Abstract
AS1411 is a first-in-class anticancer agent, currently in Phase II clinical trials. It is a quadruplex-forming oligodeoxynucleotide that binds to nucleolin as an aptamer, but its mechanism of action is not completely understood. Mechanistic insights could lead to clinically useful markers for AS1411 response and to novel targeted therapies. Previously, we proposed a model where cell surface nucleolin serves as the receptor for AS1411, leading to selective uptake in cancer cells. Here, we compare uptake of fluorophore-labeled AS1411 (FL-AS1411) in DU145 prostate cancer cells (sensitive to AS1411) and Hs27 non-malignant skin fibroblasts (resistant to AS1411). Uptake of FL-AS1411 occurred by endocytosis in both cell types and was much more efficient than an inactive, non-quadruplex oligonucleotide. Unexpectedly, uptake of FL-AS1411 was lower in cancer cells compared to Hs27 cells. However, the mechanism of uptake was different, occurring by macropinocytosis in cancer cells, but by a non-macropinocytic pathway in Hs27 cells. Additionally, treatment of various cancer cells with AS1411 caused hyperstimulation of macropinocytosis, provoking an increase in its own uptake, whereas no stimulation was observed for non-malignant cells. Nucleolin was not required for initial FL-AS1411 uptake in DU145 cells, but was necessary for induced macropinocytosis and FL-AS1411 uptake at later times. Our results are inconsistent with the previous mechanistic model, but confirm that nucleolin plays a role in mediating AS1411 effects. The data suggest a new model for AS1411 action, as well as a new role for nucleolin in stimulating macropinocytosis, a process with potential applications in drug delivery.
Keywords: AS1411, macropinocytosis, nucleolin, endocytosis, aptamer
INTRODUCTION
AS1411 is a 26-base guanine-rich oligonucleotide (GRO) with an unmodified (phosphodiester) DNA backbone. This molecule and its related analogs can inhibit proliferation and induce cell death in many types of cancer cells, but have little effect on normal cells (1–4). Biological effects of this class of molecules in cancer cells include cell cycle arrest, inhibition of NF-κB signaling, induction of tumor suppressor gene expression, and reduction of bcl-2 expression (3–6). A Phase I clinical trial of AS1411 (formerly known as AGRO100) in patients with metastatic cancer has indicated no serious adverse effects with promising clinical activity, and AS1411 is now being tested in Phase II trials (2).
Our previous work has revealed that antiproliferative G-rich oligonucleotides (GROs) such as AS1411 can form stable G-quadruplex structures, which imparts an unusual resistance to cellular and serum nucleases. We have also demonstrated that GROs bind directly and selectively to nucleolin, and that the growth inhibitory activity of GROs is positively correlated with their ability to bind this protein (1, 7, 8). In addition, several biological effects of AS1411 have been shown to result from its ability to alter the subcellular localization of certain nucleolin-containing complexes, or to interfere with the molecular interactions of nucleolin (3, 5, 6). Therefore, we have concluded that AS1411 acts as an aptamer to nucleolin.
Despite its molecular target having been identified, the mechanism of action for AS1411 and the reasons for its marked tumor selectivity have not yet been fully elucidated. Nucleolin is a multifunctional protein present in the nucleolus and nucleus of most cells, as well as in the cytoplasm and on the surface for some cells, including cancer cells and angiogenic endothelial cells (2, 9, 10). This protein has been reported to control a wide range of fundamental cellular processes (11), as well as having important roles in malignant transformation and cancer progression (12). Based on the knowledge that cell surface nucleolin mediates the endocytosis of a wide range of ligands, we had originally speculated that nucleolin could act as a receptor for AS1411 and that, due to the elevated expression of nucleolin by cancer cells, uptake of AS1411 would occur in a tumor-selective manner (13).
In this study, we evaluated uptake of fluorescently labeled AS1411 in several cell types and compared the mechanism of uptake between DU145 prostate cancer cells, which are sensitive to AS1411, and Hs27 non-malignant skin fibroblasts, which are resistant to AS1411. We now describe the results of this research, including our unexpected findings with regard to the uptake of AS1411 and the role of nucleolin in its mechanism of action.
MATERIALS AND METHODS
Materials
Oligodeoxynucleotides were purchased from Invitrogen (Carlsbad, CA). Sequences used for this study were: AS1411, 5’-d(GGTGGTGGTGGTTGTGGTGGTGGTGG); FL-AS1411 (fluorophore-labeled AS1411), 5’-Fluor-d(TTTGGTGGTGGTGGTTGTGGTGGTGGTGG), where Fluor is either 5-carboxyfluorescein (FAM, used for flow cytometry) or Alexa Fluor 488 (used for confocal microscopy); tAS1411, 5’-d(TTTGGTGGTGGTGGTTGTGGTGGTGGTGG); FL-CRO, 5’-Fluor-d(TTTCCTCCTCCTCCTTCTCCTCCTCCTCC); CRO, 5’-d(CCTCCTCCTCCTTCTCCTCCTCCTCC); and tCRO, 5’-d(TTTCCTCCTCCTCCTTCTCCTCCTCCTCC). Unmodified oligonucleotides were purchased in the desalted form, whereas fluorescently labeled sequences were HPLC purified. The 29-mer sequences were used for some experiments because quenching of the fluorophore occurred when it was located adjacent at the 5’-terminal base of AS1411 (Reyes-Reyes and Bates, unpublished observations), so a spacer consisting of 3 thymidines was added. We confirmed that the antiproliferative activities of 29-mer sequences, with and without the fluorophore, were comparable to the synthesized 26-mer AS1411 sequence, and to AS1411 obtained from Antisoma (Supplementary Figure S1A). The dextran, 10,000 MW, anionic fixable (dextran-10K) and transferrin (Tf) conjugated with Alexa Fluor 488 or Alexa Fluor 594 were purchased from Invitrogen. Anti-rabbit and anti-mouse antibodies linked to horseradish peroxidase, anti-histone 3 rabbit polyclonal and anti-pan cadherin (C19) goat polyclonal antibodies were from Santa Cruz Biotech (Santa Cruz, CA). Anti-nucleolin monoclonal antibodies were from Stressgen (4E2) and Santa Cruz (MS-3). The anti-nucleolin mAb (D3) was a generous gift from Dr. Jau-Shyong Deng, University of Pittsburgh School of Medicine. Cytochalasin D, dynasore, and amiloride were from Calbiochem (San Diego, CA). Triton X-100 was from Sigma (Saint Louis, MO), paraformaldehyde was from Electron Microscopy Sciences (Hatfield, PA), and dimethylsulfoxide (DMSO) was from the American Type Culture Collection (ATCC, Manassas, VA).
Cell Culture and Treatment
All cells were obtained from ATCC and routinely grown in a humidified incubator at 37°C with 5% CO2. Hs27 (non-malignant human foreskin fibroblasts), DU145 (hormone-refractory prostate cancer), HeLa (cervical adenocarcinoma), MCF-7 (hormone-dependent breast cancer) and MDA-MB-231 (hormone-independent breast cancer) cells were grown in DMEM supplemented with 10% fetal bovine serum (FBS; Life Technologies), 62.5 µg/mL penicillin and 100 µg/mL streptomycin (Hyclone Laboratories, Logan, Utah). MCF-10A cells (immortalized human breast epithelial cells) were grown in MEBM supplemented with all the components of MEGM bullet kit (Lonza, Allendale, NJ) except for the GA-1000. We did not carry out additional testing to authenticate cell lines, but their morphology and behavior were consistent with ATCC descriptions. Cells were plated at 50% confluence and incubated 18 h to allow adherence, and then the medium was changed for fresh supplemented medium and treated by addition of oligodeoxynucleotides directly to the culture medium to give the final concentration indicated in the figure legends. Dynasore and cytochalasin D were dissolved in DMSO. Amiloride was dissolved in serum-free medium. Cells were pre-treated with inhibitors in serum-free medium for either 30 min (cytochalasin D, dynasore) or 60 min (amiloride). Cells for biochemical analyses were lysed in lysis buffer (150 mM NaCl, 2 mM EDTA, 50 mM Tris-HCl, 0.25% deoxycholic acid, 1% IGEPAL CA-630, pH 7.5) containing protease and phosphatase inhibitor cocktails (Calbiochem) for 20 min at 4°C and then cleared by centrifugation at 16,000g for 10 min at 4°C. All protein concentrations were determined using the BCA assay (Pierce, Rockford, IL).
Flow cytometric assays
Cells (2 × 105) in fresh complete medium were plated into 6-well plates for 18 h. After complete adhesion, cells were incubated as indicated in the figures or legends. Cells were washed once with ice-cold PBS, incubated with 1 µg/ml 7-amino-actinomycin D (7-AAD) or 1 µg/ml propidium iodide (PI) for 5 min on ice to allow exclusion of non-viable cells, and washed twice with ice-cold PBS. To harvest, cells were treated with 0.01% trypsin/ 0.5 mM EDTA (300 µl) for 3 min prior addition 3 ml supplemented culture medium. Cells were then centrifuged and resuspended in 0.5 ml of 1% paraformaldehyde for analysis by flow cytometry using a FACScalibur cytometer (BD Biosciences, Mountain View, CA).
