Abstract
Human immunodeficiency virus type 1 (HIV) and Mycobacterium tuberculosis have become intertwined over the past few decades in a “syndemic” that exacerbates the morbidity and mortality associated with each pathogen alone. The severity of the coinfection has been extensively examined in clinical studies. The extrapolation of peripheral evidence from clinical studies has increased our basic understanding of how HIV increases susceptibility to TB. These studies have resulted in multiple hypotheses of how HIV exacerbates TB pathology through the manipulation of granulomas. Granulomas can be located in many tissues, most prominently the lungs and associated lymph nodes, and are made up of multiple immune cells that can actively contain M. tuberculosis. Granuloma-based research involving both animal models and clinical studies is needed to confirm these hypotheses, which will further our understanding of this coinfection and may lead to better treatment options. This review examines the data that support each hypothesis of how HIV manipulates TB pathology while emphasizing a need for more tissue-based experiments.
The emergence of human immunodeficiency virus type 1 (HIV) has exacerbated an already enormous number of cases of tuberculosis (TB) worldwide. TB affects HIV+ individuals throughout all phases of HIV infection and is the leading killer of HIV+ people (31). Of the 9.4 million individuals with new cases of active TB each year, 1.4 million are HIV+ (39). It is widely accepted that HIV causes a depletion of CD4 T cells, which is likely to contribute to the susceptibility of coinfected persons to TB, as this T cell subset is important in the control of TB. However, HIV has effects on other cells, including macrophages, and influences cytokine production, which may also prevent a host from containing an initial or latent Mycobacterium tuberculosis infection. In this review, we highlight gaps in the human coinfection literature that must be addressed to gain a more complete understanding of the interaction between the pathogens M. tuberculosis and HIV.
M. tuberculosis infects via the respiratory tract, encountering alveolar macrophages in the airways and transiting to the lung parenchyma, where innate and adaptive immune responses cooperate to generate a granuloma. The granuloma is a structure composed of macrophages, lymphocytes, dendritic cells, neutrophils, and sometimes fibroblasts, often with a necrotic center. This structure serves to contain the bacilli and acts as an immune microenvironment for cellular interactions that limit M. tuberculosis replication. However, simple formation of a granuloma is not sufficient for control of infection, as persons with active TB have multiple granulomas in the lungs and possibly other tissues. Instead, the granuloma must have optimal immunologic function to contain or eliminate the bacilli. As a highly evolved pathogen, M. tuberculosis has devised strategies for persisting within the granuloma and avoiding elimination by the host response. In latent infection, the host and bacillus coexist, with the granuloma serving as the site of bacterial persistence and host resistance. Disruption of the structure or function of the granuloma is likely to lead to reactivation of latent M. tuberculosis infection, dissemination, and active disease.
The current human HIV/M. tuberculosis literature provides a solid foundation for our current understanding of how these pathogens interact in vitro and in vivo. Several hypotheses have been generated to identify how HIV increases the risk of TB and how M. tuberculosis infection may exacerbate HIV infection. Here, we summarize the data that underlie these hypotheses (Table 1). However, it must be noted that these hypotheses are based on indirect evidence, extrapolated from experimentally tractable peripheral sampling to events in M. tuberculosis-infected tissues. Although many of these hypotheses are likely valid, confirming the events occurring in granulomas is a necessary next step. Focusing on confirming these hypotheses at the tissue level in future HIV/M. tuberculosis coinfection studies may identify new mechanisms that drugs and vaccines can target to prevent or cure TB in coinfected people.
TABLE 1.
How does HIV increase TB risk?
| Hypothesis and exptl evidence | Reference(s) |
|---|---|
| HIV replication is increased at sites of M. tuberculosis infection, leading to increased pathology | |
| Greater HIV p24 levels and viral loads in BAL fluid cells from TB-involved lungs than in BAL | |
| fluid from uninvolved lungs | 69 |
| Greater HIV loads in pleural fluid than in plasma from individuals with pleural TB | 52 |
| Greater HIV replication in stimulated macrophages infected with M. tuberculosis and HIV | |
| than in macrophages infected with HIV alone | 43, 44 |
| HIV induces primary or reactivated TB through killing of CD4 T cells within granulomas | |
| HIV+ individuals with fewer peripheral CD4 T cells are more prone to TB than HIV+ | |
| individuals with more CD4 T cells | 51 |
| Correlation between acute peripheral CD4 T cell count and reactivation of latent TB | 23 |
| Coinfected individuals have fewer BAL fluid CD4 T cells than individuals with TB alone | 46, 49 |
| Monkeys coinfected with SIVmac251 and M. tuberculosis have fewer CD4 T cells in granulomatous | |
| tissue than monkeys with active TB alone | 23 |
| HIV manipulation of macrophage function prevents M. tuberculosis killing | |
| HIV/M. tuberculosis-coinfected macrophages induce less TNF-dependent apoptosis than | |
| macrophages infected with only M. tuberculosis | 48, 72, 73 |
| Coinfected macrophages release less TNF than macrophages infected with only M. tuberculosis | 48, 73 |
| HIV decreases the ability of M. tuberculosis-infected macrophages to acidify vesicles | 22, 67 |
| HIV induces functional changes in M. tuberculosis-specific T cells that decrease their ability | |
| to contain M. tuberculosis | |
| Fewer IFN-γ+M. tuberculosis-specific memory CD4 T cells after HIV infection in individuals | |
| with latent TB | 29, 30 |
| Fewer IFN-γ-TNF-IL-2 polyfunctional BCG-specific CD4 T cells in airways of HIV+ individuals | |
| than in those of HIV− individuals | 46 |
| Lower IFN-γ mRNA production and cellular proliferation in airways of patients with AIDS and | |
| TB than in those of individuals with TB alone | 4, 17 |
| Lower IFN-γ, TNF, and IL-2 production and cellular proliferation in M. tuberculosis-specific peripheral T cells | |
| in HIV+ individuals than in HIV− individuals with active TB | 30, 40, 65, 111 |
HIV/M. TUBERCULOSIS PATHOLOGY
It is well established that HIV impairs the ability to control M. tuberculosis infection (32, 89, 95, 96, 108). Clinical studies provide compelling evidence that HIV leads to an increased risk of developing TB shortly after HIV infection. Among miners in South Africa, HIV+ individuals were 2 to 3 times more likely to develop TB than HIV− miners within 2 years of HIV seroconversion (32, 95) and after 11 years, half of the HIV+ miners developed TB (32). Although HIV+ individuals in these studies are more prone to developing TB, half of the cases of TB were attributed to time and not HIV due to the high incidence rate of TB among South African miners. It was not determined whether TB was the result of reactivation of latent infection or newly acquired M. tuberculosis infection. It is important to differentiate between reactivation and newly acquired TB because the mechanisms by which the human host controls primary and latent infections, and the effects of HIV on these mechanisms, may differ. Evidence from DNA fingerprinting (typing for IS6110 restriction fragment length polymorphism) studies indicates that HIV+ people in regions where the disease is endemic, such as South Africa and Malawi, are developing TB primarily by new infection rather than by reactivation of a latent infection (18, 96). In this type of study, the pattern of IS6110 sequences among M. tuberculosis isolates from patients within the cohort indicates whether the TB case is newly acquired or a relapse of latent TB. HIV+ individuals are between 2.2 (18) and 5.5 (96) times more likely to develop TB from a new source than are HIV-negative individuals.
Not only are HIV+ individuals at greater risk of acquiring M. tuberculosis and developing active TB, they have an increased risk of death due to TB (107, 108). Although it has been well known over the past 25 years that HIV/M. tuberculosis coinfection is remarkably detrimental (70, 75, 89), the mechanisms by which HIV disrupts function in both established and newly forming granulomas, leading to the increased morbidity and mortality of coinfected people compared to those of people with TB alone, remain to be determined (50).
EFFECTS OF HIV ON THE M. TUBERCULOSIS GRANULOMA
It has been proposed that the increase in pathology associated with HIV/M. tuberculosis coinfection is caused by a functional disruption of the local immune response within the granuloma (3, 20, 50, 83, 91). These disruptions presumably decrease the ability of the granuloma to contain M. tuberculosis, leading to increased bacterial growth with more mycobacterial dissemination and severe pathology. The cause of the disruption can be divided into general and overlapping processes, including (i) an increase in the viral load within involved tissue, leading to (ii) a decrease in the total number of CD4 T cells, along with (iii) a disruption of macrophage function and (iv) a perturbation of M. tuberculosis-specific T cell function that lead to functional and detrimental changes within granulomas. Here we review the available data on these HIV-induced changes (Table 1).
HIV REPLICATION AT SITES OF M. TUBERCULOSIS INFECTION
Hypothesis: HIV replication increases at sites of M. tuberculosis infection, which leads to a reduction in the containment of M. tuberculosis.
HIV preferentially replicates within activated CD4 T cells and macrophages. Because CD4 T cells and macrophages are major components of the granuloma and some proportion of these T cells are likely to be activated (an ideal situation for HIV uptake and replication), sites of M. tuberculosis infection are considered ideal for HIV replication. An increased viral load within involved tissue would likely cause a disruption in equilibrium between granuloma function and mycobacterial growth. The available literature supports the idea that M. tuberculosis infection leads to increased viral replication in vitro, ex vivo, and in vivo.
