Hodgkinson and colleagues review the current status of knowledge with respect to the genetic modifications being explored as a means to improve mesenchymal stem cell therapy for human diseases, with a particular focus on cardiovascular diseases.
Abstract
The use of stem cells for tissue regeneration and repair is advancing both at the bench and bedside. Stem cells isolated from bone marrow are currently being tested for their therapeutic potential in a variety of clinical conditions including cardiovascular injury, kidney failure, cancer, and neurological and bone disorders. Despite the advantages, stem cell therapy is still limited by low survival, engraftment, and homing to damage area as well as inefficiencies in differentiating into fully functional tissues. Genetic engineering of mesenchymal stem cells is being explored as a means to circumvent some of these problems. This review presents the current understanding of the use of genetically engineered mesenchymal stem cells in human disease therapy with emphasis on genetic modifications aimed to improve survival, homing, angiogenesis, and heart function after myocardial infarction. Advancements in other disease areas are also discussed.
Introduction
Stem cell therapy for tissue repair and regeneration holds great therapeutic potential. Various embryonic and adult stem or progenitors cells have been isolated from different tissues including brain, heart, and kidney (Teo and Vallier, 2010). Of these, adult stem cells from the bone marrow are the most widely used and characterized. Adult bone marrow contains a heterogeneous population of cells, including hematopoietic stem cells, macrophages, erythrocytes, fibroblasts, adipocytes, and endothelial cells (Salem and Thiemermann, 2010). One of these populations, commonly referred to as mesenchymal stem cells (MSCs) or marrow stromal stem cells, contains a subset of nonhematopoietic stem cells that have the potential to originate various terminally differentiated cell types including muscle, blood, vascular, and bone cells, among others (Salem and Thiemermann, 2010). The ability to develop into various cell types, and the ease with which MSCs can be expanded in culture, have led to a great deal of interest in their use as therapeutic agents to treat a wide range of diseases. To date, mesenchymal stem cells have been investigated in the treatment of diverse diseases such as myocardial infarction, Parkinson's disease, Crohn's disease, and cancer, amongst others (Dimmeler et al., 2005; Loebinger et al., 2009; Aguayo-Mazzucato and Bonner-Weir, 2010; Kumar et al., 2010; Pistoia and Raffaghello, 2010). Results obtained in clinical trials are summarized in Table 1. The results of these preliminary studies have been encouraging. Indeed, at present more than 100 clinical trials using MSCs are active in the United States (data from ClinicalTrials.gov).
Table 1.
Mesenchymal Stem Cell Clinical Trials
| Disease | Ref. | Notes |
|---|---|---|
| Amyotrophic lateral sclerosis | Mazzini et al. (2010) | Feasibility study |
| Acute graft-versus-host disease | Ball et al. (2008); Le Blanc et al. (2008) | |
| Arima et al. (2010) | No beneficial effect | |
| Ning et al. (2008) | Malignancies | |
| Cardiovascular disease | Assmus et al. (2002); Chen et al. (2001); Katritsis et al. (2005); Perin et al. (2003); Stamm et al. (2003); Strauer et al. (2002); Wollert et al. (2004); Hare et al. (2009); Bang et al. (2005) | |
| Improvement in left-ventricular ejection fraction only | ||
| Cardiomyopathy | Arguero et al. (2006); Moviglia et al. (2006) | |
| Crohn's disease | Garcia-Olmo et al. (2009) | |
| Diabetic foot | Vojtassak et al. (2006) | |
| Hurler syndrome | Koc et al. (2002) | |
| Jaw defect | Meijer et al. (2008) | Improvement seen in one subject |
| Limb ischemia | Lasala et al. (2010) | |
| Liver cirrhosis | Kharaziha et al. (2009) | |
| Nonhealing ulcers | Dash et al. (2009) | |
| Osteogenesis imperfecta | Horwitz et al. (2001) | |
| Parkinson's disease | Venkataramana et al. (2010) | Feasibility study |
| Scleroderma | Tyndall and Furst (2007) | |
| Spinal cord injury | Moviglia et al. (2006) | |
| Systemic lupus erythematosus | Sun et al. (2009) |
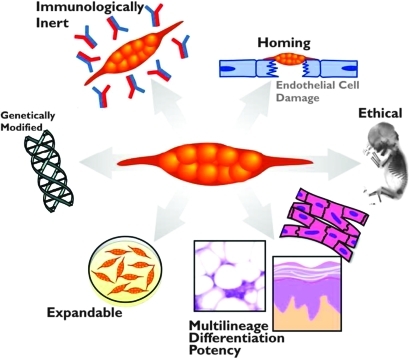
MSCs offer several benefits that make them ideal therapeutic agents for regenerative medicine (Pittenger et al., 1999; Boyle et al., 2010; Griffin et al., 2010; Salem and Thiemermann, 2010). An overview is shown in Fig. 1. MSCs can give rise to a variety of cell types, such as bone cells (osteocytes), cartilage cells (chondrocytes), fat cells (adipocytes), as well as blood, brain, and nerve cells and cardiac and skeletal muscle cells. In addition, MSCs are easily isolated, and can be greatly expanded ex vivo without loss of phenotype or differentiative capacity. Moreover, they are easily transfectable and amenable to genetic modification in vitro. Once transplanted, they are endogenously recruited and home to sites of inflammation and injury. Importantly, MSCs are also immunologically inert and, therefore, transplantation into an allogeneic host may not require immunosuppression (Rasmusson et al., 2003; DelaRosa and Lombardo, 2010). Furthermore, MSCs are easily obtained from a variety of tissue sources. Although most studies have been performed with bone marrow-derived MSCs, these cells have also been identified in cord blood, adipose tissue, muscle, cartilage, and skin (Rebelatto et al., 2008). It has been postulated that MSCs from these different sources may have diverse therapeutic properties (Rebelatto et al., 2008; van der Bogt et al., 2009). Last, as MSCs can be extracted from adult tissue, they do not pose the same ethical concerns as embryonic stem cells.
FIG. 1.
Advantages of mesenchymal stem cell (MSC) therapy. The cellular characteristics of MSCs are important for their therapeutic uses. These characteristics include being easily isolated and manipulated ex vivo, being immunologically inert, homing into the injury area, and having multilineage differentiation capacity. Genetic engineering of MSCs is aimed at improving their survival and engraftment as well as enhancing their repair mechanisms.
Despite tremendous advancements, major unresolved issues about their therapeutic application still exist. Injected MSCs suffer from poor survival and engraftment into the host tissue; in addition, in vitro culturing conditions can affect MSC pluripotency as well as expression of homing receptors (Wagner and Ho, 2007). In some cases, such as when transplanted into cardiac tissue, MSCs do not show high efficiency of transdifferentiation into functional cardiomyocytes (Noiseux et al., 2006). More importantly, there is limited knowledge about the optimal time and mode of MSC administration and there are concerns that MSC treatment has only marginal and transient effects. Meyer and colleagues found, in patients who underwent successful percutaneous coronary intervention for acute myocardial infarction (MI), an improvement in left ventricular function 6 months after intracoronary transfer of autologous bone marrow cells (Wollert et al., 2004). However, follow-up studies showed that there was no sustainable improvement at the long-term mark (Meyer et al., 2009; Schaefer et al., 2010).
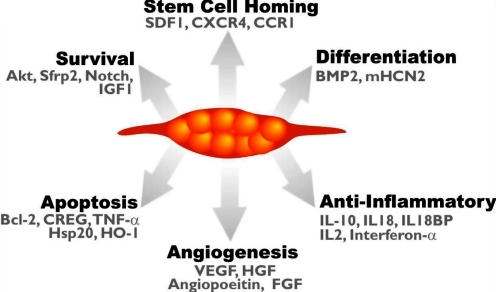
It is evident therefore that these issues need to be addressed if MSCs are to be used in the clinical setting. Moreover, the precise mechanisms underlying mesenchymal stem cell “plasticity,” or pluripotency, as well as the full spectrum of the molecular processes involved in their therapeutic capacity, are not yet fully understood. For instance, after transplantation, MSCs can participate in tissue repair not only by direct transdifferentiation but also by reducing cell damage and activating endogenous mechanisms of tissue regeneration. An overview of these mechanisms is shown in Fig. 2. The ability therefore to understand and/or regulate these processes could be important to the replacement and repair of diseased tissue.
FIG. 2.
Genetic modification of MSCs enhances their therapeutic potential. Genetic modification of MSCs is aimed toward enhancing different cellular process such as MSC survival after transplantation, homing, differentiation, angiogenesis, and antiinflammatory effects. Some of the genetic targets used for this purpose are highlighted.
In the light of this, various researchers have sought to improve MSC therapy. One emergent technology is the genetic engineering of MSCs to express proteins that improve their ability to be therapeutic agents; this avenue has generated much interest because of the positive results arising from these studies (Kode et al., 2009; Griffin et al., 2010). Engineering of MSCs has improved their ability to home to sites of disease, and to promote their survival and engraftment as well as their differentiation. Moreover, as MSCs often exert their beneficial effects through the release of paracrine factors, genetic modification can also contribute significantly to the MSC-mediated paracrine effects (Gnecchi et al., 2005, 2008).
This review aims to outline the current status of our knowledge about the genetic modifications being explored as a means to improve the MSC therapy of human diseases, focusing on cardiovascular diseases. Summaries for other disease areas are also presented.
Cardiovascular Disease
Cardiovascular disease is one of the major causes of death in the world. This is due to the series of unwanted changes such as cell death, mechanical and electrical dysfunction, changes in heart structure, and scarring that follow a heart attack (Yellon and Hausenloy, 2007; Frangogiannis, 2008). MSCs have been reported to contribute to cardiac repair by promoting neovascularization, protecting the myocardium from ischemic cell death, promoting reparative processes, and enhancing cardiomyocyte regeneration (Gnecchi et al., 2008; Nesselmann et al., 2008). At present, the challenges for MSC cardiac therapy lie in improving their survival and engraftment into the injured myocardium as well as enhancing their capacity to promote cardiac regeneration (Balsam et al., 2004; Caplice and Deb, 2004; Murry et al., 2004; Dimmeler et al., 2005). These objectives have formed the basis for the majority of published material on the genetic engineering of MSCs.
Survival
It is evident that the majority of injected MSCs die within several hours of delivery (Hofmann et al., 2005; Hou et al., 2005; Freyman et al., 2006). Therefore methods to improve their survival will be important therapeutically.
In that context, multiple genetic engineering strategies have been applied to stem cells to increase their cell viability both in vitro and in vivo (Tang et al., 2005). For instance, overexpression of fibroblast growth factor (FGF)-2 improved the viability of MSCs implanted into injured myocardium, increasing expression of cardiac markers such as cardiac troponin and the calcium channel CaV2.1 as well as promoting angiogenesis (Song et al., 2005).
MSCs exposed to hypoxic conditions before transplantation also show a higher survival rate. Multiple lines of evidence suggest that this is due in part to upregulation of survival proteins such as Akt (Hu et al., 2008; Rosova et al., 2008; Y.L. Tang et al., 2009). Work in our laboratory has explored the use of MSCs genetically engineered to overexpress Akt, using a viral approach as a means to improve MSC survival in the injured myocardium. Injection of these modified MSCs (Akt-MSCs) into the heart after MI showed that they had a higher survival rate in the ischemic heart and resulted in major improvements in cardiac function (Mangi et al., 2003; Gnecchi et al., 2005). Further research revealed that Akt-MSCs promoted their therapeutic effects by releasing paracrine signals that support the survival of organ cells, as well as by initiating angiogenesis (Mangi et al., 2003; Gnecchi et al., 2005). Further validation of this paracrine model came from in vivo experiments using a rat coronary occlusion model, where administration of concentrated conditional medium from Akt-MSCs had an effect similar to that following administration of Akt-MSC cells, reducing infarct size and cardiac cell apoptosis (Gnecchi et al., 2005). Additional work from our laboratory identified several genes that were overexpressed by Akt-MSCs, leading potentially to the secretion of vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), hepatocyte growth factor (HGF), insulin-like growth factor (IGF)-1, and secreted frizzled-related protein-2 (Sfrp2) (Mirotsou et al., 2007). In this study, we identified Sfrp2 as one of the key paracrine factors released by Akt-MSCs and playing a critical role in the survival of ischemic cardiac myocytes (Mirotsou et al., 2007). The protective effects of Sfrp2 involved the canonical Wnt3a pathway (Zhang et al., 2009). Importantly, Alfaro and colleagues have shown that intramyocardial injection of MSCs overexpressing SFRP2 acts to reduce infarct size by promoting MSC engraftment, and improves cardiac function and vascular density (Alfaro et al., 2008).
