Abstract
Previous studies have demonstrated that molecules of the Ras signaling pathway are present in intracellular compartments, including early endosomes, the endoplasmic reticulum (ER), and the Golgi, and suggested that mitogens can regulate Ras activity in these endomembranes. In this study, we investigated the effect of angiotensin II (AngII) on intracellular Ras activity in living HEK293 cells expressing angiotensin type 1 receptors (AT1-Rs) using newly developed bioluminescence resonance energy transfer biosensors. To investigate the subcellular localization of AngII-induced Ras activation, we targeted our probes to various intracellular compartments, such as the trans-Golgi network (TGN), the ER, and early endosomes. Using these biosensors, we detected AngII-induced Ras activation in the TGN and ER, but not in early endosomes. In cells expressing a cytoplasmic tail deletion AT1-R mutant, the AngII-induced response was enhanced, suggesting that receptor internalization and β-arrestin binding are not required for AngII-induced Ras activation in endomembranes. Although we were able to demonstrate EGF-induced Ras activation in the plasma membrane and TGN, but not in other endomembranes, AG1478, an EGF receptor inhibitor, did not affect the AngII-induced response, suggesting that the latter is independent of EGF receptor transactivation. AngII was unable to stimulate Ras activity in the studied compartments in cells expressing a G protein coupling-deficient AT1-R mutant (125DRY127 to 125AAY127). These data suggest that AngII can stimulate Ras activity in the TGN and ER with a G protein-dependent mechanism, which does not require β-arrestin-mediated signaling, receptor internalization, and EGF receptor transactivation.
Keywords: Endoplasmic Reticulum (ER), Endosomes, G Protein-coupled Receptors (GPCRs), G Proteins, Golgi, Growth Factors, Signal Transduction, Angiotensin II, Arrestin, Ras Signaling
Introduction
The small G proteins, such as Ras, Rho, and Rac, are central players of many signal transduction pathways that regulate a wide variety of cell functions. The small G proteins are monomeric GTPases, serve as molecular switches, and regulate a wide variety of functions, such as gene expression, cell proliferation, cytoskeleton dynamics, the cell cycle, and vesicular transport (1). The >100 mammalian small G proteins are classified into several families, including the Ras and Rho families. Generally, their GDP-bound form is inactive, and upon stimulatory effect, GDP will be changed to GTP, and this activated form will interact with downstream signaling partners. GDP/GTP conversion is stimulated by a guanine nucleotide exchange factor, whereas small G protein inactivation is facilitated by GTPase-activating proteins (2, 3). Small G proteins are active in association with a cellular membrane, and according to the well established model, an extracellular stimulus causes the activation of the Ras and Rho proteins via plasma membrane receptors (3). Activated Ras proteins activate Raf protein kinases, leading to MAPK pathway signalization (4). Raf proteins are the main and direct effectors of Ras proteins, and activated Raf kinases stimulate MEKs.
Membrane localization on the cytoplasmic leaflet of cellular membranes is mediated by post-translational modifications with a lipid moiety on their C-terminal tail. The C-terminal hypervariable regions of Ras isoforms contain the motifs for different plasma membrane signaling nanocluster associations. K-Ras localizes in a cholesterol-independent nanocluster with the help of its polybasic domain, which associates electrostatically with plasma membrane lipids. This association can be disrupted by PKC phosphorylation of Ras, which also causes mitochondrial translocation (5, 6). Recent studies have shown that Ras isoforms can also associate with different cellular membranes (7). It has been shown that targeting of Ras proteins to the plasma membrane is preceded by their localization to endomembranes, such as the endoplasmic reticulum (ER)3 and Golgi (8). Early studies also demonstrated that elements of Ras signaling can be detected in endosomes (9) and have an important role in the MAPK signaling of growth factor receptors, including the EGF receptor (10–12). Furthermore, it was shown that internalized growth factor receptors can selectively activate endosomal H-Ras (but not K-Ras) signaling (13). Although it is generally accepted that the Ras signal generation predominantly occurs at the plasma membrane (14), it seems that the plasma membrane is not the sole platform for Ras signal generation (15). In addition to the well established role of the endosomal compartment in growth factor receptor signaling, recent data suggest that Ras signaling in other endomembranes may also have physiological roles. For example, a recent study proposed that Ras signaling in the Golgi has an effect on thymocyte selection (16). The demonstration of the presence of various elements of Ras signaling in intracellular compartments, including the Golgi and ER, raises the possibility that Ras signal generation can also occur in these compartments (7).
Ras also can be activated by G protein-coupled receptors (GPCRs). Angiotensin II (AngII) is an octapeptide hormone that is the main effector of the renin-angiotensin system and mediates its physiological and pathological effects. AngII can bind and activate two types of angiotensin receptors (angiotensin type 1 and 2 receptors). The angiotensin type 1 receptor (AT1-R) is a typical seven-transmembrane GPCR, and the G protein-mediated “classical” signaling mechanisms are responsible for the majority of AngII-evoked cellular responses. These signaling mechanisms have a wide spectrum, including second messenger generation (Ca2+ signal via inositol 1,4,5-trisphosphate and diacylglycerol), activation of small G proteins and cytoplasmic tyrosine kinases, regulation of ion channels, and transactivation of growth factor receptors. After binding to the AT1-R, a heterotrimeric G protein (Gq) mediates the hydrolysis of phosphatidylinositol 4,5-bisphosphate by phosphoinositide-specific phospholipase Cβ, resulting in the generation of second messengers (17). It is well established that extracellular stimulus of the AT1-R can lead to activation of various small G proteins, including Ras, either through transactivation of a tyrosine kinase receptor (mainly via the EGF receptor) or through transactivation independently (18–20). Not only does endocytosis of GPCRs regulate the activity of plasma membrane receptors (21, 22), but it can also initiate signaling pathways, such as MAPK activation (23). In this study, we investigated whether agonist stimulation of a GPCR, the AT1-R, can lead to intracellular Ras activation and compared these responses with those of the EGF receptor.
