Abstract
Viral respiratory illnesses associated with wheezing are extremely common during early life and remain a frequent cause of morbidity and hospitalization in young children. Although many children who wheeze with respiratory viruses during infancy outgrow the problem, most children with asthma and reductions in lung function at school age begin wheezing during the first several years of life. Whether symptomatic viral infections of the lower respiratory tract are causal in asthma development or simply identify predisposed children remains a controversial issue. Wheezing illnesses caused by respiratory syncytial virus (RSV), particularly those severe enough to lead to hospitalization, have historically been associated with an increased risk of asthma at school age. However, with the development of molecular diagnostics, human rhinovirus (HRV) wheezing illnesses have been recognized more recently as a stronger predictor of school-age asthma than RSV. In this article, the authors review the impact of virus infections during early life, focusing primarily on RSV and HRV, and their potential roles in asthma inception.
Keywords: Human rhinovirus, Respiratory syncytial virus, Wheezing, Asthma, Children, Interferons
Viral respiratory illnesses associated with wheezing are extremely common during early life and remain a frequent cause of morbidity and hospitalization in young children.1 Although many children who wheeze with respiratory viruses during infancy outgrow the problem, most children with asthma and reductions in lung function at school age begin wheezing during the first several years of life.2 Whether symptomatic viral infections of the lower respiratory tract are causal in asthma development or simply identify predisposed children remains a controversial issue. Wheezing illnesses caused by respiratory syncytial virus (RSV), particularly those severe enough to lead to hospitalization, have historically been associated with an increased risk of asthma at school age.3, 4 However, with the development of molecular diagnostics, human rhinovirus (HRV) wheezing illnesses have been recognized more recently as a stronger predictor of school-age asthma than RSV.5, 6 In this article, the authors review the impact of virus infections during early life, focusing primarily on RSV and HRV, and their potential roles in asthma inception.
Epidemiology of wheezing during early life
Infections with respiratory viruses are the leading cause of wheezing during early childhood. In fact, with current molecular diagnostics, a viral pathogen can be identified in at least 90% of wheezing episodes during the first several years of life.6 All children are infected with respiratory viruses during early life, and up to 50% have a lower respiratory tract illness with wheezing at least once before school age.2 Several environmental factors have been linked to risk of wheezing illnesses during early life. The Tucson Children’s Respiratory Study (TCRS), a prospective unselected birth cohort, identified several risk factors for wheezing during early childhood. Older siblings and daycare, both associated with increased exposure to respiratory viruses, and tobacco smoke exposure were associated with increased risk of early-life wheezing illnesses, whereas breastfeeding was protective.7
The most common viruses identified during early-life wheezing illnesses are RSV, HRV, and multiple viruses. Less common causes include parainfluenza, metapneumovirus, coronavirus, influenza, bocavirus, and adenovirus.
RSV is seasonal, with peak infection during winter in the United States. RSV is ubiquitous, with nearly all children infected by 2 years of age.8 A subset of these children has more severe illnesses, and infection with RSV leading to bronchiolitis and/or pneumonia is a primary cause of hospitalization during the first year of life. Children hospitalized with RSV tend to be younger than children hospitalized for other respiratory viruses. Other risk factors of RSV hospitalization include prematurity, male gender, daycare attendance, and tobacco exposure.9
In contrast to RSV, HRV infections occur throughout the year. HRV is the leading cause of bronchiolitis leading to hospitalization in infants outside of the winter bronchiolitis season.10 Compared with RSV, children hospitalized with HRV tend to be older and are more likely to have wheezed previously.11 They also often have more atopic risk factors or characteristics, including eczema, allergic sensitization, and parental asthma.
Traditionally, HRVs have been divided into 2 groups (A and B) with about 100 known serotypes. Many HRVs do not grow well in traditional cell culture, and this is particularly true of a novel group of HRVs categorized as Group C HRVs. Molecular diagnostics and characterization of HRVs have demonstrated that the number of HRV species has previously been dramatically underestimated.12 In fact, numerous recent reports have identified novel Group C HRVs and implicated them in upper and lower respiratory tract illnesses, frequently including illnesses severe enough to lead to hospitalization.12, 13, 14, 15, 16
Virus infection and subsequent asthma development
The Role of RSV in Asthma
RSV lower respiratory tract illnesses, particularly those severe enough to lead to hospitalization, are associated with an increased risk of asthma at school age.3, 4 This observation has prompted the idea that RSV lower respiratory tract illness may be causal in asthma development. Sigurs and colleagues4 used a case-control methodology to examine the risk of asthma and allergy development after RSV bronchiolitis severe enough to lead to hospitalization. They found that severe RSV bronchiolitis was associated with a significantly increased risk of asthma and allergy at 13 years of age, although some have suggested that the rate of asthma and allergy in their control population was lower than expected.
