Abstract
The normal function of poly (ADP-ribose) polymerase-1 (PARP-1) is the routine repair of DNA damage by adding poly (ADP ribose) polymers in response to a variety of cellular stresses. Recently, it has become widely appreciated that PARP-1 also participates in diverse physiological and pathological functions from cell survival to several forms of cell death and has been implicated in gene transcription, immune responses, inflammation, learning, memory, synaptic functions, angiogenesis and aging. In the CNS, PARP inhibition attenuates injury in pathologies like cerebral ischemia, trauma and excitotoxicity demonstrating a central role of PARP-1 in these pathologies. PARP-1 is also a preferred substrate for several 'suicidal' proteases and the proteolytic action of suicidal proteases (caspases, calpains, cathepsins, granzymes and matrix metalloproteinases (MMPs)) on PARP-1 produces several specific proteolytic cleavage fragments with different molecular weights. These PARP-1 signature fragments are recognized biomarkers for specific patterns of protease activity in unique cell death programs. This review focuses on specific suicidal proteases active towards PARP-1 to generate signature PARP-1 fragments that can identify key proteases and particular forms of cell death involved in pathophysiology. The roles played by some of the PARP-1 fragments and their associated binding partners in the control of different forms of cell death are also discussed.
Introduction
PARP-1 is a nuclear protein with a wide range of physiological as well as pathological functions. Initially identified as an enzyme that performs central roles in the repair of damaged DNA, PARP-1 participates in initiating base excision repair (BER) (PARP-1-/- cells have impaired BER activity) [1,2], nucleotide excision repair, single strand base repair mediated by DNA ligase III, XRCC1, poly nucleotide kinase, proliferating cell nuclear antigen and flap endonuclease-1, and contributes to double strand base (DSB) repair in an alternate non-homologous end joining pathway with DNA ligase III [3-6]. Interestingly, over-expression of PARP-1 or DNA binding domain of PARP-1 (lacking catalytic domain) decreased DSB repair, indicating that its enzymatic activity is not essential in all repair processes [7]. Many additional functions of PARP-1 have now been demonstrated in biochemical and molecular signaling [8]. Apart from its role in repairing DNA damage, PARP-1 also plays important roles in transcription, cardiac remodeling, vasoconstriction, regulation of astrocyte and microglial function, long term memory and aging [9-17]. Progressive DNA damage and decreased PARP-1 activity in aging neurons eventually leads to programmed neuronal death and loss of memory consolidation. PARP-1's role in neuronal BER indicates that it may influence age-related memory deficits and dementia. Further, over-expression of PARP-1 and telomeric repeat binding factor-1 were also associated with age dependent telomere shortening in 'Duchenne muscular dystrophy' [18]. PARP-1 influences ~3.5% of the total transcriptome of embryonic liver and stem cells and regulates ~60-70% of genes controlling cell metabolism, cell cycle and transcription. Gene expression is dysregulated in PARP-1 deficient fibroblasts and PARP-1 deficient mice are more susceptible to skin diseases [19-21] reflecting the role of PARP-1 against UV-induced DNA damage. PARP-1 also interacts with, and modulates the function of several transcription factors including NF-κB, NFAT, E2F-1, and ELK-1 [22-28]. PARP-1 is also involved in modulating endothelial cell adhesion molecule expression (e.g. during atherogenesis) via its binding partner NF-κB [16,29,30]. PARP-1, 2 and 3 can activate CNS immune responses by promoting astrocyte production of inflammatory cytokines like TNF-α, IL-1β, nitric oxide and the chemokine CCL2 after challenge with Staphylococcus aureus, a common CNS infectious agent [14].
The PARP family consists of 17 members which have different structures and diverse functions in cells [31]. PARP-1, the canonical representative of this superfamily has become the major focus of research due to its multifaceted roles in many cellular activities. This review focuses on the interactions between PARP-1 and suicidal proteases like caspase, calpain, granzyme, and MMPs that lead to the formation of PARP-1 proteolytic signature fragments associated with particular pathological conditions.
PARP-1 is an abundant nuclear enzyme with approximately 1-2 million copies in the cell which account for ~85% of total cellular PARP activity [31-34]. Post-translation modification involving poly (ADP-ribosyl)ation plays a central role in cellular homeostasis [35]. Protein modifications involving phosphorylation, acetylation, methylation and poly (ADP ribosyl)ation are vital cellular processes that are required for cell signaling, survival and functioning [34,36-39]. This form of post translational modification is mainly mediated by PARP-1 which catalyzes the formation of chains approximately 200 units long linear or branched poly (ADP ribose) units from donor NAD+ molecules frequently linked by esterification to glutamate, and less commonly to aspartate or lysine resides on target molecules [34,40]. Poly (ADP-ribosyl)ation is therefore an important mechanism for maintaining genome integrity, replication, transcription, protein degradation, differentiation and in the repair process following DNA damage [13,34,41-43].
PARP-1 has several important domains: a 54-kD catalytic domain (CD) at the carboxyl terminus that polymerizes linear or branched poly-ADP ribose units (from NAD+) on target proteins, a 46-kD DNA binding domain (DBD) containing 2 zinc finger motifs (at the NH2 terminus), and 22-kD auto-modification domain (AMD) that functions as a target for direct covalent auto-modification in its central region [34]. For example, high affinity binding of PARP-1 to specific DNA motifs like double-strand breaks, cruciforms, cross-overs and nucleosomes require the DBD for active modification [44-46]. While the 2 zinc finger motifs at the N-terminus facilitate tight binding of PARP-1 to DNA and promotes the activation of the catalytic domain at the C-terminus, a 3rd zinc finger motif located between 2nd zinc finger motif and AMD also plays an important role in the inter-domain interactions and is vital for PARP-1 enzymatic action [47]. AMD contains a BRCT fold (a motif also found in many DNA repair proteins) that is involved in protein-protein interactions which promotes the recruitment of DNA repair enzymes to the site of DNA damage [48,49]. These PARP-1 domains play different roles in various pathological cell death processes mediated by PARP-1 cleavage by suicide proteases. These suicidal proteases (caspases, calpains, cathepsins, granzymes and MMPs) cleave PARP-1, creating PARP-1 fragments with diverse, exposed structural domains mediating specific forms of cell death. The present review focuses on the different actions of these proteases towards PARP-1, the production of a variety of different PARP-1 signature fragments, and the specific patterns of cell death linked with particular PARP-1 fragments.
PARP-1 and Caspases
Apoptosis, the process of programmed cell death is essential for proper homeostatic maintenance and survival in multi-cellular organisms. Physiological apoptosis controls cell numbers, tissue and organ morphology and patterning, and removes injured or mutated cells [50,51]. Dysregulated apoptosis results in elevated or decreased cell death often leading to neurodegenerative disorders, cancer and other hyper-proliferative disorders [52,53]. One of the most common signaling cascades involved in apoptosis is the activation of a highly specialized family of cysteinyl-aspartate proteases (caspases) which are usually present as inactive zymogen forms. Once activated, caspases initiate cell death by cleaving and activating effector caspases which drive the process of apoptosis [54]. Interestingly, recent reports have shown the involvement of caspases not only in apoptosis, but also in forms of cell proliferation in a Drosophila model. One of the 2 distinct forms of 'apoptosis induced compensatory proliferation' (AICP) depends on initiator caspase Dronc (initiator caspase in Drosophila, caspase-9-like); the other is dependent on execution caspase DrICE and Dcp-1 (effector caspases in Drosophila; caspase-3-like) [55-58]. Moreover, apart from their primary function in executing apoptosis, non-apoptotic functions of caspases include hematopoiesis (erythropoiesis, monocyte, lymphocyte differentiation, platelet maturation) [59] and regulation of neuronal synaptic plasticity in long-term potentiation [60,61]. Caspase mediated apoptotic cell death is accomplished through the cleavage of several key proteins required for cellular functioning and survival [62]. PARP-1 is one of several known cellular substrates of caspases. Cleavage of PARP-1 by caspases is considered to be a hallmark of apoptosis [63,64]. Almost all caspases including caspase-1, are known to modify PARP-1 in vitro [65]. Lazenbik et al., have observed protease activity resembling interleukin converting enzyme (prICE: caspase-3) which cleaves PARP-1 after aspartate (glutamate-valine-aspartate-glycine), a substrate specificity identical to one of ICE cleavage sites in vitro to yield an 85-kD PARP-1 fragment [66]. Cleavage of PARP-1 by caspase-3 has been implicated in several neurological diseases e.g. cerebral ischemia, Alzheimer's disease, multiple sclerosis, Parkinson's disease, traumatic brain injury, NMDA-mediated excitotoxicity and brain tumors, especially gliomas [67-74]. Besides caspase-3, caspase-7 also cleaves PARP-1 in vivo. The cleavage of PARP-1 by these caspases results in the formation of 2 specific fragments: an 89-kD catalytic fragment and a 24-kD DBD [65,66]. The 89-kD fragment containing AMD and the catalytic domain of the enzyme has a greatly reduced DNA binding capacity and is liberated from the nucleus into the cytosol [75]. The 24-kD cleaved fragment with 2 zinc-finger motifs is retained in the nucleus, irreversibly binding to nicked DNA where it acts as a trans-dominant inhibitor of active PARP-1. Importantly, irreversible binding of the 24-kD PARP-1 fragment to DNA strand breaks inhibits DNA repair enzymes (including PARP-1) and attenuates DNA repair (also conserving cellular ATP pools) [76-78]. Poly (ADP-ribosyl)ation of PARP-1 following DNA breaks changes PARP-1 targeting from caspase-3 from caspase-7 in human promyelocytic leukemia cells (HL-60) treated with etoposide phosphate (VP-16). However, increased caspase-3 levels (and activity) might lead to PARP-1 cleavage irrespective of its auto-modification (by poly (ADP-ribosyl)ation) [79]. In a similar context, Margolin et al., using the 46-kD DBD of PARP-1 (lack which auto-modification sites) reported an increased affinity of caspase-3 for PARP-1, indicating a prominent role for PARP-1 automodification sites in caspase-3 mediated proteolytic cleavage [65].
