Abstract
Antibody-Dependent Cellular Cytotoxicity (ADCC) may assist in preventing HIV or delaying disease progression. Most prior studies have analyzed Env-specific ADCC responses. We hypothesized that effective ADCC-based immunity may target conserved internal viral proteins such as Pol. We analyzed the ability overlapping Pol peptides to induce activation of NK cells via ADCC. We prospectively studied ADCC responses in 83 HIV+ subjects followed for 3 years. Pol peptides were commonly targeted by ADCC responses in these chronically infected subjects (in 32 of the 83 subjects). However, Pol-specific ADCC responses declined over time and did not correlate with delayed HIV progression, measured by either baseline CD4 T cells, CD4 T cell loss over time, baseline viral load or the need to start antiretroviral therapy. Although Pol is frequently targeted by ADCC in HIV+ subjects, the strength or specificity of Pol-specific ADCC responses needs to be modulated to be effective in delaying HIV progression.
Introduction
Human immunodeficiency virus type 1 (HIV-1) is an important global pandemic with close to 3 million new infections each year. A HIV vaccine is urgently needed. Protein subunit vaccines induce only narrowly directed neutralizing antibodies, and failed to protect in human and macaque trials (Pitisuttithum et al., 2006; Stott, 1991). Vaccination with recombinant adenoviruses expressing HIV proteins elicited HIV-specific CD8+ T lymphocytes (McElrath et al., 2008), but also do not protect against infection or reduce viral load set point in those individuals who became infected during follow-up (Buchbinder et al., 2008). These disappointing results after years of HIV vaccine research suggests newer concepts in immunity to HIV should be explored (Isitman et al., 2009). Importantly, a recent efficacy trial based on a recombinant Canarypox virus prime and envelope protein boost showed partial protection from infection, despite only inducing narrow Nab responses and minimal CTL responses. High levels of non-neutralizing antibodies were induced by this regime, suggesting such responses could play a role in protective immunity (Rerks-Ngarm et al., 2009).
ADCC is an immune response combining components of innate and humoral immunity. Cells that can be activated by ADCC to provide effector functions via their Fc receptors include NK (Natural Killer) cells, neutrophils and macrophages. ADCC utilizes effector cells bearing Fc gamma (FcγR) or CD16 receptors such as NK cells attracted by antibodies of the IgG isotype to the target antigens on the surface of virus-infected cells. NK cells comprise 15% of the peripheral blood lymphocytes. Lysis of virus-infected cells occurs once the ADCC antibodies bind to surface viral antigens and interact with the FcγR of NK cells. The activation of NK cells elicits release of perforin, granzymes and cytokines including IFNγ, IL-1, TNFα and GM-CSF. A series of in vitro studies have demonstrated the presence of ADCC antibodies against HIV in the plasma of the majority of subjects infected with HIV-1 (Forthal, Landucci, and Daar, 2001; Ljunggren et al., 1990). HIV-specific ADCC responses generally correlate with delayed HIV progression (Baum et al., 1996). Importantly, Hessel and colleagues showed significant decreases in the efficacy of mutated neutralizing antibodies that were no longer able to elicit ADCC functions (Hessell et al., 2007).
Despite the potential efficacy of ADCC antibodies, little is known about the specific HIV-1 epitopes that stimulate ADCC. To date, only Env (Alsmadi et al., 1997) and Nef (Yamada et al., 2004)-specific ADCC epitopes have been well characterized in HIV-1 infected subjects. Most ADCC responses described in the literature are to the HIV-1 envelope protein (Env). Env is highly variable across HIV strains and can readily mutate to escape NAb and CTL responses. Recent data from our group shows ADCC responses to Env epitopes also force immune escape (Chung et al., 2010). Ideal ADCC epitopes expressed by HIV vaccines would be to conserved internal proteins.
Elucidating further ADCC epitopes has been slow, in part owing to the inefficient and complex nature of the historical assays for assessing ADCC responses. Typical killing-based ADCC assays measure responses to large proteins and are not suited to mapping ADCC responses. A novel ICS (Intracellular cytokine staining) ADCC method has recently been developed in our lab that allows the fine mapping of linear ADCC epitopes (Stratov, Chung, and Kent, 2008). This assay measures NK cell activation in response to ADCC antibodies targeting linear epitopes within overlapping peptide sets. Although this is not a “cytotoxicity” based assay, analysis of CD107a (a marker of cytotoxic granule release) provides a surrogate of cytotoxicity and correlates wirh a standard killing based ADCC assay (Chung et al., 2009). Compared to existing killing based ADCC assays, no artificial cell line is required, and either the patient’s NK cells or healthy donor NK cells used as the effector cells can be tested for ADCC activity along with the assessment of NK cytokine production, chemokine production and loss of perforin or granzymes (Chung et al., 2009). The ability to map linear ADCC epitopes from within large pools of overlapping peptides permits the identification of the particular ADCC antibody.