Immunofluorescence microscopy
Cells (4 × 104) in fresh complete culture medium were plated on 18 mm diameter glass cover slips for 18 h. The medium was replaced with serum-free medium and cells were treated as described in the figure legends. After incubation, cells were washed 3 times with ice-cold PBS, fixed in 4% paraformaldehyde in PBS for 30 min at room temperature, and washed three times with PBS. After washing, the cover slips were mounted on glass slides with ProLong Antifade (Molecular Probes) according to the manufacturer's directions. Immunofluorescence was documented with an LSM 510 inverted confocal laser-scanning microscope (Carl Zeiss, Oberkochen, Germany) equipped with an Omnichrome argon–krypton laser. Images were obtained with a Zeiss Plan-Apo 63× oil immersion objective (1.4 NA).
Biotinylation and purification of cell-surface proteins
Cells were washed three times with ice-cold PBS and a freshly prepared solution of a cell-impermeable biotinylating agent (sulfo-NHS-biotin, Pierce, Rockford, IL) was added (0.5 mg/ml in PBS). After 30 min incubation at 4°C, cells were washed once with ice-cold TBS (50 mM Tris–HCl, 150 mM NaCl, pH 7.5), incubated with ice-cold complete medium for 10 min at 4 °C, and then washed twice with TBS. Biotinylated proteins were precipitated by incubating with high capacity Neutravidin agarose (Pierce) for 2 h at 4 °C with gentle agitation, and then washed with ice-cold lysis buffer.
RNA interference
Nucleolin siRNA duplexes corresponding to the sequences: 5’-GGUCGUCAUACCUCAGAAGtt (NCL1); 5’-GGCAAAGCAUUGGUAGCAAtt (NCL2); and 5’-CGGUGAAAUUGAUGGAAAUtt (NCL3), were chemically synthesized and annealed by Ambion Inc. (Austin, TX). BLAST analysis showed no homology of the siRNA sequences to any other sequence in the Human Genome Database. The siRNAs were transfected using Lipofectamine 2000 (Invitrogen), according to the manufacturer’s directions. The scrambled siRNA used as a negative control was from Ambion.
Immunoblotting
Samples were resolved by 10% SDS–Tris polyacrylamide gel electrophoresis and then electrotransferred onto polyvinylidine fluoride membranes (Millipore, Bedford, MA) in Tris–glycine buffer containing 20% methanol. Proteins were detected by immunoblotting as described (14). In some cases, membranes were stripped of bound antibodies using 62.5 mM Tris–HCl, pH 6.7, 100 mM 2-mercaptoethanol, 2% SDS for 30 min at 60°C and then reprobed as described in figure legends.
Densitometry and statistical analysis
Densitometry was used to measure band intensities by scanning autoradiographic films and using UN-SCAN-IT gel software (Silk Scientific Corporation). Band intensities were normalized as indicated in the figure legends. The statistical comparisons between AS1411-treated and control groups were performed using Student’s t test.
RESULTS
Uptake of FL-AS1411 occurs through an active uptake process
To first identify suitable conditions for our study, we analyzed the timing and serum-dependence of uptake in DU145 prostate cancer cells, which are sensitive to AS1411. Using flow cytometry with gating to exclude non-viable cells, we compared uptake of FL-AS1411, a fluorescently labeled version of the active aptamer, with that of FL-CRO, a fluorescently labeled control oligonucleotide with no antiproliferative activity. It has not been possible to identify a scrambled or point-mutated analog of AS1411 that lacks activity because of the very high proportion of guanines in the sequence. Therefore, a sequence where the guanines are substitututed with cytosines (FL-CRO) is used simply as a control phosphodiester oligdeoxyonucleotide of the same length. We first confirmed that the fluorophore signal was from internalized, rather than surface-bound, material by showing that cell-associated fluorescence was not influenced by washing the cells with dextran sulfate to remove the extracellular oligonucleotide or by adding trypan blue to quench external fluorescent signals prior to flow cytometry, (Supplementary Figure S2A).
FL-AS1411 uptake was detected as early as 5 min, with maximum signal between 2 h and 4 h under these conditions (Figure 1A). FL-CRO uptake was consistently much lower than FL-AS1411 and followed different kinetics. These data are in accord with our recent finding that G-rich phosphodiester sequences, such as AS1411, are taken up more efficiently than other non-G-rich sequences (15). As shown in Figure 1B, uptake of FL-AS1411 was independent of the presence of serum in the medium.
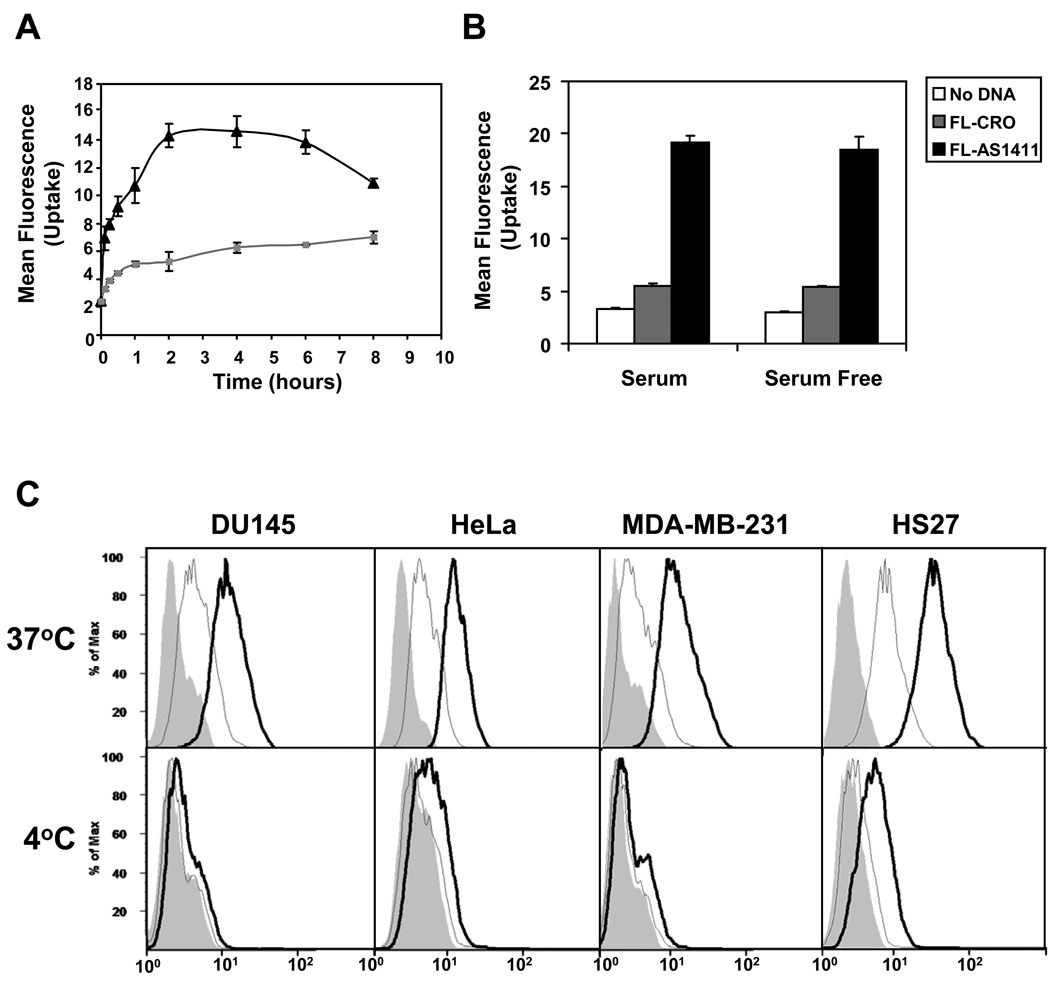
Figure 1. AS1411 cell internalization is an active process.
Cells were treated as described below and analyzed by flow cytometry. (A) DU145 cells were incubated at 37°C with complete medium containing 10 µM FL-AS1411 (black line) or 10 µM FL-CRO (gray line) for the time indicated. (B) DU145 cells were incubated for 2 h at 37°C in complete or serum-free medium containing 10 µM FL-AS1411, or 10 µM FL-CRO, or no oligonucleotide. (C) Various cell lines were incubated with 10 µM FL-AS1411 (black outline), 10 µM FL-CRO (gray outline) or without DNA (solid gray) at 37°C or 4°C for 2 h. Mean fluorescent intensities (MFI) at 37°C were: DU145, 10.8; HeLa, 10.7; MDA-MB-231, 11.9; Hs27, 35.2. All experiments were repeated at least three times. Data are mean of three independent samples; bars, SE.
