Abstract
Up to one in four lung-transplanted patients develop pulmonary infiltrates and impaired oxygenation within the first days after lung transplantation. Known as primary graft dysfunction (PGD), this condition increases mortality significantly. Complex interactions between donor lung and recipient immune system are the suspected cause. We took an integrative, systems-level approach by first exploring whether the recipient's immune response to PGD includes the development of long-lasting autoreactivity. We next explored whether proteins displaying such differential autoreactivity also display differential gene expression in donor lungs that later develop PGD compared with those that did not. We evaluated 39 patients from whom autoantibody profiles were already available for PGD based on chest radiographs and oxygenation data. An additional nine patients were evaluated for PGD based on their medical records and set aside for validation. From two recent donor lung gene expression studies, we reanalysed and paired gene profiles with autoantibody profiles. Primary graft dysfunction can be distinguished by a profile of differentially reactive autoantibodies binding to 17 proteins. Functional analysis showed that 12 of these proteins are part of a protein–protein interaction network (P=3 × 10−6) involved in proliferative processes. A nearest centroid classifier assigned correct PGD grades to eight out of the nine patients in the validation cohort (P=0·048). We observed significant positive correlation (r=0·63, P=0·011) between differences in IgM reactivity and differences in gene expression levels. This connection between donor lung gene expression and long-lasting recipient IgM autoantibodies towards a specific set of proteins suggests a mechanism for the development of autoimmunity in PGD.
Keywords: autoantibodies, lung transplantation, microarrays, primary graft dysfunction
Introduction
Development of pulmonary infiltrates and impaired oxygenation within the first 3 days after lung transplantation, defined as primary graft dysfunction (PGD), affects an estimated 10–25% of transplanted patients.1 Patients with PGD have markedly worse 90-day post-operative mortality and 3-year survival.2 The specific aetiology and pathogenesis of PGD is not well understood but is thought to be the result of complex interactions between donor lung and recipient immune system.3 Injuries to pulmonary epithelium and endothelium by reactive oxygen species, initiation of aggressive inflammatory cascades, and increases in pro-coagulant and vasoconstriction factors have all been implicated.3–6
Autoimmunity, specifically T-cell autoreactivity towards type V collagen (COL5), has been associated with the development of PGD.6 It is well established that reactivity towards this protein is also associated with the development of obliterative bronchiolitis.7 Recently, the autoantibody repertoires in the blood of recipients at various stages of chronic lung rejection in the form of obliterative bronchiolitis were studied using an antigen microarray containing hundreds of self-molecules.8 It was found that a profile of autoantibodies binding to 28 proteins or their peptides could differentiate between mild and severe chronic rejection. Here, we explored whether the recipients’ immune response to PGD also includes a long-lasting, informative repertoire of autoantibodies.
Comparing donor lungs developing PGD with those that did not has identified significantly different expression for hundreds of genes involved in both signalling and stress-activated pathways.9,10 We reasoned that such differential expression of genes encoding naturally autoreactive proteins might trigger altered levels of autoantibodies against these proteins. Such a correlation would be consistent with the hypothesis that the natural autoantibody repertoire reflects the immunogenic body state – the immunological homunculus.11
We took an integrative, systems-level analysis approach by evaluating 39 patients, for whom autoantibody profiles were already available, for PGD based on chest radiographs and oxygenation data. We found that 19 patients had no indication of PGD whereas 20 patients manifested PGD grade 1 or higher. We paired the autoantibody profiles with gene expression profiles from two recent studies comparing donor lungs that developed PGD with those that did not. We report that PGD can be differentiated by a profile of differentially reactive autoantibodies, most of which are connected in a protein–protein interaction network involved in proliferative processes such as regulation of development and cell communication. Furthermore, for the implicated proteins, we observed significant positive correlation between differential IgM reactivity and differential gene expression levels in the presence or absence of PGD (increased expression associated with increased reactivity and vice versa).