Recently, a wide range of FRET-based approaches were used to examine Ras and other signal transduction mechanisms (24, 25). To investigate Ras activation in various locations inside cells upon agonist stimulation of the AT1-R, we have developed several intra- and intermolecular probes for bioluminescence resonance energy transfer (BRET) measurements in living cell experiments based on previous FRET-based studies (26, 27). We show that Ras signal generation can be detected not only in the plasma membrane but also in intracellular compartments, such as the trans-Golgi and ER, in response to AngII stimulation.
EXPERIMENTAL PROCEDURES
Materials
Molecular biology enzymes were obtained from Fermentas (Burlington, Canada), Stratagene (La Jolla, CA), and Invitrogen. Cell culture dishes and plates for BRET measurements were purchased from Geiner Bio-One GmbH (Kremsmunster, Austria). Lipofectamine 2000 and coelenterazine h were from Invitrogen. Unless stated otherwise, all other chemicals and reagents were purchased from Sigma. HEK293 cells were from American Type Culture Collection (Manassas, VA).
Molecular Biology
For the construction of our intramolecular BRET probes, we followed the design of the Raichu (Ras and interacting protein chimeric unit) FRET probes from the Matsuda laboratory (26, 27). The cDNA parts of the constructs were generated by amplification either from a respective expressed sequence tag clone (German Resource Center for Genome Research (RZPD), Berlin, Germany) or from H295R human cell line cDNA by PCR. For the intramolecular BRET probes, the order of the domains was similar to that in the Raichu probes (26). We used the pEYFP-C1 plasmid backbone (Clontech, Mountain View, CA), and the fluorescent acceptor protein (YFP) was followed by H-Ras, the Ras-binding domain (RBD) of Raf-1, and luciferase (see Fig. 1 for schematic representation). We have previously shown that the Raf-1 RBD (amino acids 51–131) is able to recognize activated Ras but is not capable of being recruited to the plasma membrane (28), which makes this RBD suitable for utilizing in targeted biosensors. We used Renilla luciferase as the energy donor by subcloning the codon-humanized Renilla luciferase from pRluc-N2 (PerkinElmer Life Sciences). The intermolecular BRET probes were derived from their respective intramolecular probes by splitting them between Ras and the RBD of Raf-1 by adding suitable start and stop codon sequences. Targeting was achieved by placing a targeting signal at either the N- or C-terminal end of the probe. We used several previously described targeting motifs to target our BRET probes (29). Targeting to the plasma membrane was achieved using various distinct tags. We fused CAAX motifs from Ras proteins C-terminally as described (26, 30). Briefly, the tK tag (K-Ras CAAX targeting motif, tail K-Ras), KMSKDGKKKKKKSKTKCVIM, consists of the membrane-targeting CAAX motif and hypervariable regions of K-Ras (GenBank accession number NM_004985). The tHL tag (tail H-Ras long, KLNPPDESGPGCMSCKCVLS) consists of the membrane-targeting CAAX motif and hypervariable regions of H-Ras (accession number NM_005343), whereas the tHS tag (tail H-Ras short, CMSCKCVLS) consists of the membrane-targeting CAAX motif but without the hypervariable regions of H-Ras. The MP (MyrPalm) and PP (PalmPalm) tags targeting to plasma membrane rafts were based on the design of Zacharias et al. (31), where the MP tag is the N-terminal amino acid sequence (MGCIKSKRKDNLNDDE) from Lyn kinase (accession number NM_002350), and the PP tag is the N-terminal amino acid sequence (MLCCMRRTKQ) from GAP43 (accession number NM_002045) (31). Targeting to the Golgi surface was achieved by fusing the full-length trans-Golgi network protein 2 (also known as TGN38; accession number NM_006464) (32), and targeting to the ER surface was achieved by adding segment 233–250 of the yeast Ubc6 protein (accession number NM_001178991) or the C-terminal segment 521–587 of the Sac1 phosphoinositide phosphatase (accession number NM_014016) (29, 33). Endosomal targeting was done by fusing the probes C-terminally with the FYVE domain (segment 1253–1411) of EEA1 (early endosome antigen 1; accession number NM_3566). Mutagenesis was performed using standard mutagenesis conditions. After verifying the mutations with dideoxy sequencing, the mutated fragment was exchanged between the wild-type and mutated portions with suitable restriction sites to avoid the generation of unwanted mutations outside the sequenced regions. pcDNA3.1-AT1A-R, pcDNA3.1-AT1A-RΔ319 (with Tyr319 substituted with a stop codon), and pcDNA3.1-DRY/AAY-AT1A-R (with the highly conserved 125DRY127 sequence mutated to 125AAY127) were described previously (34–36).
FIGURE 1.
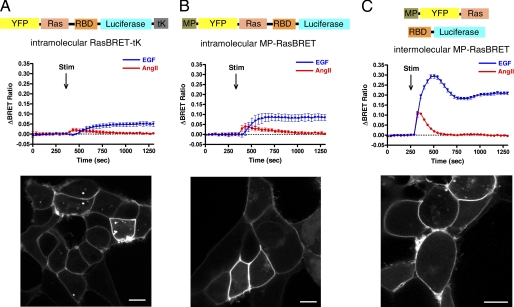
Ras activation upon AngII and EGF stimulation in HEK293 cells. A, intramolecular RasBRET-tK; B, intramolecular MP-RasBRET; C, intermolecular MP-RasBRET. Upper rows, schematic representation of the arrangement of the RasBRET probes. Middle rows, BRET assay of plasma membrane-targeted RasBRET probes. HEK293 cells were transfected with the plasmids of the indicated probes and with the AT1A-R, and after 24 h, the serum-starved cells were exposed to 50 ng/ml EGF (blue traces), 100 nm AngII (red traces), or vehicle (dotted lines) at the indicated time points. The BRET records are the average of at least three independent experiments. The means ± S.E. are shown (n = 3). Lower rows, the representative confocal micrographs show the localization and cellular distribution of the indicated probes in HEK293 cells. YFP fluorescence was detected using a Zeiss LSM 510 confocal microscope. Stim, stimulation. Scale bars = 10 μm.