Under basal conditions, the primary function of PARP-1 is to detect and repair DNA damage. However, cells with severely damaged DNA have amplified PARP-1 activity resulting in high NAD+ consumption (depleting ATP pools). If unchecked, this activity inevitably leads to passive necrotic cell death (resulting from prolonged ATP depletion) [80,81]. This process is blocked by rapid cleavage and inactivation of PARP-1 by the action of caspases [81,82]. However, insults which initiate necrosis cause PARP-1 overactivation that proceeds unchecked due to inadequate caspase activation [82-84], lower PARP-1 cleavage and less PARP-1 24-kD fragment formation. It is therefore possible that exogenous addition of PARP-1 24-kD fragments could attenuate PARP-1 overactivation possibly blocking cell death. Exogenous administration of 24-kD PARP-1 fragments might attenuate PARP-1 overactivation and divert necrosis towards apoptotic cell death. Taken together, these findings suggest that the PARP-1 24-kD fragment can also serve as a powerful therapy for CNS disorders like cerebral ischemia where necrotic cell death predominates within the infarct core. PARP-1 cleavage by caspases and the resulting specific fragmentation patterns are indicated in figure 1 and Additional file 1: Table-1.
Figure 1.
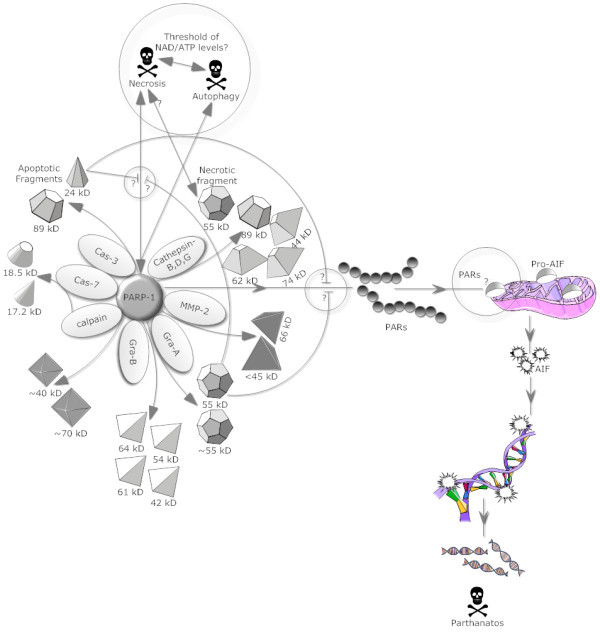
PARP-1 interactions with cell death proteases. PARP-1 cleavage by various suicidal proteases like caspases, calpain, cathepsins and granzymes liberates fragments with specific molecular weights and are shown in this schematic representation. Importantly, 21-kD and 55-kD PARP-1 fragments generated by caspase 3/7 and Gra-A respectively function as an inhibitor of PARP-1 activity and might also play important roles in reducing necrosis and/or parthanatos. Further, PARP-1 and PARP-1 fragment's involvement in various forms of cell death e.g. autophagy, necrosis and parthanatos are also indicated.
PARP-1 and Calpains
Calpains are a family of 14 Ca2+-activated, non-lysosomal cysteinyl proteases active at neutral pH [85]. Of all the calpain isoforms, m-calpain and μ-calpain (activated at μM-mM Ca2+ concentrations) are the best understood. Alterations in intracellular calcium levels are known to initiate calpain activation often exacerbating pathological conditions [86]. For example, pathologic calpain activation aggravates neurodegenerative disorders like cerebral ischemia, neonatal hypoxia, cerebral malaria, Alzheimer's disease, Parkinson's disease, Huntington's disease, multiple sclerosis, and also contributes to injury in brain tumors, such as gliomas [68-70,87-92]. Translocation of calpains to the plasma membrane is required for optimal calpain activation. However in neurons, NMDA-mediated excitotoxicity involves calpain activation without a requirement for membrane translocation [93,94]. Calpains also play prominent roles in normal physiology e.g. neuron synaptic signaling, myoblast differentiation, cell migration, embryonic development (where m-calpain deficiency is embryonic lethal) and angiogenesis [95-98]. VEGF mediated angiogenesis involves increased Ca2+ uptake in cells to activate m-calpain and VEGF induced retinal angiogenesis in vitro could be reversed by the calpain inhibitor SNJ-1945 [99,100]. Interestingly, PARP-1 inhibition also been shown to decrease VEGF induced angiogenesis [101]. Hence, even though calpain mediated PARP-1 proteolysis can decrease angiogenesis; calpains might still promote angiogenesis by augmenting VEGF expression [100] indicating that a bimodal activation pattern of calpain activation proportional to the magnitude of calpain mediated PARP-1 proteolysis is possible. More studies are still required to address this issue and understanding the roles played by PARP-1, calpains and VEGFs in post-ischemic cerebral angiogenesis is an area of drug development, particularly for stroke therapy.
Calcium, apart from activating calpains, also promotes PARP-1 hyper-activation through production of ROS and peroxynitrite [102]. The anti-tumor drug β-lapachone induces PARP-1 dependent cell death independent of caspases via metabolic starvation. β-lapachone treated cells also exhibit increased cytosolic Ca2+ (by release of endoplasmic reticulum Ca2+ stores) and ROS, resulting in PARP-1 hyper-activation. PARP-1 hyper-activation by β-lapachone was blocked by BAPTA-AM (a cytosolic Ca2+ chelator) indicating that Ca2+ induced ROS generation leads to PARP-1 hyper-activation and cell death [103]. Conversely, cellular Ca2+ levels were altered by PARP-1 in doxorubicin-induced myocardial injury. Szenczi et al., have shown that both cardiac contractile capacity and intracellular Ca2+ loading was reduced in doxorubicin-treated hearts, an effect blocked by PARP-1 inhibition. These responses may be indirect through the effects of PARP-1 on the transcription of proteins involved in Ca2+ handling and Ca2+ pumps [104]. PARP-1 has also been shown to modulate neuronal mitochondrial Ca2+ levels [105]. PARP-1 activity raises intra-mitochondrial Ca2+ levels, activating μ-calpain to release mitochondrial apoptosis inducing factor (AIF) [106]. However, because activated calpains mediate proteolytic breakdown of PARP-1, the temporal association of PARP-1 and calpain in AIF nuclear translocation may be phase-dependent, more studies will be required to address this. The roles of PARP-1 in Ca2+ handling and excitotoxicity point towards pleiotropic functions of PARP-1 in pathological settings.
Calpains play crucial roles in both caspase-dependent and independent forms of apoptotic cell death and are key mediators of necrotic cell death [107,108]. A variety of cellular insults can activate calpains including increased cytosolic Ca2+, decreased calpastatin levels, interactions with calpain activator protein, phospholipids, and caspase activation [109-112]. Several cell substrates have been identified as calpain targets resulting in proteolytic breakdown to signature fragments of specific molecular weights [69,91,113]. Appearance of distinctive cleavage ('signature') fragments is considered to represent specific forms of calpain activation. Among the various cellular substrates of calpain, PARP-1 cleavage gives rise to a 40-kD N-terminal fragment in neuroblastoma cells (SH-5YSY) challenged with maitotoxin [114]. μ-calpain isolated from calf thymus generates ~40-70-kD N-terminal PARP-1 fragments [115]. Calpain induced 40-kD fragments were recognized by both C-2-10 and N-20 antibodies (which detect PARP-1 fragments bearing the N-terminal domain/DBD). Depending on observed molecular weight and immunoreactivity it can be presumed that this PARP-1 fragment might have DBD domain, but not AMD or catalytic domains. Whether this 40-kD fragment plays any role in PARP-1 auto-modification (as shown in studies on the 24-kD PARP-1 DBD fragment) is not yet known. This fragment appears specifically when PARP-1 is digested with calpain, and μ-calpain inhibitor reduces its appearance, indicating that it is a μ-calpain-specific PARP-1 signature fragment. We don't know whether this fragment represents a byproduct formed during calpain mediated necrotic cell death or if it can actively attenuate necrosis. Based on the findings that the zinc finger motifs present in the DBD domain of PARP-1 can facilitate irreversible binding of DBD to DNA nicks and promote transdominant inhibition of activated PARP-1, the appearance of this fragment would be consistent with its ability to attenuate PARP-1 activity during calpain mediated necrosis. Calpains are known to mediate apoptotic cell death. Whether this fragment appears only during necrotic conditions or also during calpain-mediated (caspase independent) apoptosis is unknown. Future studies on the molecular biology of this fragment and its roles in specific cell death pathways will be needed to determine its role in neuronal pathologies. So far, these fragments remain most convincingly related to necrotic modes of cell death mediated by calpain.
Apart from PARP-1, caspase-7 has also been shown to be a target of μ-calpain. μ-calpain cleaves caspase-7 to active 18.5 and 17.2-kD fragments. This calpain generated caspase-7's 17.2-kD fragment is ~18 fold more active than the 20-kD caspase-3 generated fragment of caspase-7 [116]. Whether these fragments are involved in preferential PARP-1 processing over caspase-3 is not yet known. PARP-1 cleavage by calpains results in specific fragmentation patterns which are described in figure 1 and Additional file 1: Table-1.
PARP-1 and Cathepsins
Lysosomes were initially described as participants in catabolic autophagic cell death when exhausted or damaged organelles were digested and secreted into the cytoplasm [117]. Lysosomes and lysosomal proteases also participate in apoptotic and necrotic forms of cell death [118-120]. Cathepsins belong to the family of lysosomal proteases which are active at acidic pH. These are stored in lysosomes as inactive precursors and activated by stimuli that lower cell pH, resulting in the release of their catalytically active forms which cleave multiple targets [121]. Cathepsins can initiate apoptotic cell death independent of caspases, and are known to be key proteases in necrosis and autophagy. Increased autophagic protease levels can aggravate post-ischemic neuropathologic with injury [122]. Recent reports clearly show that the biogenesis of lysosomal cathepsins is required for necrotic cell death [118]. One of the important differences between apoptotic and necrotic forms of cell death is the protein synthesis pattern. During apoptotic cell death, normal protein synthetic processes are rapidly shut down, an event which conserves ATP, which is used to accomplish apoptosis. Conversely, the persistence of protein synthesis during necrotic cell death can deplete energy reserves accelerating necrotic cell death [118-123]. Since lysosomal biogenesis is required for necrotic cell death, persistent protein synthesis might help in lysosomal-cathepsin synthesis, which eventually spills into the cytosol, cleaving PARP-1 and contributing to cell death [118,123]. However, the numerous targets and temporal specificities of cathepsins and PARP-1 contributing to cell death mechanisms require further study.