In addition, overexpression of a single heat shock protein, Hsp20, in MSCs, using a viral approach, improved their survival under ischemic conditions. Akt, VEGF, FGF-2, and IGF-1 are substrates of Hsp20, and in Hsp20-MSCs these proteins are refolded, with their survival and angiogenic effects restored after a stress episode (X. Wang et al., 2009). The expression of heat shock proteins is increased during cellular stress events, playing a role in refolding damaged proteins and so restoring their function; as such they are key contributors to cell survival. Conditioned medium from Hsp20-MSCs improved the survival of cardiomyocytes under oxidative stress. As might be expected from these results, injection of Hsp20-MSCs into rat hearts after MI promoted cardiac repair and function. In addition to restoring the function of Akt, Hsp20 overexpression in MSCs increased the ratio of B cell lymphoma (Bcl)-2 to Bax, which acted to inhibit apoptosis of these cells (X. Wang et al., 2009).
Antiapoptosis
Whereas some groups have taken the approach of engineering MSCs to express proliferative proteins such as Akt, others have considered whether modulating the expression of proteins known to play important roles in the cellular pathways governing apoptosis would be beneficial. To date, Bcl-2 (W. Li et al., 2007), cellular repressor of EA1-stimulated genes (CREG) (Deng et al., 2010), heme oxygenase-1 (Zeng et al., 2008a,b), and survivin (Fan et al., 2009) have all been used with the aim of improving cell survival and resistance to apoptosis.
Bcl-2 is a key antiapoptotic protein, well known for its inhibitory role in the oligomerization of proapoptotic proteins Bax and Bak, inhibits mitochondrial outer membrane permeabilization (Tsujimoto et al., 1984; Korsmeyer, 1999; Chipuk and Green, 2008). Overexpression of Bcl-2 in adult rat bone marrow-derived MSCs improved their survival, aiding heart tissue repair and organ recovery after MI (W. Li et al., 2007). Bcl-2-MSCs have increased expression of VEGF, which in itself promotes angiogenesis. Although in vivo therapy using Bcl-2-MSCs has been shown to be beneficial there are questions regarding their safety as Bcl-2 overexpression underpins leukemia development (Tsujimoto et al., 1984).
Cellular repressor of EA1-stimulated genes (CREG) is an inhibitor of apoptosis (Deng et al., 2010), and its overexpression in MSCs improves survival of the stem cells. The beneficial effects appear to be mediated by Akt activation and degradation of p53. Increased Akt activity in these modified MSCs promotes the expression of VEGF, which in turn enhances proliferation and angiogenesis (Deng et al., 2010).
Heme oxygenase (HO-1) is involved in the oxidative cleavage of heme and is known to be protective against apoptosis (Idriss et al., 2008). The protein itself could have therapeutic potential to treat heart disease (Liu et al., 2006, 2007). Interestingly, MSCs engineered to overexpress HO-1 improved organ recovery and function, and decreased ventricular remodeling after transplantation in injured rat hearts. Overexpression of HO-1 improved the survival of the injected MSCs and increased levels of secreted VEGF and bFGF as compared with unmodified cells (Zeng et al., 2008a,b). In addition, decreased expression of the proapoptotic protein Bax and reductions in the levels of proinflammatory molecules tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and IL-6 were also observed (Zeng et al., 2008a,b). Moreover, MSCs in which HO-1 was transiently overexpressed also showed enhanced antiapoptotic and antioxidative properties leading to enhanced repair of the myocardium (Tsubokawa et al., 2010). Additional experiments have taken the idea of HO-1 overexpression further by producing MSCs that overexpress both HO-1 and inducible nitric oxide synthase (iNOS). The authors of this study showed that these modified MSCs improved repair of the heart after MI; furthermore, they suggested that inhibition of either protein completely removed the protective action of MSCs (Chabannes et al., 2007).
TNF-α is a proapoptotic and proinflammatory molecule with an important role in the detrimental effects of heart failure. The TNF-α receptors, TNFR1 and TNFR2, have different roles. TNFR1 promotes apoptosis, decreasing proliferation; on the other hand, TNFR2 promotes proliferation and survival (Zeller et al., 2009). A study has shown that virus-induced overexpression of TNFR:Fc, a fusion of TNFR and immunoglobulin Fc, by MSCs removes the detrimental activity of TNF-α and improves heart recovery and function. This was due in part to reduced expression and release of proinflammatory molecules such as IL-6, TNF-α, and IL-1β (Bao et al., 2008).
Stem cell homing
Chemokines are a group of secreted cytokines that induce chemotaxis in nearby cells (Richmond, 2010). After injury damaged cells secrete chemokines that act as attractants to recruit immune and stem cells to start the process of repair. Therefore another option that can be used to improve MSC therapy is to enhance homing of the MSCs to the injured myocardium (Chavakis et al., 2008).
Stromal cell-derived factor (SDF)-1 and its receptor CXC chemokine receptor-4 (CXCR4) are important mediators of stem cell recruitment after myocardial infarction or ischemia (Zaruba and Franz, 2010). Levels of SDF-1 correlate with rehabilitation of patients after coronary events (Hu et al., 2007; B.C. Lee et al., 2009) and increased engraftment of MSCs overexpressing IGF-1 was found to be dependent on SDF-1 (Haider et al., 2008). Penn's group found that MSCs expressing SDF-1 had beneficial effects on heart function after acute myocardial infarction, in part through cardiac myocyte preservation (Zhang et al., 2007). Although this study could not detect any cardiac regeneration from endogenous stem cells, further work did show the recruitment of small cardiac myosin-expressing cells, which despite being unable to differentiate into mature cardiac myocytes within 5 weeks of myocardial infarction did appear capable of depolarizing (Unzek et al., 2007). SDF-1 also appears to promote the differentiation of MSCs into endothelial cells (J. Tang et al., 2009b), which may involve dipeptidylpeptidase IV (Zaruba et al., 2009; Zaruba and Franz, 2010).
One of the issues associated with using SDF-1 overexpression as therapy is that the expression of its receptor, CXCR4, in progenitor cells is low (Penn, 2009; Y.L. Tang et al., 2009). Indeed, MSCs overexpressing CXCR4 home to the damaged infarct region of myocardium in greater numbers than do unmodified MSCs (Cheng et al., 2008).
C-C chemokine receptor type 1 (CCR1) is a G protein-coupled receptor involved in the recruitment of immune cells to site of inflammation (Kitamura and Taketo, 2007; Zernecke et al., 2008). This receptor is not expressed on MSCs; however, its ligands are significantly upregulated in the injured myocardium (Ip et al., 2007; Shimizu et al., 2009). Our laboratory has demonstrated that when overexpressed in MSCs, CCR1 increased MSC migration induced by chemokines as well as protected the cells against apoptosis in vitro (Huang et al., 2010). In addition, CCR1-modified MSCs injected into the myocardium after coronary ligation were found to accumulate in the damaged area in greater numbers than did unmodified cells. This was also associated with an improvement in cardiac function as shown by reduced infarct size, improved cardiomyocyte survival, and a denser capillary network. (Huang et al., 2010).
Angiogenesis
Neovascularization is the process whereby new blood vessels are formed. This highly regulated process is known to involve more than 20 separate proteins (Asahara et al., 1995) and is an important physiological response after MI that allows the heart to recover. Various groups have shown that MSCs secrete proangiogenic factors (Kinnaird et al., 2004a,b; Gnecchi et al., 2005, 2008). Indeed, both in vitro and in vivo models have shown that MSCs can enhance new blood vessel growth (Kinnaird et al., 2004a). Porcine autologous MSCs, when injected after chronic ischemia, showed enhancement of angiogenesis (Zhou et al., 2009). Increasing the secretion of angiogenic factors by MSCs by genetic engineering has been linked to improvements in cardiac function via enhanced new blood vessel growth.
One of the most extensively characterized angiogenic factors is VEGF. Because transplanted heart cells overexpressing VEGF increased capillary density in the border zone of a myocardial scar (Yau et al., 2001), researchers have attempted to repeat this finding with MSCs. Transplantation of MSCs overexpressing VEGF inhibited progression of left ventricular hypertrophy induced by chronic pressure overload in swine. This was associated with significant angiogenesis and improved heart function (Wang et al., 2006). In addition, after injection into mouse ischemic hind limbs, VEGF-modified MSCs promoted angiogenesis and limb retention with concomitant reduced muscle degeneration and tissue fibrosis (Yang et al., 2010). Furthermore, MSCs overexpressing a human form of the VEGF gene increased angiogenesis in the infarct region after acute MI (Gao et al., 2007; Matsumoto et al., 2005). Additional beneficial effects on infarct size and heart function, such as ejection fraction and E wave/A wave ratio, were also observed (Matsumoto et al., 2005; Gao et al., 2007). The most important member of the VEGF family is VEGF-A, of which 16 splice variants are currently known. A paper by Lin and colleagues raises the possibility that the therapeutic potential of VEGF-expressing MSCs can be modulated by the splice variant employed (Lin et al., 2008). The authors of this study expressed three VEGF-A splice variants, VEGF120, VEGF164, and VEGF188, in MSCs and measured the effects on proliferation, differentiation, and survival. Whereas all three variants strongly promoted MSC proliferation there were also clear differences. VEGF120 and VEGF188 were strongly associated with amplification of growth factor and cytokine expression, whereas VEGF164 was linked to genes involved in endothelial differentiation. In addition, VEGF188 preferentially facilitated MSC osteogenesis and enhanced cell death arising from serum starvation (Lin et al., 2008). In addition to the important role that VEGF plays in promoting angiogenesis it appears that this growth factor can also promote homing of stem cells. The protein acts to recruit cKit+ CD31+ progenitor cells to damaged myocardium in a process involving phosphatidylinositol-3-kinase (PI3K) and Akt (Wragg et al., 2008; J. Tang et al., 2009a; Zisa et al., 2009).
Other angiogenic factors have also been successfully employed for MSC genetic engineering. Hepatocyte growth factor-overexpressing MSCs injected into a rat heart after an infarct increased the density of capillaries and reduced the area of damage (Duan et al., 2003). Similarly, MSCs expressing angiopoietin have protective effects in both cerebral (Onda et al., 2008) and myocardial (Sun et al., 2007; Chen et al., 2009) models. In the cerebral artery occlusion model intravenous delivery of angiopoietin-expressing MSCs increased angiogenesis around the border of the lesion but had little or no effect on lesion volume (Onda et al., 2008). By combining overexpression of angiopoietin and Akt, these modified MSCs not only increased angiogenesis but also simultaneously inhibited apoptosis (S. Jiang et al., 2006). Three months after injection a significant number of these cells had survived and differentiated into mature muscle cells, producing fibers that were aligned and electrically coupled to the host muscle (Shujia et al., 2008).
The secretion of paracrine factors is not the only mechanism by which MSCs could promote angiogenesis. Newly formed blood vessels are stabilized by MSCs acting as pericytes. A clear link between MSCs and pericytes has been demonstrated by the finding that perivascular cells from several human organs expressed MSC markers (Caplan, 2008; Crisan et al., 2008). Migration towards pepsin- and papain-digested extracellular matrix (ECM) suggests that these pericytes/MSCs can home to sites of injury (Crisan et al., 2008). These studies open the door for future possibilities in engineering MSCs to promote new blood vessel growth.
Antiinflammatory action
Inflammatory processes arising in the heart after MI underpin the formation of scar tissue, which acts to degrade heart function. Unmodified MSCs themselves appear to activate antiinflammatory pathways. Burchfield and colleagues have shown that the beneficial effects of MSCs are partly dependent on the secretion of IL-10, an antiinflammatory cytokine (Burchfield et al., 2008). Engineering MSCs such that they inhibit post-MI inflammatory mechanisms would therefore have clear benefits.
One of the proinflammatory cytokines released from damaged myocardium is IL-18 (Venkatachalam et al., 2009). This cytokine promotes cell death in MSCs and limits their secretion of VEGF. IL-18 may therefore partly explain why the effects of MSCs are limited in vivo. IL-18-binding protein (IL-18BP), the naturally occurring inhibitor of IL-18 activity, decreases the severity of inflammation in response to injury; MSCs overexpressing IL-18BP were protected from cell death. Intramyocardial injection of IL-18BP-expressing MSCs improved various parameters of heart function and decreased infarct size in a coronary ligation model when compared with normal MSCs (M. Wang et al., 2009).
Cardiac pacemakers
Cardiac pacemaker cells initiate the beating cycle of the heart. It is important that implanted MSCs that differentiate into cardiomyocytes develop pacemaker potential and synchronize with preexisting tissue. In that context, human mesenchymal stem cells (hMSCs) transfected with murine hyperpolarization-activated cyclic nucleotide-gated potassium channel 2 (mHCN2), a cardiac pacemaker protein, affected the beating rate of cocultured neonatal rat ventricular myocytes. Injection of these modified hMSCs into the left ventricle of canines caused the development of spontaneous rhythms originating from the left-hand side of the heart. Furthermore, gap junctions formed between these hMSCs and native adjacent cardiomyocytes (Potapova et al., 2004). In a follow-up study Plotnikov and collaborators showed that hMSCs genetically engineered with mHCN2, when used to treat dogs after heart block, improved pacemaker function and did not show signs of cell rejection (Plotnikov et al., 2007).