A large unselected retrospective cohort study in Tennessee recently reported findings in support of a causal role for RSV bronchiolitis in asthma inception. In this study, Wu and colleagues17 reported that children born 120 days before the peak of the RSV season had the greatest risk of hospitalization for lower respiratory tract illness and of asthma between 4 and 5.5 years of age.
However, several studies argue against a causal role for RSV in asthma inception. A large twin registry in Denmark was used to assess the relationships between severe RSV illness and asthma development.18, 19 The data from this study suggest that severe RSV illnesses and asthma share a common predisposition, and although RSV can lead to short-term recurrent wheeze, it is not causal in long-term asthma development. Similarly, although the TCRS identified RSV wheezing illnesses during the first 3 years of life as an independent risk factor of wheezing at age 6 years, this relationship weakened over time and was no longer significant by age 13 years.3 Thus, early RSV wheeze may lead to a recurrent wheeze phenotype, but it is less commonly associated with lifelong asthma. It would be interesting to determine whether the relationships reported from the Tennessee population-based study between 4 and 5.5 years of age persist over time.
The Role of HRV in Asthma
Recently, molecular diagnostics have allowed several groups to demonstrate that wheezing illnesses caused by HRV are potentially a more robust predictor of asthma development than episodes caused by RSV. Kotaniemi and colleagues5 reported that children who were hospitalized with HRV wheezing illnesses during the first 2 years of life were at approximately fourfold increased risk of childhood asthma when compared with children hospitalized with wheezing associated with other viruses.
Two birth cohort studies have recently identified outpatient HRV wheezing illnesses as important predictors of childhood asthma development as well. The Childhood Origins of ASThma (COAST) study, a high-risk birth cohort examining the role of respiratory viruses and immune dysregulation in the development of asthma and other allergic diseases,20 identified HRV wheezing illnesses during the first year of life as significant predictors of wheezing in the third year of life21 and asthma at age 6 years.6 In a similar high-risk birth cohort in Australia, Kusel and colleagues22 reported that HRV wheezing illnesses during the first year of life were associated with increased asthma risk at age 5 years, although this finding was restricted to children who developed aeroallergen sensitization by age 2 years. In the COAST study, although HRV wheezing illnesses during infancy were an independent predictor of asthma development, children who wheezed with HRV and had aeroallergen sensitization during infancy had the greatest risk of asthma at school age.6
Age of Wheezing and Subsequent Asthma Risk
The COAST study has continued to evaluate specific viral cause and timing of wheezing illnesses as the children have progressed through early childhood. It seems that the age at which HRV wheezing illnesses occur has significant prognostic value with regard to subsequent asthma risk (Table 1 ). Children who wheezed with HRV during the first year of life had about a threefold risk of having asthma at age 6 years. HRV wheezing during the second year of life was associated with a more pronounced, about a sevenfold, increase in asthma risk, whereas wheezing with HRV infection during the third year of life was associated with a dramatic (odds ratio about 32) increase in asthma at school age. Nearly 90% of children who wheezed with HRV during the third year of life had asthma when they reached school age.6 Long-term follow-up of these children to determine whether these relationships persist into the teenage years and beyond would prove very informative.
Table 1.
