Abstract
The creation of complex tissues and organs is the ultimate goal in tissue engineering. Engineered morphogenesis necessitates spatially controlled development of multiple cell types within a scaffold implant. We present a novel method to achieve this by adhering nanoparticles containing different small-interfering RNAs (siRNAs) into nanostructured scaffolds. This allows spatial retention of the RNAs within nanopores until their cellular delivery. The released siRNAs were capable of gene silencing BCL2L2 and TRIB2, in mesenchymal stem cells (MSCs), enhancing osteogenic and adipogenic differentiation, respectively. This approach for enhancing a single type of differentiation is immediately applicable to all areas of tissue engineering. Different nanoparticles localized to spatially distinct locations within a single implant allowed two different tissue types to develop in controllable areas of an implant. As a consequence of this, we predict that complex tissues and organs can be engineered by the in situ development of multiple cell types guided by spatially restricted nanoparticles.
Introduction
Tissue engineering has the potential to alleviate disease by producing abundant and tolerated replacement organs.1 The standard approach is to seed patient-derived terminally differentiated cells on porous three-dimensional cell supports (scaffolds). When it comes to generating tissues with multiple cell types, however, this method is limited to scaffold structures that can be loaded with cells in physically separated locations such as spheres (bladders2) and tubes (larynx3). A similar rapid prototyping approach is cell printing where different cell populations are deposited into three-dimensional shapes.4,5 Unfortunately, this approach presents limitations6 including cell alterations from induced mechanotransduction during processing. Moreover, reconstruction with a priori differentiated cells is problematic as cells perform important functions such as preparing extracellular matrix while undergoing differentiation and loose this ability when fully differentiated.7 Consequently, we explored the seeding of stem cells onto scaffolds before cell specialization. A limitation with this approach is that conventional global provision of differentiation cues fails to differentiate stem cells into multiple cell types in discrete locations. Here, we present a novel strategy where nanostructured scaffolds are coated with different nanoparticles in spatially discrete parts directing the differentiation pathway of one homogenously seeded stem cell population into multiple cell types in situ.
Several different drugs have been used to steer differentiation on scaffolds including proteins,8 plasmids,9,10 and viruses.11 We believe a more versatile drug type to be small-interfering RNA (siRNA). Once introduced into cells, siRNAs can silence synthesis of a specific protein by base pairing with its mRNA sequence.12 When applied to cultured monolayers of mesenchymal stem cells (hMSCs), commonly used in tissue engineering,13 siRNAs were able to enhance their differentiation into bone,14 cartilage,15 fat,16 muscle,17 liver,18 and nerve cells.19 A nanoparticle-based implant coating, capable of delivering siRNAs into stem cells situated in a scaffold, therefore, has broad applications in tissue engineering.20,21
Here, we present a novel technology, siRNA-enhanced scaffolds (siRESs), composed of biodegradable nanostructured poly-ε-caprolactone (PCL) scaffolds functionalized with a lyophilized polymer/lipid-based nanoparticulate siRNA delivery system.22 We investigate, the particle localization and retention properties as well as the silencing and in vitro and in vivo enhancement of differentiation of the system, using hMSCs as progenitor cells and siRNAs targeted to enhanced green fluorescent protein (EGFP), tribbles homolog 2 (TRIB2, also known as TRB2), and BCL2 like 2 (BCL2L2, also known as BCL-w). We demonstrate successful enhancement of differentiation, and importantly, that tailored cell specialization can be affected differently in discrete locations within a composite scaffold by controlled deposition of BCL2L2 siRNA and TRIB2 siRNA containing nanoparticles.
Results
Monolayer culture
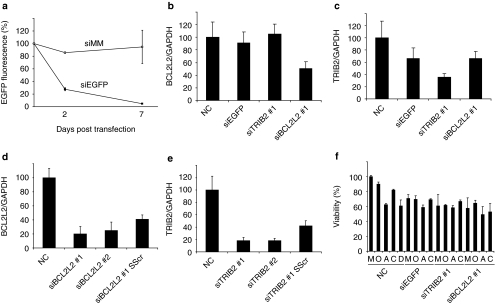
The potential of reverse transfecting hMSCs with siRNA was initially studied in monolayer culture. Tissue culture plates coated by a lyophilization process with TransIT-TKO/siRNA particles with hydrodynamic diameter (259 ± 14 nm) and ζ potential (12.6 ± 0.5 mV) were seeded with telomerase-immortalized hMSCs.23 siRNA targeting EGFP (EGFP-expressing hMSCs were used in this case24), BCL2L2, and TRIB2 (Figure 1a–c, respectively) were used. Flow cytometry and quantitative PCR (qPCR) revealed that the delivery system was capable of reducing expression of all siRNA targeted genes by at least 50% after 2 days. EGFP protein levels were reduced by over 95% 7 days post-transfection. Histograms of cellular EGFP fluorescence with or without EGFP knockdown showed that the majority of the EGFP silenced cells had an equal reduction in EGFP approximately corresponding to the average decline in EGFP (Supplementary Figure S1). The specificity of the siRNAs was investigated using siRNAs targeting different regions of TRIB2 and BCL2L2 and by scrambling part of the seed sequences (Figure 1d,e). Targeting a different region of the mRNA resulted in the same degree of knockdown, whereas partial scrambling of the siRNA seed sequence led to a significant decrease in knockdown. The influence of the siRNA transfection on cell viability was studied by growing hMSCs for 2 days on siRNA-coated plates in maintenance medium followed by 12 days in various differentiation mediums (Figure 1f). Transfected cell viability was slightly reduced (~30, ~40, and ~45% reduction in viability for EGFP, TRIB2, and BCL2L2 siRNA) in maintenance medium. This reduction was comparable to that induced by differentiation medium. To confirm that osteogenic and adipogenic differentiation could take place in the presence of siRNA particles, we performed alkaline phosphatase (ALP), alizarin red, and oil red O staining after transfection in maintenance medium and culturing in differentiation medium (Supplementary Figure S2), the stains showed that the transfection process did not adversely affect the ability of the stem cells to differentiate. In conclusion, freeze-dried TransIT-TKO/siRNA particles were an effective transfection agent for hMSCs in monolayer culture.
Figure 1.