To determine whether AS1411 uptake occurs through an active uptake process, we evaluated the temperature-dependence of AS1411 uptake in various cancer cell types and non-malignant Hs27 skin fibroblasts. In all cell types examined, the uptake of FL-AS1411 and FL-CRO showed strong temperature dependence. However, in contrast to our original hypothesis, Hs27 cells appeared to have a higher uptake of AS1411 than any of the cancer cells analyzed (Figure 1C). The dose-response curve for FL-AS1411 uptake in DU145 cells (Supplementary Figure S2B) suggested at least two components: uptake involving a high affinity, low capacity receptor, which was saturated at high nanomolar concentrations, and a non-saturable uptake that was predominant at micromolar concentrations (the curve presented an almost linear increase between 1.25 µM and 40 µM). Concentrations higher than 40 µM resulted in obvious cytotoxicity, even at this early time point. We believe that the non-saturable uptake component is most relevant for AS1411 activity, because this corresponds to concentrations at which the biological effects are observed in cultured cancer cells and to the serum concentrations in patients treated with AS1411.
FL-AS1411 uptake occurs through different endocytic mechanisms in cancer cells and in non-malignant cells
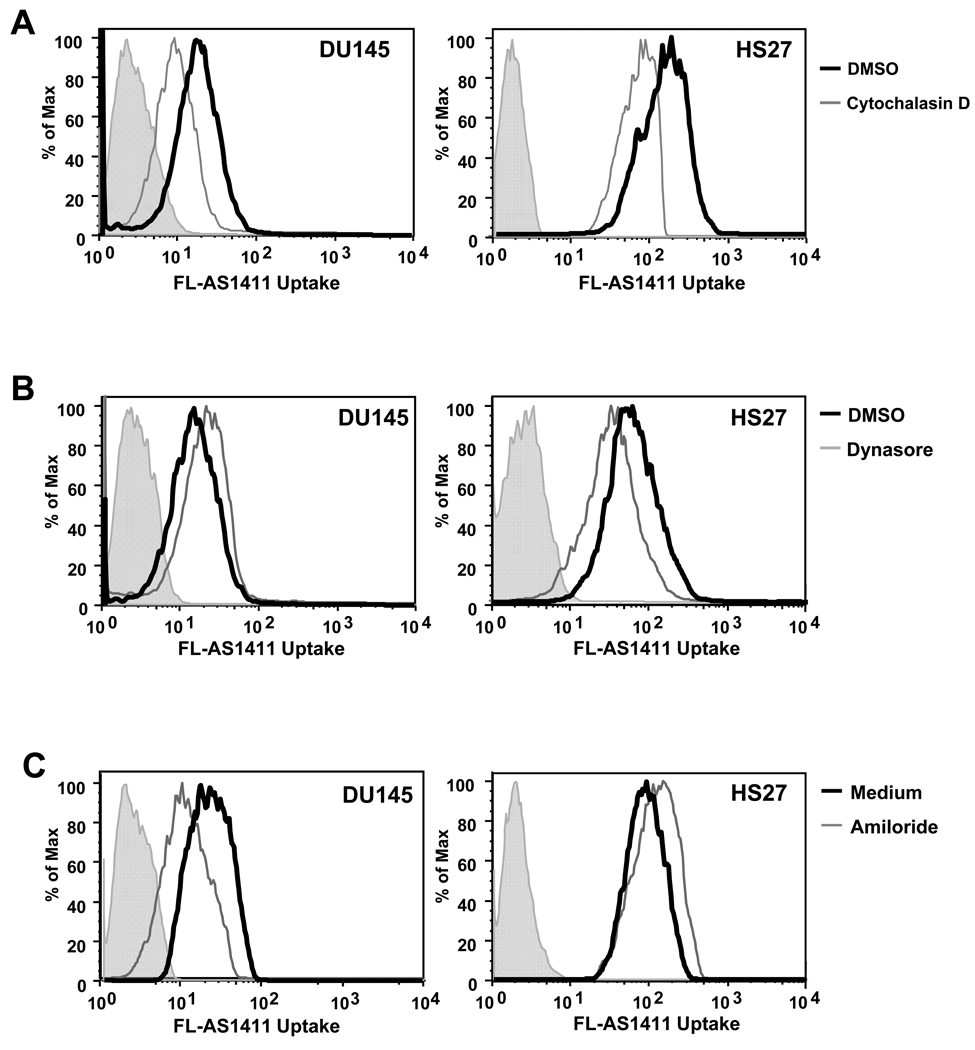
To confirm that uptake of AS1411 occurs by endocytosis, we tested for involvement of the actin cytoskeleton, which has been implicated in regulating endocytic pathways. To this end, DU145 and Hs27 were pre-treated with an actin polymerization inhibitor, cytochalasin D, and assessed for FL-AS1411 uptake by flow cytometry. Cytocholasin D-treated cells showed a decrease in FL-AS1411 uptake compared with the untreated cells (Figure 2A), consistent with endocytic uptake. Recognized pathways of endocytosis include classical clathrin-mediated endocytosis, caveolae-mediated endocytosis, clathrin- and caveolae-independent endocytosis, and macropinocytosis (16). The GTPase dynamin is required for clathrin- and caveolae-mediated endocytosis and some clathrin and caveolae-independent pathways (16), so we next investigated the effect of dynasore, a potent inhibitor of dynamin function (17), on FL-AS1411 uptake in DU145 cancer cells and nonmalignant Hs27 cells (Figure 2B). Pre-treatment of Hs27 cells with dynasore decreased their uptake of AS1411 (Figure 2B). In contrast, pre-treatment of DU145 cells slightly increased their uptake of FL-AS1411. To rule out the possibility that DU145 cells were unresponsive to dynasore, we demonstrated that uptake of transferrin, a well-established ligand of clathrin-dependent endocytosis, was inhibited in DU145 cells pre-treated with dynasore, (Supplementary Figure S3A). These results indicate that AS1411 may be taken up by a predominantly clathrin or caveolae-dependent route of entry in Hs27 cells, but not in DU145 cells.
Figure 2. AS1411 is internalized by different endocytic mechanisms in DU145 cancer cells and non-malignant Hs27 cells.
DU145 or Hs27 cells were pre-treated as described below with inhibitor (gray histogram) or the corresponding vehicle control (black histogram) before addition of 10 µM FL-AS1411 and incubation at 37°C for 2 h. Cells were then harvested and analyzed by flow cytometry. Pre-treatment conditions were at 37°C with: (A) 5µM cytochalasin D for 30 min; (B) 80µM dynasore for 30 min; or (C) 3 mM amiloride for 1 h. All experiments were repeated at least three times and representative data are shown. Solid gray histograms represent background autofluorescence of unstained cells.
Macropinocytosis is the predominant mechanism of uptake for AS1411 in cancer cells
Recent work has showed that internalization of DNA can be mediated through macropinocytosis (18–20), an actin-driven, ligand-independent mechanism in which cells “gulp” the surrounding medium and any macromolecules it contains. This endocytic mechanism has been shown to be sensitive to amiloride, a specific inhibitor of Na+/H exchange (21), so we tested the effect of amiloride on FL-AS1411 uptake. We found that amiloride pre-treatment caused a reduction in FL-AS1411 uptake in DU145 cancer cells, but a slight increase in the non-malignant Hs27 cells (Figure 2C). There was little effect of amiloride treatment on uptake of FL-CRO in either DU145 or Hs27 cells (Supplementary Figure S3B). Amiloride treatment also decreased AS1411 uptake in other cancer cells (MCF7 and MDA-MB-231, data not shown). These data imply that macropinocytosis is responsible for the internalization of AS1411 in cancer cells.
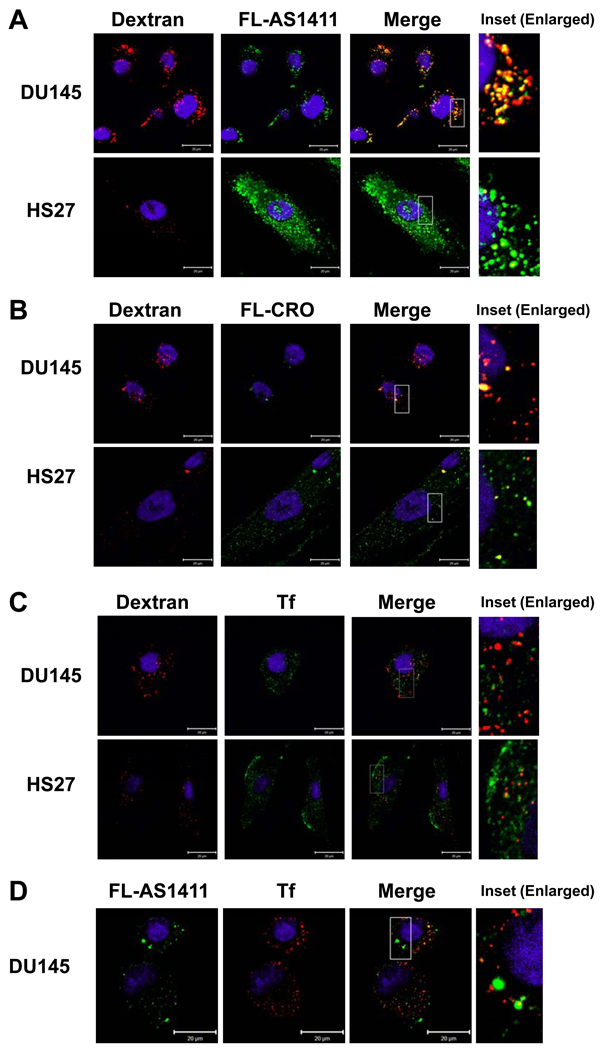
To confirm these results without using chemical inhibitors, uptake was also examined by confocal microscopy. This showed that FL-AS1411 was localized in confined structures in the cytoplasm of both cancer and non-malignant cells (Figure 3A). As expected, uptake of FL-CRO was much lower than FL-AS1411 (Figure 3B). Interestingly, our studies showed that macropinocytosis (indicated by dextran uptake) is much more active in DU145 cancer cells than in the non-malignant Hs27 cells (Figure 3). Moreover, internalized FL-AS1411 was strongly co-localized with the macropinocytic marker, dextran, in DU145 cells (Figure 3A), but not in Hs27 cells (Figure 3A).