Rhinovirus and RSV wheezing illnesses in years 1, 2, and 3, and risk of asthma at age 6 years
| Wheezing Illness | n | Asthma Age 6 Years (n) | Asthma Age 6 Years (%) | OR | 95% CI | P Value | |
|---|---|---|---|---|---|---|---|
| First year of life | |||||||
| RSV | No | 211 | 55 | 26 | 1.0 | ||
| Yes | 48 | 18 | 38 | 1.7 | (0.9, 3.3) | 0.11 | |
| RV | No | 214 | 52 | 24 | 1.0 | ||
| Yes | 45 | 21 | 47 | 2.7 | (1.4,5.3) | 0.003 | |
| RV and RSV | Neither | 192 | 46 | 24 | 1.0 | ||
| RSV only | 22 | 6 | 27 | 1.2 | (0.4, 3.2) | 0.73 | |
| RV only | 19 | 9 | 47 | 2.9 | (1.1, 7.5) | 0.03 | |
| Both | 26 | 12 | 46 | 2.7 | (1.2, 6.3) | 0.02 | |
| Second year of life | |||||||
| RSV | No | 231 | 61 | 26 | 1.0 | ||
| Yes | 28 | 12 | 43 | 2.1 | (0.9, 4.7) | 0.07 | |
| RV | No | 222 | 49 | 22 | 1.0 | ||
| Yes | 37 | 24 | 65 | 6.5 | (3.1, 13.7) | <0.0001 | |
| RV and RSV | Neither | 203 | 44 | 22 | 1.0 | ||
| RSV only | 19 | 5 | 26 | 1.3 | (0.4, 3.8) | 0.64 | |
| RV only | 28 | 17 | 61 | 5.6 | (2.4, 12.8) | <0.000l | |
| Both | 9 | 7 | 78 | 12.6 | (2.5, 63.1) | 0.002 | |
| Third year of life | |||||||
| RSV | No | 242 | 60 | 25 | 1.0 | ||
| Yes | 17 | 13 | 76 | 9.9 | (3.1, 31.4) | 0.0001 | |
| RV | No | 225 | 43 | 19 | 1.0 | ||
| Yes | 34 | 30 | 88 | 31.7 | (10.6, 94.9) | <0.0001 | |
| RV and RSV | Neither | 214 | 35 | 16 | 1.0 | ||
| RSV only | 11 | 8 | 73 | 13.6 | (3.4, 54.0) | 0.0002 | |
| RV only | 28 | 25 | 89 | 42.6 | (12.2, 148.9) | <0.0001 | |
| Both | 6 | 5 | 83 | 25.6 | (2.9, 225.6) | 0.004 | |
Abbreviations: CI, confidence interval; OR, odds ratio; RV, rhinovirus.
Data From Jackson DJ, Gangnon RE, Evans MD, et al. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am J Respir Crit Care Med 2008;178(7):667–72.
Progression from virus-induced wheezing to asthma
Preschool children with virus-induced wheezing use a disproportionate amount of health care resources compared with older children and adults with asthma,1 in part because they do not respond as well to conventional asthma therapies. This, no doubt, is also due to the heterogeneity of this population, including children who outgrow their wheezing and those who continue to wheeze into school age and beyond.
In the Preventing Early Asthma in Kids study, a trial comparing treatment with low-dose fluticasone to placebo in high-risk children with recurrent wheezing, it was observed that for children who did not outgrow their wheezing, lower respiratory symptom burden progressively increased as they advanced through the preschool years.23 Multiple cohort studies have demonstrated that there are long-lasting effects on lung function for children who begin wheezing before age 3 years and develop persistent disease. In the TCRS, children who began wheezing before age 3 years and continued to wheeze at school age had reductions in lung function at age 6 years2 that persisted at least to the teenage years.24 Illi and colleagues,25 through careful assessment of allergic sensitization and exposure, demonstrated that children who wheezed during the preschool years, were sensitized to aeroallergens, and continued to wheeze had impairment in lung function to at least age 13 years.
However, partly because of the invasive nature of the procedures necessary to directly study the lower airway, there is a paucity of data to help us understand the underlying pathogenesis of loss of lung function in children who start with recurrent virus-induced wheezing but develop persistent disease. Nonetheless, a few studies may shed some light on the inflammatory processes that occur in these children. Krawiec and colleagues26 performed bronchoscopy and bronchoalveolar lavage on children of median age 15 months with recurrent wheezing and to a group of healthy controls. These studies demonstrated that children with persistent wheezing had increased inflammatory cells and markers of inflammation compared with healthy controls but did not have the characteristic eosinophil predominance seen in older children and adults with asthma. Similarly, Saglani and colleagues27 performed bronchoscopy and biopsy in children of median age 12 months with persistent wheezing and were unable to demonstrate either eosinophilic inflammation or reticular basement membrane (RBM) thickening, both common features of asthma seen in older children and adults. In contrast, in further studies of older children with recurrent wheezing of median age 29 months, they were able to demonstrate the presence of eosinophilic inflammation and RBM thickening when compared with a group of controls. This suggests that inflammatory changes and remodeling typical of asthma develop between 1 and 3 years of age in recurrently wheezing children and highlights the potential importance of environmental exposures, such as virus infections, during this period.