Monolayer transfection of human mesenchymal stem cells (hMSCs) grown on tissue culture polystyrene plates, either noncoated (NC) or coated with freeze-dried TransIT-TKO nanoparticles containing EGFP-targeted siRNA (siEGFP), EGFP mismatch siRNA (siMM), TRIB2-targeted siRNA (siTRIB2 #1), or BCL2L2-targeted siRNA (siBCL2L2 #1). (a) The relative fluorescence level of EGFP in hMSCs (EGFP+) after 2 and 7 days of reverse transfection was determined by flow cytometry. Displayed is the average geometric mean fluorescence normalized to nontransfected cells which were set to 100%, the standard deviation is indicated by error bar (N = 3). (b) The relative expression level of BCL2L2 mRNA normalized to GAPDH mRNA after 48 hours of reverse transfection of hMSCs (EGFP−). The graph shows average normalized to untransfected cells which were set to 100%, the standard deviation is indicated by error bars (N = 3). (c) The relative expression level of TRIB2 mRNA normalized to GAPDH mRNA after 48 hours of reverse transfection of hMSCs (EGFP−). The graph shows average normalized to untransfected cells which were set to 100%, the standard deviation is indicated by error bars (N = 3). (d,e) The relative expression level of BCL2L2 mRNA and TRIB2 mRNA normalized to GAPDH mRNA after 48 hours of reverse transfection of hMSCs (EGFP−). The graph shows average normalized to untransfected cells which were set to 100%, the standard deviation is indicated by error bars (N = 5). siRNA #1 corresponds to the same sequence as in graph b and c, although a different siRNA supplier was used. siRNAs #2 targeted different regions of the genes representing a completely different sequence. SScr denotes siRNA #1 where the sequence of four nucleic acids in the seed sequence (position 3–6) has been scrambled. (f) Displays viability after hMSCs (EGFP−) were cultured for 2 days in maintenance medium followed by 12 days in the indicated mediums (M, maintenance medium; O, osteogenic medium without vitamin D3; A, adipogenic medium; C, complex medium; D, osteogenic medium with vitamin D3). The graph shows average normalized to nontransfected cells in maintenance media which were set to 100%, the standard deviation is indicated by error bars (N = 4). EGFP, enhanced green fluorescent protein; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
siRNA coating of scaffolds
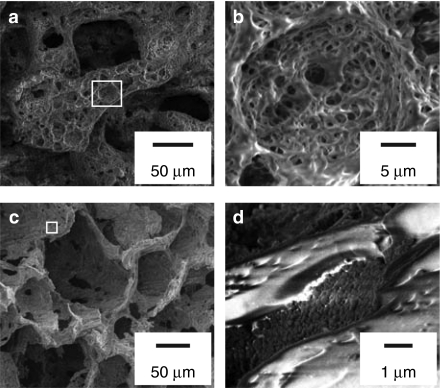
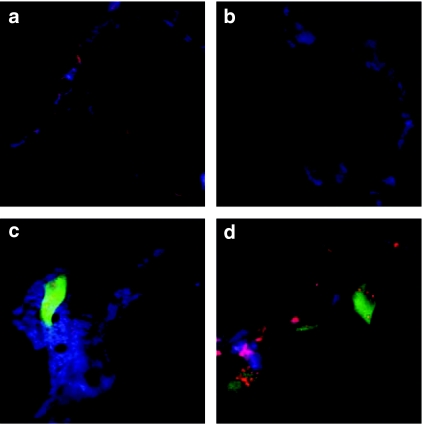
Hierarchically organized scaffolds were produced through thermally induced phase separation of PCL in 1,4-dioxane and H2O (ref. 25) and a nanoroughened hydrophilic surface was subsequently introduced by partial chemical degradation.26 When visualized with scanning electron microscopy (Figure 2a,b), the scaffolds appeared bimodal in pore size distribution. Large pores (diameter >50 µm) were separated by walls (thickness 10–40 µm) containing smaller cavities of decreasing size (the smallest structural features extended <15 nm). These nano-features greatly increase the surface area. When coated with nanoparticles (Figure 2c,d, respectively), the smaller cavities were filled with siRNA nanoparticles with a maintained ~200 nm size range. In contrast, the protruding structures contained no visible nanoparticles. Fluorescent siRNA particles were adsorbed onto scaffolds to investigate particle adherence. When medium and serum were added to the coated scaffolds the siRNA continued to locate to the walls (Figure 3a) even after 24 hours (Figure 3b). Scaffolds containing cells showed siRNA uptake, limited to a few cells up to 24 hours but in most of the population after 72 hours (Figure 3c,d, respectively). The coating process resulted in deposition of intact adherent particles onto the scaffold walls and allowed siRNA internalization into seeded cells.
Figure 2.
Scanning electron microscopy of scaffolds. (a) Scaffold before coating with nanoparticles. (b) High magnification of scaffold structure indicated in a. (c) Scaffold after coating with TransIT-TKO/BCL2L2 siRNA nanoparticles. (d) High magnification of scaffold structure indicated in c.
Figure 3.
Fluorescence confocal laser scanning microscopy of scaffolds. (a) Scaffold coated with Cy5-labeled small-interfering RNA (siRNA). (b) Scaffold coated with Cy5-labeled siRNA after 24 hours in maintenance medium at 37 °C. (c,d) Scaffolds coated with Cy5-labeled siRNA and seeded with enhanced green fluorescent protein (EGFP)-expressing human mesenchymal stem cells (EGFP+) after (c) 24 hours and (d) 72 hours incubation in maintenance medium. Blue is scaffold autofluorescence, red is Cy5 fluorescence, and green is EGFP fluorescence. All images were acquired with the same settings for the red channel and green channel at ×40 magnification. Image sizes are 100 µm × 100 µm.
siRNA induced silencing on scaffolds
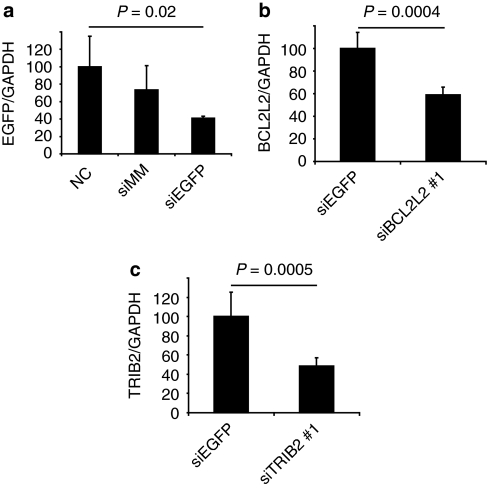
To quantify knockdown, scaffolds were coated with particles containing EGFP siRNA and seeded with EGFP-expressing hMSCs. After 48 hours, mRNA expression was measured by qPCR (Figure 4a). EGFP expression was reduced on EGFP siRNA–coated scaffolds (60% reduction compared to noncoated scaffolds, P = 0.02, 35% reduction compared to scaffolds coated with mismatched siRNA, P = 0.05). Next, hMSCs were seeded on scaffolds containing siRNA particles targeted to either BCL2L2 or TRIB2. After 72 hours, mRNA expression was measured by qPCR (Figure 4b,c). Both BCL2L2 siRNA and TRIB2 siRNA reduced the expression of BCL2L2 (by 41%, P = 0.0004) and TRIB2 (by 51%, P = 0.0005), respectively, in comparison with EGFP siRNA. In conclusion, the siRNA is taken up by cells and induces gene silencing.
Figure 4.
In vitro knockdown on scaffolds. (a) Human mesenchymal stem cells (hMSCs; EGFP+) were seeded onto noncoated (NC) scaffolds and TransIT-TKO particle–coated scaffolds made with EGFP mismatch siRNA (siMM) or EGFP-specific siRNA (siEGFP). After 48 hours, the ratio of EGFP mRNA to GAPDH mRNA was characterized by qPCR. (b,c) hMSCs (EGFP−) were seeded onto scaffolds coated with EGFP-targeted siRNA (siEGFP), BCL2L2-targeted siRNA (siBCL2L2 #1), or TRIB2-targeted siRNA (siTRIB2 #1). After 72 hours, the ratio of (b) BCL2L2 or (c) TRIB2 mRNA to GAPDH mRNA was found. Averages are shown with standard deviation on error bars (n = 5), the values were normalized to (a) noncoated or (b,c) siEGFP control scaffolds which were set to 100%. EGFP, enhanced green fluorescent protein; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
siRNA enhanced differentiation on scaffolds
To study whether siRES can influence differentiation, hMSCs were added to scaffolds precoated with siRNA against either TRIB2 or BCL2L2. At 48 hours after transfection in maintenance medium, adipogenic or osteogenic differentiation was induced using differentiation medium. At various time points, samples were collected and subjected to qPCR, micro computed tomography (CT) and histological analysis.
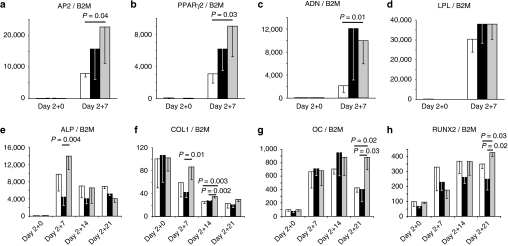
In the adipogenic differentiation experiment, the expression of the early adipogenic marker, apolipoprotein 2 (AP2; Figure 5a), the adipogenic transcription factor, peroxisome proliferator–activated receptor-γ isoform 2 (PPARγ2; Figure 5b), and the late adipogenic markers, adiponectin (Figure 5c), and lipoprotein lipase (Figure 5d), increased with time in noncoated scaffolds and EGFP or TRIB2 siRES. At day 7, the expression of all markers, except lipoprotein lipase, was specifically increased in the TRIB2 siRES compared to noncoated scaffolds (PPARγ2: 2.9 times higher, P = 0.03; AP2: 2.8 times higher, P = 0.04; adiponectin: 4.7 times higher, P = 0.01).