Cell Culture and Transfection
The experiments were performed on the HEK293 cell line. The cells were cultured in DMEM with penicillin/streptomycin (Invitrogen) and 10% heat-inactivated FBS in 5% CO2 at 37 °C. The cells were cultured in plastic dishes, trypsinized prior to transfection, and transiently transfected using Lipofectamine 2000 (Invitrogen), and plated on polylysine-pretreated white 96-well plates at a density of 1 × 105 cells/well for BRET measurements. The DNA amount was 0.2–0.4 μg/well; the amount of Lipofectamine 2000 was 0.5 μl/well.
BRET Measurements
We monitored the molecular interactions of either two parts of a biosensor (intramolecular probes) or two proteins (intermolecular probes) by BRET measurements. The measurements were performed after 24 h of transfection on white 96-well plates. Before BRET measurements, the HEK293 cells were switched to serum-free medium (DMEM with 0.1% BSA) for several hours (4–6 h) to render the cells quiescent. The medium of the cells was changed prior to measurements to a modified Krebs-Ringer buffer containing 120 mm NaCl, 4.7 mm KCl, 1.8 mm CaCl2, 0.7 mm MgSO4, 10 mm glucose, and 10 mm NaHEPES (pH 7.4), and the BRET measurements were performed at 37 °C. The BRET measurements were started after the addition of the cell-permeable substrate coelenterazine at a final concentration of 5 μm, and the counts were recorded using a Berthold Mithras LB 940 multilabel reader, which allows for the detection of signals using filters at 485- and 530-nm wavelengths. The detection time was 0.25–0.5 s. The BRET ratios were calculated as the 530/485 nm ratio. The measurements were done in triplicate. The BRET records are the average of at least three independent experiments. BRET ratios were base line-corrected to the vehicle curve using GraphPad Prism software. The approximate BRET ratio of the YFP-deficient intramolecular RasBRET-tK probe was ∼0.8 (data not shown).
Confocal Microscopy
The localization and distribution of the targeted probes were analyzed using a Zeiss LSM 510 confocal laser scanning microscope in living cells plated on polylysine-pretreated glass coverslips (3 × 105 cells/35-mm dish).
RESULTS
Generation and Optimization of RasBRET Probes to Measure Ras Activation upon AngII Stimulation in HEK293 cells
To study Ras activation after AngII or EGF stimulation in intracellular compartments, we first established a BRET-based Ras activity measurement using newly developed probes. For the detection of Ras activation, HEK293 cells were chosen for our studies because this cell line is broadly used for angiotensin receptor studies (20, 23, 37). Moreover, this cell type has endogenous EGF receptors, and Ras activation upon EGF treatment could be detected using BRET measurements. Stimulation of endogenous EGF receptors by EGF yielded a robust and prolonged response in Ras activation as assessed in the plasma membrane by the intramolecular RasBRET-tK probe (see details under “Experimental Procedures”) (Fig. 1A), which was designed as the BRET version of the Raichu-Ras fusion protein (26). The probes indicate when the respective Ras becomes loaded with GTP because the RBD of Raf-1 increases its affinity dramatically for Ras upon GTP loading. This results in conformational changes that bring the energy acceptor close to the donor, as was shown by the developers of the corresponding FRET probes (38). Compared with EGF treatment, Ras activation upon AngII stimulation resulted in a transient and smaller response in HEK293 cells (Fig. 1A).
To optimize the probe, tags were used to target it to different plasma membrane microdomains, including non-raft domains (tK), caveolae/rafts (tHS and tHL) (39), and plasma membrane raft domains (MP and PP) (31). The localization and distribution of the targeted probes in HEK293 cells were verified by confocal microscopy (Fig. 1, lower panels). Among these targeting sequences, the MP-targeted version of the intramolecular probe yielded the most sensitive probe (Fig. 1B) (data not shown for the others). To further improve the sensitivity of the detection of Ras activation in the plasma membrane, an intermolecular variant of the MP-targeted RasBRET probe (the energy donor and acceptor are in different polypeptide chains; MP-YFP-Ras + RBD-luciferase) (see “Experimental Procedures” and Fig. 1C) was constructed. Fig. 1C shows that this setup yielded a greatly improved and very sensitive probe for measuring Ras activation. The higher signal-to-noise ratio of the intermolecular biosensor can be explained by the high basal BRET signal of the intramolecular probes (∼1.4 versus ∼1.1) because, in these constructs, the donor and acceptor are in the same molecule. The cytosolic (spatially unbiased) intermolecular plasmid pair for RasBRET (non-targeted YFP-Ras plus RBD-luciferase) had the lowest absolute level of the BRET signal (∼0.9).
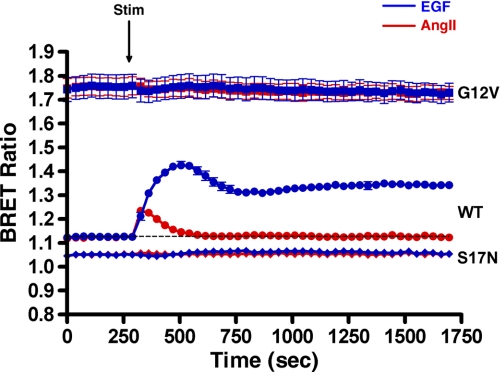
We validated that the change in the BRET signal using the intermolecular MP-RasBRET probe in our experimental protocol is dependent on the activation state of Ras (hence, the interaction of Ras and the Raf RBD) by performing measurements with mutated Ras domains in the probe. Fig. 2 shows that, by utilizing the “active” form of the intermolecular MP-RasBRET probe pair (G12V), we detected the maximal absolute level of the BRET signal. In contrast, when we applied the “inactive” Ras S17N mutation, the extent of the BRET signal was lower than the base line of the wild-type probe, showing that Ras was partially active in control cells. Because the optimization of BRET biosensors showed that the intermolecular MP-RasBRET probe resulted in the best Ras activation biosensors, we used the intermolecular BRET approach in our later work, and therefore, all subsequent data represent results using intermolecular BRET biosensors.
