Abstract
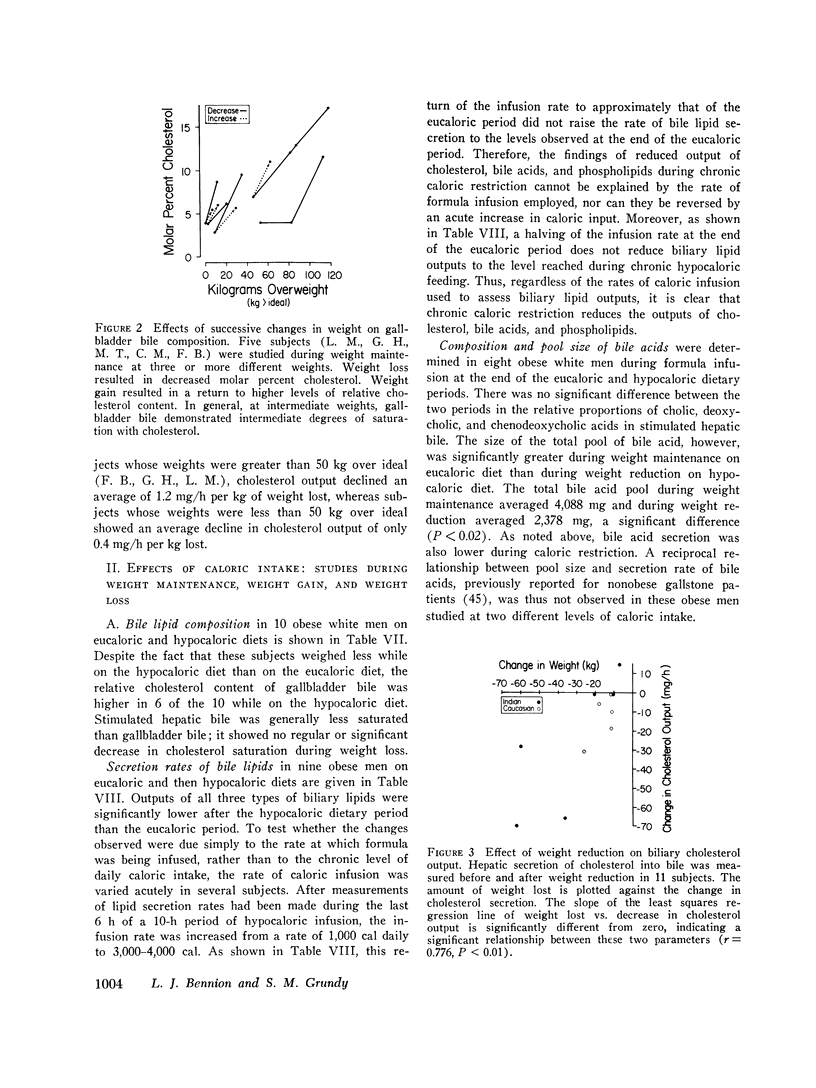
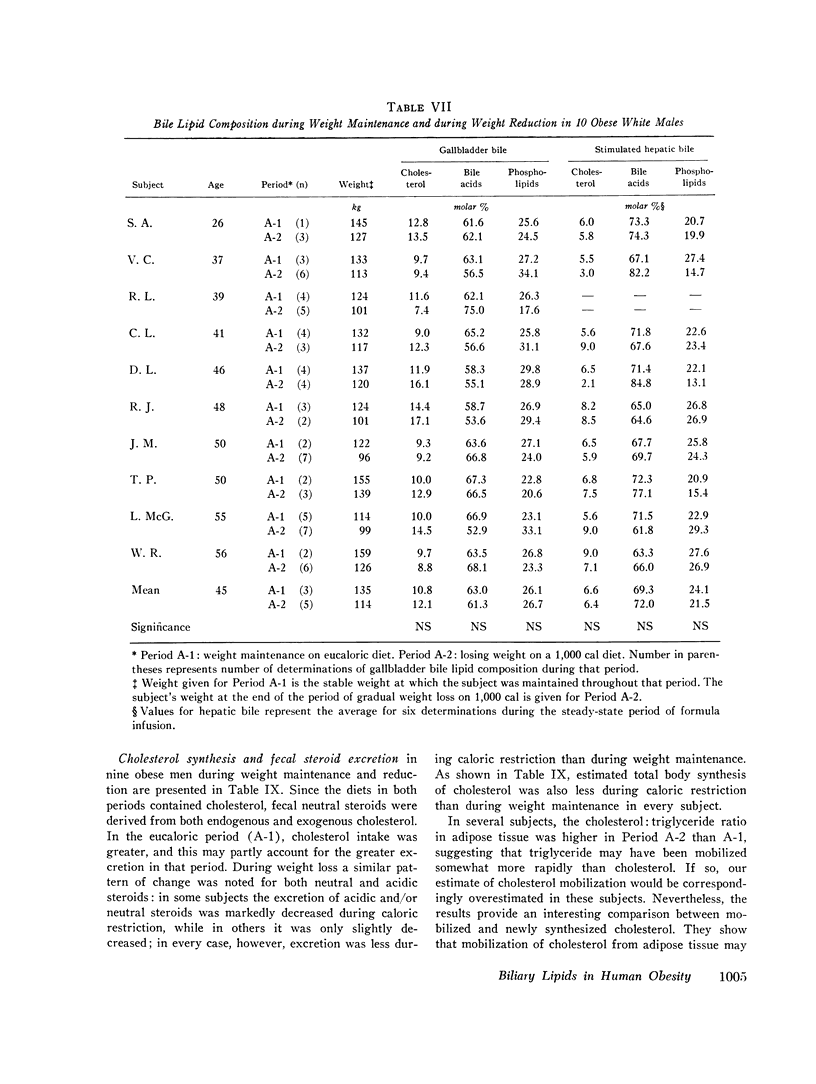
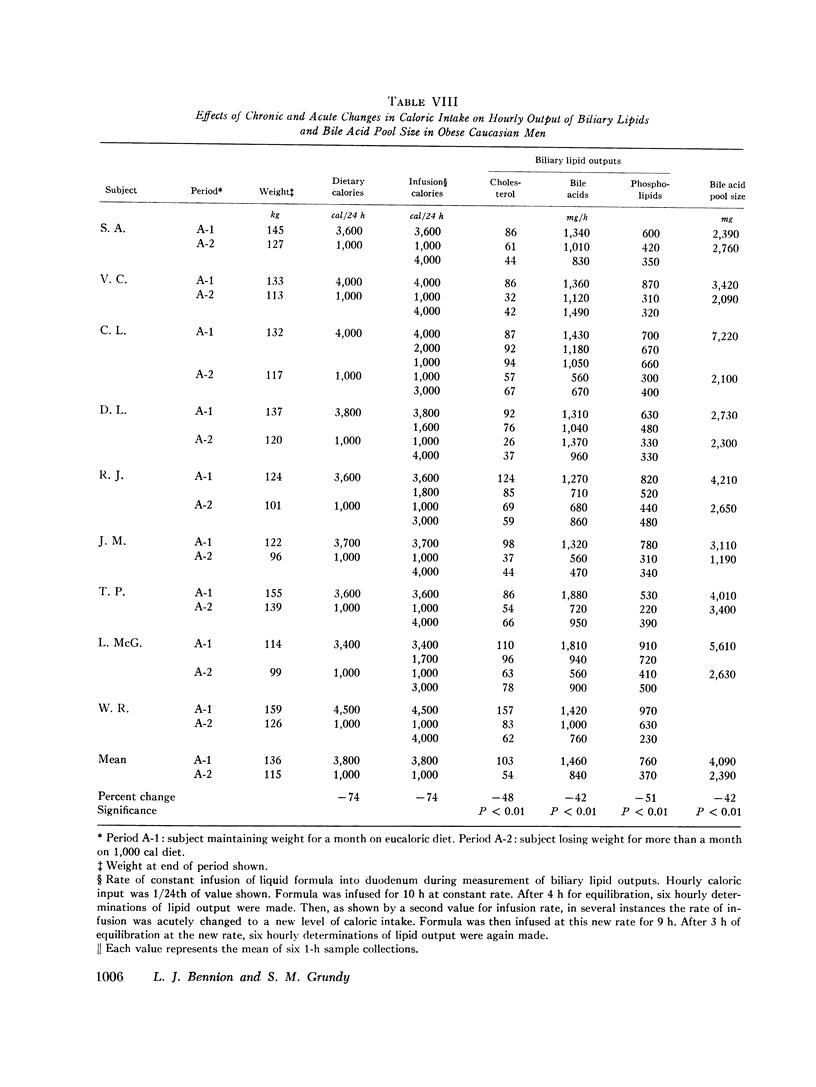
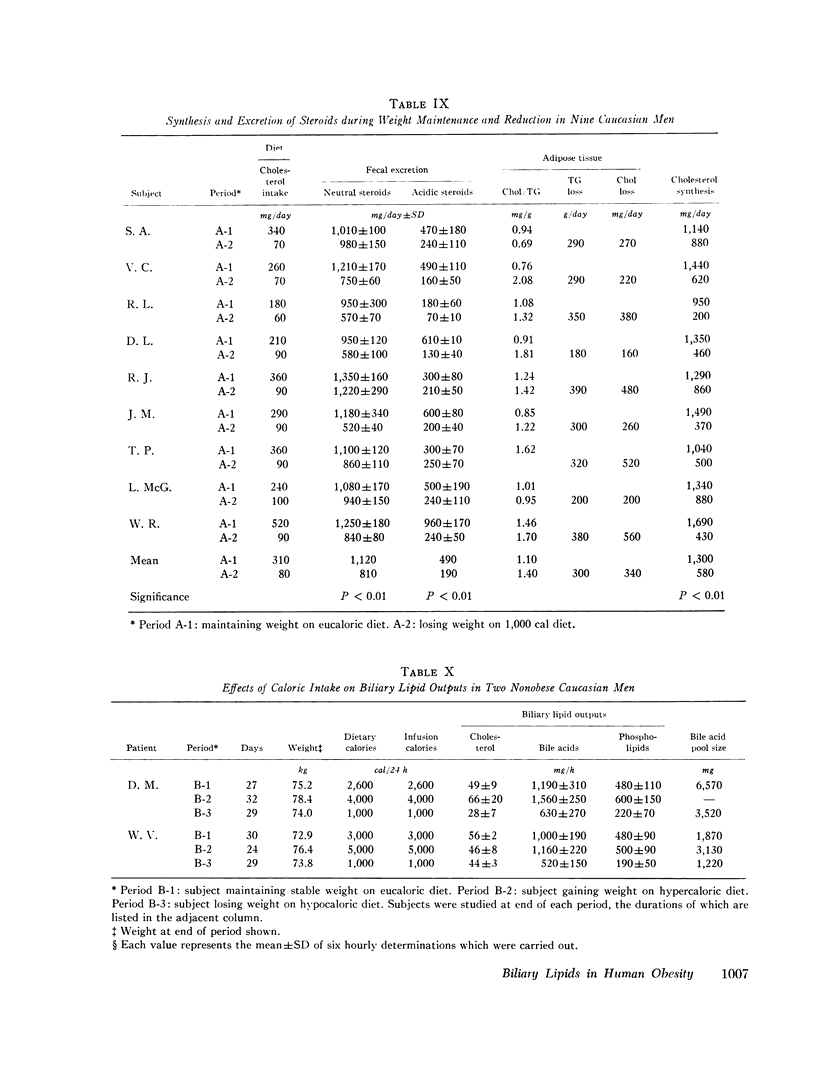
The effects of obesity and caloric intake on biliary lipid metabolism were investigated in a series of related studies. The degree of saturation of gallbladder bile with cholesterol was found to be significantly higher in a group of 23 obese healthy subjects than in a group of 23 nonobese controls matched for age, sex, and race. Bile was also significantly more saturated in 11 obese subjects before than after weight reduction. To determine whether supersaturated bile in obesity is due to excessive secretion of cholesterol or to deficient secretion of bile acids and phospholipids, the hepatic outputs of these three lipids were measured during constant duodenal infusion of formula in the same 11 subjects before and after weight reduction. Weight reduction resulted in significant reduction of cholesterol output but not of bile acid or phospholipid output. Moreover, very obese subjects were found to have cholesterol secretion rates markedly higher than less obese subjects previously studied by the same method. In obese subjects, bile was supersaturated with cholesterol despite increased bile acid pool sizes and increased secretion rates of bile acids and phospholipids. Supersaturated bile in the obese could therefore be attributed to a single defect in lipid secretion, namely, an excessive output of cholesterol. To determine whether the rate of caloric intake can account for the effects of obesity on biliary lipid composition and secretion, nine obese white men were studied on a weight maintenance diet and then during weight reduction on a 1,000 cal diet. As compared to weight maintenance, chronic caloric restriction resulted in reduced outputs of cholesterol, bile acids, and phospholipids, reduced bile acid pool size, and reduced synthesis and fecal excretion of cholesterol. Saturation of bile with cholesterol did not decrease during weight reduction, evidently because of the mobilization of cholesterol from adipose stores and the marked reduction in bile acid and phospholipid output observed during chronic caloric restriction. Acute