Cathepsins are a large family of lysosomal enzymes comprising 11 cysteinyl cathepsins (and aspartyl protease cathepsin-D) which are also active at neutral pH. These have a relatively short biological half-life, but acidification of the cytosol will increase their residence time. However, due to the abundance of cathepsin B, D and L, these are most often used as markers for the involvement of lysosomes in particular forms of cell death [119]. Cathepsins B, S and G are also involved in tumor invasion (glioblastoma multiforme, breast cancer bone metastasis and colorectal tumors), endothelial proliferation and in angiogenesis (via TGF-β, VEGF and MCP-1) [124,125]. It is important to note that necrosis is common in tumor tissue and is usually accompanied by PARP-1 hyper activation. Considering that PARP-1 inhibition reduces angiogenesis, it is possible that hyperactivated PARP-1 might in part drive tumor angiogenesis and metastasis. Cathepsins and TGF-β are also involved in caspase-independent PARP-1 cleavage (an ~85-kD caspase independent PARP-1 fragment is produced by TGF-β) [126]. How these mediators drive angiogenesis or can be used in diagnosis or therapy needs further study.
Moreover, TGF-β and cathepsin-G mediated angiogenesis depends on the upregulation of VEGF [124]. Hence, breakdown of PARP-1 (which would reduce the angiogenic program) might be balanced by increased VEGF production by cathepsins. It is important to identify whether the breakdown products of PARP-1 produced by calpains or cathepsins can substitute for the PARP-1 function in angiogenic programs. More importantly, the effect of transdominant inhibition of PARP-1 by DBD in modulating angiogenic programs is worth investigating. These findings indicate that angiogenesis associated with PARP-1 signaling is highly complex, tightly controlled and pathology specific. The extent of PARP-1 and PARP-1 cleavage products' involvement in modulating angiogenic programs has yet to be fully determined.
Cysteine cathepsins also share several common targets along with PARP-1. PARP-1 was initially found to produce a 50-kD fragment (necrotic fragment) during necrotic cell death. Necrotic PARP-1 fragmentation was later characterized and the appearance of these PARP-1 fragments shown to be mediated by lysosomes and lysosomal specific proteases cathepsin-B, -D and -G. During necrosis, induced by H2O2, 10% ethanol, HgCl2, lysosomal extracts and cathepsin-B, PARP-1 gave rise to fragments ranging from 42-89-kD [127]. Cathepsin-B and D produce active PARP-1 fragments with molecular weights of 55-kD and 42-kD, similar to the fragments obtained from the lysosomal extracts. Inactive PARP-1 fragments with molecular weights of 74 and 62-kD are also liberated by the action of these proteases. Cathepsin-G, another lysosomal protease, also produced a similar fragmentation pattern, but with lower intensity (requiring longer incubation periods vs. cathepsin-B and D). Moreover, the 89-kD PARP-1 fragment which appears during apoptotic cell death could also be produced by cathepsin-B and D, but is not generated by cathepsin-G [127]. Cathepsin specific PARP-1 fragmentation patterns are shown in figure 1 and Additional file 1: Table-1.
PARP-1 and granzymes
The immune system maintains an internal homeostasis by recognizing and reacting to foreign particles, abnormal or infected cells to restrict their further persistence and penetration within the host. Immune processes use highly specialized and cell-specific mechanisms and molecules to accomplish this. Induction of cytotoxicity in target cells is a multifactorial process accomplished by cytotoxic T lymphocytes (CTLs) and natural killer (NK) cells that secrete toxic and lytic proteases of the serine protease family (granzymes), perforins and granulysin [128]. About 90% of cytolytic granules stored in CTLs and NK cells contain granzymes. Granzymes are subdivided into 3 categories based upon their enzymatic similarity to chymotrypsin (chymase locus), trypsin (tryptase locus) and those that cleave after unbranched hydrophilic residues particularly after methionine (Met-ase-locus). A total of 10 granzymes have been found in the mouse (granzymes A-G and K-M), 7 in rats (A, B, C, I, J, K and M) and 5 in humans (granzymes A, B, H which are specific to human, M and tryptase2/granzyme-3). Human homologs of granzymes C-G have still not been found [129].
Granzymes are secreted from cytotoxic cells upon recognizing their target cells, with several physiological or pathophysiological consequences. Physiologically, granzymes promote extracellular matrix degradation, lymphocyte migration, cytokine production and attenuate tumor cell migration[130]. Pathological granzyme actions are important in inflammatory vascular disorders (atherosclerosis, transplant vascular disease, systemic lupus erythrematosus, autoimmune vasculitis) [131-133], chronic allergic or autoimmune diseases (arthritis, chronic allergic asthma, hypersensitive pneumonitis) [134-136] and neurodegenerative disorders (spinal cord injury, cerebral ischemia, multiple sclerosis, brain tumors like gliomas) [68,70,137-139]. Of the family of granzymes, granzyme-B (Gra-B) is considered to be the most potent apoptogenic molecule, even though granzyme-A (Gra-A) and Gra-B are the most abundant proteases in lytic granules [140]. Other members of the granzyme family perform functions outside of apoptosis and independent of caspases.
Gra-A has been shown to induce caspase-independent, but morphologically indistinguishable apoptosis. Induction of Gra-A mediated apoptosis involves activation of Gra-A activated DNase to induce single stranded nicks in DNA, breakdown of oxidative repair protein 'apurinic/apyrimidinic (AP) endonuclease' (APE), KU-70 and mitochondrial complex I protein [141-144]. Apart from lamin, which is the common substrate for Gra-A, Gra-B and caspase-3, PARP-1 is also cleaved by these 3 proteases resulting in different fragmentation patterns [66,145-147]. Importantly, even though PARP-1 is a direct substrate for Gra-A it can only cleave intracellular PARP-1 in the presence of perforins [145]. This finding also underscores the necessity of perforin for Gra-A to enter the cell to execute cell death, unlike Gra-B. Gra-A activity towards PARP-1 results in its breakdown with high efficiency after Lys498 leading to the formation of a C-terminal 55-kD inactive fragment and an N-terminal fragment of similar molecular weight. Cleavage of PARP-1 at Lys498 residue by Gra-A results in decreased auto-modification of PARP-1, poly (ADP ribosyl)ation and DNA repair. Gra-A mediated formation of N-terminal active 55-kD PARP-1 (after Lys498) may cause cells to undergo caspase-independent apoptotic cell death, rather than necrotic cell death, by decreasing the efficiency of ADP ribosylation. Since Gra-A cleaves oxidative repair enzymes and increases ROS production, it will indirectly activate PARP-1; decreased poly (ADP-ribosyl)ation counteracts these deleterious effects [145]. However, whether Gra-A has any role in 'parthanatos' (cell death mediated by poly (ADP ribose) polymers) or necrosis is a topic for future studies. Even though cathepsin-B and Gra-A produce similar 55-kD C-terminal PARP-1 fragments, they differ in recognized cleavage sites. For example, cathepsins cleave PARP-1 at leucine525 residues, but Gra-A cleaves preferentially at lysine498 [127,145] resulting in the formation of N-terminal fragments that attenuate PARP-1 depletion of ATP. Unlike Gra-A, cathepsin mediated PARP-1 cleavage at Leu525 results in the formation of 55-kD active C-terminal and 62-kD inactive N-terminal fragments [127]. Consequently cleavage-site specificity plays a major role in the generation of fragments derived from particular domains that differentially modulate forms of cell death (apoptosis vs. necrosis).
Gra-B was the first serine protease discovered to cleave PARP-1 during induction of cell death. Gra-B can translocate into cells in both a perforin-dependent and independent manner. Unlike Gra-A, Gra-B shares similar substrate specificity with caspase-3 (i.e. it cleaves after aspartate residues) and induces more rapid cell death [140]. Further, Gra-B can mediate apoptosis independent of caspases, or by directly activating caspases or indirectly by activating Bid [148,149].
Apart from various other cellular substrates, Gra-B cleaves PARP-1 into 64-kD and 61-kD N-terminal fragments and C-terminal 54-kD and 42-kD fragments. Of these, the 54-kD and 42-kD PARP-1 fragments were found to be catalytically active, while the 64-kD and 61-kD fragments were inactive. This was mainly based on the presence of intact catalytic domain at the C-terminus [147]. Using activity western blots, it has been shown that the fragments 54-kD and 42-kD fragments are catalytically active. The 42-kD fragment was detected in activity western blots but not when immunoblotted with N-terminus specific antibody, indicating that it is C-terminus fragment. N-terminal analysis of the 54-kD fragment has shown that the cleavage site follows Asp537. It was further suggested that the appearance of 42-kD fragment is due to the secondary cleavage of 54-kD and 61-kD fragment was due to the secondary cleavage of 64-kD fragment [147].
The appearance of PARP-1 fragments with multiple molecular weights clearly distinguishes these proteases from each other, and suggests the participation of specific proteases during different phases and forms of pathology. The presence of some PARP-1 fragments, like the 50-kD and 55-kD, which can be formed by either necrotic cathepsins or by Gra-A, need to be carefully interpreted [127,145]. The PARP-1 fragments created by granzyme action are indicated in figure 1 and Additional file 1: Table-1. Based on the roles of PARP-1 in angiogenesis, whether Gra-B cleavage of PARP-1 and the resulting PARP-1 fragments have any role in modulating angiogenesis during forms of neurodegeneration is a potential opportunity for therapy.
PARP-1 and matrix metalloproteinases
MMPs are a family of 28 zinc-dependent endopeptidases that play significant roles in angiogenesis, embryogenesis and in several pathological cardiovascular diseases like myocardial ischemia, cerebral ischemia, and atherosclerosis [150-152]. Within this family of MMPs, MMP-2 has recently been shown to be capable of cleaving PARP-1. Kwan et al. have reported that MMP-2 along with MMP-9 is present in the nuclear fraction of heart and liver, and is able to cleave PARP-1 in a concentration dependent manner. Cleavage of PARP-1 by MMP-2 produces a 66-kD and a >48-kD fragment (seen in silver stained gels and western blotting) [153] which differ in their appearance. MMP-2 produced a 66-kD PARP-1 fragment in a concentration-dependent fashion whereas there was no difference in levels of other >48-kD forms from control conditions observed in silver stained gels. Interestingly western blot analysis did not show any 66-kD PARP-1 fragments but showed an increase in >48-kD fragments in a concentration-dependent fashion. The MMP inhibitors TIMP-2 and doxycycline were able to block PARP-1 degradation to a 66-kD fragment. The reason for the appearance of a prominent ~66-kD fragment in TIMP-2 treated conditions and a faint band of the same molecular weight in doxycycline treated samples is curious. Why this fragment is specifically detected in MMP-2 inhibited samples is also unclear. Though the differences in the appearance of >48-kD and 66-kD fragments may relate to the PARP-1 antibody used (either C-terminus or N-terminus specific), the reason for the appearance of ~66-kD fragment in samples with inhibited MMP-2 (but not in untreated controls) are unclear.