Neurological Disease
Unmodified MSCs have been used as a potential therapy for various neurological disorders including Parkinson's (Studer et al., 1998), Huntington's (Armstrong et al., 2000), stroke (Modo et al., 2002; Zhao et al., 2002; Chen et al., 2003; Kurozumi et al., 2004), and multiple sclerosis (Akiyama et al., 2002). Studies using genetically modified MSCs for the treatment of these diseases are currently limited; however, there is plenty of potential for modification, especially by increasing differentiation and paracrine effects.
MSCs possess neuron markers such as glial fibrillary acidic protein (GFAP), neuron-specific enolase (Mareschi et al., 2006), nestin, and Tuj-1 (Tondreau et al., 2004). Multiple reports have indicated that MSCs can develop neuronal morphology and expression patterns, using simple treatments such as cyclic AMP (cAMP) (Deng et al., 2001; Kim et al., 2006), epidermal growth factor (EGF)/bFGF (Kim et al., 2006), 2-mercaptoethanol (Mareschi et al., 2006), or specialized media (Woodbury et al., 2000; Tondreau et al., 2004). It has been suggested that more complex regimens that mimic the regulated stepwise differentiation of precursors into adult neurons are probably required for the generation of neuronal cells from MSCs. In that context, Dezawa and colleagues were able to produce neuronal cells from MSCs by a step-by-step approach (Dezawa et al., 2004). MSCs transfected with the intracellular domain of Notch, a key regulator of the terminal differentiation of neurons and glial cells, displayed neural stem cell markers. Subsequent treatment with glial cell line-derived neurotrophic factor (GDNF) gave rise to cells that could produce dopamine and were tyrosine hydroxylase positive. Importantly, transplantation of these cells into a rat model of Parkinson's gave rise to functional recovery in the animals (Dezawa et al., 2004). In addition, MSCs transfected with the intracellular domain of Notch1 became SB623 neuroprogenitor cells. These SB623 cells derived from Notch1-MSCs, when injected, engrafted and subsequently promoted dense fiber formation with serotonin immunoreactivity in a rodent model of Parkinson's disease (Dezawa et al., 2004). More recently, the same group has shown that these effects were at least partially mediated by enhanced secretion of GDNF (Glavaski-Joksimovic et al., 2009).
Cancer
Factors released by MSCs have antitumor properties reducing the proliferation of glioma, melanoma, lung cancer, hepatoma, and breast cancer cells (Maestroni et al., 1999; Nakamura et al., 2004; Qiao et al., 2008). However, it is the ability of MSCs to migrate to cancer tissue (Nakamizo et al., 2005; Menon et al., 2007; Xin et al., 2007; Loebinger et al., 2009) that has generated the most interest, as this homing ability of MSCs suggests they may be useful as delivery agents to target tumors (Loebinger et al., 2009).
The cancer tissue-homing mechanism employed by MSCs is currently unknown. Cancer cells do secrete SDF-1, which may indicate a role for the SDF-1:CXCR4 axis (Orimo et al., 2005; Dwyer et al., 2007). However, as mentioned earlier, CXCR4 expression is low in MSCs. Considering that there are nearly 20 chemokine receptors that have been reported to be expressed on MSCs (Honczarenko et al., 2006; Ponte et al., 2007; Ringe et al., 2007), elucidating the mechanism is likely to be challenging.
At present, MSCs have been used as delivery agents for a variety of molecules that can inhibit tumor growth. Bone marrow-derived MSCs expressing interferon (IFN)-α reduced proliferation of transformed cells via an increase in apoptosis in a melanoma lung metastasis model (Ren et al., 2008a). IFN-α delivery by MSCs, which leads to accumulation of cells in S phase and increased apoptosis, was found to be beneficial in several cancer models including glioma (D.H. Lee et al., 2009), melanoma (Studeny et al., 2002), and prostate cancer (Ren et al., 2008b). Similarly, IFN-α released from MSCs inhibited leukemia cell proliferation in vitro (Li et al., 2006). Delivery of IL-12 and IL-18 has been adopted in order to recruit T cells and natural killer cells. IL-12-engineered MSCs prevented metastasis into the lymph nodes and other internal organs as well as increased tumor cell apoptosis (Chen et al., 2008). Similarly, MSCs transduced with a vector carrying IL-18 have been shown to be protective against glioma (Xu et al., 2009). Antiglioma effects have also been observed with MSCs expressing IL-2 (Nakamura et al., 2004), which is known to increase T cell proliferation. MSCs engineered to express CX3CL1, a strong T cell chemoattractant, reduced metastasis to the lung after injection of cancer cells (Xin et al., 2007). These results are interesting in light of the strong immunosuppressive effects of MSCs, with T and B cells arrested in G0/G1 (Glennie et al., 2005; Corcione et al., 2006) and regulatory T cells activated (Wolf and Wolf, 2008). TNF-related apoptosis-inducing ligand (TRAIL), a member of the tumor necrosis factor family, is a highly selective protein able to induce apoptosis in cancerous cells but leaving normal cells unaffected (Wiley et al., 1995; Pitti et al., 1996). TRAIL-expressing MSCs induced apoptosis in various cancer cell lines in vitro and cleared lung metastases in about 40% of mice compared with 0% in controls (Loebinger et al., 2009). MSCs have also been employed to deliver adenovirus; replication of the virus within malignant cells destroyed the tumors (Komarova et al., 2006; Hakkarainen et al., 2007; Stoff-Khalili et al., 2007).
However, the use of MSCs in cancer therapy is not without its disadvantages; MSCs have been implicated in promoting the growth of certain cancers. For example, IL-6 and CCL5, proteins produced by MSCs, have been shown to increase the growth and metastasis of breast cancer cells, respectively (Karnoub et al., 2007; Sasser et al., 2007).
Bone Formation
MSCs have also been used in the treatment of bone disease and repair. The incidence of bone fractures failing to heal is dependent on the fracture site and can be as high as 20% (Undale et al., 2009; Chanda et al., 2010). Loading of MSCs onto structural scaffolds has been shown to be useful in the repair of fractures. MSCs loaded onto ceramic cylinders and implanted into rat femora formed bone by 8 weeks (Kadiyala et al., 1997). These hydroxyapatite ceramic cylinders have also successfully generated bone in canine and sheep (Bruder et al., 1998; Kon et al., 2000).
There has been an increase in the use of genetically engineered MSCs to enhance osteoblast lineage commitment, bone formation, fracture repair, and spinal fusion. MSCs expressing bone morphogenetic protein-2 (BMP2), transforming growth factor (TGF)-β1, latent membrane protein (LMP)-1, IGF-1, or growth differentiation factor (GDF)-5 have enhanced cartilage, bone, and tendon repair (Nixon et al., 2007). Specifically, MSCs overexpressing IGF-1 improved tendon healing in a collagenase model of tendinitis (Schnabel et al., 2009). Ultimate tensile strength was not directly increased by MSC injection; however, tendon architecture was improved in the early period after injury, and appropriate choice of integrating nonimmunogenic vectors should further improve growth factor residence time in the tendon.
In addition, a number of studies have also demonstrated that genetic modification of MSCs by enforced expression of either telomerase reverse transcriptase (TERT) (Shi et al., 2002; Gronthos et al., 2003; Abdallah et al., 2005), Wnt4 (Chang et al., 2007), or BMP (Jiang et al., 2005; X. Jiang et al., 2006) enhanced their proliferation capacity and improved bone formation and repair of bone defects, with no adverse effects on the endogenous tissue in response to viral transfer or tumor development (Arthur et al., 2009).
Furthermore, MSCs genetically modified to express BMP2, with a further modification to express α4-integrin to enhance homing of the cells, increased bone mineral density and mineral content. This was due in part to enhanced recruitment of endogenous progenitors involved in bone formation (Kumar et al., 2010). Similar results have been obtained in other studies (H. Li et al., 2007; Tai et al., 2008). An in-depth study of the properties of BMP2-MSC-induced bone formation indicated that this new bone is structurally similar to that of native adult bone (Tai et al., 2008).
More recently, in vitro studies have shown that MSCs genetically engineered to overexpress the longer, VEGF188 isoform facilitated BMP7-mediated MSC osteogenesis. Whether these cells will also show enhanced osteogenic properties in vivo remains to be tested (Lin et al., 2008).
Renal Failure
Many kidney disorders involve both ischemic/inflammatory and immunologic injury. Therefore cell-based therapies such as those using MSCs that function through multiple mechanisms and have the potential to target the inflammatory and immunologic pathways have been considered a clinically relevant solution, in contrast to pharmacologic agents that target only a single event or pathway in the pathophysiology of a given disease. Experimental evidence suggests that administering exogenous mesenchymal stem cells during acute and chronic kidney injury may improve functional and structural recovery of the tubular, glomerular, and interstitial kidney compartments (Asanuma et al., 2010). Eliopoulos and colleagues have generated murine MSCs genetically modified to secrete erythropoietin (EPO) and they were tested to determine whether they can improve anemia in mice with mild to moderate chronic renal failure (Eliopoulos et al., 2006). Their data showed that these cells led to an increase in hematocrit when admixed in a bovine collagen matrix and implanted by subcutaneous injection in mice. More recently, the same group has demonstrated that delivery of MSCs overexpressing a combination of EPO and IGF-1 resulted in enhanced hematocrit elevation, as well as improved cardiac function, compared with animals receiving MSCs expressing EPO alone (Kucic et al., 2008).
Future Directions
MSC therapy has been shown to be beneficial in the treatment of a diverse range of diseases. However, problems with poor survival, engraftment, and differentiation have hampered the routine use of MSCs in the clinic. Genetic engineering of MSCs has the potential to overcome these challenges. Research on engineered MSCs revealed that many of the effects attributable to MSC therapy appear to be mediated by secreted proteins. This opens the door to the possibility of directly administering these proteins in a clinical setting without the need for injecting cells. Nevertheless, genetically engineered MSCs and their applicability as therapeutic agents remains an emerging and developing field. Additional research into the streamlining of protocols for the optimal isolation and expansion of those cells in vitro, as well as for their administration in vivo, is currently in progress, and has been enhanced by the use of novel biomaterials (Kobayashi et al., 2004; Mouw et al., 2007; Zhao et al., 2007; Potier et al., 2010). Importantly, the genetic modification of MSCs, such as the use of a new generation of viral vectors or the use of RNA silencing, alone or in combination with traditional gene modification protocols, is currently under investigation (Nixon et al., 2007). As more becomes understood about MSC biology the engineering of MSCs will become more refined and useful in the treatment of a greater number of diseases. Progress in these areas might make the prospect of “off-the-shelf” MSC biotherapeutic products achievable.
Acknowledgments
The authors express appreciation to Steven Conlon (Department of Pathology, Duke University) for his artistic contribution to the illustrations in this review. Research conducted by our group and mentioned in this review was supported by National Heart, Lung, and Blood Institute grants RO1 HL35610, HL81744, HL72010, and HL73219 (to V.J.D.); the Edna Mandel Foundation (to V.J.D. and M.M.); and the Leducq Foundation (to V.J.D.). M.M. is also supported by an American Heart Association National Scientist Development Award (10SDG4280011).
Author Disclosure Statement
No disclosures.