alterations in caloric infusion rates did not fully reproduce the effects of chronic administration of high and low calorie diets. Likewise, chronic intake of hypercaloric diets by nonobese subjects did not reproduce the cholesterol hypersecretion characteristic of the obese. Thus, increased cholesterol secretion in obese subjects could not be fully explained by the amount of calories they ingested to maintain stable weight. It is concluded that obesity is characterized by excessive hepatic secretion of cholesterol which results in supersaturated bile.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Admirand W. H., Small D. M. The physicochemical basis of cholesterol gallstone formation in man. J Clin Invest. 1968 May;47(5):1043–1052. doi: 10.1172/JCI105794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagdade J. D., Bierman E. L., Porte D., Jr The significance of basal insulin levels in the evaluation of the insulin response to glucose in diabetic and nondiabetic subjects. J Clin Invest. 1967 Oct;46(10):1549–1557. doi: 10.1172/JCI105646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell C. C., Jr, McCormick W. C., 3rd, Gregory D. H., Law D. H., Vlahcevic Z. R., Swell L. Relationship of bile acid pool size to the formation of lithogenous bile in male Indians of the Southwest. Surg Gynecol Obstet. 1972 Mar;134(3):473–478. [PubMed] [Google Scholar]
- Bell C. C., Jr, Vlahcevic Z. R., Prazich J., Swell L. Evidence that a diminished bile acid pool precedes the formation of cholesterol gallstones in man. Surg Gynecol Obstet. 1973 Jun;136(6):961–965. [PubMed] [Google Scholar]
- Bhathena S. J., Avigan J., Schreiner M. E. Effect of insulin on sterol and fatty acid synthesis and hydroxymethylglutaryl CoA reductase activity in mammalian cells grown in culture. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2174–2178. doi: 10.1073/pnas.71.6.2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett W. The epidemiology of gall stones. Tijdschr Gastroenterol. 1971;14(2):79–89. [PubMed] [Google Scholar]
- Danzinger R. G., Gordon H., Schoenfield L. J., Thistle J. L. Lithogenic bile in siblings of young women with cholelithiasis. Mayo Clin Proc. 1972 Oct;47(10):762–766. [PubMed] [Google Scholar]
