Abstract
The drug salubrinal has been identified as an inhibitor of phosphatases that act on the eukaryotic translation initiation factor 2 subunit (eIF2α). The resulting maintenance of protein phosphorylation results in enhanced protection from the adverse effects of initiators of the unfolded protein response. We found that salubrinal can also interact with the anti-apoptotic protein Bcl-2, inhibiting binding of the non-peptidic antagonist HA14-1 and of a porphycene that can catalyze Bcl-2 photodamage. As a result, salubrinal offers protection from the apoptotic and autophagic effects that can result from loss of Bcl-2 function.
Keywords: Apoptosis, Autophagy, Bcl-2, Photodynamic, Salubrinal
Promotion of eIF2α phosphorylation results in a halt in protein synthesis, permitting cells to recover from consequences of endoplasmic reticulum (ER) stress that can otherwise lead to apoptosis [1]. The drug salubrinal was identified as a selective inhibitor of phosphatases that act on eIF2α [2], thereby maintaining protein phosphorylation and offering protection from the adverse effects of ER stress, e.g., as induced by the drug tunicamycin.
We have now examined the ability of salubrinal to protect murine leukemia L1210 cells from another inducer of ER stress: photodamage mediated by a porphycene termed ‘CPO’ that binds to the ER [3]. Irradiation of cells containing CPO initiates apoptosis as a result of photodamage to the anti-apoptotic protein Bcl-2 associated with the ER [3,4]. Effects of ER photodamage on other stress-related phenomena have not been characterized. We now report that salubrinal protects cells from the pro-apoptotic effect of ER photodamage, a phenomenon that is, however, not associated with effects on eIF2α phosphorylation. We then examined the possibility that salubrinal could protect Bcl-2 from photodamage.
Additional studies were carried out using HA14-1, a non-peptidic antagonist of the anti-apoptotic functions of Bcl-2 family proteins. A computer screening approach was used to identify this agent as a ligand for the surface pocket on the Bcl-2 protein [5]. If the protection offered by salubrinal from the pro-apoptotic effects of ER photodamage was associated with a direct protective effect on Bcl-2, we considered it possible that this drug might also protect Bcl-2 from both pro-apoptotic [6] and pro-autophagic [7] effects of Bcl-2 inactivation by HA14-1.
Materials and methods
Chemicals and biologicals
Amino acids and tissue culture media were purchased from Sigma–Aldrich (St. Louis, MO), sterile horse serum from Atlanta Biologicals (Lawrenceville, GA), and fluorescent probes from Molecular Probes (Eugene, OR). The Bcl-2 antagonist HA14-1 was obtained from Ryan Scientific Inc. (Isle of Palms, SC). This agent gradually loses activity in the presence of water. Solutions were made up in anhydrous dimethyl sulfoxide and snap-frozen in small aliquots at −20 °C under nitrogen. Salubrinal was purchased from Calbiochem (La Jolla, CA); 10 mM solutions were prepared in anhydrous DMSO and stored at −20 °C. The porphycene CPO was prepared by Dr. G. Craça Vicente, Department of Chemistry, Louisiana State University, Baton Route, LA. The chlorin NPe6 was synthesized by Prof. Kevin M. Smith, also from LSU.
Cells and maintenance
Murine leukemia L1210 cells were grown in Fisher’s medium (Sigma–Aldrich) containing 10% horse serum and 1 mM glutamine, 1 mM mercaptoethanol, and gentamicin. Since, Fisher’s medium is no longer commercially available, we supplemented the α-MEM formulation (Sigma–Aldrich) with MgCl2 (45 mg/l), methionine (75 mg/l), phenylalanine (30 mg/l), valine (30 mg/l), and folic acid (9 mg/l). Clonogenic assays were used to determine loss of viability (LD90 values) after a 60 min exposure to HA14-1. Serial dilutions of cell suspensions were plated on soft agar. After 7–9 day growth in a humidified chamber under 5% CO2, colonies were counted and compared with untreated control values. All such experiments were carried out in triplicate. The cloning efficiency of L1210 cells is approx. 70%.