FIGURE 2.
Validation of the BRET measurement approach for agonist-stimulated Ras activation by mutated MP-RasBRET probe pairs. HEK293 cells were transfected with intermolecular RasBRET pairs. The wild-type (●), active G12V (■), and inactive S17N (♦) MP-RasBRET probes are shown. HEK293 cells were transfected with the plasmids of the indicated probes and with the AT1A-R, and after 24 h, the serum-starved cells were exposed to 50 ng/ml EGF (blue trace), 100 nm AngII (red trace), or vehicle (dashed line) at the indicated time points. The BRET records are an average of at least three independent experiments. The means ± S.E. are shown (n = 3). Stim, stimulation.
Ras Activation in the Plasma Membrane after AngII Stimulation of Different AT1-R Mutants
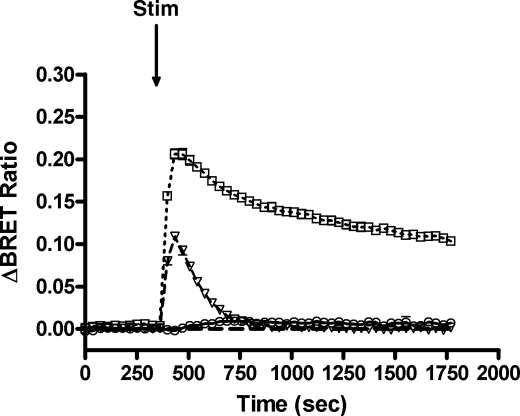
After optimization and validation of our BRET strategy, experiments were performed with RasBRET probes in HEK293 cells cotransfected with the wild-type AT1A-R, the tail deletion AT1A-RΔ319 mutant (which is unable to internalize) (40), or the AT1-R-DRY/AAY mutant (which is unable to couple to Gαq and initiate inositol phosphate signaling) (35, 37). As shown in Fig. 3, AngII stimulation of the wild-type AT1-R caused transient Ras activation in the plasma membrane, which was rapidly terminated because of the receptor desensitization and internalization. However, when the desensitization or internalization was reduced in cells expressing the AT1A-RΔ319 mutant, Ras activation was enhanced and prolonged. We did not detect significant Ras activation when utilizing the AT1-R-DRY/AAY mutant, which suggests that the AngII-induced Ras activation is predominantly G protein-mediated.
FIGURE 3.
Ras activation upon agonist stimulation of different AT1-R mutants. HEK293 cells were cotransfected with intermolecular MP-RasBRET and the wild-type AT1A-R, AT1A-RΔ319, or AT1A-R-DRY/AAY plasmid. The serum-starved cells were exposed to 100 nm AngII (open triangles, dashed line for the wild-type AT1A-R, open rectangles, dotted line for AT1A-RΔ319, and open circles for AT1A-R-DRY/AAY) or vehicle (dashed line) at the indicated time points. The BRET records are an average of at least three independent experiments. The means ± S.E. are shown (n = 3). Stim, stimulated.
Subcellular Localization of Ras Activation
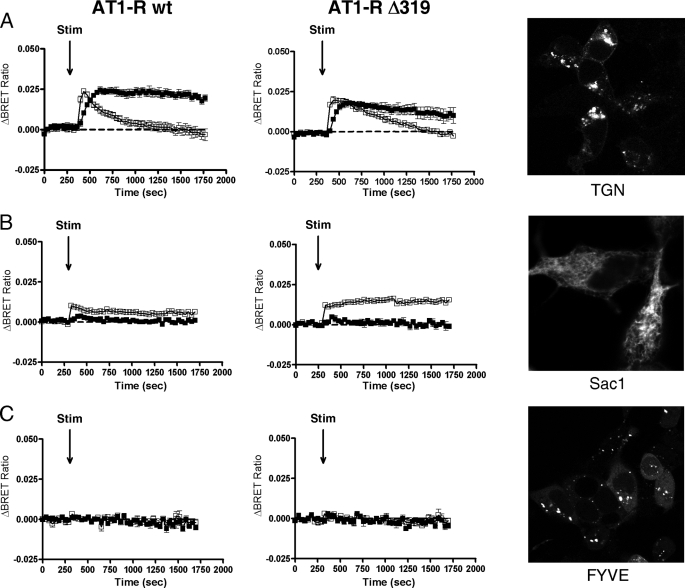
In the next set of experiments, the effect of extracellular stimuli on intracellular Ras activation was examined. To investigate specifically the possible compartmentalized Ras signaling processes, we targeted our probes to the surface of intracellular organelles using previously described targeting motifs (29). These motifs were placed at either the N or C terminus of the respective probes as described under “Experimental Procedures.” We used intermolecular probe pairs in which one of the interacting partners (either YFP-Ras or RBD-luciferase) was tagged with the targeting sequence while the other polypeptide was not targeted to organelles and was therefore expressed in the cytosol. The micrographs (confocal microscopic images) in Fig. 4 demonstrate that all probes were properly expressed in the targeted compartment. HEK293 cells were cotransfected with the AT1A-R (wild-type AT1A-R, AT1A-RΔ319 or AT1A-R-DRY/AAY) and with the indicated intermolecular probes targeted to various compartments, such as the trans-Golgi network (TGN), the ER, and endosomes.
FIGURE 4.