MMPs actions are modulated by the cytokines and endogenous MMP inhibitors in both pro- and anti-angiogenic programs affecting extracellular and basement membrane remodeling [154]. MMP-2 is known to be a key protease involved in neovascularization, and MMP-2-/- mice show defects in neovascularization. Conversely, MMP-9 may limit collagenase-induced intracerebral hemorrhage [155,156]. Moreover, conditioned medium enriched in MMP-2 and 9 (from mouse brain endothelial cells) increases the migration of neural precursor cells to sites of brain injury via ERK1/2 and PI3/AKT signaling [157]. However, several CNS pathologies such as cerebral ischemia, multiple sclerosis and Devic's neuromyelitis optica are also associated with increased MMP-2 and-9 levels and MMP-9 inhibition is beneficial against cerebral ischemia [151,158]. Nicolescu et al., have recently reported that PARP-1 inhibitors can also inhibit MMPs [159], indicating that at least part of the protection afforded by PARP-1 inhibitors during stroke might be due to MMP inhibition. PARP-1 inhibition undoubtedly rescues cells from necrotic cell death, but the role(s) played by MMP inhibition in stroke clearly deserve further study.
Although acute PARP-1 and MMP inhibition may effectively attenuate ischemic stroke, the roles of MMPs in neuronal progenitor migration, and PARP-1's role in angiogenesis programs during restitution need some clarification. Importantly, whether 66-kD and >48-kD PARP-1 fragments produced by MMP proteolysis have activity or modulate PARP-1 activity is still unknown. Because PARP-1 also regulates angiogenesis, the effect of PARP-1 proteolysis by MMPs during angiogenesis may be important to investigate for cancer and chronic inflammation therapy.
Conclusions
The molecular mechanisms involved in balancing life and death decisions controlled by PARP-1 are highly complex and incompletely understood. A delicate PARP-1 equilibrium exists within cells where any deviation, either hyper- or hypo-activity can induce or exacerbate pathology. Many related proteases including caspases, calpains, cathepsins, granzymes and MMPs also directly and indirectly mediate the effects of PARP-1 in these phenomena. Recent reports indicate that PARP-1 contributions to cell death are insult- and context-dependent. PARP-1 can also promote tissue survival by shifting the balance of cell death programs between autophagy and necrosis. PARP-1's role in driving autophagy from necrosis is distinctive from its classical role and highlights its importance in cell survival decisions. It also points out an important potential application through which modulation of PARP-1 may be applied therapeutically. Because of PARP-1's dual nature in life: death control, PARP inhibitors should be used with great caution. Furthermore, physiological and pathological roles of poly (ADP-ribosyl)ation in parthanatos and long term memory suggest that prolonged inhibition of PARP-1 may be ultimately deleterious. Conversely, the 24-kD N-terminal PARP-1 apoptotic fragment that attenuates necrotic cell death might eventually be developed for therapy. The appearance of specific PARP-1 fragments will not only help us understand the involvement of specific cell death proteases in particular processes, but also different types of cell death. These recent findings clearly indicate that a great deal remains be defined about PARP-1, the roles of its fragments and the specific molecular mechanisms they regulate during various forms of cell death.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
GVC wrote and edited the manuscript, JSA edited the manuscript and PPB edited and communicated the manuscript. All authors read and approved the final manuscript.
Supplementary Material
Table -1. PARP-1 signature fragments. Action of various proteases results in the generation of PARP-1 fragments with specific molecular weights that can be correlated with the action of specific proteases. PARP-1 signature fragments generated by various proteases are listed above.
Contributor Information
Ganta Vijay Chaitanya, Email: vganta@lsuhsc.edu.
Jonathan S Alexander, Email: JAlexa@lsuhsc.edu.
Phanithi Prakash Babu, Email: ppbsl@uohyd.ernet.in.
Acknowledgements
The authors are grateful for the excellent editorial help of Ms. Merilyn Jennings, Department of Molecular and Cellular Physiology, LSUHSC-Shreveport, LA in the preparation of this review. The authors thank Prof. K. Subba Rao, INSA Senior Scientist, JNTU, Hyderabad, India for critical suggestions. Post-doctoral fellowship from the Malcolm Feist Cardiovascular Research Endowment- LSU Health Sciences Center-Shreveport to Ganta Vijay Chaitanya is acknowledged. Funding from DBT, DST and ICMR, New Delhi, Government of India to Prof. Prakash Babu is acknowledged.
References
- Dantzer F, de La RG, Menissier-de Murcia J, Hostomsky Z, de Murcia G, Schreiber V. Base excision repair is impaired in mammalian cells lacking Poly(ADP-ribose) polymerase-1. Biochemistry. 2000;39:7559–7569. doi: 10.1021/bi0003442. [DOI] [PubMed] [Google Scholar]
- Dantzer F, Schreiber V, Niedergang C, Trucco C, Flatter E, de La RG. et al. Involvement of poly(ADP-ribose) polymerase in base excision repair. Biochimie. 1999;81:69–75. doi: 10.1016/S0300-9084(99)80040-6. [DOI] [PubMed] [Google Scholar]
- Audebert M, Salles B, Calsou P. Involvement of poly(ADP-ribose) polymerase-1 and XRCC1/DNA ligase III in an alternative route for DNA double-strand breaks rejoining. J Biol Chem. 2004;279:55117–55126. doi: 10.1074/jbc.M404524200. [DOI] [PubMed] [Google Scholar]
- Benjamin RC, Gill DM. Poly(ADP-ribose) synthesis in vitro programmed by damaged DNA. A comparison of DNA molecules containing different types of strand breaks. J Biol Chem. 1980;255:10502–10508. [PubMed] [Google Scholar]
- Fisher AE, Hochegger H, Takeda S, Caldecott KW. Poly(ADP-ribose) polymerase 1 accelerates single-strand break repair in concert with poly(ADP-ribose) glycohydrolase. Mol Cell Biol. 2007;27:5597–5605. doi: 10.1128/MCB.02248-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Wu W, Wu W, Rosidi B, Zhang L, Wang H. et al. PARP-1 and Ku compete for repair of DNA double strand breaks by distinct NHEJ pathways. Nucleic Acids Res. 2006;34:6170–6182. doi: 10.1093/nar/gkl840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susse S, Scholz CJ, Burkle A, Wiesmuller L. Poly(ADP-ribose) polymerase (PARP-1) and p53 independently function in regulating double-strand break repair in primate cells. Nucleic Acids Res. 2004;32:669–680. doi: 10.1093/nar/gkh227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh DW, Dawson TM, Dawson VL. Poly(ADP-ribosyl)ation regulation of life and death in the nervous system. Cellular and Molecular Life Sciences. 2005;62:760–768. doi: 10.1007/s00018-004-4508-y. [DOI] [PubMed] [Google Scholar]
- Albadawi H, Crawford RS, Atkins MD, Watkins MT. Role of poly(ADP-ribose) polymerase during vascular reconstruction. Vascular. 2006;14:362–365. doi: 10.2310/6670.2006.00061. [DOI] [PubMed] [Google Scholar]
- Burkle A, Brabeck C, Diefenbach J, Beneke S. The emerging role of poly(ADP-ribose) polymerase-1 in longevity. Int J Biochem Cell Biol. 2005;37:1043–1053. doi: 10.1016/j.biocel.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Chiarugi A, Moskowitz MA. Poly(ADP-ribose) polymerase-1 activity promotes NF-kappaB-driven transcription and microglial activation: implication for neurodegenerative disorders. J Neurochem. 2003;85:306–317. doi: 10.1046/j.1471-4159.2003.01684.x. [DOI] [PubMed] [Google Scholar]
- Hernandez AI, Wolk J, Hu JY, Liu J, Kurosu T, Schwartz JH. et al. Poly-(ADP-ribose) polymerase-1 is necessary for long-term facilitation in Aplysia. J Neurosci. 2009;29:9553–9562. doi: 10.1523/JNEUROSCI.1512-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus WL, Lis JT. PARP goes transcription. Cell. 2003;113:677–683. doi: 10.1016/S0092-8674(03)00433-1. [DOI] [PubMed] [Google Scholar]
- Phulwani NK, Kielian T. Poly (ADP-ribose) polymerases (PARPs) 1-3 regulate astrocyte activation. J Neurochem. 2008;106:578–590. doi: 10.1111/j.1471-4159.2008.05403.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung YJ, Ambron RT. PolyADP-ribose polymerase-1 (PARP-1) and the evolution of learning and memory. Bioessays. 2004;26:1268–1271. doi: 10.1002/bies.20164. [DOI] [PubMed] [Google Scholar]
- von Lukowicz T, Hassa PO, Lohmann C, Boren J, Braunersreuther V, Mach F. et al. PARP1 is required for adhesion molecule expression in atherogenesis. Cardiovasc Res. 2008;78:158–166. doi: 10.1093/cvr/cvm110. [DOI] [PubMed] [Google Scholar]
- Xiao CY, Chen M, Zsengeller Z, Li H, Kiss L, Kollai M. et al. Poly(ADP-Ribose) polymerase promotes cardiac remodeling, contractile failure, and translocation of apoptosis-inducing factor in a murine experimental model of aortic banding and heart failure. J Pharmacol Exp Ther. 2005;312:891–898. doi: 10.1124/jpet.104.077164. [DOI] [PubMed] [Google Scholar]