Mechanisms by which viruses may promote asthma inception
Several animal models have suggested mechanisms by which virus infection in early life could cause asthma inception. The COAST study was based on a rat model of virus-induced airway dysfunction in which a histologic and physiologic asthma phenotype could be induced only in a genetically predisposed Th2-biased strain when they were infected at a critical time in the development of the lung and/or the immune system (weanling age).28 However, there is limited data in humans demonstrating distinct mechanisms for causality of virus infections in asthma inception. Several recent human studies suggest plausible mechanisms by which HRV could be involved in airway remodeling and asthma inception.
Leigh and colleagues29 demonstrated that in vitro infection of airway epithelial cells with HRV led to increased production of several mediators involved in airway remodeling, including amphiregulin, activin A, and vascular endothelial growth factor (VEGF). The authors also found increased VEGF in nasal lavage fluid of volunteers with natural HRV infections. In another epithelial cell model, Zhu and colleagues30 found that HRV infection led to toll-like receptor 3-dependent mucin production and upregulation of epidermal growth factor receptor, a prominent component of epithelial repair. Finally, epithelial cell gene expression studies have identified several potential novel pathways involved in the response to HRV infection. Proud and colleagues31 found that HRV infection led to upregulation of many genes, perhaps most notably in interferon and airway repair and remodeling pathways. Bochkov and colleagues32 similarly demonstrated that HRV infection led to upregulation of a number of genes involved in interferon and airway remodeling pathways. In this study, differences between asthmatics and controls were similar before and during HRV infection. In summary, although data that demonstrates causality of HRV infection in asthma inception are limited, several plausible mechanisms by which recurrent HRV infection could lead to airway damage and remodeling have been identified and are deserving of further study.
Host factors associated with wheezing and asthma
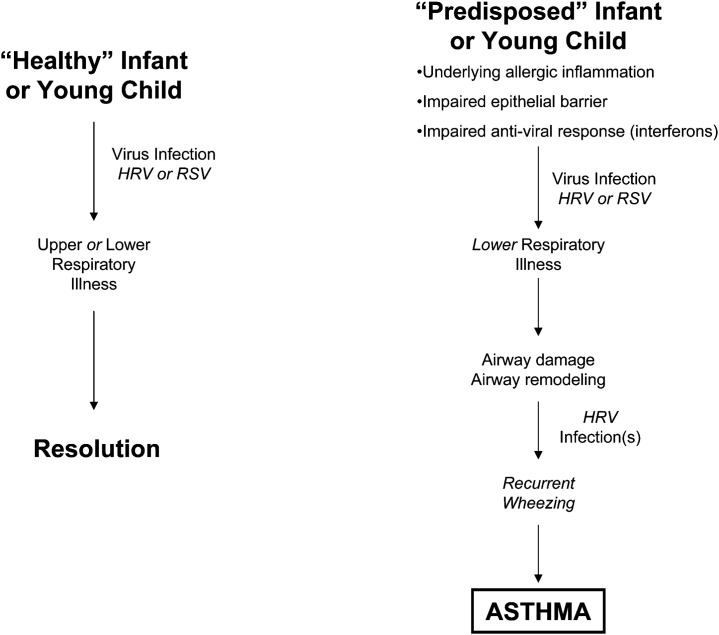
If viruses are causal in asthma inception, this is likely to be particularly true in susceptible hosts (Fig. 1 ). A so-called two-hit hypothesis has been proposed,20 whereby children with immune dysregulation (favoring an allergic phenotype) who develop a lower respiratory viral illness during a critical time in development progress to an asthma phenotype. Allergic sensitization during early childhood has been identified by several studies as an important risk factor of asthma development.6, 22, 25 In older children with asthma, allergic sensitization is a risk factor of more severe upper and lower respiratory viral illnesses.33, 34 Several observations may help explain host susceptibility to virus-induced wheezing and asthma exacerbations.
