Abstract
The astrocytic enzyme adenosine kinase (ADK) is a key negative regulator of the brain’s endogenous anticonvulsant adenosine. Astrogliosis with concomitant upregulation of ADK is part of the epileptogenic cascade and contributes to seizure generation. To molecularly dissect the respective roles of astrogliosis and ADK-expression for seizure generation, we used a transgenic approach to uncouple ADK-expression from astrogliosis: in Adk-tg mice the endogenous Adk-gene was deleted and replaced by a ubiquitously expressed Adk-transgene with novel ectopic expression in pyramidal neurons, resulting in spontaneous seizures. Here, we followed a unique approach to selectively injure the CA3 of these Adk-tg mice. Using this strategy, we had the opportunity to study astrogliosis and epileptogenesis in the absence of the endogenous astrocytic Adk-gene. After triggering epileptogenesis we demonstrate astrogliosis without upregulation of ADK, but lack of seizures, whereas matching wild-type animals developed astrogliosis with upregulation of ADK and spontaneous recurrent seizures. By uncoupling ADK-expression from astrogliosis, we demonstrate that global expression levels of ADK rather than astrogliosis per se contribute to seizure generation.
Keywords: astrogliosis, seizures, kainic acid, epileptogenesis, CA3
Introduction
Astrogliosis is a pathophysiological hallmark of the epileptic brain and a ubiquitous constituent of the epileptogenic cascade (Pitkanen and Lukasiuk, 2009). Several lines of evidence suggest that astrocyte dysfunction contributes to epileptogenesis and to seizure expression in epilepsy (Tian et al., 2005; Haydon and Carmignoto, 2006; Halassa et al., 2007; Boison, 2008b; Oberheim et al., 2008; Vezzani, 2008; Wetherington et al., 2008). Thus, in epilepsy, failure of glia to buffer extracellular glutamate, or dysfunctional release of glutamate by glia were shown to contribute to the maintenance of the paroxysmal depolarizing shift that characterizes neuronal dysfunction in epilepsy (Tian et al., 2005). Astrocytes also play a key role in regulating the extracellular availability of the endogenous anticonvulsant adenosine (Pascual et al., 2005; Boison, 2008a) and an astrocyte-based adenosine-cycle has been proposed (Boison, 2008a). Astrocytes can release ATP – a precursor of adenosine – via a synaptic mechanism (Pascual et al., 2005), but they can also release adenosine directly (Martin et al., 2007). The astrocyte-specific enzyme adenosine kinase (ADK) constitutes a metabolic reuptake system for adenosine and extracellular levels of adenosine are largely under the control of ADK (Boison, 2008a; Wetherington et al., 2008). Thus, increased levels of ADK are associated with seizures and increased susceptibility to brain injury (Li et al., 2007a; Pignataro et al., 2007; Li et al., 2008), whereas decreased levels of ADK lead to increases in adenosine (Pignataro et al., 2008) and, consequently, to seizure suppression and neuroprotection (Li et al., 2007a; Li et al., 2008).
Recently, we characterized a novel mouse model of CA3-selective epileptogenesis that is triggered by unilateral intraamygdaloid injection of the excitotoxin kainic acid (KA) (Li et al., 2007a; Li et al., 2008). As a consequence of the KA-injection, status epilepticus (SE) develops which is terminated after 30 minutes with lorazepam. Within 24 hours CA3-selective neuronal cell loss develops that constitutes a trigger for subsequent epileptogenesis. Within 3 weeks after the injury CA3-selective astrogliosis develops with associated upregulation of ADK and CA3-selective spontaneous seizures (Li et al., 2007a; Li et al., 2008). In these studies we identified ADK in astrocytes as a molecular link between astrogliosis and neuronal dysfunction in epilepsy. To molecularly dissect the respective roles of astrogliosis and ADK expression for seizure generation we generated a line of transgenic mice, Adk-tg, that lacks the endogenous regulatable Adk-gene (Boison et al., 2002), but overexpresses a ubiquitously and constitutively expressed Adk-transgene (Fedele et al., 2005) on top of this Adk-knockout background. These mice are characterized by global overexpression of ADK in brain that includes a novel ectopic expression of ADK in pyramidal neurons resulting in spontaneous hippocampal seizures (Li et al., 2008).
Objective
We tested the hypothesis that global expression levels of ADK rather than astrogliosis per se contribute to seizure generation.
Methods
Animals
All animal procedures were performed in an AAALAC-accredited facility in accordance with protocols approved by the Legacy IACUC and the principles outlined in the NIH Guide for the Care and Use of Laboratory Animals. Animals were kept in a 12-hour light/dark cycle with lights on from 7 am to 7 pm in individually ventilated isolator cages and food and water were supplied ad libitum. Adk-tg mice were maintained on a C57Bl/6 background as described (Li et al., 2008). These animals are based on a homozygous genetic deletion (i.e. “classical knockout”) of the endogenous Adk-gene (Boison et al., 2002), and co-expression of a ubiquitously expressed Ubi-Adk transgene (Fedele et al., 2005). The resulting Adk-tg mice are thus characterized by lack of the endogenous astrocyte-specific ADK that is subject to endogenous regulatory mechanisms (Li et al., 2007a; Li et al., 2008; Pignataro et al., 2008), and by ubiquitous (in astrocytes and neurons) constitutive overexpression of ADK that includes ectopic expression in hippocampal pyramidal neurons (Li et al., 2007a; Li et al., 2008).