- Aguennouz M, Vita GL, Messina S, Cama A, Lanzano N, Ciranni A, Telomere shortening is associated to TRF1 and PARP1 overexpression in Duchenne muscular dystrophy. Neurobiol Aging. 2010. in press . [DOI] [PubMed]
- Wang ZQ, Auer B, Stingl L, Berghammer H, Haidacher D, Schweiger M. et al. Mice lacking ADPRT and poly(ADP-ribosyl)ation develop normally but are susceptible to skin disease. Genes Dev. 1995;9:509–520. doi: 10.1101/gad.9.5.509. [DOI] [PubMed] [Google Scholar]
- Simbulan-Rosenthal CM, Ly DH, Rosenthal DS, Konopka G, Luo R, Wang ZQ. et al. Misregulation of gene expression in primary fibroblasts lacking poly(ADP-ribose) polymerase. Proc Natl Acad Sci USA. 2000;97:11274–11279. doi: 10.1073/pnas.200285797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogino H, Nozaki T, Gunji A, Maeda M, Suzuki H, Ohta T. et al. Loss of Parp-1 affects gene expression profile in a genome-wide manner in ES cells and liver cells. BMC Genomics. 2007;8:41. doi: 10.1186/1471-2164-8-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zingarelli B, Hake PW, O'Connor M, Denenberg A, Kong S, Aronow BJ. Absence of poly(ADP-ribose)polymerase-1 alters nuclear factor-kappa B activation and gene expression of apoptosis regulators after reperfusion injury. Mol Med. 2003;9:143–153. doi: 10.2119/2003-00011.Zingarelli. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simbulan-Rosenthal CM, Rosenthal DS, Luo R, Samara R, Espinoza LA, Hassa PO. et al. PARP-1 binds E2F-1 independently of its DNA binding and catalytic domains, and acts as a novel coactivator of E2F-1-mediated transcription during re-entry of quiescent cells into S phase. Oncogene. 2003;22:8460–8471. doi: 10.1038/sj.onc.1206897. [DOI] [PubMed] [Google Scholar]
- Olabisi OA, Soto-Nieves N, Nieves E, Yang TT, Yang X, Yu RY. et al. Regulation of transcription factor NFAT by ADP-ribosylation. Mol Cell Biol. 2008;28:2860–2871. doi: 10.1128/MCB.01746-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassa PO, Hottiger MO. A role of poly (ADP-ribose) polymerase in NF-kappaB transcriptional activation. Biol Chem. 1999;380:953–959. doi: 10.1515/BC.1999.118. [DOI] [PubMed] [Google Scholar]
- Hassa PO, Hottiger MO. The functional role of poly(ADP-ribose)polymerase 1 as novel coactivator of NF-kappaB in inflammatory disorders. Cell Mol Life Sci. 2002;59:1534–1553. doi: 10.1007/s00018-002-8527-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Armon M, Visochek L, Rozensal D, Kalal A, Geistrikh I, Klein R. et al. DNA-independent PARP-1 activation by phosphorylated ERK2 increases Elk1 activity: a link to histone acetylation. Mol Cell. 2007;25:297–308. doi: 10.1016/j.molcel.2006.12.012. [DOI] [PubMed] [Google Scholar]
- Cohen-Armon M. PARP-1 activation in the ERK signaling pathway. Trends Pharmacol Sci. 2007;28:556–560. doi: 10.1016/j.tips.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Oshima T, Pavlick KP, Laroux FS, Verma SK, Jordan P, Grisham MB. et al. Regulation and distribution of MAdCAM-1 in endothelial cells in vitro. Am J Physiol Cell Physiol. 2001;281:C1096–C1105. doi: 10.1152/ajpcell.2001.281.4.C1096. [DOI] [PubMed] [Google Scholar]
- Sharp C, Warren A, Oshima T, Williams L, Li JH, Alexander JS. Poly ADP ribose-polymerase inhibitors prevent the upregulation of ICAM-1 and E-selectin in response to Th1 cytokine stimulation. Inflammation. 2001;25:157–163. doi: 10.1023/A:1011032313445. [DOI] [PubMed] [Google Scholar]
- Ame JC, Spenlehauer C, de Murcia G. The PARP superfamily. Bioessays. 2004;26:882–893. doi: 10.1002/bies.20085. [DOI] [PubMed] [Google Scholar]
- Yamanaka H, Penning CA, Willis EH, Wasson DB, Carson DA. Characterization of human poly(ADP-ribose) polymerase with autoantibodies. J Biol Chem. 1988;263:3879–3883. [PubMed] [Google Scholar]
- Woodhouse BC, Dianov GL. Poly ADP-ribose polymerase-1: an international molecule of mystery. DNA Repair (Amst) 2008;7:1077–1086. doi: 10.1016/j.dnarep.2008.03.009. [DOI] [PubMed] [Google Scholar]
- D'Amours D, Desnoyers S, D'Silva I, Poirier GG. Poly(ADP-ribosyl)ation reactions in the regulation of nuclear functions. Biochem J. 1999;342(Pt 2):249–268. [PMC free article] [PubMed] [Google Scholar]
- Scovassi AI. The poly(ADP-ribosylation) story: a long route from Cinderella to Princess. Riv Biol. 2007;100:351–360. [PubMed] [Google Scholar]
- Althaus FR, Richter C. ADP-ribosylation of proteins. Enzymology and biological significance. Mol Biol Biochem Biophys. 1987;37:1–237. [PubMed] [Google Scholar]
- Choudhary C, Kumar C, Gnad F, Nielsen ML, Rehman M, Walther TC. et al. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science. 2009;325:834–840. doi: 10.1126/science.1175371. [DOI] [PubMed] [Google Scholar]
- Perrot R, Berges R, Bocquet A, Eyer J. Review of the multiple aspects of neurofilament functions, and their possible contribution to neurodegeneration. Mol Neurobiol. 2008;38:27–65. doi: 10.1007/s12035-008-8033-0. [DOI] [PubMed] [Google Scholar]
- Shukla A, Chaurasia P, Bhaumik SR. Histone methylation and ubiquitination with their cross-talk and roles in gene expression and stability. Cell Mol Life Sci. 2009;66:1419–1433. doi: 10.1007/s00018-008-8605-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolli V, O'Farrell M, Menissier-de Murcia J, de Murcia G. Random mutagenesis of the poly(ADP-ribose) polymerase catalytic domain reveals amino acids involved in polymer branching. Biochemistry. 1997;36:12147–12154. doi: 10.1021/bi971055p. [DOI] [PubMed] [Google Scholar]
- Chang P, Jacobson MK, Mitchison TJ. Poly(ADP-ribose) is required for spindle assembly and structure. Nature. 2004;432:645–649. doi: 10.1038/nature03061. [DOI] [PubMed] [Google Scholar]
- Herceg Z, Wang ZQ. Functions of poly(ADP-ribose) polymerase (PARP) in DNA repair, genomic integrity and cell death. Mutat Res. 2001;477:97–110. doi: 10.1016/s0027-5107(01)00111-7. [DOI] [PubMed] [Google Scholar]
- Ullrich O, Diestel A, Bechmann I, Homberg M, Grune T, Hass R. et al. Turnover of oxidatively damaged nuclear proteins in BV-2 microglial cells is linked to their activation state by poly-ADP-ribose polymerase. FASEB J. 2001;15:1460–1462. doi: 10.1096/fj.00-0540fje. [DOI] [PubMed] [Google Scholar]
- Kun E, Kirsten E, Mendeleyev J, Ordahl CP. Regulation of the enzymatic catalysis of poly(ADP-ribose) polymerase by dsDNA, polyamines, Mg2+, Ca2+, histones H1 and H3, and ATP. Biochemistry. 2004;43:210–216. doi: 10.1021/bi0301791. [DOI] [PubMed] [Google Scholar]
- Potaman VN, Shlyakhtenko LS, Oussatcheva EA, Lyubchenko YL, Soldatenkov VA. Specific binding of poly(ADP-ribose) polymerase-1 to cruciform hairpins. J Mol Biol. 2005;348:609–615. doi: 10.1016/j.jmb.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Wacker DA, Ruhl DD, Balagamwala EH, Hope KM, Zhang T, Kraus WL. The DNA binding and catalytic domains of poly(ADP-ribose) polymerase 1 cooperate in the regulation of chromatin structure and transcription. Mol Cell Biol. 2007;27:7475–7485. doi: 10.1128/MCB.01314-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langelier MF, Servent KM, Rogers EE, Pascal JM. A third zinc-binding domain of human poly(ADP-ribose) polymerase-1 coordinates DNA-dependent enzyme activation. J Biol Chem. 2008;283:4105–4114. doi: 10.1074/jbc.M708558200. [DOI] [PubMed] [Google Scholar]
- El Khamisy SF, Masutani M, Suzuki H, Caldecott KW. A requirement for PARP-1 for the assembly or stability of XRCC1 nuclear foci at sites of oxidative DNA damage. Nucleic Acids Res. 2003;31:5526–5533. doi: 10.1093/nar/gkg761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson M, Niedergang C, Schreiber V, Muller S, Menissier-de Murcia J, de Murcia G. XRCC1 is specifically associated with poly(ADP-ribose) polymerase and negatively regulates its activity following DNA damage. Mol Cell Biol. 1998;18:3563–3571. doi: 10.1128/mcb.18.6.3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galluzzi L, Joza N, Tasdemir E, Maiuri MC, Hengartner M, Abrams JM. et al. No death without life: vital functions of apoptotic effectors. Cell Death Differ. 2008;15:1113–1123. doi: 10.1038/cdd.2008.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberst A, Bender C, Green DR. Living with death: the evolution of the mitochondrial pathway of apoptosis in animals. Cell Death Differ. 2008;15:1139–1146. doi: 10.1038/cdd.2008.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter TG. Apoptosis and cancer: the genesis of a research field. Nat Rev Cancer. 2009;9:501–507. doi: 10.1038/nrc2663. [DOI] [PubMed] [Google Scholar]
- Yakovlev AG, Faden AI. Caspase-dependent apoptotic pathways in CNS injury. Mol Neurobiol. 2001;24:131–144. doi: 10.1385/MN:24:1-3:131. [DOI] [PubMed] [Google Scholar]
- Li J, Yuan J. Caspases in apoptosis and beyond. Oncogene. 2008;27:6194–6206. doi: 10.1038/onc.2008.297. [DOI] [PubMed] [Google Scholar]
