Abstract
Relative up-regulation of the Ikaros family transcription factor Helios in natural regulatory T cells (Tregs) has been reported by several groups. However, a role for Helios in regulatory T cells has not yet been described. Here, we show that Helios is upregulated in CD4+CD25+ regulatory T cells. Chromatin Immunoprecipitation (ChIP) experiments indicated that Helios binds to the FoxP3 promoter. These data were further corroborated by experiments showing that knocking-down Helios with siRNA oligonucleotides results in down-regulation of FoxP3. Functionally, we found that suppression of Helios message in CD4+CD25+ T cells significantly attenuates their suppressive function. Taken together, these data suggest that Helios may play an important role in regulatory T cell function and support the concept that Helios may be a novel target to manipulate Treg activity in a clinical setting.
Keywords: Regulatory T cells, Helios, FoxP3
1. Introduction
Naturally occurring CD4+CD25+ regulatory T cells (Tregs) represent an important population of T cells that can prevent immunologic pathology(Chatila, 2005; Coombes et al., 2005; Sakaguchi, 2004); however, this population may also function to inhibit immunity to tumors or certain pathogens(Chatila, 2005; Curiel, 2008). Since several microarray studies(Fontenot et al., 2005; Sugimoto et al., 2006), including our own(Getnet et al., 2009), showed relative up-regulation of the Ikaros family transcription factor Helios in Tregs, we hypothesized that this transcription factor might play a role in Treg generation and/or function. Helios is a highly conserved transcription factor expressed in early hematopoietic progenitors of the bone marrow and subsets of developing thymocytes, while expression in the periphery is mainly but not exclusively restricted to single positive T cells (Hahm et al., 1998; Rebollo and Schmitt, 2003; Sridharan and Smale, 2007). Helios is known to interact with nuclear remodeling proteins, HDACs, and other transcriptional elements (Sridharan and Smale, 2007). We tested the relationship between Helios and FoxP3, a lineage marker which distinguishes Tregs from other T cell subsets(Fontenot et al., 2003; Hori et al., 2003), as well as the functional role of Helios in regulatory T cells.
2. Materials and methods
2.1. Lymphocyte isolation
Lymphocytes were purified from healthy donors under a Johns Hopkins IRB approved protocol, J002. Briefly, peripheral blood was diluted with Hanks Buffered Saline Solution (Invitrogen) to 50%, overlaid on a ficoll gradient (GE Healthcare) and spin-separated at 1800 RPM. A human regulatory T cell isolation kit (Miltenyi Biotec) was used according to manufacturer's instructions to enrich CD4+CD25+ T cells for FACS sorting or separate cells for in vitro suppression assays.
2.2. Activation and skewing conditions
T cells were isolated, activated or skewed towards Tregs as described previously (Sfanos et al., 2008). Briefly, human regulatory T cell isolation kit (Miltenyi Biotec) was used according to manufacturer's instructions to enrich for CD4+CD25− and depleted again using to CD25+ microbeads supplied by manufacturer to CD4+CD25+ T cells to eliminate traces of activated T cells. Cells were activated by plate coated 0.5 ug/mL anti-CD3e (ebiosciences, Clone OKT3) and soluble 1 ug/mL anti CD28 (ebiosciences, Clone CD28.2). During this activation, rTGFβ (25ng/mL), anti-IL6 (10ug/mL), anti-IL4 (1ug/mL) and anti-IFNγ (5ug/mL) were added to skew cells towards regulatory T cells (R and D systems). Murine CD4+FoxP3GFP− T cells were FACS sorted from B10.d2 FoxP3GFP mice. Cells were skewed towards Tregs by the addition of rTGFβ (2ng/mL) and rIL2 (2U/mL) upon activation with immobilized anti-CD3e (5ug/mL) and soluble anti-CD28 (1ug/mL) as described previously (Chen et al., 2003; Zheng et al., 2007). All Treg skewing reagents were acquired from R&D systems unless and otherwise stated.