Model of CA3 selective epileptogenesis
Adult male wt or Adk-tg mice weighing 25-30g underwent seizures induced by unilateral stereotaxic microinjection of kainic acid (KA) into the basolateral amygdala nucleus based on stereotactic coordinates relative to bregma: AP = −0.94 mm, L = −2.85 mm, V = −3.75 mm as described (Li et al., 2007a; Li et al., 2008). Briefly, under anesthesia with 68.5% N2O, 30% O2, and 1.5% isoflurane, mice were affixed with three recording electrodes (Plastics One, Inc.) and a 26-gauge steel guide cannula over the intact dura using dental cement. To perform the subsequent EEG recordings and drug injections in wake animals, the mice were placed into a plexiglass restrainer. Anesthesia was discontinued, EEG recordings commenced, and then a 31-gauge internal cannula was inserted into the lumen of the guide to inject 0.3 γg KA in a volume of 0.2 γl PBS, pH 7.4, into the amygdala. The EEG was monitored for 20 min (in Adk-tg mice) or 30 min (in wild-type controls) using a Nervus video-EEG recording device until lorazepam (6 mg/kg) was administered intravenously to terminate seizures. The EEG was further monitored for up to 30 min to ensure seizure cessation. An observer unaware of the experimental treatment performed quantification of EEG records, and the cumulative duration of type IV seizure activity (during 20 and 30 min, respectively) was calculated. At the time of, or three weeks after KA-injection a bipolar, coated, stainless steel electrode (0.20 mm in diameter, Plastics One, Roanoke, VA) was implanted into the ipsilateral CA3 region and fixed with a pedestal of dental acrylate. Coordinates for the CA3 electrodes were (toothbar at 0): AP = −2.18 mm, L = −2.5 mm, and V = −2.25 mm. A monopolar reference and ground electrode was affixed above the cerebellum. 24h after electrode implantation each animal was subjected to one 20-hour session (from 4PM to 12PM) of continuous EEG monitoring. An observer unaware of the experimental treatment performed quantification of EEG records.
Histology
Brains obtained 24h following the KA injection were immediately frozen in 2-methylbutane (−30°C) and sectioned at 12 γm on a cryostat. Coronal sections at the level of bregma −1.7 mm were air dried, postfixed in 10% formalin (15 min), washed twice in PBS, and then processed for histopathology (cresyl violet staining) or for detection of DNA fragmentation (TUNEL) as described (Shinoda et al., 2004). The average number of TUNEL positive cells per brain slice was quantified by counting TUNEL positive cells from 3 adjacent brain sections, encompassing the entire CA1/3 field, derived from 3 animals from each genotype.
Alternatively, 24 hours or three weeks after KA-injection the mice were transcardially perfused with 4% paraformaldehyde in phosphate buffer (0.15M, pH 7.4). The brains were post fixed in the same fixative at 4°C for 6 h and cryoprotected in 10% DMSO in PBS (v/v) before being cut into 40 μm coronal sections. 6 sections from each brain representing different levels of the hippocampal formation encompassing the complete rostrocaudal extent of the hippocampus were mounted onto gelatin-coated slides and stained with Cresyl violet. For the immunohistochemical detection of GFAP and ADK we followed our previously published procedures and stained sections adjacent to the Cresyl violet stained sections (Studer et al., 2006). GFAP and ADK expression was quantified by analyzing fields of 200 × 300 γm encompassing the entire CA3a region. Corresponding fields from two sections from each animal (n = 6 animals per group) were analyzed by scanning ADK immunofluorescence on DAB stained slices using a Kodak imaging device and by counting individual GFAP positive cells. Data were analyzed by ANOVA with Student-Newman-Keuls test.
RESULTS
Transgenic overexpression of ADK triggers seizures in Adk-tg mice
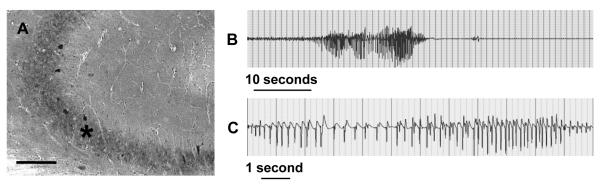
Transgenic Adk-tg mice that overexpress a constitutively and ubiquitously expressed Adk-transgene (Fedele et al., 2005) on top of a genetic disruption (Boison et al., 2002) of adenosine kinase (ADK) have been described previously (Li et al., 2007a; Li et al., 2008). These animals are characterized by spontaneous recurrent hippocampal seizures that are subclinical in nature. To generate baseline data for the subsequent experiments, 6 adult male Adk-tg mice were subjected to intrahippocampal EEG recordings using bipolar electrodes implanted into the CA3 area. 24 h of cumulative EEG recordings yielded a seizure frequency of 4.7 ± 1.5 seizures per hour, with each seizure lasting on average 25.8 ± 13.9 seconds (Fig. 1B,C, Table 1). ADK immunohistochemistry performed after completion of the EEG recordings confirmed ubiquitous and diffuse overexpression of ADK with high ectopic expression of ADK in CA3 neurons (Fig. 1A). These data suggest that overexpression of ADK as such is sufficient to trigger spontaneous seizures in the absence of any epileptogenesis triggering event.
Figure 1.
Spontaneous seizures in Adk-tg mice. A, Part of coronal brain section of an adult Adk-tg mouse stained with diaminobenzidine hydrochloride (DAB) for ADK immunoreactivity. Note strong ADK-immunoreactivity in the CA3 pyramidal cells (asterisk); Scale bar: 150 γm. B, C, Spontaneous seizure recorded from the CA3 area of an Adk-tg mouse. The trace shows the intrahippocampal representation from a bipolar electrode. For quantitative seizure data, please refer to Table 1.
Table 1.
Spontaneous electroencephalographic seizures recorded from the CA3 region. Seizure data were obtained from continuous 20 h recordings using bipolar electrodes implanted within the CA3 region (n = 6 animals per group). Untreated Adk-tg mice displayed spontaneous CA3 seizures in the absence of KA administration (baseline control). Spontaneous seizures in the contralateral CA3 of Adk-tg mice, recorded 24 h and 3 weeks after KA injection, were not affected by this manipulation. A similar pattern of ipsilateral spontaneous seizures was detected in wild type mice 3 weeks after KA-injection. Numbers of seizures per hour, as well as averaged seizure durations remained similar across treatment groups (P > 0.05, ANOVA). In contrast, not a single seizure was detected in the ipsilateral CA3 of Adk-tg mice neither 24 h nor 3 weeks after KA-injection.