Figure 3. AS1411 co-localizes with the macropinocytic marker dextran.
DU145 or Hs27 cells were incubated with the reagents indicated below, then washed and fixed. Nuclei were stained with DAPI (blue) and visualized by confocal microscopy. (A) 10 µM AS1411 labeled with Alexa Fluor 488 (green) and 0.2 mg/ml dextran-10K labeled with Alexa Fluor 594 (red) for 2 h at 37°C. (B) Similar experiments using FL-CRO in place of FL-AS1411. (C) Cells incubated with 5 µg/ml transferrin labeled with Alexa Fluor 488 (green) and 0.2 mg/ml dextran-10K labeled with Alexa Fluor 594 (red) for 30 min at 37°C. (D) DU145 cells incubated with 5 µg/ml transferrin labeled with Alexa Fluor 594 (red) and 10 µM AS1411 labeled with Alexa Fluor 488 (green) for 30 min at 37°C. Scale bars, 20 µm, and the magnified insets represent areas of 10 µm by 20 µm.
Further experiments were performed to confirm the identity of the vesicles containing FL-AS1411 as macropinosomes, which can be distinguished from other endosomes by their comparative inability to concentrate receptors (22). Therefore, we compared localization of transferrin, a ligand for the transferrin receptor, with that of dextran or FL-AS1411. We observed that transferrin and dextran were localized mainly in distinct non-overlapping vesicles in DU145 and Hs27 cells (Figure 3C) and there was very little overlap between transferrin and AS1411 in DU145 cells (Figure 3D). These combined data confirm that the endocytic process regulating the internalization of AS1411 in DU145 cancer cells is macropinocytosis.
It is also notable that no FL-AS1411 was observed in the nuclear region in these studies. This was in contrast to our earlier (unpublished) studies using fluorescence microscopy, where we had observed some cells with intense nuclear fluorescence after 24 h or more treatment with 10 µM FAM-labeled AS1411. This pattern was observed in cancer cell lines, but not in the non-responsive Hs27 cells. We initially interpreted this result as preferential uptake in cancer cells, but later realized that the cells with strong nuclear staining were likely dying in response to AS1411 and were therefore permeable, whereas that the lack of intense nuclear staining in Hs27 cells reflected that they did not die in response to AS1411. The current confocal microscopy and flow cytometry studies (which are gated to exclude dead or dying cells that are propidium iodide positive due to loss of plasma membrane integrity) demonstrate that overall uptake is not cancer-specific and that AS1411 has a non-nuclear localization in viable cells.
AS1411 stimulates macropinocytosis in cancer cells
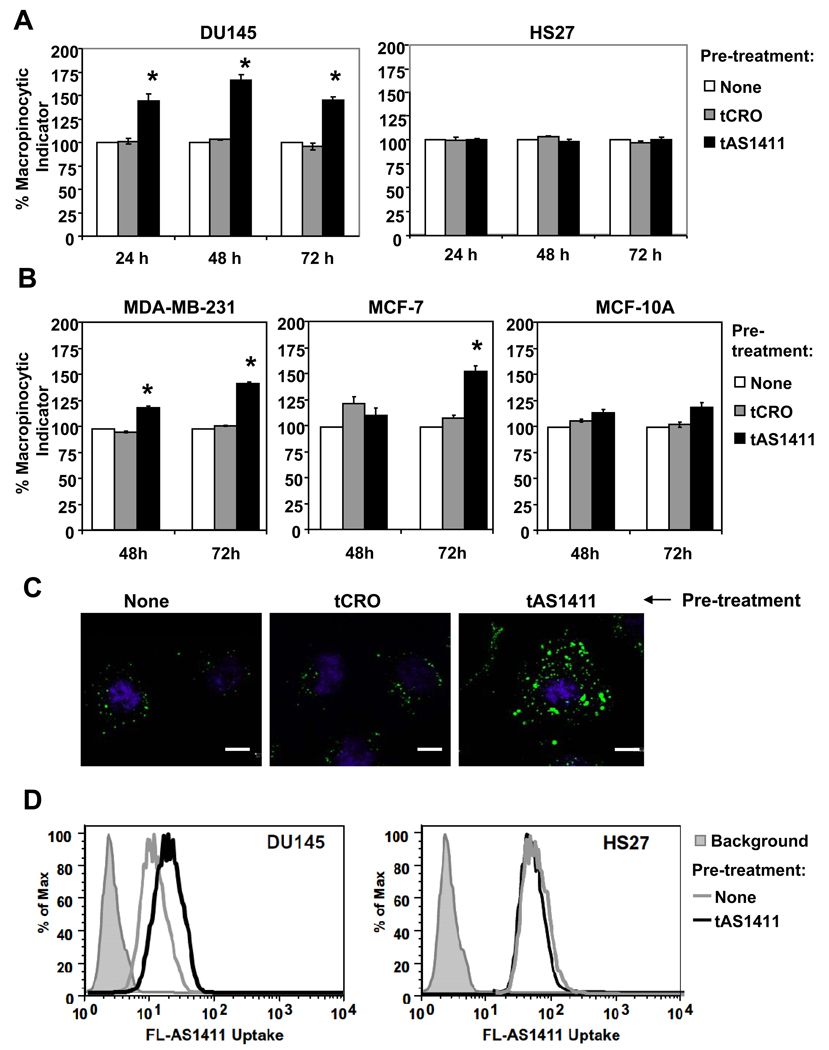
Several molecules that are internalized by macropinocytosis, including some cell penetrating peptides and viruses, are known to stimulate macropinocytosis and thereby enhance their own uptake (23–25). Moreover, several research groups have recently demonstrated that hyperstimulation of macropinocytosis can cause a novel form of cell death, characterized by vacuolization, irregular nuclei, and swollen cells (26–29), and AS1411 causes changes in cancer cell morphology with exactly these features (4). Therefore, we investigated whether AS1411 could stimulate macropinocytosis in DU145 cells or non-malignant Hs27 cells. Flow cytometry experiments indicated a significant increase in the uptake of the macropinocytic marker, dextran, in DU145 cells treated with tAS1411 (which is FL-AS1411 without the fluorescent label) for 24, 48, or 72 h, whereas there was no increase in the Hs27 cells (Figure 4A). As in all our flow cytometry experiments, cells were gated to exclude permeable cells, discounting the possibility that this increase was due to cell death. No changes in dextran uptake were observed in DU145 cells treated with the control oligonucleotide, tCRO, (Figure 4A) or with AS1411 for shorter times (1h, 2h, and 4h, data not shown). The tAS1411 was also able to induce hyperstimulation of macropinocytosis in other cancer cells lines (MCF-7 and MDA-MB-231) and had a reduced effect in another non-malignant cell type (MCF-10A) (Figure 4B), suggesting that these novel observations may represent a general difference between the response of cancer cells and normal cells, although further studies are needed to verify this idea. Confocal microscopy confirmed that DU145 cells treated with tAS1411 presented a higher dextran uptake compared to untreated or CRO-treated cells (Figure 4C). Additional experiments (Supplementary Figure S4) confirmed that the 26-mer version of AS1411 was able to induce the same response as tAS1411 (which has three additional nucleotides for reasons described in the Methods section).
Figure 4. AS1411 stimulates macropinocytosis in DU145 cancer cells, but not in non-malignant Hs27 cells.
(A) DU145 or Hs27 cells were treated with 10 µM tAS1411, 10 µM tCRO, or no oligonucleotide in complete DMEM medium at 37°C for the time indicated. After treatment, medium was replaced with fresh medium containing 0.2 mg/ml dextran-10K labeled with Alexa Fluor 488, for 30 min at 37°C, then cells were harvested and analyzed by flow cytometry to determine dextran uptake. Data are mean of three independent samples; bars, SE; * indicates p < 0.05 compared to controls. (B) The same experiment was performed using MCF7 and MDA-MB-231 breast cancer cells, or MCF10A non-malignant breast epithelial cells. (C) DU145 cells were treated for 48 h as described above, then washed with cold PBS and incubated in PBS containing 5 µg/ml PI and incubated on ice for 5 min. After washing with cold PBS, cells were fixed and the distribution of macropinocytic marker (green) was visualized by confocal microscopy. Nuclei were stained with DAPI. Scale bars, 10 µm. (D) DU145 and Hs27 cells were incubated without oligonucleotide (gray line) or with 10 µM tAS1411 (black line) for 48 h, then washed and incubated at 37°C with fresh complete medium containing 10 µM FL-AS1411 for 2 h before harvesting and analysis by flow cytometry. Solid gray histograms represent background autofluorescence of unstained cells.