Materials and methods
Autoantibody profiling data
Patients attending scheduled visits during a half-year period in the out-patient clinic at the Danish National Lung Transplant Programme were included in the study. The transplant programme has been described in detail previously.8,12 For 39 patients, PGD could be evaluated retrospectively from chest radiographs and oxygenation data pertaining to the first 72 post-operative hours. Table 1 presents clinical characteristics for this patient cohort. An additional nine patients for whom reactivity data were also available, but whose original chest radiographs had been discarded, were set aside for validation. In this validation cohort, the presence or absence of PGD was ascertained from patient journals (which included day-to-day observations from chest radiographs describing the presence or absence of pulmonary oedema and infiltrates during the first 72 hr as well as documentation for treatment with nasal oxygen when this had been used).
Table 1.
Clinical characteristics of patients. Comparison of clinical parameters and primary graft dysfunction (PGD) grades
| All (n=39) | PGD 0 (n=19) | PGD ≥ 1 (n=20) | P | |
|---|---|---|---|---|
| Recipient age (years) | ||||
| < 40 | 5 | 1 | 4 | ns |
| 40–49 | 5 | 2 | 3 | |
| 50–59 | 13 | 9 | 4 | |
| 60–69 | 12 | 6 | 6 | |
| ≥ 70 | 4 | 1 | 3 | |
| Recipient sex | ||||
| Male | 20 | 13 | 7 | 0·06 |
| Female | 19 | 6 | 13 | |
| Donor age (years) | ||||
| < 20 | 5 | 3 | 2 | ns |
| 20–29 | 7 | 3 | 4 | |
| 30–39 | 7 | 5 | 2 | |
| 40–49 | 11 | 4 | 7 | |
| ≥ 50 | 9 | 4 | 5 | |
| Donor sex | ||||
| Male | 23 | 16 | 7 | 0·003 |
| Female | 16 | 3 | 13 | |
| Primary diagnosis | ||||
| COPD | 14 | 6 | 8 | ns |
| A1AT | 15 | 10 | 5 | |
| CF | 6 | 2 | 4 | |
| Other | 4 | 1 | 3 | |
| Antihypertensive treatment | ||||
| + | 35 | 17 | 18 | ns |
| − | 4 | 2 | 2 | |
| Number of treated rejections > A1 | ||||
| 0 | 6 | 1 | 5 | ns |
| 1 | 7 | 4 | 3 | |
| 2 | 9 | 3 | 6 | |
| 3 | 7 | 4 | 3 | |
| ≥ 4 | 10 | 7 | 3 | |
| BOS grade | ||||
| 0, 0-p, 1 | 24 | 14 | 10 | ns |
| 2, 3 | 15 | 5 | 10 | |
| Months after transplant | ||||
| Average | 72 | 79 | 66 | ns |
P-values were calculated using Fisher's exact test (ns: P-value > 0·1), except for the average number of months after transplantation, where the Wilcoxon rank sum test were used.
A1AT, alpha-1-anti-trypsin; BOS, bronchiolitis obliterans syndrome; COPD, chronic obstructive pulmonary disease; CF, cystic fibrosis.
Reactivity data for IgG and IgM antibody binding in sera from these patients were retrieved from http://www.nanotech.dtu.dk/Research/Theory/SSS/Research/LungTransplant.aspx. Antigen microarray preparation, incubation of serum and fluorescent anti-IgG and anti-IgM antibodies, laser scanning and data pre-processing have been described previously.8 Briefly, 504 antigens were judged positive for IgG antibody binding (signal-to-noise ratio > 2 in at least four patients) and 610 antigens were judged positive for IgM antibody binding (473 antigens overlapping). These antigens cover 272 recombinant proteins and synthetic peptides from the sequences of key proteins. The log2-transformed, median centred, measured intensity of an antigen is denoted the reactivity of the antigen.