Two of the most conserved HIV proteins are the Gag and Pol proteins. Gag encodes capsid protein and Pol encodes for the replication enzymes including Protease (PR), Integrase (IN) and Reverse Transcriptase (RT). CTL responses that target Gag and Pol are often highly effective and escape mutations often result in large reductions in viral replicative capacity (large “fitness cost”) (Hue et al., 2009) (Clavel, Race, and Mammano, 2000). Similarly, drug resistance mutations within Pol proteins frequently result in fitness costs (Rangel et al., 2009). ADCC epitopes within these Gag and Pol proteins could be valuable vaccine targets.
We prospectively studied ADCC responses to linear Gag and Pol peptides in a cohort of 83 HIV-infected subjects using our ICS-based ADCC assay. We found that Pol was commonly targeted by ADCC responses in these chronically infected subjects but Pol-specific ADCC responses did not correlate with delayed HIV progression.
Materials and Methods
Study Subjects
To study Pol-specific ADCC responses in HIV-infected adults we recruited 83 HIV positive individuals not on antiretroviral therapy prospectively to donate blood samples in 2006 to 2007 with a follow-up period of 2 years (range 1–3yrs). Subjects were recruited through the Melbourne Sexual Health Centre and the Alfred Hospital, Melbourne, Australia. All subjects provided informed consent. Blood samples were analysed at the start of follow-up and 2 years after recruitment. The subjects provided 9–18 ml of Na-heparin anticoagulated blood for analysis using the ADCC-ICS assay. During the follow up period 45 of the 83 subjects started antiretroviral therapy for HIV progression at the discretion of the treating physician and were censored for follow up at that time. The relevant human research ethics committee approved all studies.
Peptide antigens
ADCC responses were detected using 249 Consensus B subtype Pol peptides (15-mers, overlapping by 11 amino acids, solubilized in pure dimethyl sulfoxide (DMSO), divided into two pools (Pol 1–124, Pol 125–249), spanning all HIV-1 Pol proteins using a consensus subtype B (kindly supplied by the NIH AIDS Reagent Repository). Pol-specific responses were further mapped by creating sub-pools of 20 or 21 individual peptides and further mapped to single Pol 15mer peptides. Fine mapping of Pol-specific ADCC responses was conducted approximately two years after enrolment of subjects in the study. Pol-specific responses were compared to responses to a pool of 212 consensus B subtype Env and 123 Gag 15mer overlapping peptide pools (NIH AIDS Reagent Repository)
Analysis of Pol-specific ADCC responses
The NK cell activation ADCC ICS assay was performed on all subjects using fresh sodium-heparinized whole blood to measure responses to Pol 1 and Pol 2 peptide pools. To map Pol-specific ADCC responses we studied stored plasma mixed with fresh healthy donor whole blood as previously described (Stratov, Chung, and Kent, 2008). Samples were incubated at 37°C for 5 hrs with peptides at a final concentration of 1 µg/ml of peptide in the presence of Brefeldin A (0.25mg/ml) and Monensin (5 mg/ml) (Sigma). Negative (DMSO alone) and positive control (combined SEB (Staphylococcus enterotoxin B) Sigma) wells were included. Cells were surface stained with antibodies obtained from BD Biosciences CD2-FITC (fluorescein isothiocyanate), CD3-PE (phycoerythrin), CD56-PerCP (peridinin chlorophyll protein) and CD107a-APC (allophycocyanin). Lysis was performed on the stained cells and permeabilized, and stained intracellularly with IFN- AF700 (AlexaFlour700). Data was collected using the FACS Canto analyser and data analysis was performed on FlowJo 9.0.2 software for CD56+ and CD2+ NK cells expressing IFN? and CD107a within a CD3- lymphocyte gate. A Pol specific response was defined into two parts with a Pol response to Pol 1 (Pol 1–124) as > 0.65% and to Pol 2 (Pol 125–249) as >0.32%. This criteria was based on greater than 2 standard deviations above the mean response to Pol 1–124 and Pol 125–249 in HIV-1 negative subjects (n=12).
Statistical Analysis
All statistical analyses were performed using Prism 4.0c (GraphPad). Analysis of correlation between CD4 counts and viral load with percent IFN? and CD107a expression of NK cells to Pol peptides were evaluated using the linear regression test (figure 4A and 4B). Calculations of CD4 count slope (figure 4C) were determined in Excel (Microsoft) before assessing the significance of results using the unpaired t test and the Fisher’s exact test for figure 4D.
