Abstract
Accumulation of reactive oxygen species has been implicated in various diseases and aging. However, the precise physiological effects of accumulating oxidants are still largely undefined. Here, we applied a short-term peroxide stress treatment to young Caenorhabditis elegans and measured behavioral, physiological, and cellular consequences. We discovered that exposure to peroxide stress causes a number of immediate changes, including loss in mobility, decreased growth rate, and decreased cellular adenosine triphosphate levels. Many of these alterations, which are highly reminiscent of changes in aging animals, are reversible, suggesting the presence of effective antioxidant systems in young C. elegans. One of these antioxidant systems involves the highly abundant protein peroxiredoxin 2 (PRDX-2), whose gene deletion causes phenotypes symptomatic of chronic peroxide stress and shortens lifespan. Applying the quantitative redox proteomic technique OxICAT to oxidatively stressed wild-type and prdx-2 deletion worms, we identified oxidation-sensitive cysteines in 40 different proteins, including proteins involved in mobility and feeding (e.g., MYO-2 and LET-75), protein translation and homeostasis (e.g., elongation factor 1 [EFT-1] and heat shock protein 1), and adenosine triphosphate regeneration (e.g., nucleoside diphosphate kinase). The oxidative modification of some of these redox-sensitive cysteines may contribute to the physiological and behavioral changes observed in oxidatively stressed animals. Antioxid. Redox Signal. 14, 1023–1037.
Introduction
Reactive oxygen species (ROS), like superoxide radicals (O2•−) and hydrogen peroxide (H2O2), occur during normal metabolism, often because of incomplete electron transfer within the respiratory chain (2). To cope with these oxidants, cells harbor ROS-detoxifying enzymes and redox-balancing systems to maintain the appropriate reducing potential (8). When ROS concentrations exceed the antioxidant capacity of the cell, severe DNA, lipid, and protein damage can occur and organisms suffer from a condition termed oxidative stress (17). The damaging effects of accumulating ROS have been implicated in neurodegenerative diseases, cancer, atherosclerosis, diabetes, and heart disease (1, 11). However, the specific physiological consequences of oxidative stress conditions in higher eukaryotes remain largely unknown.
The predominant cellular targets of ROS are the amino acids of proteins. Side chain modifications, which include oxidation of cysteines and methionines as well as carbonylation reactions, often cause the inactivation of the affected proteins (43). This oxidative inactivation of specific cellular proteins has been proposed to constitute one of the major mechanisms that links oxidative stress to loss of critical physiological functions (41). Although the damaging effects of ROS have long been recognized, the pivotal role that specific ROS play in the post-translational regulation of redox-sensitive proteins has only recently started to emerge (36). ROS were found to reversibly modify structurally and functionally important cysteines in numerous redox-sensitive proteins. These thiol modifications often cause profound functional changes in proteins involved in transcription, translation, metabolism, stress protection, signal transduction, and apoptosis, and these changes contribute to or may even cause the immediate and long-term responses of organisms to oxidative stress (5).
Several different proteomic techniques have been established with the goals of identifying redox-sensitive proteins in eukaryotic tissues and understanding the effects of oxidative stress in greater detail (36). However, most of these methods have limitations, including the inability to identify the redox-sensitive cysteine(s) and, even more importantly, to precisely quantify the extent of oxidative thiol modifications. While identification of the redox-sensitive cysteine is crucial in evaluating how oxidative modification might affect the structure and function of the respective protein, determination of the extent of oxidative modification is essential in determining whether or not these oxidative modifications are physiologically relevant. We have recently developed a quantitative thiol trapping technology, termed OxICAT, which enables us to determine the precise oxidation status of hundreds of cellular proteins in a single experiment (30). By using OxICAT, we identified several novel redox-sensitive Escherichia coli proteins, which were found to play pivotal roles in the oxidative stress resistance of bacteria. We have now decided to use this methodology to gain insights into the physiological and redox proteomic effects of oxidative stress in higher eukaryotes.
Here, we exposed synchronized young wild-type Caenorhabditis elegans on day 0 of adulthood to a short treatment with H2O2 and monitored subsequent behavior and physiology. Although most animals eventually recovered from this oxidative stress regimen, they first reacted with dramatic behavioral changes, including nearly complete loss of mobility and reduction in growth rate and cellular adenosine triphosphate (ATP) levels. OxICAT analysis revealed a number of physiologically relevant eukaryotic target proteins, which harbor highly conserved cysteines that significantly change their redox status upon oxidative stress treatment. These included proteins involved in muscle contraction, feeding behavior, and ATP regeneration. Identification of these redox-regulated proteins substantially increased our understanding of redox-regulated responses in eukaryotes and will form the foundation for future studies aimed to understand the physiological consequences of oxidative stress in aging and disease.
Materials and Methods
Strains and culture conditions
The Bristol strain N2 (wild-type), and the original isolate VC289 prdx-2(gk169)II were provided by the Caenorhabditis Genetics Center. The prdx-2 strain was backcrossed three times to strain N2. Strains were maintained and cultured under standard conditions using E. coli OP50 as a food source (32). For large-scale C. elegans cultivation, see Supplementary Data (available online at www.liebertonline.com/ars).
Oxidative stress treatment
Synchronized worms at day 0 of adulthood were collected by centrifugation in M9 medium and washed. One hundred microliters of worms (∼30,000 worms) was incubated in 2 ml M9 with the indicated concentration of H2O2 at room temperature in a rotating roller drum for 30 min. After treatment, the worms were collected by centrifugation and the oxidant was washed away with M9 medium. The animals were seeded on fresh nematode growth medium plates and immediately singled. Singling and scoring was performed blinded for the day 0 evaluation.
Lifespan, movement, and brood size analysis
All behavioral studies were conducted, scored, and statistically analyzed as previously described (15). Details are found in Supplementary Data.
ATP measurements
One hundred microliters of synchronized young adults was treated with the indicated concentrations of H2O2 in 1.8 ml of M9 media for 30 min. After the treatment, worms were washed with M9 medium, shock-frozen in liquid N2, thawed, transferred into boiling 4 M Guanidinium-HCl, and boiled for 15 min. After centrifugation (30 min, 4°C, 13,200 g), the protein concentration of the supernatant was determined using the BioRad assay (Bio-Rad Laboratories). Samples were diluted 1:200 into ATP buffer (40 mM HEPES-KOH and 4 mM magnesium sulfate, pH 7.8) and mixed with the same volume of assay buffer containing 200 nM luciferase (Roche), 140 μM luciferin (Biotium Inc.), 0.1 mg/ml BSA, 200 mM KH2PO4, 50 mM glycylglycine, and 0.4 mM ethylenediaminetetraacetic acid, pH 7.8. Chemiluminescence was measured using a FLUOstar Omega (BMG Labtech). ATP concentrations were determined according to an ATP standard curve and normalized over protein content.
Sample preparation for OxICAT
To prepare protein extracts for the redox proteomic analysis, 50–100 μl stress-treated worms were harvested onto 10% (w/v) trichloracetic acid (TCA) and shock-frozen in liquid nitrogen to induce breaking and lysis; after thawing, they were homogenized for 2 min with Power Gen 125 (Fisher Scientific). The TCA-treated protein extract from C. elegans was centrifuged (13,000 g, 4°C, 30 min) and the resulting pellet was washed once with 500 μl 10% (w/v) TCA and once with 200 μl 5% (w/v) TCA. The supernatant was completely removed and the sample was transferred to the anaerobic chamber for the first OxICAT alkylation step. All subsequent steps of the OxICAT labeling were performed according to the published protocol (30). Mass spectrometry (MS) was performed at the Michigan Proteome Consortium. Data were analyzed as described (30) and details can be found in Supplementary Data.
Results
Peroxide treatment leads to reversible behavioral defects in C. elegans
The cellular accumulation of ROS has been attributed to many different physiological and pathological alterations, yet the precise effects that sublethal concentrations of oxidants such as H2O2 exert on the physiology of multicellular organisms have not been well defined. We therefore decided to investigate the effects of specific oxidants on the behavior and redox proteome of C. elegans. We chose C. elegans because it is a very well-characterized organism that can be easily synchronized, and it has a multitude of age-related behavioral traits, such as mobility, brood size, and pharyngeal pumping, that can be quantitatively assessed along with size and lifespan. In previous oxidative stress resistance tests in C. elegans, the minimal lethal oxidant concentrations have been determined, but investigations of physiological effects and/or potential recovery have remained relatively unexplored (28).