Transcript profiling data
Data from two gene expression studies (GSE8021 and GSE9102)9,10 were retrieved from the Gene Expression Omnibus database.13 Both studies contrasted samples from donor lungs that later developed PGD against donor lungs that did not. For the GSE9102 study, cDNA microarray data as pre-processed by the authors were used, and covered expression measurements for 6727 Ensembl build 55 human genes (http://jul2009.archive.ensembl.org). When several probes were available for the same gene, the probe displaying the most significant differential expression was selected to represent that gene. For the GSE8021 study, the original raw data were processed as follows. Affymetrix Human Genome U133A 2.0 Array probes were remapped to 11894 different Ensembl build 55 human genes.14 Using these redefined probe sets, probe intensities were summarized and made comparable between arrays by quantile normalization as implemented in the Robust Multi-Array Average expression measure.15 It was possible to identify corresponding gene expression for 242 of the 272 proteins on the antigen microarray (89%).
Identification of differentially reactive proteins and differentially expressed genes
For each antigen and detection antibody, differential reactivity between patients without PGD (n=19) and patients with PGD (n=20) was evaluated by calculating ratios (fold-changes), t-statistics and P-values. For each gene measured, differential expression between donor lungs developing PGD (16 and 10) and those that did not (34 and 16) were similarly evaluated by ratios, t-statistics and P-values. Multiple testing was controlled using the false discovery rate.16
Constructing a high-confidence network of human protein interactions
A human protein interaction network was created by pooling human interaction data from several of the largest databases.17 Coverage was further increased by transferring data from model organisms. A network-wide confidence score for all interactions, based on network topology, experimental type and interaction reproducibility, was then established. The reliability of this score as a measure of interaction confidence was confirmed by fitting a calibration curve of the score against a high-confidence set of about 35 000 human interactions. As previously described,8 all interactions with a confidence score above 0·154 were included, resulting in a network containing approximately 154 000 unique interactions between approximately 12 500 human proteins. Out of the 272 proteins on the antigen microarray, 260 (96%) were among these.
Significance and biological themes of networks
As described previously,8 the statistical significance of the number of proteins in a network (the size) extracted from a given larger set of proteins, was estimated by randomly selecting sets of proteins of the same size, each time recording the size of the largest network possible to extract. For 107 such randomizations, the proportion of random sets of proteins for which equally sized or larger networks could be extracted, establishes the P-value of the network extracted from the original protein set. Over-represented biological processes among proteins in networks were identified by hypergeometric testing of gene ontology terms.
Results
Antibody reactivities reflect PGD grade
Out of the 48 patients for which IgG and IgM reactivity data were available,8 we could grade 39 patients according to PGD using chest radiographs and oxygenation data. In this cohort, each antigen included was tested for differential reactivity between patients having had PGD (n=20) and patients without PGD (n=19) using Student's t-test. The baseline clinical characteristics of the two groups were well matched except that there were a higher proportion of female donors in the PGD group than in the group without PGD (see Table 1).
At a significance threshold of P<0·001 (equal to false discovery rate < 0·15), we identified only a single antigen, telomerase-associated protein 1, displaying fourfold increased reactivity in patients with PGD. Comparing changes in IgG reactivity with changes in IgM reactivity for each antigen included on the microarray, however, we observed that the lower the P-values for these changes, the more frequently they changed in the same direction, see Supporting Information for Fig. S1. Requiring P<0·05 for the differential reactivity of both IgG and IgM, 16 different proteins (corresponding to 46 different antigens, because several peptides from the same protein were usually detected), were identified.
With these significance thresholds, 17 proteins were identified in all (Table 2). For each protein, the reactivity changes listed are for the most significant antigen identified.
Table 2.