2.3. Cell lines and transfections
EL4 and Jurkat T cells were maintained in Jurkat/EL4 cell media (RPMI, 10% FCS, and antibiotics) under 1×10^6 cells/mL and split the night before transfection. Both cell lines were electroporated using the Equibio Easyject Electorporator (Wolf laboratories) with settings of 280V, 975 uF, and 335 R. Cells were mixed with DNA, shocked, and placed immediately in antibiotic free media supplemented with 20% FCS (Jurkats: 12×10^6 cells/ 300uL/8ug of DNA; EL4: 5×10^6 cells/ 300uL/ 5ug of DNA). Results are representative of three or more experiments.
2.4. Apoptosis assay
Apoptosis was quantified using the BD Apoptosis kit (Becton Dickinson). Jurkat cells exposed to UV light were used as a positive control, and flow cytometry was performed using a FACS caliber instrument (Becton Dickinson).
2.5. Chromotin immunoprecipitation assay
The Upstate-Millipore chromatin-immunoprecipitation assay kit was used according to the manufacturer's instructions with minor modification. Briefly, EL4 cells were transfected with the indicated overexpression constructs (pEf1α-MycHis C, Invitrogen), rested overnight, and stimulated with anti-CD3e (5ug/mL, immobilized), anti-CD28 (1ug/mL, soluble), and rTGFβ (2ng/mL) for 24hrs. Cells were fixed with 1% formaldehyde at 37°C for 10 minutes, lysed with 1% SDS lysis buffer, sonicated 15 to 20 times (10 sec pulse,30 sec rest) on ethanol dry ice mixture, Micrococcal nuclease (New England Biolabs) treated for 10 min at 37°C and immunoprecipitated with an anti-Myc antibody (Abcam, Clone 9E10) and Protein A/G sepharose beads. After DNA extraction, input was diluted 1 to 15 and the following primers pairs were used for target amplification starting from furthest target from Transcriptional Start Site (TSS): Fwd 5′-actttctcttcctcaggcct-3′ and Rvs 5′-ctgtcataattttggtagcc-3′; Fwd 5′-ctttttctttttacacggaatctgg-3′ and Rvs 5′-cccccacaaattcacagaat-3′; Fwd 5′- cttttttctccatgaattgc-3′ and Rvs 5′-ctcatgagaaaccacaattt-3′;Fwd 5′- gatttgacttattttccctc-3′ and Rvs 5′ gcttttataccgagaagaaa-3′. ChIP assay results represent three independent experiments.
2.6. In Vitro Suppression Assay
Lymphocytes were isolated as described above. Non-CD4 T cells (flow-through) were immediately frozen in 5% DMSO and autologous serum – these cells served as feeder cells in a suppression assay. CD4+CD25− T cells were maintained in complete RPMI supplemented with 10% autologous serum. CD4+CD25+ T cells were nucleofected with the indicated siRNA oligonucleotide (siRNA ID#: 116127 and Ambion Control siRNA) (Ambion) using Amaxa kits (Amaxa) according to manufactures instructions and incubated for 60-72 hrs in complete RPMI supplemented with 10% autologous serum. Suppression assays were performed by admixing 2× 105 irradiated (8000 rad) feeder cells with CD4+CD25− T cell responders and CD4+CD25+ T cell suppressors in 2:1:1 ratio in serum free media. Cells were stimulated with 0.5ug/mL of anti-CD3e (ebiosciences, Clone OKT3) and 1ug/mL of anti-CD28 (ebiosciences, Clone CD28.2) incubated for 5 days and pulsed with 1 uCi H3-thymidine (Amersham Life sciences) for 12 to 16 hrs. H3-thymidine incorporation was measured after harvesting cells using a Packard Micromate cell harvester and counts quantified using a Packard Matrix 96 direct beta counter (Packard Biosciences, Meriden, CT). H3-incorporation suppression assay results represent three experiments from three different donors.