Figure 5.
In vitro adipogenic (a–d) and osteogenic (e–h) differentiation of human mesenchymal stem cells (EGFP−) seeded on noncoated scaffolds (white) and scaffolds coated with TransIT-TKO nanoparticles containing EGFP-targeted siRNA (black) or (a–d) TRIB2 #1- or (e–h) BCL2L2 #1-targeted siRNA (grey). After 2 days of culturing in maintenance medium, differentiation was induced with (a–d) adipogenic or (e–h) osteogenic medium for the indicated number of days. The ratio of (a) AP2, (b) PPARγ2, (c) ADN, (d) LPL, (e) ALP, (f) COL1, (g) OC, and (h) RUNX2 mRNA to B2M mRNA was characterized. Averages are shown with standard deviation indicated by error bars (n = 3 or 4). The values were normalized to the noncoated control scaffold at day 2 + 0 group which was set to 100%. Days denote days of culturing in maintenance medium plus days in differentiation medium. ADN, adiponectin; ALP, alkaline phosphatase; AP2, apolipoprotein 2; B2M, β-2 microglobulin; COL1, collagen type I; EGFP, enhanced green fluorescent protein; LPL, lipoprotein lipase; OC, osteocalcin; PPARγ2, peroxisome proliferator–activated receptor-γ isoform 2.
In the osteogenic differentiation experiment, the expression of the early osteogenic marker, ALP, increased up to 140-fold peaking at day 7 (Figure 5e). At day 7, there was a higher expression of ALP in the BCL2L2 siRNA–coated scaffolds than in the EGFP siRNA–coated scaffolds (3.4 times higher, P = 0.004). The expression of an alternative early osteogenic marker, collagen type I, decreased up to 80% during the experiment (Figure 5f). However, the reduction was slowest in the BCL2L2 siRNA–coated scaffolds, and collagen type I levels were highest in this group at day 7 and 12 (day 7: 2.1 times higher than EGFP siRNA coated, P = 0.01; day 12: 1.4 times higher than noncoated; P = 0.004 and 1.3 times higher than EGFP siRNA coated, P = 0.002). The expression of the late osteogenic marker, osteocalcin (OC; Figure 5g), and the osteogenic transcription factor, RUNX2 (Figure 5h), increased during the time course with insignificant differences between the samples at day 7 and 14. At day 21, the expression of both genes had decreased in both controls, while remaining higher in the BCL2L2 siRNA–coated scaffolds (RUNX2: 1.2 times higher than noncoated and 1.7 times higher than EGFP siRNA coated, P = 0.03 and P = 0.02, respectively. OC: 2.1 times higher than noncoated and 2.2 times higher than EGFP siRNA coated, P = 0.02 and P = 0.03, respectively).
To investigate the sequence specificity of siRNA for enhanced differentiation, we coated scaffolds with a siRNA targeted to different regions of either BCL2L2 or TRIB2 mRNA and included corresponding control siRNAs with a partially scrambled seed sequences. The siRNAs against TRIB2 increased the expression of the adipogenic marker AP2 while the scrambled siRNA decreased the expression of the adipogenic transcription factor PPARγ2 as compared to noncoated scaffolds (Supplementary Figure S3b). The siRNAs against BCL2L2 decreased the expression of the early temporary osteogenic marker ALP and increased the expression of the late osteogenic marker OC as compared to noncoated scaffolds (Supplementary Figure S3a) while the expression of collagen type I and RUNX2 was unaltered. Because both targeted siRNAs (with completely dissimilar sequences) give similar enhancements of differentiation in both the adipogenic and osteogenic experiments, we conclude the effects are target specific and not a result of off-target effects. The partially scrambled siRNAs also appear to increase differentiation, this is not surprising as these siRNAs also give considerable knockdown (Supplementary Figure S1).
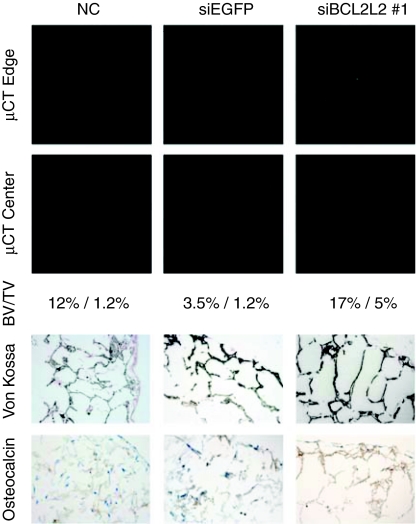
Micro CT, staining, and immunohistochemistry was performed on siRES cultured in osteogenic medium for 21 days to confirm the qPCR findings (Figure 6). Micro CT and von Kossa staining showed successful mineralization throughout the scaffolds, while OC immunohistochemistry demonstrated deposition of OC in the extracellular matrix. For all three assays, the bone matrix appeared most developed in the scaffolds coated with BCL2L2 siRNA. A negative control cultivated in maintenance medium showed no von Kossa or OC staining. Together, these in vitro results suggest that coating of scaffold implants with BCL2L2 siRNA increases their osteogenic development while TRIB2 siRNA coating enhances their adipogenic development.
Figure 6.
In vitro osteogenic differentiation of hMSCs (EGFP−) seeded on noncoated scaffolds (NC) and scaffolds coated with TransIT-TKO nanoparticles containing EGFP-targeted siRNA (siEGFP) or BCL2L2-targeted siRNA (siBCL2L2 #1). Scaffolds were cultured 2 days in maintenance medium and 21 days in osteogenic medium. Shown are two representative micro computed tomography slices from the edge and center of each scaffold as well as paraffin sections from the edge of each scaffold (with the edge in the upper right corner) stained with von Kossa (mineralization appears black) or by immunohistochemistry for osteocalcin (osteocalcin appears brown). Cells were counterstained with H&E. The average bone volume/total volume (BV/TV) of two consecutive experiments is also shown.
In vivo implantation of siRNA-coated scaffolds
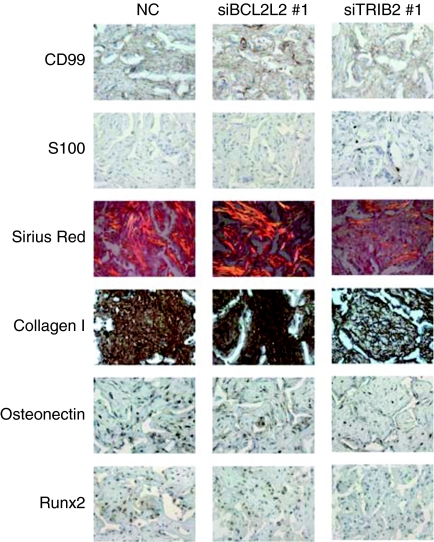
To study the effect of siRNA coating on the development of tissue in vivo, we subcutaneously implanted noncoated scaffolds as well as TRIB2 or BCL2L2 siRES into nonobese diabetic/severe combined immunodeficient (NOD/SCID) mice. Before implantation, the scaffolds were seeded with hMSCs for 16 hours in maintenance medium. After 8 weeks the scaffolds were taken out and studied using immunohistochemistry and staining (Figure 7). All scaffolds showed neovascularization, and positive human-specific CD99 staining throughout the scaffolds confirmed that the majority of the cells were human. Positive staining with the early adipocytic marker S100 was only observed in the TRIB2 siRNA–coated scaffolds indicating that silencing this gene allowed a subset of cells to start adipogenic differentiation. Collagen in the BCL2L2 siRNA–coated scaffold was stained densely with Sirius red and birefringence under polarized light indicated it had a mature structure. In contrast, there was less intense birefringence and Sirius red staining in the noncoated control and almost no staining or birefringence in the TRIB2 siRNA–coated scaffolds. Furthermore, collagen type I specific staining supported the Sirius red findings confirming that the deposited collagen was osteogenic type one. Osteonectin and RUNX2 staining were more pronounced in the BCL2L2 siRNA–coated scaffolds than in the TRIB2 siRNA–coated scaffolds. No evidence of mineralization was observed. These in vivo results demonstrate that siBCL2L2 and siTRIB2 can initiate early osteogenic and adipogenic differentiation, respectively, but also indicate they cannot drive terminal differentiation as the sole differentiation cue.