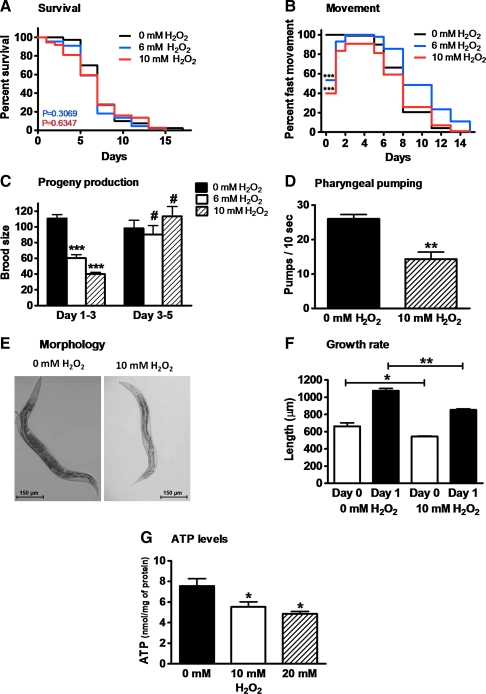
We initiated our studies by exposing synchronized C. elegans on day 0 of adulthood to different H2O2 concentrations for 30 min. We then removed the oxidant and monitored immediate and long-term behavior. Our goal was to find oxidant concentrations that affect the majority of the worm population without killing them; this information would be used for subsequent quantitative redox proteomic analysis to reveal oxidative stress-specific target proteins in C. elegans. We found that short-term exposure of C. elegans to 6 or 10 mM H2O2 fulfills these criteria. These treatments are nonlethal (survival rate >95% compared to control group) and do not affect the mean lifespan of the organism (Fig. 1A). At the same time, they cause very distinct behavioral defects in the majority of animals. The most obvious and immediate effects of H2O2 treatment that we observed were mobility defects, which were expressed as decreased thrashing in H2O2-supplemented liquid media (data not shown). This movement defect remained after washing the worms and seeding them on oxidant-free food plates. We found that highly impaired movement, ranging from no movement to slow and uncoordinated motility, occurred in ∼20% of the worms treated with 6 mM H2O2 and in ∼60% of worms treated with 10 mM H2O2 (Fig. 1B). Importantly, within <48 h after treatment, the vast majority of worms regained fast, sinusoidal body movement, which they maintained until the typical age-related motility decline set in (Fig. 1B). This result suggests that young worms have effective antioxidant systems that reverse the effects of exogenous oxidative stress and promote their recovery.
FIG. 1.
Short-term hydrogen peroxide (H2O2) treatment causes reversible behavioral defects in Caenorhabditis elegans. Synchronized wild-type C. elegans at day 0 of adulthood were treated with 0 (black trace), 6 (blue trace), or 10 mM H2O2 (red trace) for 30 min in liquid M9 media. Then, the oxidant was removed and 50 worms per treatment were singled and scored for (A) survival and lifespan, (B) fast movement, (C) progeny production, (D) pharyngeal pumping (day 1), (E) morphology (day 1), and (F) growth rate (from day 0 to 1 of adulthood) at 25°C. (G) To determine intracellular adenosine triphosphate (ATP) levels, 100 μl of worms was treated with the indicated concentrations of H2O2 for 30 min, and ATP levels were measured before and after oxidative stress treatment. As seen in (A), no significant difference in the mean lifespan of oxidatively stressed worms was observed in five independent experiments. A representative life span is shown here with p = 0.3069, χ2 = 1.044 for 6 mM H2O2 and p = 0.6347, χ2 = 2.258 for 10 mM H2O2 in comparison with the control group (0 mM H2O2) (nonparametric log rank test). The movement plot shown in (B) is an average of at least three independent experiments and the direct comparison by repeated measures analysis of variance (ANOVA) and Bonferroni post hoc test reveals a difference in the movement behavior on day 0 after the oxidative stress treatment (p < 0.001). The symbols above the bars in (C–G) represent the p-values obtained using t-test or one-way ANOVA: #p > 0.05, *p < 0.05, **p < 0.01, ***p < 0.001. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
Another consequence of exposing C. elegans to H2O2 stress was a significant reduction in the total progeny production. This decrease was not due to differences in the length of the fertile period but instead due to a severe decline in egg production in the first 3 days after the oxidative stress treatment (Fig. 1C). As observed with the mobility defects, the animals recovered from the H2O2 treatment and showed a progeny production very similar to the progeny production of the control group after 72 h (Fig. 1C). In addition, we found that peroxide-treated animals showed reduced pharyngeal pumping (Fig. 1D), a significant decrease in body length (Fig. 1E), and a significantly reduced growth rate (Fig. 1F).
Peroxide stress has been shown to lead to a massive decline in intracellular ATP levels due to the oxidative inactivation of key enzymes involved in energy-generating pathways, that is, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and ATP synthases (16). Because decreased ATP levels might explain at least some of the observed behavioral changes in C. elegans, we measured intracellular ATP concentrations after 30 min of exposure to peroxide stress. As shown in Figure 1G, we found that peroxide-treated worms do indeed suffer from a significant drop in intracellular ATP levels. These results suggest that a change in the cellular energy charge might be at least in part responsible for some of the behavioral changes observed in peroxide-treated worms.
Peroxiredoxin 2 promotes recovery from peroxide stress-induced motility and egg-laying defects
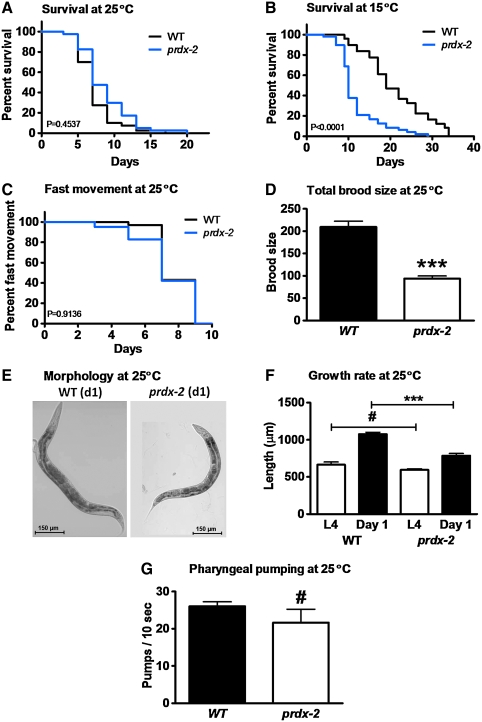
One important player in the H2O2 detoxification system of C. elegans is the 2-Cys peroxiredoxin 2 (PRDX-2) (35), a highly abundant protein that constitutes ∼0.5% of the total C. elegans proteome (Supplementary Fig. S1; see Supplementary Data available online at www.liebertonline.com/ars). Deletion of prdx-2 has been shown to increase the sensitivity of C. elegans toward exogenous peroxide treatment and to cause a significant decrease in the lifespan of these worms, especially at lower cultivation temperatures (35) (Fig. 2A, B). Moreover, the phenotypes of prdx-2 mutant worms, especially after the larval stages (18, 35), were highly reminiscent of the behavioral changes that we observed when we treated wild-type strains with H2O2 (Fig. 2C–F). Adult prdx-2 deletion strains show a reduced brood size, and remain significantly smaller during their adult lifespan. These results not only indicate that prdx-2 worms suffer from endogenous oxidative stress but also suggest that short-term peroxide treatment can be used to mimic reversible peroxide stress in vivo.
FIG. 2.
