | 0 | 0 | 175 | 2 | 0 | 0 |
| Dyspnea | 180 | 1 | 0 | 0 | 175 | 2 | 0 | 0 |
| Fatigue/malaise/lethargy | 177 | 4 | 0 | 0 | 168 | 9 | 0 | 0 |
| Febrile neutropenia | 168 | 11 | 2 | 0 | 177 | 0 | 0 | 0 |
| GI mucositis, NOS | 181 | 0 | 0 | 0 | 175 | 2 | 0 | 0 |
| Hand-foot skin reaction | 181 | 0 | 0 | 0 | 152 | 25 | 0 | 0 |
| Headache | 181 | 0 | 0 | 0 | 175 | 2 | 0 | 0 |
| Hyperglycemia | 177 | 4 | 0 | 0 | 171 | 6 | 0 | 0 |
| Hypokalemia | 180 | 0 | 1 | 0 | 176 | 1 | 0 | 0 |
| Hyponatremia | 178 | 2 | 1 | 0 | 177 | 0 | 0 | 0 |
| Infection without grade 3 to 4 neutropenia | 177 | 4 | 0 | 0 | 169 | 8 | 0 | 0 |
| Infection with grade 3 to 4 neutropenia | 178 | 2 | 1 | 0 | 176 | 1 | 0 | 0 |
| Leukopenia | 119 | 39 | 23 | 0 | 161 | 11 | 5 | 0 |
| Liver, clinical | 181 | 0 | 0 | 0 | 176 | 0 | 0 | 1 |
| Lymphopenia | 176 | 5 | 0 | 0 | 173 | 4 | 0 | 0 |
| Nausea | 168 | 13 | 0 | 0 | 174 | 3 | 0 | 0 |
| Neutropenia/granulocytopenia | 91 | 32 | 58 | 0 | 148 | 14 | 15 | 0 |
| Pain, other | 180 | 1 | 0 | 0 | 174 | 3 | 0 | 0 |
| Sensory neuropathy | 181 | 0 | 0 | 0 | 174 | 3 | 0 | 0 |
| Stomatitis/pharyngitis | 178 | 3 | 0 | 0 | 157 | 19 | 1 | 0 |
| Syncope | 179 | 2 | 0 | 0 | 177 | 0 | 0 | 0 |
| Thrombocytopenia | 173 | 5 | 3 | 0 | 172 | 4 | 1 | 0 |
| Thrombosis/embolism | 179 | 2 | 0 | 0 | 177 | 0 | 0 | 0 |
| Vomiting | 171 | 10 | 0 | 0 | 172 | 5 | 0 | 0 |
Abbreviation: NOS, not otherwise stated.
A total of 63 patients (35%) on the standard arm experienced grade 4 hematologic toxicities, including five who also experienced grade 4 nonhematologic toxicities: febrile neutropenia,2 depression, hypokalemia, and infection (lip). An additional patient on the standard arm experienced grade 4 hyponatremia.
Sixteen patients (10%) on the continuous arm experienced grade 4 hematologic toxicities, with one additional patient experiencing grade 4 stomatitis/pharyngitis. There was one death due to liver failure on the continuous arm deemed possibly related to treatment.
Paclitaxel toxicity was evaluated across treatment arms for 298 patients (data not shown), with 85 patients (29%) experiencing a grade 3 event. Fifteen patients (5%) had a grade 4 event: 13 hematologic toxicities, one sensory neuropathy, and one sensory neuropathy and hyperglycemia.
pCR Rates by Treatment Arm
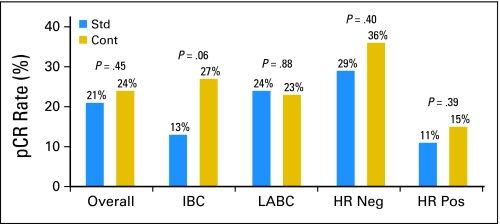
The analysis of pCR for the 356 eligible patients is presented in Table 3 and Figure 2. Overall, a pCR was detected in 80 patients (22.5%): 37 patients (20.7%) in the standard arm compared with 43 patients (24.3%) in the continuous arm (exact P = .45).