Figure 7.
In vivo differentiation of human mesenchymal stem cells (EGFP−) seeded on noncoated scaffolds (left column), scaffolds coated with TransIT-TKO nanoparticles prepared with BCL2L2 #1- (middle column) or TRIB2 #1 (right column)-targeted siRNA. After 16 hours of in vitro culturing in maintenance medium scaffolds were implanted subcutaneously in nonobese diabetic/severe combined immunodeficient mice for 8 weeks after which they were surgically removed and studied by histology using the indicated stain or antibody. Representative pictures are shown. In all cases, hematoxylin and eosin staining was used as a counterstain. Positive immunohistochemical staining appears brown. On the Sirius red–stained sections collagen deposition appears red and birefringence appears orange. Pictures are 318-µm wide and 238-µm high.
Dual differentiation in scaffolds
To construct a tissue with two cell types, scaffold cylinders were cut in half, and each part was coated with TRIB2 or BCL2L2 siRNA. The two sides were then joined together and hMSCs were added.
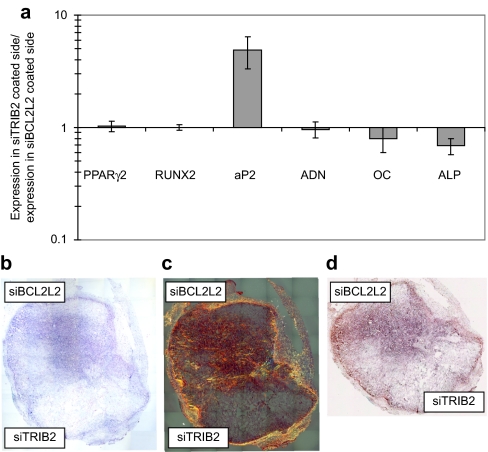
For in vitro testing, the scaffolds were cultured 2 days in maintenance medium and 8 days in complex differentiation medium. The two sides were then separated and the mRNA levels of differentiation markers measured for each part (Figure 8a). The expression of PPARγ2, RUNX2, adiponectin, and OC were within statistical variations equal in the two sides. However, AP2 was nearly fourfold upregulated in the TRIB2 siRNA–coated part, and ALP was 45% upregulated in the BCL2L2 siRNA–coated part.
Figure 8.
In vitro and in vivo double differentiation of human mesenchymal stem cells (hMSCs; EGFP−) seeded on scaffolds where one side was coated with TransIT-TKO nanoparticles containing TRIB2-targeted siRNA (#1) and the other side with TransIT-TKO nanoparticles containing BCL2L2-targeted siRNA (#1). (a) In vitro double differentiation. The dual-coated scaffolds were seeded with hMSCs and cultured 2 days in maintenance medium and 8 days in complex medium. The sides were then separated and for each side the ratios of the mRNA levels of PPARγ2, RUNX2, AP2, ADN, OC, and ALP relative to the mRNA level of B2M were determined. The ratios of the marker expression between the side with the TRIB2-targeted siRNA (#1) and the side with the BCL2L2-targeted siRNA (#1) were characterized and plotted. Averages are graphed with error bars representing standard deviation (n = 3 or 4). The y-axis is logarithmic and that markers that were upregulated in the side coated with TRIB2 targeted siRNA compared to side coated with the BCL2L2-targeted siRNA will have values above 1 and vice versa. (b–d) In vivo differentiation on a dual-coated scaffold. The dual-coated scaffolds were seeded with hMSCs for 16 hours in maintenance medium. They were then implanted subcutaneously in nonobese diabetic/severe combined immunodeficient mice for 2 weeks after which they were surgically removed and studied by histology. Whole scaffold sections were stained with (b) hematoxylin and eosin, (c) Sirius red, (d) and von Kossa. Mosaic pictures of whole scaffold sections at ×10 magnification are displayed with the BCL2L2 siRNA–coated side in the top of the pictures, host tissue can be seen surrounding the implant. On the Sirius red–stained section collagen deposition appears red and birefringence appears orange. Images in b and c are 7.6-mm wide and 8.4-mm high and d is 7.6-mm wide and 6.5-mm high. ADN, adiponectin; ALP, alkaline phosphatase; AP2, apolipoprotein 2; B2M, β-2 microglobulin; COL1, collagen type I; EGFP, enhanced green fluorescent protein; LPL, lipoprotein lipase; OC, osteocalcin; PPARγ2, peroxisome proliferator–activated receptor-γ isoform 2; siRNA, small-interfering RNA.
For in vivo evaluation, the combined scaffolds were seeded with hMSCs, cultured 16 hours in maintenance medium, and implanted subcutaneously for 2 weeks in mice. The scaffolds were then surgically removed, sectioned, and stained using hematoxylin and eosin, Sirius red and von Kossa staining (Figure 8b–d, respectively). Visualization with hematoxylin and eosin revealed that both scaffold parts contained cells but had developed very different morphologies. The BCL2L2 siRNA–coated side appeared dense while the TRIB2 siRNA–coated side was spongy with large holes. The Sirius red staining showed extensive deposition of organized birefringent collagen under polarized light in the BCL2L2 siRNA–coated side, while no birefringence was observed in the TRIB2 siRNA–coated side. The von Kossa staining showed no mineralization in either side. These results indicate that cells can be induced to commit to alternative differentiation pathways in specific locations within the same implant in vitro and in vivo by placing different siRNAs in distinct locations.
Discussion
This work presents a nanoparticle decorated nanostructured scaffold, capable of retaining and delivering siRNA, with broad applications for controlling stem cell differentiation in vitro and in vivo. When functionalized with two different siRNAs such a scaffold was shown to promote two alternative differentiation pathways in specific locations, both in vitro and in vivo.
siRNA has previously been delivered to cells growing within a three-dimensional matrix either by embedding the siRNA in flexible hydrogels27 or by adding the siRNA to the culturing medium28,29 alternatively, cells have been transfected with siRNA before seeding onto implants.30 None of these methods, however, is applicable to delivery of siRNA in specific locations in order to generate multiple cell types within a matrix. This necessitates stable binding of siRNA to scaffold walls until subsequent delivery directly to attaching cells.
DNA delivery from scaffolds has been explored more extensively than siRNA. These studies have been performed by adsorbing naked plasmid DNA,31 DNA containing nanoparticles10 and viral vectors11 onto scaffolds. The transfection efficiency of naked nucleic acids is usually very low32 and naked siRNA delivery is further complicated by instability to RNAses.33 As viral vectors have disadvantages due to oncogenicity34 and immunogenicity,35 nonviral vectors offer an attractive delivery solution. Lyoprotected, dried siRNA nanoparticles can normally achieve high silencing22 but common lyoprotectants, such as glucose, can interfere with differentiation.36 Importantly, TransIT-TKO/siRNA complexes can be freeze-dried without a lyoprotectant, stored dry for prolonged periods and still retain high knockdown efficiency in serum promoting their use for scaffold delivery.22
When lyophilized onto the scaffolds, the nanoparticles retained their prelyophilization morphology and located to the smaller cavities within the scaffold walls. NaOH-treated PCL is negatively charged because of exposed carboxylic acid25 and we found TransIT-TKO/siRNA nanoparticles to be positively charged. The observed interaction is therefore most likely electrostatic in nature. The nanostructured holes presumably provide a greatly increased surface area with which the cationic TransIT-TKO component can interact through multiple ionic interactions. Furthermore, these nanopores can be expected to protect against fluid flow displacement. The stability of the interaction is indicated by the continued retention of the nanoparticles in the walls after 24 hours in serum containing medium. This stable adherence enables localization of siRNA nanoparticles and the regional knockdown they induce.