Phenotypes of prdx-2 deletion mutants. Synchronized wild-type (black trace) and prdx-2 mutant (blue trace) worms (n = 50) were singled and scored for (A, B) survival at 25°C and 15°C (C) fast movement at 25°C and (D) progeny production at 25°C. The prdx-2 mutants are significantly short-lived (p < 0.001) at 15°C (B) but not at 25°C (p = 0.4537) (A). There is no difference in the fast movement span at 25°C (p = 0.9136) (C) and in the pharyngeal pumping rate on day 1 at 25°C (p = 0.2668) (G), but prdx-2 worms produce fewer offspring (p < 0.001) (D). Worms were imaged on day 0 and on day 1 after oxidative stress treatment to assess morphology (day 1) at 25°C (E) and growth rate from L4 stage to day 1 of adulthood (F). The symbols above the bars represent the p-values obtained: #p > 0.05, ***p < 0.001. Similar results were obtained in at least three independent experiments. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
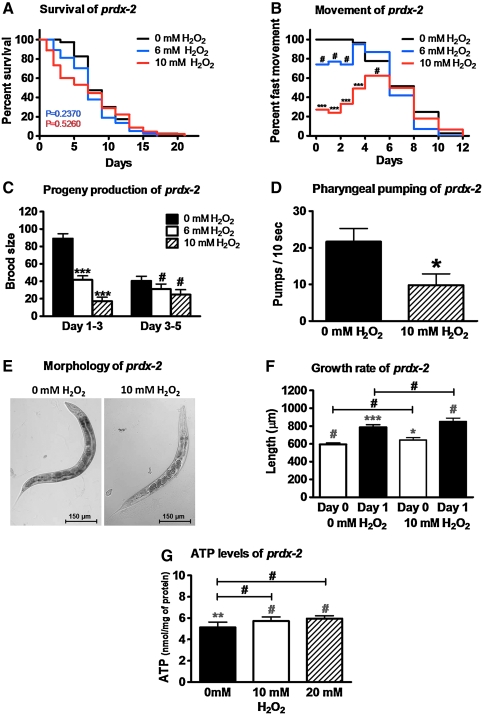
To assess the effects of exogenous oxidative stress in worms lacking PRDX-2, we exposed synchronized prdx-2 mutant worms on day 0 of adulthood to our short-term peroxide stress treatment. Although we did not detect any change in the mean adult lifespan, we did notice a reproducible increase in the initial mortality rate and a decreased minimal lifespan (Fig. 3A). This result suggests that lack of PRDX-2 might lead to a prolonged period of peroxide stress in worms, which leads to early death in a susceptible subfraction of the animals. We also analyzed movement and progeny production of prdx-2 mutants upon peroxide treatment. Although exogenous peroxide stress treatment caused only a slight but reproducibly more severe immediate motility defect in the prdx-2 mutant strain as compared to wild-type C. elegans (compare Fig. 1B with Fig. 3B), recovery in the 10 mM H2O2-treated group was severely impaired in the mutant worms, with many worms never regaining their original mobility (Fig. 3B). These results suggest that lack of PRDX-2 lengthens the exposure of cellular macromolecules to the damaging effects of peroxide stress. Very similar results were obtained when we analyzed the effects of peroxide stress on the progeny production of prdx-2 worms; brood size dropped to <20% of the values obtained in the untreated prdx-2 mutants and never recovered (Fig. 3C). In contrast to mobility, progeny production, and pharyngeal pumping of prdx-2 worms, which were all additionally affected by exogenous oxidative stress treatment (Fig. 3B–D), neither size, growth rate, nor intracellular ATP levels of prdx-2 mutant animals were further reduced by exogenous peroxide treatment (Fig. 3E–G). These results suggest that some cellular processes might already be maximally affected by the oxidative stress that is caused by the absence of PRDX-2.
FIG. 3.
Recovery from exogenous H2O2 stress is mediated by PRDX-2. Synchronized prdx-2 C. elegans (day 0 of adulthood) were incubated with 0 (black trace), 6 (blue trace), or 10 mM H2O2 (red trace) as in Figure 1. Then, the oxidant was removed and 50 worms per treatment were singled and scored for (A) survival and lifespan, (B) fast movement, (C) progeny production, (D) pharyngeal pumping (day 1), (E) morphology (day 1), and (F) growth rate (from day 0 to 1 of adulthood) at 25°C. (G) To determine intracellular ATP levels, 100 μl of worms were treated with the indicated concentrations of H2O2 for 30 min, and ATP levels were measured before and after oxidative stress treatment. As seen in (A), no significant difference in the mean lifespan of oxidatively stressed worms was observed in four independent experiments. A representative life span is shown here with p = 0.2370, χ2 = 1.3990 for 6 mM H2O2 and p = 0.5260, χ2 = 0.4020 for 10 mM H2O2 in comparison with the control group (0 mM H2O2) (nonparametric log rank test). The movement plot shown in (B) is an average of at least three independent experiments and the direct comparison by repeated measures ANOVA and Bonferroni post hoc test reveals a difference in the movement behavior on day 0 through day 3 after the oxidative stress treatment with 10 mM H2O2 (p < 0.001) but not with 6 mM H2O2. The symbols above the bars in (C–G) represent the p-values obtained using t-test or one-way ANOVA: #p > 0.05, *p < 0.05, **p < 0.01, ***p < 0.001. The additional symbols (light gray) above the bars in (F–G) represent the p-values obtained for the comparison of prdx-2 worms and the respective wild-type worms presented in Figure 1F and G using two-way ANOVA and Bonferroni post hoc test: #p > 0.05, *p < 0.05, **p < 0.01, ***p < 0.001. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
Quantitative redox proteomics identifies redox-sensitive C. elegans proteins
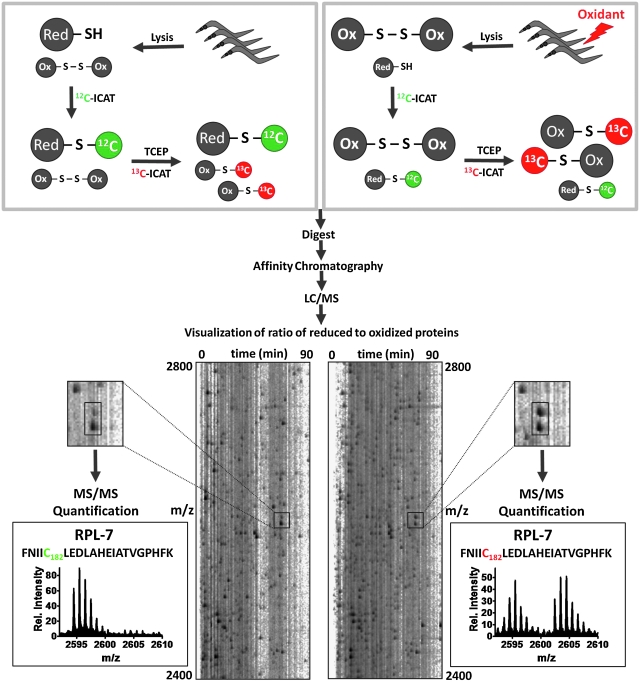
Peroxide stress conditions affect physiological processes, presumably through the oxidative modification of specific cellular targets. We therefore decided to use OxICAT to reveal particularly stress-sensitive proteins whose redox-regulated function might be responsible for some of the observed behavioral and physiological effects of peroxide stress. The OxICAT technology is based on the differential trapping of reduced and oxidized cysteines with two versions of the isotope coded affinity tag (ICAT): an isotopically light 12C-form (i.e., light ICAT) and a 9 Da heavier, isotopically heavy 13C-form (i.e., heavy ICAT) (30) (for details, see Fig. 4). All in vivo-reduced cysteines are alkylated with the light ICAT reagent, whereas all in vivo-oxidized cysteines are, upon their reduction, labeled with the heavy ICAT reagent. After tryptic digest and enrichment using affinity chromatography, the ICAT-labeled peptides are separated by liquid chromatography and identified by MS. Because light- and heavy-labeled peptides are chemically identical, the relative ion intensities of the two peaks represents the relative abundance of reduced and oxidized protein species in cells, making this method independent of absolute protein amounts. This makes the OxICAT technique uniquely suited to quantitatively describe changes in the thiol status of hundreds of individual proteins in a single experiment.
FIG. 4.
Identification of redox-sensitive C. elegans proteins using OxICAT. For the OxICAT analysis, a population of ∼100,000 worms (day 0 of adulthood) was either left untreated (left panel) or treated with 10 mM H2O2 for 30 min (right panel). The worms were washed and lysed. Proteins were incubated with isotopically light 12C-ICAT reagent (green) under denaturing conditions to irreversibly label all reduced cysteines. Then, all reversibly oxidized cysteines were reduced with Tris(2-carboxyethyl)phosphine (TCEP) and subsequently irreversibly labeled with the 9 Da heavier 13C-ICAT reagent (red). The proteins were digested, and isotope coded affinity tag (ICAT)-labeled peptides were purified by affinity chromatography and analyzed using liquid chromatography (LC)/mass spectrometry (MS). MSInspect was used to illustrate the LC/MS run [for details, see Leichert et al. (30)]. The mass spectra of a typical ICAT pair harboring one oxidative stress-sensitive cysteine are shown. The mass signal with the m/z value at 2594.5 Da has incorporated one light ICAT molecule and represents the reduced form of the peptide. The mass signal with the higher m/z value of 2603.5 Da (spectra on the right) has incorporated one heavy ICAT molecule and represents the oxidized form of the peptide. MS/MS analysis revealed the identity of the protein (i.e., RPL-7) and of the redox-sensitive cysteine (i.e., Cys182). Analysis of the peak intensity revealed that oxidative stress treatment increased the oxidation status of this cysteine from 6% to 51%.