Fig. 1.
The potential consequences of virus infections in young children and how recurrent virus infection, particularly with HRV, may be causal in asthma pathogenesis in predisposed children.
Interferons are known to play an important role in the host response to virus infections, and there is growing data to support a prominent role for interferons in the pathogenesis of virus-induced wheezing and asthma. Gern and colleagues35 linked immature peripheral blood interferon-γ responses to mitogens and viruses at birth with recurrent wheezing during infancy. Similarly, Stern and colleagues36 connected reduced peripheral blood interferon-γ responses to mitogen at 9 months of age to an increased risk of wheezing in toddlers and school-aged children. Peripheral blood mononuclear cells from adult asthmatics produce less interferon-γ in response to HRV infection than controls,37 and the degree of impairment correlates with airway hyper-responsiveness and degree of airway obstruction.38
Several other investigators, through experiments on airway epithelial cells and in peripheral blood, have identified deficiencies in interferons as an important potential mechanism for asthmatics’ susceptibility to more severe HRV illnesses. Wark and colleagues.39 demonstrated that bronchial epithelial cells from atopic asthmatics infected with HRV in vitro have deficient interferon-β (a type I interferon) responses when compared with controls. In this study, the decreased interferon-β response was associated with delayed apoptosis and increased viral replication, which was overcome with the addition of exogenous interferon-β. Additionally, lower levels of interferon-α (another type I interferon) responses have been demonstrated in the peripheral blood of children40 and adults41 with atopic asthma versus nonatopic controls. Finally, Contoli and colleagues42 found that bronchial epithelial cells and alveolar macrophages from atopic asthmatics had impaired interferon-λ (a type III interferon) responses to HRV infection and that these responses correlated with decline of forced expiratory volume in the first second of expiration during exacerbations. Although these studies have identified defects in interferon pathways as potential important contributors to early childhood wheezing and asthma, unfortunately, attempts to replicate these findings have not always been successful. Two recent studies found no difference in epithelial cell responses between asthmatics and controls.32, 43
Another important component of the innate immune response to virus infection is barrier function. Barrier function of the skin is important in the pathogenesis of atopic dermatitis, with filaggrin mutations identified in several patients with severe atopic dermatitis.44 Ongoing allergic inflammation may lead to a local or acquired filaggrin deficit.45 Several studies have highlighted how disruption of the airway epithelium could alter host antiviral responses and severity of lower respiratory illnesses. In fact, filaggrin mutations have been identified as a risk factor of asthma and asthma exacerbations in children.46, 47 A unique model of airway injury took this observation one step further in demonstrating the potential consequences of impairment in airway barrier function through physical injury. Jakiela and colleagues,48 using in vitro epithelial cell culture, showed that mechanical injury to the epithelia resulted in increased replication of HRV. This is a potential mechanism by which various host factors or environmental exposures, such as allergen, tobacco smoke, and previous virus infection, could lead to impairment in barrier function, increased viral replication, and more severe lower respiratory illnesses.
Summary
Respiratory viruses are a major cause of morbidity in young children. Wheezing illnesses caused by viruses during early life are the most common initial presentation of childhood asthma. Recent evidence suggests that wheezing with HRV is the most robust predictor of subsequent asthma. However, whether HRV is causal in asthma inception is an open question. Plausible mechanisms of causality have been identified but are in need of further study. The most definitive way to prove causality would be to prevent HRV infection and demonstrate reductions in asthma development, but vaccines for HRV have proven elusive, in part due to the nearly 150 existing species of HRV. Studies attempting to identify more virulent or asthmogenic forms of HRV are ongoing and could prove very informative. Novel preventive and therapeutic strategies are sorely needed to prevent or interrupt the progression from virus-induced wheezing to persistent asthma.
Footnotes
Supported by NIH grants 1UL1RR025011, T32 AI007635, P01 HL70831, and by the AAAAI/GSK Allergy Fellow Career Development Award.