In vitro we observed that BCL2L2 silencing enhanced osteogenic differentiation when combined with osteogenic medium. This confirms a previous study done with in vitro monolayer cuture.14 This study showed that silencing the antiapoptotic factors BCL2L2, BCL2, TBX3, and BIRC4 increased osteogenic differentiation, although the exact mechanism remains unknown. In vivo, we found no evidence of mineralization, confirming previously published in vitro findings14 that BCL2L2 knockdown alone does not induce mineralization. We did, however, find that BCL2L2 knockdown alone enhanced early osteogenic differentiation with regard to collagen type I deposition and organization in vivo.
TRIB2 silencing increased adipogenic differentiation in vitro when combined with adipogenic medium. This confirms several in vitro monolayer studies demonstrating that the TRIB family members repress adipogenic differentiation.16,37,38 TRIB2 inhibits adipogenesis by inhibiting Akt1 activation while increasing C/EBPβ degradation. EGFP-specific siRNA appeared to increase adipogenic differentiation, although not as much as TRIB2 siRNA. This confirms an earlier study showing that siRNAs induce weak sequence independent adipogenesis.39 The in vivo study showed that TRIB2 knockdown alone was enough to abolish collagen birefringence while allowing a subset to differentiate into S100 positive adipocytes after 8 weeks.
A single siRNA sequence has previously been shown to stimulate terminal MSC differentiation,18,19 but our results indicate that silencing BCL2L2 or TRIB2, while enhancing differentiation, is insufficient to drive terminal osteogenic and adipogenic specialization, respectively, without further stimuli. The few S100 positive adipocytes observed in vivo, may be a result of a weak adipogenic stimulus from the scaffolds or the implantation site. To achieve more effective differentiation, siRNA cocktails may be used.14 Alternatively, microRNAs (miRNAs) or miRNA inhibitors (antimirs) could be used. miRNAs are endogenous RNA molecules related to siRNAs, and they simultaneous regulate multiple genes. miRNAs increasing osteogenic40 and adipogenic41 differentiation have been found. Considering that the TransIT-TKO delivery system can deliver miRNA42 and antimirs, the system presented here should also be directly applicable for scaffold delivery of miRNA regulators.
We were able to develop a nanostructured scaffold that preferentially stimulated adipocytic and osteoblastic differentiation in distinct regions using localized coating with siRNAs against TRIB2 and BCL2L2. This was confirmed in vitro by the differential expression of the markers AP2 and ALP. In the same experiment RUNX2, PPARγ2, adiponectin, and OC expression was equal between the two sides at day 7. Adipogenesis and osteogenesis are two oppositely regulated pathways and differentiation enhancers are known to downregulate genes relating to the opposite pathway.43 We find it conceivable that this effect may delay the upregulation of certain markers and account for their equal expression in the two sides. It does not, however, seem to affect the development of distinct tissues in vivo. In the in vivo incubated scaffolds, the adjacent parts developed markedly different morphologies and collagen deposition and organization was highly increased in the siBCL2L2-coated side. Based on these results, we can imagine engineering tissues containing multiple cell types, by assembling differentially coated building blocks. Such cocultures are of great interest in tissue engineering44 as they form the basis of most organs. Although we have focused on siRNA nanoparticles due to known versatility, spatial localization of other differentiation cues could possibly be used to achieve the same result. In addition, other assembly techniques such as rapid prototyping of different drugs to distinct locations could conceivably be used instead of manually assembling building blocks.
To our knowledge, we have developed the first example of an siRNA nanoparticle functionalized scaffold capable of modulating stem cell differentiation. We demonstrate that the siRNA is delivered into seeded cells and induces sequence specific gene silencing. As clinically relevant examples, we show that specific targeting of TRIB2 and BCL2L2 on implants leads to enhanced adipogenic and osteogenic differentiation, respectively. Scaffold coating with a single type of siRNA is thus a versatile and effective method for enhancing the development of single cell type tissues and represents a method for conducting gene knockout studies in three dimension. Importantly, the nanostructured nature of the scaffolds enables nanoparticle retention and localization of different siRNAs to distinct parts of an implant. This made it possible to guide stem cells into alternate differentiation in specified locations. We believe this method will provide a realistic strategy to engineer tissues and organs that contain multiple cell types in the future.
Materials and Methods
Materials. Phosphate-buffered saline, minimum essential medium, fetal bovine serum, antibiotics, Trizol, MMLV RT, and SYBR Green qPCR kits were from Invitrogen (Carlsbad, CA). TransIT-TKO was from Mirus Bio (Madison, WI). Primers were from DNA Technology (Risskov, Denmark). PCL (grade 6506) was from Solvay (Warrington, UK). 1,4-Dioxane, insulin, 3-isobutyl-1-methylxanthine, dexamethasone, paraformaldehyde, vitamin D3, vitamin C, and β-glycerophosphate were from Sigma (St Louis, MO). MTS assay was from Promega (Madison, WI). Ultra-low cell adhesion plates were from Corning (Corning, NY). EGFP siRNAs were from Dharmacon (Lafayette, CO). siRNAs against BCL2L2 and TRIB2 were from DNA Technology or Ribotask (Odense, Denmark). Sequences can be found in Supplementary Tables S1 and S2.
Complex formulation. TransIT-TKO/siRNA complexes were made by mixing 3 µl TransIT-TKO with 25 µl minimum essential medium, and after 5 minutes 3 µl of 5 µmol/l siRNA was added. After 15 minutes, the complexes were used or evaluated for hydrodynamic diameter and ζ potential. All measurements were performed in triplicates on a Zetasizer Nano ZS (Malvern, Malvern, UK).
Scaffold production. PCL scaffolds were prepared by thermally induced phase separation of 41.8 mg PCL in 1 g 1,4-dioxane and 100 ng H2O. After lyophilization, the scaffolds were frozen in liquid N2, and scaffold discs (Ø = 8 mm, h = 4 mm) were cut. The discs were washed with EtOH and double-distilled H2O. The scaffolds were then etched in 0.25 mol/l NaOH for 16 hours and 0.25 mol/l HCl for 1 hour. Afterward they were neutralized, dehydrated, and soaked with TransIT-TKO/siRNA solution followed by lyophilization at −20 °C for 24 hours.
Cell culture. The hMSCs were maintained in minimum essential medium with 10% fetal bovine serum and 1% penicillin/streptomycin at 37 °C, 5% CO2, and 100% humidity, and were split when confluent. The hMSCs used had been passaged between 59 and 62 times. Coated scaffolds were placed in ultra-low cell adhesion plates and were soaked in maintenance medium with 4,000 cells/µl. After 15 minutes, 2 ml medium was added. The medium was replaced after 24 hours and twice every week. The adipogenic medium contained 100 nmol/l dexamethasone, 450 nmol/l 3-isobutyl-1-methylxanthine, and 10 nmol/l insulin. The osteogenic medium contained 10 mmol/l β-glycerophosphate, 10 nmol/l dexamethasone, 290 nmol/l vitamin C, and 10 nmol/l vitamin D3. The complex medium contained 27.5 nmol/l dexamethasone, 112.5 nmol/l 3-isobutyl-1-methylxanthine, 2.5 nmol/l insulin, 2.5mmol/l β-glycerophosphate, 2.5 nmol/l vitamin D3, and 290 nmol/l vitamin C.
Monolayer transfection. Monolayer reverse transfection experiments were performed by adding 25,000 cells/cm2 in 125 µl/cm2 to wells precoated with 12.5 µl/cm2 lyophilized siRNA reagent giving an active well concentration of 25 nmol/l siRNA. After 24 hours, the medium was removed and replaced with 250 µl/cm2 fresh medium. The medium was changed twice every week.
Flow cytometry. The flow cytometry experiments were performed with 24-well plates. The cells were harvested from wells using a standard phosphate-buffered saline and trypsin procedure. The harvested cells were then centrifuged for 5 minutes at 1,500 r.p.m., washed with phosphate-buffered saline, centrifuged again, and finally fixed in 1% paraformaldehyde in phosphate-buffered saline. The cells were processed on a FACScalibur (BD Biosciences, San Jose, CA). The cell population was selected using forward and side scatter, the geometric mean of this population in the FL1 channel was used as a measure of EGFP fluorescence. Each sample type was performed in three biological replicates.