Protocols
Studies with HA14-1 involved a 60 min exposure of cells to an LD90 drug concentration (40 μM). In some studies, a 50 μM concentration of salubrinal was also present. Photodynamic ER perturbation involved a 30 min incubation of cells at 37 °C with a 2 μM concentration of the porphycene CPO. The cells were then irradiated with an LD90 light dose (135 mJ/cm2, 600–650 nm) as described in Ref. [6]. Where specified, 50 μM salubrinal was present at all times. DEVDase activity was assessed after a 10 min incubation at 37 °C following irradiation of photosensitized cells, or after a 60 min incubation with HA14-1.
DEVDase activity
The fluorogenic substrate zDEVD-R110 was used to measure the activation of procaspase-3/7 [8]. This substrate releases the fluorescent dye Rhodamine 110 upon enzymatic hydrolysis. The increase in fluorescence as a function of time was measured with a Fluoreskan fluorescence plate reader using 485 nm excitation and 510 nm emission.
Western blots for Bcl-2, phosphorylated and non-phosphorylated eIF2α, and LC-3
Cells were lysed and extracts prepared for Western blot analysis [9]. Phosphatase inhibitors (5 mM NaF, 10 mM Na4P2O7, and 5 mM Na3VO4) were present during extraction procedures. The assay for LC3 processing also incorporated two protease inhibitors, E64d and pepstatin A, to inhibit protein degradation by lysosomal proteases [10]. Aliquots containing 40 μg of protein were used for these assays. Immunofluorescence signals were detected with Vistra ECF Western blot reagent (GE-Amersham Biosciences Corp., Piscataway, NJ) using the Storm imaging system (Molecular Dynamics, Sunnyvale, CA). An antibody to eIF2α was obtained from BD Biosciences Inc. (San Jose, CA). An antibody to LC3 was kindly provided by Dr. Masahiro Shibata, Osaka University, Japan.
Formation of liposomes and FRET studies
Binding of CPO to Bcl-2 in a liposomal environment was determined as described in Ref. [9]. Dioleoyl phosphatidylcholine liposomes were incubated with 1 μM CPO for 10 min at room temperature, and fluorescence emission was assessed upon excitation at 280 nm. Bcl-2 was then added (900 ng/ml) and an additional emission spectrum was obtained. The effect of salubrinal was determined in a separate experiment where a 50 μM concentration was added to lipososomes containing Bcl-2 10 min before the addition of CPO. Excitation of Bcl-2 at 280 nm can lead to fluorescence that could excite CPO if the molecules are in sufficiently close proximity, resulting in CPO fluorescence at 645 nm. These studies were carried out in an SLM 48000 fluorescence system.
Results and discussion
Incubation of L1210 cells with LD50 or LD90 concentrations of the Bcl-2 antagonist HA14-1 is known to result in a prompt apoptotic response leading to cell death, as does Bcl-2 photodamage [6]. We found that the presence of 50 μM salubrinal during exposure of cells to HA14-1, or irradiation of photosensitized cells, offers protection from loss of viability as determined by clonogenic assays (Table 1). This was accompanied by impaired activation of caspase-3, a precursor to apoptosis (Table 1).
Table 1.
Effects of salubrinal on DEVDase activity and loss of viability
| System | DEVDase activity |
Viability (%) |
||
|---|---|---|---|---|
| No additions | + Salubrinal | No additions | + Salubrinal | |
| Control | 14 ± 2.1 | 12 ± 1.9 | ||
| HA14-1 LD90 conditions | 225 ± 18 | 70 ± 3 | 13 ± 2 | 48 ± 2 |
| PDT LD50 conditions | 60 ± 7 | 22 ± 4 | 48 ± 2 | 98 ± 3 |
| PDT LD90 conditions | 244 ± 16 | 85 ± 9 | 11 ± 3 | 53 ± 4 |
Cells were treated with HA14-1 (LD90 concentration) or exposed to the porphycene CPO and irradiated under LD50 or LD90 conditions. DEVDase activity (nmol/mg protein/h) was assessed 60 min after addition of HA14-1 or 10 min after irradiation of photosensitized cells. Viability was determined by clonogenic assays. Data represent the mean ± SD of three determinations.
