Abstract
The severe complications of dengue virus infections, hemorrhagic manifestations and shock, are more commonly observed during secondary dengue virus infections than during primary infections. It has been speculated that these complications are mediated by cross-reactive host-immune responses. We have begun to analyze human T cell responses to dengue antigens in vitro to explain the possible role of T lymphocytes in the pathogenesis of these complications. Dengue antigens induce proliferative responses of PBMC from dengue antibody-positive donors, but do not induce specific proliferative responses of PBMC from dengue antibody-negative donors. IFN gamma is detected in the culture fluids of dengue-immune PBMC stimulated with dengue antigens. The cells that proliferate in the dengue antigen-stimulated bulk cultures have CD3+, CD4+, CD8-, CD16-, and CD20- phenotypes. Dengue-specific T cell lines were established using limiting dilution techniques. They have CD3+, CD4+, and CD8- phenotypes, and produce IFN gamma in response to dengue antigens. Culture fluids from dengue-immune PBMC stimulated with dengue antigens, which contain IFN gamma, augment dengue virus infection of human monocytes by dengue virus-antibody complexes. These results indicate that PBMC from dengue-immune donors contain CD4+ T cells that proliferate and produce IFN gamma after stimulation with dengue antigens, and suggest that the IFN gamma that is produced by these stimulated dengue-specific T cells may contribute to the pathogenesis of dengue hemorrhagic fever and dengue shock syndrome by increasing the number of dengue virus-infected monocytes in the presence of cross-reactive anti-dengue antibodies.
Full text
PDF

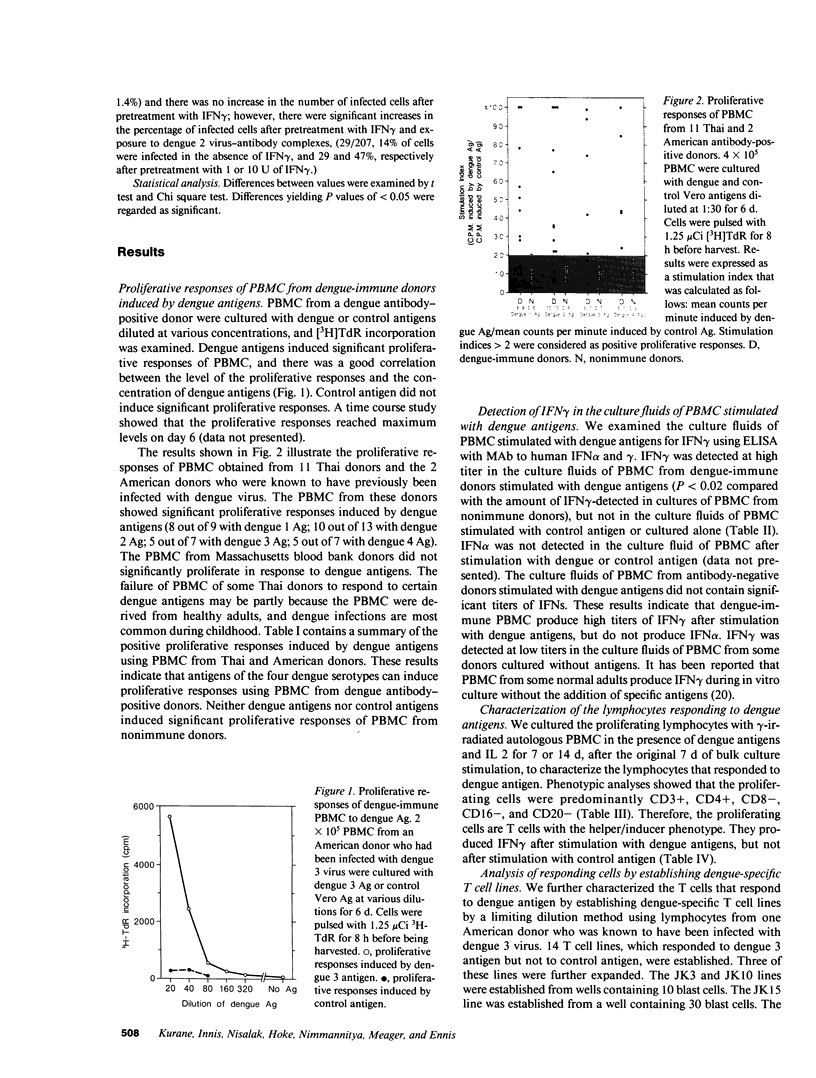
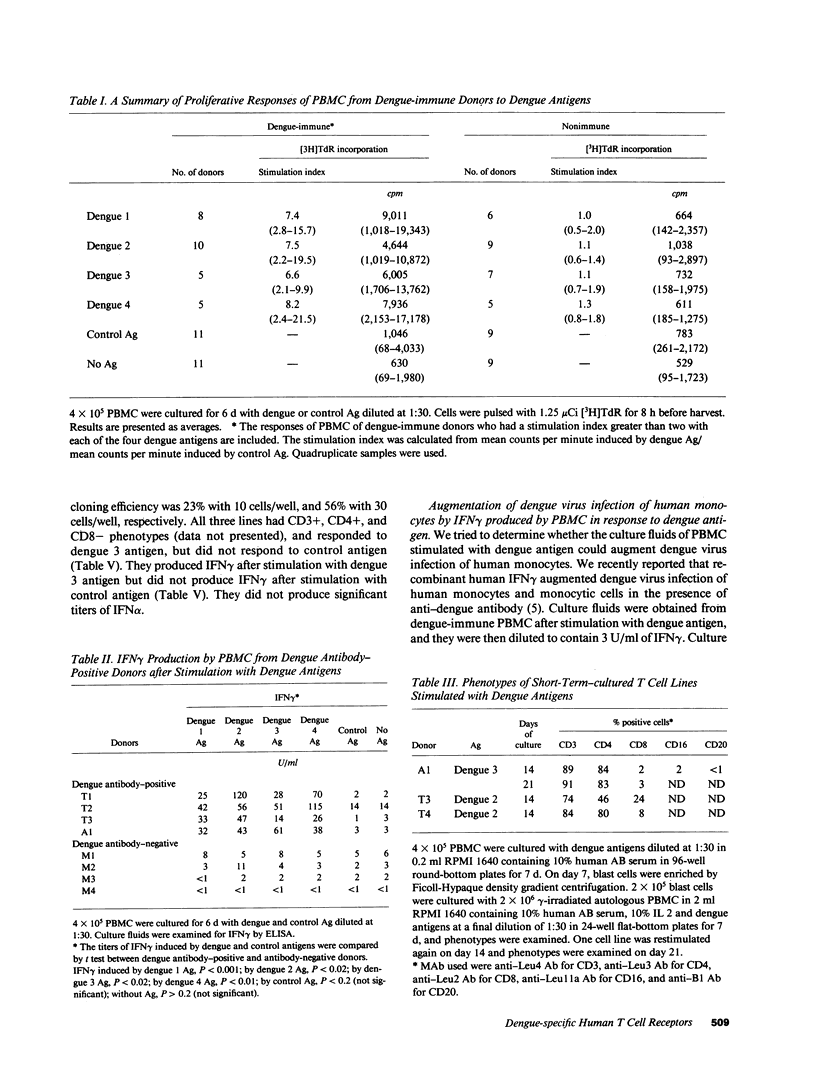
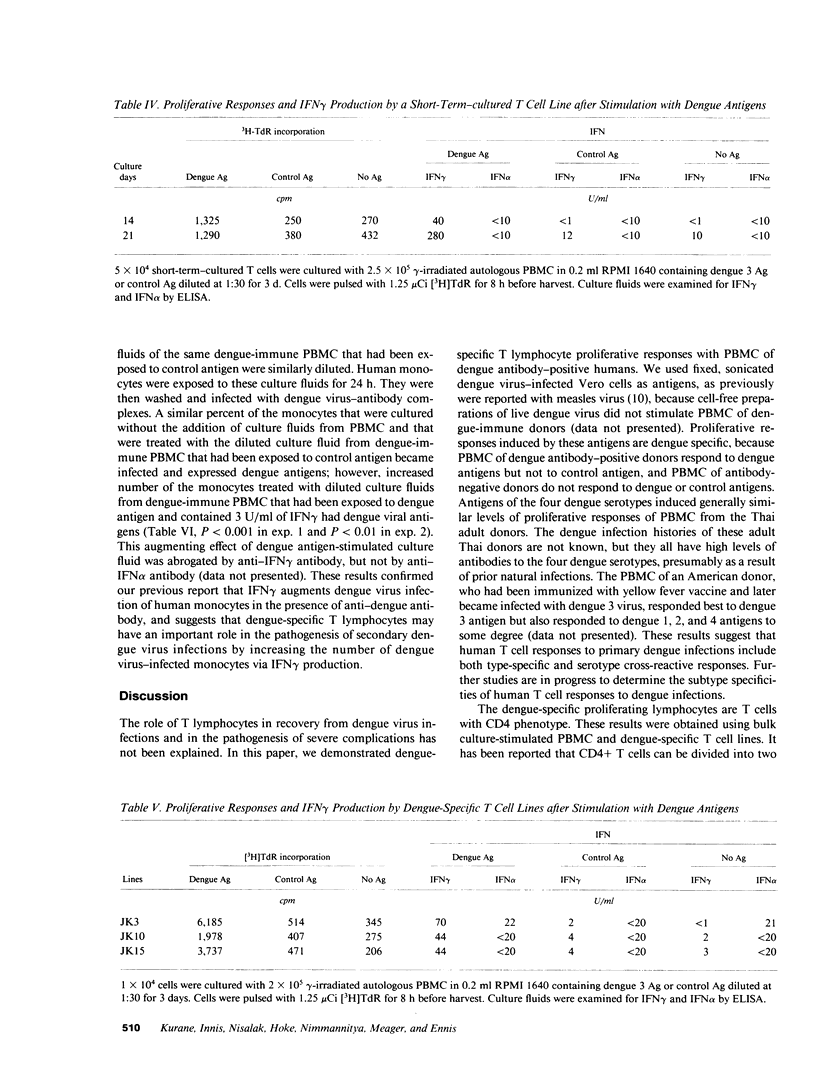



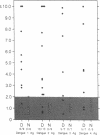
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borysiewicz L. K., Morris S., Page J. D., Sissons J. G. Human cytomegalovirus-specific cytotoxic T lymphocytes: requirements for in vitro generation and specificity. Eur J Immunol. 1983 Oct;13(10):804–809. doi: 10.1002/eji.1830131005. [DOI] [PubMed] [Google Scholar]