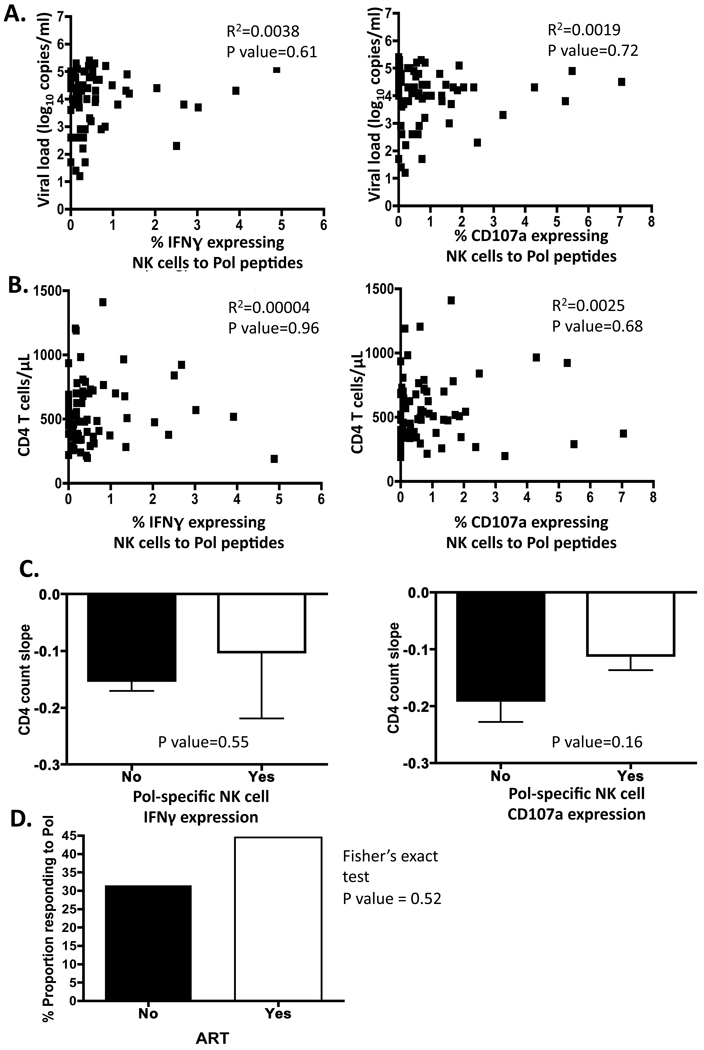
Figure 4. Correlation of Pol-specific ADCC responses with HIV progression.
Correlation of Pol responses, NK cells expressing IFN? and CD107a to A. viral loads and B. CD4 T cell at recruitment to the study were undertaken. No correlation was evident with all parameters analysed against Pol-specific ADCC responses. C. The unpaired t test was used to assess the relationship between the loss of CD4 T cell (CD4 count slope) over time and whether or not subjects had a detectable Pol-specific ADCC response. The decline in CD4 T cell count over time did not correlate with the presence of an ADCC response to Pol. D. The effects of ART on subjects with a response to Pol were also calculated. Of the subjects, who progressed to require ART, 45% were Pol responders and 31% of ART naïve subjects were also responding to Pol (P=0.52).
Results
Pol is a target for ADCC responses
Envelope is the most well recognised ADCC target (Ahmad and Menezes, 1996; Alsmadi and Tilley, 1998; Baum et al., 1996), but ADCC responses to other more conserved HIV proteins such as Pol proteins could help mediate control of viremia. We studied ADCC responses to Pol peptide pools Pol 1 (Pol 1–124) and/or Pol 2 (125–249) using our novel ADCC-ICS assay (figure 1). Subject responses were mapped by gating on the lymphocyte population analysing the expression of IFN? and CD107a from CD56+ and CD2+ CD3- NK cells (figure 2A). Thirty-two (39%) subjects of the eighty-three assessed showed NK cells activated by one of the Pol overlapping peptide pools (figure 1). Similarly to our previous studies on ADCC responses to other HIV antigens (Chung et al., 2009), we found that Pol peptides were required to be present on presenting cells. The activation of NK cells was not diminished if free peptides were washed out prior to adding serum. The proportion of IFNγ expressing NK cells in response to Pol peptides was 0.24% when added directly to donor whole blood and serum, and 0.42% when peptides were first added to whole blood for 1hr then washed twice before adding serum.
Figure 1. Flowchart of Pol-specific ADCC studies.
83 Subjects were initially tested for Pol-specific ADCC responses, 32 showed a positive Pol response. Further mapping of these 32 subjects was then undertaken.
Figure 2. Intracellular cytokine staining ADCC assay and mapping process.
A. Gating strategy: lymphocytes from all cells identified and isolated CD3- cells to allow assessment of CD2+ and CD56+ NK cells expressing IFN?
B. Subject response to Pol, Pol pool 1–124 and 125–249, mapped to Pol pool 231–249 and fine mapped to Pol peptide Pol 233. Responses for DMSO, Rev, Pol pool 126–146, and Pol 232 are included as irrelevant peptides or pools indicating negative ADCC responses.