| Adk-tg untreated control |
Adk-tg 24h after KA contralateral |
Adk-tg 24h after KA ipsilateral |
Adk-tg 3 wks after KA contralateral |
Adk-tg 3 wks after KA ipsilateral |
wt 3 wks after KA contralateral |
wt 3 wks after KA ipsilateral |
|
|---|---|---|---|---|---|---|---|
| Seizure | 25.8 ± 13.9 | 23.2 ± 13.5 | 0 | 24.7 ± 14.2 | 0 | 0 | 17.1 ± 6.2 |
| duration (s) | |||||||
| Number of seizures / h |
4.7 ± 1.5 | 4.5 ± 1.4 | 0 | 4.6 ± 1.6 | 0 | 0 | 4.1 ± 1.2 |
ADK-expression levels control severity of status epilepticus and extent of acute cell death
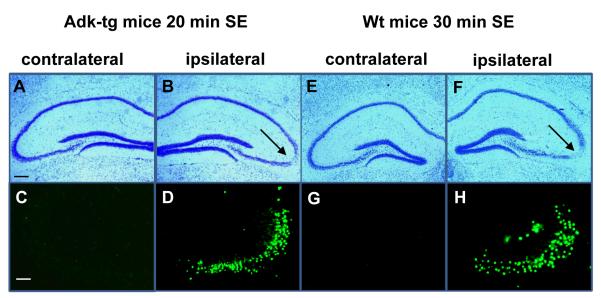
In wild-type mice 30 minutes of KA-induced status epilepticus (SE) causes CA3-selective neuronal cell death and constitutes a trigger to subsequent CA3-selective epileptogenesis (Li et al., 2008). In contrast, 30 minutes of KA-induced SE is lethal in Adk-tg mice; all animals die within 72 hours thus precluding subsequent studies on epileptogenesis (Li et al., 2008). In order to study epileptogenesis in Adk-tg mice we aimed to recreate wild-type like SE and a wild-type like degree of acute CA3-selective neuronal cell loss by reducing the seizure time. Adk-tg mice and wild-type control mice (n=6, each) received intraamygdaloid injection of KA, which triggered SE in both groups. Using intravenous injection of lorazepam (6 mg/kg) SE was terminated in Adk-tg mice after 20 minutes and in wild-type controls after 30 minutes. The total duration of type IV seizure activity during the permitted seizure time (20 versus 30 min) was quantified. According to our aim, Adk-tg mice and wild-type mice had similar type IV seizure times of 418 ± 130 seconds and 443 ± 147 seconds, respectively (P>0.05). 24 h after KA-injection all animals were euthanized and subjected to histological analysis of their brains. In all animals comparable CA3-selective neuronal cell loss became evident that was restricted to the CA3 ipsilateral to the KA-injection (Fig. 2B,D,F,H), whereas the contralateral hippocampus was spared from injury (Fig. 2A,C,E,G). The degree of the acute seizure-induced injury was quantified by counting TUNEL positive cells. Cell counts of TUNEL positive cells in Adk-tg mice 24h after 20min of SE (75.3±20.1) were similar (P>0.05, n=6) to cell counts of TUNEL positive cells in wt mice 24h after 30min of SE (73.8±17.1). These findings demonstrate that (i) the rate of cell death in CA3 reflects the ADK-dependent seizure-“dose”, and that (ii) wild-type like acute injury can be re-created in Adk-tg mice by reducing the duration of the KA-induced SE from 30 to 20 minutes.
Figure 2.
Intraamygdaloid KA –induced SE terminated after 20 min with lorazepam in Adk-tg mice causes wt-like acute injury. Coronal brain sections were prepared 24 h after status epilepticus (SE). A, B, Cresyl violet staining showing acute injury restricted to the ipsilateral CA3 induced by SE terminated after 20 min with lorazepam in Adk-tg mice. C, D, TUNEL positive cells in Adk-tg mice subjected to 20 min of SE. E, F, Cresyl violet staining showing ipsilateral CA3 damage induced by SE terminated after 30 min with lorazepam in wild-type (Wt) mice. G, H, TUNEL positive cells in wt mice 24 h after 30 min of SE. Scale bars: A,B,E,F, 300μm, C,D,G,H, 75μm. For quantitative and statistical analysis, please refer to main text.
Unilateral CA3-selective injury in Adk-tg mice
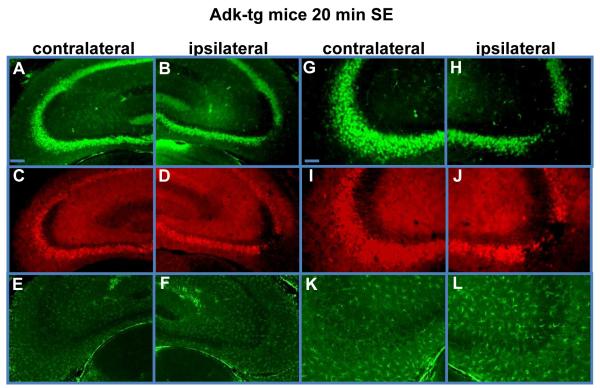
The acute CA3-selective injury described above is expected to constitute a trigger for subsequent epileptogenesis. This neuronal cell loss should at the same time abolish neuronal ADK expression in the CA3 ipsilateral to the KA-injection in Adk-tg mice, while maintaining the transgene-driven overexpression of ADK in the contralateral CA3. To assess the degree and cell-type specificity of the CA3-selective injury in Adk-tg mice subjected to 20 min of SE, we stained brain sections obtained 24 hours after KA-injection (using a new set of 6 Adk-tg mice) for the expression of the neuronal marker NeuN, the astrocyte-specific marker GFAP, and for ADK. While the expression of NeuN and ADK was not affected in the contralateral hippocampus (Fig. 3A,G,C,I), the contralateral hippocampus was characterized by concomitant loss of NeuN and ADK immunoreactivity that was selective for the injured CA3. In contrast, astrocytes (GFAP immunoreactivity) were not affected by the acute CA3-injury (Fig. 3E,F,K,L). In conclusion, 20 min of KA-induced SE in Adk-tg mice causes wild-type like CA3-selective injury that is characterized by site-specific ADK-deficiency. This is an important finding, as it allows us to study seizure generation within the context of focal ADK-deficiency.