Ras activation in intracellular organelles. HEK293 cells were cotransfected with the indicated intermolecular RasBRET probes along with either the wild-type AT1A-R (left panels) or AT1A-RΔ319 (middle panels) plasmid. HEK293 cells were exposed to 50 ng/ml EGF (filled symbols), 100 nm AngII (open symbols), or vehicle (dashed lines) at the indicated time points. The BRET records are an average of at least three independent experiments, each performed in triplicate. The means ± S.E. are shown (n = 3). A–C, Ras activation in the TGN, the ER, and endosomes, respectively. The representative confocal micrographs (right panels) show the localization and cellular distribution of the various probes in HEK293 cells that were transfected with the plasmids of the indicated probes. YFP fluorescence was detected using a Zeiss LSM 510 confocal microscope. Because the endosome- and ER-targeting sequences are located at the C terminus of RBD-luciferase, the micrographs for endosomes and the ER show the cellular distribution of the intramolecular versions of RasBRET-Sac1 and RasBRET-FYVE, respectively. Stim, stimulated.
EGF- and AngII-induced Ras activation was detected in the Golgi using YFP-Ras targeted to the TGN (the targeting sequence was fused to the N terminus of YFP-Ras, and RBD-luciferase was cytosolic) (Fig. 4A). AngII caused a rapid and transient Ras activation in the TGN (Fig. 4A). In contrast to the Ras activation in the plasma membrane, where AngII stimulation resulted in a much smaller response compared with EGF-evoked activation, the changes in the BRET ratios in the TGN were quite similar for EGF and AngII, suggesting that the relative Ras activation efficacy of AngII may be higher than that of EGF in this compartment. It is also important to note that the response to AngII consistently occurred more rapidly then the EGF-induced response. To assess the compartmentalized Ras signaling upon agonist stimulation, we compared the data obtained using the wild-type AT1-R and an internalization-deficient AT1-R mutant (AT1A-RΔ319) coexpressed with various BRET probes in HEK293 cells. Our data show that the AngII-induced Ras activation in the TGN is not a consequence of receptor internalization because the internalization-deficient AT1-R mutant showed prolonged, rather than inhibited, Ras activation compared with the wild-type AT1-R (Fig. 4A).
We also detected Ras activation on the surface of the ER using both Ubc6- and Sac1- targeted intermolecular probes. In these constructs, the ER-targeting sequences were fused to the C terminus of the RBD-luciferase part while the YFP-Ras part was cytosolic. Interestingly, only AngII stimulation activated Ras in the ER, whereas EGF was not able to show this effect (Fig. 4B). The kinetics and characteristics of the AngII-induced Ras activation in the ER were indistinguishable when Sac1- or Ubc6-targeting sequences were used (Fig. 4B; only the results with the Sac1-targeted probe are shown). These data also suggest that the AngII-induced response is independent of EGF receptor transactivation. Similar to Ras activation detected in the TGN, the AngII-induced Ras activation was more sustained in the ER in cells expressing the internalization-deficient AT1A-RΔ319 mutant compared with the wild-type receptor.
Although EGF receptor activation in endosomes was detected by resonance energy transfer measurements in previous studies (11), our RasBRET probe targeted with the FYVE domain to endosomes (the targeting sequence was fused to the C terminus of RBD-luciferase while YFP-Ras is cytosolic) was not able to detect EGF- or AngII-induced Ras activation in endosomes (Fig. 4C). Based on these data, it is possible that endosomal Ras activation occurs away from the targeted phosphatidylinositol 3-phosphate-containing microdomain of this organelle.
Mechanism of AngII-induced Ras Activation
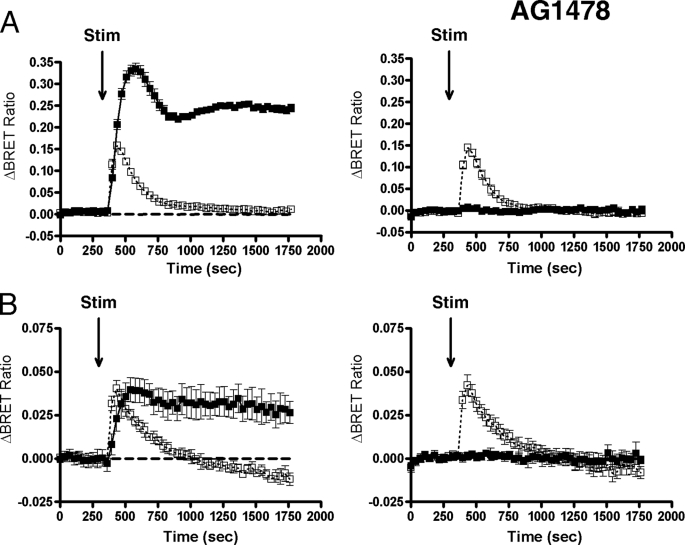
In the cells of the cardiovascular system, AngII-induced Ras activation (and hence, MAPK activation) is mostly the consequence of the transactivation of receptor tyrosine kinases, such as the EGF receptor. However, in some other cell types, AngII-induced ERK activation is independent of EGF receptor transactivation (17). It has been demonstrated that phosphorylation of ERK1/2 upon AngII stimulation in HEK293 cells, but not in C9 hepatic cells, is EGF receptor transactivation-independent, but Ras activation was not investigated (20). In our experiments using HEK293 cells, the EGF receptor kinase inhibitor AG1478 abolished the EGF-evoked Ras activation in the plasma membrane, but it had no inhibitory effect on the AngII-induced Ras activation in the plasma membrane (Fig. 5A). The AngII-induced Ras activation in the TGN was not affected (Fig. 5 B). These data suggest that Ras activation in HEK293 cells is primarily independent of EGF receptor transactivation but requires G protein coupling because AngII stimulation of cells expressing the AT1-R-DRY/AAY mutant failed to initiate Ras signaling in the plasma membrane (Fig. 3) and in the intracellular organelles studied (data not shown).
FIGURE 5.