References
- Abdallah B.M. Haack-Sorensen M. Burns J.S. Elsnab B. Jakob F. Hokland P. Kassem M. Maintenance of differentiation potential of human bone marrow mesenchymal stem cells immortalized by human telomerase reverse transcriptase gene despite [corrected] extensive proliferation. Biochem. Biophys. Res. Commun. 2005;326:527–538. doi: 10.1016/j.bbrc.2004.11.059. [DOI] [PubMed] [Google Scholar]
- Aguayo-Mazzucato C. Bonner-Weir S. Stem cell therapy for type 1 diabetes mellitus. Nat. Rev. Endocrinol. 2010;6:139–148. doi: 10.1038/nrendo.2009.274. [DOI] [PubMed] [Google Scholar]
- Akiyama Y. Radtke C. Kocsis J.D. Remyelination of the rat spinal cord by transplantation of identified bone marrow stromal cells. J. Neurosci. 2002;22:6623–6630. doi: 10.1523/JNEUROSCI.22-15-06623.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfaro M.P. Pagni M. Vincent A. Atkinson J. Hill M.F. Cates J. Davidson J.M. Rottman J. Lee E. Young P.P. The Wnt modulator sFRP2 enhances mesenchymal stem cell engraftment, granulation tissue formation and myocardial repair. Proc. Natl. Acad. Sci. U.S.A. 2008;105:18366–18371. doi: 10.1073/pnas.0803437105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arguero R. Careaga-Reyna G. Castano-Guerra R. Garrido-Garduno M.H. Magana-Serrano J.A. de Jesus Nambo-Lucio M. Cellular autotransplantation for ischemic and idiopathic dilated cardiomyopathy: Preliminary report. Arch. Med. Res. 2006;37:1010–1014. doi: 10.1016/j.arcmed.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Arima N. Nakamura F. Fukunaga A. Hirata H. Machida H. Kouno S. Ohgushi H. Single intra-arterial injection of mesenchymal stromal cells for treatment of steroid-refractory acute graft-versus-host disease: A pilot study. Cytotherapy. 2010;12:265–268. doi: 10.3109/14653240903390795. [DOI] [PubMed] [Google Scholar]
- Armstrong R.J. Watts C. Svendsen C.N. Dunnett S.B. Rosser A.E. Survival, neuronal differentiation, and fiber outgrowth of propagated human neural precursor grafts in an animal model of Huntington's disease. Cell Transplant. 2000;9:55–64. doi: 10.1177/096368970000900108. [DOI] [PubMed] [Google Scholar]
- Arthur A. Zannettino A. Gronthos S. The therapeutic applications of multipotential mesenchymal/stromal stem cells in skeletal tissue repair. J. Cell. Physiol. 2009;218:237–245. doi: 10.1002/jcp.21592. [DOI] [PubMed] [Google Scholar]
- Asahara T. Bauters C. Zheng L.P. Takeshita S. Bunting S. Ferrara N. Symes J.F. Isner J.M. Synergistic effect of vascular endothelial growth factor and basic fibroblast growth factor on angiogenesis in vivo. Circulation. 1995;92:II365–II371. doi: 10.1161/01.cir.92.9.365. [DOI] [PubMed] [Google Scholar]
- Asanuma H. Meldrum D.R. Meldrum K.K. Therapeutic applications of mesenchymal stem cells to repair kidney injury. J. Urol. 2010;184:26–33. doi: 10.1016/j.juro.2010.03.050. [DOI] [PubMed] [Google Scholar]
- Assmus B. Schachinger V. Teupe C. Britten M. Lehmann R. Dobert N. Grunwald F. Aicher A. Urbich C. Martin H. Hoelzer D. Dimmeler S. Zeiher A.M. Transplantation of Progenitor Cells and Regeneration Enhancement in Acute Myocardial Infarction (TOPCARE-AMI) Circulation. 2002;106:3009–3017. doi: 10.1161/01.cir.0000043246.74879.cd. [DOI] [PubMed] [Google Scholar]
- Ball L.M. Bernardo M.E. Locatelli F. Egeler R.M. Potential role of mesenchymal stromal cells in pediatric hematopoietic SCT. Bone Marrow Transplant. 2008;42(Suppl. 2):S60–S66. doi: 10.1038/bmt.2008.286. [DOI] [PubMed] [Google Scholar]
- Balsam L.B. Wagers A.J. Christensen J.L. Kofidis T. Weissman I.L. Robbins R.C. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature. 2004;428:668–673. doi: 10.1038/nature02460. [DOI] [PubMed] [Google Scholar]
- Bang O.Y. Lee J.S. Lee P.H. Lee G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann. Neurol. 2005;57:874–882. doi: 10.1002/ana.20501. [DOI] [PubMed] [Google Scholar]
- Bao C. Guo J. Lin G. Hu M. Hu Z. TNFR gene-modified mesenchymal stem cells attenuate inflammation and cardiac dysfunction following MI. Scand. Cardiovasc. J. 2008;42:56–62. doi: 10.1080/14017430701543556. [DOI] [PubMed] [Google Scholar]
- Boyle A.J. McNiece I.K. Hare J.M. Mesenchymal stem cell therapy for cardiac repair. Methods Mol. Biol. 2010;660:65–84. doi: 10.1007/978-1-60761-705-1_5. [DOI] [PubMed] [Google Scholar]
- Bruder S.P. Kraus K.H. Goldberg V.M. Kadiyala S. The effect of implants loaded with autologous mesenchymal stem cells on the healing of canine segmental bone defects. J. Bone Joint Surg. Am. 1998;80:985–996. doi: 10.2106/00004623-199807000-00007. [DOI] [PubMed] [Google Scholar]
- Burchfield J.S. Iwasaki M. Koyanagi M. Urbich C. Rosenthal N. Zeiher A.M. Dimmeler S. Interleukin-10 from transplanted bone marrow mononuclear cells contributes to cardiac protection after myocardial infarction. Circ. Res. 2008;103:203–211. doi: 10.1161/CIRCRESAHA.108.178475. [DOI] [PubMed] [Google Scholar]
- Caplan A.I. All MSCs are pericytes? Cell Stem Cell. 2008;3:229–230. doi: 10.1016/j.stem.2008.08.008. [DOI] [PubMed] [Google Scholar]
- Caplice N.M. Deb A. Myocardial-cell replacement: The science, the clinic and the future. Nat. Clin. Pract. Cardiovasc. Med. 2004;1:90–95. doi: 10.1038/ncpcardio0051. [DOI] [PubMed] [Google Scholar]
- Chabannes D. Hill M. Merieau E. Rossignol J. Brion R. Soulillou J.P. Anegon I. Cuturi M.C. A role for heme oxygenase-1 in the immunosuppressive effect of adult rat and human mesenchymal stem cells. Blood. 2007;110:3691–3694. doi: 10.1182/blood-2007-02-075481. [DOI] [PubMed] [Google Scholar]
- Chanda D. Kumar S. Ponnazhagan S. Therapeutic potential of adult bone marrow-derived mesenchymal stem cells in diseases of the skeleton. J. Cell Biochem. 2010;111:249–257. doi: 10.1002/jcb.22701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J. Sonoyama W. Wang Z. Jin Q. Zhang C. Krebsbach P.H. Giannobile W. Shi S. Wang C.Y. Noncanonical Wnt-4 signaling enhances bone regeneration of mesenchymal stem cells in craniofacial defects through activation of p38 MAPK. J. Biol. Chem. 2007;282:30938–30948. doi: 10.1074/jbc.M702391200. [DOI] [PubMed] [Google Scholar]
- Chavakis E. Urbich C. Dimmeler S. Homing and engraftment of progenitor cells: A prerequisite for cell therapy. J. Mol. Cell. Cardiol. 2008;45:514–522. doi: 10.1016/j.yjmcc.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Chen J. Li Y. Wang L. Lu M. Zhang X. Chopp M. Therapeutic benefit of intracerebral transplantation of bone marrow stromal cells after cerebral ischemia in rats. J. Neurol. Sci. 2001;189:49–57. doi: 10.1016/s0022-510x(01)00557-3. [DOI] [PubMed] [Google Scholar]
- Chen J. Li Y. Katakowski M. Chen X. Wang L. Lu D. Lu M. Gautam S.C. Chopp M. Intravenous bone marrow stromal cell therapy reduces apoptosis and promotes endogenous cell proliferation after stroke in female rat. J. Neurosci. Res. 2003;73:778–786. doi: 10.1002/jnr.10691. [DOI] [PubMed] [Google Scholar]
- Chen S.L. Zhu C.C. Liu Y.Q. Tang L.J. Yi L. Yu B.J. Wang D.J. Mesenchymal stem cells genetically modified with the angiopoietin-1 gene enhanced arteriogenesis in a porcine model of chronic myocardial ischaemia. J. Int. Med. Res. 2009;37:68–78. doi: 10.1177/147323000903700108. [DOI] [PubMed] [Google Scholar]
- Chen X. Lin X. Zhao J. Shi W. Zhang H. Wang Y. Kan B. Du L. Wang B. Wei Y. Liu Y. Zhao X. A tumor-selective biotherapy with prolonged impact on established metastases based on cytokine gene-engineered MSCs. Mol. Ther. 2008;16:749–756. doi: 10.1038/mt.2008.3. [DOI] [PubMed] [Google Scholar]
- Cheng Z. Ou L. Zhou X. Li F. Jia X. Zhang Y. Liu X. Li Y. Ward C.A. Melo L.G. Kong D. Targeted migration of mesenchymal stem cells modified with CXCR4 gene to infarcted myocardium improves cardiac performance. Mol. Ther. 2008;16:571–579. doi: 10.1038/sj.mt.6300374. [DOI] [PubMed] [Google Scholar]
- Chipuk J.E. Green D.R. How do BCL-2 proteins induce mitochondrial outer membrane permeabilization? Trends Cell Biol. 2008;18:157–164. doi: 10.1016/j.tcb.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcione A. Benvenuto F. Ferretti E. Giunti D. Cappiello V. Cazzanti F. Risso M. Gualandi F. Mancardi G.L. Pistoia V. Uccelli A. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006;107:367–372. doi: 10.1182/blood-2005-07-2657. [DOI] [PubMed] [Google Scholar]
- Crisan M. Yap S. Casteilla L. Chen C.W. Corselli M. Park T.S. Andriolo G. Sun B. Zheng B. Zhang L. Norotte C. Teng P.N. Traas J. Schugar R. Deasy B.M. Badylak S. Buhring H.J. Giacobino J.P. Lazzari L. Huard J. Péault B. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Dash N.R. Dash S.N. Routray P. Mohapatra S. Mohapatra P.C. Targeting nonhealing ulcers of lower extremity in human through autologous bone marrow-derived mesenchymal stem cells. Rejuvenation Res. 2009;12:359–366. doi: 10.1089/rej.2009.0872. [DOI] [PubMed] [Google Scholar]
- DelaRosa O. Lombardo E. Modulation of adult mesenchymal stem cells activity by Toll-like receptors: Implications on therapeutic potential. Mediators Inflamm. 2010;2010:865601. doi: 10.1155/2010/865601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng J. Han Y. Yan C. Tian X. Tao J. Kang J. Li S. Overexpressing cellular repressor of E1A-stimulated genes protects mesenchymal stem cells against hypoxia- and serum deprivation-induced apoptosis by activation of PI3K/Akt. Apoptosis. 2010;15:463–473. doi: 10.1007/s10495-009-0434-7. [DOI] [PubMed] [Google Scholar]
- Deng W. Obrocka M. Fischer I. Prockop D.J. In vitro differentiation of human marrow stromal cells into early progenitors of neural cells by conditions that increase intracellular cyclic AMP. Biochem. Biophys. Res. Commun. 2001;282:148–152. doi: 10.1006/bbrc.2001.4570. [DOI] [PubMed] [Google Scholar]
- Dezawa M. Kanno H. Hoshino M. Cho H. Matsumoto N. Itokazu Y. Tajima N. Yamada H. Sawada H. Ishikawa H. Mimura T. Kitada M. Suzuki Y. Ide C. Specific induction of neuronal cells from bone marrow stromal cells and application for autologous transplantation. J. Clin. Invest. 2004;113:1701–1710. doi: 10.1172/JCI20935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimmeler S. Zeiher A.M. Schneider M.D. Unchain my heart: The scientific foundations of cardiac repair. J. Clin. Invest. 2005;115:572–583. doi: 10.1172/JCI24283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan H.F. Wu C.T. Wu D.L. Lu Y. Liu H.J. Ha X.Q. Zhang Q.W. Wang H. Jia X.X. Wang L.S. Treatment of myocardial ischemia with bone marrow-derived mesenchymal stem cells overexpressing hepatocyte growth factor. Mol. Ther. 2003;8:467–474. doi: 10.1016/s1525-0016(03)00186-2. [DOI] [PubMed] [Google Scholar]
- Dwyer R.M. Potter-Beirne S.M. Harrington K.A. Lowery A.J. Hennessy E. Murphy J.M. Barry F.P. O'Brien T. Kerin M.J. Monocyte chemotactic protein-1 secreted by primary breast tumors stimulates migration of mesenchymal stem cells. Clin. Cancer Res. 2007;13:5020–5027. doi: 10.1158/1078-0432.CCR-07-0731. [DOI] [PubMed] [Google Scholar]