To investigate which C. elegans proteins are sensitive to oxidative modification, we exposed a synchronized population of ∼100,000 young adult wild-type C. elegans N2 or prdx-2 deletion mutants to our previously established oxidative stress regimen and quantified the thiol oxidation status of proteins before and after the stress treatment. We found a considerable number of ICAT-labeled peptides whose masses changed by 9 or 18 Da, indicating that they contain either one or two cysteine residues whose redox status changed upon peroxide treatment. We only considered C. elegans peptides to be redox sensitive if their stress-induced changes in thiol oxidation status were reproducibly >1.5-fold and the oxidized population exceeded 20%, the thresholds we previously used to identify redox-sensitive proteins in E. coli (30). Both the extent of oxidation as well as the total number of identified oxidation-sensitive proteins could be underestimates because only ∼60% of the treated worms showed behavioral changes upon stress treatment (Fig. 1B). The remaining 40% of worms revealed no obvious defects, suggesting that their redox proteome might not be affected. We then used MS/MS analysis to identify the peptides and the oxidation-sensitive cysteines. We found a total of 40 different proteins whose thiol oxidation status changed at least 1.5-fold and up to 9-fold upon peroxide stress treatment in wild-type and/or prdx-2 mutant strains (Table 1). Of those, 22 proteins were found to be oxidation sensitive in both strain backgrounds, confirming their general oxidation sensitivity. Noteworthy, many of these proteins showed very similar absolute oxidation levels in the two strains, both before and after stress treatment. We identified only two proteins whose cysteines were found to be approximately threefold more oxidized in nonstress-treated prdx-2 worms compared to wild-type worms: the highly conserved Cys307 of the Hsp70 homolog heat shock protein 1 (HSP-1) and Cys204 of the small subunit ISW-1 of the chromatin remodeling factor (Table 1). This increase in thiol oxidation might reflect subcellular, localized changes in the peroxide levels of prdx-2 mutant worms.
Table 1.
Thiol Oxidation Status of Caenorhabditis elegans Proteins Before and After Peroxide Treatment
| |
Peptide annotation |
Cysteine oxidation status [%] |
||||
|---|---|---|---|---|---|---|
| ACC number | Peptide sequence | Protein | N2 | N2 + H2O2 | prdx-2 | prdx-2 + H2O2 |
| Protein translation | ||||||
| CE00664 | TC199SKLYPLQEVYIR | 40S ribosomal protein S3a (RPS-1) | 14 | 39 | 15 | 25 |
| CE00821 | IGRIEDVTPIPSDC140TR | 40S ribosomal protein S14 (RPS-14) | 14 | 28 | 17a | 34 |
| CE26948 | VC35DEVAIIGSKPLR | 40S ribosomal protein S17 (RPS-17) | 11 | 30 | 11 | 37 |
| CE13265 | GVAPNHFQTSAGNC99LR/Kb | 40S ribosomal protein S19 (RPS-19) | 9/13c | 34/27c | 23 | 34/34c |
| CE30779 | YAIC57GAIR/Rb | 40S ribosomal protein S21 (RPS-21) | 14/22c | 39/42c | 20 | 47 |
| CE21842 | TGSQGQC22TQVRVEFINDQNNR | 40S ribosomal protein S28 (RPS-28) | 18 | 36 | —d | —d |
| CE07669 | QK/LGPVVIYGQDAEC216ARb | Ribosomal protein RL4L4 (RPL-4) | 6/12c | 29/34c | 12a/18a | 25/34c |
| CE11024 | FNIIC182LEDLAHEIATVGPHFK | 60S ribosomal protein L7 (RPL-7) | 6 | 51 | —d | —d |
| CE04102 | FNVEC27KNPVEDGILR | 60S ribosomal protein L22 (RPL-22) | 10 | 31 | 16a | 37 |
| CE15900 | LLEPVYLVEIQC745PEAAVGGIYGVLNR/Rb | Elongation factor 2 (EFT-2) | 7/9c | 31/27c | 13 | 35 |
| CE01270 | SGDAGIVELIPTKPLC377VESFTDYAPLGR | Elongation factor EF-1 alpha (EFT-4) | —d | —d | 15 | 36 |
| CE20412 | SIYDTFSLFGNILSC149K | Polyadenylate-binding protein (PAB-1) | 17 | 34 | —d | —d |
| Protein homeostasis | ||||||
| CE09682 | FEELC307ADLFR | Heat shock 70 kDa protein A (HSP-1) | 7 | 27 | 24 | 42 |
| CE02262 | SIHDALC410VIR | TCP-1, delta subunit (CCT-4) | 13a | 41 | 16a | 36 |
| CE01234 | LADHITEC171VVDAVLAIR | TCP-1, zeta subunit (CCT-6) | 17 | 33 | —d | —d |
| CE27244 | LIQDVANKANEEAGDGTTC106ATVLAR | Cpn60 (HSP-60) | 10 | 38 | 11 | 48 |
| CE05402 | AAAPC577VLFFDELDSIAK | AAA+-type ATPase (CDC-48.2) | 10 | 22 | 14a | 30 |
| CE14954 | FLEFVKPFC13GFVPEVSKPER | Sec61, alpha-subunit (SEC-61) | —d | —d | 11 | 30 |
| CE03482 | IYHPNINSNGSIC85LDILR | Ubiquitin-protein ligase (UBC-2) | 12 | 36 | 13 | 29 |
| Motility | ||||||
| CE06253 | C280YHIFYQIYSDFRPELK | Myosin class II heavy chain (LET-75) | 8 | 33 | —d | —d |
| CE09349 | LASC702DIEHYLLEK | Myosin class II heavy chain (UNC-54) | —d | —d | 10 | 26 |
| CE31619 | ASGVIDAGLVLNQLTC708NGVLEGIR | Myosin-2 (MYO-2) | —d | —d | 20 | 31 |
| CE04994 | NFLAAVC219R | Troponin T (MUP-2) | 18 | 25 | 17 | 30 |
| CE31204 | C704LSHDIDILR | Intermediate filament protein (IFC-2) | — | — | 14 | 29 |
| CE27706 | KPQILQQTSAGGEPAIC550FDIGYSAR | Immunoglobin-like protein (DIM-1) | 6 | 46 | — | — |
| ATP homeostasis | ||||||
| CE22210 | LAANNPLLC218GQR | Vacuolar H+-ATPase V1, subunit A (VHA-13) | 18 | 25 | 13 | 35 |
| CE04424 | LIYQTVC33GVNGPLVILNDVKFPQFSEIVK | Vacuolar H+-ATPase V1, subunit B (VHA-12) | 7 | 26 | —d | —d |
| CE09650 | GDFC109IQTGR | Nucleoside diphosphate kinase (F25H2.5) | 24 | 41 | —d | —d |
| CE09650 | NIC117HGSDAVDSANR | Nucleoside diphosphate kinase (F25H2.5) | 9 | 34 | —d | —d |
| CE37112 | SIC341DGLKLQIR | Creatine kinase (F46H5.3) | —d | —d | 13 | 30 |
| Metabolism and others | ||||||
| CE01225 | C153VLNIGTHTPSHLGMLENANVLAR | Fructose-biphosphate aldolase (ALDO-2) | 21 | 45 | 26 | 47 |
| CE12728 | GIFIC76DGSQHEADELIDKLIER | PEP-carboxykinase (R11A5.4) | —d | —d | 8 | 39 |
| CE01130 | NTC391PGDVSALRPGGIR | Gly/Ser hydroxymethyltransferase (MEL-32) | 30 | 46 | 32a | 53 |
| CE25005 | TAVPSTIHC123DHLIEAQKGGAQDLAR | Aconitase (ACO-2) | —d | —d | 10 | 43 |
| CE29792 | WC204PSINAVVLIGDEAAR | Chromatin remodeling complex (ISW-1) | 13 | 22 | 34 | 50 |
| CE17691 | GPDAGYIATSGC374VLSAALTLIR | Uncharacterized membrane protein (F22F7.1) | 20 | 43 | 23 | 28 |
| CE17154 | HFELLPNDAIVC299NVGHFDC306EIDVKe | S-adenosylhomocysteine hydrolase (AHCY-1) | 27 | 42 | 23a | 43 |
| CE23530 | LISDIEDEC666GGVHIRFPSEK | Vigilin (C08H9.2) | 26 | 36 | 13 | 29 |
| CE00913 | TAVC354DIPPR | Tubulin beta-2 chain (TBB-1) | 11 | 25 | —d | —d |
| CE01431 | GLGTDEAVLIEILC112SR | Annexin (NEX-1) | 8 | 23 | 11 | 27 |
| CE41898 | TGLGLC919IR | Similarity to human attractin (TAG-53) | — | — | 20 | 36 |
Peptide was identified once only.
Two peptides differing in their cleavage site were identified.
Average oxidation status in the respective peptides is shown.
Peptide was either not identified or the spectrum quality was too poor to determine precise oxidation status.
Both cysteines in this peptide were found to be oxidized.
All other redox-sensitive peptides that are listed in Table 1 were detected either only in wild-type or prdx-2 deletion strains. That we did not detect these peptides in both strain backgrounds might be due to differences in protein expression levels or due to limitations of the liquid chromatography/MS analysis. The fact, however, that we confirmed so many of the peptides to be sensitive to peroxide stress in both strain backgrounds makes us confident that the listed proteins are indeed peroxide sensitive.