Mechanism of Ras activation upon AngII stimulation is independent of EGF receptor transactivation in HEK293 cells. HEK293 cells were transfected with the AT1A-R and with the plasmid pair of the intermolecular MP-RasBRET (A) or TGN-RasBRET (B), and after 24 h, the serum-starved cells were pretreated for 30 min prior to BRET measurement either with vehicle (left panels) or with 1 μm AG1478 (right panels). After the pretreatment, the cells were exposed to 50 ng/ml EGF (filled symbols), 100 nm AngII (open symbols, dotted lines), or vehicle (dashed traces) at the indicated time points. The BRET records are an average of at least three independent experiments, each performed in triplicate. The means ± S.E. are shown (n = 3). Stim, stimulated.
DISCUSSION
Small G proteins are central players in many signal transduction pathways and regulate a wide variety of cell functions. It is well known that stimulation of GPCRs, including the AT1-R, can lead to activation of various small G proteins, such as Ras (1, 17). Although it is generally believed that the signal generation of GPCRs occurs at the plasma membrane, the presence of small G proteins in intracellular organelles has already been demonstrated (7), and in this study, we examined whether AngII-induced Ras activation can be detected on the surface of intracellular membranes by targeting biosensors to various compartments. BRET and FRET probes report energy transfer from an energy donor (Renilla luciferase in BRET and cyan fluorescent protein in FRET applications) to YFP when Ras becomes loaded with GTP and interacts with its effector during Ras activation. In our studies, we used BRET measurements because BRET has several advantages compared with FRET microscopy. For example, BRET is a more quantitative method because it can be performed on a large population of cells. BRET is also more sensitive compared with FRET because the excitation light causes a high background in FRET measurements. Our BRET probes were targeted to plasma membrane microdomains and intracellular organelles, including the ER and Golgi, using specific targeting sequences to assess the effect of hormonal stimulation on Ras activity in these compartments.
AngII induced a transient Ras activation in the plasma membrane, which was much smaller compared with the EGF-induced response (Fig. 1). This finding is consistent with the fact that EGF is a more effective Ras activator than AngII; however, it is possible that the incomplete transfection efficiency of the angiotensin receptor-containing plasmid also contributed to this finding. Kinetically, EGF receptor activation also produced more prolonged Ras activation compared with the AngII-induced response. However, it is also noteworthy that our initial studies with intramolecular BRET probes showed that the AngII-induced Ras activation in the plasma membrane was more rapid than the EGF-induced response (Fig. 1, A and B).
The sensitivity of intramolecular RasBRET probes was not sufficient to detect Ras activation in intracellular organelles (data not shown). In the intramolecular BRET probes, the energy donor and acceptor are in the same molecule, and their molecular proximity causes high initial (background) levels and a low dynamic range of these biosensors. On the other hand, in intermolecular RasBRET probes, the energy donor and acceptor are in distinct polypeptide chains, which results in a much better signal-to-noise ratio (Fig. 1). In this study, we were able to demonstrate that AngII stimulation not only increased Ras activity in the plasma membrane but also activated it in intracellular compartments, such as the TGN and ER (Fig. 4). Endocytosis of receptors, including both tyrosine kinase receptors and GPCRs, not only regulates their activity but also initiates signaling pathways, such as MAPK activation (23). Although AT1-R- and EGF receptor-generated signaling in endosomes has been reported (11, 41), our endosomal targeted probes were not able to demonstrate Ras signaling on endosomes (Fig. 4C). This fact calls for cautious evaluation of the BRET and FRET measurements because negative results with BRET or FRET biosensors certainly do not report the nonexistence of the investigated events but rather reflect the unsuitable or non-optimal arrangement of interacting partners and energy donors and acceptors of the applied biosensors. It is also possible that the FYVE domain-targeted biosensor and the elements of Ras signaling are localized in distinct microdomains of endosomes, preventing the interactions of these probes.
Mitogen-induced Ras activation in the Golgi has been demonstrated using a Raf-1-based FRET sensor (42). Chiu et al. have shown that rapid and transient plasma membrane Ras activation is followed by a slower and sustained Ras response in the Golgi. A possible explanation for the different kinetics of EGF-induced Ras activation in the Golgi in this report compared with the more rapid response observed in our study is the difference in the targeting strategies because our probe was targeted to the TGN. We also detected AngII-induced rapid and transient Ras activation in the TGN. AngII was a relatively efficient activator of Ras in the TGN because the amplitude of the AngII and EGF responses in the TGN was comparable, whereas EGF caused a much larger response in the plasma membrane. AngII caused a more prolonged activation of Ras in the TGN in cells expressing the internalization-deficient AT1A-RΔ319 mutant, which is consistent with the impaired desensitization of this receptor (43) and argues against the role of receptor endocytosis in AngII-induced Ras activation in the TGN. Similarly, as was shown previously, EGF-induced endomembrane-associated Ras activation is also independent of EGF receptor endocytosis in COS-1 cells (42).
The Ras activation in the ER is surprising in many respects. It is remarkable that only AngII stimulation activated Ras, but EGF receptor stimulation was not able to produce this effect (Fig. 4B). This result provides evidence that the effect of AngII in the ER is independent of EGF receptor transactivation. Although mitogen-induced Ras signaling in the ER has been reported in COS-1 cells (42), we did not observe a major effect of EGF receptor activation on Ras activity in the ER in HEK293 cells (Fig. 4B). Similar to the TGN, AngII caused a more prolonged activation of ER-targeted Ras in cells expressing the internalization-deficient AT1A-RΔ319 mutant, suggesting that the effect of AngII on Ras activation is also internalization-independent in this compartment.
Our data demonstrate that AngII stimulates Ras activity in the Golgi and ER, which raises the question as to the function of Ras activation in these compartments. The most obvious answer is that Ras has multiple and widespread functions, and the compartmentalized signaling can provide the complexity of downstream pathways with different kinetics. Activation of small G proteins in endosomes is not surprising because this cellular membrane compartment is derived from the plasma membrane, and it is possible that the signaling continues after internalization of the plasma membrane receptor (and extracellular ligand) on the cytosolic surface of the endosomes. Activated GPCRs also bind β-arrestin molecules. In the case of many GPCRs, including the AT1-R, β-arrestin binding of the internalized receptor persist in the endosomes. Because β-arrestin can facilitate the activation of signaling complexes, this mechanism can also cause signaling, including activation of MAPKs, on the surface of endosomes (17, 21, 41). Ras activation on the surface of endomembranes, such as the ER and Golgi, is much more surprising because these compartments are not derived from the plasma membrane. Because the presence of agonist-bound receptors in the Golgi and ER is unlikely after a short stimulation of the receptor, it can be assumed that Ras activation in these compartments occurs with a different mechanism compared with the plasma membrane.