For CFSE dilution experiments, lymphocytes were purified, nucleofected and incubated or frozen as described above. CD4+CD25− responding T cells were CFSE labeled with 2.5 uM CFSE in 1ml PBS for 45 sec at room temperature. CFSE dilution suppression assays were set up by admixing 105 irradiated (8000 rad) feeder cells with 104 CD4+CD25− T cell responders and CD4+CD25+ T cell suppressors in 10:1:1 ratio in serum free media. Results represent suppression assays from four separate donors from H3-thymidine incorporation assay.
2.7. Quantitative PCR
qRT-PCR was performed as previously described(Huang et al., 2004). Cells were pelleted and frozen in Trizol reagent. Total RNA was extracted using the RNeasy kit (Qiagen) and cDNA was synthesized using Superscript III kit (Invitrogen). All primers and probes for quantitative RT-PCR were obtained from Applied Biosystems inventory. Each sample was assayed in triplicates for the target gene together with 18S rRNA as the internal reference in a 25 ul final reaction volume, using the Taq man Universal PCR Master Mix and the ABI Prism 7900 Sequence Detection system. The relative mRNA frequency was determined by normalization to the internal control 18S RNA. Relative mRNA frequencies were calculated as 2ΔΔCt where ΔΔCt=(ΔCtcalibration−ΔCtsample).
Results and discussion
3.1. Helios expression in regulatory T cells
As several transcriptional profiles of regulatory T cells (Treg), including ours, documented upregulation of Helios in antigen-experienced regulatory T cells in mice (Fontenot et al., 2005; Getnet et al., 2009; Sugimoto et al., 2006), we tested whether Helios mRNA expression in Tregs parallels the microarray studies. qRT-PCR determination of transcript levels from FACS sorted CD4+CD25− and CD4+CD25+ T cells showed that Helios expression is increased in the CD25+ compartment of both human and mouse CD4 T cells; correlating well with FoxP3+ expression (Fig. 1A). These data demonstrate that Helios expression is relatively restricted to the activated phenotype (CD25+) of CD4 T cells, a compartment enriched for regulatory T cells as determined by sustained FoxP3 expression (Hori et al., 2003).
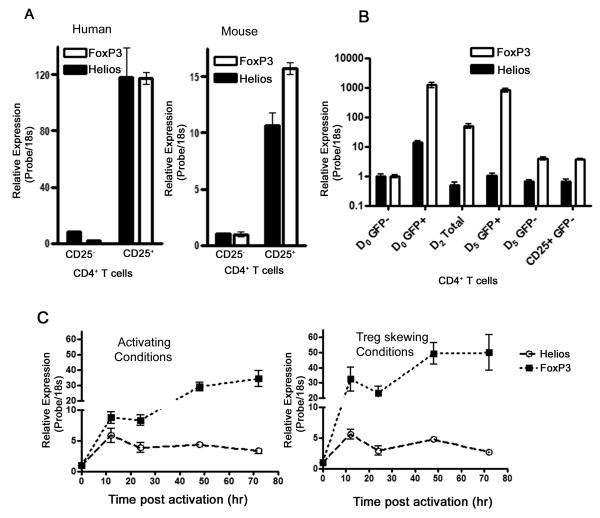
Fig. 1.
Helios expression correlates with FoxP3 expression in CD4 T cells. CD4 T cells were enriched using magnetic beads and CD25+ and CD25− populaitons FACS sorted. RT-PCR was used to assess message levels of Helios and FoxP3. (A) Relative Helios and Foxp3 message levels in CD25− and CD25+ subsets of human and mouse CD4+ T cells. (B) Helios and FoxP3 message comparison in FoxP3GFP− CD4+CD25− T cells skewed towards Regulatory T cells using TGFb and IL2. (C) Relative expression of Helios and FoxP3 message in naïve T cells post activation (left panel) or post Treg induction (right panel).