Figure 3.
Selective ADK-deficiency in the ipsilateral CA3. Coronal brain sections were prepared 24 h after 20 min of status epilepticus (SE) in Adk-tg mice. A, B, G, H, NeuN immunofluorescence staining (green) shows neuronal cell loss that is selective for the ipsilateral CA3. C, D, I, J, ADK immunofluorescence staining (red) shows ADK-deficiency in the ipsilateral CA3 that corresponds to the loss of NeuN expressing neurons. E, F, K, L, GFAP immunofluorescence (green) shows maintenance of astrocytes within the injured CA3. Note that the lack of ADK-immunoreactivity within the injured CA3 is a function of the loss of ADK-expressing neurons and the transient downregulation of ADK as a consequence of acute injury. Scale bars, A-D, 150μm, E-H, 75μm. For quantitative and statistical analysis, please refer to Figure 6.
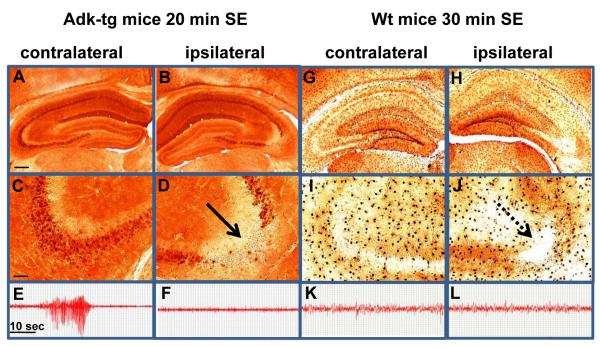
CA3-selective injury prevents spontaneous seizures in Adk-tg mice
If spontaneous CA3-seizures in Adk-tg mice (Fig. 1) are due to overexpression of ADK in CA3, then a CA3-selective injury should abolish these seizures. When analyzed 24 h after 20 min SE, Adk-tg mice displayed significant ADK-loss in the CA3 ipsilateral to the injury (Fig. 4B,D, Fig. 6A, P<0.01), while ADK overexpression in the contralateral CA3 (Fig. 4A,C) was maintained. A parallel set of Adk-tg mice (n = 6) subjected to 20 min SE was equipped with bipolar EEG-recording electrodes inserted into the CA3 of both hemispheres. 20 hours of continuous EEG recording performed from 24 hours to 44 hours after SE revealed regular seizures (a total of >500 seizures) within the ADK-overexpressing contralateral CA3 (Fig. 4E, Table 1), while not a single seizure was detected in the ipsilateral CA3 (Fig. 4F). Thus, local ADK-deficiency caused by CA3-selective injury in Adk-tg mice effectively abolished seizures. In contrast to the diffuse and ubiquitous overexpression of ADK in Adk-tg mice, in wild-type mice the enzyme displays a distinct pattern of expression within the cell bodies of astrocytes (Fig. 4G,I). 24 h after 30 min SE the astrocytic expression of native ADK was significantly attenuated (Fig. 4H,J and Fig. 6A, P<0.01) in line with our previous findings of a biphasic ADK-regulation, during which astrocytic ADK is transiently downregulated as an acute response to ischemic (Pignataro et al., 2008) or epileptic (Gouder et al., 2004) brain injury. In contrast to overexpression of ADK (Fig. 4A,C,E) normal or reduced levels of ADK expression (Fig. 4B,D,F-L) were not associated with seizures. Together, these studies demonstrate that transgenic overexpression of ADK is sufficient to trigger seizures.
Figure 4.
Prevention of spontaneous seizures in Adk-tg mice. Intra-CA3 EEG recordings, followed by immunohistochemical analysis were performed 24 h after 20 min of SE in Adk-tg mice or 30 min of SE in wild-type mice. A-D, Coronal brain section of Adk-tg mice stained with diaminobenzidine hydrochloride (DAB) for ADK immunoreactivity reveal ADK deficiency that is restricted to the ipsilateral CA3 in Adk-tg mice. E, F, Spontaneous seizures recorded from the CA3 area of Adk-tg mice are restricted to the contralateral hippocampus (E) that is characterized by ADK-overexpressing pyramidal neurons (A,C), whereas the ipsilateral CA3, characterized by lack of ADK-expressing neurons (B,D), is devoid of any seizure activity (F). G-J, ADK immunoreactivity in wild-type mice is largely restricted to the cell bodies of astrocytes. Note downregulation of ADK as an adaptive response to acute injury. K, L, EEG recordings taken from the ipsi- and contralateral CA3 of wild-type mice do not reveal any seizure activity. Scale bars, A,B,G, H, 300μm, C,D,I,J, 75μm. For quantitative and statistical analysis, please refer to Figure 6.
Figure 6.
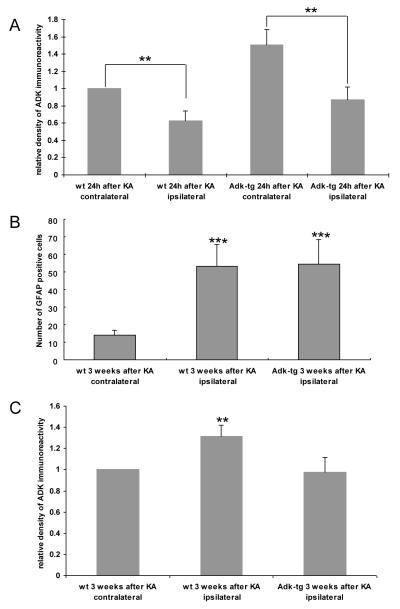
Quantification of ADK immunodensities and GFAP-expressing cells in the CA3 region. A, Relative density of ADK immunoreactivity was determined on DAB-stained sections by scanning CA3a fields of 300 × 200 μm on 2 sections each from n = 6 animals per treatment group 24 h after KA-injection. Data were normalized to the contralateral hippocampus of KA-injected wt mice. B, Total number of GFAP-positive cells in corresponding CA3a fields of 300 × 200 μm was determined by counting GFAP-positive cells on 2 sections each from n = 6 animals per treatment group. Note the similar degree of astrogliosis (i.e. increase in GFAP positive cells) in wt and in Adk-tg mice 3 weeks after KA-injection. C, Relative density of ADK immunoreactivity normalized to the contralateral hippocampus of KA-injected wt mice 3 weeks after KA-injection. Note a significant increase in ADK immunoreactivity in the ipsilateral CA3 of wild-type mice, while ADK-immunoreactivity in the CA3 of Adk-tg mice was comparable to wild-type control. Data analysis was done by ANOVA; mean ± SD. **P < 0.01; ***P < 0.001.