Table 3.
pCR Rates by Disease Subtype, Receptor Status, Stage, and Treatment Arm
| Variable | Standard Arm |
Continuous Arm |
Total |
P† | |||
|---|---|---|---|---|---|---|---|
| No.* | % | No.* | % | No.* | % | ||
| Overall | .45 | ||||||
| pCR | 37/179 | 20.7 | 43/177 | 24.3 | 80/356 | 22.5 | |
| No pCR or no surgery | 142/179 | 79.3 | 134/177 | 75.7 | 276/356 | 77.5 | |
| Inflammatory breast cancer | .06 | ||||||
| pCR | 7/56 | 12.5 | 15/55 | 27.3 | 22/111 | 19.8 | |
| No pCR or no surgery | 49/56 | 87.5 | 40/55 | 72.7 | 88/111 | 80.2 | |
| Locally advanced breast cancer | .88 | ||||||
| pCR | 30/123 | 24.4 | 28/122 | 23.0 | 58/245 | 23.7 | |
| No pCR or no surgery | 93/123 | 75.6 | 94/122 | 77.0 | 187/245 | 76.3 | |
| Receptor-negative breast cancer | .40 | ||||||
| pCR | 25/86 | 29.0 | 26/73 | 35.6 | 51/159 | 32.1 | |
| No pCR or no surgery | 61/86 | 71.0 | 47/73 | 64.4 | 108/159 | 67.9 | |
| Receptor-positive breast cancer | .39 | ||||||
| pCR | 9/84 | 10.7 | 15/98 | 15.3 | 24/182 | 13.2 | |
| No pCR or no surgery | 75/84 | 89.3 | 83/98 | 84.7 | 158/182 | 86.8 | |
| Stage IIB | .23 | ||||||
| pCR | 13/35 | 37.1 | 4/21 | 19.1 | 17/56 | 30.4 | |
| No pCR or no surgery | 22/35 | 62.9 | 17/21 | 80.9 | 39/56 | 59.6 | |
| Stage IIIA | .67 | ||||||
| pCR | 16/54 | 29.6 | 14/56 | 25.0 | 30/110 | 27.3 | |
| No pCR or no surgery | 38/54 | 70.4 | 42/56 | 75.0 | 80/110 | 72.7 | |
| Stage IIIB | .0057 | ||||||
| pCR | 8/86 | 9.3 | 24/93 | 25.8 | 32/179 | 17.9 | |
| No pCR or no surgery | 78/86 | 90.7 | 69/93 | 74.2 | 147/179 | 82.1 | |
Abbreviation: pCR, pathologic complete response.
No. refers to No. achieving indicated result (pCR v no pCR) over total evaluated for this end point.
All P values are two sided.
Fig 2.
Pathologic complete response (pCR) rates overall and by subgroup. Rates and associated two-sided P value for the comparison of the standard arm (Std) to the continuous therapy arm (Cont) are shown for the group overall, then by disease type (inflammatory breast cancer [IBC] or locally advanced breast cancer [LABC]), and then by hormone receptor (HR) status. Neg, negative; Pos, positive.
A test of pCR by treatment group stratified by disease type yielded an exact P value of .42, although there is an indication that the effects of treatment on pCR differ for the two disease types (interaction P = .075). Therefore, the effect of treatment was considered in subsets of disease type, hormone receptor status, and tumor stage (Table 3).
For 111 patients with IBC, the overall pCR rate was 19.8%. Specifically, a pCR was detected in seven patients (12.5%) in the standard arm compared with 15 patients (27.3%) in the continuous arm (exact P = .06). The direction was in favor of the continuous arm with an absolute difference in pCR rates of 14.8% (95% CI, 0.1% to 29.4%). For LABC (n = 245), the overall pCR rate was 23.7%: 30 patients (24.4%) in the standard arm compared with 28 patients (23.0%) in the continuous arm (exact P = .88).