- Burke D. S., Nisalak A., Johnson D. E., Scott R. M. A prospective study of dengue infections in Bangkok. Am J Trop Med Hyg. 1988 Jan;38(1):172–180. doi: 10.4269/ajtmh.1988.38.172. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Cunningham A. L., Merigan T. C. Leu-3+ T cells produce gamma-interferon in patients with recurrent herpes labialis. J Immunol. 1984 Jan;132(1):197–202. [PubMed] [Google Scholar]
- Engleman E. G., Benike C. J., Glickman E., Evans R. L. Antibodies to membrane structures that distinguish suppressor/cytotoxic and helper T lymphocyte subpopulations block the mixed leukocyte reaction in man. J Exp Med. 1981 Jul 1;154(1):193–198. doi: 10.1084/jem.154.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennis F. A., Meager A. Immune interferon produced to high levels by antigenic stimulation of human lymphocytes with influenza virus. J Exp Med. 1981 Nov 1;154(5):1279–1289. doi: 10.1084/jem.154.5.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman R. M., Vogel S. N. Interferons with special emphasis on the immune system. Adv Immunol. 1983;34:97–140. doi: 10.1016/s0065-2776(08)60378-8. [DOI] [PubMed] [Google Scholar]
- Guyre P. M., Morganelli P. M., Miller R. Recombinant immune interferon increases immunoglobulin G Fc receptors on cultured human mononuclear phagocytes. J Clin Invest. 1983 Jul;72(1):393–397. doi: 10.1172/JCI110980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halstead S. B. In vivo enhancement of dengue virus infection in rhesus monkeys by passively transferred antibody. J Infect Dis. 1979 Oct;140(4):527–533. doi: 10.1093/infdis/140.4.527. [DOI] [PubMed] [Google Scholar]
- Halstead S. B., O'Rourke E. J., Allison A. C. Dengue viruses and mononuclear phagocytes. II. Identity of blood and tissue leukocytes supporting in vitro infection. J Exp Med. 1977 Jul 1;146(1):218–229. doi: 10.1084/jem.146.1.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halstead S. B., O'Rourke E. J. Dengue viruses and mononuclear phagocytes. I. Infection enhancement by non-neutralizing antibody. J Exp Med. 1977 Jul 1;146(1):201–217. doi: 10.1084/jem.146.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halstead S. B. The Alexander D. Langmuir Lecture. The pathogenesis of dengue. Molecular epidemiology in infectious disease. Am J Epidemiol. 1981 Nov;114(5):632–648. doi: 10.1093/oxfordjournals.aje.a113235. [DOI] [PubMed] [Google Scholar]