This result also implies that AS1411 might actually promote its own internalization by cancer cells. To test this idea, we pre-treated DU145 cells for 24 h with or without tAS1411, then added FL-AS1411 and evaluated uptake after an additional 2 h using flow cytometry. As predicted, DU145 cells pre-treated with tAS1411, but not those that received control pre-treatment, showed an increase in the uptake of FL-AS1411 in DU145 cells, whereas there was no comparable increase in AS1411-treated Hs27 cells (Figure 4D). All of these results indicate that initial AS1411 uptake leads to the stimulation of macropinocytosis, inducing an increase of its own uptake. This idea is not necessarily inconsistent with the time course data (Figure 1A) because the measured fluorescence signal may decrease over time for a number of reasons, including exocytosis of the ligand or fluorescence quenching due to environmental factors such as protein binding or pH.
Having demonstrated that AS1411-induced macropinocytosis could lead to enhanced uptake of a nucleic acid (AS1411) and a polysaccharide (dextran), we also evaluated uptake of a protein, namely, fluorescently labeled transferrin. We observed that pre-treatment with AS1411 led to increased uptake of transferrin in DU145 cells (Supplementary Figure S5). Although uptake of transferrin in untreated cells occurs by dynamin-dependent receptor-mediated endocytosis (Supplementary Figures S3 and S5), the additional uptake induced by AS1411 was apparently due to macropinocytosis because it was completely inhibited by amiloride (Supplementary Figure S5).
Initial uptake of AS1411 is independent of nucleolin
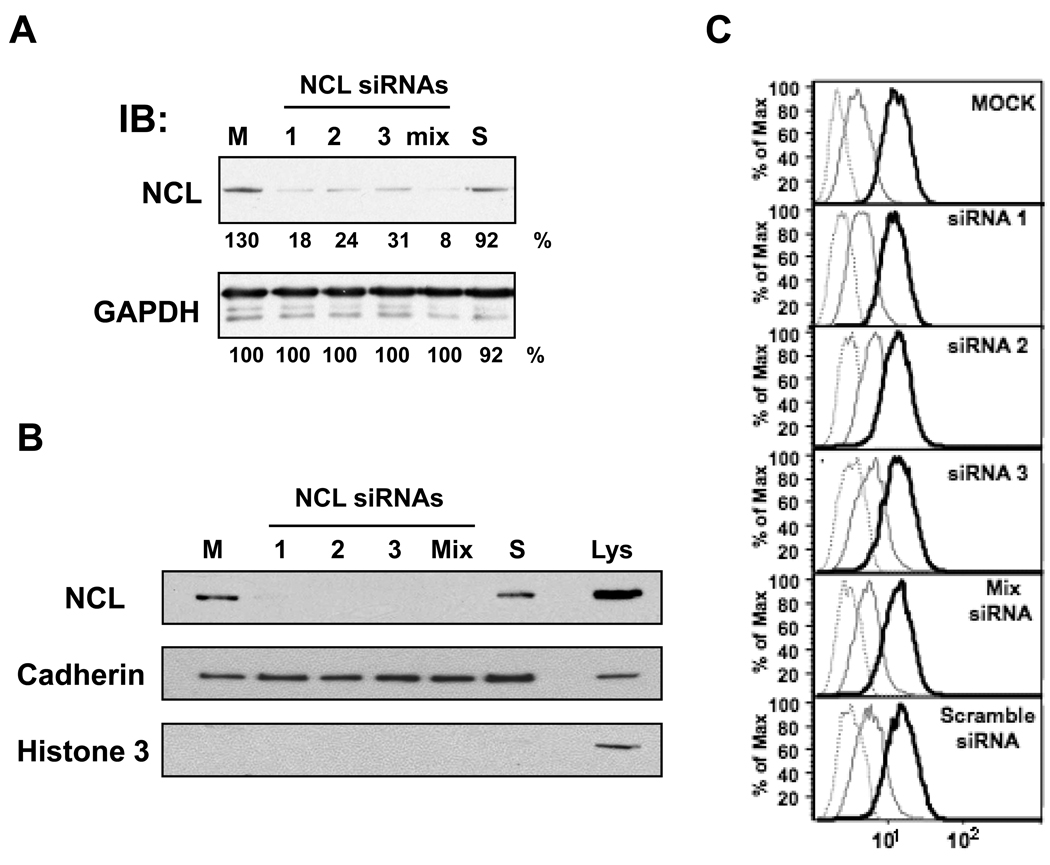
We have shown that nucleolin is the primary molecular target of AS1411 and had previously hypothesized that surface nucleolin may serve as a receptor for AS1411 (2, 13). However, the new data are not consistent with that hypothesis because they indicate that uptake occurs, not by classical receptor-mediated endocytosis, but by macropinocytosis. Therefore, we were curious to learn whether nucleolin played a role in AS1411 uptake. We first assessed the effect of an anti-nucleolin mAb (D3, which we confirmed could bind to surface nucleolin) on uptake of FL-AS1411 in DU145 cells after 2 h incubation and found no effect (Supplementary Figure S6). Next, we carried out similar experiments using siRNAs to knockdown expression of nucleolin. Immunoblot analyses confirmed that expression of total nucleolin could be reduced by more than 80% in cells transfected with nucleolin siRNAs compared with control-transfected cells (Figure 5A). We also showed that these siRNAs could effectively knockdown the cell surface form of nucleolin (Figure 5B), using techniques described in the Methods section. We next used the transfected DU145 cells to assess the uptake of FL-AS1411 after 2 h by flow cytometry analysis and found that knockdown of nucleolin had no effect on FL-AS1411 uptake under these conditions (Figure 5C).
Figure 5. AS1411 uptake after 2 h is not affected by knockdown of nucleolin expression.
DU145 cells were transfected for 48 h without siRNA (mock, M), or with 30 nM of one of three different nucleolin siRNAs (NCL1, NCL2, NCL3) or a control siRNA (scramble, S), or contransfected with 10 nM of each nucleolin siRNAs (mix). (A) Cells were lysed and total cell lysates were analyzed by immunoblotting using the antibodies shown. (B) Cell-surface proteins from intact transfected DU145 cells were labeled and captured as described in Methods, then analyzed by blotting with anti-nucleolin antibody (NCL). After stripping, the membrane was reprobed with antibodies for a plasma membrane marker (anti-pan Cadherin) and a nuclear marker (histone 3) to confirm fractionation. Total lysate (Lys) was used as control. (C) The medium of transfected cells was replaced by fresh complete medium containing no oligonucleotide (gray dashed line) or 10 µM FL-CRO (gray solid line) or 10 µM FL-AS1411 (black solid line) and incubated at 37°C for 2 h before harvesting and analysis by flow cytometry.
Nucleolin regulates AS1411-induced stimulation of macropinocytosis
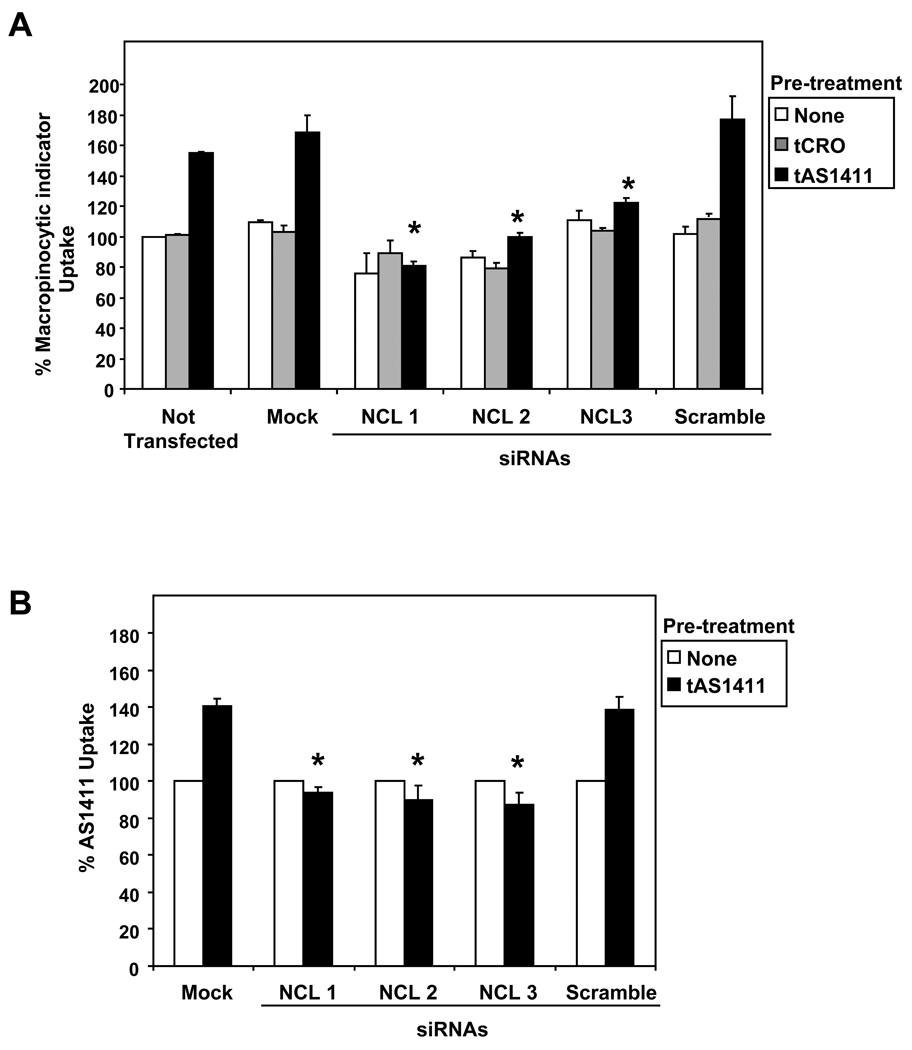
Our results (Figure 4) suggest that induction of macropinocytosis may be an important component of AS1411 activity. Therefore, we also determined whether nucleolin knockdown affects the tAS1411-mediated stimulation of macropinocystosis in DU145 cells. As shown in Figure 6A, inhibition of nucleolin expression by specific siRNAs had only a marginal effect on the baseline macropinocytosis, but caused a significant decrease in AS1411-induced macropinocytosis, almost completely blocking this process. Accordingly, the tAS1411-induced uptake of FL-AS1411 was also completely blocked in DU145 cells transfected with nucleolin siRNAs (Figure 6B). These results indicate that, whereas nucleolin does not appear to play a role in the initial macropinocytic uptake of AS1411, it is essential for the AS1411-induced stimulation of macropinocytosis. Consequently, nucleolin is also essential for the induced uptake of AS1411 that occurs at later time points.