Prevention of chronic seizures but not of astrogliosis in Adk-tg mice subjected to 20 min SE
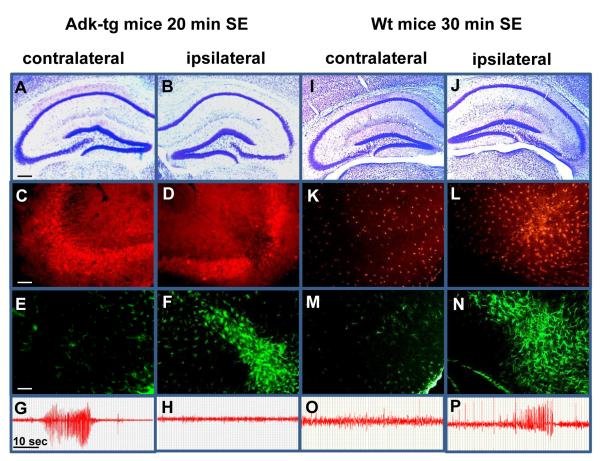
In contrast to the transient downregulation of ADK as an acute response to brain injury (Fig. 4), astrogliosis and concomitant upregulation of ADK constitute a chronic response to injury and are characteristic hallmarks of epileptogenesis (Li et al., 2007a; Boison, 2008a; Li et al., 2008). Since in wild-type mice astrogliosis and ADK expression levels are tightly linked, Adk-tg mice subjected to 20 min SE constitute an ideal model to molecularly separate astrogliosis from ADK-expression during epileptogenesis. We therefore studied epileptogenesis in Adk-tg mice. A new set of Adk-tg mice and respective wild-type control mice was subjected to either 20 or 30 min of intraamygdaloid KA-induced SE (n = 6, each). Three weeks after SE all animals were implanted bilaterally with bipolar CA3 recording electrodes and subjected to 20 h of continuous EEG recording. After completion of EEG recording all animals were subjected to histological analysis (Fig. 5). Seizures were recorded in the contralateral CA3 of Adk-tg mice (Fig. 5G), consistent with the ectopic overexpression of ADK in pyramidal neurons. In contrast, however, not a single seizure was detected in the ipsilateral CA3 of Adk-tg mice based on cumulative EEG recordings of 120 h. This lack of seizures is in marked contrast to the presence of CA3-injury (Fig. 5B) and profound astrogliosis in these animals as evidenced by a significant increase in the number of GFAP-positive cells compared to control (Fig. 5F, Fig. 6B, P<0.001), but corresponds to ADK-deficiency within the ipsilateral CA3. In contrast, wild-type animals developed astrogliosis plus astrogliotic upregulation of ADK (as evidenced by a >30% increase in ADK immunodensity compared to control, P<0.01, Fig. 6C) and recurrent spontaneous seizures that were all restricted to the ipsilateral CA3 (Fig. 5L,N,P) in accordance with our previous findings (Li et al., 2007a; Li et al., 2008). These findings demonstrate that astrogliosis in the absence of ADK overexpression is not sufficient to generate spontaneous seizures.
Figure 5.
Lack of ADK-upregulation and lack of spontaneous seizures in Adk-tg mice 3 weeks after KA. Adk-tg mice subjected to 20 min of SE or wild-type mice subjected to 30 min of SE were analyzed 3 weeks after KA-injection. A, B, Coronal brain sections of Adk-tg mice stained with Cresyl violet reveal the original IPI (neuronal cell loss) that is restricted to the ipsilateral CA3. C, D, ADK immunoreactivity (red) reveals ADK-deficiency in the ipsilateral CA3. Note the lack of upregulated ADK in the ipsilateral CA3. E, F, GFAP immunoreactivity (green) reveals prominent astrogliosis that is restricted to the ipsilateral CA3. G, H, Electroencephalograms recorded from the CA3. Note the lack of spontaneous seizures in the ipsilateral CA3 despite the presence of prominent astrogliosis. I, J, Coronal brain sections of wild-type mice stained with Cresyl violet demonstrate a similar degree of the original IPI. K, L, ADK immunoreactivity reveals upregulation of (astrocytic) ADK in the ipsilateral CA3. M, N, GFAP immunoreactivity reveals prominent astrogliosis restricted to the ipsilateral CA3. O, P, Electroencephalograms recorded from the CA3 demonstrate seizure activity only within the astrogliotic ADK-overexpressing CA3. Scale bars, A, B, I, J, 300μm, C-F, K-N, 75μm. For quantitative and statistical analysis, please refer to Table 1 and Figure 6.
CONCLUSIONS
Global expression levels of ADK (in neurons or astrocytes) contribute to seizure generation.
Astrogliosis without overexpression of ADK is not sufficient to trigger seizures.
ADK deficiency may preclude epileptogenesis.
Overexpression of ADK in epilepsy is a cause, not a consequence, of chronic seizures.
DISCUSSION
In healthy adult brain adenosine levels are largely regulated by an astrocyte-based adenosine cycle and the activity of its major metabolic enzyme adenosine kinase (ADK) (Boison, 2008a), an evolutionary conserved enzyme belonging to the ribokinase family of proteins (Park and Gupta, 2008). Since ADK activity is regulated by all of its substrates and metabolites the enzyme is ideally suited to serve both as a sensor for the energy state of a cell as well as a switch to adjust energy consumption to supplies. Astrogliosis and associated upregulation of ADK are part of the epileptogenic cascade and directly responsible for seizure generation (Li et al., 2007a; Li et al., 2008). By uncoupling ADK expression from astrogliosis in Adk-tg mice, we demonstrate here that global expression levels of ADK (in neurons or astrocytes) determine seizure expression. We also demonstrate that astrogliosis without overexpression of ADK is not sufficient to trigger seizures. These are important new findings that need to be discussed within the context of the role of astrocytes and ADK in epilepsy.