However, it was not clear from these experiments whether Helios is expressed in thymic-derived regulatory T cells (natural Treg) or Helios expression defines a population of peripherally induced regulatory T cells(Curotto de Lafaille and Lafaille, 2009). This issue is particularly complex in human Treg population where the compartment is relatively less well-defined(Roncador et al., 2005). To examine whether Helios is expressed in induced Treg, we utilized the FoxP3-GFP reporter mice originally described by Fonenot et. al (2005). CD4+CD25− FoxP3-GFP T cells from these animals could convert from FoxP3-GFP negative to FoxP3-GFP positive in vitro providing a source of induced Tregs (Zheng et al., 2007). We thus sorted out a pure population of FoxP3-GFP negative CD4+CD25− T cells and skewed them towards Treg development by TCR engagement in the presence of TGFβ and IL-2 in vitro. After 5 days of in vitro skewing of FoxP3-GFP negative cells, both GFP positive and negative populations were collected FACS sorting. To our surprise, in vitro skewed regulatory T cells (FoxP3-GFP positive cells) did not appear to upregulate Helios suggesting that Helios expression may be a characteristic of natural Treg as opposed to induced Treg (Fig 1B).
Previous work has shown that transient FoxP3 expression is a property of activated human CD4 T cells (Pillai et al., 2007; Wang et al., 2007), while human Treg in vitro show a more sustained up-regulation of FoxP3(Allan et al., 2007; Roncador et al., 2005). We thus examined whether transient Helios up-regulation is a property of naïve human CD4+CD25− T cells during activation. CD4 T cells were highly depleted of CD25+ cells using magnetic beads and stimulated with anti-CD3e/anti-CD28 antibodies. As shown in Figure 1c (left panel), we confirmed relative up-regulation of FoxP3 in naïve human CD4 T cells activated in vitro. However, Helios upregulation in activated naïve CD4 T cells was moderate compared to FoxP3 message and reached its peak within 12hrs of activation. We next tested whether Helios up-regulation would correlate with FoxP3 expression as naïve human CD4 T cells are skewed towards Treg induction in vitro. Naïve human CD4 T cells were activated with anti-CD3e/anti-Cd28 antibodies in the presence of TGFβ, anti-IL6, anti-IL4, anti-INF γ. Similar to the activating conditions, Helios message increased early in Treg skewing conditions and declined subsequently with its message peaking within 12hrs as opposed to FoxP3 message, which peaked at a much later time point after activation and remained elevated. Taken together, these data demonstrate that sustained Helios expression in T cells is selective and restricted to a subset within the pre-existing CD4+ CD25+ population, suggesting that this transcription factor is more likely to play a role in natural Treg as opposed to induced Treg.
3.2 Forced Helios expression induces apoptosis
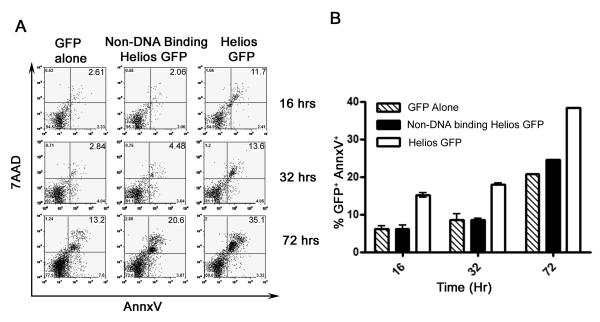
To better elucidate the relationship between FoxP3 expression and Helios in CD4 T cells, we created several Helios Lenti virus constructs and transduced T cells with Helios. Unfortunately, the yeild of Helios-transduced primary cells was surprisingly low. Thus, we were unable to further study the phenotype of this transduced population. To further understand this reduced survival of Helios positive cells, Jurkat T cells were transfected with a full-length isoform of Helios as well as a non-DNA binding isoform of Helios (Zhang et al., 2007) and assayed for apoptosis by 7AAD and Annexin V staining (Fig. 2). Very low total GFP positive percentages and high numbers of apoptotic cells were detected in wells transfected with complete Helios starting from early time points while the reverse (low apoptotic cells but high total number of GFP positive cells) was observed for controls (Fig 2A). By 72 hrs post transfection, the GFP positive fraction of Helios transfected Jurkat cells stained consistently high for 7AAD and annexin V indicating increased apoptosis driving the progressive loss of GFP positive cells in sample. These data indicate that Helios expression mediates apoptosis and such an over-expression alone appears to be insufficient to generate regulatory T cell characteristics.