- Fan Y, Bergmann A. Apoptosis-induced compensatory proliferation. The Cell is dead. Long live the Cell! Trends Cell Biol. 2008;18:467–473. doi: 10.1016/j.tcb.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Bergmann A. Distinct mechanisms of apoptosis-induced compensatory proliferation in proliferating and differentiating tissues in the Drosophila eye. Dev Cell. 2008;14:399–410. doi: 10.1016/j.devcel.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells BS, Yoshida E, Johnston LA. Compensatory proliferation in Drosophila imaginal discs requires Dronc-dependent p53 activity. Curr Biol. 2006;16:1606–1615. doi: 10.1016/j.cub.2006.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo S, Senoo-Matsuda N, Hiromi Y, Miura M. DRONC coordinates cell death and compensatory proliferation. Mol Cell Biol. 2006;26:7258–7268. doi: 10.1128/MCB.00183-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droin N, Jacquel A, Guery L, Dufour E, Garrido C, Solary E. Various functions of caspases in hematopoiesis. Front Biosci. 2009;14:2358–2371. doi: 10.2741/3383. [DOI] [PubMed] [Google Scholar]
- Gulyaeva NV. Non-apoptotic functions of caspase-3 in nervous tissue. Biochemistry (Mosc) 2003;68:1171–1180. doi: 10.1023/B:BIRY.0000009130.62944.35. [DOI] [PubMed] [Google Scholar]
- Gulyaeva NV, Kudryashov IE, Kudryashova IV. Caspase activity is essential for long-term potentiation. J Neurosci Res. 2003;73:853–864. doi: 10.1002/jnr.10730. [DOI] [PubMed] [Google Scholar]
- Fischer U, Janicke RU, Schulze-Osthoff K. Many cuts to ruin: a comprehensive update of caspase substrates. Cell Death Differ. 2003;10:76–100. doi: 10.1038/sj.cdd.4401160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann SH, Desnoyers S, Ottaviano Y, Davidson NE, Poirier GG. Specific proteolytic cleavage of poly(ADP-ribose) polymerase: an early marker of chemotherapy-induced apoptosis. Cancer Res. 1993;53:3976–3985. [PubMed] [Google Scholar]
- Tewari M, Quan LT, O'Rourke K, Desnoyers S, Zeng Z, Beidler DR. et al. Yama/CPP32 beta, a mammalian homolog of CED-3, is a CrmA-inhibitable protease that cleaves the death substrate poly(ADP-ribose) polymerase. Cell. 1995;81:801–809. doi: 10.1016/0092-8674(95)90541-3. [DOI] [PubMed] [Google Scholar]
- Margolin N, Raybuck SA, Wilson KP, Chen W, Fox T, Gu Y. et al. Substrate and inhibitor specificity of interleukin-1 beta-converting enzyme and related caspases. J Biol Chem. 1997;272:7223–7228. doi: 10.1074/jbc.272.11.7223. [DOI] [PubMed] [Google Scholar]
- Lazebnik YA, Kaufmann SH, Desnoyers S, Poirier GG, Earnshaw WC. Cleavage of poly(ADP-ribose) polymerase by a proteinase with properties like ICE. Nature. 1994;371:346–347. doi: 10.1038/371346a0. [DOI] [PubMed] [Google Scholar]
- Bhaskara VK, Panigrahi M, Challa S, Babu PP. Comparative status of activated ERK1/2 and PARP cleavage in human gliomas. Neuropathology. 2005;25:48–53. doi: 10.1111/j.1440-1789.2004.00585.x. [DOI] [PubMed] [Google Scholar]
- Bhaskara VK, Challa S, Panigrahi M, Babu PP. Differential PARP cleavage: An indication for existence of multiple forms of cell death in human gliomas. Neurol India. 2009;57:264–268. doi: 10.4103/0028-3886.53265. [DOI] [PubMed] [Google Scholar]
- Chaitanya GV, Babu PP. Activation of calpain, cathepsin-b and caspase-3 during transient focal cerebral ischemia in rat model. Neurochem Res. 2008;33:2178–2186. doi: 10.1007/s11064-007-9567-7. [DOI] [PubMed] [Google Scholar]
- Chaitanya GV, Babu PP. Differential PARP cleavage: an indication of heterogeneous forms of cell death and involvement of multiple proteases in the infarct of focal cerebral ischemia in rat. Cell Mol Neurobiol. 2009;29:563–573. doi: 10.1007/s10571-009-9348-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliams-Francis KL, Quaye AA, Naegele JR. PARP cleavage, DNA fragmentation, and pyknosis during excitotoxin-induced neuronal death. Exp Neurol. 2003;184:359–372. doi: 10.1016/j.expneurol.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Kanthasamy AG, Anantharam V, Zhang D, Latchoumycandane C, Jin H, Kaul S. et al. A novel peptide inhibitor targeted to caspase-3 cleavage site of a proapoptotic kinase protein kinase C delta (PKCdelta) protects against dopaminergic neuronal degeneration in Parkinson's disease models. Free Radic Biol Med. 2006;41:1578–1589. doi: 10.1016/j.freeradbiomed.2006.08.016. [DOI] [PubMed] [Google Scholar]
- Lau A, Arundine M, Sun HS, Jones M, Tymianski M. Inhibition of caspase-mediated apoptosis by peroxynitrite in traumatic brain injury. J Neurosci. 2006;26:11540–11553. doi: 10.1523/JNEUROSCI.3507-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng QL, Buz'Zard AR, Lau BH. Pycnogenol protects neurons from amyloid-beta peptide-induced apoptosis. Brain Res Mol Brain Res. 2002;104:55–65. doi: 10.1016/S0169-328X(02)00263-2. [DOI] [PubMed] [Google Scholar]
- Soldani C, Lazze MC, Bottone MG, Tognon G, Biggiogera M, Pellicciari CE. et al. Poly(ADP-ribose) polymerase cleavage during apoptosis: when and where? Exp Cell Res. 2001;269:193–201. doi: 10.1006/excr.2001.5293. [DOI] [PubMed] [Google Scholar]
- D'Amours D, Sallmann FR, Dixit VM, Poirier GG. Gain-of-function of poly(ADP-ribose) polymerase-1 upon cleavage by apoptotic proteases: implications for apoptosis. J Cell Sci. 2001;114:3771–3778. doi: 10.1242/jcs.114.20.3771. [DOI] [PubMed] [Google Scholar]
- Smulson ME, Pang D, Jung M, Dimtchev A, Chasovskikh S, Spoonde A. et al. Irreversible binding of poly(ADP)ribose polymerase cleavage product to DNA ends revealed by atomic force microscopy: possible role in apoptosis. Cancer Res. 1998;58:3495–3498. [PubMed] [Google Scholar]
- Alvarez-Gonzalez R, Spring H, Muller M, Burkle A. Selective loss of poly(ADP-ribose) and the 85-kDa fragment of poly(ADP-ribose) polymerase in nucleoli during alkylation-induced apoptosis of HeLa cells. J Biol Chem. 1999;274:32122–32126. doi: 10.1074/jbc.274.45.32122. [DOI] [PubMed] [Google Scholar]
- Germain M, Affar EB, D'Amours D, Dixit VM, Salvesen GS, Poirier GG. Cleavage of automodified poly(ADP-ribose) polymerase during apoptosis. Evidence for involvement of caspase-7. J Biol Chem. 1999;274:28379–28384. doi: 10.1074/jbc.274.40.28379. [DOI] [PubMed] [Google Scholar]
- Eguchi Y, Shimizu S, Tsujimoto Y. Intracellular ATP levels determine cell death fate by apoptosis or necrosis. Cancer Res. 1997;57:1835–1840. [PubMed] [Google Scholar]
- Lemaire C, Andreau K, Souvannavong V, Adam A. Inhibition of caspase activity induces a switch from apoptosis to necrosis. FEBS Lett. 1998;425:266–270. doi: 10.1016/S0014-5793(98)00252-X. [DOI] [PubMed] [Google Scholar]
- Herceg Z, Wang ZQ. Failure of poly(ADP-ribose) polymerase cleavage by caspases leads to induction of necrosis and enhanced apoptosis. Mol Cell Biol. 1999;19:5124–5133. doi: 10.1128/mcb.19.7.5124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aikin R, Rosenberg L, Paraskevas S, Maysinger D. Inhibition of caspase-mediated PARP-1 cleavage results in increased necrosis in isolated islets of Langerhans. J Mol Med. 2004;82:389–397. doi: 10.1007/s00109-004-0540-5. [DOI] [PubMed] [Google Scholar]
- Los M, Mozoluk M, Ferrari D, Stepczynska A, Stroh C, Renz A. et al. Activation and caspase-mediated inhibition of PARP: a molecular switch between fibroblast necrosis and apoptosis in death receptor signaling. Mol Biol Cell. 2002;13:978–988. doi: 10.1091/mbc.01-05-0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Wang KK. The calpain family and human disease. Trends Mol Med. 2001;7:355–362. doi: 10.1016/S1471-4914(01)02049-4. [DOI] [PubMed] [Google Scholar]
- Verkhratsky A. Calcium and cell death. Subcell Biochem. 2007;45:465–480. doi: 10.1007/978-1-4020-6191-2_17. full_text. [DOI] [PubMed] [Google Scholar]
- Bhupanapadu S, Swain U, Babu PP. Cell death is associated with reduced base excision repair during chronic alcohol administration in adult rat brain. Neurochem Res. 2008;33:1117–1128. doi: 10.1007/s11064-007-9560-1. [DOI] [PubMed] [Google Scholar]
- Bizat N, Hermel JM, Boyer F, Jacquard C, Creminon C, Ouary S. et al. Calpain is a major cell death effector in selective striatal degeneration induced in vivo by 3-nitropropionate: implications for Huntington's disease. J Neurosci. 2003;23:5020–5030. doi: 10.1523/JNEUROSCI.23-12-05020.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goni-Oliver P, Avila J, Hernandez F. Memantine Inhibits Calpain-Mediated Truncation of GSK-3 Induced by NMDA: Implications in Alzheimer's Disease. J Alzheimers Dis. 2009;18:843–848. doi: 10.3233/JAD-2009-1190. [DOI] [PubMed] [Google Scholar]
- Samantaray S, Ray SK, Banik NL. Calpain as a potential therapeutic target in Parkinson's disease. CNS Neurol Disord Drug Targets. 2008;7:305–312. doi: 10.2174/187152708784936680. [DOI] [PubMed] [Google Scholar]