Figure 6. Nucleolin regulates AS1411-induced stimulation of macropinocytosis.
DU145 cells were not transfected or transfected without siRNA (mock), or with 30 nM of one of three different nucleolin siRNAs (NCL1, NCL2, NCL3) or control siRNA (scramble). After 48 h transfection, cells were incubated with 10 µM tAS1411, 10 µM tCRO, or no oligonucleotide in complete medium at 37°C for 24 h. (A) After treatment, medium was replaced with fresh complete medium containing 0.2 mg/ml dextran-10K labeled with Alexa Fluor 488 and incubated at 37°C for 30 min. (B) After treatment, medium was replaced with fresh complete medium containing 10 µM FL-AS1411 and incubated at 37°C for 2 h. Cells were harvested and analyzed by flow cytometry and mean fluorescence was normalized to “not transfected” control (panel A) or to no pre-treatment control (panel B). Data are mean of three independent samples; bars, SE; * indicates p < 0.05 compared to mock-transfected cells pre-treated with tAS1411.
DISCUSSION
AS1411 is a first-in-class experimental drug that has already been tested in early clinical trials with promising results. The primary goal of our current research is to gain a better understanding about the mechanism of AS1411 activity, which we believe is important for several reasons. Knowledge of the factors that determine response to AS1411 might ultimately be translated into clinical tests that could identify patients with the best chance of benefiting from treatment with AS1411. Given the difficulties of achieving clinically relevant dosing of AS1411 in animal models (i.e., a 7-day continuous intravenous infusion), understanding how AS1411 works in cultured cancer cells will likely be the most expeditious route to identify candidate biomarkers, which can be then be evaluated retrospectively or prospectively in human clinical trials of AS1411. In addition, comprehending how AS1411 is able to preferentially affect cancer cells compared to non-malignant cells may lead to new insights into cancer biology. In particular, AS1411 has turned out to be a valuable tool to probe the functions of nucleolin, a protein that is increasingly being recognized as an important target in cancer therapy. Already, our investigations of AS1411 have revealed previously unrecognized functions for nucleolin in NF-κB signaling (3) and PRMT5 localization (6), and have now led to the discovery of another novel role in stimulating macropinocytosis.
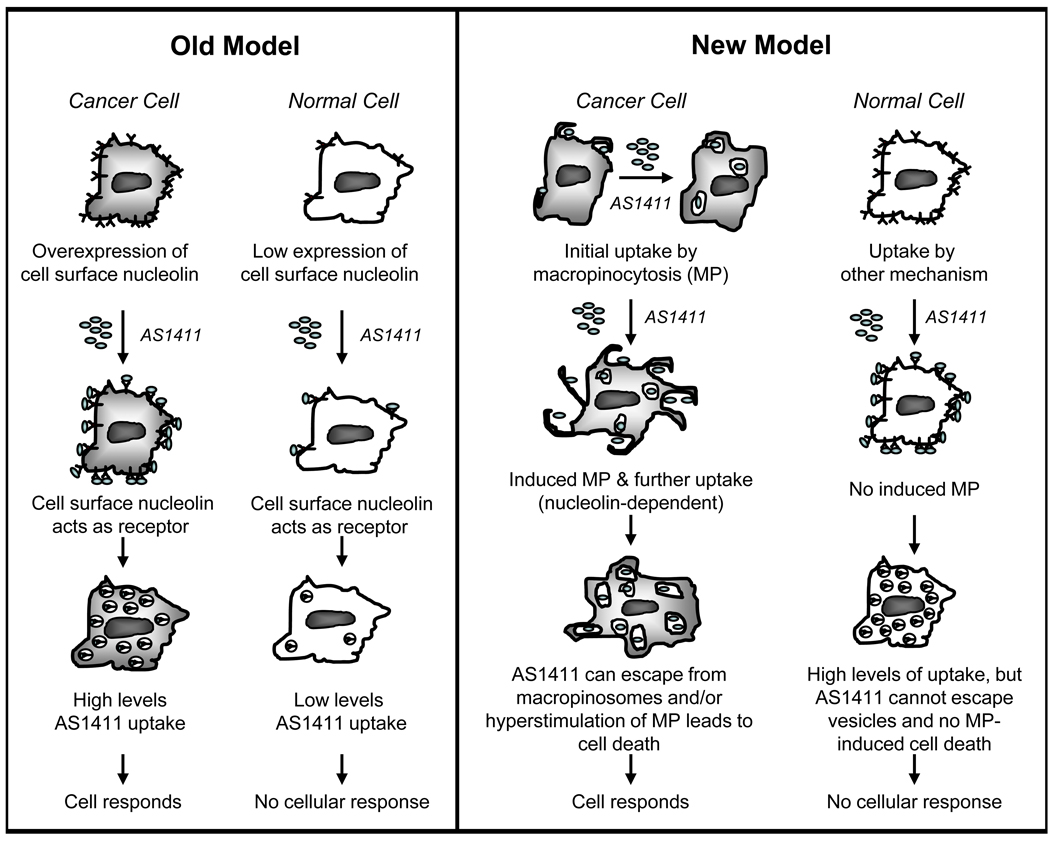
Previously, we identified nucleolin as the target of AS1411 and subsequently predicted that it would act as a receptor for AS1411 (2, 13). This hypothesis was attractive because it provided a possible explanation for the tumor-selectivity of AS1411 based on the reasoning that nucleolin expression is higher in cancer cells compared to normal cells, and therefore, we expected there would be selective uptake of AS1411 in cancer cells. However, the new data presented here show that these hypotheses were incorrect. We found that initial uptake of AS1411 is not higher in cancer cells compared to Hs27 non-malignant skin fibroblasts that are not responsive to AS1411. Instead, it appears that initial uptake of AS1411 in cancer cells occurs via macropinocytosis, whereas uptake of AS1411 occurs by other mechanisms in Hs27 cells. These intriguing results suggest it may be the mechanism of uptake, as opposed to the level of uptake, that is key to determining whether a cell is sensitive to AS1411 activity. Therefore, we now advocate a new model to explain the cancer-selectivity of AS1411, which involves uptake by macropinocytosis in cancer cells (Figure 7). Studies using other cancer cell lines and non-malignant cell types are ongoing to determine whether the results observed here can be generalized. So far, we have observed uptake by macropinocytosis and subsequent stimulation of macropinocytosis in all cells that respond to AS1411 (E. M. Reyes-Reyes and P. J. Bates, manuscript in preparation). Although we do not yet know why uptake by macropinocytosis and stimulation of macropinocytosis are so important for AS1411 activity, there are a number of possibilities that seem plausible. Macropinosomes are believed to be leaky compared to other endosomal vesicles and their cargo can bypass degradative processing (22, 25, 30). Therefore, the response of cells that take up AS1411 by macropinocytosis could be related to the ability of AS1411 to escape from macropinosomes in these cells, whereas uptake by non-macropinocytic pathways is expected to lead to endosomal trapping and/or lysosomal degradation. Alternatively (or in addition), it is possible that the selective effects of AS1411 on cancer cells occurs because treatment of these cells leads to hyperstimulation of macropinocytosis, which has been recently described as a novel form of cell death (27–29). Final proof of these ideas must await the development of new approaches for long-term inhibition of macropinocytosis because the current inhibitors are toxic when applied to cells for more than a few hours. However, there is precedent for the idea that drug activity can depend on the mechanism of uptake, rather than uptake efficiency, including a recent example showing that biological activity of an antisense oligonucleotide was dependent upon the route of endocytic internalization (31).