- Davignon J., Simmonds W. J., Ahrens E. H. Usefulness of chromic oxide as an internal standard for balance studies in formula-fed patients and for assessment of colonic function. J Clin Invest. 1968 Jan;47(1):127–138. doi: 10.1172/JCI105703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietschy J. M., Brown M. S. Effect of alterations of the specific activity of the intracellular acetyl CoA pool on apparent rates of hepatic cholesterogenesis. J Lipid Res. 1974 Sep;15(5):508–516. [PubMed] [Google Scholar]
- Edwards P. A., Muroya H., Gould R. G. In vivo demonstration of the circadian thythm of cholesterol biosynthesis in the liver and intestine of the rat. J Lipid Res. 1972 May;13(3):396–401. [PubMed] [Google Scholar]
- Friedman G. D., Kannel W. B., Dawber T. R. The epidemiology of gallbladder disease: observations in the Framingham Study. J Chronic Dis. 1966 Mar;19(3):273–292. doi: 10.1016/0021-9681(66)90132-9. [DOI] [PubMed] [Google Scholar]
- GRUNDY S. M., AHRENS E. H., Jr, MIETTINEN T. A. QUANTITATIVE ISOLATION AND GAS--LIQUID CHROMATOGRAPHIC ANALYSIS OF TOTAL FECAL BILE ACIDS. J Lipid Res. 1965 Jul;6:397–410. [PubMed] [Google Scholar]
- Garbutt J. T., Kenney T. J. Effect of cholestyramine on bile acid metabolism in normal man. J Clin Invest. 1972 Nov;51(11):2781–2789. doi: 10.1172/JCI107100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy S. M., Ahrens E. H., Jr, Salen G. Dietary beta-sitosterol as an internal standard to correct for cholesterol losses in sterol balance studies. J Lipid Res. 1968 May;9(3):374–387. [PubMed] [Google Scholar]
- Grundy S. M., Duane W. C., Adler R. D., Aron J. M., Metzger A. L. Biliary lipid outputs in young women with cholesterol gallstones. Metabolism. 1974 Jan;23(1):67–73. doi: 10.1016/0026-0495(74)90105-x. [DOI] [PubMed] [Google Scholar]
- Grundy S. M. Effects of polyunsaturated fats on lipid metabolism in patients with hypertriglyceridemia. J Clin Invest. 1975 Feb;55(2):269–282. doi: 10.1172/JCI107930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy S. M., Metzger A. L. A physiological method for estimation of hepatic secretion of biliary lipids in man. Gastroenterology. 1972 Jun;62(6):1200–1217. [PubMed] [Google Scholar]