The role of astrocytes in epilepsy
Astrogliosis is a pathological hallmark of the epileptic brain and an astrocytic basis for epilepsy has recently been proposed (Tian et al., 2005; Boison, 2008b; Kumaria et al., 2008; Oberheim et al., 2008). Astrocytes not only play an important role in controlling seizure initiation, but they are also important mediators of seizure termination (Lado and Moshe, 2008). Astrocytes are known to synthesize proinflammatory cytokines during seizures that can affect extracellular glutamate levels as well as inflammatory reactions in epilepsy (Ravizza et al., 2008). Astrocytes can modulate neuronal function in epilepsy in many ways. Seizures can be triggered by excessive astrocytic glutamate release or defective glutamate reuptake (Tian et al., 2005). Experimental models of epilepsy are characterized by a loss of astrocytic domain organization that is involved in neurovascular coupling (Iadecola and Nedergaard, 2007). Thus, astrocytes are key regulators of neuronal function and of neurovascular coupling by a mechanism known as gliotransmission (Pascual et al., 2005; Haydon and Carmignoto, 2006). Astrocytic ATP release is tightly linked to the glial calcium wave. This can either increase synaptic strength across the tripartite synapse directly (a pro-epileptogenic effect) or constitute a source for the extracellular generation of adenosine, which would provide the net-effect of global inhibition of adjacent neurons (Kumaria et al., 2008). Through their capacity to release both the adenosine precursor ATP, as well as adenosine as such, astrocytes play an essential role in regulating the extracellular availability of the brain’s own anticonvulsant adenosine (Haydon and Carmignoto, 2006; Halassa et al., 2007; Martin et al., 2007; Bjorklund et al., 2008; Boison, 2008a). Previous studies from our laboratory have demonstrated an essential role of the astrocytic enzyme ADK in regulating neuronal excitability by providing a metabolic reuptake system for extracellular adenosine (Boison, 2008a; Li et al., 2008). Remarkably, astrocytic ADK regulates basal synaptic adenosine levels and seizure activity but not activity-dependent adenosine release in the hippocampus (Wetherington et al., 2008).
The role of ADK in epilepsy
ADK, which in adult brain is predominantly expressed in astrocytes (Studer et al., 2006), is considered to be the key regulator of the endogenous anticonvulsant adenosine (Boison, 2006). Adenosine deficiency in epilepsy has been identified as a major contributor to seizure generation (Rebola et al., 2003; Gouder et al., 2004; Li et al., 2007a; Li et al., 2008), and, consequently, adenosine augmenting cell therapies constitute an effective tool for seizure control (Boison, 2007). Recent work in the mouse model of CA3 selective epileptogenesis forms the basis of the current study: acute CA3-selective neuronal cell loss leads to spatial and temporal coincidence of astrogliosis (a pathological hallmark of the epileptic brain) and spontaneous electrographic seizures, all restricted to the injured CA3 (Li et al., 2007a; Li et al., 2008). The focal nature of these CA3 seizures was demonstrated on two different levels: (i) Only bipolar electrodes implanted directly into the affected CA3 yielded spontaneous electrographic seizure activity, while electrodes implanted elsewhere (e.g. CA1 or cortex) yielded no seizure activity (Li et al., 2008). While pyramidal cell bodies in CA3 were lost as a result of the acute brain injury, the ipsilateral CA3 was characterized by a dense network of NeuN-positive neuronal processes derived from neuronal cell bodies located elsewhere (Li et al., 2007a); the spontaneous CA3-selective seizures are likely derived from this neuronal network that is under-inhibited by deficiency of adenosine in this ADK-overexpressing brain region. (ii) The temporal coincidence of overexpression of ADK and the first emergence of spontaneous seizures 12 days after the IPI further support the focal nature of the seizures and their link to ADK.
Transgenic Adk-tg mice that overexpress ADK in neurons and astrocytes of the brain are characterized by spontaneous electrographic seizures that are highly similar to those encountered in the CA3-selective model of epileptogenesis (Li et al., 2007a). Focal CA3-selective ADK-deficiency was induced in these mutants as a consequence of acute CA3-injury and demonstrated here 24 hours after KA-injection (Fig. 3D,J, and Fig. 4B,D,H,J). Our data suggest that the acute ADK-deficiency described here is a function of two separate mechanisms: (i) ablation of ADK-expressing CA3-neurons (Fig. 3A,B,G,H), and (ii) downregulation of ADK in astrocytes as acute response to brain injury (Fig. 4). The transient downregulation of ADK as an acute (and protective) response to brain injury has been described in models of epilepsy (Gouder et al., 2004) and stroke (Pignataro et al., 2008) and extends from 2 to 24 hours following acute injury. Most importantly, the focal CA3-selective deficiency of ADK expression in Adk-tg mice abolished spontaneous seizures (Fig. 4) indicating that spontaneous seizures in Adk-tg mice are related to ADK expression levels rather than to altered hippocampal circuitry. Our findings also indicate that either neuronal or astrocytic overexpression of ADK is sufficient to trigger seizures irrespective of astrogliosis. The unique design of the current study allowed us to assess cell-type specific roles of increased ADK for seizure generation. While pharmacological inhibition of astrocytic ADK in epilepsy is sufficient to prevent seizures (Gouder et al., 2004), in the present study we demonstrate seizure elimination in Adk-tg mice after induction of a CA3-selective injury (Fig. 4). Together, these findings suggest that ADK likely has no cell-type specific direct roles in seizure control, but rather influences seizure activity indirectly by acting as a paracrine regulator of ambient concentrations of adenosine.