- Eliopoulos N. Gagnon R.F. Francois M. Galipeau J. Erythropoietin delivery by genetically engineered bone marrow stromal cells for correction of anemia in mice with chronic renal failure. J. Am. Soc. Nephrol. 2006;17:1576–1584. doi: 10.1681/ASN.2005101035. [DOI] [PubMed] [Google Scholar]
- Fan L. Lin C. Zhuo S. Chen L. Liu N. Luo Y. Fang J. Huang Z. Lin Y. Chen J. Transplantation with survivin-engineered mesenchymal stem cells results in better prognosis in a rat model of myocardial infarction. Eur. J. Heart Fail. 2009;11:1023–1030. doi: 10.1093/eurjhf/hfp135. [DOI] [PubMed] [Google Scholar]
- Frangogiannis N.G. The immune system and cardiac repair. Pharmacol. Res. 2008;58:88–111. doi: 10.1016/j.phrs.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freyman T. Polin G. Osman H. Crary J. Lu M. Cheng L. Palasis M. Wilensky R.L. A quantitative, randomized study evaluating three methods of mesenchymal stem cell delivery following myocardial infarction. Eur. Heart J. 2006;27:1114–1122. doi: 10.1093/eurheartj/ehi818. [DOI] [PubMed] [Google Scholar]
- Gao F. He T. Wang H. Yu S. Yi D. Liu W. Cai Z. A promising strategy for the treatment of ischemic heart disease: Mesenchymal stem cell-mediated vascular endothelial growth factor gene transfer in rats. Can. J. Cardiol. 2007;23:891–898. doi: 10.1016/s0828-282x(07)70845-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Olmo D. Herreros D. Pascual M. Pascual I. De-La-Quintana P. Trebol J. Garcia-Arranz M. Treatment of enterocutaneous fistula in Crohn's disease with adipose-derived stem cells: A comparison of protocols with and without cell expansion. Int. J. Colorectal Dis. 2009;24:27–30. doi: 10.1007/s00384-008-0559-0. [DOI] [PubMed] [Google Scholar]
- Glavaski-Joksimovic A. Virag T. Chang Q.A. West N.C. Mangatu T.A. McGrogan M.P. Dugich-Djordjevic M. Bohn M.C. Reversal of dopaminergic degeneration in a parkinsonian rat following micrografting of human bone marrow-derived neural progenitors. Cell Transplant. 2009;18:801–814. doi: 10.3727/096368909X470801. [DOI] [PubMed] [Google Scholar]
- Glennie S. Soeiro I. Dyson P.J. Lam E.W. Dazzi F. Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood. 2005;105:2821–2827. doi: 10.1182/blood-2004-09-3696. [DOI] [PubMed] [Google Scholar]
- Gnecchi M. He H. Liang O.D. Melo L.G. Morello F. Mu H. Noiseux N. Zhang L. Pratt R.E. Ingwall J.S. Dzau V.J. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat. Med. 2005;11:367–368. doi: 10.1038/nm0405-367. [DOI] [PubMed] [Google Scholar]
- Gnecchi M. Zhang Z. Ni A. Dzau V.J. Paracrine mechanisms in adult stem cell signaling and therapy. Circ. Res. 2008;103:1204–1219. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin M. Greiser U. Barry F. O'Brien T. Ritter T. Genetically modified mesenchymal stem cells and their clinical potential in acute cardiovascular disease. Discov. Med. 2010;9:219–223. [PubMed] [Google Scholar]
- Gronthos S. Chen S. Wang C.Y. Robey P.G. Shi S. Telomerase accelerates osteogenesis of bone marrow stromal stem cells by upregulation of CBFA1, osterix, and osteocalcin. J. Bone Miner. Res. 2003;18:716–722. doi: 10.1359/jbmr.2003.18.4.716. [DOI] [PubMed] [Google Scholar]
- Haider H. Jiang S. Idris N.M. Ashraf M. IGF-1-overexpressing mesenchymal stem cells accelerate bone marrow stem cell mobilization via paracrine activation of SDF-1α/CXCR4 signaling to promote myocardial repair. Circ. Res. 2008;103:1300–1308. doi: 10.1161/CIRCRESAHA.108.186742. [DOI] [PubMed] [Google Scholar]
- Hakkarainen T. Sarkioja M. Lehenkari P. Miettinen S. Ylikomi T. Suuronen R. Desmond R.A. Kanerva A. Hemminki A. Human mesenchymal stem cells lack tumor tropism but enhance the antitumor activity of oncolytic adenoviruses in orthotopic lung and breast tumors. Hum. Gene Ther. 2007;18:627–641. doi: 10.1089/hum.2007.034. [DOI] [PubMed] [Google Scholar]
- Hare J.M. Traverse J.H. Henry T.D. Dib N. Strumpf R.K. Schulman S.P. Gerstenblith G. DeMaria A.N. Denktas A.E. Gammon R.S. Hermiller J.B., Jr. Reisman M.A. Schaer G.L. Sherman W. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J. Am. Coll. Cardiol. 2009;54:2277–2286. doi: 10.1016/j.jacc.2009.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann M. Wollert K.C. Meyer G.P. Menke A. Arseniev L. Hertenstein B. Ganser A. Knapp W.H. Drexler H. Monitoring of bone marrow cell homing into the infarcted human myocardium. Circulation. 2005;111:2198–2202. doi: 10.1161/01.CIR.0000163546.27639.AA. [DOI] [PubMed] [Google Scholar]
- Honczarenko M. Le Y. Swierkowski M. Ghiran I. Glodek A.M. Silberstein L.E. Human bone marrow stromal cells express a distinct set of biologically functional chemokine receptors. Stem Cells. 2006;24:1030–1041. doi: 10.1634/stemcells.2005-0319. [DOI] [PubMed] [Google Scholar]
- Horwitz E.M. Prockop D.J. Gordon P.L. Koo W.W. Fitzpatrick L.A. Neel M.D. McCarville M.E. Orchard P.J. Pyeritz R.E. Brenner M.K. Clinical responses to bone marrow transplantation in children with severe osteogenesis imperfecta. Blood. 2001;97:1227–1231. doi: 10.1182/blood.v97.5.1227. [DOI] [PubMed] [Google Scholar]
- Hou D. Youssef E.A. Brinton T.J. Zhang P. Rogers P. Price E.T. Yeung A.C. Johnstone B.H. Yock P.G. March K.L. Radiolabeled cell distribution after intramyocardial, intracoronary, and interstitial retrograde coronary venous delivery: Implications for current clinical trials. Circulation. 2005;112:I150–156. doi: 10.1161/CIRCULATIONAHA.104.526749. [DOI] [PubMed] [Google Scholar]
- Hu X. Dai S. Wu W.J. Tan W. Zhu X. Mu J. Guo Y. Bolli R. Rokosh G. Stromal cell derived factor-1α confers protection against myocardial ischemia/reperfusion injury: Role of the cardiac stromal cell derived factor-1α CXCR4 axis. Circulation. 2007;116:654–663. doi: 10.1161/CIRCULATIONAHA.106.672451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X. Yu S.P. Fraser J.L. Lu Z. Ogle M.E. Wang J.A. Wei L. Transplantation of hypoxia-preconditioned mesenchymal stem cells improves infarcted heart function via enhanced survival of implanted cells and angiogenesis. J. Thorac. Cardiovasc. Surg. 2008;135:799–808. doi: 10.1016/j.jtcvs.2007.07.071. [DOI] [PubMed] [Google Scholar]
- Huang J. Zhang Z. Guo J. Ni A. Deb A. Zhang L. Mirotsou M. Pratt R.E. Dzau V.J. Genetic modification of mesenchymal stem cells overexpressing CCR1 increases cell viability, migration, engraftment, and capillary density in the injured myocardium. Circ. Res. 2010;106:1753–1762. doi: 10.1161/CIRCRESAHA.109.196030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idriss N.K. Blann A.D. Lip G.Y. Hemoxygenase-1 in cardiovascular disease. J. Am. Coll. Cardiol. 2008;52:971–978. doi: 10.1016/j.jacc.2008.06.019. [DOI] [PubMed] [Google Scholar]
- Ip J.E. Wu Y. Huang J. Zhang L. Pratt R.E. Dzau V.J. Mesenchymal stem cells use integrin β1 not CXC chemokine receptor 4 for myocardial migration and engraftment. Mol. Biol. Cell. 2007;18:2873–2882. doi: 10.1091/mbc.E07-02-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang S. Haider H. Idris N.M. Salim A. Ashraf M. Supportive interaction between cell survival signaling and angiocompetent factors enhances donor cell survival and promotes angiomyogenesis for cardiac repair. Circ. Res. 2006;99:776–784. doi: 10.1161/01.RES.0000244687.97719.4f. [DOI] [PubMed] [Google Scholar]
- Jiang X. Gittens S.A. Chang Q. Zhang X. Chen C. Zhang Z. The use of tissue-engineered bone with human bone morphogenetic protein-4-modified bone-marrow stromal cells in repairing mandibular defects in rabbits. Int. J. Oral Maxillofac. Surg. 2006;35:1133–1139. doi: 10.1016/j.ijom.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Jiang X.Q. Chen J.G. Gittens S. Chen C.J. Zhang X.L. Zhang Z.Y. The ectopic study of tissue-engineered bone with hBMP-4 gene modified bone marrow stromal cells in rabbits. Chin. Med. J. (Engl) 2005;118:281–288. [PubMed] [Google Scholar]
- Kadiyala S. Young R.G. Thiede M.A. Bruder S.P. Culture expanded canine mesenchymal stem cells possess osteochondrogenic potential in vivo and in vitro. Cell Transplant. 1997;6:125–134. doi: 10.1177/096368979700600206. [DOI] [PubMed] [Google Scholar]
- Karnoub A.E. Dash A.B. Vo A.P. Sullivan A. Brooks M.W. Bell G.W. Richardson A.L. Polyak K. Tubo R. Weinberg R.A. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–563. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- Katritsis D.G. Sotiropoulou P.A. Karvouni E. Karabinos I. Korovesis S. Perez S.A. Voridis E.M. Papamichail M. Transcoronary transplantation of autologous mesenchymal stem cells and endothelial progenitors into infarcted human myocardium. Catheter Cardiovasc. Interv. 2005;65:321–329. doi: 10.1002/ccd.20406. [DOI] [PubMed] [Google Scholar]
- Kharaziha P. Hellstrom P.M. Noorinayer B. Farzaneh F. Aghajani K. Jafari F. Telkabadi M. Atashi A. Honardoost M. Zali M.R. Soleimani M. Improvement of liver function in liver cirrhosis patients after autologous mesenchymal stem cell injection: A phase I–II clinical trial. Eur. J. Gastroenterol. Hepatol. 2009;21:1199–1205. doi: 10.1097/MEG.0b013e32832a1f6c. [DOI] [PubMed] [Google Scholar]
- Kim S. Honmou O. Kato K. Nonaka T. Houkin K. Hamada H. Kocsis J.D. Neural differentiation potential of peripheral blood- and bone-marrow-derived precursor cells. Brain Res. 2006;1123:27–33. doi: 10.1016/j.brainres.2006.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnaird T. Stabile E. Burnett M.S. Lee C.W. Barr S. Fuchs S. Epstein S.E. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ. Res. 2004a;94:678–685. doi: 10.1161/01.RES.0000118601.37875.AC. [DOI] [PubMed] [Google Scholar]
- Kinnaird T. Stabile E. Burnett M.S. Shou M. Lee C.W. Barr S. Fuchs S. Epstein S.E. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation. 2004b;109:1543–1549. doi: 10.1161/01.CIR.0000124062.31102.57. [DOI] [PubMed] [Google Scholar]
- Kitamura T. Taketo M.M. Keeping out the bad guys: Gateway to cellular target therapy. Cancer Res. 2007;67:10099–10102. doi: 10.1158/0008-5472.CAN-07-2100. [DOI] [PubMed] [Google Scholar]
- Kobayashi N. Yasu T. Ueba H. Sata M. Hashimoto S. Kuroki M. Saito M. Kawakami M. Mechanical stress promotes the expression of smooth muscle-like properties in marrow stromal cells. Exp. Hematol. 2004;32:1238–1245. doi: 10.1016/j.exphem.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Koc O.N. Day J. Nieder M. Gerson S.L. Lazarus H.M. Krivit W. Allogeneic mesenchymal stem cell infusion for treatment of metachromatic leukodystrophy (MLD) and Hurler syndrome (MPS-IH) Bone Marrow Transplant. 2002;30:215–222. doi: 10.1038/sj.bmt.1703650. [DOI] [PubMed] [Google Scholar]
- Kode J.A. Mukherjee S. Joglekar M.V. Hardikar A.A. Mesenchymal stem cells: Immunobiology and role in immunomodulation and tissue regeneration. Cytotherapy. 2009;11:377–391. doi: 10.1080/14653240903080367. [DOI] [PubMed] [Google Scholar]
- Komarova S. Kawakami Y. Stoff-Khalili M.A. Curiel D.T. Pereboeva L. Mesenchymal progenitor cells as cellular vehicles for delivery of oncolytic adenoviruses. Mol. Cancer Ther. 2006;5:755–766. doi: 10.1158/1535-7163.MCT-05-0334. [DOI] [PubMed] [Google Scholar]