The mechanism of AngII-induced Ras activation in the plasma membrane is cell type-dependent (17). In vascular smooth muscle cells and many other cell types, transactivation of EGF receptors plays a major role in the mitogen effects of AngII (17, 44). In our study, AG1478, an EGF receptor kinase inhibitor, eliminated all EGF-induced responses but had no effect on AngII-induced Ras activation in the Golgi and ER, similar to that in the plasma membrane, in HEK293 cells (Fig. 5). This finding is consistent with previous studies that demonstrated that AngII-induced ERK activation and mitogen signaling are independent of EGF receptor transactivation in these cells (20). We did not detect significant AngII-induced Ras activation in cells expressing the AT1-R-DRY/AAY mutant, suggesting that the response, both in the plasma membrane (Fig. 3) and in endomembranes (data not shown), is predominantly G protein-mediated. Considering its rapid timing, it is likely that the effect of AngII on Ras activation in the TGN and ER is mediated by soluble messengers, similar to the previously reported similar effects of growth factors and lysophosphatidic acid (7, 45, 46). In cells expressing the internalization-deficient AT1A-RΔ319 mutant, AngII caused more prolonged Ras activation both in the plasma membrane (Fig. 3) and in endomembranes (Fig. 4), which is probably caused by the impaired desensitization of this mutant receptor (43). These data are also consistent with our conclusion that AngII-induced Ras activation in the Golgi and ER is G protein-mediated and does not require receptor internalization or β-arrestin-mediated signaling.
This study has demonstrated the wide-scale effects of AngII on Ras activation and emphasizes that Ras can signal from distinct compartments of cellular membranes to ensure functional diversity but not redundancy of its signaling. The better understanding of the signaling properties of the AT1-R may provide additional clues to improve the therapeutic potential of drugs that target this receptor.
Acknowledgments
The excellent technical assistance of Judit Bakacsiné Rácz and Mártonné Schultz is greatly appreciated.
This work was supported in part by Hungarian Scientific Research Fund (OTKA) Grant NK72661, Hungarian Ministry of Public Health (ETT) Grant 337/2009, and National Development Agency of Hungary (NFÜ TÁMOP) Grant 4.2.2-08/1/KMR.
- ER
- endoplasmic reticulum
- GPCR
- G protein-coupled receptor
- AngII
- angiotensin II
- AT1-R
- angiotensin type 1 receptor
- BRET
- bioluminescence resonance energy transfer
- RBD
- Ras-binding domain
- TGN
- trans-Golgi network.
REFERENCES
- 1. Takai Y., Sasaki T., Matozaki T. (2001) Physiol. Rev. 81, 153–208 [DOI] [PubMed] [Google Scholar]
- 2. García-Mata R., Burridge K. (2007) Trends Cell Biol. 17, 36–43 [DOI] [PubMed] [Google Scholar]
- 3. Buday L., Downward J. (2008) Biochim. Biophys. Acta 1786, 178–187 [DOI] [PubMed] [Google Scholar]
- 4. Vojtek A. B., Hollenberg S. M., Cooper J. A. (1993) Cell 74, 205–214 [DOI] [PubMed] [Google Scholar]
- 5. Bivona T. G., Quatela S. E., Bodemann B. O., Ahearn I. M., Soskis M. J., Mor A., Miura J., Wiener H. H., Wright L., Saba S. G., Yim D., Fein A., Pérez de Castro I., Li C., Thompson C. B., Cox A. D., Philips M. R. (2006) Mol. Cell 21, 481–493 [DOI] [PubMed] [Google Scholar]
- 6. Plowman S. J., Ariotti N., Goodall A., Parton R. G., Hancock J. F. (2008) Mol. Cell. Biol. 28, 4377–4385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mor A., Philips M. R. (2006) Annu. Rev. Immunol. 24, 771–800 [DOI] [PubMed] [Google Scholar]
- 8. Choy E., Chiu V. K., Silletti J., Feoktistov M., Morimoto T., Michaelson D., Ivanov I. E., Philips M. R. (1999) Cell 98, 69–80 [DOI] [PubMed] [Google Scholar]
- 9. Di Guglielmo G. M., Baass P. C., Ou W. J., Posner B. I., Bergeron J. J. (1994) EMBO J. 13, 4269–4277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang Y., Pennock S., Chen X., Wang Z. (2002) Mol. Cell. Biol. 22, 7279–7290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sorkin A., McClure M., Huang F., Carter R. (2000) Curr. Biol. 10, 1395–1398 [DOI] [PubMed] [Google Scholar]
- 12. Vieira A. V., Lamaze C., Schmid S. L. (1996) Science 274, 2086–2089 [DOI] [PubMed] [Google Scholar]
- 13. Roy S., Wyse B., Hancock J. F. (2002) Mol. Cell. Biol. 22, 5128–5140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Augsten M., Pusch R., Biskup C., Rennert K., Wittig U., Beyer K., Blume A., Wetzker R., Friedrich K., Rubio I. (2006) EMBO Rep. 7, 46–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Plowman S. J., Hancock J. F. (2005) Biochim. Biophys. Acta 1746, 274–283 [DOI] [PubMed] [Google Scholar]