- Jacobson S., Richert J. R., Biddison W. E., Satinsky A., Hartzman R. J., McFarland H. F. Measles virus-specific T4+ human cytotoxic T cell clones are restricted by class II HLA antigens. J Immunol. 1984 Aug;133(2):754–757. [PubMed] [Google Scholar]
- Kaplan D. R., Griffith R., Braciale V. L., Braciale T. J. Influenza virus-specific human cytotoxic T cell clones: heterogeneity in antigenic specificity and restriction by class II MHC products. Cell Immunol. 1984 Oct 1;88(1):193–206. doi: 10.1016/0008-8749(84)90064-9. [DOI] [PubMed] [Google Scholar]
- Kaufman B. M., Summers P. L., Dubois D. R., Eckels K. H. Monoclonal antibodies against dengue 2 virus E-glycoprotein protect mice against lethal dengue infection. Am J Trop Med Hyg. 1987 Mar;36(2):427–434. doi: 10.4269/ajtmh.1987.36.427. [DOI] [PubMed] [Google Scholar]
- Kliks S. C., Nimmanitya S., Nisalak A., Burke D. S. Evidence that maternal dengue antibodies are important in the development of dengue hemorrhagic fever in infants. Am J Trop Med Hyg. 1988 Mar;38(2):411–419. doi: 10.4269/ajtmh.1988.38.411. [DOI] [PubMed] [Google Scholar]
- Kontny U., Kurane I., Ennis F. A. Gamma interferon augments Fc gamma receptor-mediated dengue virus infection of human monocytic cells. J Virol. 1988 Nov;62(11):3928–3933. doi: 10.1128/jvi.62.11.3928-3933.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurane I., Hebblewaite D., Brandt W. E., Ennis F. A. Lysis of dengue virus-infected cells by natural cell-mediated cytotoxicity and antibody-dependent cell-mediated cytotoxicity. J Virol. 1984 Oct;52(1):223–230. doi: 10.1128/jvi.52.1.223-230.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurane I., Hebblewaite D., Ennis F. A. Characterization with monoclonal antibodies of human lymphocytes active in natural killing and antibody-dependent cell-mediated cytotoxicity of dengue virus-infected cells. Immunology. 1986 Jul;58(3):429–436. [PMC free article] [PubMed] [Google Scholar]
- Lanier L. L., Phillips J. H., Warner N. L., Babcock G. F. A human natural killer cell-associated antigen defined by monoclonal antibody anti-Leu (NKP-15): functional and two-color flow cytometry analysis. J Leukoc Biol. 1984 Jan;35(1):11–17. doi: 10.1002/jlb.35.1.11. [DOI] [PubMed] [Google Scholar]