pCR Rates by Hormone Receptor Status, HER2 Status, and Tumor Stage
Despite random assignment, more tumors in the continuous arm were hormone receptor–positive (57.3%) compared with those in the standard arm (49.4%). Since pCR was much less likely in hormone receptor–positive tumors (P < .001), this imbalance could affect the difference between the arms. However, as described in Table 3, there was no significant difference in pCR rates by treatment for patients with hormone receptor–negative tumors (n = 159; exact P = .40) and those with hormone receptor–positive tumors (n = 182; exact P = .39). Treatment results also did not differ by HER2 status: patients with HER2-negative breast cancer (n = 230; exact P = .51) or those with HER2-positive tumors (n = 90; exact P = 1.00) had similar pCR rates (results not shown). In Table 3, there is no difference between the two treatment groups for stage IIB and IIIA, but there is a large significant difference for stage IIIB tumors (P = .0057) with a higher pCR rate in the continuous arm. The interaction of stage and treatment was statistically significant (P = .0081), testing the homogeneity of the treatment effect across the three stages.
The probability of pCR by treatment group, disease type, and other factors was modeled by using logistic regression. The unadjusted odds ratio (OR) was in favor of the continuous arm (OR, 1.23; 95% CI, 0.75 to 2.03; P = .41) but was not statistically significant. For stage IIIB, the OR favored the continuous arm (OR, 3.86; 95% CI, 1.53 to 9.72; P = .004) adjusting for disease subtype and hormone receptor status. In contrast, for stages IIB and IIIC, the difference was in favor of standard therapy, but it was not significant (OR, 0.77; 95% CI, 0.37 to 1.63; P = .50) after adjustment.
Survival Analysis
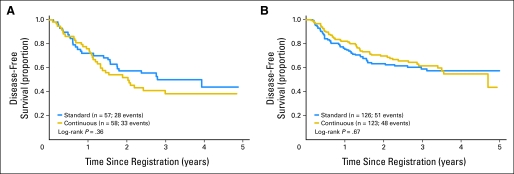
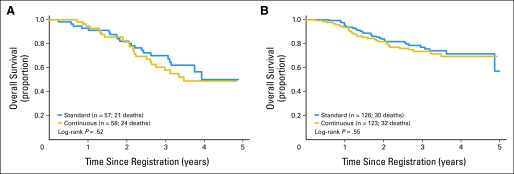
Overall, there was no evidence that DFS differed by treatment. The unadjusted HR for the continuous arm versus the standard arm was 1.03 (95% CI, 0.75 to 1.40; P = .87). Adjusting for disease type did not change the overall results. Figure 3 shows no significant effect of treatment on DFS in IBC (P = .36) or LABC (P = .67). However, IBC has poorer DFS than LABC (P = .021). Similarly, there was no evidence that OS differed by treatment. The unadjusted HR for the continuous arm versus the standard arm was 1.19 (95% CI, 0.81 to 1.74; P = .37). Separate evaluation of OS by disease type showed no difference due to treatment (Fig 4).
Fig 3.
Disease-free survival by treatment group by disease subtype. The graphs show Kaplan-Meier estimates of disease-free survival from time of registration to progression, disease recurrence, or death as a result of any cause. The log-rank P values compare the two randomized arms within disease subtype. (A) Inflammatory breast cancer and (B) locally advanced breast cancer.
Fig 4.
Overall survival by treatment group by disease subtype. The graphs show Kaplan-Meier estimates of overall survival from time of registration to death as a result of any cause. The log-rank P values compare the two randomized arms within disease subtype. (A) Inflammatory breast cancer and (B) locally advanced breast cancer.
DISCUSSION
This trial represents a unique effort to compare systemic therapies in women who meet the commonly used clinical definitions of LABC or IBC. Randomization was stratified by disease type since IBC has worse outcomes.10 Cristofanilli et al,11 reported 5-year progression-free survival of approximately 35% for a small group with IBC in which paclitaxel was incorporated. This outcome was similar to that in a larger historical study from the same institution, but paclitaxel was given as part of neoadjuvant therapy only in those who had a poor response to initial anthracycline-based treatment, then used in all patients after surgery. From a more comprehensive summary by Hortobagyi et al,12 on the basis of the FAC regimen and variations, it appears that approximately 45% of patients with LABC and 35% of those with IBC achieve 5-year DFS, and that after 5 years, there is a near plateau in both groups, suggesting the possibility of cure in a substantial minority. Thus, the overall outcome of the SWOG 0012 study appears at least comparable to historical data and may serve as a suitable comparison for modern chemotherapy.