Viability assay. The viability was assessed using a tetrazolium based MTS-based assay in 96-well plates. After 14 days of cell culture, wells were added fresh maintenance medium with 20% cytotoxicity assay solution, the wells were then incubated at 37 °C until color development had taken place (~30 minutes). OD490nm and OD650nm were then measured for each well, OD490nm was then corrected for OD650nm and was subtracted the average value of four wells without cells. The resulting number was then used as a measure of viability. All samples were run in four biological replicates.
Scanning electron microscopy. For scanning electron microscopy, the scaffolds were placed on aluminum stubs and visualized using a NovaSEM system (low vacuum detector, spot size: 3, voltage: 3 kV, working distance <5 mm).
Confocal laser scanning microscopy. Fluorescence microscopy was performed using an inverted Zeiss LSM510 META NLO laser scanning confocal microscope system. Images were acquired with a ×40 NA 1.2 water objective with Z-directional slice thickness of 1 µm. The scaffolds were visualized by means of two-photon excitation at 760 nm using a femtosecond laser (MaiTai Broadband; Spectra Physics, Santa Clara, CA). The scaffold autofluorescence signal was detected in the 405–490 nm interval using the META detector. Fluorescence signals from EGFP and Cy5 were imaged on the other detectors using 488 and 633 nm excitation, respectively, and appropriate bandpass filters. Images were acquired sequentially (multitrack mode) with pin holes set 1 airy unit for the EGFP and Cy5 detection channels but left fully open for the scaffold detection channel.
qPCR. RNA was extracted from scaffolds using Trizol. Complementary DNA was produced using an MMLV RT kit. qPCR was performed on an mxpro3005 system, Stratagene (La Jolla, CA), using a SYBR Green kit. CT values were found using the dR method. The PCR efficiency was measured using standard curves. The relative mRNA levels were calculated using the following equation:
The mRNA levels were normalized to GAPDH or β-2 microglobulin. GAPDH is upregulated in hypoxic hMSCs45 and was not used for differentiation experiments as deposited extracellular matrix could interfere with oxygen diffusion. The qPCR experiments were conducted on at least biological triplicates in technical duplicates. A two-tailed t-test was performed to test for difference of means. Primer sequences can be found in Supplementary Table S2.
Immunohistochemistry and staining. The scaffolds were fixed in 4% formaldehyde at 4 °C for 24 hours and stored at 4 °C in 0.9% NaCl. Samples were paraffin embedded and 4-µm thick sections were mounted on slides, deparaffinized with xylene, and rehydrated by stepwise washing in ethanol with increasing quantities of water. Slides were demasked by boiling in a microwave oven. Staining was performed, following the EnVision+ protocol (Dako, Glostrup, Denmark). See Supplementary Table S3 for target proteins, primary antibodies, dilutions, and suppliers used. Slides were incubated with primary antibody for 1 hour, washed with TNT-buffer (0.1 mol/l Tris HCl, 0.15 mol/l NaCl, 0.05% Tween-20, pH 7.5), and incubated with secondary antibody; peroxidase-labeled EnVision+ polymer, for 30 minutes. After washing with TNT-buffer, slides were incubated with 3,3-diaminobenzidine for 10 minutes to produce the stain. Slides were counterstained with Mayer's hematoxylin and mounted on a cover slip with Harleco Aquatex mounting medium (EMD Chemicals, Gibbstown, NJ).
Von Kossa staining was performed on deparaffinized sections in 10% silver nitrate for 20 minutes in the dark. Slides were then placed in sunlight for 45 minutes, rinsed in 5% pyragallol for 2 minutes, and immersed in 5% sodium thiosulphate for 5 minutes before counterstaining in eosin and mounting. Sections were stained with picro-Sirius red and visualized under circular polarized light. Whole-section images were obtained at ×100 magnification using a Leica microscope, DFC480 camera, and Survey 5.5.4.3 automated specimen scanning software.
Micro CT scanning. The scaffold discs were fixed with 4% formaldehyde for 24 hours at 4 °C and washed for four times with double-distilled H2O before being dehydrated and stacked in a cylinder. Tomographic reconstructions (6-µm voxel resolution) were performed on the cylinder using a µCT-40 from Scanco Medical (Brüttisellen, Switzerland). The maximum signal strength from a scaffold without cells was used as threshold X-ray absorbance value for “bone” tissue. The threshold was then applied equally to all slices in all samples to establish the degree of mineralization (bone volume/total volume).
Implantation. The in vivo implantation was modified from previous.46 Half-discs containing either BCL2L2 or TRIB2 siRNA were sutured (Ethilon EH7446 monofilament) together at each end of their flat diameter to generate a combined scaffold disc with a juxtaposed central diameter. The BCL2L2 side received an additional suture for identification. Composite scaffolds or single siRNA-coated scaffolds were seeded with 800,000 cells and maintained overnight in maintenance medium. Scaffolds were implanted subcutaneously in 8-week-old female NOD/LTSz-Prkdcscid mice.
SUPPLEMENTARY MATERIAL Figure S1. Monolayer transfections of hMSCs (EGFP+) were carried out with EGFP match or mismatch siRNA for 2 days as detailed in Figure 1a. EGFP fluorescence was investigated using a histogram. Representative pictures are shown. Figure S2. Alkaline phosphatase (a), oil red O (b) and alizarin red (c) staining of hMSCs (EGFP-) grown for 2 days in maintenance medium in non-coated wells (NC) or wells coated with siEGFP, siBCL2L2 #1 or siTRIB2 #1 followed by 12 days in maintenance or differentiation medium. Positive staining appears red, counter staining appears blue (H&E staining, only applied to a & b). MM, OM and AM denote maintenance, osteogenic and adipogenic media, respectively. All samples were performed in triplicate. Note that the transfected samples contain fewer cells than the non-transfected controls. Figure S3. In vitro osteogenic (a) and adipogenic (b) differentiation of hMSCs (EGFP-) seeded on non-coated scaffolds and scaffolds coated with TransIT-TKO nanoparticles containing siRNA targeted to two regions of BCL2L2 or TRIB2, respectively. After 3 days of culturing in maintenance medium, differentiation was induced with osteogenic (a) or adipogenic (b) medium for 7 days. The ratio of ALP, COL1, RUNX2 and OC (a) or AP2 and PPARγ2 (b) mRNA to GAPDH mRNA was characterized. Averages are shown with standard deviation indicated by error bars. The samples represent n = 5, the values were normalized to the non-coated control scaffolds which were set to 100, “*” denotes that the sample average is significantly different (p < 0.05) from the non-coated control group. Table S1. siRNA sequences used. Table S2. Primers used for quantitative real time PCR. Table S3. Antibodies for immunohistochemistry.
Acknowledgments
We thank Claus Bus, Rita Rosendahl Hansen, Bianca Jørgensen, Tina Kamilla Nielsen, Lone Christiansen, and Nicholas Ditzel for technical help. We also thank Ulrik Lytt Rahbek, Lea Bjerre, and Sys Glud Zoffman for useful advice and help with various methods. The project was financed by grants from the Faculty of Science (University of Aarhus), the Danish Medical Research Council, the Danish Ministry of Science, Technology and Innovation, the EU (FP6 Osteocord), the Lundbeck Foundation and NABIIT (number 2106-04-0029). MØA planned the studies, was involved in all experiments, and wrote the paper; JVN helped planning the studies, produced the scaffolds, and performed the µCT; JSB planned and performed the implantation studies and the histology; MKR performed the confocal microscopy. JRN, CB, FB, KAH, MK, and JK supervised the project and helped drafting the manuscript.
Supplementary Material
Monolayer transfections of hMSCs (EGFP+) were carried out with EGFP match or mismatch siRNA for 2 days as detailed in Figure 1a. EGFP fluorescence was investigated using a histogram. Representative pictures are shown.
Alkaline phosphatase (a), oil red O (b) and alizarin red (c) staining of hMSCs (EGFP-) grown for 2 days in maintenance medium in non-coated wells (NC) or wells coated with siEGFP, siBCL2L2 #1 or siTRIB2 #1 followed by 12 days in maintenance or differentiation medium. Positive staining appears red, counter staining appears blue (H&E staining, only applied to a & b). MM, OM and AM denote maintenance, osteogenic and adipogenic media, respectively. All samples were performed in triplicate. Note that the transfected samples contain fewer cells than the non-transfected controls.