Protein translation is a major target of oxidative modifications
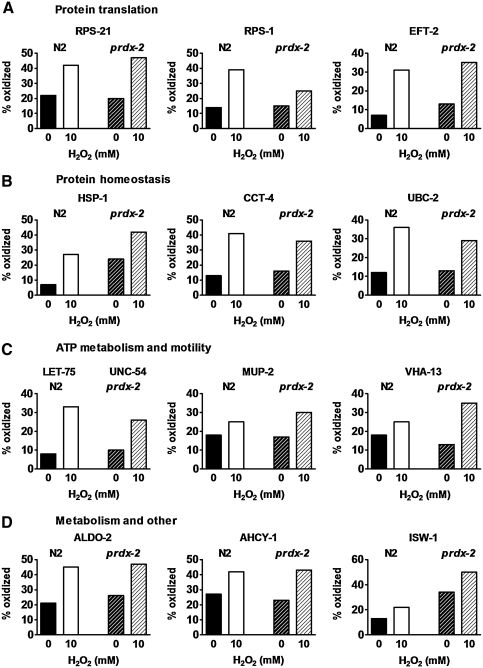
Proteins involved in translation appear to be among those most heavily targeted by H2O2 stress (Table 1), a result that agrees well with previous observations that protein translation is a peroxide-sensitive process in vivo (40). We identified peroxide-sensitive cysteines in six small (S3A, S14, S17, S19, S21, and S28) and three large (L4, L7, and L22) ribosomal subunits, in the polyadenylate-binding protein (PAB)-1, and in two elongation factors, EFT-2 and EF-1α (EFT-4). One of the most peroxide-sensitive cysteines that we identified is the lone Cys182 of the large ribosomal subunit protein L7; the oxidation status of this cysteine changed from 6% to over 50% upon peroxide treatment (Fig. 4, inset). Although this cysteine is highly conserved in eukaryotes, no studies have addressed its role in L7 function. It is conceivable that formation of sulfenic acid or of an intermolecular disulfide with either the small tripeptide glutathione (i.e., S-glutathionylation) or another protein leads to conformational and functional changes in this important component of the eukaryotic translation machinery.
The small subunit S21 (RPS-21) is another ribosomal protein that we found to contain a highly peroxide-sensitive cysteine (i.e., Cys57) in both wild-type and prdx-2 mutant strains (Fig. 5A). Its activity is required for embryonic and germline development and for the overall health of the animal (www.wormbase.org, release WS204, 29 July 2009). We identified two Cys57-containing RPS-21 peptides in wild-type worms that only differ in their respective cleavage site. Cys57 was oxidized to either 39% or 42% depending on the respective peptide (Table 1 and Fig. 5A), a result that nicely illustrates the accuracy of our OxICAT technique. Similarly, we identified two Cys745-containing peptides of the EFT-2 whose oxidation status changed from either 7% to 31% or from 6% to 27% upon peroxide treatment (Fig. 5A). This result strongly suggests that this cysteine, which is highly conserved and located in one of the three highly mobile transfer RNA mimicking C-terminal domains of EFT-2, is peroxide sensitive. This notion is consistent with previous studies, which revealed that EFT-2 in Jurkat cells contains peroxide stress-sensitive cysteine(s) (3) and that EFT-2 in macrophages and smooth muscle cells undergoes S-nitrosylation reactions (13). While the peroxide-sensitive cysteine(s) in EFT-2 have not yet been located, Cys567 has been identified as a target of S-nitrosylation (13). We found that a significant proportion of EFT-2 is reproducibly and significantly oxidized at the equally conserved Cys745. Additional studies are necessary to determine if this oxidative cysteine modifications plays indeed a regulatory role in EFT-2 function. Finally, we identified Cys149, one of three conserved cysteines in the PAB-1, to be sensitive to peroxide-mediated oxidation. Expression of PAB-1 has very recently been shown to suppress peroxide-induced cell death in NIH/3T3 cells, suggesting that it has an oxidative-stress protective function in vivo (33).
FIG. 5.
Oxidation status of select redox-sensitive C. elegans proteins. The oxidation state of cysteines in select redox-sensitive proteins involved in (A) protein translation, (B) protein homeostasis, (C) ATP metabolism and motility, and (D) metabolism and other functions is shown.
Peroxide treatment targets proteins involved in protein homeostasis
Oxidative modification and inactivation of proteins involved in protein translation is considered to be an effective strategy to rapidly downregulate new protein synthesis under stress conditions that cause harm to newly synthesized and existing proteins. In contrast, molecular chaperones, such as bacterial Hsp33 (20) or eukaryotic PRDX (21), which are involved in maintaining protein homeostasis, use oxidative thiol modifications as a mechanism to rapidly activate their chaperone function. This effectively prevents the aggregation of stress-unfolded proteins and increases oxidative stress resistance (27). Here, we identified several additional chaperones whose function might be redox regulated. One of these chaperones is the Hsp70 homolog HSP-1, a highly conserved family of ATP-dependent chaperones whose members play important roles in de novo protein folding, stress-induced unfolding, protein transport, and protein degradation [reviewed in Liberek et al. (31)]. We found that Cys307, one of the three highly conserved cysteines in eukaryotic Hsp70s, becomes significantly oxidized in both wild-type and prdx-2 deletion strains (Table 1 and Fig. 5B). S-glutathionylation of at least one Hsp70 homolog has been previously shown to increase its in vitro chaperone function, suggesting that oxidative cysteine modification might serve as post-translational regulation of Hsp70's chaperone activity (14). It remains now to be determined whether oxidation of the highly conserved Cys307 is involved in this regulation.
In addition to HSP-1, we also identified one conserved cysteine in the delta subunit of the TCP/TriC chaperonin complex (i.e., CCT-4) to be highly sensitive to peroxide-mediated oxidation (Fig. 5B). The TCP/TriC complex supports de novo folding of actin and myosin, as well as numerous other β-sheet-rich multidomain proteins (47). The oxidation-sensitive cysteine that we identified is part of a highly conserved Cys-X3-Cys motif, which is common for redox-sensitive and/or metal-binding proteins (24). We also identified a peroxide-sensitive cysteine in the zeta subunit CCT-6 of the TCP/TriC chaperonin complex, suggesting that several members of this complex might be redox sensitive.
In addition to identifying these potentially redox-sensitive chaperones, we also noted that peroxide stress targets a set of cysteine-containing proteins involved in protein targeting and degradation. One of these proteins is the class I ubiquitin-conjugating enzyme UBC-2, whose active-site cysteine was found to become significantly oxidized upon peroxide stress treatment (Table 1). The C. elegans ortholog UBC-2, which is encoded by the essential let-70 gene, has been implicated in the ubiquitin-mediated degradation of many short-lived proteins (50). UBC-2 belongs to the highly conserved class of E2-conjugating enzymes. All members of this class share the presence of one active-site cysteine (e.g., Cys85 in UBC-2), which catalyzes the transfer of small peptidic modifiers, such as ubiquitin or SUMO, from specific E1-activating enzymes to protein substrates via the engagement of distinct E3 ligases. These post-translational modifications modify and affect protein–protein interactions, localization, activity, and/or stability of hundreds of different proteins in eukaryotic cells [for recent review, see Ye and Rape (49)]. Our study clearly demonstrates that the redox state of the active-site cysteine of UBC-2 changes from a predominantly reduced state before oxidative stress treatment to a >30% oxidized state upon treatment (Fig. 5B). This result is in excellent agreement with previous reports on the redox-regulated activity of both ubiquitin- and SUMO-conjugating enzymes E2 (4, 19). Because oxidation of the active-site cysteine is known to cause the inactivation of the conjugating enzyme, and by extension, the complete pathway, these results suggest significant changes in the proteostasis network during oxidative stress conditions in C. elegans.
In addition to UBC-2, our study revealed at least one more redox-sensitive protein that plays a role in ubiquitin-mediated protein degradation: the highly conserved AAA-protein CDC-48 (i.e., p99, VCP). CDC-48 is an ATP-dependent molecular chaperone whose primary function appears to be the chaperoning of retro-translocated, ubiquitylated ER proteins to the proteasome [reviewed in Dreveny et al. (9)]. CDC-48 harbors several cysteine residues, of which only three are conserved between yeast CDC-48 and human VCP. One of these conserved cysteines is the highly oxidation-sensitive Cys105 that we identified in our study (Table 1). So far, it is unclear how oxidation of Cys105, which resides in the N-terminal substrate-binding domain of CDC-48, affects the functionality of the chaperone. The C-terminal ATPase activity of mammalian CDC-48 has been shown to be redox regulated (34), suggesting that CDC-48 is an intrinsically redox-sensitive protein. The last protein that we identified in this group of redox-sensitive proteins involved in proteostasis is the ER translocation channel protein SEC-61. It has been suggested that SEC-61 mediates the retro-translocation of ubiquitylated ER proteins into the cytoplasm and thereby the transfer to CDC-48 (22). These results suggest that peroxide stress conditions lead to the reversible downregulation of ubiquitin-mediated protein degradation. It remains to be tested whether this mechanism prevents the degradation of oxidatively modified proteins during sublethal oxidative stress conditions, which would eliminate the possibility of quickly regenerating their protein activity once reducing conditions have been restored.