- Ledbetter J. A., Evans R. L., Lipinski M., Cunningham-Rundles C., Good R. A., Herzenberg L. A. Evolutionary conservation of surface molecules that distinguish T lymphocyte helper/inducer and cytotoxic/suppressor subpopulations in mouse and man. J Exp Med. 1981 Feb 1;153(2):310–323. doi: 10.1084/jem.153.2.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackow E., Makino Y., Zhao B. T., Zhang Y. M., Markoff L., Buckler-White A., Guiler M., Chanock R., Lai C. J. The nucleotide sequence of dengue type 4 virus: analysis of genes coding for nonstructural proteins. Virology. 1987 Aug;159(2):217–228. doi: 10.1016/0042-6822(87)90458-2. [DOI] [PubMed] [Google Scholar]
- Martinez-Maza O., Andersson U., Andersson J., Britton S., De Ley M. Spontaneous production of interferon-gamma in adult and newborn humans. J Immunol. 1984 Jan;132(1):251–255. [PubMed] [Google Scholar]
- Meager A., Berg K. Epitope localization of a monoclonal antibody, LO-22, with broad specificity for interferon-alpha subtypes. J Interferon Res. 1986 Dec;6(6):729–736. doi: 10.1089/jir.1986.6.729. [DOI] [PubMed] [Google Scholar]
- Meager A., Parti S., Barwick S., Spragg J., O'Hagan K. Detection of hybridomas secreting monoclonal antibodies to human gamma interferon using a rapid screening technique and specificity of certain monoclonal antibodies to gamma interferon. J Interferon Res. 1984 Fall;4(4):619–625. doi: 10.1089/jir.1984.4.619. [DOI] [PubMed] [Google Scholar]
- Morimoto C., Letvin N. L., Boyd A. W., Hagan M., Brown H. M., Kornacki M. M., Schlossman S. F. The isolation and characterization of the human helper inducer T cell subset. J Immunol. 1985 Jun;134(6):3762–3769. [PubMed] [Google Scholar]
- Morimoto C., Letvin N. L., Distaso J. A., Aldrich W. R., Schlossman S. F. The isolation and characterization of the human suppressor inducer T cell subset. J Immunol. 1985 Mar;134(3):1508–1515. [PubMed] [Google Scholar]
- Ndumbe P. M., Levinsky R. J. Generation of phenotypically different T cell populations by cell-free or cell-bound preparations of varicella zoster virus. Immunol Lett. 1986 Oct;13(4):191–195. doi: 10.1016/0165-2478(86)90054-4. [DOI] [PubMed] [Google Scholar]