The main goal of this trial was to determine whether there was a difference in pCR at the primary site between standard intermittent AC and a more continuous form of AC, which required hematopoietic growth factor support, followed by weekly paclitaxel in both groups. We were unable to show such a difference, with overall pCR rates of 21% on the standard and 24% on the continuous arm. Unfortunately, despite random assignment, there was an imbalance in the distribution of hormone receptor–positive disease, so that more patients on the continuous arm had positive tumors than on the standard arm. This may affect the interpretation of the outcome, since the receptor status by pCR (collapsed over treatment) showed a pCR rate of 32% for hormone receptor–negative tumors and 13% for hormone receptor–positive tumors (P < .001), confirming the findings of other studies.12–15 Although there was a suggestion of a benefit of continuous therapy in stage IIIB disease, we recognize this was an unplanned subset analysis and the finding could be due to chance. Consequently, our trial fails to demonstrate a sufficient magnitude of benefit for the continuous arm to recommend that a continuous therapy regimen replace the current standard therapy regimen.
DFS and OS also did not differ by treatment group. In a landmark analysis confined to patients who were survivors at 1 year (data not shown), there was clear evidence of improved DFS for those who achieved pCR versus those who did not (P < .001). This justifies the continued use of pCR as a surrogate end point for survival after neoadjuvant therapy. However, multiple factors contribute to survival that may obscure any effect of pCR. In our study, as in others, LABC had better DFS than IBC (P = .040), and patients with hormone receptor–positive tumors had better DFS than those with hormone receptor–negative breast cancer (P < .001), despite having worse pCR rates.
What accounted for the failure of our continuous metronomic program to improve on the standard? One possibility is that paclitaxel on a weekly schedule, previously shown to improve outcome over anthracycline-based therapy alone in the neoadjuvant setting, may be a great leveler, obscuring the effect of scheduling for AC. Second, in the absence of an added drug specifically targeting angiogenesis, any antiangiogenic effects of our continuous approach with chemotherapy may be too weak to be of clinical benefit, or there may be rapid recovery and even overshoot in angiogenesis in remaining tumor after cessation of the therapy, as suggested in preclinical models.16 Finally, it is possible that there is a deleterious effect that results from the administration of G-CSF in the continuous regimen, due to an upregulation in circulating endothelial progenitor cells, as suggested by Natori et al.17 This might vitiate negative direct effects on endothelial cell viability.
There was a marginally significant effect on pCR rate in IBC, which is known to have a greater reliance on angiogenesis.18 It is possible that this approach, if it makes a net contribution to antiangiogenesis (eg, via thrombospondin upregulation), may have greater potency than standard intermittent therapy when combined with an agent specifically targeting angiogenesis by another mechanism (eg, vascular endothelial growth factor inhibition). Finally, given microscopic residual disease after surgery rather than bulk, high-volume disease before surgery, a beneficial effect of continuous versus standard chemotherapy might be seen only in the adjuvant setting. For example, significant benefit for comparison of docetaxel plus AC versus FAC emerged in the adjuvant setting but not in the setting of more advanced disease.19,20 Such an adjuvant trial (SWOG-S0221) is currently ongoing as an Intergroup study in North America and is nearing completion of accrual.
Appendix
The first 27 patients accrued to this trial were treated with the doxorubicin and cyclophosphamide portion of therapy alone, before the trial was amended to include weekly paclitaxel as the second component of neoadjuvant therapy. We report briefly on these first 27 patients (17 inflammatory breast cancer; 10 locally advanced breast cancer). Twelve were randomly assigned to standard therapy and 15 to continuous therapy. Receptor status was known for 25 patients, 84% of whom were receptor negative. Pathologic complete response (pCR) was not evaluated for four patients. None of the 12 patients who received standard therapy had a pCR, although six of 11 patients who received metronomic therapy had a pCR (exact P = .005). The first 27 patients were more likely to have inflammatory breast cancer and be receptor negative than patients enrolled after the amendment. Their subsequent survival was poorer, as would be expected for patients with these tumor characteristics.