Autoreactivity and expression changes for the significant proteins
| IgG reactivity | IgM reactivity | mRNA GSE8021 | mRNA GSE9102 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Gene symbol | log2 ratio | P | log2 ratio | P | log2 ratio | P | log2 ratio | P | Gene Name |
| EGFR | 1·73 | 0·0029 | 1·30 | 0·011 | −0·04 | 0·55 | −0·33 | 0·083 | Epidermal growth factor receptor |
| MBP | 0·93 | 0·0047 | 0·53 | 0·015 | 0·05 | 0·47 | Myelin basic protein | ||
| MLANA | 0·73 | 0·0027 | 0·88 | 0·0070 | 0·03 | 0·42 | −0·15 | 0·31 | Melan-A |
| MUC1 | 4·36 | 0·024 | 2·09 | 0·045 | 0·02 | 0·88 | Mucin 1, cell surface associated | ||
| MYCL1 | 2·35 | 0·041 | 0·94 | 0·0057 | 0·14 | 0·064 | 0·32 | 0·090 | v-myc myelocytomatosis viral oncogene 1 |
| PLCG1 | 2·03 | 0·018 | 0·86 | 0·018 | −0·04 | 0·53 | Phospholipase C, gamma 1 | ||
| PRKCA | 1·63 | 0·021 | 2·40 | 0·028 | 0·12 | 0·067 | 0·24 | 0·021 | Protein kinase C, alpha |
| HSP90AA1 | 0·91 | 0·0015 | −1·14 | 0·0060 | −0·12 | 0·27 | Heat shock protein 90kDa alpha, A1 | ||
| IGF1R | 2·98 | 0·013 | −0·58 | 0·018 | −0·16 | 0·33 | Insulin-like growth factor 1 receptor | ||
| RB1 | 0·73 | 0·035 | −0·67 | 0·019 | −0·06 | 0·59 | Retinoblastoma 1 (including osteosarcoma) | ||
| CERK | −0·50 | 0·040 | 0·96 | 0·0035 | 0·16 | 0·098 | 0·02 | 0·87 | Ceramide kinase |
| HSPD1 | −0·66 | 0·0043 | 2·49 | 0·0047 | Heat shock 60kDa protein 1 (chaperonin) | ||||
| TEP1 | −1·40 | 0·20 | 2·16 | 0·0009 | 0·04 | 0·51 | Telomerase-associated protein 1 | ||
| CYP3A4 | −1·07 | 0·0084 | −0·52 | 0·026 | 0·03 | 0·59 | Cytochrome P450, 3A4 | ||
| SOCS3 | −0·47 | 0·0065 | −0·83 | 0·023 | −0·27 | 0·17 | −0·56 | 0·050 | Suppressor of cytokine signaling 3 |
| TARP | −0·37 | 0·0013 | 1·37 | 0·013 | TCR gamma alt. reading frame protein | ||||
| TP53 | −0·56 | 0·028 | −0·60 | 0·049 | −0·01 | 0·97 | Tumor protein p53 | ||
The 17 proteins displaying significant IgG and/or IgM reactivity changes between patients having developed primary graft dysfunction compared with those that did not are listed.
For each protein, the log2 transformed reactivity ratio and P-value (Student's t-test) for the most significant antigen are shown. Gene expression changes between donor lungs developing PGD compared with those that do not, as measured in two independent studies, are also listed (log2 transformed expression ratio and P-value from Student's t-test).
Out of the 17 proteins identified in this manner, six proteins (HSPD1, HSP90AA1, IGF1R, PRKCA, TARP, and TP53) were previously found to be differentially reactive in connection with bronchiolitis obliterans syndrome (BOS).8 Two-factor analysis of variance for these proteins, with PGD and BOS as the factors, still identified all proteins except TP53 (P=0·11) as displaying significant differences for PGD (P<0·05), see Table 3 and Supporting Information for Fig. S2.
Table 3.
Analysis of autoreactivities including both bronchiolitis obliterans syndrome (BOS) and primary graft dysfunction (PGD) status
| Gene symbol | P (BOS IgG) | P (PGD IgG) | P (BOS IgM) | P (PGD IgM) |
|---|---|---|---|---|
| HSPD1 | 0·031 | 0·016 | 0·064 | 0·0087 |
| HSP90AA1 | 0·10 | 0·0042 | 0·048 | 0·016 |
| IGF1R | 0·18 | 0·020 | 0·050 | 0·050 |
| PRKCA | 0·041 | 0·049 | 0·28 | 0·040 |
| TARP | 0·032 | 0·0052 | 0·0085 | 0·030 |
| TP53 | 0·021 | 0·090 | 0·095 | 0·11 |
Autoreactivities from the 39 patients were analysed using two-way analysis of variance for the six antigens also identified previously.8
The table lists the resulting P-values for each detection antibody (IgG and IgM) for BOS and PGD. See Fig. S2 for distributions of reactivities for the six antigens.