- Kon E. Muraglia A. Corsi A. Bianco P. Marcacci M. Martin I. Boyde A. Ruspantini I. Chistolini P. Rocca M. Giardino R. Cancedda R. Quarto R. Autologous bone marrow stromal cells loaded onto porous hydroxyapatite ceramic accelerate bone repair in critical-size defects of sheep long bones. J. Biomed. Mater. Res. 2000;49:328–337. doi: 10.1002/(sici)1097-4636(20000305)49:3<328::aid-jbm5>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Korsmeyer S.J. BCL-2 gene family and the regulation of programmed cell death. Cancer Res. 1999;59:1693s–1700s. [PubMed] [Google Scholar]
- Kucic T. Copland I.B. Cuerquis J. Coutu D.L. Chalifour L.E. Gagnon R.F. Galipeau J. Mesenchymal stromal cells genetically engineered to overexpress IGF-I enhance cell-based gene therapy of renal failure-induced anemia. Am. J. Physiol. Renal Physiol. 2008;295:F488–F496. doi: 10.1152/ajprenal.00044.2008. [DOI] [PubMed] [Google Scholar]
- Kumar S. Nagy T.R. Ponnazhagan S. Therapeutic potential of genetically modified adult stem cells for osteopenia. Gene Ther. 2010;17:105–116. doi: 10.1038/gt.2009.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurozumi K. Nakamura K. Tamiya T. Kawano Y. Kobune M. Hirai S. Uchida H. Sasaki K. Ito Y. Kato K. Honmou O. Houkin K. Date I. Hamada H. BDNF gene-modified mesenchymal stem cells promote functional recovery and reduce infarct size in the rat middle cerebral artery occlusion model. Mol. Ther. 2004;9:189–197. doi: 10.1016/j.ymthe.2003.10.012. [DOI] [PubMed] [Google Scholar]
- Lasala G.P. Silva J.A. Gardner P.A. Minguell J.J. Combination stem cell therapy for the treatment of severe limb ischemia: Safety and efficacy analysis. Angiology. 2010;61:551–556. doi: 10.1177/0003319710364213. [DOI] [PubMed] [Google Scholar]
- Le Blanc K. Frassoni F. Ball L. Locatelli F. Roelofs H. Lewis I. Lanino E. Sundberg B. Bernardo M.E. Remberger M. Dini G. Egeler R.M. Bacigalupo A. Fibbe W. Ringdén O. Developmental Committee of the European Group for Blood and Marrow Transplantation. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: A phase II study. Lancet. 2008;371:1579–1586. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- Lee B.C. Hsu H.C. Tseng W.Y. Su M.Y. Chen S.Y. Wu Y.W. Chien K.L. Chen M.F. Effect of cardiac rehabilitation on angiogenic cytokines in postinfarction patients. Heart. 2009a;95:1012–1018. doi: 10.1136/hrt.2008.153510. [DOI] [PubMed] [Google Scholar]
- Lee D.H. Ahn Y. Kim S.U. Wang K.C. Cho B.K. Phi J.H. Park I.H. Black P.M. Carroll R.S. Lee J. Kim S.K. Targeting rat brainstem glioma using human neural stem cells and human mesenchymal stem cells. Clin. Cancer Res. 2009b;15:4925–4934. doi: 10.1158/1078-0432.CCR-08-3076. [DOI] [PubMed] [Google Scholar]
- Li H. Dai K. Tang T. Zhang X. Yan M. Lou J. Bone regeneration by implantation of adipose-derived stromal cells expressing BMP-2. Biochem. Biophys. Res. Commun. 2007;356:836–842. doi: 10.1016/j.bbrc.2007.02.165. [DOI] [PubMed] [Google Scholar]
- Li W. Ma N. Ong L.L. Nesselmann C. Klopsch C. Ladilov Y. Furlani D. Piechaczek C. Moebius J.M. Lützow K. Lendlein A. Stamm C. Li R.K. Steinhoff G. Bcl-2 engineered MSCs inhibited apoptosis and improved heart function. Stem Cells. 2007;25:2118–2127. doi: 10.1634/stemcells.2006-0771. [DOI] [PubMed] [Google Scholar]
- Li X. Lu Y. Huang W. Xu H. Chen X. Geng Q. Fan H. Tan Y. Xue G. Jiang X. In vitro effect of adenovirus-mediated human Gamma Interferon gene transfer into human mesenchymal stem cells for chronic myelogenous leukemia. Hematol. Oncol. 2006;24:151–158. doi: 10.1002/hon.779. [DOI] [PubMed] [Google Scholar]
- Lin H. Shabbir A. Molnar M. Yang J. Marion S. Canty J.M., Jr. Lee T. Adenoviral expression of vascular endothelial growth factor splice variants differentially regulate bone marrow-derived mesenchymal stem cells. J. Cell. Physiol. 2008;216:458–468. doi: 10.1002/jcp.21414. [DOI] [PubMed] [Google Scholar]
- Liu X. Pachori A.S. Ward C.A. Davis J.P. Gnecchi M. Kong D. Zhang L. Murduck J. Yet S.F. Perrella M.A. Pratt R.E. Dzau V.J. Melo L.G. Heme oxygenase-1 (HO-1) inhibits postmyocardial infarct remodeling and restores ventricular function. FASEB J. 2006;20:207–216. doi: 10.1096/fj.05-4435com. [DOI] [PubMed] [Google Scholar]
- Liu X. Simpson J.A. Brunt K.R. Ward C.A. Hall S.R. Kinobe R.T. Barrette V. Tse M.Y. Pang S.C. Pachori A.S. Dzau V.J. Ogunyankin K.O. Melo L.G. Preemptive heme oxygenase-1 gene delivery reveals reduced mortality and preservation of left ventricular function 1 yr after acute myocardial infarction. Am. J. Physiol. Heart Circ. Physiol. 2007;293:H48–H59. doi: 10.1152/ajpheart.00741.2006. [DOI] [PubMed] [Google Scholar]
- Loebinger M.R. Eddaoudi A. Davies D. Janes S.M. Mesenchymal stem cell delivery of TRAIL can eliminate metastatic cancer. Cancer Res. 2009;69:4134–4142. doi: 10.1158/0008-5472.CAN-08-4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestroni G.J. Hertens E. Galli P. Factor(s) from nonmacrophage bone marrow stromal cells inhibit Lewis lung carcinoma and B16 melanoma growth in mice. Cell. Mol. Life Sci. 1999;55:663–667. doi: 10.1007/s000180050322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangi A.A. Noiseux N. Kong D. He H. Rezvani M. Ingwall J.S. Dzau V.J. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat. Med. 2003;9:1195–1201. doi: 10.1038/nm912. [DOI] [PubMed] [Google Scholar]
- Mareschi K. Novara M. Rustichelli D. Ferrero I. Guido D. Carbone E. Medico E. Madon E. Vercelli A. Fagioli F. Neural differentiation of human mesenchymal stem cells: Evidence for expression of neural markers and eag K+ channel types. Exp. Hematol. 2006;34:1563–1572. doi: 10.1016/j.exphem.2006.06.020. [DOI] [PubMed] [Google Scholar]
- Matsumoto R. Omura T. Yoshiyama M. Hayashi T. Inamoto S. Koh K.R. Ohta K. Izumi Y. Nakamura Y. Akioka K. Kitaura Y. Takeuchi K. Yoshikawa J. Vascular endothelial growth factor-expressing mesenchymal stem cell transplantation for the treatment of acute myocardial infarction. Arterioscler. Thromb. Vasc. Biol. 2005;25:1168–1173. doi: 10.1161/01.ATV.0000165696.25680.ce. [DOI] [PubMed] [Google Scholar]
- Mazzini L. Ferrero I. Luparello V. Rustichelli D. Gunetti M. Mareschi K. Testa L. Stecco A. Tarletti R. Miglioretti M. Fava E. Nasuelli N. Cisari C. Massara M. Vercelli R. Oggioni G.D. Carriero A. Cantello R. Monaco F. Fagioli F. Mesenchymal stem cell transplantation in amyotrophic lateral sclerosis: A phase I clinical trial. Exp. Neurol. 2010;223:229–237. doi: 10.1016/j.expneurol.2009.08.007. [DOI] [PubMed] [Google Scholar]
- Meijer G.J. de Bruijn J.D. Koole R. van Blitterswijk C.A. Cell based bone tissue engineering in jaw defects. Biomaterials. 2008;29:3053–3061. doi: 10.1016/j.biomaterials.2008.03.012. [DOI] [PubMed] [Google Scholar]
- Menon L.G. Picinich S. Koneru R. Gao H. Lin S.Y. Koneru M. Mayer-Kuckuk P. Glod J. Banerjee D. Differential gene expression associated with migration of mesenchymal stem cells to conditioned medium from tumor cells or bone marrow cells. Stem Cells. 2007;25:520–528. doi: 10.1634/stemcells.2006-0257. [DOI] [PubMed] [Google Scholar]
- Meyer G.P. Wollert K.C. Lotz J. Pirr J. Rager U. Lippolt P. Hahn A. Fichtner S. Schaefer A. Arseniev L. Ganser A. Drexler H. Intracoronary bone marrow cell transfer after myocardial infarction: 5-year follow-up from the randomized-controlled BOOST trial. Eur. Heart J. 2009;30:2978–2984. doi: 10.1093/eurheartj/ehp374. [DOI] [PubMed] [Google Scholar]
- Mirotsou M. Zhang Z. Deb A. Zhang L. Gnecchi M. Noiseux N. Mu H. Pachori A. Dzau V. Secreted frizzled related protein 2 (Sfrp2) is the key Akt-mesenchymal stem cell-released paracrine factor mediating myocardial survival and repair. Proc. Natl. Acad. Sci. U.S.A. 2007;104:1643–1648. doi: 10.1073/pnas.0610024104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modo M. Stroemer R.P. Tang E. Patel S. Hodges H. Effects of implantation site of stem cell grafts on behavioral recovery from stroke damage. Stroke. 2002;33:2270–2278. doi: 10.1161/01.str.0000027693.50675.c5. [DOI] [PubMed] [Google Scholar]
- Mouw J.K. Connelly J.T. Wilson C.G. Michael K.E. Levenston M.E. Dynamic compression regulates the expression and synthesis of chondrocyte-specific matrix molecules in bone marrow stromal cells. Stem Cells. 2007;25:655–663. doi: 10.1634/stemcells.2006-0435. [DOI] [PubMed] [Google Scholar]
- Moviglia G.A. Fernandez Vina R. Brizuela J.A. Saslavsky J. Vrsalovic F. Varela G. Bastos F. Farina P. Etchegaray G. Barbieri M. Martinez G. Picasso F. Schmidt Y. Brizuela P. Gaeta C.A. Costanzo H. Moviglia-Brandolino M.T. Merino S. Pes M.E. Veloso M.J. Rugilo C. Tamer I. Shuster G.S. Combined protocol of cell therapy for chronic spinal cord injury: Report on the electrical and functional recovery of two patients. Cytotherapy. 2006;8:202–209. doi: 10.1080/14653240600736048. [DOI] [PubMed] [Google Scholar]
- Murry C.E. Soonpaa M.H. Reinecke H. Nakajima H. Nakajima H.O. Rubart M. Pasumarthi K.B. Virag J.I. Bartelmez S.H. Poppa V. Bradford G. Dowell J.D. Williams D.A. Field L.J. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature. 2004;428:664–668. doi: 10.1038/nature02446. [DOI] [PubMed] [Google Scholar]
- Nakamizo A. Marini F. Amano T. Khan A. Studeny M. Gumin J. Chen J. Hentschel S. Vecil G. Dembinski J. Andreeff M. Lang F.F. Human bone marrow-derived mesenchymal stem cells in the treatment of gliomas. Cancer Res. 2005;65:3307–3318. doi: 10.1158/0008-5472.CAN-04-1874. [DOI] [PubMed] [Google Scholar]
- Nakamura K. Ito Y. Kawano Y. Kurozumi K. Kobune M. Tsuda H. Bizen A. Honmou O. Niitsu Y. Hamada H. Antitumor effect of genetically engineered mesenchymal stem cells in a rat glioma model. Gene Ther. 2004;11:1155–1164. doi: 10.1038/sj.gt.3302276. [DOI] [PubMed] [Google Scholar]
- Nesselmann C. Ma N. Bieback K. Wagner W. Ho A. Konttinen Y.T. Zhang H. Hinescu M.E. Steinhoff G. Mesenchymal stem cells and cardiac repair. J. Cell. Mol. Med. 2008;12:1795–1810. doi: 10.1111/j.1582-4934.2008.00457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ning H. Yang F. Jiang M. Hu L. Feng K. Zhang J. Yu Z. Li B. Xu C. Li Y. Wang J. Hu J. Lou X. Chen H. The correlation between cotransplantation of mesenchymal stem cells and higher recurrence rate in hematologic malignancy patients: Outcome of a pilot clinical study. Leukemia. 2008;22:593–599. doi: 10.1038/sj.leu.2405090. [DOI] [PubMed] [Google Scholar]
- Nixon A.J. Goodrich L.R. Scimeca M.S. Witte T.H. Schnabel L.V. Watts A.E. Robbins P.D. Gene therapy in musculoskeletal repair. Ann. N. Y. Acad. Sci. 2007;1117:310–327. doi: 10.1196/annals.1402.065. [DOI] [PubMed] [Google Scholar]
- Noiseux N. Gnecchi M. Lopez-Ilasaca M. Zhang L. Solomon S.D. Deb A. Dzau V.J. Pratt R.E. Mesenchymal stem cells overexpressing Akt dramatically repair infarcted myocardium and improve cardiac function despite infrequent cellular fusion or differentiation. Mol. Ther. 2006;14:840–850. doi: 10.1016/j.ymthe.2006.05.016. [DOI] [PubMed] [Google Scholar]