- Shukla M, Rajgopal Y, Babu PP. Activation of calpains, calpastatin and spectrin cleavage in the brain during the pathology of fatal murine cerebral malaria. Neurochem Int. 2006;48:108–113. doi: 10.1016/j.neuint.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Zhou M, Xu W, Liao G, Bi X, Baudry M. Neuroprotection against neonatal hypoxia/ischemia-induced cerebral cell death by prevention of calpain-mediated mGluR1alpha truncation. Exp Neurol. 2009;218:75–82. doi: 10.1016/j.expneurol.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michetti M, Salamino F, Tedesco I, Averna M, Minafra R, Melloni E. et al. Autolysis of human erythrocyte calpain produces two active enzyme forms with different cell localization. FEBS Lett. 1996;392:11–15. doi: 10.1016/0014-5793(96)00775-2. [DOI] [PubMed] [Google Scholar]
- Hewitt KE, Lesiuk HJ, Tauskela JS, Morley P, Durkin JP. Selective coupling of mu-calpain activation with the NMDA receptor is independent of translocation and autolysis in primary cortical neurons. J Neurosci Res. 1998;54:223–232. doi: 10.1002/(SICI)1097-4547(19981015)54:2<223::AID-JNR10>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Dutt P, Croall DE, Arthur JS, Veyra TD, Williams K, Elce JS. et al. m-Calpain is required for preimplantation embryonic development in mice. BMC Dev Biol. 2006;6:3. doi: 10.1186/1471-213X-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang YC, Yeh JY, Forsberg NE, Ou BR. Involvement of mu- and m-calpains and protein kinase C isoforms in L8 myoblast differentiation. Int J Biochem Cell Biol. 2006;38:662–670. doi: 10.1016/j.biocel.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Wu HY, Lynch DR. Calpain and synaptic function. Mol Neurobiol. 2006;33:215–236. doi: 10.1385/MN:33:3:215. [DOI] [PubMed] [Google Scholar]
- Franco SJ, Huttenlocher A. Regulating cell migration: calpains make the cut. J Cell Sci. 2005;118:3829–3838. doi: 10.1242/jcs.02562. [DOI] [PubMed] [Google Scholar]
- Ma H, Tochigi A, Shearer TR, Azuma M. Calpain inhibitor SNJ-1945 attenuates events prior to angiogenesis in cultured human retinal endothelial cells. J Ocul Pharmacol Ther. 2009;25:409–414. doi: 10.1089/jop.2009.0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Y, Cui Z, Li Z, Block ER. Calpain-2 regulation of VEGF-mediated angiogenesis. FASEB J. 2006;20:1443–1451. doi: 10.1096/fj.05-5354com. [DOI] [PubMed] [Google Scholar]
- Rajesh M, Mukhopadhyay P, Batkai S, Godlewski G, Hasko G, Liaudet L. et al. Pharmacological inhibition of poly(ADP-ribose) polymerase inhibits angiogenesis. Biochem Biophys Res Commun. 2006;350:352–357. doi: 10.1016/j.bbrc.2006.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virag L, Scott GS, Antal-Szalmas P, O'Connor M, Ohshima H, Szabo C. Requirement of intracellular calcium mobilization for peroxynitrite-induced poly(ADP-ribose) synthetase activation and cytotoxicity. Mol Pharmacol. 1999;56:824–833. [PubMed] [Google Scholar]
- Bentle MS, Reinicke KE, Bey EA, Spitz DR, Boothman DA. Calcium-dependent modulation of poly(ADP-ribose) polymerase-1 alters cellular metabolism and DNA repair. J Biol Chem. 2006;281:33684–33696. doi: 10.1074/jbc.M603678200. [DOI] [PubMed] [Google Scholar]
- Szenczi O, Kemecsei P, Holthuijsen MF, van Riel NA, van der Vusse GJ, Pacher P. et al. Poly(ADP-ribose) polymerase regulates myocardial calcium handling in doxorubicin-induced heart failure. Biochem Pharmacol. 2005;69:725–732. doi: 10.1016/j.bcp.2004.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan Y, Gross RA, Sheu SS. Ca2+-dependent generation of mitochondrial reactive oxygen species serves as a signal for poly(ADP-ribose) polymerase-1 activation during glutamate excitotoxicity. J Physiol. 2007;585:741–758. doi: 10.1113/jphysiol.2007.145409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosler PS, Sun D, Wang S, Gao Y, Kintner DB, Signore AP. et al. Calcium dysregulation induces apoptosis-inducing factor release: Cross-talk between PARP-1- and calpain- signaling pathways. Exp Neurol. 2009;218:213–220. doi: 10.1016/j.expneurol.2009.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Xing D, Chen WR. mu-Calpain regulates caspase-dependent and apoptosis inducing factor-mediated caspase-independent apoptotic pathways in cisplatin-induced apoptosis. Int J Cancer. 2009;15;125(12):2757–66. doi: 10.1002/ijc.24626. [DOI] [PubMed] [Google Scholar]
- Liu X, Van Vleet T, Schnellmann RG. The role of calpain in oncotic cell death. Annu Rev Pharmacol Toxicol. 2004;44:349–370. doi: 10.1146/annurev.pharmtox.44.101802.121804. [DOI] [PubMed] [Google Scholar]
- Melloni E, Michetti M, Salamino F, Minafra R, Pontremoli S. Modulation of the calpain autoproteolysis by calpastatin and phospholipids. Biochem Biophys Res Commun. 1996;229:193–197. doi: 10.1006/bbrc.1996.1779. [DOI] [PubMed] [Google Scholar]
- Melloni E, Averna M, Salamino F, Sparatore B, Minafra R, Pontremoli S. Acyl-CoA-binding protein is a potent m-calpain activator. J Biol Chem. 2000;275:82–86. doi: 10.1074/jbc.275.1.82. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Sorimachi H. A novel aspect of calpain activation. FEBS Lett. 1998;433:1–4. doi: 10.1016/S0014-5793(98)00856-4. [DOI] [PubMed] [Google Scholar]
- Neumar RW, Xu YA, Gada H, Guttmann RP, Siman R. Cross-talk between calpain and caspase proteolytic systems during neuronal apoptosis. J Biol Chem. 2003;278:14162–14167. doi: 10.1074/jbc.M212255200. [DOI] [PubMed] [Google Scholar]
- Wang KK. Calpain and caspase: can you tell the difference? Trends Neurosci. 2000;23:20–26. doi: 10.1016/S0166-2236(99)01479-4. [DOI] [PubMed] [Google Scholar]
- McGinnis KM, Gnegy ME, Park YH, Mukerjee N, Wang KK. Procaspase-3 and poly(ADP)ribose polymerase (PARP) are calpain substrates. Biochem Biophys Res Commun. 1999;263:94–99. doi: 10.1006/bbrc.1999.1315. [DOI] [PubMed] [Google Scholar]
- Buki KG, Bauer PI, Kun E. Isolation and identification of a proteinase from calf thymus that cleaves poly(ADP-ribose) polymerase and histone H1. Biochim Biophys Acta. 1997;1338:100–106. doi: 10.1016/s0167-4838(96)00189-6. [DOI] [PubMed] [Google Scholar]
- Gafni J, Cong X, Chen SF, Gibson BW, Ellerby LM. Calpain-1 cleaves and activates caspase-7. J Biol Chem. 2009;284:25441–25449. doi: 10.1074/jbc.M109.038174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saftig P, Klumperman J. Lysosome biogenesis and lysosomal membrane proteins: trafficking meets function. Nat Rev Mol Cell Biol. 2009;10:623–635. doi: 10.1038/nrm2745. [DOI] [PubMed] [Google Scholar]
- Artal-Sanz M, Samara C, Syntichaki P, Tavernarakis N. Lysosomal biogenesis and function is critical for necrotic cell death in Caenorhabditis elegans. J Cell Biol. 2006;173:231–239. doi: 10.1083/jcb.200511103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova S, Repnik U, Bojic L, Petelin A, Turk V, Turk B. Lysosomes in apoptosis. Methods Enzymol. 2006;442:183–199. doi: 10.1016/S0076-6879(08)01409-2. full_text. [DOI] [PubMed] [Google Scholar]
- Pacheco FJ, Servin J, Dang D, Kim J, Molinaro C, Daniels T. et al. Involvement of lysosomal cathepsins in the cleavage of DNA topoisomerase I during necrotic cell death. Arthritis Rheum. 2005;52:2133–2145. doi: 10.1002/art.21147. [DOI] [PubMed] [Google Scholar]
- He C, Klionsky DJ. Regulation Mechanisms and Signaling Pathways of Autophagy. Annu Rev Genet. 2009;43:67–93. doi: 10.1146/annurev-genet-102808-114910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen YD, Sheng R, Zhang LS, Han R, Zhang X, Zhang XD. et al. Neuronal injury in rat model of permanent focal cerebral ischemia is associated with activation of autophagic and lysosomal pathways. Autophagy. 2008;4:762–769. doi: 10.4161/auto.6412. [DOI] [PubMed] [Google Scholar]
- Saelens X, Festjens N, Parthoens E, Vanoverberghe I, Kalai M, van Kuppeveld F. et al. Protein synthesis persists during necrotic cell death. J Cell Biol. 2005;168:545–551. doi: 10.1083/jcb.200407162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TJ, Nannuru KC, Futakuchi M, Singh RK. Cathepsin G-mediated enhanced TGF-beta signaling promotes angiogenesis via upregulation of VEGF and MCP-1. Cancer Lett. 2009;28;288(2):162–9. doi: 10.1016/j.canlet.2009.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burden RE, Gormley JA, Jaquin TJ, Small DM, Quinn DJ, Hegarty SM. et al. Antibody-mediated inhibition of cathepsin S blocks colorectal tumor invasion and angiogenesis. Clin Cancer Res. 2009;15:6042–6051. doi: 10.1158/1078-0432.CCR-09-1262. [DOI] [PubMed] [Google Scholar]
- Yang Y, Zhao S, Song J. Caspase-dependent apoptosis and -independent poly(ADP-ribose) polymerase cleavage induced by transforming growth factor beta1. Int J Biochem Cell Biol. 2004;36:223–234. doi: 10.1016/S1357-2725(03)00215-2. [DOI] [PubMed] [Google Scholar]
- Gobeil S, Boucher CC, Nadeau D, Poirier GG. Characterization of the necrotic cleavage of poly(ADP-ribose) polymerase (PARP-1): implication of lysosomal proteases. Cell Death Differ. 2001;8:588–594. doi: 10.1038/sj.cdd.4400851. [DOI] [PubMed] [Google Scholar]