Muscle contraction and growth rate are major targets of peroxide stress in C. elegans
Another large group of C. elegans proteins that is targeted by peroxide stress include muscle-specific proteins and enzymes involved in ATP homeostasis. This result agrees well with our earlier observations that peroxide-treated worms show a massive decline in motility (Fig. 1). We found increased cysteine oxidation in three different paralogs of the myosin class II heavy chain: Cys280 of the pharynx-specific LET-75, Cys702 of UNC-54, and the equivalent Cys708 of MYO-2 (Fig. 5C). The fact that we independently identified the same cysteine to be redox sensitive in two different myosin-isoforms (UNC-54, MYO-2) illustrates the general oxidation sensitivity of this specific cysteine residue. This is particularly significant because this cysteine is highly conserved from yeast to human myosin and is located adjacent to a second conserved cysteine, which has been proposed to be redox sensitive and involved in the ATPase activity of myosin (25). Oxidative inactivation of UNC-54, the major C. elegans myosin heavy chain required for locomotion and egg-laying, and MYO-2, which is exclusively expressed in the pharynx, might contribute to the observed peroxide-mediated defects in motility, pharyngeal pumping, and egg-laying. In addition to the three myosin class 2 paralogs, we also found MUP-2, a homolog of the invertebrate troponin T, the muscle-organizing protein DIM-1, and the intermediate filament IFC-2 (i.e., lamin) to contain at least one significantly peroxide-sensitive cysteine (Table 1 and Fig. 5C). Like myosin, troponin T is involved in muscle contraction and normal growth rate, whereas IFC-2 is required for movement, growth rate, body size, and body shape (www.wormbase.org, release WS204, 29 July 2009). It is therefore very likely that oxidative modification of one or more of these proteins contributes to the severe defects in movement, pharyngeal pumping, and/or growth rate observed in oxidatively stressed animals.
Identification of peroxide-sensitive thiols in ATPases and enzymes catalyzing transphosphorylation reactions
Like the defects in C. elegans motility, which became immediately obvious in oxidatively stressed animals, we measured a dramatic loss in intracellular ATP levels within 30 min of peroxide treatment in wild-type worms (Fig. 1G) and found decreased steady-state ATP levels in prdx-2-deficient worms (Fig. 3G). A similar decrease in cellular ATP levels was previously observed in peroxide-stress-treated bacteria and yeast and has, at least in part, been attributed to the oxidative inactivation of GAPDH and F-type ATPases (16). Although we could not detect the cysteine-containing active-site peptides of C. elegans GAPDH or F-type ATPase in our mass spectra, we found redox-sensitive cysteines in the A and B subunits of the V-type ATPase and in proteins involved in transphosphorylation reactions [e.g., nucleoside diphosphate kinase [NDPK] and creatine kinase] (Table 1 and Fig. 5C). V-type ATPases deplete cellular ATP levels by hydrolyzing ATP to acidify lysosomal and endosomal organelles, whereas NDPK transfers phosphoryl groups from ATP to other nucleoside diphosphates. Creatine kinase, on the other hand, generates ATP by transferring phosphoryl groups from the phosphocreatine pool of the muscle cells to adenosine diphosphate. Importantly, redox sensitivity has been previously reported for all three proteins, which agrees well with our observed results. It has been shown, for instance, that mammalian and plant V-type ATPases undergo reversible disulfide bond formation in response to H2O2 treatment, which leads to the inactivation of the proton pump (10, 45). Whereas some of the redox-sensitive cysteines in subunit A have already been identified (i.e., Cys 254 and Cys532) (10), we have now also discovered a highly conserved Cys33 in subunit B to be oxidation sensitive. Inactivation of C. elegans V-type ATPase has been recently shown to exert neuroprotective effects against necrosis (44), suggesting that oxidation-mediated inactivation of V-type ATPases might not only save already scarce ATP resources during oxidative stress, but may be a strategy to protect against necrosis.
We confirmed previous reports that the active-site Cys109 of NDPK is highly susceptible to oxidative modification (Table 1) and that the multiple activities of the mammalian NDPK homolog Nm23 in endocytosis and tumor suppression are redox regulated (29). In addition, we detected the nearby Cys117 to be highly oxidation sensitive as well (Table 1), suggesting that peroxide stress leads to intramolecular disulfide bond formation in NDPK. In the case of creatine kinase, we were unable to identify the peptide containing the active-site Cys283, which has also previously been reported to be redox sensitive (38). Instead, we detected a nearby second cysteine to be highly oxidation sensitive, implying again the possibility of an intramolecular disulfide bond. In either case, oxidative thiol modification has been demonstrated to cause the inactivation of both kinases, which might contribute to the observed changes in C. elegans' ATP homeostasis and/or serve as a protective measure to prevent further ATP depletion. Unfortunately, we did not detect the corresponding active-site cysteine-containing peptides in the mass spectra of our prdx-2 deficient worms, making it impossible for us to confirm whether oxidative modification of any of these proteins is responsible for the significantly decreased steady state ATP levels observed in prdx-2 deficient worms.
In addition to the redox-sensitive proteins that we identified to be involved in protein translation, protein homeostasis, muscle function, and ATP homeostasis, we also found redox-sensitive proteins involved in a variety of other functions, including metabolism (e.g., aldolase and aconitase), signal transduction (e.g., annexin), chromatin remodeling (e.g., ISW-1 complex), and heterochromatin formation (e.g., vigilin) (Table 1 and Fig. 5D). Again, some of these proteins have previously been shown to be redox sensitive (e.g., annexin) (6), whereas many others have not, to our knowledge, been reported to undergo reversible oxidative modifications (e.g., DIM-1, vigilin, and tubulin). However, the fact that we confirmed the oxidation-sensitive cysteines in many of the known redox-sensitive proteins makes us very confident that the newly identified proteins are redox sensitive as well. Potential functional or structural changes that occur upon the oxidative modification of these proteins will likely contribute to the behavioral and physiological changes that accompany oxidative stress in C. elegans and possibly higher eukaryotes.
Discussion
The accumulation of oxidative damage to biomolecules has been implicated in the pathogenesis of a variety of different diseases and is thought to be one potential cause of aging (1, 26). The major drawback in analyzing the role of oxidative stress in aging and disease, however, is the inability to define if, when, and which oxidants become physiologically relevant. To start to address some of these questions, we decided to expose synchronized young C. elegans to a sublethal short-term treatment of peroxide stress, monitor the physiological effects, and determine potential eukaryotic target proteins. When we studied the behavioral changes in peroxide-treated worms, we made the surprising observation that the majority of worms suffer from severe, yet fully reversible behavioral changes that are highly reminiscent of well-known age-related changes, such as declines in body movement, pharyngeal pumping, and reproduction, as well as morphological changes and reduced metabolic activity (7). In contrast to old worms, however, the peroxide-treated young worms fully recovered from this damage.
We identified the highly conserved enzyme PRDX-2 as a repair system that appears to be involved in the recovery from exogenous peroxide stress. Together with catalases, PRDX are known to keep intracellular peroxide concentrations low. Lack of either prdx-2 or the cytosolic catalase ctl-2 has been shown, both here and elsewhere, to cause a number of so-called progeric (age-related) phenotypes (35, 37), which are very similar to the changes that we observed in peroxide-treated animals, strongly suggesting that these mutant animals suffer from chronic peroxide stress. Deletion of either one of the two genes significantly shortens the lifespan of C. elegans, providing evidence that peroxide-mediated damage contributes to lifespan. These results are in stark contrast to observations made in strains lacking one or more paralogs of superoxide dismutase (SOD)-1 through SOD-5, which detoxify the second major physiological oxidant, superoxide. Whereas individual sod deletion mutants suffer from increased oxidative damage as assessed by protein carbonylation, the worms did not exhibit progeric phenotypes or shortened lifespan (48). These results raise the intriguing possibility that H2O2 and its reaction products play more prominent roles in aging and lifespan determination than superoxide, which might allow for the development of more targeted antioxidant strategies.
As previously noted, the analysis of protein carbonylation is a widely used method for assessing cellular oxidative damage. One major disadvantage of measuring protein carbonylation is that this oxidative side chain modification is irreversible, which makes its quantification very challenging (42). It is difficult, for instance, to distinguish whether one protein molecule is carbonylated at 100 different sites or whether 100 protein molecules are carbonylated at one site, the two scenarios having very different implications to overall protein function. Thus, conclusions about the extent of oxidative damage are often problematic. These limitations might have contributed to some conflicting findings. For example, mutants defective in SOD do not reveal any lifespan defect despite increased levels of protein carbonylation (48). In this study, we therefore decided to use the quantitative thiol trapping method OxICAT to determine (i) which proteins undergo oxidative thiol modification, (ii) what specific cysteine residues are involved, and (iii) how much of a protein population is affected (30). In a single set of experiments, we confirmed or discovered the oxidation sensitivity of many known and unknown redox-sensitive proteins and identified cysteine residues targeted by peroxide stress. We found that most of the identified redox-sensitive cysteines are highly conserved, implying that they likely play structural and/or functional roles in the respective proteins and that their modification reversibly affects the activity of the protein.