Figure 7. A new model to explain the cancer-selective antiproliferative activity of AS1411.
A cartoon comparing the previous model for AS1411 uptake with a revised model that is consistent with the new results. Key features of the new model are that it is the mechanism of uptake (rather than the levels of uptake) that determine response to AS1411 and that uptake occurs by macropinocytosis in cancer cells. In the new model, nucleolin does not act as a classical cell surface receptor, but is essential for the stimulation of macropinocytosis by AS1411, which leads to further uptake of the aptamer. We hypothesize that, in cancer cells, uptake by macropinocytosis allows endosomal escape of AS1411 (because macropinosomes are relatively leaky), and/or that AS1411 induces cell death due to hyperstimulation of macropinocytosis. We propose that the lack of response in normal cells may be due to the different AS1411 uptake route leading to endosomal entrapment or lysosomal degradation, and/or the inability of AS1411 to stimulate macropinocytosis in these cells because they have lower levels of nucleolin. Further research is required to test these hypotheses.
Direct confirmation of the role of nucleolin in mediating the antiproliferative effects of AS1411 is similarly challenging because long-term inhibition of nucleolin is also cytotoxic, but our new data clearly point to an essential function for nucleolin in AS1411’s ability of to stimulate macropinocytosis in cancer cells. The precise mechanism by which nucleolin regulates AS1411-induced macropinocytosis in cancer cells remains unknown. Macropinocytosis stimulation was not an early cellular response to AS1411, but was typically observed after 24 to 48 h of treatment, suggesting that it might require changes in protein expression. Thus, it may be relevant that nucleolin can regulate gene expression by various transcriptional and post-transcriptional mechanisms (5, 12, 32–35). Our confocal images showed that AS1411 was never localized in the nuclear region of cells (except dead cells) even after treatment for more two days (data not shown). Presumably then, the effects of AS1411 are on extranuclear nucleolin functions, such as shuttling, signal transduction, or modulating mRNA stability, which could also affect protein expression. This idea is entirely consistent with our previously proposed hypothesis that the effects of AS1411 stem from it binding to a subset of nucleolin complexes and thereby interfering with certain functions of nucleolin (2, 13). Interestingly, nucleolin has been implicated in the uptake of plasmids and DNA nanoparticles in previous independent studies (20, 36), although the relevance of these to AS1411 is unknown at present. Another recent publication has reported nucleolin as a receptor for AS1411 (37). These authors reported that binding of AS1411 to the surface of MV-4-11 leukemia cells was inhibited by pre-incubation with a nucleolin antibody (MS3), and that uptake (measured at 2 – 6 h) was reduced in MCF-7 cells that were stably transfected with nucleolin shRNA (37). These results appear to be at variance with our findings in DU145 cells, where neither an antibody that binds surface nucleolin (D3) nor nucleolin siRNA had any inhibitory effect on AS1411 uptake at 2 h (Figure 5 and Supplementary Figure S5).
Further research to explore the mechanism and applications of AS1411-induced macropinocytosis appears to be worthwhile. Macropinocytosis has been most widely explored in immune cells, such as macrophages and dendritic cells, where it serves to “sample” the extracellular fluid for the presence of pathogens, but is also induced by oncogenes and growth factor signaling (16, 28, 29, 38, 39), so it is perhaps unsurprising that it occurs in cancer cells. Moreover, macropinocytosis is emerging as a key mechanism for intracellular delivery of various physiological, pathological, and therapeutic cargoes. This process has been reported as an entry portal for a range of macromolecules, including numerous cell penetrating peptides (23, 40), intact proteins (41–43), bacterial virulence factors (44), gram-negative bacterial lipopolysaccharide (45), naked DNA plasmid (18–20), and many viruses (46). Remarkably, the uptake and effects of AS1411 are somewhat reminiscent of some cell penetrating peptides (also called protein transduction domains) and viruses, which can be taken up by macropinocytosis and then cause stimulation of macropinocytosis (23–25). Furthermore, there is substantial interest in the uptake mechanisms of oligonucleotides in general, largely because poor cellular delivery is a major hurdle in developing oligonucleotide-based therapeutics, including siRNAs (47). Interestingly, one of the most successful methods for in vivo siRNA to date may involve macropinocytosis (30). Thus, studying the uptake of AS1411—one of the few oligonucleotides that has activity in cultured cells without the need for transfection agents and which has progressed to clinical testing—may well lead to insights that will help create novel strategies for siRNA delivery. Similarly, our results may be relevant to efforts to improve delivery of plasmid DNA, because several recent reports have suggested that macropinocytosis is the major mechanism for uptake of naked DNA in cells and in mice (18–20). In summary, our novel findings regarding AS1411 might have broad implications for drug delivery and gene therapy strategies.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Drs. Ulf Brunk, John Eaton, Chuan Hu, Robert Mitchell, and Brian Wattenberg for reading the draft manuscript and providing constructive comments. We thank the anonymous reviewers for their helpful suggestions regarding the current studies and future directions.
Financial Support: Supported by National Institutes of Health grant R01 CA122383 to PJB.
Footnotes
Disclosures: PJB and EMR are co-inventors on issued or pending patents related to AS1411. PJB has financial interests in Antisoma PLC, as shareholder, consultant, and/or recipient of research support.
REFERENCES
- 1.Bates PJ, Kahlon JB, Thomas SD, Trent JO, Miller DM. Antiproliferative activity of G-rich oligonucleotides correlates with protein binding. J Biol Chem. 1999;274:26369–26377. doi: 10.1074/jbc.274.37.26369. [DOI] [PubMed] [Google Scholar]
- 2.Bates PJ, Laber DA, Miller DM, Thomas SD, Trent JO. Discovery and development of the G-rich oligonucleotide AS1411 as a novel treatment for cancer. Exp Mol Pathol. 2009;86:151–164. doi: 10.1016/j.yexmp.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Girvan AC, Teng Y, Casson LK, et al. AGRO100 inhibits activation of nuclear factor-kappaB (NF-kappaB) by forming a complex with NF-kappaB essential modulator (NEMO) and nucleolin. Mol Cancer Ther. 2006;5:1790–1799. doi: 10.1158/1535-7163.MCT-05-0361. [DOI] [PubMed] [Google Scholar]
- 4.Xu X, Hamhouyia F, Thomas SD, et al. Inhibition of DNA replication and induction of S phase cell cycle arrest by G-rich oligonucleotides. J Biol Chem. 2001;276:43221–43230. doi: 10.1074/jbc.M104446200. [DOI] [PubMed] [Google Scholar]
- 5.Soundararajan S, Chen W, Spicer EK, Courtenay-Luck N, Fernandes DJ. The nucleolin targeting aptamer AS1411 destabilizes Bcl-2 messenger RNA in human breast cancer cells. Cancer Res. 2008;68:2358–2365. doi: 10.1158/0008-5472.CAN-07-5723. [DOI] [PubMed] [Google Scholar]
- 6.Teng Y, Girvan AC, Casson LK, et al. AS1411 alters the localization of a complex containing protein arginine methyltransferase 5 and nucleolin. Cancer Res. 2007;67:10491–10500. doi: 10.1158/0008-5472.CAN-06-4206. [DOI] [PubMed] [Google Scholar]
- 7.Dapić V, Abdomerović V, Marrington R, et al. Biophysical and biological properties of quadruplex oligodeoxyribonucleotides. Nucleic Acids Res. 2003;31:2097–2107. doi: 10.1093/nar/gkg316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dapić V, Bates PJ, Trent JO, Rodger A, Thomas SD, Miller DM. Antiproliferative activity of G-quartet-forming oligonucleotides with backbone and sugar modifications. Biochemistry. 2002;41:3676–3685. doi: 10.1021/bi0119520. [DOI] [PubMed] [Google Scholar]
- 9.Christian S, Pilch J, Akerman ME, Porkka K, Laakkonen P, Ruoslahti E. Nucleolin expressed at the cell surface is a marker of endothelial cells in angiogenic blood vessels. J Cell Biol. 2003;163:871–878. doi: 10.1083/jcb.200304132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ginisty H, Sicard H, Roger B, Bouvet P. Structure and functions of nucleolin. J Cell Sci. 1999;112:761–772. doi: 10.1242/jcs.112.6.761. [DOI] [PubMed] [Google Scholar]