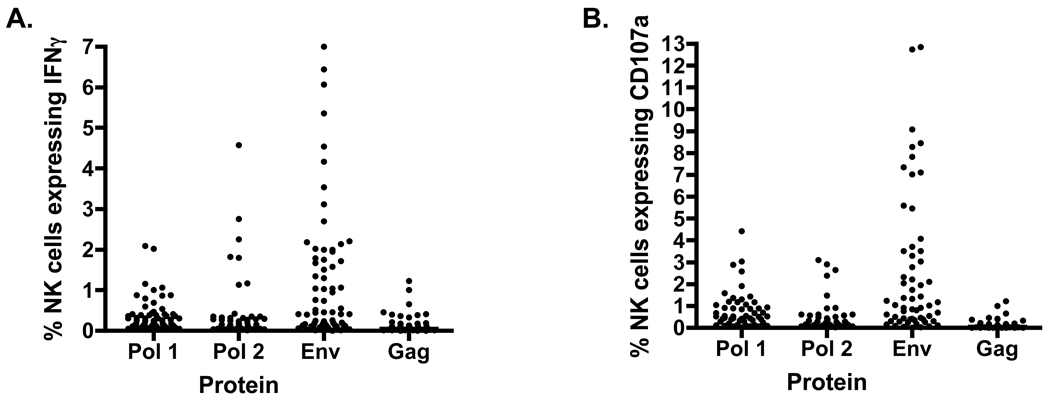
We also measured responses to Gag and Env peptides and compared how frequent and robust ADCC responses to Pol were in comparison to Env and Gag. Comparing Pol to Env and Gag we found 11 subjects had CD3− CD2+ cells expressing IFN? to Pol 1 (Mean ± SE = 0.30±0.05), and 14 subjects showed responses to Pol 2 (Mean ± SE = 0.30±0.08), 34 responded to Env (Mean ± SE = 1.165±0.19) and only 3 to Gag (Mean ± SE = 0.06±0.05) (figure 3A). As compared to CD3- CD2+ IFN?+ expressing cells, CD3- CD2+ CD107a+ cells responded as follows: 23 responses to Pol 1(Mean ± SE = 0.63±0.11) and 17 responses to Pol 2 (Mean ± SE = 0.29±0.09), 48 responded to Env (Mean ± SE = 2.32±0.40) and 11 to Gag (Mean ± SE = 0.23±0.08) (figure 3B).
Figure 3. Env, Gag and Pol specific ADCC responses.
Initial ADCC responses to Pol were identified when ADCC responses were mapped for HIV proteins (Env, Gag and Pol). Significant ADCC responses for env were ascertained as well as for Pol 1 and or Pol 2 by a number of subjects.
Significant ADCC responses to overlapping peptides of Pol 1 or Pol 2 were detected in a number of subjects (figure 3). The use of overlapping peptide pools as antigens allows the mapping of responses to smaller sets of peptides. We mapped responses to smaller pools of Pol consisting of 19–21 Pol peptides per pool. Nine subjects from the 32 Pol responders were mapped to at least one pool of Pol peptides. We were further able to fine map responses from 2 subjects to single Pol peptides (figure 2B). Subject 29 showed ADCC responses to Pol pool 231–249, which was then mapped to Pol peptides 233 (figure 2B).
Correlation of Pol specific ADCC responses with HIV progression
We detected Pol-specific ADCC responses in just under half of the ART naïve, HIV positive subjects. We next asked whether Pol-specific ADCC responses correlated with markers of HIV disease progression. Pol-specific ADCC responses, as measured by NK cells expressing IFN? and CD107a in response to Pol peptides, were correlated to viral loads (figure 4A) and CD4 T cell counts were assessed (figure 4B). No correlation was evident between the subject’s baseline viral load or CD4 T cell count and ADCC response to Pol using the ADCC ICS assay, either by IFN? or CD107a expression analysis. We followed the subjects for a mean of 3 years (2006–2010) after enrolment, during which time they had regular CD4 T cell counts. The unpaired t test was used to assess the relationship between the loss of CD4 T cell (CD4 count slope) over time and Pol-specific ADCC responses (figure 4C). The decline in CD4 T cell count over time did not correlate with the presence of ADCC responses to Pol.
During follow up, the HIV infection of 45 subjects progressed such that ART was initiated. The effects of ART on subjects with a response to Pol were also calculated (figure 4D). Of the subjects, who progressed to require ART, 45% were Pol responders and 31% of ART naïve subjects were also responding to Pol (P=0.52). Overall, we found that Pol-specific ADCC responses did not illustrate a significant correlation with progressive HIV disease.
ADCC Pol-specific responses decline over time
Interestingly a number of subjects with initial responses to Pol and Pol pools (Table 1) were unable to be fine mapped to individual Pol peptides at subsequent blood samples (figure 5). For example subject 2 had a Pol response of approximately 1% in 2006–2008, which was mapped to Pol pools 168–188 (1.33%) and 210–230 (0.66%). (Table 1.) However we were unable to later fine map these responses to the individual Pol peptides, since the ADCC response to Pol declined to <0.1% in 2009–2010. This decline in Pol response over the years was evident in most of the subjects (figure 5). Inability to fine map some subjects to specific Pol peptides may be explained by this decline in response.