M. tuberculosis increases HIV replication in stimulated coinfected macrophages in vitro.
Multiple studies have been performed to determine whether M. tuberculosis influences HIV replication. The data on the replication of HIV within M. tuberculosis-coinfected macrophages is controversial, supporting both increases (34, 43, 44, 80) and decreases (33) in viral replication. In vitro studies have demonstrated the importance of the macrophage activation state and the presence of proinflammatory cytokines in inducing HIV replication. THP-1 macrophages in contact with lymphocytes or neutrophils induce viral replication (43, 44). These coinfected macrophages increase HIV replication when the CCAAT enhancer binding protein beta (C/EBPb) transcription factor is inhibited and an increase in NF-κB production is induced, and NF-κB binds to the HIV long terminal repeat and initiates viral transcription. HIV replication decreased in the presence of neutralizing antibodies to tumor necrosis factor (TNF) and interleukin-6 (IL-6) and increased in the presence of antibodies to IL-10 and transforming growth factor β (34), which further supports the idea that coinfected activated macrophages increase HIV replication. However, the increase in viral replication may be M. tuberculosis strain specific, since clinical strains of M. tuberculosis can manipulate replication of HIV to different degrees. For example, strain CDC1551, which is a clinical strain but is considered less virulent (81), induces more HIV replication than the more virulent clinical strain HN878 in coinfected peripheral blood mononuclear cells (PBMC) (80). M. tuberculosis has also been shown to decrease HIV replication in coinfected macrophages. Monocyte-derived macrophages incubated with heat-inactivated M. tuberculosis prior to HIV infection prevented viral replication despite an increase in CCR5, a coreceptor used by HIV to infect cells (33). However, most of the data support the increase in HIV replication, and this may be deleterious to the coinfected individual because the increase in HIV replication enhances the transmission of HIV to autologous T cells in vitro (59) and M. tuberculosis-specific CD4 T cells in vivo (29). The increase in HIV transmission may be caused by an increase in T cell proliferation and viral release occurring when T cells are incubated with HIV/M. tuberculosis-coinfected macrophages rather than macrophages infected with HIV alone (59).
M. tuberculosis microenvironments increase HIV replication ex vivo and in vivo.
The immune environment created by M. tuberculosis also promotes viral replication ex vivo. M. tuberculosis causes an increase in inflammatory cytokines in vivo, which can lead to activation of T cells and macrophages, which induces replication of HIV. Garrait et al. determined that incubation of HIV-infected PBMC with pleural fluid from individuals with TB induces more replication than incubation with pleural fluid from individuals without TB (28). This increase in replication was dependent on IL-6 and TNF, which supports the notion that activated cells induced by proinflammatory environments may increase HIV replication. This supports work that confirmed that HIV replication increases in activated CD4 T cells (101) and CD14+ macrophages (52), which are prevalent at sites of M. tuberculosis infection.
High viral titers are inversely proportional to peripheral CD4 T cell counts and correlated with susceptibility to various opportunistic infections (1, 9, 66) and advancement to AIDS. One study concluded that there was a 5- to 160-fold increase in plasma viral titers during acute infection with M. tuberculosis (35), while another determined that viral titers were 2.5 times higher in HIV+ individuals upon TB diagnosis (103). A transient increase in viral titers may occur in coinfected people during acute TB due to an increase in activated CD4 T cells. However, most of the clinical research has demonstrated that plasma viral titers do not correlate with susceptibility to active TB in coinfected people (51) or in monkeys infected with simian immunodeficiency virus (SIV) and M. tuberculosis (23, 83). Likewise, treatment of TB does not necessarily lead to a reduction in plasma viral loads (47); further supporting the idea that the peripheral viral load does not by itself represent susceptibility to TB.
Although little correlation between the plasma viral titer and reactivation of TB has been observed in coinfected individuals, high viral loads within involved tissues have been suggested as a cause of the functional disruption within the granuloma (16, 35, 50). Nakata et al. determined that HIV replicates within the lungs by measuring viral loads and p24 levels in bronchoalveolar lavage (BAL) fluid cells from coinfected individuals (69). BAL fluid represents the airway environment, which may be an initial site of M. tuberculosis replication, and is also a site of M. tuberculosis replication during active TB but may not accurately represent events within the granulomas in the lung parenchyma. BAL fluid cells sampled from the airways of involved lungs of individuals coinfected with HIV and M. tuberculosis (radiographic evidence of TB infiltrate) had higher viral titers and p24 levels than cells from the airways of uninvolved lungs of the same persons (69). In this same study, the viral load within BAL fluid cells was greater than that in plasma. This study was one of the first to demonstrate that HIV may replicate more at sites of disease. Other studies have confirmed that sites of M. tuberculosis infection have increased viral replication in coinfected patients (15, 16, 102). Pleural fluid from coinfected subjects has higher viral titers (102) and greater HIV heterogeneity (16) than plasma from the same patients. Increases in viral titer and heterogeneity within M. tuberculosis-involved tissue may increase viral fitness (15) and decrease the ability to contain both infections. On the contrary, granulomas from cynomolgus macaques coinfected with SIVmac251 and M. tuberculosis displayed SIV viral loads similar to those of uninvolved tissues, albeit with substantial variability (23). Since these monkeys had very low plasma and PBMC viral loads, they may not represent exactly what is occurring within coinfected humans.
These studies provide a basic framework for our understanding of how M. tuberculosis manipulates HIV replication. However, no clinical studies have demonstrated that granulomas provide this ideal environment for HIV replication, which emphasizes the need for clinical researchers to determine whether the granuloma environment is influencing virus replication directly and the need for animal models of coinfection.
CHANGES IN THE T CELL NUMBER WITHIN GRANULOMAS
Hypothesis: HIV induces active or reactivated TB by reducing CD4 T cells within granulomas.
CD4 T cells are essential for the containment of M. tuberculosis and the long-term survival of infected mice, which was demonstrated by a significant decrease in survival time and an increase in the bacterial burden in major histocompatibility complex class II and CD4−/− mice (10). HIV and SIV cause substantial reductions in peripheral, mucosal, and gut CD4 T cells shortly after infection by preferentially infecting activated CD4 T cells (11, 62, 86, 105) and resting memory CD4 T cells (8, 55). Studies have determined that SIV, and presumably HIV, kills up to 60% of the gut CD4 T cells within the first 10 days of infection, with an 80% reduction of these cells by 2 weeks postinfection (11, 62). The affected cells are mostly effector memory T cells (55, 105), which are abundant at these sites. Because HIV depletes these cells within the periphery, gut, and mucosal tissue (86, 104), it has been hypothesized that HIV-induced depletion of CD4 T cells within granulomas leads to a direct disruption of the containment of M. tuberculosis infection (49, 50).
HIV-induced decreases in peripheral CD4 T cells correlate with susceptibility to TB.
The peripheral CD4 T cell count is a standard measure of disease progression in HIV-infected individuals, and this has been reported for many HIV/M. tuberculosis coinfection studies (7, 23, 30, 40, 51). HIV+ individuals are more susceptible to TB than HIV− people are, regardless of their peripheral CD4 T cell counts (88, 89), although susceptibility increases with decreasing peripheral CD4 T cell counts. HIV+ individuals with <200 CD4 T cells/μl blood are more susceptible to TB than HIV+ individuals with >500 CD4 T cells/μl blood, regardless of antiretroviral therapy (51). Similarly, an acute and transient decrease in peripheral CD4 T cells after SIVmac251 inoculation in monkeys with latent TB significantly correlated with time to development of reactivation TB (23). The reduction in CD4 T cell counts was not confined to the periphery, as coinfected monkeys with reactivated latent TB within 17 weeks of SIVmac251 inoculation had a trend toward fewer BAL fluid CD4 T cells than monkeys with reactivated latent TB after 26 weeks. A lower frequency of BAL fluid CD4 T cells has also been noted in HIV+ individuals with TB than in HIV− individuals with TB (7, 46, 49) and also in HIV+ individuals without TB who live in areas with high TB incidence rates than in HIV− individuals in the same community (46).
Depletion of T cells in TB granulomas of AIDS patients and SIV-coinfected monkeys.
Histological analysis was used to provide the first data on CD4 counts in granulomas in AIDS patients with TB (91). Lymph node biopsy specimens from patients with AIDS and tuberculous adenitis had fewer CD4 T cells than individuals without AIDS and tuberculous adenitis. In the absence of CD4 T cells within the granulomas, CD8 T cells were distributed throughout the granuloma without being confined to the periphery, as is normally observed. This suggests that CD4 T cells help maintain the architecture and integrity of the granuloma during coinfection. The reduction in CD4 T cell counts within granulomas of AIDS patients is not surprising because one characteristic of AIDS patients is having <200 CD4 T cells/μl blood and likely reflects a long duration of CD4 T cell depletion. Another study demonstrated that similar numbers of granulomas were observed in HIV+ and HIV− patients with pleural TB (41). No difference in the number of bacilli assessed by staining of culture-positive tissues was observed between the two groups. However, individuals with <100 CD4 T cells/μl blood were more likely to have acid-fast bacilli within biopsied granulomas than individuals with >100 CD4 T cells/μl. A threshold CD4 T cell count may be needed to prevent bacterial growth in coinfected individuals. Taken together, these studies demonstrate that the peripheral decrease in T cells correlates with a decrease within the granuloma during AIDS. If this correlation also occurs throughout the course of HIV infection, this may also explain why coinfected individuals with fewer CD4 T cells are more prone to developing active TB (51). Many coinfected people present with TB well before the development of AIDS, so these granulomas may not represent what is occurring in most coinfected people. We observed significantly fewer CD4 and CD8 T cells within lung granulomas of coinfected monkeys than in granulomas from those with active TB alone (23). The decrease in lung T cell numbers was independent of peripheral CD4 T cell counts, which means that HIV may selectively kill T cells directly involved in maintaining granulomas (i.e., activated T cells at the site) prior to loss of peripheral T cells and signs of AIDS.