References
- 1.Akinbami L.J., Moorman J.E., Garbe P.L. Status of childhood asthma in the United States, 1980–2007. Pediatrics. 2009;123(Suppl 3):S131–S145. doi: 10.1542/peds.2008-2233C. [DOI] [PubMed] [Google Scholar]
- 2.Martinez F.D., Wright A.L., Taussig L.M. Asthma and wheezing in the first six years of life. N Engl J Med. 1995;332:133–138. doi: 10.1056/NEJM199501193320301. [DOI] [PubMed] [Google Scholar]
- 3.Stein R.T., Sherrill D., Morgan W.J. Respiratory syncytial virus in early life and risk of wheeze and allergy by age 13 years. Lancet. 1999;354:541–545. doi: 10.1016/S0140-6736(98)10321-5. [DOI] [PubMed] [Google Scholar]
- 4.Sigurs N., Gustafsson P.M., Bjarnason R. Severe respiratory syncytial virus bronchiolitis in infancy and asthma and allergy at age 13. Am J Respir Crit Care Med. 2005;171(2):137–141. doi: 10.1164/rccm.200406-730OC. [DOI] [PubMed] [Google Scholar]
- 5.Kotaniemi-Syrjanen A., Vainionpaa R., Reijonen T.M. Rhinovirus-induced wheezing in infancy–the first sign of childhood asthma? J Allergy Clin Immunol. 2003;111(1):66–71. doi: 10.1067/mai.2003.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jackson D.J., Gangnon R.E., Evans M.D. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am J Respir Crit Care Med. 2008;178(7):667–672. doi: 10.1164/rccm.200802-309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taussig L.M., Wright A.L., Holberg C.J. Tucson children’s respiratory study: 1980 to present. J Allergy Clin Immunol. 2003;111(4):661–675. doi: 10.1067/mai.2003.162. [DOI] [PubMed] [Google Scholar]
- 8.Openshaw P.J.M. Immunological mechanisms in respiratory syncytial virus disease. Springer Semin Immunopathol. 1995;17:187–201. doi: 10.1007/BF00196165. [DOI] [PubMed] [Google Scholar]
- 9.Simoes E.A. Environmental and demographic risk factors for respiratory syncytial virus lower respiratory tract disease. J Pediatr. 2003;143(Suppl 5):S118–S126. doi: 10.1067/s0022-3476(03)00511-0. [DOI] [PubMed] [Google Scholar]
- 10.Miller E.K., Lu X., Erdman D.D. Rhinovirus-associated hospitalizations in young children. J Infect Dis. 2007;195(6):773–781. doi: 10.1086/511821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jartti T., Lehtinen P., Vuorinen T. Bronchiolitis: age and previous wheezing episodes are linked to viral etiology and atopic characteristics. Pediatr Infect Dis J. 2009;28(4):311–317. doi: 10.1097/INF.0b013e31818ee0c1. [DOI] [PubMed] [Google Scholar]
- 12.Lee W.M., Kiesner C., Pappas T. A diverse group of previously unrecognized human rhinoviruses are common causes of respiratory illnesses in infants. PLoS One. 2007;2(10):e966. doi: 10.1371/journal.pone.0000966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller E.K., Edwards K.M., Weinberg G.A. A novel group of rhinoviruses is associated with asthma hospitalizations. J Allergy Clin Immunol. 2009;123(1):98–104. doi: 10.1016/j.jaci.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller E.K., Khuri-Bulos N., Williams J.V. Human rhinovirus C associated with wheezing in hospitalised children in the Middle East. J Clin Virol. 2009;46(1):85–89. doi: 10.1016/j.jcv.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tan B.H., Loo L.H., Lim E.A. Human rhinovirus group C in hospitalized children, Singapore. Emerg Infect Dis. 2009;15(8):1318–1320. doi: 10.3201/eid1508.090321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McErlean P., Shackelton L.A., Andrews E. Distinguishing molecular features and clinical characteristics of a putative new rhinovirus species, human rhinovirus C (HRV C) PLoS One. 2008;3(4):e1847. doi: 10.1371/journal.pone.0001847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu P., Dupont W.D., Griffin M.R. Evidence of a causal role of winter virus infection during infancy in early childhood asthma. Am J Respir Crit Care Med. 2008;178(11):1123–1129. doi: 10.1164/rccm.200804-579OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomsen S.F., van der S.S., Stensballe L.G. Exploring the association between severe respiratory syncytial virus infection and asthma: a registry-based twin study. Am J Respir Crit Care Med. 2009;179(12):1091–1097. doi: 10.1164/rccm.200809-1471OC. [DOI] [PubMed] [Google Scholar]