Uncoupling of ADK expression from astrogliosis
During epileptogenesis astrogliosis is coupled with overexpression of ADK. Is overexpression of ADK cause for or consequence of astrogliosis? Here, we used a transgenic strategy to uncouple astrogliosis from ADK expression. This was made possible by using Adk-tg mice which lack the endogenous Adk-gene that generates increased levels of ADK during astrogliosis and epileptogenesis. In Adk-tg mice the endogenous gene is replaced by a constitutively expressed transgene that does not respond to regulatory cues (Li et al., 2007a; Li et al., 2008). This mouse model allowed us to address the question, whether astrogliosis can develop in the absence of the endogenous Adk-gene. Our data clearly demonstrate that lack of the endogenous astrocytic Adk-gene does not preclude astrogliosis (Fig. 5 & 6). This is an important finding indicating that astrogliotic upregulation of ADK is consequence of rather than cause for astrogliosis.
As outlined above, astrocytes may trigger seizures through several different mechanisms (deficient regulation of glutamate, cytokines, and adenosine). Here, we demonstrate that a local disruption of ADK expression is sufficient to prevent spontaneous seizures in the presence of astrogliosis (Fig. 5). Thus, ADK expression levels, rather than astrogliosis per se determine seizure generation in the epileptic brain. This finding is of significance, since it rules out that other astrogliotic components of epileptogenesis (e.g. glutamate and cytokine dysregulation) by themselves are not sufficient to trigger seizures in the absence of ADK. We conclude that ADK appears to be a dominant regulator for seizure generation.
Does ADK-deficiency prevent epileptogenesis?
Previous data derived from fb-Adk-def mice, which have reduced ADK expression in forebrain, suggest that ADK-deficiency renders the brain resistant to epileptogenesis (Li et al., 2007a; Li et al., 2008). Despite creating a wild-type like CA3-injury as potential trigger for subsequent epileptogenesis, fb-Adk-def mice failed to develop spontaneous seizures three weeks after injury, whereas wild-type control mice reproducibly developed seizures around day 12 after injury (Li et al., 2007a; Li et al., 2008). In addition, fb-Adk-def mice were characterized by reduced astrogliosis and lack of upregulated ADK. These findings were interpreted as potential antiepileptogenic effect of ADK-deficiency. In the present study, we recreated wild-type like acute injury in Adk-tg mice by reducing the acute seizure time from 30 to 20 minutes. Remarkably, despite having been exposed to an acute IPI (Fig. 5) and despite developing astrogliosis within 3 weeks, these animals fail to express spontaneous seizures in the affected CA3. These findings suggest that ADK expression is a necessary prerequisite for epileptogenesis and the development and expression of spontaneous seizures. In line with these conclusions, focal adenosine augmentation therapies that are based on intracerebral transplantation of adenosine releasing cells or polymer have antiepileptogenic properties: (i) adenosine-releasing embryonic stem cell derived infrahippocampal implants not only suppressed kindling epileptogenesis (Li et al., 2007b), but also reduced astrogliosis, prevented upregulation of ADK, and prevented the development of spontaneous seizures in the mouse model of CA3-selective epileptogenesis (Li et al., 2008). Most recently, the antiepileptogenic effect of adenosine was confirmed by kindling rats in the presence of silk-based polymers engineered to release defined doses of adenosine during a limited time span of 10 days (Szybala et al., 2009). Animals kindled during sustained release of 1000 ng adenosine per day failed to develop convulsive seizures (during 30 kindling stimulations). However, when kindling was resumed after expiration of adenosine release from the polymers kindled seizures gradually developed with a time course similar to kindling development in recipients of control polymers that did not release adenosine. Together, these findings (Szybala et al., 2009) and results from the present study suggest that adenosine augmentation and reductions in ADK have antiepileptogenic effects. These findings could have major implications for epilepsy following traumatic brain injury or stroke and could lead to novel therapies aimed at the prevention of epilepsy.
ACKNOWLEDGEMENTS
The present study was supported by NIH Grant R01NS061844 from the National Institute of Neurological Disorders and Stroke (NINDS).
Footnotes
STATEMENT OF INTEREST: - NONE -
REFERENCES
- Bjorklund O, Shang MM, Tonazzini I, Dare E, Fredholm BB. Adenosine A(1) and A(3) receptors protect astrocytes from hypoxic damage. European Journal of Pharmacology. 2008;596:6–13. doi: 10.1016/j.ejphar.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Boison D. Adenosine kinase, epilepsy and stroke: mechanisms and therapies. Trends Pharmacol Sci. 2006;27:652–658. doi: 10.1016/j.tips.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Boison D. Adenosine-based cell therapy approaches for pharmacoresistant epilepsies. Neurodegener Dis. 2007;4:28–33. doi: 10.1159/000100356. [DOI] [PubMed] [Google Scholar]
- Boison D. The adenosine kinase hypothesis of epileptogenesis. Progress in Neurobiology. 2008a;84:249–262. doi: 10.1016/j.pneurobio.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boison D. Astrogliosis and adenosine kinase: a glial basis of epilepsy. Future Neurology. 2008b;3:221–224. [Google Scholar]
- Boison D, Scheurer L, Zumsteg V, Rülicke T, Litynski P, Fowler B, Brandner S, Mohler H. Neonatal hepatic steatosis by disruption of the adenosine kinase gene. Proc Natl Acad Sci USA. 2002;99:6985–6990. doi: 10.1073/pnas.092642899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedele DE, Gouder N, Güttinger M, Gabernet L, Scheurer L, Rulicke T, Crestani F, Boison D. Astrogliosis in epilepsy leads to overexpression of adenosine kinase resulting in seizure aggravation. Brain. 2005;128:2383–2395. doi: 10.1093/brain/awh555. [DOI] [PubMed] [Google Scholar]