The processes that we found to be targeted by peroxide stress involve protein translation, protein homeostasis, and metabolism (Table 1). All these cellular processes have previously been implied to be peroxide sensitive. We find that many individual players in a single process are simultaneously targeted and that the targeting occurs to similar extents. These results suggest that the oxidative modification of these proteins might not simply be a passive, nonspecific reaction to oxidants but an active regulatory mechanism, potentially involved in combating oxidative stress. Oxidative inactivation of protein translation, for instance, will not only save scarce energy resources needed to fight oxidative damage but will actively prevent production of nascent polypeptides under potentially error-prone conditions. This response is used to actively fight oxidative stress and has been shown to extend lifespan in a variety of different organisms (23). Oxidative inactivation of proteins involved in targeted degradation, such as UBC-2, SEC-61, and CDC-48, might prevent proteolysis of proteins whose oxidative modifications are reversible once reducing conditions have been restored. Oxidative modification of critical cysteines in chaperones such as the Hsp70 homolog HSP-1, on the other hand, might be an effective strategy to increase chaperone function under conditions in which protein unfolding and aggregation is likely to occur (14). This strategy finds precedent in bacteria, where peroxide-mediated activation of the redox-sensitive chaperone Hsp33 is used to combat protein unfolding and aggregation during severe oxidative stress (46). Oxidative inactivation of enzymes involved in glycolysis (e.g., aldolase, ALDO-2) will redirect glucose to the pentose phosphate pathway, which is an effective mechanism to increase NADPH levels, necessary to restore redox homeostasis (12). Finally, transient oxidative inactivation of ATP-depleting enzymes, such as the muscle-related proteins myosin and troponin, or the vacuolar V-type ATPase and NDPK, would avoid energy expenditure for processes that are not immediately essential for survival. Based on these considerations, it is now tempting to speculate that peroxide stress, although eventually toxic, might initially be used to actively downregulate nonessential functions, thereby conserving energy, combating oxidative stress, and, by these means, extending an otherwise even shorter lifespan. This hypothesis would still uphold Harman's free radical theory of aging, which states that the progressive decline observed in aging organisms is due to increased oxidative damage. However, it would also include the potentially beneficial aspects of ROS as modulatory second messengers that affect stress resistance and longevity early in life. These conclusions are supported by recent observations by Ristow and coworkers, who reported that lifespan extending regimens in C. elegans, such as glucose restriction, involve transient ROS accumulation, causing increased oxidative stress resistance and hormetic life extension (39).
Supplementary Material
Abbreviations Used
- ATP
adenosine triphosphate
- Cys
cysteine
- EFT
elongation factor
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- H2O2
hydrogen peroxide
- HSP
heat shock protein
- ICAT
isotope coded affinity tag
- LC
liquid chromatography
- let
lethal
- MLS
mean lifespan
- MS
mass spectrometry
- NDPK
nucleoside diphosphate kinase
- PAB
polyadenylate-binding protein
- PRDX
peroxiredoxin-2
- ROS
reactive oxygen species
- RPL
large ribosomal protein
- RPS
small ribosomal subunit
- SE
standard error
- SOD
superoxide dismutase
- TCA
trichloracetic acid
- TCEP
Tris(2-carboxyethyl)phosphine
Acknowledgments
We thank the Caenorhabditis Genetics Center, which is funded by the NIH National Center for Research Resources, for providing us with strains, and Dr. I. Bruchhaus for providing us with the PRDX-2 expression vector. We thank the Michigan Proteome Consortium for conducting the mass spectrometric analysis. We thank Dr. Bardwell for critically reading the article. This work was supported by the National Institute of Aging grant AG027349 and an OVPR Faculty grant to U.J. and by a Boehringer Ingelheim Ph.D. fellowship to C.K.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Aliev G. Smith MA. Seyidov D. Neal ML. Lamb BT. Nunomura A. Gasimov EK. Vinters HV. Perry G. LaManna JC. Friedland RP. The role of oxidative stress in the pathophysiology of cerebrovascular lesions in Alzheimer's disease. Brain Pathol. 2002;12:21–35. doi: 10.1111/j.1750-3639.2002.tb00419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Apel K. Hirt H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol. 2004;55:373–399. doi: 10.1146/annurev.arplant.55.031903.141701. [DOI] [PubMed] [Google Scholar]
- 3.Baty JW. Hampton MB. Winterbourn CC. Proteomic detection of hydrogen peroxide-sensitive thiol proteins in Jurkat cells. Biochem J. 2005;389:785–795. doi: 10.1042/BJ20050337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bossis G. Melchior F. Regulation of SUMOylation by reversible oxidation of SUMO conjugating enzymes. Mol Cell. 2006;21:349–357. doi: 10.1016/j.molcel.2005.12.019. [DOI] [PubMed] [Google Scholar]
- 5.Brandes N. Schmitt S. Jakob U. Thiol-based redox switches in eukaryotic proteins. Antioxid Redox Signal. 2009;11:997–1014. doi: 10.1089/ars.2008.2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caplan JF. Filipenko NR. Fitzpatrick SL. Waisman DM. Regulation of annexin A2 by reversible glutathionylation. J Biol Chem. 2004;279:7740–7750. doi: 10.1074/jbc.M313049200. [DOI] [PubMed] [Google Scholar]
- 7.Collins JJ. Huang C. Hughes S. Kornfeld K. The measurement and analysis of age-related changes in Caenorhabditis elegans. Worm Book. 2008;24:1–21. doi: 10.1895/wormbook.1.137.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deneke SM. Thiol-based antioxidants. Curr Top Cell Regul. 2000;36:151–180. doi: 10.1016/s0070-2137(01)80007-8. [DOI] [PubMed] [Google Scholar]
- 9.Dreveny I. Pye VE. Beuron F. Briggs LC. Isaacson RL. Matthews SJ. McKeown C. Yuan X. Zhang X. Freemont PS. p97 and close encounters of every kind: a brief review. Biochem Soc Trans. 2004;32:715–720. doi: 10.1042/BST0320715. [DOI] [PubMed] [Google Scholar]
- 10.Feng Y. Forgac M. Inhibition of vacuolar H(+)-ATPase by disulfide bond formation between cysteine 254 and cysteine 532 in subunit A. J Biol Chem. 1994;269:13224–13230. [PubMed] [Google Scholar]
- 11.Finkel T. Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 12.Godon C. Lagniel G. Lee J. Buhler JM. Kieffer S. Perrot M. Boucherie H. Toledano MB. Labarre J. The H2O2 stimulon in Saccharomyces cerevisiae. J Biol Chem. 1998;273:22480–22489. doi: 10.1074/jbc.273.35.22480. [DOI] [PubMed] [Google Scholar]
- 13.Greco TM. Hodara R. Parastatidis I. Heijnen HF. Dennehy MK. Liebler DC. Ischiropoulos H. Identification of S-nitrosylation motifs by site-specific mapping of the S-nitrosocysteine proteome in human vascular smooth muscle cells. Proc Natl Acad Sci U S A. 2006;103:7420–7425. doi: 10.1073/pnas.0600729103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoppe G. Chai YC. Crabb JW. Sears J. Protein s-glutathionylation in retinal pigment epithelium converts heat shock protein 70 to an active chaperone. Exp Eye Res. 2004;78:1085–1092. doi: 10.1016/j.exer.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 15.Huang C. Xiong C. Kornfeld K. Measurements of age-related changes of physiological processes that predict lifespan of Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2004;101:8084–8089. doi: 10.1073/pnas.0400848101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hyslop PA. Hinshaw DB. Halsey WA., Jr. Schraufstatter IU. Sauerheber RD. Spragg RG. Jackson JH. Cochrane CG. Mechanisms of oxidant-mediated cell injury. The glycolytic and mitochondrial pathways of ADP phosphorylation are major intracellular targets inactivated by hydrogen peroxide. J Biol Chem. 1988;263:1665–1675. [PubMed] [Google Scholar]
- 17.Imlay JA. Pathways of oxidative damage. Annu Rev Microbiol. 2003;57:395–418. doi: 10.1146/annurev.micro.57.030502.090938. [DOI] [PubMed] [Google Scholar]