- Grundy S. M., Metzger A. L., Adler R. D. Mechanisms of lithogenic bile formation in American Indian women with cholesterol gallstones. J Clin Invest. 1972 Dec;51(12):3026–3043. doi: 10.1172/JCI107130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOFMANN A. F. CLINICAL IMPLICATIONS OF PHYSICOCHEMICAL STUDIES ON BILE SALTS. Gastroenterology. 1965 Apr;48:484–494. [PubMed] [Google Scholar]
- Hegardt F. G., Dam H. The solubility of cholesterol in aqueous solutions of bile salts and lecithin. Z Ernahrungswiss. 1971 Apr;10(3):223–233. doi: 10.1007/BF02020933. [DOI] [PubMed] [Google Scholar]
- Holzbach R. T., Marsh M., Olszewski M., Holan K. Cholesterol solubility in bile. Evidence that supersaturated bile is frequent in healthy man. J Clin Invest. 1973 Jun;52(6):1467–1479. doi: 10.1172/JCI107321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISAKSSON B. On the dissolving power of lecithin and bile salts for cholesterol in human bladder bile. Acta Soc Med Ups. 1954 Sep 30;59(5-6):296–306. [PubMed] [Google Scholar]
- Kottke B. A. Difference in bile acid excretion. Primary hypercholesteremia compared to combined hypercholesteremia and hypertriglyceridemia. Circulation. 1969 Jul;40(1):13–20. doi: 10.1161/01.cir.40.1.13. [DOI] [PubMed] [Google Scholar]
- MIETTINEN T. A., AHRENS E. H., Jr, GRUNDY S. M. QUANTITATIVE ISOLATION AND GAS--LIQUID CHROMATOGRAPHIC ANALYSIS OF TOTAL DIETARY AND FECAL NEUTRAL STEROIDS. J Lipid Res. 1965 Jul;6:411–424. [PubMed] [Google Scholar]
- MIETTINEN T. A., AHRENS E. H., Jr, GRUNDY S. M. QUANTITATIVE ISOLATION AND GAS--LIQUID CHROMATOGRAPHIC ANALYSIS OF TOTAL DIETARY AND FECAL NEUTRAL STEROIDS. J Lipid Res. 1965 Jul;6:411–424. [PubMed] [Google Scholar]
- Marinovic I., Guerra C., Larach G. Incidencia de litiasis biliar en material de autopsias y análisis de composición de los cálculos. Rev Med Chil. 1972 Nov;100(11):1320–1327. [PubMed] [Google Scholar]
- Metzger A. L., Adler R., Heymsfield S., Grundy S. M. Diurnal variation in biliary lipid composition. Possible role in cholesterol gallstone formation. N Engl J Med. 1973 Feb 15;288(7):333–336. doi: 10.1056/NEJM197302152880702. [DOI] [PubMed] [Google Scholar]
- Miettinen T. A. Cholesterol production in obesity. Circulation. 1971 Nov;44(5):842–850. doi: 10.1161/01.cir.44.5.842. [DOI] [PubMed] [Google Scholar]