- 19.Stensballe L.G., Simonsen J.B., Thomsen S.F. The causal direction in the association between respiratory syncytial virus hospitalization and asthma. J Allergy Clin Immunol. 2009;123(1):131–137. doi: 10.1016/j.jaci.2008.10.042. [DOI] [PubMed] [Google Scholar]
- 20.Lemanske R.F., Jr. The childhood origins of asthma (COAST) study. Pediatr Allergy Immunol. 2002;13(Suppl):1538–1543. doi: 10.1034/j.1399-3038.13.s.15.8.x. [DOI] [PubMed] [Google Scholar]
- 21.Lemanske R.F., Jr., Jackson D.J., Gangnon R.E. Rhinovirus illnesses during infancy predict subsequent childhood wheezing. J Allergy Clin Immunol. 2005;116(3):571–577. doi: 10.1016/j.jaci.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 22.Kusel M.M., de Klerk N.H., Kebadze T. Early-life respiratory viral infections, atopic sensitization, and risk of subsequent development of persistent asthma. J Allergy Clin Immunol. 2007;119(5):1105–1110. doi: 10.1016/j.jaci.2006.12.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guilbert T.W., Morgan W.J., Zeiger R.S. Long-term inhaled corticosteroids in preschool children at high risk for asthma. N Engl J Med. 2006;354(19):1985–1997. doi: 10.1056/NEJMoa051378. [DOI] [PubMed] [Google Scholar]
- 24.Morgan W.J., Stern D.A., Sherrill D.L. Outcome of asthma and wheezing in the first 6 years of life: follow-up through adolescence. Am J Respir Crit Care Med. 2005;172(10):1253–1258. doi: 10.1164/rccm.200504-525OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Illi S., von Mutius E., Lau S. Perennial allergen sensitisation early in life and chronic asthma in children: a birth cohort study. Lancet. 2006;368(9537):763–770. doi: 10.1016/S0140-6736(06)69286-6. [DOI] [PubMed] [Google Scholar]
- 26.Krawiec M.E., Westcott J.Y., Chu H.W. Persistent wheezing in very young children is associated with lower respiratory inflammation. Am J Respir Crit Care Med. 2001;163(6):1338–1343. doi: 10.1164/ajrccm.163.6.2005116. [DOI] [PubMed] [Google Scholar]
- 27.Saglani S., Malmstrom K., Pelkonen A.S. Airway remodeling and inflammation in symptomatic infants with reversible airflow obstruction. Am J Respir Crit Care Med. 2005;171(7):722–727. doi: 10.1164/rccm.200410-1404OC. [DOI] [PubMed] [Google Scholar]
- 28.Rosenthal L.A., Amineva S.P., Szakaly R.J. A rat model of picornavirus-induced airway infection and inflammation. Virol J. 2009;6:122. doi: 10.1186/1743-422X-6-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leigh R., Oyelusi W., Wiehler S. Human rhinovirus infection enhances airway epithelial cell production of growth factors involved in airway remodeling. J Allergy Clin Immunol. 2008;121(5):1238–1245. doi: 10.1016/j.jaci.2008.01.067. [DOI] [PubMed] [Google Scholar]
- 30.Zhu L., Lee P.K., Lee W.M. Rhinovirus-induced major airway mucin production involves a novel TLR3-EGFR-dependent pathway. Am J Respir Cell Mol Biol. 2009;40(5):610–619. doi: 10.1165/rcmb.2008-0223OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Proud D., Turner R.B., Winther B. Gene expression profiles during in vivo human rhinovirus infection: insights into the host response. Am J Respir Crit Care Med. 2008;178(9):962–968. doi: 10.1164/rccm.200805-670OC. [DOI] [PubMed] [Google Scholar]