Table 1.
Mapping of Pol-specific ADCC responses to pools of 20 overlapping peptides
| Pol Peptide Pools | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subject | 1–20 | 21–41 | 42–62 | 63–83 | 84–104 | 105–125 | 126–146 | 147–167 | 168–188 | 189–209 | 210–230 | 231–249 |
| 2 | 1.33* | 0.66* | ||||||||||
| 11 | 0.68# | 0.63+ | ||||||||||
| 29 | 0.7+ | 0.8^ | 1.31+ | 1.71+ | ||||||||
| 46 | 0.37+ | 0.35+ | ||||||||||
| 53 | 2.45^ | 0.92^ | 0.65^ | 1.202^ | 0.862^ | 0.621^ | 1.102^ | 0.872^ | 1.042^ | 0.752^ | 0.658^ | |
| 62 | 0.7^ | |||||||||||
| 65 | 0.445^ | |||||||||||
| 67 | 0.59^ | 0.43* | 0.57# | 1.32^ | ||||||||
| 80 | 0.383^ | 0.368^ | 0.362^ | 0.51^ | ||||||||
Maximum response for each pool shown based on gating
CD56+ CD107a+;
CD56+ IFNγ+;
CD2+ IFNγ+;
CD2+ CD107a+
Figure 5. Changes in Pol-specific ADCC responses over time.
A decline in Pol-specific ADCC responses over time occurred in the majority of subjects.
Discussion
Until HIV-specific broadly neutralizing antibodies to envelope can be reliably induced, it will be important to identify functional immune responses to conserved HIV proteins, which can effectively be induced by vaccination. Although some Gag-specific CD8+ CTLs are associated with reduced HIV viral loads, only a subset of individuals have the HLA class I alleles that can present the most effective CTL responses. Effector antibody responses such as ADCC that recognize conserved internal proteins, such as Gag and Pol, is a potential method of targeting more broadly acting useful immune responses.
This is the first study to investigate in detail ADCC immune responses to HIV-1 Pol and Gag proteins. We employed a recently described ICS assay that assessed NK cell activation in response to overlapping HIV peptides and ADCC antibodies in plasma (Chung et al., 2009; Stratov, Chung, and Kent, 2008). This assay measures both NK cell IFNγ expression and CD107a expression (a degranulation marker), as markers of cytotoxicity. This assay provides an alternate perspective on antibodies that trigger NK cell activation. We studied a cohort of 83 HIV+ subjects not on ART and found 32 subjects with ADCC responses to linear Pol peptides, although only 11 subjects targeted the Gag protein (including those that expressed either IFNγ or CD107a or both). By mapping ADCC responses to smaller pools of Pol peptides we found that Pol proteins were broadly targeted by ADCC responses. The majority of the higher responses >0.5% was to pools 126–249, which correlates with the 5’ end of the Pol protein. This region expresses RT and IN proteins. Targeting the RT and IN sections of Pol may prove useful targets for a vaccine that elicits ADCC antibodies to induce significant ADCC immune responses against HIV infection. However, we found no correlation between Pol-specific ADCC responses and several markers of HIV progression in the cohort, including loss of CD4 T cells over time and the requirement for anti-retroviral therapy. Interestingly, Pol-specific ADCC responses declined during the follow-up of this cohort.
ADCC responses to HIV are induced early during HIV infection and several studies have shown ADCC responses to Env proteins correlate with slower HIV progression (Ahmad et al., 2001; Alsmadi and Tilley, 1998; Baum et al., 1996; Chung et al., 2008; Tyler et al., 1990). Env readily mutates to escape other immune responses without significant loss in replicative capacity (i.e. minimal “fitness cost”) and is likely to mutate to avoid ADCC responses (Richman et al., 2003). Pol specific CTL epitopes can also mutate to escape immune pressure although there may be bigger fitness costs associated with escape from Pol specific ADCC responses in comparison to Env-specific responses. Similarly, Pol-specific mutations induced during the development of antiretroviral drug resistance also frequently result in significant fitness costs (Sun et al., 2009). Mapping of multiple Pol-specific responses to specific epitopes allows the assessment of immune escape mutations within the Pol-specific ADCC epitope.
Evidence of escape would suggest some immune pressure is being applied by Pol-specific ADCC responses and explain in part the lack of correlation with disease progression observed. It is important to note that our results are restricted to analysing linear rather than conformational Pol-specific ADCC responses. Conformational Pol-specific ADCC epitopes could be more effective at slowing HIV disease. Future studies could evaluate Pol-specific ADCC responses using purified whole Pol proteins.