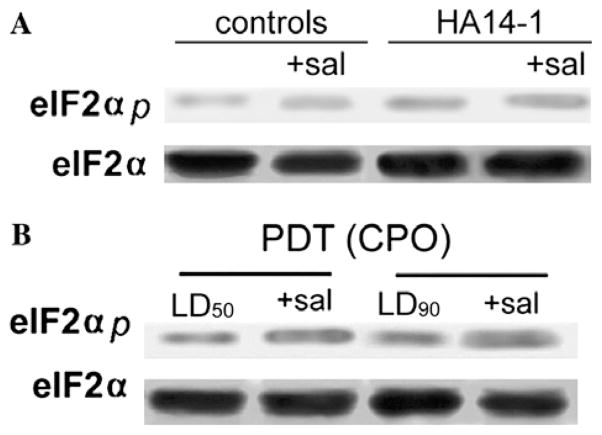
Although incubation with a 50 μM concentration salubrinal promoted maintenance of eIF2α phosphorylation in several cell lines [2], we observed no alterations in either total eIF2α or phosphorylated protein in L1210 cells during short-term incubations (Fig. 1A) or after a 24–48 h incubation (not shown). A 60-min incubation of L1210 cells with an LD90 concentration of HA14-1 did not significantly alter the level of phosphorylated eIF2α; this was only slightly enhanced when salubrinal was present. No changes in eIF2α phosphorylation were observed upon irradiation of photosensitized cells (Fig. 1B).
Fig. 1.
Western blots showing: (A) levels of eIF2α (phosphorylated and non-phosphorylated) 60 min after exposure of L1210 cells to 50 μM salubrinal or 60 min after treatment with an LD90 concentration of HA14-1. Salubrinal was present where specified. (B) Effects of LD50 and LD90 levels of ER photodamage on eIF2α 60 min after irradiation. Salubrinal (50 μM) was added during irradiation as indicated.
Results shown in Table 1 are consistent with a proposal that salubrinal can protect Bcl-2 from inactivation caused by an interaction with HA14-1, or from photodamage. Additional evidence relating to the latter effect was provided by an analysis of Bcl-2 photodamage on Western blots (Fig. 2). In this study, we used two photosensitizing agents: the chlorin termed NPe6 is known to target lysosomes but not Bcl-2, and mediates apoptosis via activation of the pro-apoptotic protein Bid [11]. The porphycene CPO does target Bcl-2 associated with the ER [3]. Irradiation of cells containing NPe6 did not alter Bcl-2 levels (Fig. 2B), but a substantial level of Bcl-2 photodamage resulted upon irradiation of cells photosensitized by CPO (Fig. 2D). This effect was markedly reduced when salubrinal was present during irradiation (Fig. 2E). These results are consistent with the proposal that salubrinal protects Bcl-2 from photodamage.
Fig. 2.
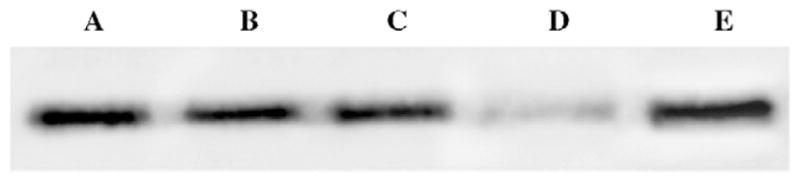
Western blots showing effects of LD90 photodynamic protocols on Bcl-2 levels. (A) Control; (B) 60 min after NPe6 + light; (C) same as (B) but with 50 μM salubrinal present during irradiation. (D) Sixty minutes after CPO + light; (D) same as (C) with salubrinal present during irradiation.