Taken together, these studies demonstrate that CD4 (and possibly CD8) T cells play a very important role in preventing the development of TB in coinfected individuals. However, more studies are needed to confirm that T cell depletion is occurring within granulomas. Future studies may also provide a correlation of peripheral, airway, and granuloma T cell counts, which may be used as a biomarker of disease progression.
CHANGES IN MACROPHAGE FUNCTION
Hypothesis: the ability of HIV to manipulate macrophage function inhibits killing of intracellular M. tuberculosis.
Alveolar macrophages are presumably the first group of cells infected with M. tuberculosis and are the primary immune cells within the airways. They can act as a reservoir for both HIV and M. tuberculosis. Following the entry of M. tuberculosis into the parenchyma, monocytes migrate to the lungs and differentiate into different macrophage types within the granuloma. All of these macrophage types may be susceptible to HIV infection, as well as M. tuberculosis infection. HIV envelope phenotyping has suggested that HIV infects activated (HLA-DR+) alveolar macrophages (CD14+ CD36+), as well as lymphocytes (CD26+), in the pleural fluid (52) or airways (45) of coinfected individuals. Since HIV has been shown to infect macrophages in vivo, HIV is likely to disrupt the function of M. tuberculosis-infected macrophages (44, 45, 67, 72, 73), leading to granuloma dysfunction and increased bacterial growth and dissemination.
HIV decreases responsiveness to M. tuberculosis ex vivo.
Macrophage apoptosis appears to be a critical immune response to M. tuberculosis during coinfection (61, 72, 73, 76). Although it is not fully understood, HIV infection of alveolar macrophages from healthy adults (73) or HIV+ adults (72) is associated with reduced M. tuberculosis-induced apoptosis compared to that of macrophages infected with M. tuberculosis alone. Exogenous HIV Nef protein added to M. tuberculosis-infected macrophages inhibits ASK1/p38 mitogen-activated protein kinase signaling, which leads to a decrease in TNF release and TNF-dependent apoptosis (48), suggesting that infectious virus is not necessary for inducing this functional change within a macrophage. This is important because HIV is a retrovirus with an error-prone reverse transcriptase that causes numerous site mutations that render most viral buds noninfectious (54). Since phagolysosome fusion is inhibited in M. tuberculosis-infected alveolar macrophages from HIV+ individuals (67), apoptosis may be used as a last resort of infected macrophages. This allows other activated macrophages to engulf the nearby apoptotic bodies, which may lead to killing of the mycobacteria and enhanced induction of T cell responses (42). M. tuberculosis-induced apoptosis in macrophages is complex and may not always be beneficial to the host. Some evidence suggests that an increase in apoptosis occurs in alveolar macrophages from AIDS patients with pulmonary TB compared to that in individuals with only pulmonary TB (77). An increase in apoptosis may be beneficial to the pathogens because it would allow them to exit macrophages capable of killing. This may also lead to increased dissemination of M. tuberculosis and HIV.
HIV appears to manipulate both apoptosis (72, 73) and the ability of macrophages to acidify M. tuberculosis-infected phagosomes (22, 67). These changes in macrophage function may increase the risk of developing active or reactivated TB in coinfected patients. One limitation of many of the studies addressing how HIV and M. tuberculosis change macrophage function is the use of cell lines or monocyte-derived macrophages (26, 35, 43) rather than alveolar macrophages (44, 67, 68, 72, 73) or macrophages from granulomatous tissue. It is possible that alveolar macrophages respond differently than macrophages recruited to granulomas, as these environments are very different and represent different stages of the infectious process.
CHANGES IN M. TUBERCULOSIS-SPECIFIC T CELL RESPONSES
Hypothesis: HIV impairs the function of M. tuberculosis-specific T cells within involved tissue.
T cell-mediated responses are essential to protection against disease due to both M. tuberculosis and HIV. T cells release cytokines, including gamma interferon (IFN-γ), TNF, and IL-2, as well as a variety of cytolytic molecules that are important in controlling both M. tuberculosis and HIV. HIV can exhaust HIV-specific and nonspecific T cells (25, 82), which has led to the hypothesis that HIV reduces the number and functionality of M. tuberculosis-specific T cells in coinfected individuals (30, 40, 46, 65, 111).
HIV decreases peripheral M. tuberculosis-specific T cell responses.
Numerous studies have examined M. tuberculosis-specific T cell responses in individuals infected with M. tuberculosis by stimulating PBMC, BAL fluid, or pleural fluid cells with purified protein derivative (PPD) or culture filtrate protein (CFP) (both are mixtures of mycobacterial proteins and lipids), killed M. tuberculosis, or peptide pools from immunogenic M. tuberculosis-specific proteins ESAT-6, CFP10, and Ag85 (4, 17, 29, 30, 40, 65, 111). Zhang and colleagues demonstrated that PBMC stimulated with heat-killed M. tuberculosis from coinfected individuals proliferated significantly less, released less IFN-γ, and expressed less IL-2 and IL-12 mRNA than those from TB-only patients (111). PBMC from HIV/PPD+ (latently infected) individuals who were stimulated with whole M. tuberculosis lysate, ESAT6, or Ag85B proliferated less and released less IFN-γ than PBMC from HIV− PPD+ individuals (65). Likewise, PBMC stimulated with killed M. tuberculosis released less TNF without a decrease in IFN-γ release in coinfected individuals with active TB than in people with TB alone (40). These decreases were not observed in mitogen- or Candida albicans antigen-stimulated cells, which supports the idea that HIV is specifically manipulating M. tuberculosis-specific T cells. It should be noted that a few studies have demonstrated that coinfected individuals can have a high number of peripheral IFN-γ-releasing M. tuberculosis-specific T cells even with a low number of CD4 T cells (13, 71, 79). However, most of these data support, at least peripherally, the idea that HIV impairs the ability of T cells to respond to M. tuberculosis. The reduction in the observable number of peripheral M. tuberculosis-specific CD4 T cells may result from their direct infection by HIV in coinfected individuals (29).
One inherent limitation of HIV/M. tuberculosis coinfection clinical research is that it is difficult to assess changes in an immunologic response before and after HIV infection. One excellent study addressed this limitation by examining changes in the number of M. tuberculosis-specific peripheral CD4 T cells in individuals with latent M. tuberculosis infection before and after HIV seroconversion (30). They determined that within 3 months after HIV seroconversion, a dramatic decrease in peripheral M. tuberculosis-specific memory (CD27+ CD45RO+) CD4 T cells releasing IFN-γ occurred in 4 out of 5 individuals. Although only 5 individuals with latent TB became HIV seropositive during this study, it is the first to demonstrate that HIV specifically reduces M. tuberculosis-specific T cells over time. Changes in the peripheral responses have provided evidence to support the hypothesis that HIV depletes and/or functionally disrupts M. tuberculosis-specific T cells. However, since TB is rarely a systemic disease, it remains to be seen whether these peripheral changes are replicated within involved tissue. An alternative hypothesis is that HIV causes increased M. tuberculosis replication in tissues and peripheral cells migrate to the lungs in response to increased antigen, which would appear as a reduction in peripheral responses but may not indicate a true loss of specific responses.
HIV reduces M. tuberculosis-specific T cell responses in the airways.
Incubation of BAL fluid cells from HIV+ individuals (previously vaccinated with BCG, an avirulent vaccine strain of M. bovis) with BCG resulted in significantly fewer IFN-γ- and TNF-releasing BCG-specific CD4 T cells than when cells from HIV− individuals were used (46). This depletion also occurred in IFN-γ+ TNF+ IL-2+ polyfunctional CD4 T cells within the HIV group. Although these individuals did not have any signs of TB at the time of collection or in the past, this study demonstrates that HIV specifically impairs the function of both mono- and polyfunctional mycobacterium (BCG)-specific T cells even without active TB.
The reduction in BAL fluid T cell responses to mycobacteria also occurs in individuals coinfected with HIV and M. tuberculosis. AIDS patients with pulmonary TB have a lower ability to produce IFN-γ mRNA in isolated BAL fluid cells than individuals with pulmonary TB alone (17). The functional changes in the context of HIV are not limited to cytokine release. Less proliferation of pulmonary lymphocytes from BAL fluid stimulated with either PPD or an avirulent M. tuberculosis strain was observed in individuals with AIDS and TB than in individuals with TB alone (4). T cell responses in AIDS patients may not recapitulate what is occurring within HIV+ individuals prior to the significant depletion of CD4 T cells associated with AIDS. However, the BAL fluid studies suggest that HIV disrupts multiple pulmonary T cell functions that may be required to prevent reactivation of latent M. tuberculosis infection.