ADCC responses generally target viral proteins expressed on the surface of infected cells (Ahmad and Menezes, 1996). Since Pol is composed of several enzymes which act intracellularly, with very little of the protein expressed within virions, it is not clear how Pol proteins would be recognised by ADCC antibodies. Alternatively, the ADCC recognition of Pol proteins may primarily be induced by recognition of viral debris form lysed infected cells – in this scenario Pol would not be expected to delay disease progression as we observed. Precisely which cells would present Pol peptides is unclear. Phagocytic cells such as neutrophils and macrophages may scavenge Pol antigens and present them to ADCC antibodies. Future studies of purified Pol-specific ADCC antibodies could assess whether HIV replication is inhibited in vitro using the antibody-dependent cellular viral inhibition assay described by Forthal et al (Forthal et al., 2006)
Interestingly, we observed a general decline in linear Pol-specific ADCC responses during follow up of the cohort. This restricted our ability to map responses to minimal epitopes. Others have also observed decline in HIV-specific ADCC responses over time (Baum et al., 1996). Decreases in numbers and function of circulating NK cells is likely to contribute in part to this decrease in ADCC activity during HIV infection (Fauci, Mavilio, and Kottilil, 2005; Kottilil et al., 2003; Mavilio et al., 2005; Mavilio et al., 2006). A decrease in the function of ADCC effector cells over time would restrict the ability of ADCC responses to slow HIV disease progression as we observed. In addition, a decline in all HIV-specific antibodies is commonly observed in late stage HIV disease (Popovic et al., 1984). However, we might expect that ADCC responses induced by vaccination (prior to HIV infection) would be fully functional and could be capable of contributing to prevention of HIV infection. A modest reduction in numbers of new infections was recently observed in the Thai RV 144 HIV vaccine efficacy trial (Rerks-Ngarm et al., 2009). The vaccine regimen infrequently induced CTL responses and induces only narrowly directed Nab responses but does induce robust ADCC responses (Karnasuta et al., 2005). Several groups have speculated that ADCC responses may be in part responsible for the protection observed by SIV vaccine regimens in macaques (Florese et al., 2006; Gomez-Roman et al., 2005).
In summary, we found Pol is a significant target for ADCC responses in HIV-1 infected subjects but that Pol-specific ADCC responses declined over time and did not correlate with slower progression of HIV infection. To further probe these findings, future studies are needed to resolve both the mechanisms of induction and presentation of Pol-specific ADCC responses and whether such responses force immune escape over time.
Acknowledgments
We are grateful to R Center, L Wren, J Silvers and the subjects studied for assistance with these studies. Supported by NHMRC awards 510448, ARC award LP0991498, the ACH2, The RACP, Ramaciotti Foundation, and NIH award R21AI081541.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmad A, Menezes J. Antibody-dependent cellular cytotoxicity in HIV infections. Faseb J. 1996;10(2):258–266. doi: 10.1096/fasebj.10.2.8641559. [DOI] [PubMed] [Google Scholar]
- Ahmad R, Sindhu ST, Toma E, Morisset R, Vincelette J, Menezes J, Ahmad A. Evidence for a correlation between antibody-dependent cellular cytotoxicity-mediating anti-HIV-1 antibodies and prognostic predictors of HIV infection. J Clin Immunol. 2001;21(3):227–233. doi: 10.1023/a:1011087132180. [DOI] [PubMed] [Google Scholar]
- Alsmadi O, Herz R, Murphy E, Pinter A, Tilley SA. A novel antibody-dependent cellular cytotoxicity epitope in gp120 is identified by two monoclonal antibodies isolated from a long-term survivor of human immunodeficiency virus type 1 infection. J Virol. 1997;71(2):925–933. doi: 10.1128/jvi.71.2.925-933.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsmadi O, Tilley SA. Antibody-dependent cellular cytotoxicity directed against cells expressing human immunodeficiency virus type 1 envelope of primary or laboratory-adapted strains by human and chimpanzee monoclonal antibodies of different epitope specificities. J Virol. 1998;72(1):286–293. doi: 10.1128/jvi.72.1.286-293.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum LL, Cassutt KJ, Knigge K, Khattri R, Margolick J, Rinaldo C, Kleeberger CA, Nishanian P, Henrard DR, Phair J. HIV-1 gp120-specific antibody-dependent cell-mediated cytotoxicity correlates with rate of disease progression. J Immunol. 1996;157(5):2168–2173. [PubMed] [Google Scholar]