- 16. Daniels M. A., Teixeiro E., Gill J., Hausmann B., Roubaty D., Holmberg K., Werlen G., Holländer G. A., Gascoigne N. R., Palmer E. (2006) Nature 444, 724–729 [DOI] [PubMed] [Google Scholar]
- 17. Hunyady L., Catt K. J. (2006) Mol. Endocrinol. 20, 953–970 [DOI] [PubMed] [Google Scholar]
- 18. Ohtsu H., Suzuki H., Nakashima H., Dhobale S., Frank G. D., Motley E. D., Eguchi S. (2006) Hypertension 48, 534–540 [DOI] [PubMed] [Google Scholar]
- 19. Higuchi S., Ohtsu H., Suzuki H., Shirai H., Frank G. D., Eguchi S. (2007) Clin. Sci. 112, 417–428 [DOI] [PubMed] [Google Scholar]
- 20. Shah B. H., Yesilkaya A., Olivares-Reyes J. A., Chen H. D., Hunyady L., Catt K. J. (2004) Mol. Endocrinol. 18, 2035–2048 [DOI] [PubMed] [Google Scholar]
- 21. Ferguson S. S. (2001) Pharmacol. Rev. 53, 1–24 [PubMed] [Google Scholar]
- 22. Hunyady L. (1999) J. Am. Soc. Nephrol. 10, Suppl. 11, S47–S56 [PubMed] [Google Scholar]
- 23. Daaka Y., Luttrell L. M., Ahn S., Della Rocca G. J., Ferguson S. S., Caron M. G., Lefkowitz R. J. (1998) J. Biol. Chem. 273, 685–688 [DOI] [PubMed] [Google Scholar]
- 24. Bivona T. G., Philips M. R. (2005) Methods 37, 138–145 [DOI] [PubMed] [Google Scholar]
- 25. Kiyokawa E., Hara S., Nakamura T., Matsuda M. (2006) Cancer Sci. 97, 8–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mochizuki N., Yamashita S., Kurokawa K., Ohba Y., Nagai T., Miyawaki A., Matsuda M. (2001) Nature 411, 1065–1068 [DOI] [PubMed] [Google Scholar]
- 27. Nakamura T., Kurokawa K., Kiyokawa E., Matsuda M. (2006) Methods Enzymol. 406, 315–332 [DOI] [PubMed] [Google Scholar]
- 28. Bondeva T., Balla A., Várnai P., Balla T. (2002) Mol. Biol. Cell 13, 2323–2333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Várnai P., Balla T. (2007) Pflugers Arch. 455, 69–82 [DOI] [PubMed] [Google Scholar]
- 30. Fukano T., Sawano A., Ohba Y., Matsuda M., Miyawaki A. (2007) Cell. Struct. Funct. 32, 9–15 [DOI] [PubMed] [Google Scholar]
- 31. Zacharias D. A., Violin J. D., Newton A. C., Tsien R. Y. (2002) Science 296, 913–916 [DOI] [PubMed] [Google Scholar]
- 32. Szentpetery Z., Várnai P., Balla T. (2010) Proc. Natl. Acad. Sci. U.S.A. 107, 8225–8230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Várnai P., Balla A., Hunyady L., Balla T. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 7859–7864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Smith R. D., Baukal A. J., Zolyomi A., Gaborik Z., Hunyady L., Sun L., Zhang M., Chen H. C., Catt K. J. (1998) Mol. Endocrinol. 12, 634–644 [DOI] [PubMed] [Google Scholar]
- 35. Gáborik Z., Jagadeesh G., Zhang M., Spät A., Catt K. J., Hunyady L. (2003) Endocrinology 144, 2220–2228 [DOI] [PubMed] [Google Scholar]
- 36. Gáborik Z., Mihalik B., Jayadev S., Jagadeesh G., Catt K. J., Hunyady L. (1998) FEBS Lett. 428, 147–151 [DOI] [PubMed] [Google Scholar]
- 37. Wei H., Ahn S., Shenoy S. K., Karnik S. S., Hunyady L., Luttrell L. M., Lefkowitz R. J. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 10782–10787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ohba Y., Kurokawa K., Matsuda M. (2003) EMBO J. 22, 859–869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Prior I. A., Harding A., Yan J., Sluimer J., Parton R. G., Hancock J. F. (2001) Nat. Cell Biol. 3, 368–375 [DOI] [PubMed] [Google Scholar]
- 40. Balmforth A. J., Lee A. J., Bajaj B. P., Dickinson C. J., Warburton P., Ball S. G. (1995) Eur. J. Pharmacol. 291, 135–141 [DOI] [PubMed] [Google Scholar]
- 41. Luttrell L. M., Roudabush F. L., Choy E. W., Miller W. E., Field M. E., Pierce K. L., Lefkowitz R. J. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 2449–2454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chiu V. K., Bivona T., Hach A., Sajous J. B., Silletti J., Wiener H., Johnson R. L., 2nd, Cox A. D., Philips M. R. (2002) Nat. Cell Biol. 4, 343–350 [DOI] [PubMed] [Google Scholar]
- 43. Olivares-Reyes J. A., Smith R. D., Hunyady L., Shah B. H., Catt K. J. (2001) J. Biol. Chem. 276, 37761–37768 [DOI] [PubMed] [Google Scholar]
- 44. Eguchi S., Numaguchi K., Iwasaki H., Matsumoto T., Yamakawa T., Utsunomiya H., Motley E. D., Kawakatsu H., Owada K. M., Hirata Y., Marumo F., Inagami T. (1998) J. Biol. Chem. 273, 8890–8896 [DOI] [PubMed] [Google Scholar]
- 45. Bivona T. G., Pérez De Castro I., Ahearn I. M., Grana T. M., Chiu V. K., Lockyer P. J., Cullen P. J., Pellicer A., Cox A. D., Philips M. R. (2003) Nature 424, 694–698 [DOI] [PubMed] [Google Scholar]
- 46. Arozarena I., Matallanas D., Berciano M. T., Sanz-Moreno V., Calvo F., Muñoz M. T., Egea G., Lafarga M., Crespo P. (2004) Mol. Cell. Biol. 24, 1516–1530 [DOI] [PMC free article] [PubMed] [Google Scholar]