In vitro osteogenic (a) and adipogenic (b) differentiation of hMSCs (EGFP-) seeded on non-coated scaffolds and scaffolds coated with TransIT-TKO nanoparticles containing siRNA targeted to two regions of BCL2L2 or TRIB2, respectively. After 3 days of culturing in maintenance medium, differentiation was induced with osteogenic (a) or adipogenic (b) medium for 7 days. The ratio of ALP, COL1, RUNX2 and OC (a) or AP2 and PPARγ2 (b) mRNA to GAPDH mRNA was characterized. Averages are shown with standard deviation indicated by error bars. The samples represent n = 5, the values were normalized to the non-coated control scaffolds which were set to 100, “*” denotes that the sample average is significantly different (p < 0.05) from the non-coated control group.
siRNA sequences used.
Primers used for quantitative real time PCR.
Antibodies for immunohistochemistry.
REFERENCES
- Langer R., and, Vacanti JP. Tissue engineering. Science. 1993;260:920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- Atala A, Bauer SB, Soker S, Yoo JJ., and, Retik AB. Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet. 2006;367:1241–1246. doi: 10.1016/S0140-6736(06)68438-9. [DOI] [PubMed] [Google Scholar]
- Macchiarini P, Jungebluth P, Go T, Asnaghi MA, Rees LE, Cogan TA, et al. Clinical transplantation of a tissue-engineered airway. Lancet. 2008;372:2023–2030. doi: 10.1016/S0140-6736(08)61598-6. [DOI] [PubMed] [Google Scholar]
- Lee W, Debasitis JC, Lee VK, Lee JH, Fischer K, Edminster K, et al. Multi-layered culture of human skin fibroblasts and keratinocytes through three-dimensional freeform fabrication. Biomaterials. 2009;30:1587–1595. doi: 10.1016/j.biomaterials.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Fedorovich NE, De Wijn JR, Verbout AJ, Alblas J., and, Dhert WJ. Three-dimensional fiber deposition of cell-laden, viable, patterned constructs for bone tissue printing. Tissue Eng Part A. 2008;14:127–133. doi: 10.1089/ten.a.2007.0158. [DOI] [PubMed] [Google Scholar]
- Mironov V, Kasyanov V, Drake C., and, Markwald RR. Organ printing: promises and challenges. Regen Med. 2008;3:93–103. doi: 10.2217/17460751.3.1.93. [DOI] [PubMed] [Google Scholar]
- Aubin JE, Liu F, Malaval L., and, Gupta AK. Osteoblast and chondroblast differentiation. Bone. 1995;17 2 Suppl:77S–83S. doi: 10.1016/8756-3282(95)00183-e. [DOI] [PubMed] [Google Scholar]
- Lee SJ. Cytokine delivery and tissue engineering. Yonsei Med J. 2000;41:704–719. doi: 10.3349/ymj.2000.41.6.704. [DOI] [PubMed] [Google Scholar]
- Jang JH, Rives CB., and, Shea LD. Plasmid delivery in vivo from porous tissue-engineering scaffolds: transgene expression and cellular transfection. Mol Ther. 2005;12:475–483. doi: 10.1016/j.ymthe.2005.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YC, Simmons C, Kaigler D, Rice KG., and, Mooney DJ. Bone regeneration in a rat cranial defect with delivery of PEI-condensed plasmid DNA encoding for bone morphogenetic protein-4 (BMP-4) Gene Ther. 2005;12:418–426. doi: 10.1038/sj.gt.3302439. [DOI] [PubMed] [Google Scholar]
- Phillips JE, Burns KL, Le Doux JM, Guldberg RE., and, García AJ. Engineering graded tissue interfaces. Proc Natl Acad Sci USA. 2008;105:12170–12175. doi: 10.1073/pnas.0801988105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tijsterman M., and, Plasterk RH. Dicers at RISC; the mechanism of RNAi. Cell. 2004;117:1–3. doi: 10.1016/s0092-8674(04)00293-4. [DOI] [PubMed] [Google Scholar]
- Abdallah BM., and, Kassem M. Human mesenchymal stem cells: from basic biology to clinical applications. Gene Ther. 2008;15:109–116. doi: 10.1038/sj.gt.3303067. [DOI] [PubMed] [Google Scholar]
- Zhao Y., and, Ding S. A high-throughput siRNA library screen identifies osteogenic suppressors in human mesenchymal stem cells. Proc Natl Acad Sci USA. 2007;104:9673–9678. doi: 10.1073/pnas.0703407104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motomura H, Niimi H, Sugimori K, Ohtsuka T, Kimura T., and, Kitajima I. Gas6, a new regulator of chondrogenic differentiation from mesenchymal cells. Biochem Biophys Res Commun. 2007;357:997–1003. doi: 10.1016/j.bbrc.2007.04.035. [DOI] [PubMed] [Google Scholar]
- Naiki T, Saijou E, Miyaoka Y, Sekine K., and, Miyajima A. TRB2, a mouse Tribbles ortholog, suppresses adipocyte differentiation by inhibiting AKT and C/EBPbeta. J Biol Chem. 2007;282:24075–24082. doi: 10.1074/jbc.M701409200. [DOI] [PubMed] [Google Scholar]
- Aziz A, Miyake T, Engleka KA, Epstein JA., and, McDermott JC. Menin expression modulates mesenchymal cell commitment to the myogenic and osteogenic lineages. Dev Biol. 2009;332:116–130. doi: 10.1016/j.ydbio.2009.05.555. [DOI] [PubMed] [Google Scholar]
- Shimomura T, Yoshida Y, Sakabe T, Ishii K, Gonda K, Murai R, et al. Hepatic differentiation of human bone marrow-derived UE7T-13 cells: Effects of cytokines and CCN family gene expression. Hepatol Res. 2007;37:1068–1079. doi: 10.1111/j.1872-034X.2007.00162.x. [DOI] [PubMed] [Google Scholar]
- Yang Y, Li Y, Lv Y, Zhang S, Chen L, Bai C, et al. NRSF silencing induces neuronal differentiation of human mesenchymal stem cells. Exp Cell Res. 2008;314:2257–2265. doi: 10.1016/j.yexcr.2008.04.008. [DOI] [PubMed] [Google Scholar]
- Cheema SK, Chen E, Shea LD., and, Mathur AB. Regulation and guidance of cell behavior for tissue regeneration via the siRNA mechanism. Wound Repair Regen. 2007;15:286–295. doi: 10.1111/j.1524-475X.2007.00228.x. [DOI] [PubMed] [Google Scholar]
- Yao Y, Wang C, Varshney RR., and, Wang DA. Antisense makes sense in engineered regenerative medicine. Pharm Res. 2009;26:263–275. doi: 10.1007/s11095-008-9772-3. [DOI] [PubMed] [Google Scholar]
- Andersen MØ, Howard KA, Paludan SR, Besenbacher F., and, Kjems J. Delivery of siRNA from lyophilized polymeric surfaces. Biomaterials. 2008;29:506–512. doi: 10.1016/j.biomaterials.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Simonsen JL, Rosada C, Serakinci N, Justesen J, Stenderup K, Rattan SI, et al. Telomerase expression extends the proliferative life-span and maintains the osteogenic potential of human bone marrow stromal cells. Nat Biotechnol. 2002;20:592–596. doi: 10.1038/nbt0602-592. [DOI] [PubMed] [Google Scholar]
- Bentzon JF, Stenderup K, Hansen FD, Schroder HD, Abdallah BM, Jensen TG, et al. Tissue distribution and engraftment of human mesenchymal stem cells immortalized by human telomerase reverse transcriptase gene. Biochem Biophys Res Commun. 2005;330:633–640. doi: 10.1016/j.bbrc.2005.03.072. [DOI] [PubMed] [Google Scholar]
- Nam YS., and, Park TG. Porous biodegradable polymeric scaffolds prepared by thermally induced phase separation. J Biomed Mater Res. 1999;47:8–17. doi: 10.1002/(sici)1097-4636(199910)47:1<8::aid-jbm2>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Vance RJ, Miller DC, Thapa A, Haberstroh KM., and, Webster TJ. Decreased fibroblast cell density on chemically degraded poly-lactic-co-glycolic acid, polyurethane, and polycaprolactone. Biomaterials. 2004;25:2095–2103. doi: 10.1016/j.biomaterials.2003.08.064. [DOI] [PubMed] [Google Scholar]