- Buchbinder SP, Mehrotra DV, Duerr A, Fitzgerald DW, Mogg R, Li D, Gilbert PB, Lama JR, Marmor M, Del Rio C, McElrath MJ, Casimiro DR, Gottesdiener KM, Chodakewitz JA, Corey L, Robertson MN. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet. 2008;372(9653):1881–1893. doi: 10.1016/S0140-6736(08)61591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung A, Isitman G, Navis M, Kent SJ, Stratov I. CROI 2010. San Francisco: 2010. Evidence of HIV Viral Escape from NK Cell-mediated ADCC. [Google Scholar]
- Chung A, Rollman E, Johansson S, Kent SJ, Stratov I. The utility of ADCC responses in HIV infection. Curr HIV Res. 2008;6(6):515–519. doi: 10.2174/157016208786501472. [DOI] [PubMed] [Google Scholar]
- Chung AW, Rollman E, Center RJ, Kent SJ, Stratov I. Rapid degranulation of NK cells following activation by HIV-specific antibodies. J Immunol. 2009;182(2):1202–1210. doi: 10.4049/jimmunol.182.2.1202. [DOI] [PubMed] [Google Scholar]
- Clavel F, Race E, Mammano F. HIV drug resistance and viral fitness. Adv Pharmacol. 2000;49:41–66. doi: 10.1016/s1054-3589(00)49023-x. [DOI] [PubMed] [Google Scholar]
- Fauci AS, Mavilio D, Kottilil S. NK cells in HIV infection: paradigm for protection or targets for ambush. Nat Rev Immunol. 2005;5(11):835–843. doi: 10.1038/nri1711. [DOI] [PubMed] [Google Scholar]
- Florese RH, Van Rompay KK, Aldrich K, Forthal DN, Landucci G, Mahalanabis M, Haigwood N, Venzon D, Kalyanaraman VS, Marthas ML, Robert-Guroff M. Evaluation of passively transferred, nonneutralizing antibody-dependent cellular cytotoxicity-mediating IgG in protection of neonatal rhesus macaques against oral SIVmac251 challenge. J Immunol. 2006;177(6):4028–4036. doi: 10.4049/jimmunol.177.6.4028. [DOI] [PubMed] [Google Scholar]
- Forthal DN, Landucci G, Cole KS, Marthas M, Becerra JC, Van Rompay K. Rhesus macaque polyclonal and monoclonal antibodies inhibit simian immunodeficiency virus in the presence of human or autologous rhesus effector cells. J Virol. 2006;80(18):9217–9225. doi: 10.1128/JVI.02746-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forthal DN, Landucci G, Daar ES. Antibody from patients with acute human immunodeficiency virus (HIV) infection inhibits primary strains of HIV type 1 in the presence of natural-killer effector cells. J Virol. 2001;75(15):6953–6961. doi: 10.1128/JVI.75.15.6953-6961.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Roman VR, Patterson LJ, Venzon D, Liewehr D, Aldrich K, Florese R, Robert-Guroff M. Vaccine-elicited antibodies mediate antibody-dependent cellular cytotoxicity correlated with significantly reduced acute viremia in rhesus macaques challenged with SIVmac251. J Immunol. 2005;174(4):2185–2189. doi: 10.4049/jimmunol.174.4.2185. [DOI] [PubMed] [Google Scholar]
- Hessell AJ, Hangartner L, Hunter M, Havenith CE, Beurskens FJ, Bakker JM, Lanigan CM, Landucci G, Forthal DN, Parren PW, Marx PA, Burton DR. Fc receptor but not complement binding is important in antibody protection against HIV. Nature. 2007;449(7158):101–104. doi: 10.1038/nature06106. [DOI] [PubMed] [Google Scholar]
- Hue S, Gifford RJ, Dunn D, Fernhill E, Pillay D. Demonstration of sustained drug-resistant human immunodeficiency virus type 1 lineages circulating among treatment-naive individuals. J Virol. 2009;83(6):2645–2654. doi: 10.1128/JVI.01556-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isitman G, Navis M, Kent SJ, Stratov I. Designing immunity to HIV: manipulating antibody-dependent cellular cytotoxicity antibodies. Future Medicine. 2009;3(6):633–640. [Google Scholar]
- Karnasuta C, Paris RM, Cox JH, Nitayaphan S, Pitisuttithum P, Thongcharoen P, Brown AE, Gurunathan S, Tartaglia J, Heyward WL, McNeil JG, Birx DL, de Souza MS. Antibody-dependent cell-mediated cytotoxic responses in participants enrolled in a phase I/II ALVAC-HIV/AIDSVAX B/E prime-boost HIV-1 vaccine trial in Thailand. Vaccine. 2005;23(19):2522–2529. doi: 10.1016/j.vaccine.2004.10.028. [DOI] [PubMed] [Google Scholar]
- Kottilil S, Chun TW, Moir S, Liu S, McLaughlin M, Hallahan CW, Maldarelli F, Corey L, Fauci AS. Innate immunity in human immunodeficiency virus infection: effect of viremia on natural killer cell function. J Infect Dis. 2003;187(7):1038–1045. doi: 10.1086/368222. [DOI] [PubMed] [Google Scholar]