Fig. 2.
Ectopic expression of Helios induces Apoptosis. Jurkat T cells were transfected with the indicated constructs and GFP positive cells were analyzed for apoptosis via Annexin V and 7AAD staining. (A) Representative FACS plot of GFP positive cells (B) graphical representation of Annexin V positive GFP positive Jurkat T cells of 2 independent experiments.
3.3 Helios binds to the FoxP3 promoter
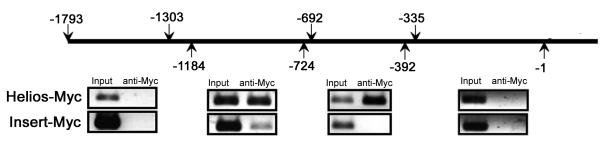
Given Helios' association with nuclear remodeling complex proteins and transcription initiation factors, we postulated that Helios might be involved in the transcriptional regulation of FoxP3 (Sridharan and Smale, 2007). To address this question, we used EL4 mouse thymoma cells, which express several isoforms of Helios and have been reported to transcribe FoxP3 upon stimulation with anti-CD3e, anti-CD28, and TGFβ (Tone et al., 2008). Myc-tagged complete Helios or Insert control were electroporated in EL4 cells and cells were rested overnight. An anti-Myc antibody was able to precipitate regions of the FoxP3 promoter in a ChIP assay 24 hrs after stimulation (Figure 3). Two separate but adjacent regions (−1184 to −724 and −692 to −335 from transcriptional start site) on the FoxP3 promoter were targeted by Helios. These data demonstrate that the DNA binding isoform of Helios is present at the promoter region during FoxP3 transcription in these thymoma cells suggesting that FoxP3 expression may be partially regulated by Helios in T cells.
Fig. 3.
Helios binds the FoxP3 promoter. EL4 cells were transfected with Myc tagged Helios or insert control and rested overnight. Chip assay performed using an anti-Myc antibody after stimulation with anti-CD3e, anti-CD28, and TGFb. One iteration of three independent experiments is shown.
3.4 Helios affects FoxP3 message levels and regulatory T cell activity
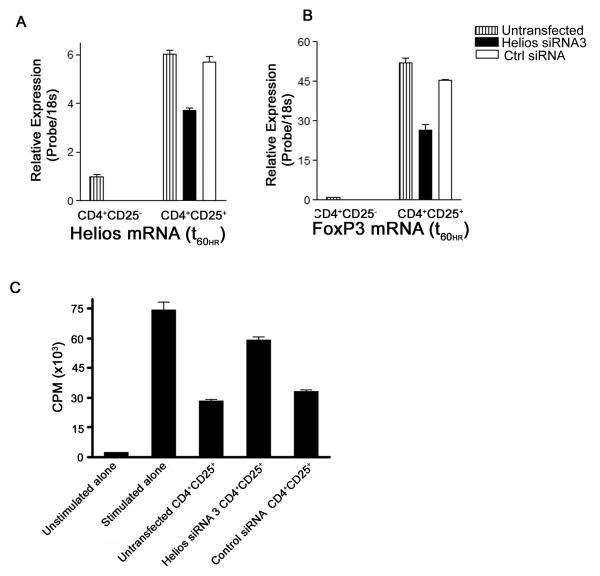
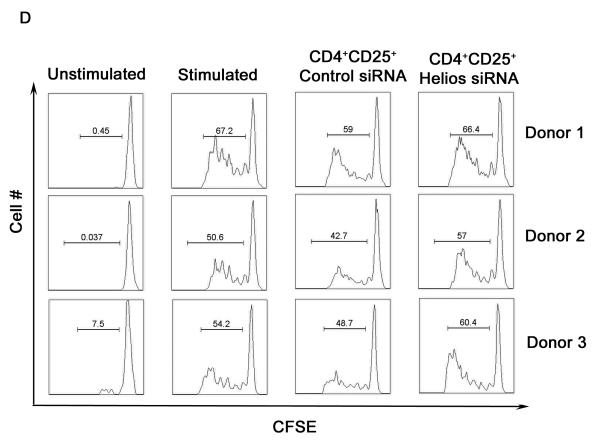
Because Helios binds to the FoxP3 promoter, we reasoned that siRNA mediated knockdown of Helios might affect FoxP3 expression. CD4+CD25+ Treg were enriched from human peripheral blood lymphocytes, nucleofected with Helios siRNA or control siRNA, incubated for 60hrs, and message levels were analyzed by quantitative RT-PCR. Helios siRNA did not completely abolish Helios expression, but did attenuate Helios message levels by approximately 40% (Fig. 4A). Interestingly, Helios knockdown also resulted in decreased levels of FoxP3 message (Fig. 4B). We ruled out non-specific activity of siRNA by using multiple siRNA to target Helios message in CD4+CD25+ T cells (data not shown). The synchronous reduction of FoxP3 by selective targeting of Helios suggested that Helios could impact regulatory T cell function. To examine the importance of Helios in the function of human regulatory T cells, we investigated the suppressive capacity of regulatory T cells after siRNA knockdown of Helios using a classical in vitro suppression assay (Thornton and Shevach, 1998). Helios siRNA transfected CD4+CD25+ T cells were incubated for 60hrs and admixed with autologous CD4+CD25− T cells and irradiated APCs(Ono et al., 2007). Untransfected and control siRNA transfected regulatory T cells suppressed the proliferation of responding cells significantly