- 11.Mongelard F, Bouvet P. Nucleolin:a multiFACeTed protein. Trends Cell Biol. 2007;17:80–86. doi: 10.1016/j.tcb.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 12.Storck S, Shukla M, Dimitrov S, Bouvet P. Functions of the histone chaperone nucleolin in diseases. Subcell Biochem. 2007;41:125–144. doi: 10.1007/1-4020-5466-1_7. [DOI] [PubMed] [Google Scholar]
- 13.Bates P, Mergny JL, Yang D. Quartets in G-major. The First International Meeting on Quadruplex DNA. EMBO Rep. 2007;8:1003–1010. doi: 10.1038/sj.embor.7401073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reyes-Reyes EM, George M, Roberts J, Akiyama S. P-selectin activates integrin-mediated colon carcinoma cell adhesion to fibronectin. Exp Cell Res. 2006;312:4056–4069. doi: 10.1016/j.yexcr.2006.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi EW, Nayak LV, PJ B. Cancer-selective antiproliferative activity is a general property of some G-rich oligodeoxynucleotides. Nucleic Acids Res. 2010;38:1623–1635. doi: 10.1093/nar/gkp1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doherty GJ, McMahon HT. Mechanisms of endocytosis. Annu Rev Biochem. 2009;78:857–902. doi: 10.1146/annurev.biochem.78.081307.110540. [DOI] [PubMed] [Google Scholar]
- 17.Macia E, Ehrlich M, Massol R, Boucrot E, Brunner C, Kirchhausen T. Dynasore, a cell-permeable inhibitor of dynamin. Dev Cell. 2006;10:839–850. doi: 10.1016/j.devcel.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 18.Basner-Tschakarjan E, Mirmohammadsadegh A, Baer A, Hengge UR. Uptake and trafficking of DNA in keratinocytes:evidence for DNA-binding proteins. Gene Ther. 2004;11:765–774. doi: 10.1038/sj.gt.3302221. [DOI] [PubMed] [Google Scholar]
- 19.Fumoto S, Nishi J, Ishii H, et al. Rac-mediated macropinocytosis is a critical route for naked plasmid DNA transfer in mice. Mol Pharm. 2009;6:1170–1179. doi: 10.1021/mp900042p. [DOI] [PubMed] [Google Scholar]
- 20.Wittrup A, Sandgren S, Lilja JBC, Gustavsson N, Mörgelin M, Belting M. Identification of proteins released by mammalian cells that mediate DNA internalization through proteoglycan-dependent macropinocytosis. J Biol Chem. 2007;282:27897–27904. doi: 10.1074/jbc.M701611200. [DOI] [PubMed] [Google Scholar]
- 21.West MA, Bretscher MS, Watts C. Distinct endocytotic pathways in epidermal growth factor-stimulated human carcinoma A431 cells. J Cell Biol. 1989;109:2731–2739. doi: 10.1083/jcb.109.6.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hewlett LJ, Prescott AR, Watts C. The coated pit and macropinocytic pathways serve distinct endosome populations. J Cell Biol. 1994;124:689–703. doi: 10.1083/jcb.124.5.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaplan IM, Wadia JS, Dowdy SF. Cationic TAT peptide transduction domain enters cells by macropinocytosis. J Control Release. 2005;102:247–253. doi: 10.1016/j.jconrel.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 24.Mercer J, Helenius A. Vaccinia virus uses macropinocytosis and apoptotic mimicry to enter host cells. Science. 2008;320:531–535. doi: 10.1126/science.1155164. [DOI] [PubMed] [Google Scholar]
- 25.Wadia JS, Stan RV, Dowdy SF. Transducible TAT-HA fusogenic peptide enhances escape of TAT-fusion proteins after lipid raft macropinocytosis. Nat Med. 2004;10:310–315. doi: 10.1038/nm996. [DOI] [PubMed] [Google Scholar]
- 26.Chi S, Kitanaka C, Noguchi K, et al. Oncogenic Ras triggers cell suicide through the activation of a caspase-independent cell death program in human cancer cells. Oncogene. 1999;18:2281–2290. doi: 10.1038/sj.onc.1202538. [DOI] [PubMed] [Google Scholar]
- 27.Kaul A, Overmeyer JH, Maltese WA. Activated Ras induces cytoplasmic vacuolation and non-apoptotic death in glioblastoma cells via novel effector pathways. Cell Signal. 2007;19:1034–1043. doi: 10.1016/j.cellsig.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li C, Macdonald JI, Hryciw T, Meakin SO. Nerve growth factor activation of the TrkA receptor induces cell death, by macropinocytosis, in medulloblastoma Daoy cells. J Neurochem. 2010;112:882–899. doi: 10.1111/j.1471-4159.2009.06507.x. [DOI] [PubMed] [Google Scholar]
- 29.Overmeyer JH, Kaul A, Johnson EE, Maltese WA. Active ras triggers death in glioblastoma cells through hyperstimulation of macropinocytosis. Mol Cancer Res. 2008;6:965–977. doi: 10.1158/1541-7786.MCR-07-2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Love KT, Mahon KP, Levins CG, et al. Lipid-like materials for low-dose, in vivo gene silencing. Proc Natl Acad Sci U S A. 2010;107:1864–1869. doi: 10.1073/pnas.0910603106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alam MR, Ming X, Dixit V, Fisher M, Chen X, Juliano RL. The Biological Effect of an Antisense Oligonucleotide Depends on Its Route of Endocytosis and Trafficking. Oligonucleotides. 2010;20:103–109. doi: 10.1089/oli.2009.0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Angelov D, Bondarenko VA, Almagro S, et al. Nucleolin is a histone chaperone with FACT-like activity and assists remodeling of nucleosomes. EMBO J. 2006;25:1669–1679. doi: 10.1038/sj.emboj.7601046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gabellini D, Green MR, Tupler R. Inappropriate gene activation in FSHD:a repressor complex binds a chromosomal repeat deleted in dystrophic muscle. Cell. 2002;110:339–348. doi: 10.1016/s0092-8674(02)00826-7. [DOI] [PubMed] [Google Scholar]
- 34.Hanakahi LA, Bu Z, Maizels N. The C-terminal domain of nucleolin accelerates nucleic acid annealing. Biochemistry. 2000;39:15493–15439. doi: 10.1021/bi001683y. [DOI] [PubMed] [Google Scholar]
- 35.Hanakahi LA, Dempsey LA, Li MJ, N M. Nucleolin is one component of the B cell-specific transcription factor and switch region binding protein, LR1. Proc Natl Acad Sci U S A. 1997;94:3605–3610. doi: 10.1073/pnas.94.8.3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen X, Kube DM, Cooper MJ, Davis PB. Cell surface nucleolin serves as receptor for DNA nanoparticles composed of pegylated polylysine and DNA. Mol Ther. 2008;16:333–342. doi: 10.1038/sj.mt.6300365. [DOI] [PubMed] [Google Scholar]
- 37.Soundararajan S, Wang L, Sridharan V, et al. Plasma membrane nucleolin is a receptor for the anticancer aptamer AS1411 in MV4-11 leukemia cells. Mol Pharmacol. 2009;76:984–991. doi: 10.1124/mol.109.055947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bar-Sagi D, Feramisco JR. Induction of membrane ruffling and fluid-phase inocytosis in quiescent fibroblasts by ras proteins. Science. 1986;233:1061–1068. doi: 10.1126/science.3090687. [DOI] [PubMed] [Google Scholar]
- 39.Kerr MC, Teasdale RD. Defining macropinocytosis. Traffic. 2009;10:364–371. doi: 10.1111/j.1600-0854.2009.00878.x. [DOI] [PubMed] [Google Scholar]
- 40.Nakase I, Niwa M, Takeuchi T, et al. Cellular uptake of arginine-rich peptides:roles for macropinocytosis and actin rearrangement. Mol Ther. 2004;10:1011–1022. doi: 10.1016/j.ymthe.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 41.Greenwood KP, Daly NL, Brown DL, Stow JL, Craik DJ. The cyclic cystine knot miniprotein MCoTI-II is internalized into cells by macropinocytosis. Int J Biochem Cell Biol. 2007;39:2252–2264. doi: 10.1016/j.biocel.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 42.Magzoub M, Sandgren S, Lundberg P, et al. N-terminal peptides from unprocessed prion proteins enter cells by macropinocytosis. Biochem Biophys Res Commun. 2006;348:379–385. doi: 10.1016/j.bbrc.2006.07.065. [DOI] [PubMed] [Google Scholar]
- 43.Noguchi H, Bonner-Weir S, Wei FY, Matsushita M, Matsumoto S. BETA2/NeuroD protein can be transduced into cells due to an arginine- and lysine-rich sequence. Diabetes. 2005;54:2859–2866. doi: 10.2337/diabetes.54.10.2859. [DOI] [PubMed] [Google Scholar]
- 44.Khelef N, Gounon P, Guiso N. Internalization of Bordetella pertussis adenylate cyclase-haemolysin into endocytic vesicles contributes to macrophage cytotoxicity. Cell Microbiol. 2001;3:721–730. doi: 10.1046/j.1462-5822.2001.00151.x. [DOI] [PubMed] [Google Scholar]
- 45.Poussin C, Foti M, Carpentier JL, Pugin J. CD14-dependent endotoxin internalization via a macropinocytic pathway. J Biol Chem. 1998;273:20285–20291. doi: 10.1074/jbc.273.32.20285. [DOI] [PubMed] [Google Scholar]
- 46.Mercer J, Helenius A. Virus entry by macropinocytosis. Nat Cell Biol. 2009;11:510–520. doi: 10.1038/ncb0509-510. [DOI] [PubMed] [Google Scholar]
- 47.Meade BR, Dowdy SF. The road to therapeutic RNA interference (RNAi):Tackling the 800 pound siRNA delivery gorilla. Discov Med. 2009;8:253–256. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.