- Chavez-Galan L, Arenas-Del Angel MC, Zenteno E, Chavez R, Lascurain R. Cell death mechanisms induced by cytotoxic lymphocytes. Cell Mol Immunol. 2009;6:15–25. doi: 10.1038/cmi.2009.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapani JA. Granzymes: a family of lymphocyte granule serine proteases. Genome Biol. 2001;2:REVIEWS3014. doi: 10.1186/gb-2001-2-12-reviews3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero V, Andrade F. Non-apoptotic functions of granzymes. Tissue Antigens. 2008;71:409–416. doi: 10.1111/j.1399-0039.2008.01013.x. [DOI] [PubMed] [Google Scholar]
- Choy JC, Cruz RP, Kerjner A, Geisbrecht J, Sawchuk T, Fraser SA. et al. Granzyme B induces endothelial cell apoptosis and contributes to the development of transplant vascular disease. Am J Transplant. 2005;5:494–499. doi: 10.1111/j.1600-6143.2004.00710.x. [DOI] [PubMed] [Google Scholar]
- Choy JC, McDonald PC, Suarez AC, Hung VH, Wilson JE, McManus BM. et al. Granzyme B in atherosclerosis and transplant vascular disease: association with cell death and atherosclerotic disease severity. Mod Pathol. 2003;16:460–470. doi: 10.1097/01.MP.0000067424.12280.BC. [DOI] [PubMed] [Google Scholar]
- Blanco P, Pitard V, Viallard JF, Taupin JL, Pellegrin JL, Moreau JF. Increase in activated CD8+ T lymphocytes expressing perforin and granzyme B correlates with disease activity in patients with systemic lupus erythematosus. Arthritis Rheum. 2005;52:201–211. doi: 10.1002/art.20745. [DOI] [PubMed] [Google Scholar]
- Saito S, Murakoshi K, Kotake S, Kamatani N, Tomatsu T. Granzyme B induces apoptosis of chondrocytes with natural killer cell-like cytotoxicity in rheumatoid arthritis. J Rheumatol. 2008;35:1932–1943. [PubMed] [Google Scholar]
- Tschopp CM, Spiegl N, Didichenko S, Lutmann W, Julius P, Virchow JC. et al. Granzyme B, a novel mediator of allergic inflammation: its induction and release in blood basophils and human asthma. Blood. 2006;108:2290–2299. doi: 10.1182/blood-2006-03-010348. [DOI] [PubMed] [Google Scholar]
- Humbert M, Magnan A, Ladurie FL, Dartevelle P, Simonneau G, Duroux P. et al. Perforin and granzyme B gene-expressing cells in bronchoalveolar lavage fluids from lung allograft recipients displaying cytomegalovirus pneumonitis. Transplantation. 1994;57:1289–1292. doi: 10.1097/00007890-199404270-00031. [DOI] [PubMed] [Google Scholar]
- Chaitanya GV, Kolli M, Babu PP. Granzyme-b mediated cell death in the spinal cord-injured rat model. Neuropathology. 2009;29:270–279. doi: 10.1111/j.1440-1789.2008.00980.x. [DOI] [PubMed] [Google Scholar]
- Wang T, Allie R, Conant K, Haughey N, Turchan-Chelowo J, Hahn K. et al. Granzyme B mediates neurotoxicity through a G-protein-coupled receptor. FASEB J. 2006;20:1209–1211. doi: 10.1096/fj.05-5022fje. [DOI] [PubMed] [Google Scholar]
- Chaitanya GV, Schwaninger M, Alexander JS, Babu PP. Granzyme-b is involved in mediating post-ischemic neuronal death during focal cerebral ischemia in rat model. Neuroscience. 2010;165:1203–1216. doi: 10.1016/j.neuroscience.2009.10.067. [DOI] [PubMed] [Google Scholar]
- Trapani JA, Sutton VR. Granzyme B: pro-apoptotic, antiviral and antitumor functions. Curr Opin Immunol. 2003;15:533–543. doi: 10.1016/S0952-7915(03)00107-9. [DOI] [PubMed] [Google Scholar]
- Martinvalet D, Thiery J. [A novel caspase-independent apoptotic pathway triggered by Granzyme A] Med Sci (Paris) 2008;24:901–903. doi: 10.1051/medsci/20082411901. [DOI] [PubMed] [Google Scholar]
- Martinvalet D, Dykxhoorn DM, Ferrini R, Lieberman J. Granzyme A cleaves a mitochondrial complex I protein to initiate caspase-independent cell death. Cell. 2008;133:681–692. doi: 10.1016/j.cell.2008.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu P, Zhang D, Chowdhury D, Martinvalet D, Keefe D, Shi L. et al. Granzyme A, which causes single-stranded DNA damage, targets the double-strand break repair protein Ku70. EMBO Rep. 2006;7:431–437. doi: 10.1038/sj.embor.7400622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beresford PJ, Zhang D, Oh DY, Fan Z, Greer EL, Russo ML. et al. Granzyme A activates an endoplasmic reticulum-associated caspase-independent nuclease to induce single-stranded DNA nicks. J Biol Chem. 2001;276:43285–43293. doi: 10.1074/jbc.M108137200. [DOI] [PubMed] [Google Scholar]
- Zhu P, Martinvalet D, Chowdhury D, Zhang D, Schlesinger A, Lieberman J. The cytotoxic T lymphocyte protease granzyme A cleaves and inactivates poly(adenosine 5'-diphosphate-ribose) polymerase-1. Blood. 2009;114:1205–1216. doi: 10.1182/blood-2008-12-195768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Beresford PJ, Greenberg AH, Lieberman J. Granzymes A and B directly cleave lamins and disrupt the nuclear lamina during granule-mediated cytolysis. Proc Natl Acad Sci USA. 2001;98:5746–5751. doi: 10.1073/pnas.101329598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froelich CJ, Hanna WL, Poirier GG, Duriez PJ, D'Amours D, Salvesen GS. et al. Granzyme B/perforin-mediated apoptosis of Jurkat cells results in cleavage of poly(ADP-ribose) polymerase to the 89-kDa apoptotic fragment and less abundant 64-kDa fragment. Biochem Biophys Res Commun. 1996;227:658–665. doi: 10.1006/bbrc.1996.1565. [DOI] [PubMed] [Google Scholar]
- Waterhouse NJ, Sedelies KA, Trapani JA. Role of Bid-induced mitochondrial outer membrane permeabilization in granzyme B-induced apoptosis. Immunol Cell Biol. 2006;84:72–78. doi: 10.1111/j.1440-1711.2005.01416.x. [DOI] [PubMed] [Google Scholar]
- Adrain C, Murphy BM, Martin SJ. Molecular ordering of the caspase activation cascade initiated by the cytotoxic T lymphocyte/natural killer (CTL/NK) protease granzyme B. J Biol Chem. 2005;280:4663–4673. doi: 10.1074/jbc.M410915200. [DOI] [PubMed] [Google Scholar]
- Krishnamurthy P, Peterson JT, Subramanian V, Singh M, Singh K. Inhibition of matrix metalloproteinases improves left ventricular function in mice lacking osteopontin after myocardial infarction. Mol Cell Biochem. 2009;322:53–62. doi: 10.1007/s11010-008-9939-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candelario-Jalil E, Yang Y, Rosenberg GA. Diverse roles of matrix metalloproteinases and tissue inhibitors of metalloproteinases in neuroinflammation and cerebral ischemia. Neuroscience. 2009;158:983–994. doi: 10.1016/j.neuroscience.2008.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrke M, Greif M, Broedl UC, Lebherz C, Laubender RP, Becker A. et al. MMP-1 serum levels predict coronary atherosclerosis in humans. Cardiovasc Diabetol. 2009;8:50. doi: 10.1186/1475-2840-8-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan JA, Schulze CJ, Wang W, Leon H, Sariahmetoglu M, Sung M. et al. Matrix metalloproteinase-2 (MMP-2) is present in the nucleus of cardiac myocytes and is capable of cleaving poly (ADP-ribose) polymerase (PARP) in vitro. FASEB J. 2004;18:690–692. doi: 10.1096/fj.02-1202fje. [DOI] [PubMed] [Google Scholar]
- Raffetto JD, Khalil RA. Matrix metalloproteinases and their inhibitors in vascular remodeling and vascular disease. Biochem Pharmacol. 2008;75:346–359. doi: 10.1016/j.bcp.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean RA, Butler GS, Hamma-Kourbali Y, Delbe J, Brigstock DR, Courty J. et al. Identification of candidate angiogenic inhibitors processed by matrix metalloproteinase 2 (MMP-2) in cell-based proteomic screens: disruption of vascular endothelial growth factor (VEGF)/heparin affin regulatory peptide (pleiotrophin) and VEGF/Connective tissue growth factor angiogenic inhibitory complexes by MMP-2 proteolysis. Mol Cell Biol. 2007;27:8454–8465. doi: 10.1128/MCB.00821-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J, Liu J, Zhou C, Alexander JS, Nanda A, Granger DN. et al. Mmp-9 deficiency enhances collagenase-induced intracerebral hemorrhage and brain injury in mutant mice. J Cereb Blood Flow Metab. 2004;24:1133–1145. doi: 10.1097/01.WCB.0000135593.05952.DE. [DOI] [PubMed] [Google Scholar]
- Wang L, Zhang ZG, Zhang RL, Gregg SR, Hozeska-Solgot A, LeTourneau Y. et al. Matrix metalloproteinase 2 (MMP2) and MMP9 secreted by erythropoietin-activated endothelial cells promote neural progenitor cell migration. J Neurosci. 2006;26:5996–6003. doi: 10.1523/JNEUROSCI.5380-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandler RN, Dencoff JD, Midani F, Ford CC, Ahmed W, Rosenberg GA. Matrix metalloproteinases and tissue inhibitors of metalloproteinases in cerebrospinal fluid differ in multiple sclerosis and Devic's neuromyelitis optica. Brain. 2001;124:493–498. doi: 10.1093/brain/124.3.493. [DOI] [PubMed] [Google Scholar]
- Nicolescu AC, Holt A, Kandasamy AD, Pacher P, Schulz R. Inhibition of matrix metalloproteinase-2 by PARP inhibitors. Biochem Biophys Res Commun. 2009;387:646–650. doi: 10.1016/j.bbrc.2009.07.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table -1. PARP-1 signature fragments. Action of various proteases results in the generation of PARP-1 fragments with specific molecular weights that can be correlated with the action of specific proteases. PARP-1 signature fragments generated by various proteases are listed above.