while the introduction of Helios siRNA into human regulatory T cells alleviated the suppression of responding cells (Fig. 4C). To eliminate the possibility that the proliferation of Helios siRNA transfected cells accounted for the increased H3-thymidine incorporation in these assays, we repeated the studies using CFSE dilution of responder cells as a readout. As shown in Figure 4D, Helios siRNA transfected Treg were less capable of mediating suppression using this readout as well, supporting the notion that Helios interacts with the FoxP3 promoter to maintain the suppressive activity of Treg.
Fig. 4.
Helios knockdown results in decreased FoxP3 message and diminished suppressive capacity of human Tregs in vitro. Primary CD4+CD25+ T cells were nucleofected with Helios siRNA and control siRNA and incubated for 60hrs; repeated x3. (A) Relative message levels of Helios (B) relative message levels FoxP3. Responder CD4+CD25− T cells admixed with untransfected or siRNA transfected suppressor CD4+CD25+ T cells in a 1:1 ratio. Proliferation of responders was assayed by H3-thymidine incorporation (C) or CFSE dilution (D) after 5 days of activation. Suppression assay repeated x3.
Taken together, these data suggest that Helios expression identifies a subset of Tregs, and also affects the function of these cells. Here, we show that ectopic Helios expression induces apoptosis, suggesting that Helios expression alone is not sufficient for the generation of regulatory T cells. Recently, the first Helios null mice from a targeted inactivation of the dimerizing domain on exon 7 were described (Cai et al., 2009). Surprisingly, those mice showed intact lypmphogenesis, although viability of knockout progeny was severely compromised. Those data are inconsistent with prior studies in which a dominant negative form of Helios was expressed in the hematopoietic compartment, resulting in T cell hyperproliferation and leukemia (Zhang et al., 2007). These knockout data are also seemingly inconsistent with our findings in human cells. However, it is possible the lack of phenotype in these Helios-null mice could represent the results of a compensatory escape mechanism, possibly through target sharing and/or the homo- or hetero-dimerization by other Ikaros family of transcription factors (Hahm et al., 1998; Kelley et al., 1998). Clearly, further studies will be required to address these issues.
In summary, we confirmed that the Ikaros family member Helios is up-regulated in human and murine Tregs. We found that direct functional studies of Helios' role in CD4 T cells are complicated by the finding that Helios expression appears to mediate apoptosis. In natural Tregs, however the co-expression of anti-apoptotic proteins such as Bcl-2 might counteract this apoptotic propensity (Getnet et al., 2009; Liu et al., 2005). On a mechanistic level, we found that Helios binds to at least two sites on the FoxP3 promoter, and that siRNA-mediated Helios knockdown attenuates FoxP3 levels. Functionally, we found that Helios knockdown mitigates the suppressive capacity of human Treg – suggesting that manipulation of Helios function or levels might serve to attenuate Treg function in settings such as tumor immunotherapy, where enhanced immunity is desired.