PGD profile is organized in a specific protein interaction network
We analysed the known interactions between the 17 proteins that displayed significant differential autoantibody reactivity (Table 2). This allowed us to examine whether the informative antigens formed networks with specific biological functions. Other large-scale data integrative methods have shown that well-defined interaction networks can often be functionally related to pathological processes and complex diseases.8,17
For 15 of the 17 proteins, interaction data were available, and we identified an interconnected network consisting of 12 proteins, which is significantly more than would be expected by chance (P=3 × 10−6) as determined by randomly selecting 15 proteins out of the 260 proteins on the array where interaction data are available, recording the largest interconnected network possible to construct from these, and repeating this 107 times. Also shown in Fig. 1 are the results of hypergeometric testing on the gene ontology biological process terms assigned to the proteins in the network. Regulation of developmental process (P<5 × 10−5) and cell communication (P<5 × 10−4) were two of the most significantly enriched terms.
Figure 1.
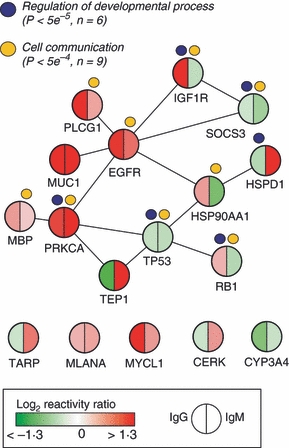
Primary graft dysfunction (PGD) network. Network of the 12 differentially reactive proteins that interact directly. Biological themes summarizing over-represented biological processes in the network are indicated. The five differentially reactive proteins not in the network are also shown for completeness.
PGD profile can be used to predict PGD status in an independent patient cohort
In the validation cohort of nine patients, six had PGD grade 1, and for the remaining three there was no evidence to suggest PGD. All patients were extubated in the first 24 hr and none qualified for a PGD grade 2 or higher. A nearest centroid classifier18 was constructed from the 17 differentially reactive proteins identified (Fig. 2a), and was used to predict the PGD grades of the nine patients in this validation cohort (Fig. 2b). Here, five out of six patients having had PGD were correctly identified (83% sensitivity), and all three patients without PGD were classified as such (100% specificity), giving an overall classification accuracy of 89% (P=0·048 by Fisher's exact test). This is comparable to the classification accuracy in the test set (85%).
Figure 2.
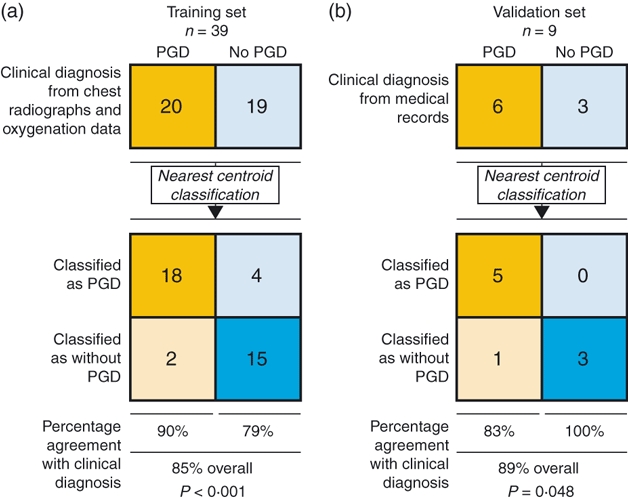
Classification and prediction of primary graft dysfunction (PGD) status. (a) The 17 proteins identified were used for PGD class prediction in the training set using a nearest centroid (NC) classification algorithm. (b) The trained NC classifier was then used for PGD class prediction in the validation set. Results are shown in modified 2 × 2 contingency tables that were used to calculate the percentage of classifications that agreed with clinical diagnosis. P-values were calculated with Fisher's exact test.
Identifying transcript differences for the proteins with altered reactivity
Two recent studies have investigated gene expression differences in donor lungs developing PGD9,10 Differential gene expression in each study was evaluated using Student's t-test. Out of the 17 differentially reactive proteins identified, 15 proteins could be paired with gene expression in the first study,9 and six with expressions from the second study10 (Table 2).