- 18.Isermann K. Liebau E. Roeder T. Bruchhaus I. A peroxiredoxin specifically expressed in two types of pharyngeal neurons is required for normal growth and egg production in Caenorhabditis elegans. J Mol Biol. 2004;338:745–755. doi: 10.1016/j.jmb.2004.03.021. [DOI] [PubMed] [Google Scholar]
- 19.Jahngen-Hodge J. Obin MS. Gong X. Shang F. Nowell TR., Jr. Gong J. Abasi H. Blumberg J. Taylor A. Regulation of ubiquitin-conjugating enzymes by glutathione following oxidative stress. J Biol Chem. 1997;272:28218–28226. doi: 10.1074/jbc.272.45.28218. [DOI] [PubMed] [Google Scholar]
- 20.Jakob U. Muse W. Eser M. Bardwell JC. Chaperone activity with a redox switch. Cell. 1999;96:341–352. doi: 10.1016/s0092-8674(00)80547-4. [DOI] [PubMed] [Google Scholar]
- 21.Jang HH. Lee KO. Chi YH. Jung BG. Park SK. Park JH. Lee JR. Lee SS. Moon JC. Yun JW. Choi YO. Kim WY. Kang JS. Cheong GW. Yun DJ. Rhee SG. Cho MJ. Lee SY. Two enzymes in one; two yeast peroxiredoxins display oxidative stress-dependent switching from a peroxidase to a molecular chaperone function. Cell. 2004;117:625–635. doi: 10.1016/j.cell.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 22.Jarosch E. Geiss-Friedlander R. Meusser B. Walter J. Sommer T. Protein dislocation from the endoplasmic reticulum—pulling out the suspect. Traffic. 2002;3:530–536. doi: 10.1034/j.1600-0854.2002.30803.x. [DOI] [PubMed] [Google Scholar]
- 23.Kennedy BK. Kaeberlein M. Hot topics in aging research: protein translation, 2009. Aging Cell. 2009;8:617–623. doi: 10.1111/j.1474-9726.2009.00522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koehler CM. The small Tim proteins and the twin Cx3C motif. Trends Biochem Sci. 2004;29:1–4. doi: 10.1016/j.tibs.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 25.Konno K. Ue K. Khoroshev M. Martinez H. Ray B. Morales MF. Consequences of placing an intramolecular crosslink in myosin S1. Proc Natl Acad Sci U S A. 2000;97:1461–1466. doi: 10.1073/pnas.030523997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kovacic P. Jacintho JD. Mechanisms of carcinogenesis: focus on oxidative stress and electron transfer. Curr Med Chem. 2001;8:773–796. doi: 10.2174/0929867013373084. [DOI] [PubMed] [Google Scholar]
- 27.Kumsta C. Jakob U. Redox-regulated chaperones. Biochemistry. 2009;48:4666–4676. doi: 10.1021/bi9003556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Larsen PL. Aging and resistance to oxidative damage in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 1993;90:8905–8909. doi: 10.1073/pnas.90.19.8905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee E. Jeong J. Kim SE. Song EJ. Kang SW. Lee KJ. Multiple functions of Nm23-H1 are regulated by oxido-reduction system. PLoS ONE. 2009;4:e7949. doi: 10.1371/journal.pone.0007949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leichert LI. Gehrke F. Gudiseva HV. Blackwell T. Ilbert M. Walker AK. Strahler JR. Andrews PC. Jakob U. Quantifying changes in the thiol redox proteome upon oxidative stress in vivo. Proc Natl Acad Sci U S A. 2008;105:8197–8202. doi: 10.1073/pnas.0707723105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liberek K. Lewandowska A. Zietkiewicz S. Chaperones in control of protein disaggregation. EMBO J. 2008;27:328–335. doi: 10.1038/sj.emboj.7601970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mains PE. McGhee JD. Biochemistry of C. elegans. In: Hope IA, editor. A Practical Approach: C. elegans. Oxford: Oxford University Press; 1999. pp. 227–231. [Google Scholar]
- 33.Nagano-Ito M. Banba A. Ichikawa S. Functional cloning of genes that suppress oxidative stress-induced cell death: TCTP prevents hydrogen peroxide-induced cell death. FEBS Lett. 2009;583:1363–1367. doi: 10.1016/j.febslet.2009.03.045. [DOI] [PubMed] [Google Scholar]
- 34.Noguchi M. Takata T. Kimura Y. Manno A. Murakami K. Koike M. Ohizumi H. Hori S. Kakizuka A. ATPase activity of p97/valosin-containing protein is regulated by oxidative modification of the evolutionally conserved cysteine 522 residue in Walker A motif. J Biol Chem. 2005;280:41332–41341. doi: 10.1074/jbc.M509700200. [DOI] [PubMed] [Google Scholar]
- 35.Olahova M. Taylor SR. Khazaipoul S. Wang J. Morgan BA. Matsumoto K. Blackwell TK. Veal EA. A redox-sensitive peroxiredoxin that is important for longevity has tissue- and stress-specific roles in stress resistance. Proc Natl Acad Sci U S A. 2008;105:19839–19844. doi: 10.1073/pnas.0805507105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paulsen CE. Carroll KS. Orchestrating redox signaling networks through regulatory cysteine switches. ACS Chem Biol. 2010;5:47–62. doi: 10.1021/cb900258z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Petriv OI. Rachubinski RA. Lack of peroxisomal catalase causes a progeric phenotype in Caenorhabditis elegans. J Biol Chem. 2004;279:19996–20001. doi: 10.1074/jbc.M400207200. [DOI] [PubMed] [Google Scholar]
- 38.Reddy S. Jones AD. Cross CE. Wong PS. Van Der Vliet A. Inactivation of creatine kinase by S-glutathionylation of the active-site cysteine residue. Biochem J. 2000;347(Pt 3):821–827. [PMC free article] [PubMed] [Google Scholar]
- 39.Schulz TJ. Zarse K. Voigt A. Urban N. Birringer M. Ristow M. Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress. Cell Metab. 2007;6:280–293. doi: 10.1016/j.cmet.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 40.Shenton D. Smirnova JB. Selley JN. Carroll K. Hubbard SJ. Pavitt GD. Ashe MP. Grant CM. Global translational responses to oxidative stress impact upon multiple levels of protein synthesis. J Biol Chem. 2006;281:29011–29021. doi: 10.1074/jbc.M601545200. [DOI] [PubMed] [Google Scholar]
- 41.Sohal RS. Role of oxidative stress and protein oxidation in the aging process. Free Radic Biol Med. 2002;33:37–44. doi: 10.1016/s0891-5849(02)00856-0. [DOI] [PubMed] [Google Scholar]
- 42.Soskic V. Groebe K. Schrattenholz A. Nonenzymatic posttranslational protein modifications in ageing. Exp Gerontol. 2008;43:247–257. doi: 10.1016/j.exger.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 43.Stadtman ER. Protein oxidation in aging and age-related diseases. Ann N Y Acad Sci. 2001;928:22–38. doi: 10.1111/j.1749-6632.2001.tb05632.x. [DOI] [PubMed] [Google Scholar]
- 44.Syntichaki P. Samara C. Tavernarakis N. The vacuolar H+-ATPase mediates intracellular acidification required for neurodegeneration in C. elegans. Curr Biol. 2005;15:1249–1254. doi: 10.1016/j.cub.2005.05.057. [DOI] [PubMed] [Google Scholar]
- 45.Tavakoli N. Kluge C. Golldack D. Mimura T. Dietz KJ. Reversible redox control of plant vacuolar H+-ATPase activity is related to disulfide bridge formation in subunit E as well as subunit A. Plant J. 2001;28:51–59. doi: 10.1046/j.1365-313x.2001.01130.x. [DOI] [PubMed] [Google Scholar]
- 46.Winter J. Ilbert M. Graf PC. Ozcelik D. Jakob U. Bleach activates a redox-regulated chaperone by oxidative protein unfolding. Cell. 2008;135:691–701. doi: 10.1016/j.cell.2008.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yam AY. Xia Y. Lin HT. Burlingame A. Gerstein M. Frydman J. Defining the TRiC/CCT interactome links chaperonin function to stabilization of newly made proteins with complex topologies. Nat Struct Mol Biol. 2008;15:1255–1262. doi: 10.1038/nsmb.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang W. Li J. Hekimi S. A measurable increase in oxidative damage due to reduction in superoxide detoxification fails to shorten the life span of long-lived mitochondrial mutants of Caenorhabditis elegans. Genetics. 2007;177:2063–2074. doi: 10.1534/genetics.107.080788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ye Y. Rape M. Building ubiquitin chains: E2 enzymes at work. Nat Rev Mol Cell Biol. 2009;10:755–764. doi: 10.1038/nrm2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhen M. Heinlein R. Jones D. Jentsch S. Candido EP. The ubc-2 gene of Caenorhabditis elegans encodes a ubiquitin-conjugating enzyme involved in selective protein degradation. Mol Cell Biol. 1993;13:1371–1377. doi: 10.1128/mcb.13.3.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.