HIV changes the cytokine profile within granulomas.
In situ hybridization and immunohistochemistry have been used to identify changes in cytokine expression within coinfected granulomas (3, 20). Although these techniques cannot determine changes in the function of M. tuberculosis-specific T cells, they provide an overall summary of how cells are responding within the context of granulomas. Bezuidenhout et al. determined that the same number of granulomas within HIV+ and HIV− individuals with pleural TB express Th1 (IFN-γ, IL-12, TNF) or Th2 (IL-4, IL-10) mRNA (3). However, it was determined that granulomas within HIV+ patients expressed more IFN-γ, TNF, IL-12, and IL-4 mRNA than granulomas from HIV− individuals. The increase in TNF mRNA expression correlated with an increase in necrotic granulomas within the coinfected patients. This does not necessarily mean that the M. tuberculosis-specific T cells are producing more cytokines in coinfected granulomas. It is possible that the increase in HIV antigens within the granulomas causes an increase in HIV-specific T cell activity too, which cannot be determined without antigen-specific functional assays. The increase in cytokine mRNA expression may also be the result of more cells within the granulomas of coinfected individuals than in those of HIV− individuals. If the increase in cytokine mRNA leads to increased inflammation, excessive pathology or changes in granuloma function and architecture may occur that inhibit the control of M. tuberculosis infection. Contrary to the previous result, another immunohistochemistry study determined that granulomas from HIV+ individuals with TB expressed less TNF and had more extensive necrosis than granulomas from individuals with TB alone (20). The decrease in TNF expression may be due to a functional disruption or a decrease in the number of T cells and infected macrophages within the granulomas. Due to the highly invasive nature of granuloma-based studies and the difficulties in obtaining autopsy tissues (and selection bias to these samples), the one solution is the use of a realistic animal model. These studies may elucidate the mechanistic changes that occur within the granuloma as a result of HIV infection and identify targets for preventive or intervention therapies.
A significant amount of evidence supports the hypothesis that HIV reduces M. tuberculosis-specific T cell functions. However, most of these studies have confirmed these changes within the periphery or BAL fluid cells, which may interact differently within the structured environment of a granuloma. No clinical studies have examined functional T cell changes within granulomatous tissue, which could be addressed with an animal model.
Antiretroviral treatment increases M. tuberculosis-specific T cell responses.
Antiretroviral treatment has been used to treat individuals who are coinfected with both HIV and M. tuberculosis. Wilkinson et al. determined that antiretroviral treatment of coinfected individuals led to an increase in the percentage of naive (CD27+ CD45RA+) CD4 T cells at 36 weeks after antiretroviral therapy and a sustained increase in central memory (CD27+ CD45RA−) CD4 T cells by 12 weeks posttreatment (109). The increase in central and naïve CD4 T cells correlates with an increase in ESAT-6/CFP10-specific T cells 48 weeks after antiretroviral treatment. Surprisingly, a decrease in IFN-γ release was observed when PPD was used as a stimulator in this study. Another study followed coinfected patients for 12 months and found an increase in polyfunctional effector memory (CD27− CD45RO+) and terminal memory (CD27− CD45RO−) CD4 T cell responses to PPD (98). Although antiretroviral therapy increases T cell responses in coinfected patients, these responses are significantly weaker than those of individuals with TB alone (85). Although antiretroviral therapy increases M. tuberculosis-specific T cell responses in coinfected individuals, this increase in M. tuberculosis-specific T cell responses may not always ameliorate TB pathology and may actually exacerbate TB (see the section on immune reconstitution inflammatory syndrome [IRIS] below) (2, 24).
IRIS further complicates the coinfection.
Highly active antiretroviral treatment (HAART) ameliorates the symptoms of HIV-induced disease through a dramatic reduction in plasma viremia and restoration of CD4 T cell levels (106). Individuals on HAART are still more susceptible to TB than HIV− individuals (51). This susceptibility to TB is inversely proportional to the peripheral CD4 T cell count (51). Individuals coinfected with HIV and TB on HAART have a delayed increase in M. tuberculosis-specific T cell responses and may not reach levels observed in HIV− adults (85).
Coinfected individuals on HAART may have excessive inflammation during immune reconstitution, and they may suffer from TB-associated IRIS, which occurs in two forms. Paradoxical TB IRIS occurs in patients on TB treatment before HAART. Unmasking TB IRIS occurs in patients who are not on TB treatment when they start HAART and may represent either reactivation of latent infection or enhanced symptoms from TB that was not previously diagnosed as active disease or was subclinical (63). This is believed to be the result of increased inflammation in the tissues, which can enhance the symptoms of TB or possibly even trigger reactivation. The available data on IRIS in M. tuberculosis-infected persons strongly suggest that excessive inflammation in the setting of subclinical or latent M. tuberculosis infection is detrimental to control of the infection (2, 6, 24, 99). The excessive inflammation may be caused by an increase in the antigenic burden, perhaps by reconstituting CD4 T cell effector function in the granuloma, which can kill M. tuberculosis and release antigen (5); dysregulation of cytokine responses (99); and/or an increase in T cell migration and activation at the site of infection (2, 6, 24). Higher concentrations of TNF, IL-6, and IFN-γ were observed in patients with TB IRIS than in individuals with TB alone (99). The increases in cytokine release may be due to the increase in Th1 responses such as IFN-γ release by T cells (6, 24) and T cell activation (HLA-DR+) (2) observed in individuals who develop IRIS shortly after HAART initiation. One possibility is that the increase in Th1 function is caused by a defect in regulatory T cell function (87); however, recent studies have reported no difference in the number of regulatory T cells between coinfected individuals with IRIS and those without IRIS (64, 100). It should be noted that it is not known whether these mechanistic changes are the cause or the result of IRIS, so no predictive clinical biomarker has been identified.
Although the mechanisms that lead to IRIS-associated TB are not fully understood and have been reviewed more fully elsewhere (53, 63, 90), this unfortunate side effect of HAART demonstrates that preventing TB is not as straightforward as simply replacing CD4 T cells in the periphery or even in the granuloma (63). Instead, a balance of pro- and anti-inflammatory responses is necessary for optimal control of M. tuberculosis at the granuloma level. The resurgence of immune responses following HAART is likely deficient in reconstituting that balance in some individuals. Again, data are lacking on granulomas and tissues in TB IRIS patients, and we currently have no animal model where this phenomenon can be studied.
ANIMAL MODELS: THEIR POTENTIAL TO ADDRESS GAPS IN THE HUMAN HIV/M. TUBERCULOSIS COINFECTION LITERATURE
The available human data have shaped our current understanding of how HIV manipulates M. tuberculosis infection and disease. Building on the solid base of knowledge these studies have provided will substantially increase our understanding of HIV/M. tuberculosis coinfection. A priority should be to increase the number of studies that focus on tissue events, especially at the granuloma level, in HIV/M. tuberculosis-coinfected people. The difficulties in obtaining such samples are obvious. Appropriate and relevant animal models may be the next best choice for determining the events that occur in the tissues and granulomas of coinfected individuals. The TB field has several experimental animal models. The advantages of an animal model include the abilities to control the timing, dose, and strain of infection, to sample or necropsy at predetermined time points, and to obtain tissue at necropsy. Currently, there are only a few animal models available for studying interactions between HIV and M. tuberculosis. These models may be able to address some of the gaps in the human HIV/M. tuberculosis coinfection literature.
Mouse models.
Mouse models have been invaluable for addressing immunological and pathogenesis questions about TB. Genetic similarities and the availability of genetically manipulated strains and reagents, low cost, and relatively easy maintenance make them ideal for most TB research facilities. A disadvantage of the mouse model is that TB in mice is a chronic infection that differs from both active and latent TB in humans. Murine models have demonstrated the importance of IFN-γ, TNF, activated macrophages, and CD4 T cells, among other factors, in controlling TB (10, 78, 84). However, mice are not susceptible to HIV and there is not a suitable homologous murine virus, so wild type mice are not ideal candidates for coinfection research.
The most basic HIV/M. tuberculosis mouse model is one in which mice are rendered CD4 T cell deficient through either antibody-mediated depletion or genetic manipulation (10, 84). Because CD4 T cells are depleted during HIV infection, it is logical to study CD4-deficient mice as a model of HIV/M. tuberculosis coinfection. CD4 T cell-deficient mice are more susceptible to advanced TB than wild type mice, supporting the importance of CD4 T cells in containing primary and chronic TB. However, the disease associated with HIV is not entirely caused by depletion of CD4 T cells. Viral particles can induce nonspecific apoptosis (110), disruption of lymph node architecture (14), and T cell anergy (82) and affect macrophages, all of which effects have been associated with HIV pathology. These aspects of HIV infection cannot be recapitulated in CD4 T cell-depleted mice.