Comparing differences in IgM reactivity with differences in gene expression levels in the first study (study GSE8021 in Table 2), 12 out of 15 change in the same direction (80% concordance, P=0·04 by Fisher's Exact Test), i.e. increased expression is significantly associated with increased reactivity and vice versa. The same conclusion is reached when calculating Pearson's product–moment correlation (r=0·63, P=0·011), see Fig. 3(a). For IgG reactivity, no significant correlation with gene expression changes was observed (r= − 0·01, P=0·98).
Figure 3.
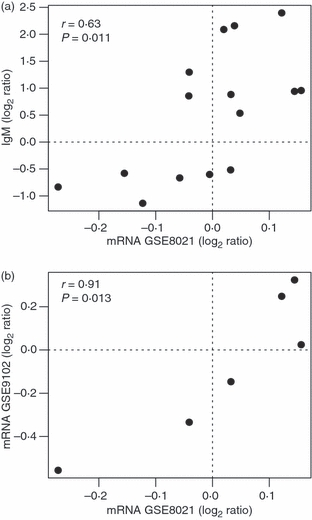
Correlation between reactivity and expression changes. (a) Scatterplot between gene expression changes (GSE8021 study) and IgM reactivity changes. (b) Scatterplot between gene expression changes measured in both mRNA studies. The Pearson correlation coefficient and its associated P-value are shown for each scatter.
Inspection of the P-values for the differential expressions (study GSE8021 in Table 2) showed that none of them had P<0·05, which is usually a standard threshold of significance. Still, five out of six genes displayed the same direction as well as magnitude of change when compared with the second gene expression study (GSE9102 in Table 2), which is a significant correlation (r=0·91, P=0·013), see Fig 3(b).
Discussion
This study demonstrates that lung transplant recipients manifest widespread IgG and IgM autoantibody reactivity, and that specific patterns of reactivity to self-antigens discriminate between patients with and without PGD.
It has been speculated that PGD may induce or accelerate chronic rejection in the form BOS, although conflicting results have been published.2 We observed no significant correlation between BOS and PGD grades among the 39 patients included in this study (Table 1). However, six (35%) out of the 17 informative proteins were also observed to be informative with respect to BOS.8 A two-factor analysis of variance including both BOS and PGD as factors in general confirms the significant differential reactivity with respect to both factors (Table 3 and Fig. S2). The association of these six proteins with both BOS and PGD could suggest a possible immunological link between early graft injury and later chronic rejection, as has been proposed by others.19
We extended the biological meaning of the profile of autoreactive proteins by integrating information about interactions between the proteins as well as their functional roles. Indeed, out of the 17 proteins identified, 12 proteins could be organized in a network with a distinct biological profile involved in regulation of development and cellular communication (Fig. 1), both of which play a role in coordinating cellular proliferation. Comparing with expression levels in donor lungs as measured in two already published studies9,10 for the genes encoding 15 of the 17 proteins, we observed significant positive correlation with autoreactivity changes in the recipients. This correlation was observed even though the gene expressions and autoreactivity were measured in different patient cohorts.
The interpretation of these correlated molecular events with respect to PGD is not straightforward. Downstream signalling from both EGFR and IGF1R, which are central components in the protein network in Fig. 1, typically includes activation of the mitogen-activated protein kinase cascade and subsequent transcriptional activation of immediate-early genes such as the activating protein 1 (AP-1) transcription factor subunits FOS and JUN.20 Indeed, AP-1 is known to regulate processes such as proliferation and transformation, which meshes well with the biological profile of the identified proteins (Fig. 1 and Table 2). Interrogation of FOS and JUN gene expression in the GSE8021 study showed that FOS displays almost two-fold lower expression and JUN 1.2-fold lower expression in donor lungs that later developed PGD compared with those that did not (both with P<0·05).