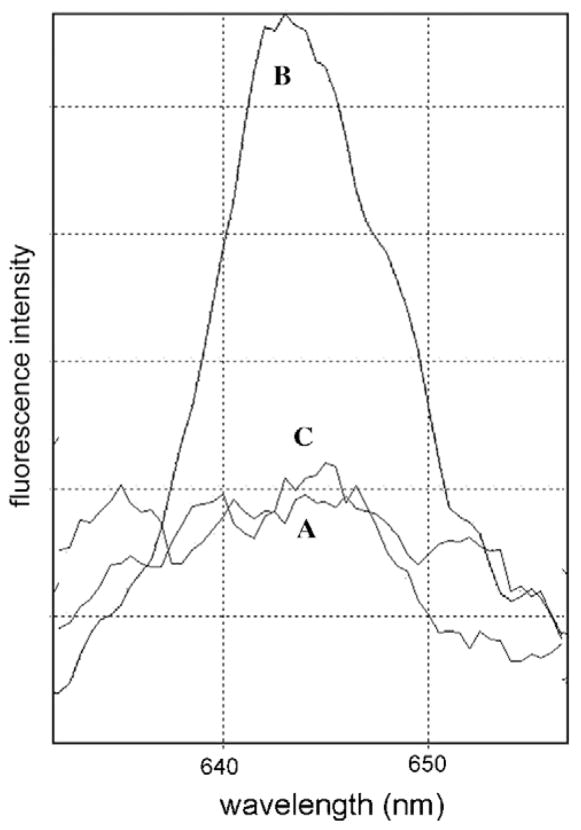
A possible mechanism for the latter effect was provided by FRET analysis, using a procedure we had previously adopted for assessing the affinity of CPO for the Bcl-2 protein in a lysosomal environment [9]. Excitation of CPO at 400 nm leads to fluorescence at 642 nm, but excitation at 280 nm does not result in any detectable fluorescence emission (Fig. 3A). Upon excitation at 280 nm, the aromatic amino acids in the Bcl-2 protein fluorescence in the vicinity of 400 nm [9]. When both Bcl-2 and CPO were simultaneously present in the liposomes, the two products bind in sufficient proximity so that excitation at 280 nm yields CPO fluorescence at 642 nm (Fig. 3B). This occurs when the 400 nm fluorescence from Bcl-2 excites nearby CPO molecules. When 50 μM salubrinal was added, fluorescence energy transfer was inhibited, suggesting that the proximity of Bcl-2 and CPO had become insufficient for energy transfer to occur (Fig. 3C). In other studies, we determined that the fluorescence of neither Bcl-2 nor CPO was quenched by the addition of salubrinal. We interpret these results to indicate that salubrinal protects Bcl-2 from photodamage by either competing with CPO for binding sites, or altering the Bcl-2 configuration so as to inhibit CPO binding. Oleinick’s group has established that photodamage to Bcl-2 can only occur when photosensitizers are bound in close proximity to this protein [12].
Fig. 3.
FRET from Bcl-2 to CPO in a liposomal environment. Fluorescence was excited at 280 nm and assessed between 630 and 660 nm. (A) CPO alone, (B) CPO + Bcl-2, and (C) CPO + Bcl-2 + 50 μM salubrinal.
Since inhibition of Bcl-2 function can lead to autophagy [7, 13], we examined the ability of salubrinal to modify the autophagic response to exposure of L1210 cells to HA14-1. As shown in Fig. 4, treatment with an LD90 concentration of HA14-1 led to an enhanced processing of the LC3-1 protein to a species (LC3-II) that migrates more rapidly during gel electrophoresis. Formation of LC3-II is considered to be one of the hallmarks of autophagy [14]. Upon the addition of salubrinal, LC3 processing was markedly reduced (lane C).
Fig. 4.

Processing of the protein LC3-I to LC3-II. (A) Control (untreated) cells, (B) cells exposed for 60 min at 37 °C to an LD90 level of HA14-1, (C) same as (B) except 50 μM salubrinal was present.
The results described here are consistent with salubrinal-induced protection of the Bcl-2 protein from the pro-apoptotic effects of both HA14-1 and the CPO-induced photo-damage. These effects do not appear to involve the modification of eIF2α phosphorylation by salubrinal described in Ref. [2], but could be explained by a conformational change in the Bcl-2 protein configuration or an association with salubrinal such that binding of both HA14-1 and CPO is markedly reduced.
Acknowledgments
Excellent technical assistance was provided by Ann Marie Santiago and Nakaiya Okan-Mensah. These studies were supported in part by Grant CA 23378 from the National Cancer Institute, NIH.