To address how viral proteins, specifically, HIV Nef, manipulate immunological responses, Nef transgenic mice have been developed (36-38). These mice express Nef in CD4 T cells, macrophages, and dendritic cells and subsequently develop an AIDS-like disease characterized by CD4 T cell depletion, as well as lung, heart, and kidney diseases (36, 37). This transgenic mouse model demonstrates that Nef expression within CD4 T cells is a major determinant of the pathogenicity of HIV infection (38). Nef expression within CD4 T cells causes increased activation and apoptosis, which eventually leads to their depletion. Future TB studies may be able to use this transgenic mouse to determine how Nef expression changes immunologic responses to TB.
Another mouse model that may increase our understanding of how HIV manipulates TB pathology involves humanized bone marrow-liver-thymus (BLT) mice reconstituted with human hematopoietic stem cells, which produces human lymphoid tissues (97). Human CD4 T cells reconstitute the gastrointestinal and female reproductive tracts, causing these mice to be susceptible to rectal (97) and vaginal (21) HIV inoculation. HIV infection results in systemic viral loads, a depletion of systemic CD4 T cells, and T cell activation similar to what is observed in humans with HIV (21, 97). Infecting these mice with HIV and M. tuberculosis may identify how HIV behaves within granulomas. Prophylaxis has been shown to reduce infection rates in these mice (21), which indicates that antiretroviral effectiveness in the context of a HIV/M. tuberculosis coinfection may be studied in these mice. The use of HAART in coinfected mice may make it possible to address how HIV induces functional changes in M. tuberculosis-specific T cells within granulomas but also how the virus changes granuloma formation and architecture. BLT mice may be used to identify immunologic targets that can be examined within more expensive primate models or clinical studies.
NHP models.
Nonhuman primates (NHP) have helped elucidate our understanding of HIV (27, 60, 74) and M. tuberculosis (12, 56-58) infections. Serial blood, BAL fluid, and lymph node biopsy samples can be obtained from NHP during the course of infection, and all tissues are available at necropsy, addressing an inherent limitation of clinical studies. This model may be ideal for approaching questions that cannot be answered in clinical studies.
Several NHP models of HIV/M. tuberculosis coinfection have been developed (19, 23, 83, 92-94, 112). SIV-infected rhesus macaques have been inoculated with BCG (19, 92-94) or M. tuberculosis (83). These models have recapitulated the decrease in peripheral mycobacterium-specific T cell responses observed in HIV/M. tuberculosis-coinfected humans. The similarities between these results and those of human coinfection studies demonstrate the validity of the NHP as an animal model of coinfection. We recently reported a cynomolgus macaque model of SIV-induced reactivation of latent TB which should be very useful in understanding how HIV manipulates TB immunology and pathology (23). This model examined aspects of coinfection that have not been addressed in human studies. For example, the severity of the initial but transient reduction in peripheral T cell numbers during acute SIV infection was correlated with time to reactivation, and reductions in T cell numbers also occurred within lung granulomas of coinfected monkeys compared to those of monkeys with active TB without SIV (23). Data extrapolated from this model and clinical studies helped elucidate a potential mechanism for the reactivation of latent TB granuloma (Fig. 1). These models have the potential to be used for immunomodulation-, vaccine-, and antiretroviral-based studies to study the efficacy of therapies against TB in the context of coinfection.
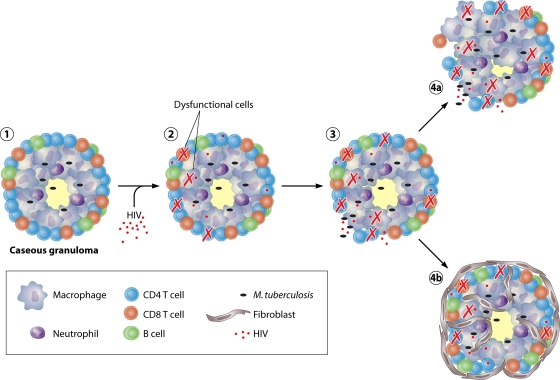
FIG. 1.
Proposed mechanism of HIV-induced reactivation of latent TB. (Stage 1) Necrotic granuloma functioning “normally” in an individual with latent TB. (Stage 2) HIV enters the granuloma and induces functional changes within T cells and macrophages. HIV also kills activated T cells. (Stage 3) The decrease in T cell number and increase in cellular dysfunction lead to a functional disruption of the granuloma. This may lead to increased dissemination. (Stage 4a) Granulomas functionally disrupted shortly after HIV infection leads to continued M. tuberculosis dissemination and early TB reactivation. (Stage 4b) Fibrotic granulomas temporarily reestablish granuloma containment, which prevents reactivation.
Animal models will allow us to assess HIV-induced changes in M. tuberculosis-specific immune responses that may lead to reactivation of TB and increased susceptibility to TB in HIV+ individuals. Although every animal model has its limitations, we hope that these new mouse and NHP models will provide evidence supporting or refuting the various hypotheses of how HIV manipulates TB pathology. Increasing our basic understanding of how these pathogens interact in vivo will help us uncover possible treatments for coinfected people.
HOW CAN TISSUE-BASED STUDIES IMPROVE TREATMENT?
Future studies may demonstrate that a high level of HIV replication within the granuloma correlates to granuloma dysfunction and mycobacterial growth. This is important because it is unknown whether antiretrovirals reduce viral loads within granulomas or even if they penetrate granulomas in the appropriate concentrations in coinfected patients. Drug concentrations in granulomas can be quantified by direct measurement in animal models, and this may be necessary to determine the best treatment for coinfected persons, taking into account the penetration of granulomas, viral loads, and granuloma types. A further important factor is that understanding the correlation between viral titers within BAL fluid and plasma with viral titers in granulomatous tissue will provide a better opportunity to assess the efficacy of HAART in coinfected individuals and possibly identify biomarkers of drug efficacy. These studies will be essential for the development of tractable biomarkers in the blood that translate to granuloma dysfunction and predict outcomes for coinfected individuals.
However, if clinical and animal-based studies demonstrate that HIV does not replicate specifically within granulomas, the functional change in the immune response may occur in the lymph nodes, where priming of the initial and perhaps ongoing T cell responses occurs. The thoracic lymph nodes are a common site of M. tuberculosis infection and may actually be a site of reactivation of latent infection (56). This may be validated by correlating changes in M. tuberculosis-specific T cell function with the viral loads within these lymph nodes. If a positive correlation is present, drug effectiveness may be determined by examining changes in the viral loads within these tissues during drug treatment.
CONCLUSION
The mechanisms by which HIV disrupts TB granuloma function and leads to increased morbidity and mortality have been extrapolated from clinical and animal studies (Fig. 1) but remain poorly understood. Changes in T cell and macrophage function within granulomas need to be examined in future clinical and animal studies to elucidate possible mechanisms by which HIV disrupts TB immune pathology. These studies may provide insight into potential drug and/or vaccine therapies. In addition, it is important to understand the parallels between responses in easily obtained samples (like blood) and tissue responses so that one can correctly interpret blood-based data. Only through studies that correlate blood and tissue responses can we begin to search for biomarkers of disease status that can be used in vaccine and drug studies.
Acknowledgments
This study was supported by the NIH (RO1 HL075845-04 and NIH RO1 A150732 to J.L.F. and T32 AI060525-05 to C.R.D.).
We thank Joshua Mattila and Philana Ling Lin for their help in the preparation of this review. We also thank Patrick Lane of scEYEnce for his help in enhancing Fig. 1.
Biography
 Collin Diedrich was born and raised in St. Louis, MO. In 2006, he received his B.S. in Cell and Molecular Biology from Bradley University. He completed his undergraduate research project focusing on how cocaine and hypoxia change neuronal mitochondrial oxygen consumption under the direction of Dr. Erich Stabenau. He currently is a fifth-year graduate student at the University of Pittsburgh School of Medicine in the Molecular Virology and Microbiology Ph.D. program. After joining Dr. JoAnne Flynn's lab, he worked with Dr. Joshua Mattila on developing an HIV/M. tuberculosis coinfection NHP animal model designed to examine how HIV induces reactivation of latent TB. This project spurred his dedication to understanding the complexity of this coinfection. He hopes to continue his work within HIV/M. tuberculosis coinfection immunology as a postdoctoral fellow.
Collin Diedrich was born and raised in St. Louis, MO. In 2006, he received his B.S. in Cell and Molecular Biology from Bradley University. He completed his undergraduate research project focusing on how cocaine and hypoxia change neuronal mitochondrial oxygen consumption under the direction of Dr. Erich Stabenau. He currently is a fifth-year graduate student at the University of Pittsburgh School of Medicine in the Molecular Virology and Microbiology Ph.D. program. After joining Dr. JoAnne Flynn's lab, he worked with Dr. Joshua Mattila on developing an HIV/M. tuberculosis coinfection NHP animal model designed to examine how HIV induces reactivation of latent TB. This project spurred his dedication to understanding the complexity of this coinfection. He hopes to continue his work within HIV/M. tuberculosis coinfection immunology as a postdoctoral fellow.
 JoAnne Flynn, Ph.D., is a Professor in the Department of Microbiology and Molecular Genetics at the University of Pittsburgh School of Medicine, with secondary appointments in Immunology, Pediatrics, and Infectious Disease. She is also a faculty member of the Center for Vaccine Research. Dr. Flynn received her B.S. from the University of California, Davis, in 1982 with a biochemistry major and her Ph.D. in microbiology and immunology from the University of California, Berkeley, in 1987. She did postdoctoral work at the Research Institute of Scripps Clinic with Dr. Magdalene So and with Dr. Barry Bloom at the Albert Einstein College of Medicine, where she was a Howard Hughes Fellow. She joined the University of Pittsburgh in 1994. Dr. Flynn has studied the immunology and pathogenesis of TB for 20 years.