In clinical studies with lung biopsies, PGD has been associated with acute alveolar damage early and fibrosis later, leading to reduced lung volumes.21 The fibrotic response in inflamed airways most probably manifests itself in part by increased airway epithelial cell proliferation rates.22 We hypothesize that such aberrant proliferation may in part be caused by growth-factor-mediated, proliferative signalling in the donor lung not in balance with the surrounding tissues and organs in the recipient, inferred by the differences in gene expression that correlate with altered autoreactivity against the encoded proteins.
The link between donor transcript levels and recipient autoantibody repertoires reported here is supported by significant statistical results on four biological levels: at the level of autoreactive protein selection, at the level of network size and biological process over-representation, at the level of classification accuracy in an independent validation cohort of nine patients, and at the level of correlation with gene expression changes in two other independent patient cohorts of 50 and 26 patients, respectively.9,10 Even random selections of 17 proteins out of the 273 present on the antigen microarray, not requiring significant differential reactivity, network size, or discriminatory power, only achieves equal or higher correlation with gene expression changes compared with that achieved by the 17 proteins reported in this study (r≥0·63) in 16 out of 1000 attempts (P=0·016), confirming its significance.
Nevertheless, our analysis is focused on hypothesis-generation, hence it is speculative in its attempt to integrate disparate observed molecular events to elucidate PGD pathogenesis. Also, this study has several limitations to its methodology, which must be addressed in the future. The antigen microarray used only screened a small fraction of all the proteins constituting the lung proteome, perhaps as few as 1%. Furthermore, this analysis gives no information about time-sequence causality of suggested processes involved. Prospective follow-up studies are needed to confirm our findings, as well as to elucidate how the reactive proteins as well as their down-stream components behave functionally over time in respect to the pathogenesis of PGD.
Acknowledgments
The authors wish to thank Dr Noam Shental for advice on statistical design and analysis and Yoni Boxman for support and advice on scientific issues. The work of P.H.H. was supported by a grant from the Lundbeck Foundation. The work of E.D. was partially supported by a grant from the Leir Charitable Foundation.
Disclosures
The authors have no financial conflicts of interest.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Figure S1. Concordance between IgG and IgM reactivity changes.
Figure S2. Distributions of autoreactivities including both bronchiolitis obliterans syndrome and primary graft dysfunction status.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Christie JD, Carby M, Bag R, Corris P, Hertz M, Weill D. Report of the ISHLT working group on primary lung graft dysfunction part II: definition. A consensus statement of the international society for heart and lung transplantation. J Heart Lung Transplant. 2005;24:1454–9. doi: 10.1016/j.healun.2004.11.049. [DOI] [PubMed] [Google Scholar]
- 2.Arcasoy SM, Fisher A, Hachem RR, Scavuzzo M, Ware LB, ISHLT Working Group on Primary Lung Graft Dysfunction Report of the ISHLT working group on primary lung graft dysfunction part V: predictors and outcomes. J Heart Lung Transplant. 2005;24:1483–8. doi: 10.1016/j.healun.2004.11.314. [DOI] [PubMed] [Google Scholar]
- 3.Lee JC, Christie JD. Primary graft dysfunction. Proc Am Thorac Soc. 2009;6:39–46. doi: 10.1513/pats.200808-082GO. [DOI] [PubMed] [Google Scholar]
- 4.Pelaez A, Force SD, Gal AA, et al. Receptor for advanced glycation end products in donor lungs is associated with primary graft dysfunction after lung transplantation. Am J Transplant. 2010;10:900–7. doi: 10.1111/j.1600-6143.2009.02995.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salama M, Andrukhova O, Hoda MA, Tagehevi S, Jaksch P, Heinze G, Klepetko W, Aharinejad S. Concomitant endothelin-1 overexpression in lung transplant donors and recipients predicts primary graft dysfunction. Am J Transplant. 2010;10:628–36. doi: 10.1111/j.1600-6143.2009.02957.x. [DOI] [PubMed] [Google Scholar]