- Miettinen T. A. Detection of changes in human cholesterol metabolism. Ann Clin Res. 1970 Dec;2(4):300–320. [PubMed] [Google Scholar]
- NORMAN A. ELECTROPHORETIC BEHAVIOR OF BILE ACIDS AND CHOLESTEROL IN GALL BLADDER AND HEPATIC BILE. Proc Soc Exp Biol Med. 1964 Apr;115:936–940. doi: 10.3181/00379727-115-29083. [DOI] [PubMed] [Google Scholar]
- Nepokroeff C. M., Lakshmanan M. R., Ness G. C., Dugan R. E., Porter J. W. Regulation of the diurnal rhythm of rat liver beta-hydroxy-beta-methylglutaryl coenzmye A reductase activity by insulin, glucagon, cyclic AMP and hydrocortisone. Arch Biochem Biophys. 1974 Feb;160(2):387–396. doi: 10.1016/0003-9861(74)90412-3. [DOI] [PubMed] [Google Scholar]
- Nestel P. J., Monger E. A. Turnover of plasma esterified cholesterol in normocholesterolemic and hypercholesterolemic subjects and its relation to body build. J Clin Invest. 1967 Jun;46(6):967–974. doi: 10.1172/JCI105603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestel P. J., Schreibman P. H., Ahrens E. H., Jr Cholesterol metabolism in human obesity. J Clin Invest. 1973 Oct;52(10):2389–2397. doi: 10.1172/JCI107428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestel P. J., Whyte H. M., Goodman D. S. Distribution and turnover of cholesterol in humans. J Clin Invest. 1969 Jun;48(6):982–991. doi: 10.1172/JCI106079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northfield T. C., Hofmann A. F. Biliary lipid secretion in gallstone patients. Lancet. 1973 Apr 7;1(7806):747–748. doi: 10.1016/s0140-6736(73)92130-2. [DOI] [PubMed] [Google Scholar]
- Northfield T. C., Hofmann A. F. Biliary lipid secretion in gallstone patients. Lancet. 1973 Apr 7;1(7806):747–748. doi: 10.1016/s0140-6736(73)92130-2. [DOI] [PubMed] [Google Scholar]
- Redinger R. N., Small D. M. Bile composition, bile salt metabolism and gallstones. Arch Intern Med. 1972 Oct;130(4):618–630. [PubMed] [Google Scholar]
- Shefer S., Hauser S., Lapar V., Mosbach E. H. Diurnal variation of HMG CoA reductase activity in rat intestine. J Lipid Res. 1972 Sep;13(5):571–573. [PubMed] [Google Scholar]