References
- 1.Lu PD, Jousse C, Marciniak SJ, Zhang Y, Novoa I, Scheuner D, Kaufman RJ, Ron D, Harding HP. Cytoprotection by preemptive conditional phosphorylation of translation initiation factor 2. EMBO J. 2004;23:69–179. doi: 10.1038/sj.emboj.7600030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boyce M, Bryant KF, Jousse C, Long K, Harding HP, Scheuner D, Kaufman RJ, Ma D, Coen DM, Ron D, Yuan YA. A selective inhibitor of eIF2α dephosphorylation protects cells from ER stress. Science. 2005;307:935–939. doi: 10.1126/science.1101902. [DOI] [PubMed] [Google Scholar]
- 3.Kessel D, Castelli M, Reiners JJ., Jr Ruthenium red-mediated suppression of Bcl-2 loss and Ca(2+) release initiated by photodamage to the endoplasmic reticulum: scavenging of reactive oxygen species. Cell Death Differ. 2005;12:502–511. doi: 10.1038/sj.cdd.4401579. [DOI] [PubMed] [Google Scholar]
- 4.Kessel D, Castelli M. Evidence that Bcl-2 is the target of three photosensitizers that induce a rapid apoptotic response. Photochem Photobiol. 2001;74:318–322. doi: 10.1562/0031-8655(2001)074<0318:etbitt>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 5.Wang JL, Liu D, Zhang ZJ, Shan S, Han X, Srinivasula SM, Croce CM, Alnemri ES, Huang Z. Structure based discovery of an organic compound that binds Bcl-2 protein and induces apoptosis of tumor cells. Proc Natl Acad Sci USA. 2000;97:7124–7129. doi: 10.1073/pnas.97.13.7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kessel D, Castelli M, Reiners JJ., Jr Apoptotic response to photodynamic therapy versus the Bcl-2 antagonist HA14-1. Photochem Photobiol. 2002;76:314–319. doi: 10.1562/0031-8655(2002)076<0314:artptv>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 7.Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, Packer M, Schneider MD, Levine B. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122:927–939. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 8.Cai SX, Zhang HZ, Guastella J, Drewe J, Yang W, Weber E. Design and synthesis of rhodamine 110 derivative and caspase-3 substrate for enzyme and cell-based fluorescent assay. Bioorg Med Chem Lett. 2001;11:39–42. doi: 10.1016/s0960-894x(00)00590-4. [DOI] [PubMed] [Google Scholar]
- 9.Castelli M, Reiners JJ, Kessel D. A mechanism for the proapoptotic activity of ursodeoxycholic acid: effects on Bcl-2 conformation. Cell Death Differ. 2004;11:906–914. doi: 10.1038/sj.cdd.4401433. [DOI] [PubMed] [Google Scholar]
- 10.Tanida I, Minematsu-Ikeguchi N, Ueno T, Kominami E. Lysosomal turnover, but not a cellular level, of endogenous LC3 is a marker for autophagy. Autophagy. 2005;1:84–91. doi: 10.4161/auto.1.2.1697. [DOI] [PubMed] [Google Scholar]
- 11.Reiners JJ, Jr, Caruso JA, Mathieu P, Chelladurai B, Yin XM, Kessel D. Release of cytochrome c and activation of pro-caspase-9 following lysosomal photodamage involves bid cleavage. Cell Death Differ. 2002;9:934–944. doi: 10.1038/sj.cdd.4401048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Usuda J, Azizuddin K, Chiu SM, Oleinick NL. Association between the photodynamic loss of Bcl-2 and the sensitivity to apoptosis caused by phthalocyanine photodynamic therapy. Photochem Photobiol. 2003;78:1–8. doi: 10.1562/0031-8655(2003)078<0001:abtplo>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 13.Levine B, Klionsky DJ. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell. 2004;6:463–477. doi: 10.1016/s1534-5807(04)00099-1. [DOI] [PubMed] [Google Scholar]
- 14.Yorimitsu T, Klionsky DJ. Autophagy: molecular machinery for self-eating. Cell Death Differ. 2005;12(Suppl 2):542–1552. doi: 10.1038/sj.cdd.4401765. [DOI] [PMC free article] [PubMed] [Google Scholar]