- Onda T. Honmou O. Harada K. Houkin K. Hamada H. Kocsis J.D. Therapeutic benefits by human mesenchymal stem cells (hMSCs) and Ang-1 gene-modified hMSCs after cerebral ischemia. J. Cereb. Blood Flow Metab. 2008;28:329–340. doi: 10.1038/sj.jcbfm.9600527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orimo A. Gupta P.B. Sgroi D.C. Arenzana-Seisdedos F. Delaunay T. Naeem R. Carey V.J. Richardson A.L. Weinberg R.A. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- Penn M.S. Importance of the SDF-1:CXCR4 axis in myocardial repair. Circ. Res. 2009;104:1133–1135. doi: 10.1161/CIRCRESAHA.109.198929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perin E.C. Dohmann H.F. Borojevic R. Silva S.A. Sousa A.L. Mesquita C.T. Rossi M.I. Carvalho A.C. Dutra H.S. Dohmann H.J. Silva G.V. Belém L. Vivacqua R. Rangel F.O. Esporcatte R. Geng Y.J. Vaughn W.K. Assad J.A. Mesquita E.T. Willerson J.T. Transendocardial, autologous bone marrow cell transplantation for severe, chronic ischemic heart failure. Circulation. 2003;107:2294–2302. doi: 10.1161/01.CIR.0000070596.30552.8B. [DOI] [PubMed] [Google Scholar]
- Pistoia V. Raffaghello L. Potential of mesenchymal stem cells for the therapy of autoimmune diseases. Expert Rev. Clin. Immunol. 2010;6:211–218. doi: 10.1586/eci.09.86. [DOI] [PubMed] [Google Scholar]
- Pittenger M.F. Mackay A.M. Beck S.C. Jaiswal R.K. Douglas R. Mosca J.D. Moorman M.A. Simonetti D.W. Craig S. Marshak D.R. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Pitti R.M. Marsters S.A. Ruppert S. Donahue C.J. Moore A. Ashkenazi A. Induction of apoptosis by Apo-2 ligand, a new member of the tumor necrosis factor cytokine family. J. Biol. Chem. 1996;271:12687–12690. doi: 10.1074/jbc.271.22.12687. [DOI] [PubMed] [Google Scholar]
- Plotnikov A.N. Shlapakova I. Szabolcs M.J. Danilo P., Jr. Lorell B.H. Potapova I.A. Lu Z. Rosen A.B. Mathias R.T. Brink P.R. Robinson R.B. Cohen I.S. Rosen M.R. Xenografted adult human mesenchymal stem cells provide a platform for sustained biological pacemaker function in canine heart. Circulation. 2007;116:706–713. doi: 10.1161/CIRCULATIONAHA.107.703231. [DOI] [PubMed] [Google Scholar]
- Ponte A.L. Marais E. Gallay N. Langonne A. Delorme B. Herault O. Charbord P. Domenech J. The in vitro migration capacity of human bone marrow mesenchymal stem cells: Comparison of chemokine and growth factor chemotactic activities. Stem Cells. 2007;25:1737–1745. doi: 10.1634/stemcells.2007-0054. [DOI] [PubMed] [Google Scholar]
- Potapova I. Plotnikov A. Lu Z. Danilo P., Jr. Valiunas V. Qu J. Doronin S. Zuckerman J. Shlapakova I.N. Gao J. Pan Z. Herron A.J. Robinson R.B. Brink P.R. Rosen M.R. Cohen I.S. Human mesenchymal stem cells as a gene delivery system to create cardiac pacemakers. Circ. Res. 2004;94:952–959. doi: 10.1161/01.RES.0000123827.60210.72. [DOI] [PubMed] [Google Scholar]
- Potier E. Noailly J. Ito K. Directing bone marrow-derived stromal cell function with mechanics. J. Biomech. 2010;43:807–817. doi: 10.1016/j.jbiomech.2009.11.019. [DOI] [PubMed] [Google Scholar]
- Qiao L. Zhao T.J. Wang F.Z. Shan C.L. Ye L.H. Zhang X.D. NF-κB downregulation may be involved the depression of tumor cell proliferation mediated by human mesenchymal stem cells. Acta Pharmacol. Sin. 2008;29:333–340. doi: 10.1111/j.1745-7254.2008.00751.x. [DOI] [PubMed] [Google Scholar]
- Rasmusson I. Ringden O. Sundberg B. Le Blanc K. Mesenchymal stem cells inhibit the formation of cytotoxic T lymphocytes, but not activated cytotoxic T lymphocytes or natural killer cells. Transplantation. 2003;76:1208–1213. doi: 10.1097/01.TP.0000082540.43730.80. [DOI] [PubMed] [Google Scholar]
- Rebelatto C.K. Aguiar A.M. Moretao M.P. Senegaglia A.C. Hansen P. Barchiki F. Oliveira J. Martins J. Kuligovski C. Mansur F. Christofis A. Amaral V.F. Brofman P.S. Goldenberg S. Nakao L.S. Correa A. Dissimilar differentiation of mesenchymal stem cells from bone marrow, umbilical cord blood, and adipose tissue. Exp. Biol. Med. (Maywood) 2008;233:901–913. doi: 10.3181/0712-RM-356. [DOI] [PubMed] [Google Scholar]
- Ren C. Kumar S. Chanda D. Chen J. Mountz J.D. Ponnazhagan S. Therapeutic potential of mesenchymal stem cells producing interferon-α in a mouse melanoma lung metastasis model. Stem Cells. 2008a;26:2332–2338. doi: 10.1634/stemcells.2008-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren C. Kumar S. Chanda D. Kallman L. Chen J. Mountz J.D. Ponnazhagan S. Cancer gene therapy using mesenchymal stem cells expressing interferon-β in a mouse prostate cancer lung metastasis model. Gene Ther. 2008b;15:1446–1453. doi: 10.1038/gt.2008.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond A. Chemokine modulation of the tumor microenvironment. Pigment Cell Melanoma Res. 2010;23:312–313. doi: 10.1111/j.1755-148X.2010.00714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringe J. Strassburg S. Neumann K. Endres M. Notter M. Burmester G.R. Kaps C. Sittinger M. Towards in situ tissue repair: Human mesenchymal stem cells express chemokine receptors CXCR1, CXCR2 and CCR2, and migrate upon stimulation with CXCL8 but not CCL2. J. Cell. Biochem. 2007;101:135–146. doi: 10.1002/jcb.21172. [DOI] [PubMed] [Google Scholar]
- Rosova I. Dao M. Capoccia B. Link D. Nolta J.A. Hypoxic preconditioning results in increased motility and improved therapeutic potential of human mesenchymal stem cells. Stem Cells. 2008;26:2173–2182. doi: 10.1634/stemcells.2007-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salem H.K. Thiemermann C. Mesenchymal stromal cells: Current understanding and clinical status. Stem Cells. 2010;28:585–596. doi: 10.1002/stem.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasser A.K. Sullivan N.J. Studebaker A.W. Hendey L.F. Axel A.E. Hall B.M. Interleukin-6 is a potent growth factor for ER-α-positive human breast cancer. FASEB J. 2007;21:3763–3770. doi: 10.1096/fj.07-8832com. [DOI] [PubMed] [Google Scholar]
- Schaefer A. Zwadlo C. Fuchs M. Meyer G.P. Lippolt P. Wollert K.C. Drexler H. Long-term effects of intracoronary bone marrow cell transfer on diastolic function in patients after acute myocardial infarction: 5-year results from the randomized-controlled BOOST trial—an echocardiographic study. Eur. J. Echocardiogr. 2010;11:165–171. doi: 10.1093/ejechocard/jep191. [DOI] [PubMed] [Google Scholar]
- Schnabel L.V. Lynch M.E. van der Meulen M.C. Yeager A.E. Kornatowski M.A. Nixon A.J. Mesenchymal stem cells and insulin-like growth factor-I gene-enhanced mesenchymal stem cells improve structural aspects of healing in equine flexor digitorum superficialis tendons. J. Orthop. Res. 2009;27:1392–1398. doi: 10.1002/jor.20887. [DOI] [PubMed] [Google Scholar]
- Shi S. Gronthos S. Chen S. Reddi A. Counter C.M. Robey P.G. Wang C.Y. Bone formation by human postnatal bone marrow stromal stem cells is enhanced by telomerase expression. Nat. Biotechnol. 2002;20:587–591. doi: 10.1038/nbt0602-587. [DOI] [PubMed] [Google Scholar]
- Shimizu K. Minami M. Shubiki R. Lopez-Ilasaca M. MacFarlane L. Asami Y. Li Y. Mitchell R.N. Libby P. CC chemokine receptor-1 activates intimal smooth muscle-like cells in graft arterial disease. Circulation. 2009;120:1800–1813. doi: 10.1161/CIRCULATIONAHA.109.859595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shujia J. Haider H.K. Idris N.M. Lu G. Ashraf M. Stable therapeutic effects of mesenchymal stem cell-based multiple gene delivery for cardiac repair. Cardiovasc. Res. 2008;77:525–533. doi: 10.1093/cvr/cvm077. [DOI] [PubMed] [Google Scholar]
- Song H. Kwon K. Lim S. Kang S.M. Ko Y.G. Xu Z. Chung J.H. Kim B.S. Lee H. Joung B. Park S. Choi D. Jang Y. Chung N.S. Yoo K.J. Hwang K.C. Transfection of mesenchymal stem cells with the FGF-2 gene improves their survival under hypoxic conditions. Mol. Cells. 2005;19:402–407. [PubMed] [Google Scholar]
- Stamm C. Westphal B. Kleine H.D. Petzsch M. Kittner C. Klinge H. Schumichen C. Nienaber C.A. Freund M. Steinhoff G. Autologous bone-marrow stem-cell transplantation for myocardial regeneration. Lancet. 2003;361:45–46. doi: 10.1016/S0140-6736(03)12110-1. [DOI] [PubMed] [Google Scholar]
- Stoff-Khalili M.A. Rivera A.A. Mathis J.M. Banerjee N.S. Moon A.S. Hess A. Rocconi R.P. Numnum T.M. Everts M. Chow L.T. Douglas J.T. Siegal G.P. Zhu Z.B. Bender H.G. Dall P. Stoff A. Pereboeva L. Curiel D.T. Mesenchymal stem cells as a vehicle for targeted delivery of CRAds to lung metastases of breast carcinoma. Breast Cancer Res. Treat. 2007;105:157–167. doi: 10.1007/s10549-006-9449-8. [DOI] [PubMed] [Google Scholar]
- Strauer B.E. Brehm M. Zeus T. Kostering M. Hernandez A. Sorg R.V. Kogler G. Wernet P. Repair of infarcted myocardium by autologous intracoronary mononuclear bone marrow cell transplantation in humans. Circulation. 2002;106:1913–1918. doi: 10.1161/01.cir.0000034046.87607.1c. [DOI] [PubMed] [Google Scholar]
- Studeny M. Marini F.C. Champlin R.E. Zompetta C. Fidler I.J. Andreeff M. Bone marrow-derived mesenchymal stem cells as vehicles for interferon-β delivery into tumors. Cancer Res. 2002;62:3603–3608. [PubMed] [Google Scholar]
- Studer L. Tabar V. McKay R.D. Transplantation of expanded mesencephalic precursors leads to recovery in parkinsonian rats. Nat. Neurosci. 1998;1:290–295. doi: 10.1038/1105. [DOI] [PubMed] [Google Scholar]
- Sun L. Cui M. Wang Z. Feng X. Mao J. Chen P. Kangtao M. Chen F. Zhou C. Mesenchymal stem cells modified with angiopoietin-1 improve remodeling in a rat model of acute myocardial infarction. Biochem. Biophys. Res. Commun. 2007;357:779–784. doi: 10.1016/j.bbrc.2007.04.010. [DOI] [PubMed] [Google Scholar]
- Sun L. Akiyama K. Zhang H. Yamaza T. Hou Y. Zhao S. Xu T. Le A. Shi S. Mesenchymal stem cell transplantation reverses multiorgan dysfunction in systemic lupus erythematosus mice and humans. Stem Cells. 2009;27:1421–1432. doi: 10.1002/stem.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai K. Pelled G. Sheyn D. Bershteyn A. Han L. Kallai I. Zilberman Y. Ortiz C. Gazit D. Nanobiomechanics of repair bone regenerated by genetically modified mesenchymal stem cells. Tissue Eng. Part A. 2008;14:1709–1720. doi: 10.1089/ten.tea.2007.0241. [DOI] [PubMed] [Google Scholar]
- Tang J. Wang J. Kong X. Yang J. Guo L. Zheng F. Zhang L. Huang Y. Wan Y. Vascular endothelial growth factor promotes cardiac stem cell migration via the PI3K/Akt pathway. Exp. Cell Res. 2009a;315:3521–3531. doi: 10.1016/j.yexcr.2009.09.026. [DOI] [PubMed] [Google Scholar]
- Tang J. Wang J. Yang J. Kong X. Zheng F. Guo L. Zhang L. Huang Y. Mesenchymal stem cells over-expressing SDF-1 promote angiogenesis and improve heart function in experimental myocardial infarction in rats. Eur. J. Cardiothorac. Surg. 2009b;36:644–650. doi: 10.1016/j.ejcts.2009.04.052. [DOI] [PubMed] [Google Scholar]