- Krebs MD, Jeon O., and, Alsberg E. Localized and sustained delivery of silencing RNA from macroscopic biopolymer hydrogels. J Am Chem Soc. 2009;131:9204–9206. doi: 10.1021/ja9037615. [DOI] [PubMed] [Google Scholar]
- Carlson MA, Prall AK., and, Gums JJ. RNA interference in human foreskin fibroblasts within the three-dimensional collagen matrix. Mol Cell Biochem. 2007;306:123–132. doi: 10.1007/s11010-007-9561-z. [DOI] [PubMed] [Google Scholar]
- Plasilova M, Schonmeyr B, Schonmyer B, Fernandez J, Clavin N, Soares M, et al. Accelerating stem cell proliferation by down-regulation of cell cycle regulator p21. Plast Reconstr Surg. 2009;123 2 Suppl:149S–157S. doi: 10.1097/PRS.0b013e318191c82b. [DOI] [PubMed] [Google Scholar]
- Nho RS, Xia H, Kahm J, Kleidon J, Diebold D., and, Henke CA. Role of integrin-linked kinase in regulating phosphorylation of Akt and fibroblast survival in type I collagen matrices through a beta1 integrin viability signaling pathway. J Biol Chem. 2005;280:26630–26639. doi: 10.1074/jbc.M411798200. [DOI] [PubMed] [Google Scholar]
- Shea LD, Smiley E, Bonadio J., and, Mooney DJ. DNA delivery from polymer matrices for tissue engineering. Nat Biotechnol. 1999;17:551–554. doi: 10.1038/9853. [DOI] [PubMed] [Google Scholar]
- Cherng JY, vd Wetering P, Talsma H, Crommelin DJ., and, Hennink WE. Stabilization of polymer-based gene delivery systems. Int J Pharm. 1999;183:25–28. doi: 10.1016/s0378-5173(99)00037-x. [DOI] [PubMed] [Google Scholar]
- Wu SY, Putral LN, Liang M, Chang HI, Davies NM., and, McMillan NA. Development of a novel method for formulating stable siRNA-loaded lipid particles for in vivo use. Pharm Res. 2009;26:512–522. doi: 10.1007/s11095-008-9766-1. [DOI] [PubMed] [Google Scholar]
- Baum C, Kustikova O, Modlich U, Li Z., and, Fehse B. Mutagenesis and oncogenesis by chromosomal insertion of gene transfer vectors. Hum Gene Ther. 2006;17:253–263. doi: 10.1089/hum.2006.17.253. [DOI] [PubMed] [Google Scholar]
- Marshal E. Gene Therapy Death Prompts Review of Adenovirus Vector. Science. 1999;286:2244–2245. doi: 10.1126/science.286.5448.2244. [DOI] [PubMed] [Google Scholar]
- Aguiari P, Leo S, Zavan B, Vindigni V, Rimessi A, Bianchi K, et al. High glucose induces adipogenic differentiation of muscle-derived stem cells. Proc Natl Acad Sci USA. 2008;105:1226–1231. doi: 10.1073/pnas.0711402105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezy O, Vernochet C, Gesta S, Farmer SR., and, Kahn CR. TRB3 blocks adipocyte differentiation through the inhibition of C/EBPbeta transcriptional activity. Mol Cell Biol. 2007;27:6818–6831. doi: 10.1128/MCB.00375-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y, Ohoka N, Hayashi H., and, Sato R. TRB3 suppresses adipocyte differentiation by negatively regulating PPARgamma transcriptional activity. J Lipid Res. 2008;49:880–892. doi: 10.1194/jlr.M700545-JLR200. [DOI] [PubMed] [Google Scholar]
- Xu Y, Mirmalek-Sani SH, Lin F, Zhang J., and, Oreffo RO. Adipocyte differentiation induced using nonspecific siRNA controls in cultured human mesenchymal stem cells. RNA. 2007;13:1179–1183. doi: 10.1261/rna.527207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoolmeesters A, Eklund T, Leake D, Vermeulen A, Smith Q, Force Aldred S, et al. Functional profiling reveals critical role for miRNA in differentiation of human mesenchymal stem cells. PLoS ONE. 2009;4:e5605. doi: 10.1371/journal.pone.0005605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Li YC, Wang J, Kong J, Qi Y, Quigg RJ, et al. miR-17-92 cluster accelerates adipocyte differentiation by negatively regulating tumor-suppressor Rb2/p130. Proc Natl Acad Sci USA. 2008;105:2889–2894. doi: 10.1073/pnas.0800178105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foshay KM., and, Gallicano GI. miR-17 family miRNAs are expressed during early mammalian development and regulate stem cell differentiation. Dev Biol. 2009;326:431–443. doi: 10.1016/j.ydbio.2008.11.016. [DOI] [PubMed] [Google Scholar]
- Kong J., and, Li YC. Molecular mechanism of 1,25-dihydroxyvitamin D3 inhibition of adipogenesis in 3T3-L1 cells. Am J Physiol Endocrinol Metab. 2006;290:E916–E924. doi: 10.1152/ajpendo.00410.2005. [DOI] [PubMed] [Google Scholar]
- Hendriks J, Riesle J., and, van Blitterswijk CA. Co-culture in cartilage tissue engineering. J Tissue Eng Regen Med. 2007;1:170–178. doi: 10.1002/term.19. [DOI] [PubMed] [Google Scholar]
- Fink T, Lund P, Pilgaard L, Rasmussen JG, Duroux M., and, Zachar V. Instability of standard PCR reference genes in adipose-derived stem cells during propagation, differentiation and hypoxic exposure. BMC Mol Biol. 2008;9:98. doi: 10.1186/1471-2199-9-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdallah BM, Ditzel N., and, Kassem M. Assessment of bone formation capacity using in vivo transplantation assays: procedure and tissue analysis. Methods Mol Biol. 2008;455:89–100. doi: 10.1007/978-1-59745-104-8_6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Monolayer transfections of hMSCs (EGFP+) were carried out with EGFP match or mismatch siRNA for 2 days as detailed in Figure 1a. EGFP fluorescence was investigated using a histogram. Representative pictures are shown.
Alkaline phosphatase (a), oil red O (b) and alizarin red (c) staining of hMSCs (EGFP-) grown for 2 days in maintenance medium in non-coated wells (NC) or wells coated with siEGFP, siBCL2L2 #1 or siTRIB2 #1 followed by 12 days in maintenance or differentiation medium. Positive staining appears red, counter staining appears blue (H&E staining, only applied to a & b). MM, OM and AM denote maintenance, osteogenic and adipogenic media, respectively. All samples were performed in triplicate. Note that the transfected samples contain fewer cells than the non-transfected controls.
In vitro osteogenic (a) and adipogenic (b) differentiation of hMSCs (EGFP-) seeded on non-coated scaffolds and scaffolds coated with TransIT-TKO nanoparticles containing siRNA targeted to two regions of BCL2L2 or TRIB2, respectively. After 3 days of culturing in maintenance medium, differentiation was induced with osteogenic (a) or adipogenic (b) medium for 7 days. The ratio of ALP, COL1, RUNX2 and OC (a) or AP2 and PPARγ2 (b) mRNA to GAPDH mRNA was characterized. Averages are shown with standard deviation indicated by error bars. The samples represent n = 5, the values were normalized to the non-coated control scaffolds which were set to 100, “*” denotes that the sample average is significantly different (p < 0.05) from the non-coated control group.
siRNA sequences used.
Primers used for quantitative real time PCR.
Antibodies for immunohistochemistry.