- Small D. M., Rapo S. Source of abnormal bile in patients with cholesterol gallstones. N Engl J Med. 1970 Jul 9;283(2):53–57. doi: 10.1056/NEJM197007092830201. [DOI] [PubMed] [Google Scholar]
- Sturdevant R. A., Pearce M. L., Dayton S. Increased prevalence of cholelithiasis in men ingesting a serum-cholesterol-lowering diet. N Engl J Med. 1973 Jan 4;288(1):24–27. doi: 10.1056/NEJM197301042880106. [DOI] [PubMed] [Google Scholar]
- Swell L., Bell C. C., Jr, Vlahcevic Z. R. Relationship of bile acid pool size to biliary lipid excretion and the formation of lithogenic bile in man. Gastroenterology. 1971 Nov;61(5):716–722. [PubMed] [Google Scholar]
- Thistle J. L., Schoenfield L. J. Lithogenic bile among young Indian women. N Engl J Med. 1971 Jan 28;284(4):177–181. doi: 10.1056/NEJM197101282840404. [DOI] [PubMed] [Google Scholar]
- Thomas P. J., Hofmann A. F. Letter: A simple calculation of the lithogenic index of bile: expressing biliary lipid composition on rectangular coordinates. Gastroenterology. 1973 Oct;65(4):698–700. [PubMed] [Google Scholar]
- Vlahcevic Z. R., Bell C. C., Jr, Buhac I., Farrar J. T., Swell L. Diminished bile acid pool size in patients with gallstones. Gastroenterology. 1970 Aug;59(2):165–173. [PubMed] [Google Scholar]
- Vlahcevic Z. R., Bell C. C., Jr, Gregory D. H., Buker G., Juttijudata P., Swell L. Relationship of bile acid pool size to the formation of lithogenic bile in female Indians of the southwest. Gastroenterology. 1972 Jan;62(1):73–83. [PubMed] [Google Scholar]
- Vlahcevic Z. R., Bell C., Jr, Swell L. Significance of the liver in the production of lithogenic bile in man. Gastroenterology. 1970 Jul;59(1):62–69. [PubMed] [Google Scholar]
- Wheeler M., Hills L. L., Laby B. Cholelithiasis: a clinical and dietary survey. Gut. 1970 May;11(5):430–437. doi: 10.1136/gut.11.5.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyte H. M., Nestel P. J., Goodman D. S. Cholesterol distribution and turnover in obesity in man. Isr J Med Sci. 1969 Jul-Aug;5(4):644–647. [PubMed] [Google Scholar]
- van der LINDEN Some biological traits in female gallstone-disease patients. A study of body build, parity and serum cholesterol level with a discussion of some problems of selection in observational hospital data. Acta Chir Scand Suppl. 1961;Suppl 269:1–94. [PubMed] [Google Scholar]