- Gouder N, Scheurer L, Fritschy J-M, Boison D. Overexpression of adenosine kinase in epileptic hippocampus contributes to epileptogenesis. J Neurosci. 2004;24:692–701. doi: 10.1523/JNEUROSCI.4781-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halassa MM, Fellin T, Haydon PG. The tripartite synapse: roles for gliotransmission in health and disease. Trends Mol Med. 2007;13:54–63. doi: 10.1016/j.molmed.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Haydon PG, Carmignoto G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiological Reviews. 2006;86:1009–1031. doi: 10.1152/physrev.00049.2005. [DOI] [PubMed] [Google Scholar]
- Iadecola C, Nedergaard M. Glial regulation of the cerebral microvasculature. Nat Neurosci. 2007;10:1369–1376. doi: 10.1038/nn2003. [DOI] [PubMed] [Google Scholar]
- Kumaria A, Tolias C, Burnstock G. ATP signalling in epilepsy. Purinergic Signalling. 2008;4:339–346. doi: 10.1007/s11302-008-9115-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lado FA, Moshe SL. How do seizures stop? Epilepsia. 2008;49:1651–1664. doi: 10.1111/j.1528-1167.2008.01669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Lan JQ, Fredholm BB, Simon RP, Boison D. Adenosine dysfunction in astrogliosis: cause for seizure generation? Neuron Glia Biology. 2007a;3:353–366. doi: 10.1017/S1740925X0800015X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Ren G, Lusardi T, Wilz A, Lan JQ, Iwasato T, Itohara S, Simon RP, Boison D. Adenosine kinase is a target for the prediction and prevention of epileptogenesis in mice. J Clin Inv. 2008;118:571–582. doi: 10.1172/JCI33737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Steinbeck JA, Lusardi T, Koch P, Lan JQ, Wilz A, Segschneider M, Simon RP, Brustle O, Boison D. Suppression of kindling epileptogenesis by adenosine releasing stem cell-derived brain implants. Brain. 2007b;130:1276–1288. doi: 10.1093/brain/awm057. [DOI] [PubMed] [Google Scholar]
- Martin ED, Fernandez M, Perea G, Pascual O, Haydon PG, Araque A, Cena V. Adenosine released by astrocytes contributes to hypoxia-induced modulation of synaptic transmission. Glia. 2007;55:36–45. doi: 10.1002/glia.20431. [DOI] [PubMed] [Google Scholar]
- Oberheim NA, Tian GF, Han X, Peng W, Takano T, Ransom B, Nedergaard M. Loss of astrocytic domain organization in the epileptic brain. J Neurosci. 2008;28:3264–3276. doi: 10.1523/JNEUROSCI.4980-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Gupta RS. Adenosine kinase and ribokinase - the RK family of proteins. Cellular and Molecular Life Sciences. 2008;65:2875–2896. doi: 10.1007/s00018-008-8123-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual O, Casper KB, Kubera C, Zhang J, Revilla-Sanchez R, Sul JY, Takano H, Moss SJ, McCarthy K, Haydon PG. Astrocytic purinergic signaling coordinates synaptic networks. Science. 2005;310:113–116. doi: 10.1126/science.1116916. [DOI] [PubMed] [Google Scholar]
- Pignataro G, Maysami S, Studer FE, Wilz A, Simon RP, Boison D. Downregulation of hippocampal adenosine kinase after focal ischemia as potential endogenous neuroprotective mechanism. J Cereb Blood Flow Metab. 2008;28:17–23. doi: 10.1038/sj.jcbfm.9600499. [DOI] [PubMed] [Google Scholar]
- Pignataro G, Simon RP, Boison D. Transgenic overexpression of adenosine kinase aggravates cell death in ischemia. J Cereb Blood Flow Metab. 2007;27:1–5. doi: 10.1038/sj.jcbfm.9600334. [DOI] [PubMed] [Google Scholar]
- Pitkanen A, Lukasiuk K. Molecular and cellular basis of epileptogenesis in symptomatic epilepsy. Epilepsy Behav. 2009;14(Suppl 1):16–25. doi: 10.1016/j.yebeh.2008.09.023. [DOI] [PubMed] [Google Scholar]
- Ravizza T, Gagliardi B, Noe F, Boer K, Aronica E, Vezzani A. Innate and adaptive immunity during epileptogenesis and spontaneous seizures: evidence from experimental models and human temporal lobe epilepsy. Neurobiol Dis. 2008;29:142–160. doi: 10.1016/j.nbd.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Rebola N, Coelho JE, Costenla AR, Lopes LV, Parada A, Oliveira CR, Soares-da-Silva P, de Mendonca A, Cunha RA. Decrease of adenosine A1 receptor density and of adenosine neuromodulation in the hippocampus of kindled rats. Eur J Neurosci. 2003;18:820–828. doi: 10.1046/j.1460-9568.2003.02815.x. [DOI] [PubMed] [Google Scholar]
- Shinoda S, Schindler CK, Meller R, So NK, Araki T, Yamamoto A, Lan JQ, Taki W, Simon RP, Henshall DC. Bim regulation may determine hippocampal vulnerability after injurious seizures and in temporal lobe epilepsy. J Clin Invest. 2004;113:1059–1068. doi: 10.1172/JCI19971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studer FE, Fedele DE, Marowsky A, Schwerdel C, Wernli K, Vogt K, Fritschy J-M, Boison D. Shift of adenosine kinase expression from neurons to astrocytes during postnatal development suggests dual functionality of the enzyme. Neuroscience. 2006;142:125–137. doi: 10.1016/j.neuroscience.2006.06.016. [DOI] [PubMed] [Google Scholar]
- Szybala C, Pritchard EM, Wilz A, Kaplan DL, Boison D. Antiepileptic effects of silk-polymer based adenosine release in kindled rats. Exp Neurol. 2009 doi: 10.1016/j.expneurol.2009.05.018. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian GF, Azmi H, Takano T, Xu QW, Peng WG, Lin J, Oberheim N, Lou NH, Wang XH, Zielke HR, Kang J, Nedergaard M. An astrocytic basis of epilepsy. Nature Medicine. 2005;11:973–981. doi: 10.1038/nm1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezzani A. Epileptogenic role of astrocyte dysfunction. Epilepsy Curr. 2008;8:46–47. doi: 10.1111/j.1535-7511.2008.00233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherington J, Serrano G, Dingledine R. Astrocytes in the epileptic brain. Neuron. 2008;58:168–178. doi: 10.1016/j.neuron.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]