- 6.Bobadilla JL, Love RB, Jankowska-Gan E, et al. Th-17, monokines, collagen type V, and primary graft dysfunction in lung transplantation. Am J Respir Crit Care Med. 2008;177:660–8. doi: 10.1164/rccm.200612-1901OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sumpter TL, Wilkes DS. Role of autoimmunity in organ allograft rejection: a focus on immunity to type V collagen in the pathogenesis of lung transplant rejection. Am J Physiol Lung Cell Mol Physiol. 2004;286:L1129–39. doi: 10.1152/ajplung.00330.2003. [DOI] [PubMed] [Google Scholar]
- 8.Hagedorn PH, Burton CM, Carlsen J, et al. Chronic rejection of a lung transplant is characterized by a profile of specific autoantibodies. Immunology. 2010;130:427–35. doi: 10.1111/j.1365-2567.2010.03246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ray M, Dharmarajan S, Freudenberg J, Zhang W, Patterson GA. Expression profiling of human donor lungs to understand primary graft dysfunction after lung transplantation. Am J Transplant. 2007;7:2396–405. doi: 10.1111/j.1600-6143.2007.01918.x. [DOI] [PubMed] [Google Scholar]
- 10.Anraku M, Cameron MJ, Waddell TK, et al. Impact of human donor lung gene expression profiles on survival after lung transplantation: a case–control study. Am J Transplant. 2008;8:2140–8. doi: 10.1111/j.1600-6143.2008.02354.x. [DOI] [PubMed] [Google Scholar]
- 11.Cohen IR. The cognitive paradigm and the immunological homunculus. Immunol Today. 1992;13:490–4. doi: 10.1016/0167-5699(92)90024-2. [DOI] [PubMed] [Google Scholar]
- 12.Burton CM, Milman N, Carlsen J, Arendrup H, Eliasen K, Andersen CB, Iversen M. Survival after single lung, double lung, and heart lung transplantation. J Heart Lung Transplant. 2005;24:1834–43. doi: 10.1016/j.healun.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 13.Barrett T, Troup DB, Wilhite SE, et al. NCBI GEO: mining tens of millions of expression profiles – database and tools update. Nucleic Acids Res. 2007;35:D760–5. doi: 10.1093/nar/gkl887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dai M, Wang P, Boyd AD, et al. Evolving gene/transcript definitions significantly alter the interpretation of GeneChip data. Nucleic Acids Res. 2005;33:e175. doi: 10.1093/nar/gni179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Irizarry RA, Bolstad BM, Collin F, Cope LM, Hobbs B, Speed TP. Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res. 2003;31:e15. doi: 10.1093/nar/gng015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B. 1995;57:289–300. [Google Scholar]
- 17.Lage K, Karlberg E, Størling Z, et al. A human phenome–interactome network of protein complexes implicated in genetic disorders. Nat Biotechnol. 2007;25:309–16. doi: 10.1038/nbt1295. [DOI] [PubMed] [Google Scholar]
- 18.Hastie T, Tibshirani R, Friedman J. Springer Series in Statistics. New York: Springer Verlag; 2001. The elements of statistical learning. [Google Scholar]
- 19.Bharat A, Kuo E, Steward N, et al. Immunological link between primary graft dysfunction and chronic lung allograft rejection. Ann Thorac Surg. 2008;86:189–95. doi: 10.1016/j.athoracsur.2008.03.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hess J, Angel P, Schorpp-Kistner M. AP-1 subunits: quarrel and harmony among siblings. J Cell Sci. 2004;117:5965–73. doi: 10.1242/jcs.01589. [DOI] [PubMed] [Google Scholar]
- 21.Burton CM, Iversen M, Milman N, Zemtsovski M, Carlsen J, Steinbrüchel D, Mortensen J, Andersen CB. Outcome of lung transplanted patients with primary graft dysfunction. Eur J Cardiothorac Surg. 2007;31:75–82. doi: 10.1016/j.ejcts.2006.10.024. [DOI] [PubMed] [Google Scholar]
- 22.Leigh MW, Kylander JE, Yankaskas JR, Boucher RC. Cell proliferation in bronchial epithelium and submucosal glands of cystic fibrosis patients. Am J Respir Cell Mol Biol. 1995;12:605–12. doi: 10.1165/ajrcmb.12.6.7766425. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