JoAnne Flynn, Ph.D., is a Professor in the Department of Microbiology and Molecular Genetics at the University of Pittsburgh School of Medicine, with secondary appointments in Immunology, Pediatrics, and Infectious Disease. She is also a faculty member of the Center for Vaccine Research. Dr. Flynn received her B.S. from the University of California, Davis, in 1982 with a biochemistry major and her Ph.D. in microbiology and immunology from the University of California, Berkeley, in 1987. She did postdoctoral work at the Research Institute of Scripps Clinic with Dr. Magdalene So and with Dr. Barry Bloom at the Albert Einstein College of Medicine, where she was a Howard Hughes Fellow. She joined the University of Pittsburgh in 1994. Dr. Flynn has studied the immunology and pathogenesis of TB for 20 years.
Editor: A. T. Maurelli
Footnotes
Published ahead of print on 18 January 2011.
REFERENCES
- 1.Anonymous. 1998. Antiretroviral therapy and medical management of pediatric HIV infection and 1997 USPHS/IDSA report on the prevention of opportunistic infections in persons infected with human immunodeficiency virus. Pediatrics 102(4 Pt. 2):999-1085. [PubMed] [Google Scholar]
- 2.Antonelli, L. R., et al. 2010. Elevated frequencies of highly activated CD4+ T cells in HIV+ patients developing immune reconstitution inflammatory syndrome. Blood 116:3818-3827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bezuidenhout, J., T. Roberts, L. Muller, P. van Helden, and G. Walzl. 2009. Pleural tuberculosis in patients with early HIV infection is associated with increased TNF-alpha expression and necrosis in granulomas. PLoS One 4:e4228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonecini-Almeida Mda, G., et al. 1998. Functional activity of alveolar and peripheral cells in patients with human acquired immunodeficiency syndrome and pulmonary tuberculosis. Cell. Immunol. 190:112-120. [DOI] [PubMed] [Google Scholar]
- 5.Bonham, S., D. B. Meya, P. R. Bohjanen, and D. R. Boulware. 2008. Biomarkers of HIV immune reconstitution inflammatory syndrome. Biomark. Med. 2:349-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bourgarit, A., et al. 2006. Explosion of tuberculin-specific Th1-responses induces immune restoration syndrome in tuberculosis and HIV co-infected patients. AIDS 20:F1-F7. [DOI] [PubMed] [Google Scholar]
- 7.Breen, R. A., et al. 2006. Detection of mycobacterial antigen responses in lung but not blood in HIV-tuberculosis co-infected subjects. AIDS 20:1330-1332. [DOI] [PubMed] [Google Scholar]
- 8.Brenchley, J. M., et al. 2004. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J. Exp. Med. 200:749-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bucy, R. P., and J. M. Kilby. 2001. Perspectives on inducing efficient immune control of HIV-1 replication—a new goal for HIV therapeutics? AIDS 15(Suppl. 2):S36-S42. [DOI] [PubMed] [Google Scholar]
- 10.Caruso, A. M., et al. 1999. Mice deficient in CD4 T cells have only transiently diminished levels of IFN-gamma, yet succumb to tuberculosis. J. Immunol. 162:5407-5416. [PubMed] [Google Scholar]
- 11.Centlivre, M., M. Sala, S. Wain-Hobson, and B. Berkhout. 2007. In HIV-1 pathogenesis the die is cast during primary infection. AIDS 21:1-11. [DOI] [PubMed] [Google Scholar]
- 12.Chen, C. Y., et al. 2009. A critical role for CD8 T cells in a nonhuman primate model of tuberculosis. PLoS Pathog. 5:e1000392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clark, S. A., et al. 2007. Tuberculosis antigen-specific immune responses can be detected using enzyme-linked immunospot technology in human immunodeficiency virus (HIV)-1 patients with advanced disease. Clin. Exp. Immunol. 150:238-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen, O. J., et al. 1995. Pathogenic insights from studies of lymphoid tissue from HIV-infected individuals. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 10(Suppl. 1):S6-S14. [PubMed] [Google Scholar]
- 15.Collins, K. R., M. E. Quinones-Mateu, Z. Toossi, and E. J. Arts. 2002. Impact of tuberculosis on HIV-1 replication, diversity, and disease progression. AIDS Rev. 4:165-176. [PubMed] [Google Scholar]
- 16.Collins, K. R., et al. 2002. Human immunodeficiency virus type 1 (HIV-1) quasispecies at the sites of Mycobacterium tuberculosis infection contribute to systemic HIV-1 heterogeneity. J. Virol. 76:1697-1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Condos, R., W. N. Rom, and M. Weiden. 2000. Lung-specific immune response in tuberculosis. Int. J. Tuberc. Lung Dis. 4:S11-S17. [PubMed] [Google Scholar]
- 18.Crampin, A. C., et al. 2010. Recurrent TB: relapse or reinfection? The effect of HIV in a general population cohort in Malawi. AIDS 24:417-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Croix, D. A., et al. 2000. Effect of mycobacterial infection on virus loads and disease progression in simian immunodeficiency virus-infected rhesus monkeys. AIDS Res. Hum. Retroviruses 16:1895-1908. [DOI] [PubMed] [Google Scholar]
- 20.de Noronha, A. L., A. Bafica, L. Nogueira, A. Barral, and M. Barral-Netto. 2008. Lung granulomas from Mycobacterium tuberculosis/HIV-1 co-infected patients display decreased in situ TNF production. Pathol. Res. Pract. 204:155-161. [DOI] [PubMed] [Google Scholar]
- 21.Denton, P. W., et al. 2008. Antiretroviral pre-exposure prophylaxis prevents vaginal transmission of HIV-1 in humanized BLT mice. PLoS Med. 5:e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deretic, V., et al. 2004. Endosomal membrane traffic: convergence point targeted by Mycobacterium tuberculosis and HIV. Cell. Microbiol. 6:999-1009. [DOI] [PubMed] [Google Scholar]
- 23.Diedrich, C. R., et al. 2010. Reactivation of latent tuberculosis in cynomolgus macaques infected with SIV is associated with early peripheral T cell depletion and not virus load. PLoS One 5:e9611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elliott, J. H., et al. 2009. Immunopathogenesis and diagnosis of tuberculosis and tuberculosis-associated immune reconstitution inflammatory syndrome during early antiretroviral therapy. J. Infect. Dis. 200:1736-1745. [DOI] [PubMed] [Google Scholar]
- 25.Erikstrup, C., et al. 2010. T-cell dysfunction in HIV-1-infected patients with impaired recovery of CD4 cells despite suppression of viral replication. J. Acquir. Immune Defic. Syndr. 53:303-310. [DOI] [PubMed] [Google Scholar]
- 26.Finnegan, A., et al. 1996. IL-10 cooperates with TNF-alpha to activate HIV-1 from latently and acutely infected cells of monocyte/macrophage lineage. J. Immunol. 156:841-851. [PubMed] [Google Scholar]
- 27.Gardner, M. B. 1989. SIV infected rhesus macaques: an AIDS model for immunoprevention and immunotherapy. Adv. Exp. Med. Biol. 251:279-293. [DOI] [PubMed] [Google Scholar]
- 28.Garrait, V., et al. 1997. Tuberculosis generates a microenvironment enhancing the productive infection of local lymphocytes by HIV. J. Immunol. 159:2824-2830. [PubMed] [Google Scholar]
- 29.Geldmacher, C., et al. 2010. Preferential infection and depletion of Mycobacterium tuberculosis-specific CD4 T cells after HIV-1 infection. J. Exp. Med. 207:2869-2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Geldmacher, C., et al. 2008. Early depletion of Mycobacterium tuberculosis-specific T helper 1 cell responses after HIV-1 infection. J. Infect. Dis. 198:1590-1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Getahun, H., C. Gunneberg, R. Granich, and P. Nunn. 2010. HIV infection-associated tuberculosis: the epidemiology and the response. Clin. Infect. Dis. 50(Suppl. 3):S201-S207. [DOI] [PubMed] [Google Scholar]
- 32.Glynn, J. R., et al. 2008. Effects of duration of HIV infection and secondary tuberculosis transmission on tuberculosis incidence in the South African gold mines. AIDS 22:1859-1867. [DOI] [PubMed] [Google Scholar]
- 33.Goletti, D., et al. 2004. Inhibition of HIV-1 replication in monocyte-derived macrophages by Mycobacterium tuberculosis. J. Infect. Dis. 189:624-633. [DOI] [PubMed] [Google Scholar]
- 34.Goletti, D., et al. 1998. The in vitro induction of human immunodeficiency virus (HIV) replication in purified protein derivative-positive HIV-infected persons by recall antigen response to Mycobacterium tuberculosis is the result of a balance of the effects of endogenous interleukin-2 and proinflammatory and antiinflammatory cytokines. J. Infect. Dis. 177:1332-1338. [DOI] [PubMed] [Google Scholar]
- 35.Goletti, D., et al. 1996. Effect of Mycobacterium tuberculosis on HIV replication. Role of immune activation. J. Immunol. 157:1271-1278. [PubMed] [Google Scholar]