- Tang Y.L. Tang Y. Zhang Y.C. Qian K. Shen L. Phillips M.I. Improved graft mesenchymal stem cell survival in ischemic heart with a hypoxia-regulated heme oxygenase-1 vector. J. Am. Coll. Cardiol. 2005;46:1339–1350. doi: 10.1016/j.jacc.2005.05.079. [DOI] [PubMed] [Google Scholar]
- Tang Y.L. Zhu W. Cheng M. Chen L. Zhang J. Sun T. Kishore R. Phillips M.I. Losordo D.W. Qin G. Hypoxic preconditioning enhances the benefit of cardiac progenitor cell therapy for treatment of myocardial infarction by inducing CXCR4 expression. Circ. Res. 2009;104:1209–1216. doi: 10.1161/CIRCRESAHA.109.197723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teo A.K. Vallier L. Emerging use of stem cells in regenerative medicine. Biochem. J. 2010;428:11–23. doi: 10.1042/BJ20100102. [DOI] [PubMed] [Google Scholar]
- Tondreau T. Lagneaux L. Dejeneffe M. Massy M. Mortier C. Delforge A. Bron D. Bone marrow-derived mesenchymal stem cells already express specific neural proteins before any differentiation. Differentiation. 2004;72:319–326. doi: 10.1111/j.1432-0436.2004.07207003.x. [DOI] [PubMed] [Google Scholar]
- Tsubokawa T. Yagi K. Nakanishi C. Zuka M. Nohara A. Ino H. Fujino N. Konno T. Kawashiri M.A. Ishibashi-Ueda H. Nagaya N. Yamagishi M. Impact of anti-apoptotic and anti-oxidative effects of bone marrow mesenchymal stem cells with transient overexpression of heme oxygenase-1 on myocardial ischemia. Am. J. Physiol. Heart Circ. Physiol. 2010;298:H1320–1329. doi: 10.1152/ajpheart.01330.2008. [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y. Yunis J. Onorato-Showe L. Erikson J. Nowell P.C. Croce C.M. Molecular cloning of the chromosomal breakpoint of B-cell lymphomas and leukemias with the t(11;14) chromosome translocation. Science. 1984;224:1403–1406. doi: 10.1126/science.6610211. [DOI] [PubMed] [Google Scholar]
- Tyndall A. Furst D.E. Adult stem cell treatment of scleroderma. Curr. Opin. Rheumatol. 2007;19:604–610. doi: 10.1097/BOR.0b013e3282e6f534. [DOI] [PubMed] [Google Scholar]
- Undale A.H. Westendorf J.J. Yaszemski M.J. Khosla S. Mesenchymal stem cells for bone repair and metabolic bone diseases. Mayo Clin. Proc. 2009;84:893–902. doi: 10.4065/84.10.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unzek S. Zhang M. Mal N. Mills W.R. Laurita K.R. Penn M.S. SDF-1 recruits cardiac stem cell-like cells that depolarize in vivo. Cell Transplant. 2007;16:879–886. doi: 10.3727/096368907783338271. [DOI] [PubMed] [Google Scholar]
- van der Bogt K.E. Schrepfer S. Yu J. Sheikh A.Y. Hoyt G. Govaert J.A. Velotta J.B. Contag C.H. Robbins R.C. Wu J.C. Comparison of transplantation of adipose tissue- and bone marrow-derived mesenchymal stem cells in the infarcted heart. Transplantation. 2009;87:642–652. doi: 10.1097/TP.0b013e31819609d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatachalam K. Prabhu S.D. Reddy V.S. Boylston W.H. Valente A.J. Chandrasekar B. Neutralization of interleukin-18 ameliorates ischemia/reperfusion-induced myocardial injury. J. Biol. Chem. 2009;284:7853–7865. doi: 10.1074/jbc.M808824200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkataramana N.K. Kumar S.K. Balaraju S. Radhakrishnan R.C. Bansal A. Dixit A. Rao D.K. Das M. Jan M. Gupta P.K. Totey S.M. Open-labeled study of unilateral autologous bone-marrow-derived mesenchymal stem cell transplantation in Parkinson's disease. Transl. Res. 2010;155:62–70. doi: 10.1016/j.trsl.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Vojtassak J. Danisovic L. Kubes M. Bakos D. Jarabek L. Ulicna M. Blasko M. Autologous biograft and mesenchymal stem cells in treatment of the diabetic foot. Neurol. Endocrinol. Lett. 2006;27(Suppl. 2):134–137. [PubMed] [Google Scholar]
- Wagner W. Ho A.D. Mesenchymal stem cell preparations—comparing apples and oranges. Stem Cell Rev. 2007;3:239–248. doi: 10.1007/s12015-007-9001-1. [DOI] [PubMed] [Google Scholar]
- Wang M. Tan J. Wang Y. Meldrum K.K. Dinarello C.A. Meldrum D.R. IL-18 binding protein-expressing mesenchymal stem cells improve myocardial protection after ischemia or infarction. Proc. Natl. Acad. Sci. U.S.A. 2009;106:17499–17504. doi: 10.1073/pnas.0908924106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. Hu Q. Mansoor A. Lee J. Wang Z. Lee T. From A.H. Zhang J. Bioenergetic and functional consequences of stem cell-based VEGF delivery in pressure-overloaded swine hearts. Am. J. Physiol. Heart Circ. Physiol. 2006;290:H1393–H1405. doi: 10.1152/ajpheart.00871.2005. [DOI] [PubMed] [Google Scholar]
- Wang X. Zhao T. Huang W. Wang T. Qian J. Xu M. Kranias E.G. Wang Y. Fan G.C. Hsp20-engineered mesenchymal stem cells are resistant to oxidative stress via enhanced activation of Akt and increased secretion of growth factors. Stem Cells. 2009;27:3021–3031. doi: 10.1002/stem.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley S.R. Schooley K. Smolak P.J. Din W.S. Huang C.P. Nicholl J.K. Sutherland G.R. Smith T.D. Rauch C. Smith C.A. Goodwin R.G. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995;3:673–682. doi: 10.1016/1074-7613(95)90057-8. [DOI] [PubMed] [Google Scholar]
- Wolf D. Wolf A.M. Mesenchymal stem cells as cellular immunosuppressants. Lancet. 2008;371:1553–1554. doi: 10.1016/S0140-6736(08)60666-2. [DOI] [PubMed] [Google Scholar]
- Wollert K.C. Meyer G.P. Lotz J. Ringes-Lichtenberg S. Lippolt P. Breidenbach C. Fichtner S. Korte T. Hornig B. Messinger D. Arseniev L. Hertenstein B. Ganser A. Drexler H. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: The BOOST randomised controlled clinical trial. Lancet. 2004;364:141–148. doi: 10.1016/S0140-6736(04)16626-9. [DOI] [PubMed] [Google Scholar]
- Woodbury D. Schwarz E.J. Prockop D.J. Black I.B. Adult rat and human bone marrow stromal cells differentiate into neurons. J. Neurosci. Res. 2000;61:364–370. doi: 10.1002/1097-4547(20000815)61:4<364::AID-JNR2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Wragg A. Mellad J.A. Beltran L.E. Konoplyannikov M. San H. Boozer S. Deans R.J. Mathur A. Lederman R.J. Kovacic J.C. Boehm M. VEGFR1/CXCR4-positive progenitor cells modulate local inflammation and augment tissue perfusion by a SDF-1-dependent mechanism. J. Mol. Med. 2008;86:1221–1232. doi: 10.1007/s00109-008-0390-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin H. Kanehira M. Mizuguchi H. Hayakawa T. Kikuchi T. Nukiwa T. Saijo Y. Targeted delivery of CX3CL1 to multiple lung tumors by mesenchymal stem cells. Stem Cells. 2007;25:1618–1626. doi: 10.1634/stemcells.2006-0461. [DOI] [PubMed] [Google Scholar]
- Xu G. Jiang X.D. Xu Y. Zhang J. Huang F.H. Chen Z.Z. Zhou D.X. Shang J.H. Zou Y.X. Cai Y.Q. Kou S.B. Chen Y.Z. Xu R.X. Zeng Y.J. Adenoviral-mediated interleukin-18 expression in mesenchymal stem cells effectively suppresses the growth of glioma in rats. Cell Biol. Int. 2009;33:466–474. doi: 10.1016/j.cellbi.2008.07.023. [DOI] [PubMed] [Google Scholar]
- Yang F. Cho S.W. Son S.M. Bogatyrev S.R. Singh D. Green J.J. Mei Y. Park S. Bhang S.H. Kim B.S. Langer R. Anderson D.G. Genetic engineering of human stem cells for enhanced angiogenesis using biodegradable polymeric nanoparticles. Proc. Natl. Acad. Sci. U.S.A. 2010;107:3317–3322. doi: 10.1073/pnas.0905432106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau T.M. Fung K. Weisel R.D. Fujii T. Mickle D.A. Li R.K. Enhanced myocardial angiogenesis by gene transfer with transplanted cells. Circulation. 2001;104:I218–222. doi: 10.1161/hc37t1.094896. [DOI] [PubMed] [Google Scholar]
- Yellon D.M. Hausenloy D.J. Myocardial reperfusion injury. N. Engl. J. Med. 2007;357:1121–1135. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- Zaruba M.M. Franz W.M. Role of the SDF-1–CXCR4 axis in stem cell-based therapies for ischemic cardiomyopathy. Expert Opin. Biol. Ther. 2010;10:321–335. doi: 10.1517/14712590903460286. [DOI] [PubMed] [Google Scholar]
- Zaruba M.M. Theiss H.D. Vallaster M. Mehl U. Brunner S. David R. Fischer R. Krieg L. Hirsch E. Huber B. Nathan P. Israel L. Imhof A. Herbach N. Assmann G. Wanke R. Mueller-Hoecker J. Steinbeck G. Franz W.M. Synergy between CD26/DPP-IV inhibition and G-CSF improves cardiac function after acute myocardial infarction. Cell Stem Cell. 2009;4:313–323. doi: 10.1016/j.stem.2009.02.013. [DOI] [PubMed] [Google Scholar]
- Zeller C.N. Wang Y. Markel T.A. Weil B. Abarbanell A. Herrmann J.L. Kelly M.L. Coffey A. Meldrum D.R. Role of tumor necrosis factor receptor 1 in sex differences of stem cell mediated cardioprotection. Ann. Thorac. Surg. 2009;87:812–819. doi: 10.1016/j.athoracsur.2008.12.033. [DOI] [PubMed] [Google Scholar]
- Zeng B. Chen H. Zhu C. Ren X. Lin G. Cao F. Effects of combined mesenchymal stem cells and heme oxygenase-1 therapy on cardiac performance. Eur. J. Cardiothorac. Surg. 2008a;34:850–856. doi: 10.1016/j.ejcts.2008.05.049. [DOI] [PubMed] [Google Scholar]
- Zeng B. Ren X. Lin G. Zhu C. Chen H. Yin J. Jiang H. Yang B. Ding D. Paracrine action of HO-1-modified mesenchymal stem cells mediates cardiac protection and functional improvement. Cell Biol. Int. 2008b;32:1256–1264. doi: 10.1016/j.cellbi.2008.07.010. [DOI] [PubMed] [Google Scholar]
- Zernecke A. Shagdarsuren E. Weber C. Chemokines in atherosclerosis: An update. Arterioscler. Thromb. Vasc. Biol. 2008;28:1897–1908. doi: 10.1161/ATVBAHA.107.161174. [DOI] [PubMed] [Google Scholar]
- Zhang M. Mal N. Kiedrowski M. Chacko M. Askari A.T. Popovic Z.B. Koc O.N. Penn M.S. SDF-1 expression by mesenchymal stem cells results in trophic support of cardiac myocytes after myocardial infarction. FASEB J. 2007;21:3197–3207. doi: 10.1096/fj.06-6558com. [DOI] [PubMed] [Google Scholar]
- Zhang Z. Deb A. Pachori A. He W. Guo J. Pratt R. Dzau V.J. Secreted frizzled related protein 2 protects cells from apoptosis by blocking the effect of canonical Wnt3a. J. Mol. Cell. Cardiol. 2009;46:370–377. doi: 10.1016/j.yjmcc.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao F. Chella R. Ma T. Effects of shear stress on 3-D human mesenchymal stem cell construct development in a perfusion bioreactor system: Experiments and hydrodynamic modeling. Biotechnol. Bioeng. 2007;96:584–595. doi: 10.1002/bit.21184. [DOI] [PubMed] [Google Scholar]
- Zhao L.R. Duan W.M. Reyes M. Keene C.D. Verfaillie C.M. Low W.C. Human bone marrow stem cells exhibit neural phenotypes and ameliorate neurological deficits after grafting into the ischemic brain of rats. Exp. Neurol. 2002;174:11–20. doi: 10.1006/exnr.2001.7853. [DOI] [PubMed] [Google Scholar]
- Zhou Y. Wang S. Yu Z. Hoyt R.F., Jr. Sachdev V. Vincent P. Arai A.E. Kwak M. Burkett S.S. Horvath K.A. Direct injection of autologous mesenchymal stromal cells improves myocardial function. Biochem. Biophys. Res. Commun. 2009;390:902–907. doi: 10.1016/j.bbrc.2009.10.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zisa D. Shabbir A. Mastri M. Suzuki G. Lee T. Intramuscular VEGF repairs the failing heart: Role of host-derived growth factors and mobilization of progenitor cells. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009;297:R1503–R1515. doi: 10.1152/ajpregu.00227.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]