Footnotes
Supported in part by Public Health Service Cooperative Agreement Grants No. CA32102, CA38926, CA58882, CA86780, CA14028, CA20319, CA52654, CA63845, CA379891, CA63844, CA22433, CA35431, CA76448, CA35176, CA58861, CA46441, CA35178, CA67575, CA45808, CA12644, CA11083, CA35192, CA76447, CA46282, CA45560, CA04919, CA45377, CA35090, CA74811, CA95860, CA35281, CA74647, CA76462, CA35128, CA76462, CA46136, and CA35119 awarded by the National Cancer Institute, Department of Health and Human Services.
Presented in part at the 42nd Annual Meeting of the American Society of Clinical Oncology, June 2-6, 2006, Atlanta, GA.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00016406.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Gabriel N. Hortobagyi, Novartis (C), Allergan (C), Genentech (C), Merck (C), sanofi-aventis (C), Taivex Therapeutics (U) Stock Ownership: None Honoraria: Georgiana K. Ellis, Amgen Research Funding: Julie R. Gralow, Bristol-Myers Squibb, Amgen; Gabriel N. Hortobagyi, Novartis Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Georgiana K. Ellis, Robert B. Livingston
Administrative support: Gabriel N. Hortobagyi
Provision of study materials or patients: Georgiana K. Ellis, Julie R. Gralow, Christy A. Russell, Melanie E. Royce, Edith A. Perez,Robert B. Livingston
Collection and assembly of data: Georgiana K. Ellis, William E. Barlow, Danika Lew
Data analysis and interpretation: Georgiana K. Ellis, William E. Barlow, Edith A. Perez, Robert B. Livingston
Manuscript writing:Georgiana K. Ellis, William E. Barlow, Julie R. Gralow, Gabriel N. Hortobagyi, Christy A. Russell, Melanie E. Royce, Edith A. Perez, Danika Lew, Robert B. Livingston
Final approval of manuscript: Georgiana K. Ellis, William E. Barlow, Julie R. Gralow, Gabriel N. Hortobagyi, Christy A. Russell, Melanie E. Royce, Edith A. Perez, Danika Lew, Robert B. Livingston
REFERENCES
- 1.Hryniuk W, Bush H. The importance of dose intensity in chemotherapy of metastatic breast cancer. J Clin Oncol. 1984;2:1281–1288. doi: 10.1200/JCO.1984.2.11.1281. [DOI] [PubMed] [Google Scholar]
- 2.Hryniuk WM, Levine MN, Levin L. Analysis of dose intensity for chemotherapy in early (stage II) and advanced breast cancer. NCI Monogr. 1986;1:87–94. [PubMed] [Google Scholar]
- 3.Hryniuk W, Levine MN. Analysis of dose intensity for adjuvant chemotherapy trials in stage II breast cancer. J Clin Oncol. 1986;4:1162–1170. doi: 10.1200/JCO.1986.4.8.1162. [DOI] [PubMed] [Google Scholar]
- 4.Ellis GK, Livingston RB, Gralow JR, et al. Dose-dense anthracycline-based chemotherapy for node-positive breast cancer. J Clin Oncol. 2002;20:3637–3643. doi: 10.1200/JCO.2002.12.113. [DOI] [PubMed] [Google Scholar]
- 5.Ellis G, Green S, Livingston R, et al. Neoadjuvant doxorubicin, cyclophosphamide and G-CSF (AC+G) for locally advanced breast cancer (LABC), a Southwest Oncology Group phase II study. Proc Am Soc Clin Oncol. 2000;19(suppl):85a. abstr 326. [Google Scholar]
- 6.Citron ML, Berry DA, Cirrincione C, et al. Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: First report of Intergroup Trial C9741/Cancer and Leukemia Group B Trial 9741. J Clin Oncol. 2003;21:1431–1439. doi: 10.1200/JCO.2003.09.081. [DOI] [PubMed] [Google Scholar]
- 7.Man S, Bocci G, Francia G, et al. Antitumor effects in mice of low-dose (metronomic) cyclophosphamide administered continuously through the drinking water. Cancer Res. 2002;62:2731–2735. [PubMed] [Google Scholar]