Acknowledgements
CGD is a Damon Runyon-Lilly Clinical Investigator. This work was also supported by National Institutes of Health R01 CA127153 (CGD), K08 CA096948 (CGD), and the Patrick C. Walsh Fund. DMP is a Januey Scholar, holds the Seraph Chair for Cancer Research, and is supported in part by gifts from William and Betty Toperer, Dorothy Needle, and the Commonwealth Foundation. We wish to thank Dr. Stephen Smale for providing Helios cDNA, Dr. Christopher A Klug for providing retroviral constructs of Helios, and Dr. Joseph Barbi for assisting in experiments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allan SE, Crome SQ, Crellin NK, Passerini L, Steiner TS, Bacchetta R, Roncarolo MG, Levings MK. Activation-induced FOXP3 in human T effector cells does not suppress proliferation or cytokine production. Int Immunol. 2007;19:345–54. doi: 10.1093/intimm/dxm014. [DOI] [PubMed] [Google Scholar]
- Cai Q, Dierich A, Oulad-Abdelghani M, Chan S, Kastner P. Helios deficiency has minimal impact on T cell development and function. J Immunol. 2009;183:2303–11. doi: 10.4049/jimmunol.0901407. [DOI] [PubMed] [Google Scholar]
- Chatila TA. Role of regulatory T cells in human diseases. J Allergy Clin Immunol. 2005;116:949–59. doi: 10.1016/j.jaci.2005.08.047. quiz 960. [DOI] [PubMed] [Google Scholar]
- Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–86. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombes JL, Robinson NJ, Maloy KJ, Uhlig HH, Powrie F. Regulatory T cells and intestinal homeostasis. Immunol Rev. 2005;204:184–94. doi: 10.1111/j.0105-2896.2005.00250.x. [DOI] [PubMed] [Google Scholar]
- Curiel TJ. Regulatory T cells and treatment of cancer. Curr Opin Immunol. 2008;20:241–6. doi: 10.1016/j.coi.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curotto de Lafaille MA, Lafaille JJ. Natural and adaptive foxp3+ regulatory T cells: more of the same or a division of labor? Immunity. 2009;30:626–35. doi: 10.1016/j.immuni.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–6. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–41. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Getnet D, Maris CH, Hipkiss EL, Grosso JF, Harris TJ, Yen HR, Bruno TC, Wada S, Adler A, Georgantas RW, Jie C, Goldberg MV, Pardoll DM, Drake CG. Tumor recognition and self-recognition induce distinct transcriptional profiles in antigen-specific CD4 T cells. J Immunol. 2009;182:4675–85. doi: 10.4049/jimmunol.0803400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahm K, Cobb BS, McCarty AS, Brown KE, Klug CA, Lee R, Akashi K, Weissman IL, Fisher AG, Smale ST. Helios, a T cell-restricted Ikaros family member that quantitatively associates with Ikaros at centromeric heterochromatin. Genes Dev. 1998;12:782–96. doi: 10.1101/gad.12.6.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–61. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- Huang CT, Workman CJ, Flies D, Pan X, Marson AL, Zhou G, Hipkiss EL, Ravi S, Kowalski J, Levitsky HI, Powell JD, Pardoll DM, Drake CG, Vignali DA. Role of LAG-3 in regulatory T cells. Immunity. 2004;21:503–13. doi: 10.1016/j.immuni.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Kelley CM, Ikeda T, Koipally J, Avitahl N, Wu L, Georgopoulos K, Morgan BA. Helios, a novel dimerization partner of Ikaros expressed in the earliest hematopoietic progenitors. Curr Biol. 1998;8:508–15. doi: 10.1016/s0960-9822(98)70202-7. [DOI] [PubMed] [Google Scholar]