- Ljunggren K, Moschese V, Broliden PA, Giaquinto C, Quinti I, Fenyo EM, Wahren B, Rossi P, Jondal M. Antibodies mediating cellular cytotoxicity and neutralization correlate with a better clinical stage in children born to human immunodeficiency virus-infected mothers. J Infect Dis. 1990;161(2):198–202. doi: 10.1093/infdis/161.2.198. [DOI] [PubMed] [Google Scholar]
- Mavilio D, Lombardo G, Benjamin J, Kim D, Follman D, Marcenaro E, O'Shea MA, Kinter A, Kovacs C, Moretta A, Fauci AS. Characterization of CD56-/CD16+ natural killer (NK) cells: a highly dysfunctional NK subset expanded in HIV-infected viremic individuals. Proc Natl Acad Sci U S A. 2005;102(8):2886–2891. doi: 10.1073/pnas.0409872102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavilio D, Lombardo G, Kinter A, Fogli M, La Sala A, Ortolano S, Farschi A, Follmann D, Gregg R, Kovacs C, Marcenaro E, Pende D, Moretta A, Fauci AS. Characterization of the defective interaction between a subset of natural killer cells and dendritic cells in HIV-1 infection. J Exp Med. 2006;203(10):2339–2350. doi: 10.1084/jem.20060894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElrath MJ, De Rosa SC, Moodie Z, Dubey S, Kierstead L, Janes H, Defawe OD, Carter DK, Hural J, Akondy R, Buchbinder SP, Robertson MN, Mehrotra DV, Self SG, Corey L, Shiver JW, Casimiro DR. HIV-1 vaccine-induced immunity in the test-of-concept Step Study: a case-cohort analysis. Lancet. 2008;372(9653):1894–1905. doi: 10.1016/S0140-6736(08)61592-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitisuttithum P, Gilbert P, Gurwith M, Heyward W, Martin M, van Griensven F, Hu D, Tappero JW, Choopanya K. Randomized, double-blind, placebo-controlled efficacy trial of a bivalent recombinant glycoprotein 120 HIV-1 vaccine among injection drug users in Bangkok, Thailand. J Infect Dis. 2006;194(12):1661–1671. doi: 10.1086/508748. [DOI] [PubMed] [Google Scholar]
- Popovic M, Sarngadharan MG, Read E, Gallo RC. Detection, isolation, and continuous production of cytopathic retroviruses (HTLV-III) from patients with AIDS and pre-AIDS. Science. 1984;224(4648):497–500. doi: 10.1126/science.6200935. [DOI] [PubMed] [Google Scholar]
- Rangel HR, Garzaro D, Fabro R, Fernandez D, Gutierrez CR, Martinez N, Pujol FH. Comparative analysis of polymorphisms in the HIV type 1 pol gene in the proviral DNA and viral RNA in the peripheral compartment. AIDS Res Hum Retroviruses. 2009;25(8):837–841. doi: 10.1089/aid.2009.0023. [DOI] [PubMed] [Google Scholar]
- Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, Premsri N, Namwat C, de Souza M, Adams E, Benenson M, Gurunathan S, Tartaglia J, McNeil JG, Francis DP, Stablein D, Birx DL, Chunsuttiwat S, Khamboonruang C, Thongcharoen P, Robb ML, Michael NL, Kunasol P, Kim JH. Vaccination with ALVAC and AIDSVAX to Prevent HIV-1 Infection in Thailand. N Engl J Med. 2009;361:2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- Richman DD, Wrin T, Little SJ, Petropoulos CJ. Rapid evolution of the neutralizing antibody response to HIV type 1 infection. Proc Natl Acad Sci U S A. 2003;100(7):4144–4149. doi: 10.1073/pnas.0630530100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stott EJ. Anti-cell antibody in macaques. Nature. 1991;353(6343):393. doi: 10.1038/353393a0. [DOI] [PubMed] [Google Scholar]
- Stratov I, Chung A, Kent SJ. Robust NK cell-mediated human immunodeficiency virus (HIV)-specific antibody-dependent responses in HIV-infected subjects. J Virol. 2008;82(11):5450–5459. doi: 10.1128/JVI.01952-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Ma L, Yu X, Huang Y, Yuan L, Shao Y. Replication and drug resistant mutation of HIV-1 subtype B' (Thailand B) variants isolated from HAART treatment individuals in China. Virol J. 2009;6:201. doi: 10.1186/1743-422X-6-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler DS, Stanley SD, Zolla-Pazner S, Gorny MK, Shadduck PP, Langlois AJ, Matthews TJ, Bolognesi DP, Palker TJ, Weinhold KJ. Identification of sites within gp41 that serve as targets for antibody-dependent cellular cytotoxicity by using human monoclonal antibodies. J Immunol. 1990;145(10):3276–3282. [PubMed] [Google Scholar]
- Yamada T, Watanabe N, Nakamura T, Iwamoto A. Antibody-dependent cellular cytotoxicity via humoral immune epitope of Nef protein expressed on cell surface. J Immunol. 2004;172(4):2401–2406. doi: 10.4049/jimmunol.172.4.2401. [DOI] [PubMed] [Google Scholar]