- 36.Hanna, Z., et al. 1998. Transgenic mice expressing human immunodeficiency virus type 1 in immune cells develop a severe AIDS-like disease. J. Virol. 72:121-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hanna, Z., et al. 1998. Nef harbors a major determinant of pathogenicity for an AIDS-like disease induced by HIV-1 in transgenic mice. Cell 95:163-175. [DOI] [PubMed] [Google Scholar]
- 38.Hanna, Z., et al. 2009. Selective expression of human immunodeficiency virus Nef in specific immune cell populations of transgenic mice is associated with distinct AIDS-like phenotypes. J. Virol. 83:9743-9758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harrington, M. 2010. From HIV to tuberculosis and back again: a tale of activism in 2 pandemics. Clin. Infect. Dis. 50(Suppl. 3):S260-S266. [DOI] [PubMed] [Google Scholar]
- 40.Hertoghe, T., et al. 2000. T cell activation, apoptosis and cytokine dysregulation in the (co)pathogenesis of HIV and pulmonary tuberculosis (TB). Clin. Exp. Immunol. 122:350-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heyderman, R. S., et al. 1998. Pleural tuberculosis in Harare, Zimbabwe: the relationship between human immunodeficiency virus, CD4 lymphocyte count, granuloma formation and disseminated disease. Trop. Med. Int. Health 3:14-20. [DOI] [PubMed] [Google Scholar]
- 42.Hinchey, J., et al. 2007. Enhanced priming of adaptive immunity by a proapoptotic mutant of Mycobacterium tuberculosis. J. Clin. Invest. 117:2279-2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hoshino, Y., et al. 2007. Mechanisms of polymorphonuclear neutrophil-mediated induction of HIV-1 replication in macrophages during pulmonary tuberculosis. J. Infect. Dis. 195:1303-1310. [DOI] [PubMed] [Google Scholar]
- 44.Hoshino, Y., et al. 2002. Maximal HIV-1 replication in alveolar macrophages during tuberculosis requires both lymphocyte contact and cytokines. J. Exp. Med. 195:495-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hoshino, Y., et al. 2004. Mycobacterium tuberculosis-induced CXCR4 and chemokine expression leads to preferential X4 HIV-1 replication in human macrophages. J. Immunol. 172:6251-6258. [DOI] [PubMed] [Google Scholar]
- 46.Kalsdorf, B., et al. 2009. HIV-1 infection impairs the bronchoalveolar T-cell response to mycobacteria. Am. J. Respir. Crit. Care Med. 180:1262-1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kizza, H. M., et al. 2005. Persistent replication of human immunodeficiency virus type 1 despite treatment of pulmonary tuberculosis in dually infected subjects. Clin. Diagn. Lab. Immunol. 12:1298-1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kumawat, K., S. K. Pathak, A. L. Spetz, M. Kundu, and J. Basu. 2010. Exogenous Nef is an inhibitor of Mycobacterium tuberculosis-induced tumor necrosis factor-alpha production and macrophage apoptosis. J. Biol. Chem. 285:12629-12637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Law, K. F., J. Jagirdar, M. D. Weiden, M. Bodkin, and W. N. Rom. 1996. Tuberculosis in HIV-positive patients: cellular response and immune activation in the lung. Am. J. Respir. Crit. Care Med. 153:1377-1384. [DOI] [PubMed] [Google Scholar]
- 50.Lawn, S. D., S. T. Butera, and T. M. Shinnick. 2002. Tuberculosis unleashed: the impact of human immunodeficiency virus infection on the host granulomatous response to Mycobacterium tuberculosis. Microbes Infect. 4:635-646. [DOI] [PubMed] [Google Scholar]
- 51.Lawn, S. D., L. Myer, D. Edwards, L. G. Bekker, and R. Wood. 2009. Short-term and long-term risk of tuberculosis associated with CD4 cell recovery during antiretroviral therapy in South Africa. AIDS 23:1717-1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lawn, S. D., et al. 2001. Anatomically compartmentalized human immunodeficiency virus replication in HLA-DR+ cells and CD14+ macrophages at the site of pleural tuberculosis coinfection. J. Infect. Dis. 184:1127-1133. [DOI] [PubMed] [Google Scholar]
- 53.Lawn, S. D., R. J. Wilkinson, M. C. Lipman, and R. Wood. 2008. Immune reconstitution and “unmasking” of tuberculosis during antiretroviral therapy. Am. J. Respir. Crit. Care Med. 177:680-685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Layne, S. P., et al. 1992. Factors underlying spontaneous inactivation and susceptibility to neutralization of human immunodeficiency virus. Virology 189:695-714. [DOI] [PubMed] [Google Scholar]
- 55.Li, Q., et al. 2005. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature 434:1148-1152. [DOI] [PubMed] [Google Scholar]
- 56.Lin, P. L., et al. 2010. Tumor necrosis factor neutralization results in disseminated disease in acute and latent Mycobacterium tuberculosis infection with normal granuloma structure in a cynomolgus macaque model. Arthritis Rheum. 62:340-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lin, P. L., et al. 2006. Early events in Mycobacterium tuberculosis infection in cynomolgus macaques. Infect. Immun. 74:3790-3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lin, P. L., et al. 2009. Quantitative comparison of active and latent tuberculosis in the cynomolgus macaque model. Infect. Immun. 77:4631-4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mancino, G., et al. 1997. Infection of human monocytes with Mycobacterium tuberculosis enhances human immunodeficiency virus type 1 replication and transmission to T cells. J. Infect. Dis. 175:1531-1535. [DOI] [PubMed] [Google Scholar]
- 60.Mannioui, A., et al. 2009. Dynamics of viral replication in blood and lymphoid tissues during SIVmac251 infection of macaques. Retrovirology 6:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mariani, F., et al. 2001. Macrophage response to Mycobacterium tuberculosis during HIV infection: relationships between macrophage activation and apoptosis. Curr. Mol. Med. 1:209-216. [DOI] [PubMed] [Google Scholar]
- 62.Mattapallil, J. J., et al. 2005. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature 434:1093-1097. [DOI] [PubMed] [Google Scholar]
- 63.Meintjes, G., H. Rabie, R. J. Wilkinson, and M. F. Cotton. 2009. Tuberculosis-associated immune reconstitution inflammatory syndrome and unmasking of tuberculosis by antiretroviral therapy. Clin. Chest Med. 30:797-810. [DOI] [PubMed] [Google Scholar]
- 64.Meintjes, G., et al. 2008. Type 1 helper T cells and FoxP3-positive T cells in HIV-tuberculosis-associated immune reconstitution inflammatory syndrome. Am. J. Respir. Crit. Care Med. 178:1083-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mendonça, M., et al. 2007. Deficient in vitro anti-mycobacterial immunity despite successful long-term highly active antiretroviral therapy in HIV-infected patients with past history of tuberculosis infection or disease. Clin. Immunol. 125:60-66. [DOI] [PubMed] [Google Scholar]
- 66.Merchant, R. H., and Z. A. Quadir. 2002. Management of opportunistic infections in pediatric HIV. Indian J. Pediatr. 69:973-977. [DOI] [PubMed] [Google Scholar]
- 67.Mwandumba, H. C., et al. 2004. Mycobacterium tuberculosis resides in nonacidified vacuoles in endocytically competent alveolar macrophages from patients with tuberculosis and HIV infection. J. Immunol. 172:4592-4598. [DOI] [PubMed] [Google Scholar]
- 68.Mwandumba, H. C., et al. 2007. Alveolar macrophages from HIV-infected patients with pulmonary tuberculosis retain the capacity to respond to stimulation by lipopolysaccharide. Microbes Infect. 9:1053-1060. [DOI] [PubMed] [Google Scholar]
- 69.Nakata, K., et al. 1997. Mycobacterium tuberculosis enhances human immunodeficiency virus-1 replication in the lung. Am. J. Respir. Crit. Care Med. 155:996-1003. [DOI] [PubMed] [Google Scholar]
- 70.Nambuya, A., N. Sewankambo, J. Mugerwa, R. Goodgame, and S. Lucas. 1988. Tuberculous lymphadenitis associated with human immunodeficiency virus (HIV) in Uganda. J. Clin. Pathol. 41:93-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Oni, T., et al. 2010. Enhanced diagnosis of HIV-1-associated tuberculosis by relating T-SPOT.TB and CD4 counts. Eur. Respir. J. 36:594-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Patel, N. R., K. Swan, X. Li, S. D. Tachado, and H. Koziel. 2009. Impaired M. tuberculosis-mediated apoptosis in alveolar macrophages from HIV+ persons: potential role of IL-10 and BCL-3. J. Leukoc. Biol. 86:53-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Patel, N. R., et al. 2007. HIV impairs TNF-alpha mediated macrophage apoptotic response to Mycobacterium tuberculosis. J. Immunol. 179:6973-6980. [DOI] [PubMed] [Google Scholar]
- 74.Pawar, S. N., et al. 2008. Comparison of the effects of pathogenic simian human immunodeficiency virus strains SHIV-89.6P and SHIV-KU2 in cynomolgus macaques. AIDS Res. Hum. Retroviruses 24:643-654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pitchenik, A. E., et al. 1987. Human T-cell lymphotropic virus-III (HTLV-III) seropositivity and related disease among 71 consecutive patients in whom tuberculosis was diagnosed. A prospective study. Am. Rev. Respir. Dis. 135:875-879. [DOI] [PubMed] [Google Scholar]