- 8.Green MC, Buzdar AU, Smith T, et al. Weekly paclitaxel improves pathologic complete remission in operable breast cancer when compared with paclitaxel once every 3 weeks. J Clin Oncol. 2005;23:5983–5992. doi: 10.1200/JCO.2005.06.232. [DOI] [PubMed] [Google Scholar]
- 9.Ellis GK, Green SJ, Russell CA, et al. SWOG 0012, a randomized phase III comparison of standard doxorubicin and cyclophosphamide followed by weekly paclitaxel versus weekly doxorubicin and daily oral cyclophosphamide plus G-CSF followed by weekly paclitaxel as neoadjuvant therapy for inflammatory and locally advanced breast cancer. J Clin Oncol. 2006;24(suppl):12s. doi: 10.1200/JCO.2009.27.6543. abstr LBA537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jaiyesimi IA, Buzdar AU, Hortobagyi G. Inflammatory breast cancer: A review. J Clin Oncol. 1992;10:1014–1024. doi: 10.1200/JCO.1992.10.6.1014. [DOI] [PubMed] [Google Scholar]
- 11.Cristofanilli M, Buzdar AU, Sneige N, et al. Paclitaxel in the multimodality treatment for inflammatory breast carcinoma. Cancer. 2001;92:1775–1782. doi: 10.1002/1097-0142(20011001)92:7<1775::aid-cncr1693>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 12.Hortobagyi GN, Singletary SE, Buchholz TA. Locally advanced breast cancer. In: Singletary SE, Robb GL, Hortobagyi HN, editors. Advanced Therapy of Breast Disease. ed 2. Hamilton, Ontario, Canada: BC Decker; 2000. [Google Scholar]
- 13.von Minckwitz G, Raab G, Caputo A, et al. Doxorubicin with cyclophosphamide followed by docetaxel every 21 days compared with doxorubicin and docetaxel every 14 days as preoperative treatment in operable breast cancer: The GEPARDUO study of the German Breast Group. J Clin Oncol. 2005;23:2676–2685. doi: 10.1200/JCO.2005.05.078. [DOI] [PubMed] [Google Scholar]
- 14.Liedtke C, Mazouni C, Hess KR, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol. 2008;26:1275–1281. doi: 10.1200/JCO.2007.14.4147. [DOI] [PubMed] [Google Scholar]
- 15.Stearns V, Singh B, Tsangaris T, et al. A prospective randomized pilot study to evaluate predictors of response in serial core biopsies to single agent neoadjuvant doxorubicin or paclitaxel for patients with locally advanced breast cancer. Clin Cancer Res. 2003;9:124–133. [PubMed] [Google Scholar]
- 16.Mancuso MR, Davis R, Norberg SM, et al. Rapid vascular regrowth in tumors after reversal of VEGF inhibition. J Clin Invest. 2006;116:2610–2621. doi: 10.1172/JCI24612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Natori T, Sata M, Washida M, et al. G-CSF stimulates angiogenesis and promotes tumor growth: Potential contribution of bone marrow-derived endothelial progenitor cells. Biochem Biophys Res Commun. 2002;297:1058–1061. doi: 10.1016/s0006-291x(02)02335-5. [DOI] [PubMed] [Google Scholar]
- 18.Kleer CG, van Golen KL, Merajver SD. Molecular biology of breast cancer metastasis: Inflammatory breast cancer—Clinical syndrome and molecular determinants. Breast Cancer Res. 2000;2:423–429. doi: 10.1186/bcr89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin M, Pienkowski T, Mackey J, et al. Adjuvant docetaxel for node-positive breast cancer. N Engl J Med. 2005;352:2302–2313. doi: 10.1056/NEJMoa043681. [DOI] [PubMed] [Google Scholar]
- 20.Mackey JR, Paterson A, Dirix LY, et al. Final results of the phase III randomized trial comparing docetaxel (T), doxorubicin (A) and cycclophosphamide (C) to FAC as first line chemotherapy (CT) for patients (pts) with metastatic breast cancer (MBC) Proc Am Soc Clin Oncol. 2002;21(suppl):35a. abstr 137. [Google Scholar]