- Liu R, La Cava A, Bai XF, Jee Y, Price M, Campagnolo DI, Christadoss P, Vollmer TL, Van Kaer L, Shi FD. Cooperation of invariant NKT cells and CD4+CD25+ T regulatory cells in the prevention of autoimmune myasthenia. J Immunol. 2005;175:7898–904. doi: 10.4049/jimmunol.175.12.7898. [DOI] [PubMed] [Google Scholar]
- Ono M, Yaguchi H, Ohkura N, Kitabayashi I, Nagamura Y, Nomura T, Miyachi Y, Tsukada T, Sakaguchi S. Foxp3 controls regulatory T-cell function by interacting with AML1/Runx1. Nature. 2007;446:685–9. doi: 10.1038/nature05673. [DOI] [PubMed] [Google Scholar]
- Pillai V, Ortega SB, Wang CK, Karandikar NJ. Transient regulatory T-cells: a state attained by all activated human T-cells. Clin Immunol. 2007;123:18–29. doi: 10.1016/j.clim.2006.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebollo A, Schmitt C. Ikaros, Aiolos and Helios: transcription regulators and lymphoid malignancies. Immunol Cell Biol. 2003;81:171–5. doi: 10.1046/j.1440-1711.2003.01159.x. [DOI] [PubMed] [Google Scholar]
- Roncador G, Brown PJ, Maestre L, Hue S, Martinez-Torrecuadrada JL, Ling KL, Pratap S, Toms C, Fox BC, Cerundolo V, Powrie F, Banham AH. Analysis of FOXP3 protein expression in human CD4+CD25+ regulatory T cells at the single-cell level. Eur J Immunol. 2005;35:1681–91. doi: 10.1002/eji.200526189. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–62. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- Sfanos KS, Bruno TC, Maris CH, Xu L, Thoburn CJ, DeMarzo AM, Meeker AK, Isaacs WB, Drake CG. Phenotypic analysis of prostate-infiltrating lymphocytes reveals TH17 and Treg skewing. Clin Cancer Res. 2008;14:3254–61. doi: 10.1158/1078-0432.CCR-07-5164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridharan R, Smale ST. Predominant interaction of both Ikaros and Helios with the NuRD complex in immature thymocytes. J Biol Chem. 2007;282:30227–38. doi: 10.1074/jbc.M702541200. [DOI] [PubMed] [Google Scholar]
- Sugimoto N, Oida T, Hirota K, Nakamura K, Nomura T, Uchiyama T, Sakaguchi S. Foxp3-dependent and -independent molecules specific for CD25+CD4+ natural regulatory T cells revealed by DNA microarray analysis. Int Immunol. 2006;18:1197–209. doi: 10.1093/intimm/dxl060. [DOI] [PubMed] [Google Scholar]
- Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998;188:287–96. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tone Y, Furuuchi K, Kojima Y, Tykocinski ML, Greene MI, Tone M. Smad3 and NFAT cooperate to induce Foxp3 expression through its enhancer. Nat Immunol. 2008;9:194–202. doi: 10.1038/ni1549. [DOI] [PubMed] [Google Scholar]
- Wang J, Ioan-Facsinay A, van der Voort EI, Huizinga TW, Toes RE. Transient expression of FOXP3 in human activated nonregulatory CD4+ T cells. Eur J Immunol. 2007;37:129–38. doi: 10.1002/eji.200636435. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Swindle CS, Bates JT, Ko R, Cotta CV, Klug CA. Expression of a non-DNA-binding isoform of Helios induces T-cell lymphoma in mice. Blood. 2007;109:2190–7. doi: 10.1182/blood-2005-01-031930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng SG, Wang J, Wang P, Gray JD, Horwitz DA. IL-2 is essential for TGF-beta to convert naive CD4+CD25- cells to CD25+Foxp3+ regulatory T cells and for expansion of these cells. J Immunol. 2007;178:2018–27. doi: 10.4049/jimmunol.178.4.2018. [DOI] [PubMed] [Google Scholar]