- 76.Placido, R., et al. 2006. P2X(7) purinergic receptors and extracellular ATP mediate apoptosis of human monocytes/macrophages infected with Mycobacterium tuberculosis reducing the intracellular bacterial viability. Cell. Immunol. 244:10-18. [DOI] [PubMed] [Google Scholar]
- 77.Placido, R., et al. 1997. Apoptosis of human monocytes/macrophages in Mycobacterium tuberculosis infection. J. Pathol. 181:31-38. [DOI] [PubMed] [Google Scholar]
- 78.Plessner, H. L., et al. 2007. Neutralization of tumor necrosis factor (TNF) by antibody but not TNF receptor fusion molecule exacerbates chronic murine tuberculosis. J. Infect. Dis. 195:1643-1650. [DOI] [PubMed] [Google Scholar]
- 79.Rangaka, M. X., et al. 2007. Clinical, immunological, and epidemiological importance of antituberculosis T cell responses in HIV-infected Africans. Clin. Infect. Dis. 44:1639-1646. [DOI] [PubMed] [Google Scholar]
- 80.Ranjbar, S., et al. 2009. HIV-1 replication is differentially regulated by distinct clinical strains of Mycobacterium tuberculosis. PLoS One 4:e6116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Reed, M. B., et al. 2004. A glycolipid of hypervirulent tuberculosis strains that inhibits the innate immune response. Nature 431:84-87. [DOI] [PubMed] [Google Scholar]
- 82.Rosignoli, G., C. H. Lim, M. Bower, F. Gotch, and N. Imami. 2009. Programmed death (PD)-1 molecule and its ligand PD-L1 distribution among memory CD4 and CD8 T cell subsets in human immunodeficiency virus-1-infected individuals. Clin. Exp. Immunol. 157:90-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Safi, H., et al. 2003. Spectrum of manifestations of Mycobacterium tuberculosis infection in primates infected with SIV. AIDS Res. Hum. Retroviruses 19:585-595. [DOI] [PubMed] [Google Scholar]
- 84.Scanga, C. A., et al. 2000. Depletion of CD4(+) T cells causes reactivation of murine persistent tuberculosis despite continued expression of interferon gamma and nitric oxide synthase 2. J. Exp. Med. 192:347-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schluger, N. W., D. Perez, and Y. M. Liu. 2002. Reconstitution of immune responses to tuberculosis in patients with HIV infection who receive antiretroviral therapy. Chest 122:597-602. [DOI] [PubMed] [Google Scholar]
- 86.Schneider, T., et al. 1995. Loss of CD4 T lymphocytes in patients infected with human immunodeficiency virus type 1 is more pronounced in the duodenal mucosa than in the peripheral blood. Berlin Diarrhea/Wasting Syndrome Study Group. Gut 37:524-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Seddiki, N., et al. 2009. Proliferation of weakly suppressive regulatory CD4+ T cells is associated with over-active CD4+ T-cell responses in HIV-positive patients with mycobacterial immune restoration disease. Eur. J. Immunol. 39:391-403. [DOI] [PubMed] [Google Scholar]
- 88.Selwyn, P. A., et al. 1992. Clinical manifestations and predictors of disease progression in drug users with human immunodeficiency virus infection. N. Engl. J. Med. 327:1697-1703. [DOI] [PubMed] [Google Scholar]
- 89.Selwyn, P. A., et al. 1992. High risk of active tuberculosis in HIV-infected drug users with cutaneous anergy. JAMA 268:504-509. [PubMed] [Google Scholar]
- 90.Sereti, I., A. J. Rodger, and M. A. French. 2010. Biomarkers in immune reconstitution inflammatory syndrome: signals from pathogenesis. Curr. Opin. HIV AIDS 5:504-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shen, J. Y., P. F. Barnes, T. H. Rea, and P. R. Meyer. 1988. Immunohistology of tuberculous adenitis in symptomatic HIV infection. Clin. Exp. Immunol. 72:186-189. [PMC free article] [PubMed] [Google Scholar]
- 92.Shen, Y., et al. 2004. Clinical latency and reactivation of AIDS-related mycobacterial infections. J. Virol. 78:14023-14032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shen, Y., et al. 2001. Antiretroviral agents restore Mycobacterium-specific T-cell immune responses and facilitate controlling a fatal tuberculosis-like disease in macaques coinfected with simian immunodeficiency virus and Mycobacterium bovis BCG. J. Virol. 75:8690-8696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shen, Y., et al. 2002. Induction of an AIDS virus-related tuberculosis-like disease in macaques: a model of simian immunodeficiency virus-mycobacterium coinfection. Infect. Immun. 70:869-877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sonnenberg, P., et al. 2005. How soon after infection with HIV does the risk of tuberculosis start to increase? A retrospective cohort study in South African gold miners. J. Infect. Dis. 191:150-158. [DOI] [PubMed] [Google Scholar]
- 96.Sonnenberg, P., et al. 2001. HIV-1 and recurrence, relapse, and reinfection of tuberculosis after cure: a cohort study in South African mineworkers. Lancet 358:1687-1693. [DOI] [PubMed] [Google Scholar]
- 97.Sun, Z., et al. 2007. Intrarectal transmission, systemic infection, and CD4+ T cell depletion in humanized mice infected with HIV-1. J. Exp. Med. 204:705-714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sutherland, J. S., et al. 2010. Polyfunctional CD4(+) and CD8(+) T cell responses to tuberculosis antigens in HIV-1-infected patients before and after anti-retroviral treatment. J. Immunol. 184:6537-6544. [DOI] [PubMed] [Google Scholar]
- 99.Tadokera, R., et al. 3 September 2010, posting date. Hypercytokinaemia accompanies HIV-tuberculosis immune reconstitution inflammatory syndrome. Eur. Respir. J. doi: 10.1183/09031936.00091010. [DOI] [PMC free article] [PubMed]
- 100.Tan, D. B., et al. 2008. Immunological profiles of immune restoration disease presenting as mycobacterial lymphadenitis and cryptococcal meningitis. HIV Med. 9:307-316. [DOI] [PubMed] [Google Scholar]
- 101.Tong-Starksen, S. E., P. A. Luciw, and B. M. Peterlin. 1987. Human immunodeficiency virus long terminal repeat responds to T-cell activation signals. Proc. Natl. Acad. Sci. U. S. A. 84:6845-6849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Toossi, Z., et al. 2001. Increased replication of HIV-1 at sites of Mycobacterium tuberculosis infection: potential mechanisms of viral activation. J. Acquir. Immune Defic. Syndr. 28:1-8. [DOI] [PubMed] [Google Scholar]
- 103.Toossi, Z., et al. 2001. Impact of tuberculosis (TB) on HIV-1 activity in dually infected patients. Clin. Exp. Immunol. 123:233-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Veazey, R. S., P. A. Marx, and A. A. Lackner. 2001. The mucosal immune system: primary target for HIV infection and AIDS. Trends Immunol. 22:626-633. [DOI] [PubMed] [Google Scholar]
- 105.Veazey, R. S., et al. 2000. Identifying the target cell in primary simian immunodeficiency virus (SIV) infection: highly activated memory CD4(+) T cells are rapidly eliminated in early SIV infection in vivo. J. Virol. 74:57-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wendland, T., et al. 1999. HAART in HIV-infected patients: restoration of antigen-specific CD4 T-cell responses in vitro is correlated with CD4 memory T-cell reconstitution, whereas improvement in delayed type hypersensitivity is related to a decrease in viraemia. AIDS 13:1857-1862. [DOI] [PubMed] [Google Scholar]
- 107.Whalen, C., et al. 1997. Site of disease and opportunistic infection predict survival in HIV-associated tuberculosis. AIDS 11:455-460. [DOI] [PubMed] [Google Scholar]
- 108.Whalen, C. C., et al. 2000. Impact of pulmonary tuberculosis on survival of HIV-infected adults: a prospective epidemiologic study in Uganda. AIDS 14:1219-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wilkinson, K. A., et al. 2009. Dissection of regenerating T-cell responses against tuberculosis in HIV-infected adults sensitized by Mycobacterium tuberculosis. Am. J. Respir. Crit. Care Med. 180:674-683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yoon, V., et al. 2010. The GP120 molecule of HIV-1 and its interaction with T cells. Curr. Med. Chem. 17:741-749. [DOI] [PubMed] [Google Scholar]
- 111.Zhang, M., et al. 1994. T cell cytokine responses in persons with tuberculosis and human immunodeficiency virus infection. J. Clin. Invest. 94:2435-2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhou, D., et al. 1999. Mycobacterium bovis bacille Calmette-Guerin enhances pathogenicity of simian immunodeficiency virus infection and accelerates progression to AIDS in macaques: a role of persistent T cell activation in AIDS pathogenesis. J. Immunol. 162:2204-2216. [PubMed] [Google Scholar]