- Perussia B., Dayton E. T., Lazarus R., Fanning V., Trinchieri G. Immune interferon induces the receptor for monomeric IgG1 on human monocytic and myeloid cells. J Exp Med. 1983 Oct 1;158(4):1092–1113. doi: 10.1084/jem.158.4.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richert J. R., McFarland H. F., McFarlin D. E., Johnson A. H., Woody J. N., Hartzman R. J. Measles-specific T cell clones derived from a twin with multiple sclerosis: genetic restriction studies. J Immunol. 1985 Mar;134(3):1561–1566. [PubMed] [Google Scholar]
- Sangkawibha N., Rojanasuphot S., Ahandrik S., Viriyapongse S., Jatanasen S., Salitul V., Phanthumachinda B., Halstead S. B. Risk factors in dengue shock syndrome: a prospective epidemiologic study in Rayong, Thailand. I. The 1980 outbreak. Am J Epidemiol. 1984 Nov;120(5):653–669. doi: 10.1093/oxfordjournals.aje.a113932. [DOI] [PubMed] [Google Scholar]
- Schlesinger J. J., Brandriss M. W., Walsh E. E. Protection of mice against dengue 2 virus encephalitis by immunization with the dengue 2 virus non-structural glycoprotein NS1. J Gen Virol. 1987 Mar;68(Pt 3):853–857. doi: 10.1099/0022-1317-68-3-853. [DOI] [PubMed] [Google Scholar]
- Stashenko P., Nadler L. M., Hardy R., Schlossman S. F. Characterization of a human B lymphocyte-specific antigen. J Immunol. 1980 Oct;125(4):1678–1685. [PubMed] [Google Scholar]
- Westaway E. G. Flavivirus replication strategy. Adv Virus Res. 1987;33:45–90. doi: 10.1016/s0065-3527(08)60316-4. [DOI] [PubMed] [Google Scholar]
- Yamada Y. K., Meager A., Yamada A., Ennis F. A. Human interferon alpha and gamma production by lymphocytes during the generation of influenza virus-specific cytotoxic T lymphocytes. J Gen Virol. 1986 Nov;67(Pt 11):2325–2334. doi: 10.1099/0022-1317-67-11-2325. [DOI] [PubMed] [Google Scholar]
- Yasukawa M., Zarling J. M. Human cytotoxic T cell clones directed against herpes simplex virus-infected cells. I. Lysis restricted by HLA class II MB and DR antigens. J Immunol. 1984 Jul;133(1):422–427. [PubMed] [Google Scholar]
- Zhao B., Mackow E., Buckler-White A., Markoff L., Chanock R. M., Lai C. J., Makino Y. Cloning full-length dengue type 4 viral DNA sequences: analysis of genes coding for structural proteins. Virology. 1986 Nov;155(1):77–88. doi: 10.1016/0042-6822(86)90169-8. [DOI] [PubMed] [Google Scholar]