- 32.Bochkov Y.A., Hanson K.M., Keles S. Rhinovirus-induced modulation of gene expression in bronchial epithelial cells from subjects with asthma. Mucosal Immunol. 2009;3(1):69–80. doi: 10.1038/mi.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Olenec J.P., Kim W.K., Lee W.M. Weekly monitoring of children with asthma for infections and illness during common cold seasons. J Allergy Clin Immunol. 2010;125(5):1001–1006. doi: 10.1016/j.jaci.2010.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heymann P.W., Carper H.T., Murphy D.D. Viral infections in relation to age, atopy, and season of admission among children hospitalized for wheezing. J Allergy Clin Immunol. 2004;114(2):239–247. doi: 10.1016/j.jaci.2004.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gern J.E., Brooks G.D., Meyer P. Bidirectional interactions between viral respiratory illnesses and cytokine responses in the first year of life. J Allergy Clin Immunol. 2006;117(1):72–78. doi: 10.1016/j.jaci.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 36.Stern D.A., Guerra S., Halonen M. Low IFN-gamma production in the first year of life as a predictor of wheeze during childhood. J Allergy Clin Immunol. 2007;120(4):835–841. doi: 10.1016/j.jaci.2007.05.050. [DOI] [PubMed] [Google Scholar]
- 37.Papadopoulos N.G., Stanciu L.A., Papi A. A defective type 1 response to rhinovirus in atopic asthma. Thorax. 2002;57(4):328–332. doi: 10.1136/thorax.57.4.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brooks G.D., Buchta K.A., Swenson C.A. Rhinovirus-induced interferon-gamma and airway responsiveness in asthma. Am J Respir Crit Care Med. 2003;168(9):1091–1094. doi: 10.1164/rccm.200306-737OC. [DOI] [PubMed] [Google Scholar]
- 39.Wark P.A., Johnston S.L., Bucchieri F. Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J Exp Med. 2005;201(6):937–947. doi: 10.1084/jem.20041901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bufe A., Gehlhar K., Grage-Griebenow E. Atopic phenotype in children is associated with decreased virus-induced interferon-alpha release. Int Arch Allergy Immunol. 2002;127(1):82–88. doi: 10.1159/000048173. [DOI] [PubMed] [Google Scholar]
- 41.Gehlhar K., Bilitewski C., Reinitz-Rademacher K. Impaired virus-induced interferon-alpha2 release in adult asthmatic patients. Clin Exp Allergy. 2006;36(3):331–337. doi: 10.1111/j.1365-2222.2006.02450.x. [DOI] [PubMed] [Google Scholar]
- 42.Contoli M., Message S.D., Laza-Stanca V. Role of deficient type III interferon-lambda production in asthma exacerbations. Nat Med. 2006;12:1023–1026. doi: 10.1038/nm1462. [DOI] [PubMed] [Google Scholar]
- 43.Lopez-Souza N., Favoreto S., Wong H. In vitro susceptibility to rhinovirus infection is greater for bronchial than for nasal airway epithelial cells in human subjects. J Allergy Clin Immunol. 2009;123(6):1384–1390. doi: 10.1016/j.jaci.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Palmer C.N., Irvine A.D., Terron-Kwiatkowski A. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat Genet. 2006;38(4):441–446. doi: 10.1038/ng1767. [DOI] [PubMed] [Google Scholar]
- 45.Howell M.D., Kim B.E., Gao P. Cytokine modulation of atopic dermatitis filaggrin skin expression. J Allergy Clin Immunol. 2007;120(1):150–155. doi: 10.1016/j.jaci.2007.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Basu K., Palmer C.N., Lipworth B.J. Filaggrin null mutations are associated with increased asthma exacerbations in children and young adults. Allergy. 2008;63(9):1211–1217. doi: 10.1111/j.1398-9995.2008.01660.x. [DOI] [PubMed] [Google Scholar]
- 47.McLean W.H., Palmer C.N., Henderson J. Filaggrin variants confer susceptibility to asthma. J Allergy Clin Immunol. 2008;121(5):1294–1295. doi: 10.1016/j.jaci.2008.02.039. [DOI] [PubMed] [Google Scholar]
- 48.Jakiela B., Brockman-Schneider R., Amineva S. Basal cells of differentiated bronchial epithelium are more susceptible to rhinovirus infection. Am J Respir Cell Mol Biol. 2008;38(5):517–523. doi: 10.1165/rcmb.2007-0050OC. [DOI] [PMC free article] [PubMed] [Google Scholar]